Abstract
Background
In 2011 the World Health Organization (WHO) recommended parenteral artesunate in preference to quinine as first‐line treatment for people with severe malaria. Prior to this recommendation many countries, particularly in Africa, had begun to use artemether, an alternative artemisinin derivative. This Cochrane Review evaluates intramuscular artemether compared with both quinine and artesunate.
Objectives
To assess the efficacy and safety of intramuscular artemether versus any other parenteral medication in the treatment of severe malaria in adults and children.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (the Cochrane Library), MEDLINE, Embase, and LILACS, ISI Web of Science, conference proceedings, and reference lists of articles. We also searched the WHO International Clinical Trial Registry Platform, ClinicalTrials.gov, and the metaRegister of Controlled Trials (mRCT) for ongoing trials up to 7 September 2018. We checked the reference lists of all studies identified by the search. We examined references listed in review articles and previously compiled bibliographies to look for eligible studies.
Selection criteria
Randomized controlled trials (RCTs) comparing intramuscular artemether with intravenous/intramuscular quinine or artesunate for treating severe malaria.
Data collection and analysis
The primary outcome was all‐cause death. Two review authors independently screened each article by title and abstract, and examined potentially relevant studies for inclusion using an eligibility form. Two review authors independently extracted data and assessed risk of bias of included studies. We summarized dichotomous outcomes using risk ratios (RRs) and continuous outcomes using mean differences (MDs), and have presented both measures with 95% confidence intervals (CIs). Where appropriate, we combined data in meta‐analyses and used the GRADE approach to summarize the certainty of the evidence.
Main results
We included 19 RCTs, enrolling 2874 adults and children with severe malaria, carried out in Africa (12 trials) and in Asia (7 trials).
Artemether versus quinine
For children, there is probably little or no difference in the risk of death between intramuscular artemether and quinine (RR 0.97, 95% CI 0.77 to 1.21; 13 trials, 1659 participants, moderate‐certainty evidence). Coma resolution time may be about five hours shorter with artemether (MD −5.45, 95% CI −7.90 to −3.00; six trials, 358 participants, low‐certainty evidence). Artemether may make little difference to neurological sequelae (RR 0.84, 95% CI 0.66 to 1.07; seven trials, 968 participants, low‐certainty evidence). Compared to quinine, artemether probably shortens the parasite clearance time by about nine hours (MD −9.03, 95% CI −11.43 to −6.63; seven trials, 420 participants, moderate‐certainty evidence), and may shorten the fever clearance time by about three hours (MD −3.73, 95% CI −6.55 to −0.92; eight trials, 457 participants, low‐certainty evidence).
For adults, treatment with intramuscular artemether probably results in fewer deaths than treatment with quinine (RR 0.59, 95% CI 0.42 to 0.83; four trials, 716 participants, moderate‐certainty evidence).
Artemether versus artesunate
Artemether and artesunate have not been directly compared in randomized trials in children.
For adults, mortality is probably higher with intramuscular artemether (RR 1.80, 95% CI 1.09 to 2.97; two trials, 494 participants, moderate‐certainty evidence).
Authors' conclusions
Artemether appears to be more effective than quinine in children and adults. Artemether compared to artesunate has not been extensively studied, but in adults it appears inferior. These findings are consistent with the WHO recommendations that artesunate is the drug of choice, but artemether is acceptable when artesunate is not available.
16 September 2019
Up to date
All studies incorporated from most recent search
All published trials found in the last search (7 Sep, 2018) were included, and we did not identify any ongoing trials.
Plain language summary
Artemether injection for treating people with severe malaria
What is the aim of this review?
Injection of artesunate is recommended by the World Health Organization (WHO) for treating adults and children that have severe malaria as studies have shown that it results in fewer deaths compared to quinine treatment.
Artemether is an alternative artemisinin‐based medicine but is only available as a pre‐mixed oil‐based solution for intramuscular injection. Artemether is now widely available and is used in many African countries, although it is not specifically recommended by the WHO. The aim of this review was to examine the effects of treating people that have severe malaria with artemether injected intramuscularly compared to treatment with other antimalarial medicines given intramuscularly or intravenously.
Key messages
Artemether may not be more effective than quinine at preventing deaths from severe malaria in children. However, in adults artemether is probably more effective than quinine at preventing deaths. With respect to other patient‐oriented outcomes such as fever and parasite clearance time, artemether seems to be more effective than quinine in children and adults. For adults, artemether had a large effect on death compared to quinine but other outcomes were largely not reported or showed no significant difference. Artemether has not been compared to artesunate in children. Although there is a paucity of direct evidence comparing artemether with artesunate in adults, artemether probably increases the risk of death compared to artesunate. In settings where artesunate is not available, artemether remains a better alternative to quinine for the treatment of severe malaria.
What was studied in the review?
The review authors examined the available research that evaluated the effects of treating people that have severe malaria with artemether injected intramuscularly compared to treatment with other antimalarial medicines given intramuscularly or intravenously. Nineteen studies looked at the effects of treatment with intramuscular artemether on people with severe malaria compared to treatment with other antimalarial medicines given intramuscularly or intravenously. These studies were undertaken in Africa (12 studies) and Asia (seven studies). This is an update of a 2014 Cochrane Review and includes a new trial from Central African Republic.
What are the main results of the review?
Artemether versus quinine
For children, intramuscular artemether is probably as good as quinine at preventing deaths from severe malaria (moderate‐certainty evidence). Artemether may shorten time to coma resolution by about five hours (low‐certainty evidence), and may reduce the number of children with signs of brain damage at the time of hospital discharge (low‐certainty evidence).
In older children (> 15 years) and adults, treatment with artemether probably results in fewer deaths than quinine (moderate‐certainty evidence).
Artemether versus artesunate
In adults, artemether performs worse than artesunate in terms of mortality (moderate‐certainty evidence), but no trials have been conducted in young children.
How up to date is this review?
The review authors searched for studies up to 7 September 2018.
Summary of findings
Background
Description of the condition
Malaria is a febrile illness caused by Plasmodium parasites, which are transmitted to humans through the bite of infected female anopheline mosquitoes. Five species of Plasmodium cause this disease in humans, of which Plasmodium falciparum is the most common worldwide, and is responsible for almost all of the severe disease and deaths (WHO 2000; WHO 2008).
Severe malaria is diagnosed on the basis of a positive blood slide or antigen test for malaria, plus the presence of clinical or laboratory features of vital organ dysfunction. These include impaired consciousness, coma, convulsions, respiratory distress, shock (systolic blood pressure < 70 mmHg in adults, < 50 mmHg in children), jaundice, haemoglobinuria, hypoglycaemia, severe metabolic acidosis, and anaemia (WHO 2010). Cerebral malaria is one form of severe malaria, where the patient has some impairment of consciousness and cognition. This can vary from slight disorientation through to deep coma where the patient is unconscious and unrousable. Even with correct treatment cerebral malaria can cause a mortality rate of up to 20%, and a small proportion of people that survive infection can have persistent neurological sequelae (Jaffar 1997).
People living in malaria‐endemic regions can develop a naturally acquired immunity to malaria through repeated exposure to the parasite over five to 10 years (Doolan 2009). This partial immunity is protective against the most severe forms of the disease, and as a consequence, in high transmission settings mortality from severe malaria is highest in young children and decreases with increasing age (WHO 2010).
Parenteral quinine used to be the standard treatment for severe malaria despite sporadic observations of resistance. Clinical and in vitro resistance to quinine has been observed in Southeast Asia, but not consistently in Africa (Wongsrichanalai 2002; Cui 2015). Artemisinin derivatives have become the keystone of malaria treatment and control (WHO 2015). They are effective at killing all asexual blood stages of P falciparum parasites, as well as disrupting sexual development, resulting in rapid clinical and parasitological cure and a reduction in malaria transmission rates (Tyagi 2018).
Evidence of resistance to artemisinin combination therapy has been reported for about a decade, initially in the Thai‐Cambodia border region and now increasingly in Southeast Asia. Resistance has been characterized by delayed parasite clearance time in patients with uncomplicated and severe malaria (Ashley 2014; Hawkes 2014; Menard 2016)
The World Health Organization (WHO) strongly recommends parenteral artesunate as the drug of choice and artemether in preference to quinine for the treatment of severe malaria in adults and children, followed by a complete course of an effective artemisinin‐based combination therapy (ACT) as soon as the patient can take oral medications (WHO 2015). The WHO based their recommendation of parenteral artesunate on evidence from two large multi‐centre clinical trials that demonstrated the superiority of intravenous artesunate over the standard treatment, quinine (Dondorp 2005; Dondorp 2010). A Cochrane Review of available data concluded that treating people that have severe malaria with artesunate instead of quinine would reduce the risk of death by 39% in adults and 24% in children (Sinclair 2012).
Description of the intervention
Artesunate is only one of a number of antimalarials derived from artemisinin, which is extracted from the herb Artemesia annua and is the active ingredient in a Chinese herbal remedy for fever. Once ingested or injected, artemisinin derivatives undergo conversion to dihydroartemisinin, the active metabolite, which has a broad spectrum of activity against the blood stage asexual Plasmodium parasites (ter Kuile 1993; Navaratnam 2000). Artemisinin derivatives clear parasites from the peripheral blood quicker than other antimalarials, but only artesunate has been shown to impact mortality.
Unlike artesunate, artemether is poorly soluble in water and the parenteral formulation is only available as a pre‐mixed oil‐based solution for intramuscular injection (80 mg/mL for use in adults and 40 mg/mL for children). The standard dose is 3.2 mg/kg on admission followed by 1.6 mg/kg once daily until oral therapy is tolerated (WHO 2010). Peak plasma concentrations typically occur around six hours after intramuscular injection, but in severely ill children with poor peripheral perfusion, absorption can be highly erratic (Karbwang 1997; Murphy 1997; Mithwani 2004).
Conversely, artesunate is supplied as a dry powder for mixing with sodium bicarbonate prior to either intravenous or intramuscular injection (WHO 2010). The absorption of artesunate is more reliable compared with artemether, with peak plasma concentrations following intramuscular injection occurring at around one hour (Illet 2002; Nealon 2002; Hien 2004). These more favourable pharmacokinetic properties of artesunate moved research attention away from artemether; and artesunate now has a stronger evidence base and is the preferred therapy (WHO 2010; Sinclair 2012).
How the intervention might work
Deaths from severe malaria often occur between one and two days following hospital admission. Consequently, to be effective, antimalarial drugs need to achieve rapid therapeutic blood concentrations following administration. Artemisinins, especially artesunate and artemether, result in more rapid parasite clearance (being active on gametocytes (immature forms) and are safer and simpler to administer (ter Kuile 1993; Adjuik 2004). Artemether is a potent and rapidly acting blood schizonticide which is highly efficacious in treating complicated P falciparum malaria including cerebral malaria. The drug is given by intramuscular injection in the gluteal muscle. Its quick onset of action and high efficacy in bringing down the parasite load are the properties which make this drug a suitable therapeutic option against P falciparum infection. Artemether may be an excellent alternative for treatment of severe malaria and cerebral malaria in rural areas where facilities for intravenous administration may not yet be optimal and might increase the risk of infection.
Why it is important to do this review
A number of African countries incorporated intramuscular artemether into their national guidelines prior to the WHO recommendation for artesunate. Systematic reviews concluded that intramuscular artemisinin derivatives (including both artesunate and artemether) were not inferior to quinine in preventing deaths from malaria, but were safer and easier to administer (McIntosh 2000; AQMSG 2001; Kyu 2009).
Following the WHO recommendation for artesunate as the preferred treatment for severe malaria, there is a need to re‐evaluate the role of intramuscular artemether in the management of severe malaria in adults and children.
Objectives
To assess the efficacy and safety of intramuscular artemether versus any other parenteral medication in the treatment of severe malaria in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Adults and children (< 15 years of age) with severe malaria.
Types of interventions
Intervention
Intramuscular artemether
Control
Any other parenteral medication for the treatment of severe malaria
Types of outcome measures
Primary outcomes
Death from any cause
Secondary outcomes
Coma resolution time
Neurological sequelae (such as blindness, deafness, hemiplegia and others)
Time to hospital discharge
Parasite clearance time
Fever clearance time
Need for blood transfusion
Severe anaemia
Adverse events (including hypoglycaemia, tinnitus, nausea, vomiting, haematological and cardiac‐related adverse events)
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press and in progress).
Electronic searches
Databases
We examined the following databases up to 7 September 2018 using the search terms detailed in Appendix 1: Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library; MEDLINE (PubMed); Embase, LILACS and ISI Web of Science. We also searched the WHO clinical trial registry platform, ClinicalTrials.gov and the metaRegister of Controlled Trials (mRCT) up to 7 September 2018 for ongoing trials using ‘artemether', ‘severe malaria', ‘complicated malaria', ‘artesunate', ‘arteether', and ‘child*' as search terms.
Searching other resources
Conference proceedings
We searched relevant proceedings of the following meetings for trial information: Multilateral Initiative on Malaria (MIM) Pan‐African Malaria Conference; European Congress on Tropical Medicine and International Health; and American Society of Tropical Medicine and Hygiene (7 September 2018).
Researchers
We contacted researchers working in the field and the WHO for unpublished and ongoing trials.
Reference lists
We checked the reference lists of existing reviews and of all trials identified by the above methods.
Data collection and analysis
Selection of studies
Two review authors screened the abstract of each title obtained from the search for potentially relevant studies. We retrieved the corresponding full articles of these identified studies, and two review authors assessed inclusion by using an eligibility form. We independently screened each search result, assessed each article, and resolved any discrepancies between eligibility results through discussion. Also, we listed the excluded studies and the reasons for their exclusion.
Data extraction and management
Two review authors independently extracted data from each study report onto a pre‐designed data extraction form. We discussed any discrepancies with a third review author. We contacted the corresponding publication author in the case of unclear information or missing data. For each outcome we aimed to extract the number of participants randomized and the number analysed in each treatment group. For dichotomous outcomes, we recorded the number of participants experiencing the event and the number assessed in each treatment group. For continuous outcomes, we extracted arithmetic means and standard deviations for each treatment group, together with the numbers assessed in each group.
Where baseline proportions of participants in the intervention and control arms in whom antipyretics were administered varied, we only included trials that reported fever clearance time and provided additional information about antipyretics use at baseline for participants in both intervention and control arm to avoid confounding in the summary estimate for fever clearance. Where there was significant difference between antipyretic use at baseline in intervention and control arms, we only reported fever clearance time in a table. We defined cure rates in this review as time from first dose to first negative parasite reading for two consecutive readings.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study using Cochrane's ‘Risk of bias' tool (Higgins 2011). We followed the guidance for making judgements on the risk of bias in six domains: sequence generation; allocation concealment; blinding (of participants, personnel and outcome assessors); incomplete outcome data; selective outcome reporting; and other risk of bias (such as the trial stopped early). We categorized these judgements as low risk of bias, high risk of bias, or unclear risk of bias.
Measures of treatment effect
We calculated results using risk ratios for dichotomous data, and mean difference values for continuous data, and have presented these effect estimates with 95% confidence intervals (CIs). We treated time‐to‐event outcomes as continuous data and accordingly mean difference calculated from mean time in intervention versus control groups. Where the data were not normally distributed and medians were reported, we excluded them from the meta‐analysis and reported them in additional tables.
Unit of analysis issues
For multiple arm trials, we combined all relevant experimental intervention groups of the trial into a single group, and also combined all relevant control intervention groups into a single control group. For dichotomous outcomes, both the sample sizes and the numbers of people with events were added across groups. For continuous outcomes, we combined means and standard deviations using methods described in the Cochrane Handbook for Systematic Reviews of Interventions.
Dealing with missing data
We analysed data according to the intention‐to‐treat principle (all randomized participants should be analysed in the groups to which they were originally assigned). If there were discrepancies between the number randomized and the number analysed, we calculated the percentage loss to follow‐up for each treatment group and reported this information.
However, if for some trials it was unclear whether there was loss to follow‐up, we entered the number analysed into Review Manager 2014 whenever these figures were available. By attempting to carry out a complete case analysis, we avoided making assumptions about the outcomes of participants lost to follow‐up. Where possible, we contacted authors for missing data.
Assessment of heterogeneity
We looked for statistical heterogeneity by inspecting the forest plots for overlapping CIs, applying the Chi2 test (P < 0.10 considered statistically significant) and the I2 statistic (I2 value < 50% used to denote moderate levels of heterogeneity).
Assessment of reporting biases
Since asymmetry of funnel plots may result from publication bias, heterogeneity, or poor methodological quality (Sterne 2011), we planned to examine funnel plots using Review Manager 5 (RevMan 5) but found an insufficient number of trials to do this (Review Manager 2014).
Data synthesis
We analysed the data using RevMan 5 (Review Manager 2014). In the first instance, we applied a fixed‐effect meta‐analysis. However, if we detected moderate heterogeneity but still considered it appropriate to combine the trials, we then used a random‐effects approach. Where heterogeneity was very high such that meta‐analysis was not appropriate, we displayed the results in forest plots or tables but did not combine the results. Where data were only presented as medians and ranges, we have presented the results in tables.
For both dichotomous and continuous outcomes, optimal information size was calculated. The optimal information size (OIS) can be defined as the minimum amount of information needed in a meta‐analysis to obtain reliable conclusions about an intervention. The optimal information size calculations were performed using a power calculator available at www.sealedenvelope.com/power. All calculations were performed for a power of 80% and an α error of 0.05 and the proportion in the control groups were taken from the median control group risk across trials. Also, a maximum 3% risk difference was chosen to represent equivalence (Table 4). For the continuous outcomes (coma resolution time, parasite clearance time, and fever clearance time) a six‐hour time difference was chosen to represent a clinically important benefit (Table 5).
1. Optimal information size calculations; dichotomous outcomes.
| Outcome | Type of test | Proportion in control groupa | Proportion in Intervention group | Estimated RR | Total sample sizeb,c |
| Death | Superiority | 0.15 | 0.12 | 0.80 | 4068 |
| Equivalence | 0.15 | 0.12 to 0.18d | ‐ | 5956 | |
| Neurological sequelae | Superiority | 0.25 | 0.20 | 0.80 | 2184 |
| Equivalence | 0.25 | 0.22 to 0.28d | ‐ | 8760 |
aThe proportion in the control group is taken from the median control group risk across trials. bThese calculation were performed using a power calculator available at www.sealedenvelope.com/power cAll calculations were performed for a power of 80% and an α error of 0.05. dA maximum 3% risk difference was chosen to represent equivalence.
2. Optimal information size calculations; continuous outcomes.
| Outcome | Type of test | Mean in control groupa | Mean in Intervention groupb | SD of outcome | Total sample sizec,d |
| Coma resolution time | Superiority | 25 | 19 | 20 | 350 |
| Equivalence | 25 | 19 to 31 | 20 | 382 | |
| Parasite clearance time | Superiority | 42 | 36 | 20 | 350 |
| Equivalence | 42 | 36 to 48 | 20 | 382 | |
| Fever clearance time | Superiority | 48 | 42 | 20 | 350 |
| Equivalence | 48 | 36 to 54 | 20 | 382 |
aThe mean in the control group is taken from the median control group across studies. bA six‐hour time difference was chosen to represent a clinically important benefit. cThese calculations were performed using a power calculator available at: www.sealedenvelope.com/power dAll calculation were performed for a power of 80% and an α error of 0.05.
Certainty of the evidence
We assessed the certainty of the evidence following the GRADE approach and defined ‘certainty' as an assessment of our confidence in the estimates of effect (Guyatt 2008). We rated each outcome as follows.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
As all the included studies were RCTs the evidence for each outcome started as high certainty, but we would downgrade if there were valid reasons to do so within the following five categories: risk of bias; imprecision; inconsistency; indirectness; and publication bias (Balshem 2011). We summarized the certainty of the evidence for each outcome in a ‘Summary of findings’ table.
Subgroup analysis and investigation of heterogeneity
We grouped the analysis and results by children and adults. We reported results by whether the studies were carried out in Africa or in Asia. We examined whether loading dose or quinine influenced outcomes.
Sensitivity analysis
We conducted a sensitivity analysis to investigate the robustness of the results to the risk of bias components by including only trials that concealed the allocation and had low incomplete outcome data (< 10%).
Results
Description of studies
See the Characteristics of included studies section for details of the included trials.
Results of the search
We conducted the literature search up to 7 September 2018 and identified 87 references (see Figure 1).
1.
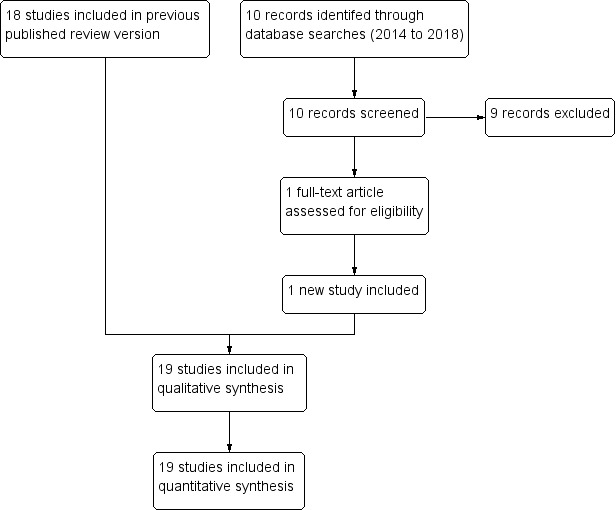
PRISMA diagram.
In the previous version of this Cochrane Review, we identified 77 titles and abstracts from the electronic search of databases and 2 additional articles after contacting researchers and screening reference lists (Esu 2014). After we removed duplicates, 77 records remained. Of these, we obtained 35 potentially eligible articles. We identified 18 studies that fulfilled the selection criteria and reported on outcomes of interest.
For this review update, we identified 10 additional titles and abstracts from electronic searches of databases. There were 87 articles after we removed duplicates. Of these, there was only one potentially eligible article. This new study met the inclusion criteria (Bobossi‐Serengbe 2015).
Included studies
In the previous version of this review, we included 18 RCTs, enrolling 2662 participants. Twelve trials enrolled children only (1447 participants aged between six months and 15 years), and six trials enrolled older children and adults (1215 participants aged between 13 and 79 years).
In this review update, we included one additional RCT enrolling 212 participants (children aged between six and 59 months) (Bobossi‐Serengbe 2015).
Location
The trials in children were primarily conducted in Africa: Nigeria (five trials), Sudan (two trials), Central African Republic (one trial), the Gambia (one trial), Kenya (one trial), Malawi (one trial), and Mali (one trial); with only one trial from Asia (India).
Five adult trials were conducted in Asia: Vietnam (three trials), and Thailand (two trials); and one in Oceania; Papua New Guinea (one trial). We have attached a three‐letter country code to each trial ID to aid forest plot interpretation.
Interventions
All 13 trials in children compared artemether with quinine. In most trials, artemether was given by intramuscular injection, with a loading dose of 3.2 mg/kg body weight followed by maintenance doses of 1.6 mg/kg for three to six days (see Table 6 for details). Only three trials followed this with oral therapy once tolerated (Murphy 1996; van Hensbroek 1996; Taylor 1998). For quinine, nine trials administered the WHO recommended loading dose of 20 mg/kg of intravenous or intramuscular quinine followed by a maintenance dose of 10 mg/kg. However, van Hensbroek 1996 administered the maintenance dose at 12‐hourly intervals instead of eight‐hour intervals (see Table 6).
3. Characteristics of trials comparing artemether and quinine in children.
| Trial ID | Year of study | Age limits | Quinine dosing schedule | Artemether dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| Adam 2002 | 2002 | ‘Children' | 20 mg/kg IV | 10 mg/kg IV every 8 hours for 72 hours | Oral quinine for 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None |
| Aguwa 2010 | 2007 | 6 months to 12 years | 20 mg/kg IV or IM | 10 mg/kg IV/IM every 8 hours | None | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 2 days | None |
| Bobossi‐Serengbe 2015 | 2010 | 6 to 59 months | 10 mg/kg IV | 10 mg/kg IV every 4 hours | Oral quinine for 7 days | 2 mg/kg IM twice daily | 2 mg/kg IM once daily for 2 days | None |
| Huda 2003 | 2001 | < 14 years | 20 mg/kg IV | 10 mg/kg IV every 8 hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 5 days | None |
| Minta 2005 | 2004 | 3 months to 15 years | 20 mg/kg IV | 10 mg/kg IV every 8 hours | Quinine 10 mg/kg every 8 hours | 3.2 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None |
| Murphy 1996 | 1996 | 5 months to 12 years | 20 mg/kg IV | 10 mg/kg IV every 8 hours | SP once | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | SP once |
| Ojuawo 1998 | 1998 | Mean age about 4 years | 10 mg/kg IV | 10 mg/kg IV every 8 hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM 12 hrs later, then once daily for 2 days | None |
| Olumese 1999 | 1999 | 11 months to 5 years | 20mg/kg IV | 10mg/kg IV every 8 hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None |
| Osonuga 2009 | 2009 | 1 to 12 years | 10 mg/kg IV | 10 mg/kg IV every 8 hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None |
| Satti 2002 | 1996 | 3 months to 15 years | 10 mg/kg IV | 10 mg/kg IV every 8 hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None |
| Taylor 1998 | 1994 | Mean age of 3 years | 20 mg/kg IV | 10 mg/kg IV every 8 hours for at least 2 doses | SP once | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 2 days at least | SP once |
| van Hensbroek 1996 | 1994 | 1 to 9 years | 20 mg/kg IV | 10 mg/kg IV every 12 hours | Quinine to complete 5 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 3 days | SP oncea |
| Walker 1993 | 1993 | 1 to 5 years | 20 mg/kg IV | 10 mg/kg IV every 8 hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None |
Abbreviations: IM = intramuscular; IV = intravenous; SP = sulphadoxine‐pyrimethamine. aOnly in the second and third years of the study.
In adults, four trials compared artemether with quinine (Karbwang 1992; Karbwang 1995; Hien 1996; Seaton 1998); and two trials compared artemether with artesunate (Vinh 1997; Phu 2010). Artemether was given intramuscularly over three to seven days with slight variations in dosing (see Table 7 and Table 8); and all four trials administered quinine at the WHO‐recommended loading dose.
4. Characteristics of trials comparing artemether and quinine in adults.
| Trial ID | Year of study | Age limits | Quinine dosing schedule | Artemether dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| Hien 1996 | 1996 | 15 to 79 years | 20 mg/kg IM | 10 mg/kg IM every 8 hours | Quinine or mefloquine to complete 7 days | 4 mg/kg IM | 2 mg/kg IM once daily for 4 days | Quinine or mefloquine to complete 7 days |
| Karbwang 1992 | 1991 | 15 to 45 years | 20 mg/kg IV | 10 mg/kg every 8 hours for 7 days | Quinine to complete 7 days | 160 mg IM | 80 mg IM once daily for 6 days | None |
| Karbwang 1995 | 1994 | 15 to 55 years | 20 mg/kg IV | 10 mg/kg every 8 hours for 7 days | Quinine to complete 7 days | 160 mg IM | 80 mg IM once daily for 6 days | None |
| Seaton 1998 | 1995 | > 12 years | 20 mg/kg IV | 10 mg/kg IV every 8 hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None |
Abbreviations: IM = intramuscular; IV = intravenous.
5. Characteristics of studies comparing artemether and artesunate in adults.
| Trial ID | Year of study | Age limits | Artemether dosing schedule | Artesunate dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| Phu 2010 | 2003 | 15 to 77 years | 3.2 mg/kg IM | 1.6 mg/kg IM daily | None | 2.4 mg/kg IM | 1.2 mg/kg IM once daily | 2 mg/kg of artesunate to complete 7 days |
| Vinh 1997 | 1994 | 15 to 66 years | 200 mg IM | 100 mg IM once daily for 3 days | Mefloquine once | 120 mg IM or IV | 60 mg IM or IV once daily for 3 days | Mefloquine once |
Abbreviations: IM = intramuscular; IV = intravenous.
Supportive care
Twelve trials in children reported measurement of blood glucose on admission, but only nine trials reported any subsequent active monitoring for hypoglycaemia.
Only one trial in adults reported measuring blood glucose on admission and monitored hypoglycaemia up to 24 hours after admission (Hien 1996).
Outcome measures
Eighteen trials reported death, a measure of coma resolution, fever clearance and parasite clearance as outcomes. Eleven trials reported neurological sequelae at discharge. Only two trials reported duration of hospital stay (Aguwa 2010; Phu 2010); and two trials reported on the number of children requiring blood transfusions (Hien 1996; Olumese 1999). Twelve trials reported on adverse events including episodes of hypoglycaemia (Karbwang 1992; Walker 1993; Karbwang 1995; Hien 1996; Murphy 1996; van Hensbroek 1996; Seaton 1998; Adam 2002; Huda 2003; Minta 2005; Phu 2010; Bobossi‐Serengbe 2015). We have listed the outcome definitions used in the included trials in Table 9.
6. Definitions of outcome measures used in the review.
| Trial ID | Coma resolution time | Fever clearance time | Parasite clearance time | Hypoglycaemia |
| Adam 2002 | Mean value (h) reported and defined as a Blantyre coma score of 5 recorded for at least 24 hours | Mean value (h) reported and defined as the time after which the temperature remained normal (axillary temperature < 37.5 °C) | Mean value (h) reported and defined as the time passed from admission and start of treatment until 2 consecutive negative smears. Blood films repeated every 8 hours. | Number of episodes (n/N) reported but not defined |
| Aguwa 2010 | Proportions with coma resolution on D3 reported but not defined | Proportions with fever clearance on D3 and D14 reported and defined as body temperature ≤ 37.5 °C after commencement of treatment | Proportions with parasite clearance on D3 and D14. Parasite clearance was taken as adequate clinical and parasitological response (ACPR) at days 3 and 14. Parasite count taken on D0, D3 and D14. | Not reported |
| Bobossi‐Serengbe 2015 | Not reported | Not reported | Proportions with parasite clearance on D3 and D7 reported but not defined. | Not reported |
| Hien 1996 | Median value (h) reported and defined as the time to reach a score of 15 on the Glasgow Coma Scale | Median value (h) reported but not defined. | Median value (h) reported and defined as the time passed from admission and start of treatment until 3 consecutive negative blood smears. Blood films repeated every 4 hours for the first 24 hours and then every 6 hours. | Number of episodes (n/N) reported but not defined |
| Huda 2003 | Glasgow coma scale was used in grading the level of consciousness of the patients every 8 hours | Mean value (h) reported and defined as time to clearance of fever | Mean value (h) reported but not defined | Not reported |
| Karbwang 1992 | Unclear if values reported are means or medians (h) | Mean value (h) reported and defined as time for the temperature to fall below 37.5 °C and remain that value for 72 hours | Mean value (h) reported and defined as the time for the parasite count to fall below the level of microscopic detection (thick film) | Not reported |
| Karbwang 1995 | Median value (h) reported and defined as the time taken for the patients to recover completely from unconciousness | Mean value (h) reported and defined as time for the temperature to fall below 37.5 °C and remain that value for 72 hours | Median value (h) reported and defined as the time taken for parasite count to fall below the level of microscopic detection (thick film) | Not reported |
| Minta 2005 | Mean value (h) reported and defined as the time to normalization of consciousness | Mean value (h) reported but not defined | Mean value (h) reported and defined as time till negative parasitaemia result | Not reported |
| Murphy 1996 | Median value (h) reported but not described | Median value (h) reported but not described | Median value (h) reported but not described. Every 4 hours until clearance | Not reported |
| Ojuawo 1998 | Mean value (h) reported and defined as the interval between onset of therapy and the attainment of full consciousness | Mean value (h) reported and defined as the interval between the onset of therapy and the time the body temperature is ≤ 37 °C and remained so | Defined as: 2 successive thick blood films done at 12 hours interval are negative for asexual forms of Plasmodium species | Not reported |
| Olumese 1999 | Mean value (h) reported and defined as time to regain full consciousness | Mean value (h) reported and defined as the time for temperature to fall below 37.5 °C and remain so for at least 48 hours | Mean value (h) reported and defined as the time from start of drug administration to the first of 2 consecutive negative thick smears remaining negative until day 7 | Not reported |
| Osonuga 2009 | Mean value (h) reported and defined as time to attainment of a Blantyre score of 5 for at least 24 hours from initiation of treatment | Mean value (h) reported but not defined | Mean value (h) reported but not defined. Thick and thin film done on D0 and repeated on Days 3, 7 and 14 | Not reported |
| Phu 2010 | Median value (h) reported and defined as time to Glasgow Coma Score of 15. | Median value (h) reported and defined as the time for temperature to fall below 37.5 °C and remain so | Median value (h) reported and defined as the time to clear all parasites | Number of episodes (n/N) reported but not defined |
| Satti 2002 | Mean value (h) reported and defined as time to regaining consciousness | Mean value (h) reported and defined as the time for temperature to fall below 37.5 °C | Mean value (h) reported and defined as time to clear parasites measured every 6 hours till clearance | Not reported |
| Seaton 1998 | Median value (h) reported but not defined | Median value (h) reported and defined as a temperature < 37.5 °C on 2 successive readings | Median value (h) reported and defined as the time at which the blood films were negative for P falciparum for at least 8 hours | Number of episodes (n/N) reported but not defined |
| Taylor 1998 | Median value (h) reported and defined as time required for a child to achieve a Blantyre Coma Score of 5 | Median value (h) reported and defined as the time at which the rectal or axillary temperature dropped below 37.5 °C and remained < 37.5 °C for 24 consecutive hours | Median value (h) reported and defined as the time at which the first of 2 negative (0 parasites/200 WBC) thick blood films was prepared. Every 4 hours till clearance | Not reported |
| van Hensbroek 1996 | Median value (h) reported and defined as time to regain full consciousness | Median value (h) reported and defined as time needed for the rectal temperature to fall below 38.0 °C for at least 24 hours | Median value (h) reported and defined as time needed for all parasites to clear relative to parasite density at admission and assessed every 12 hours till clearance | Number of episodes (n/N) reported and defined as a blood glucose level below 40 mg/dL (2.2 mmol/L) |
| Vinh 1997 | Median value (h) reported and defined as time to regain full consciousness | Median value (h) reported and defined as time for axillary temperature to fall ‒ and remain for ≥ 24 hours ‒ to 37.5 °C or lower | Median value (h) reported and defined as time to clear parasites | Not reported |
| Walker 1993 | Mean value (h) reported but not defined | Mean value (h) reported | Mean value (h) reported and defined as the time for parasitaemia to be cleared and to remain so up to Day 7. Assessed every 6 hours during period of coma and then every 12 hours. | Not reported |
Abbreviations: WBC = white blood cell.
Excluded studies
We excluded 14 trials and listed the reasons for their exclusion in the ‘Characteristics of excluded studies' section.
Risk of bias in included studies
See Figure 2 for a summary of the ‘Risk of bias' assessments. We have presented further details in the Characteristics of included studies tables.
2.
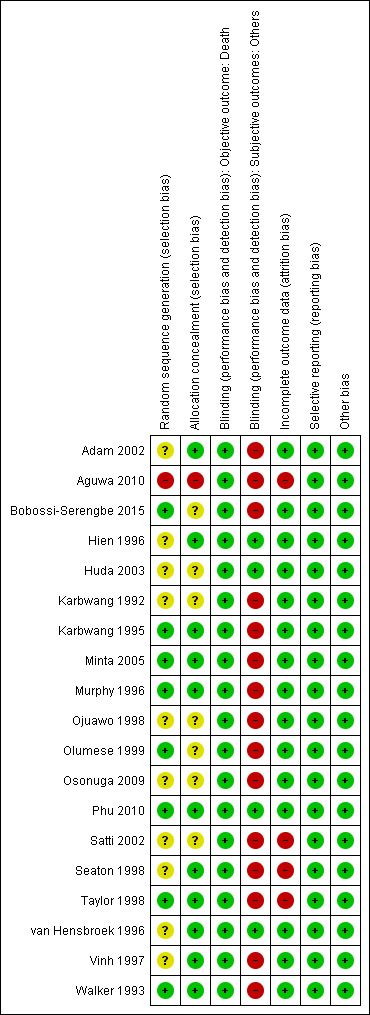
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Eight trials were at low risk of bias regarding the generation of allocation sequence while one trial was at high risk of bias (Aguwa 2010). Ten trials were at unclear risk of bias because review authors did not provide enough information to permit us to make a judgement.
Eleven trials were at low risk of bias regarding allocation concealment and the remaining trials provided insufficient information to make a judgement.
Blinding
In all trials except Hien 1996 and Phu 2010, investigators and participants were aware of treatment allocation. Participants were also not blind to the intervention as two different routes (intramuscular (artemether) and intravenous (quinine)) were used to administer the interventions. Blinding was unlikely to affect the assessment of the outcome ‘death' in all trials. In one trial, microscopists were blinded to the intervention and clinical status of the patients (Huda 2003). The other subjective outcomes were thus at high risk of bias in all open included trials or at unclear risk of bias where trials did not provide information.
Incomplete outcome data
Fifteen trials reported no losses to follow‐up. The remaining four trials reported over 10% attrition in either one or both trial arms (Seaton 1998; Taylor 1998; Satti 2002; Aguwa 2010). Two trials used the ‘per protocol' number of participants as a denominator in the analysis (Seaton 1998; Taylor 1998). The other two trials used the number of participants randomized as the denominator in the analysis.
Selective reporting
We did not detect any evidence of selective outcome reporting based on comparison between the Methods section and results of the included studies.
Other potential sources of bias
We did not identify any other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. ‘Summary of findings' table 1.
| Artemether compared with quinine for treating children with severe malaria | ||||||
| Patient or population: children with severe malaria Settings: malaria‐endemic countries Intervention: intramuscular artemether Comparison: intravenous or intramuscular quinine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with quinine | Risk with artemether | |||||
| Death | 151 per 1000 | 147 per 1000 (116 to 183) | RR 0.97 (0.77 to 1.21) | 1659 (13 trials) | ⊕⊕⊕⊝
MODERATEa,b,c,d Due to imprecision |
Artemether probably makes little or no difference to death compared to quinine. |
| Coma resolution time | The mean coma resolution time ranged across control groups from 17.4 to 42.4 hours | The mean coma resolution time in the intervention groups was 5.45 hours shorter (7.90 to 3.00 shorter) | ‐ | 358 (6 trials) | ⊕⊕⊝⊝
LOWc,e,f,g Due to risk of bias |
Artemether may reduce coma resolution time compared to quinine. |
| Neurological sequelae at discharge | 220 per 1000 | 185 per 1000 (145 to 235) | RR 0.84 (0.66 to 1.07) | 968 (7 trials) | ⊕⊕⊝⊝
LOWa,b,c,h Due to imprecision |
Artemether may lead to fewer episodes of neurological sequelae. However, the effects of artemether vary and it is possible that artemether may lead to more neurological sequelae |
| Parasite clearance time | The mean parasite clearance time ranged across control groups from 22.4 to 61.25 hours | The mean parasite clearance time in the intervention groups was 9.03 hours shorter (11.43 to 6.63 shorter) | ‐ | 420 (7 trials) | ⊕⊕⊕⊝
MODERATEa,c,g,i Due to inconsistency |
Artemether probably reduces parasite clearance time compared to quinine. |
| Fever clearance time | The mean fever clearance time ranged across control groups from 18 to 61.25 hours | The mean fever clearance time in the intervention groups was 3.73 shorter (6.55 to 0.92 shorter) | ‐ | 457 (8 trials) | ⊕⊕⊝⊝
LOWc,j,k,l Due to risk of bias and inconsistency |
Artemether may reduce fever clearance time compared to quinine. |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aNo serious risk of bias: trials were variable in their risk of bias, but exclusion of the trials at high or unclear risk of selection bias did not change this result. bNo serious inconsistency: none of the individual trials found statistically significant effects, and there was no statistical heterogeneity between trials. cNo serious indirectness: trials were from West Africa, East Africa, Central Africa and one from India. All were in children with severe malaria (aged under 15 years), and most compared the standard dose of intramuscular artemether with the WHO recommended dose of intravenous quinine. dDowngraded by 1 for serious imprecision: these trials, and the overall meta‐analysis are underpowered to detect a difference or to prove equivalence. eDowngraded by 2 for serious risk of bias: four of the six trials were at unclear risk of selection bias. When these four trials are excluded the result becomes non‐significant. fNo serious inconsistency: statistically significant differences were only seen in two of the six trials. However, statistical heterogeneity between trials was low and the overall meta‐analysis is statistically significant. gNo serious imprecision: the result is statistically significant and the overall meta‐analysis is adequately powered to detect this effect. hDowngraded by 2 for very serious imprecision: these trials, and the overall meta‐analysis are underpowered to detect a difference or to prove equivalence. The 95% CI is very wide and includes clinically important differences and no effect. iDowngraded by 1 for serious inconsistency: the mean difference in parasite clearance time ranged from a two hour increase with artemether to a 15 hour decrease. jDowngraded by 1 for serious risk of bias: four of the seven trials were at unclear risk of selection bias. When these four trials are excluded the result becomes non‐significant. kDowngraded by 1 for serious inconsistency: the mean difference in fever clearance time ranged from a 25‐hour increase with artemether to an 18‐hour decrease. lNo serious imprecision: the overall meta‐analysis is powered to detect this effect. The result is statistically significant but may not be clinically important.
Summary of findings 2. ‘Summary of findings' table 2.
| Artemether compared with quinine for treating adults with severe malaria | ||||||
| Patient or population: adults with severe malaria Settings: malaria‐endemic countries Intervention: intramuscular artemether Comparison: intravenous or intramuscular quinine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with quinine | Risk with artemether | |||||
| Death | 208 per 1000 | 123 per 1000 (87 to 173) | RR 0.59 (0.42 to 0.83) | 716 (4 trials) | ⊕⊕⊕⊝
MODERATEa,b,c,d Due to imprecision |
Artemether probably reduces the risk of death compared to quinine. |
| Coma resolution time | ‐ | ‐ | Not pooled. Little difference. | 657 (2 trials) | ⊕⊕⊝⊝
LOWa,e,f,g Due to inconsistency and imprecision |
Artemether may make little or no difference to coma resolution time compared to quinine. |
| Neurological sequelae at discharge | 4 per 1000 |
12 per 1000 (1 to 111) |
RR 2.92 (0.31 to 27.86) | 560 (1 trial) | ⊕⊕⊝⊝
LOWg,h Due to imprecision |
Artemether may increase the risk of neurological sequelae compared to quinine. However, the effects vary and it is possible artemether decreases neurological sequelae. |
| Parasite clearance time | ‐ | ‐ | Not pooled. Little difference apparent. | 716 (4 trials) | ⊕⊕⊕⊝
MODERATEa,c,f,i Due to imprecision |
Artemether probably makes no difference to parasite clearance time compared to quinine. |
| Fever clearance time | ‐ | ‐ | Not pooled. Little difference apparent. | 716 (4 trials) | ⊕⊕⊝⊝
LOWa,c,f,j Due to inconsistency and imprecision |
Artemether may make little or no difference to parasite clearance time compared to quinine. |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aNo serious risk of bias: trials are generally well conducted and at low risk of bias. bNo serious inconsistency: statistically significant differences were only seen in one of the four trials. However, statistical heterogeneity between trials was low and the overall meta‐analysis is statistically significant. cNo serious indirectness: all four trials compared intramuscular artemether with intravenous quinine in adults; two trials from Thailand, one each from Vietnam and Papua New Guinea dDowngraded by 1 for serious imprecision: these trials, and the overall meta‐analysis are very underpowered to detect a difference in mortality or to prove equivalence. eHien 1996 and Karbwang 1995 reported median coma time for artemether versus quinine (Hien 1996: 66 versus 48, P = 0.003; Karbwang 1995: 48 versus 48). Downgraded by 1 for inconsistency: one trial found a shorter median coma resolution time with quinine, and one trial found no difference. fDowngraded by 1 for imprecision: the data could not be pooled. gNo serious risk of bias: this single trial was at low risk of bias. hDowngraded by 2 for very serious imprecision: neurological sequelae in adults were uncommon. This trial is underpowered to detect or exclude clinically important differences. iTwo trials found no significant difference between parasite clearance time for artemether versus quinine (Karbwang 1992: mean 63.6 versus 61.6, P = 0.85; and Seaton 1998: median 48 versus 52, P = 0.381). Two other trials reported significantly shorter median parasite clearance times for artemether versus quinine (Hien 1996: 72 versus 90, P < 0.001 and Karbwang 1995: 54 versus 78, P = 0.007). No serious inconsistency: The two largest trials both found shorter median clearance times with artemether. jThree trials reported median fever clearance time for artemether versus quinine (127 versus 90, P < 0.001; 32 versus 48, P = 0.034 and 79 versus 84, no significant difference) (Hien 1996, Seaton 1998 and Karbwang 1995). Karbwang 1992 reported mean fever clearance time and found a statistically significant reduction of about 30 hours with artemether. Downgraded by 1 for inconsistency: One trial found a shorter median fever clearance time with quinine, and two trials found a shorter time with artemether.
Summary of findings 3. ‘Summary of findings' table 3.
| Artemether compared with artesunate for treating adults with severe malaria | ||||||
| Patient or population: adults with severe malaria Settings: malaria‐endemic countries Intervention: intramuscular artemether Comparison: intravenous or intramuscular artesunate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with artesunate | Risk with artemether | |||||
| Death | 87 per 1000 | 156 per 1000 (95 to 258) | RR 1.80 (1.09 to 2.97) | 494 (2 trials) | ⊕⊕⊕⊝
MODERATEa,b,c,d Due to imprecision |
Artemether probably increases the risk of death compared to artesunate. |
| Coma resolution time | ‐ | ‐ | Not pooled. No significant difference | 494 (2 trials) | ⊕⊕⊕⊝
MODERATEa,c,e,f Due to imprecision |
Artemether probably makes little or no difference to coma resolution time compared to artesunate. |
| Neurological sequelae at discharge | ‐ | ‐ | ‐ | 0 (0 trials) | ‐ | None of the studies looked at neurological sequelae in adults. |
| Parasite clearance time | ‐ | ‐ | Not pooled. No significant difference | 494 (2 trials) | ⊕⊕⊕⊝
MODERATEa,c,f,g Due to imprecision |
Artemether probably makes little or no difference to parasite clearance time compared to artesunate. |
| Fever clearance time | ‐ | ‐ | Not pooled. No significant difference | 494 (2 trials) | ⊕⊕⊕⊝
MODERATEa,c,f,h Due to imprecision |
Artemether probably makes little or no difference to fever clearance time compared to artesunate. |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aNo serious risk of bias: trials were generally well conducted and at low risk of bias. bNo serious inconsistency: there is no statistical heterogeneity. cNo serious indirectness: the two trials were conducted in Vietnam and Thailand and both compared intramuscular artemether with intravenous artesunate in adults. dDowngraded by 1 for serious imprecision: these trials, and the overall meta‐analysis are very underpowered to detect a difference in mortality or to prove equivalence. ePhu 2010 and Vinh 1997 reported median coma resolution time for artemether versus artesunate (Phu 2010: 72 versus 60, P = 0.11; Vinh 1997: 47 (artemether) versus 30 (artesunate IM) versus 24 (artesunate IV). No serious inconsistency: Both trials suggest an advantage with artesunate although not statistically significant. fDowngraded by 1 for serious imprecision: we could not pool these data as median data were presented for both trials. gPhu 2010 and Vinh 1997 reported median parasite clearance time (Phu 2010: 72 versus 72, P = 0.97; Vinh 1997: 30 (artemether) versus 24 (artesunate IM) versus 24 (artesunate IV). No serious inconsistency: Both trials found no difference between treatments. hPhu 2010 and Vinh 1997 reported median fever clearance time (Phu 2010: 108 versus 108, P = 0.27; Vinh 1997: 48 (artemether) versus 36 (artesunate IM) versus 30 (artesunate IV). No serious inconsistency: Both trials found no statistically significant difference between artemether and artesunate.
Artemether versus quinine
Children
Twelve trials were conducted in children; 11 in Africa and one in Asia. All used loading doses of quinine.
Death
There was no overall difference in all‐cause mortality between intramuscular artemether and intravenous quinine (RR 0.97, 95% CI 0.77 TO 1.21; 1659 participants, 13 trials, I2 = 0%, Analysis 1.1). However, these 13 trials were too small to detect or exclude clinically important differences, and the overall meta‐analysis remains significantly underpowered to prove equivalence (see Table 4 and Table 5). The current total sample size has adequate power to exclude effects as large as seven extra deaths per 100 patients. Adam 2002 reported death within 24 hours. There was no difference between intramuscular artemether and intravenous quinine (RR 0.35, 95% CI 0.02 to 8.10; 41 participants, 1 trial, Analysis 1.2).
1.1. Analysis.
Comparison 1 Artemether versus quinine, Outcome 1 Death.
1.2. Analysis.
Comparison 1 Artemether versus quinine, Outcome 2 Death: Time since admission to hospital.
Coma resolution time
The mean coma resolution time was about five hours shorter with artemether (26 hours) compared with quinine (30.55 hours) (MD −5.45 hours, 95% CI −7.90 to −3.00; 358 participants, 6 trials, I2 = 0%, Analysis 1.3). The results were not statistically significant when a sensitivity analysis excluding studies with unclear allocation concealment was done (MD −5.53 hours, 95% CI −12.39 to 1.33; 95 participants, 2 trials, Analysis 3.3). In addition, three trials reported median time to coma resolution (see Table 10). Two trials found no overall difference (Murphy 1996; Taylor 1998); and one trial found the median time to be longer with artemether (26 hours versus 20 hours, P = 0.046, van Hensbroek 1996). Two other trials reported mean coma resolution time as mean (SD) but the data were not normally distributed and so we have reported this in an additional table (Table 10) (Minta 2005; Osonuga 2009).
1.3. Analysis.
Comparison 1 Artemether versus quinine, Outcome 3 Coma resolution time (hours).
3.3. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 3 Coma resolution time (hours).
7. Additional data: Artemether versus quinine in children.
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Quinine | Comparative results reported in article |
| Coma resolution time (hours) | Median (IQR) |
Murphy 1996 | 160 | 12 (2.8 to 96) | 13 (2.8 to 96) | Not significantly different |
| Median (IQR) |
van Hensbroek 1996 | 576 | 26 (15 to 48) | 20 (12 to 43) | P = 0.046 | |
| Median (IQR) |
Taylor 1998 | 164 | 18 (8 to 30) | 20 (10 to 54) | Not significantly different | |
| Coma recovery (%) on Day 3 | Aguwa 2010 | 90 | 15.9% | 21.4% | RR = 0.763 (95% CI 0.065 to 9.015) | |
| Mean (SD) | Osonuga 2009 | 32 | 4.5 (13.05) | 9 (24.59) | P = 0.523 | |
| Mean (SD) | Minta 2005 | 67 | 30.57 (29.02) | 25.15 (31.62) | P = 0.53 | |
| Time to hospital discharge | % spending less than one week in hospital |
Aguwa 2010 | 90 | 61.76% | 71.74% | P = 0.829 |
| Fever clearance (hours) | Median (IQR) |
Murphy 1996 | 160 | 32 (4 to 86) | 32 (4 to 96) | Not significantly different |
| van Hensbroek 1996 | 576 | 30 (16 to 48) | 33 (12‐60) | P = 0.8 | ||
| Taylor 1998 | 164 | 31 (24 to 52) | 45 (33 to 60) | "Significant" | ||
| Fever clearance (%) on Day 3 | Aguwa 2010 | 90 | 90.0% | 87.7% | P = 0.753 | |
| Parasite clearance (hours) | Median (IQR) |
Murphy 1996 | 160 | 39.5 (24 to 45) | 48 (37 to 56) |
P < 0.001 |
| van Hensbroek 1996 | 576 | 48 (36 to 60) | 60 (48 to 72) | P < 0.001 | ||
| Taylor 1998 | 164 | 32 (25 to 36) | 40 (32 to 48) | ‘significant' | ||
| Parasite clearance (%) on Day 3 | Aguwa 2010 | 90 | 99.0% n = 44 |
96.8% n = 46 |
P = 0.422 | |
| Needing blood transfusion | ‐ | van Hensbroek 1996 | ‐ | ‐ | ‐ | "The two groups were similar in terms of the need for blood transfusions,and the incidence of secondary bacterial infections (data not shown)." |
Abbreviations: IQR = interquartile range.
Neurological sequelae
There was no statistically significant difference in the risk of neurological sequelae at hospital discharge between artemether (185 per 1000) and quinine (220 per 1000) (968 participants, 7 trials, I2 = 1%, Analysis 1.4). Again these trials were too small to enable us to confidently detect or exclude what may be clinically important differences between treatments (see Table 4). The overall meta‐analysis is adequately powered to exclude effects larger than eight additional sequelae per 100 patients.
1.4. Analysis.
Comparison 1 Artemether versus quinine, Outcome 4 Neurological sequelae at discharge.
Three trials continued to monitor patients with neurological sequelae after hospital discharge. Satti 2002 found no difference at day seven, Taylor 1998 found most sequelae had resolved and van Hensbroek 1996 found no difference at day 28 (Analysis 1.5).
1.5. Analysis.
Comparison 1 Artemether versus quinine, Outcome 5 Neurological sequelae at follow‐up.
Parasite clearance time
The mean parasite clearance time in children was approximately nine hours shorter with artemether (MD −9.03 hours, 95% CI −11.43 to −6.63; 420 participants, I2 = 62%, 7 trials, Analysis 1.6). The results were similar after sensitivity analysis which excluded trials at unclear or high risk of selection bias. The mean parasite clearance times for artemether and quinine were 36.25 and 43.18 hours respectively.
1.6. Analysis.
Comparison 1 Artemether versus quinine, Outcome 6 Parasite clearance time.
Three additional trials reported median parasite clearance time and all showed an overall benefit with artemether (see Table 10). Two trials expressed parasite clearance as the proportion of patients with parasite clearance at 72 hours and at seven days, with no overall differences between groups (see Analysis 1.7) (Ojuawo 1998; Bobossi‐Serengbe 2015). The inconsistency may be due to trials differing with respect to the frequency with which they repeated malaria blood smears (see Table 9).
1.7. Analysis.
Comparison 1 Artemether versus quinine, Outcome 7 Proportion with parasite clearance.
Fever clearance time
Eight trials reported mean fever clearance time with a statistically significant reduction of about three hours with artemether (MD −3.73 hours, 95% CI −6.55 to −0.92; 457 participants, 8 trials, I2 = 83%, Analysis 1.8). The mean fever clearance times for artemether and quinine were 43.69 and 46.26 hours respectively. However, only two of the individual trials showed an overall difference between the groups. Three trials in children reported median fever clearance time and two trials found no overall difference between the two groups (see Table 10). There was high inconsistency due to opposing directions of effect. Also, the definitions of fever varied across the included trials. Six trials used a cut‐off of body temperature less than 37.5 °C from initiation of treatment to define fever clearance (see Table 9).
1.8. Analysis.
Comparison 1 Artemether versus quinine, Outcome 8 Fever clearance time (hours).
Need for blood transfusion
One trial, Olumese 1999, reported on the number of patients requiring blood transfusions for severe malarial anaemia in both artemether and quinine arms (RR 1.27, 95% CI 0.62 to 2.59; 103 participants, 1 trial, I2 = 0%, Analysis 1.9). No statistically significant difference was observed between both arms.
1.9. Analysis.
Comparison 1 Artemether versus quinine, Outcome 9 Need for blood transfusion.
Adverse effects
Eight trials reported on the frequency of adverse events (Walker 1993; Murphy 1996; van Hensbroek 1996; Olumese 1999; Adam 2002; Huda 2003; Minta 2005; Bobossi‐Serengbe 2015). Olumese 1999 and Huda 2003 reported no adverse events had occurred during the trial duration (see Table 11). One trial reported the absence of adverse events in the artemether arm (Minta 2005). No trial reported discontinuation of medication.
8. Adverse event monitoring and reporting.
| Study ID | Sample size | Clinical symptoms monitoring | Biochemistry | Haematological | Electrocardiogram | Additional comments on adverse events |
| Adam 2002 | 41 | Not reported | Not reported | Not reported | Not reported | "Neurological deficits were not observed in any patient during the follow‐up period" |
| Aguwa 2010 | 90 | Not reported | Not reported | Not reported | Not reported | None |
| Hien 1996 | 560 | Clinical assessment every 4 hours for the first 24 hours and 6‐hourly afterwards | Blood glucose, lactate and cytokine levels measured 4, 8, 12 and 24 hours after admission | Full blood count (FBC) on admission | Pre‐treatment and 12 hours after initiation of treatment on Day 0, 4 hours after last dose and at discharge | None |
| Huda 2003 | 46 | Lumbar puncture Chest x‐ray on day 0 |
Blood Glucose, Renal Function Test, Liver Function Test and Serum Electrolyte on Days 0 and 3 |
FBC on Days 0 and 3 | Day 0 | "No serious side effects of either of the drugs were observed in our study...... Closer and more frequent monitoring and larger sample size would have probably revealed more subtle adverse drug effects." |
| Karbwang 1992 | 26 | Clinical evaluation daily for at least 7 days Lumbar puncture Chest x‐ray on day 0 |
Biochemistry on Days 0, 2, 4 and 7 | FBC on Days 0, 2, 4 and 7 | On admission for all patients; then once daily and every 6 hours for quinine and artemether patients respectively | "The side effects in the quinine group were dizziness and vertigo. No side effects were detected with artemether". |
| Karbwang 1995 | 102 | Clinical evaluation on admission and twice daily for at least 7 days Lumbar puncture Chest x‐ray on day 0 |
Biochemistry on Days 0, 2, 4 and 7 | FBC on Days 0, 2, 4 and 7 | On admission for all patients; then once daily and every 6 hours for quinine and artemether patients respectively | QTc prolongation and tinnitus were the major adverse events in Quinine arm. Mild transient pain at injection site for approximately 15 mins after artemether treatment. |
| Minta 2005 | 67 | Clinical examination daily on Days 1 to 7, and 14 | Blood glucose on Days 1, 2, 3, 5, 7 and 14 Urea and Serum electrolyte, transaminases, phosphatases on Days 1, 3, 5, 7 and 14 |
FBC on Days 1, 3, 5, 7 and 14 | Once daily on Days 1, 3, 5, 7 and 14 | None |
| Murphy 1996 | 160 | Clinical assessment on admission, then at 6‐, then 12‐hour intervals till discharge | Blood glucose, urea, electrolytes, blood gases and when clinically indicated | FBC on Day 0 and when clinically indicated Blood cultures on Day 0 |
On admission and at 6, 24, 30, 48 and 54 hours | None |
| Ojuawo 1998 | 37 | Clinical assessment on Day 0 | Urea and electrolyte Blood sugar and liver function test on Day 0 |
FBC on Day 0 | None | None |
| Olumese 1999 | 103 | Clinical assessments on Days 0, 3, 7, 14, 28 | Blood glucose, urea and creatinine, electrolytes on Days 0, 3, 7, 14, 28 | WBC count on Days 0, 3, 7, 14, 28 | None | "No adverse reactions to the two drugs were recorded during the study". |
| Osonuga 2009 | 32 | Clinical examination on Days 0 to 7 and 14 | None | None | None | None |
| Phu 2010 | 370 | Clinical examination on admission Chest x‐ray on admission Lumbar puncture |
Blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine transaminase, plasma lactate | FBC on admission | None | None |
| Satti 2002 | 77 | Clinical evaluation on admission and every six hours on Days 0 to 4, and then once daily on Days 14, 21 and 28 | Blood glucose, serum creatinine, serum aspartate, aminotransferase on Day 0 | WBC, haemoglobin on Days 0 and 3 | None | None |
| Seaton 1998 | 33 | Chest X‐ray on admission | Renal and liver function tests on admission, Days 3 and 7 | FBC on Days 0, 3 and 7 | None | None |
| Taylor 1998 | 183 | CSF collected on admission | Blood glucose, Blood pH, on Day 0 (every four hours for the first 24 hours |
Haematocrit every 8 hours FBC, urea, and electrolytes on Days 0, 3, 7 and 28 |
On admission, 6, 48, 54 and 96 hours | "Of the initial 127 patients on whom serial electrocardiographic tracings were made, more patients in the quinine group showed prolongation of the corrected QT intervals after treatment, but the differences were not statistically or clinically significant." "There were no significant differences between the two treatment groups in terms of adverse effects associated with antimalarial treatment (i.e. new signs and symptoms which develop within seven days of the start of treatment)." |
| van Hensbroek 1996 | 576 | Clinical examination on Day 0 Lumbar puncture on admission |
Blood glucose on admission, after 4 hours and 12 hours | PCV, haemoglobin, Blood culture on Day 0 | None | None |
| Vinh 1997 | 124 | Clinical examination on admission | Blood glucose, serum creatinine, serum bilirubin on admission | FBC on admission | None | None |
| Walker 1993 | 54 | Clinical examination twice daily Spinal taps |
Urea and Electrolyte, on days 3, 7, 14, 28 | PCV on days 3, 7, 14, 28 | On admission, at 4 and 6 hours | None |
Abbreviations: CSF: cerebrospinal fluid; FBC: full blood count; PCV: packed cell volume; WBC: white blood cells
Only two trials reported episodes of hypoglycaemia (Analysis 1.10). Two trials reported the proportion of participants with QT prolongation (Minta 2005; Murphy 1996). There was significantly higher proportion of QT prolongation in the artemether arm (RR 3.10, 95% CI 1.33 to 7.19; 229 participants, 2 trials, I2 = 52%, Analysis 1.11). Both trials varied in terms of the populations studied. Minta 2005 had fever (38 °C or more) as part of the inclusion criteria. In addition, all participants received a single dose of oral SP in addition to either IM artemether or IV quinine. Other adverse effects reported were local skin reaction at the injection site, abscess, urticarial rash, pruritus, supraventricular tachycardia, urinary tract infection, and haemoglobinuria. However, these trials were insufficiently powered to detect differences in adverse events. The trials had similar definitions of adverse events (included only adverse effects that could not be attributable to malaria).
1.10. Analysis.
Comparison 1 Artemether versus quinine, Outcome 10 Episodes of hypoglycaemia.
1.11. Analysis.
Comparison 1 Artemether versus quinine, Outcome 11 Adverse events.
Time to hospital discharge
None of the included trials reported time to discharge. One trial reported the proportion of patients that spent less than one week in hospital and found no significant difference between groups (see Table 10).
Adults
Four trials were conducted in adults: three in Asia and one in Oceania. All used loading doses of quinine.
Death
Artemether resulted in fewer deaths compared with quinine (RR 0.59, 95% CI 0.42 to 0.83; 716 participants, 4 trials, I2 = 36%, Analysis 1.1) from trials conducted mostly in Asia.
Coma resolution time
Three trials reported a measure of coma resolution time. Hien 1996 reported median coma resolution time, which was shorter in the quinine arm. Karbwang 1995 found both arms to be comparable in terms of coma resolution time. The third trial, Karbwang 1992, reported mean coma resolution time but the data were incompletely reported (see Table 12).
9. Additional data: Artemether versus quinine in adults.
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Quinine | Comparative results reported in article |
| Coma resolution time (hours) | Median (IQR) | Hien 1996 | 560 | 66 (30 to 132) | 48 (20 to 84) | P = 0.003 |
| Median (range) | Karbwang 1995 | 97 | 48 (6 to 144) | 48 (6 to 144) | Not significantly different | |
| Fever clearance (hours) | Median (IQR) | Hien 1996 | 560 | 127 (60 to 216) | 90 (54 to 144) | < 0.001 |
| Median (range) | Seaton 1998 | 33 | 32 (20 to 112) | 48 (28 to 88) | P = 0.034 | |
| Median (range) | Karbwang 1995 | 97 | 79 (16 to 147) | 84 (36 to 144) | Not significantly different | |
| Parasite clearance (hours) | Median (IQR) | Hien 1996 | 560 | 72 (54 to 102) | 90 (66 to 108) | < 0.001 |
| Median (range) | Seaton 1998 | 33 | 48(4 to 72) | 52 (12 to112) | P = 0.381 | |
| Median (range) | Karbwang 1995 | 97 | 54 (30 to 164) | 78 (18 to 168) | P = 0.007 |
Abbreviations: IQR = interquartile range.
Neurological sequelae
Only one trial reported neurological sequelae at discharge (Hien 1996). Four neurological sequelae were reported with no difference between groups (560 participants, 1 trial, Analysis 1.4).
Parasite clearance time
One trial reported mean parasite clearance time but showed no overall difference between artemether and quinine (26 participants, one trial, Analysis 1.6). Three other trials reported median parasite clearance time. Two trials reported a significantly shorter time to clearance of parasites with artemether (Karbwang 1995; Hien 1996; see Table 10). Seaton 1998 found no significant difference between artemether and quinine with respect to parasite clearance time.
Trials differed with respect to the frequency with which they repeated malaria blood smears (see Table 9).
Fever clearance time
Four trials reported a measure of fever clearance time. Karbwang 1992 reported mean fever clearance time and found an overall reduction of about 30 hours with artemether (MD −29.7 hours, 95% CI −54.14 to −5.26; 26 participants, 1 trial, Analysis 1.8). The other three trials in adults reported median fever clearance time; and two reported a statistically significant reduction in fever clearance time in favour of quinine and artemether respectively (Hien 1996; Seaton 1998). Karbwang 1995 found both groups were comparable (see Table 12).
The definitions of fever varied across the included trials. Three trials used a cut‐off of body temperature less than 37.5 °C from initiation of treatment to define fever clearance (see Table 9).
Need for blood transfusion
One trial reported on the number of patients requiring blood transfusions for severe malarial anaemia in both artemether and quinine arms (Hien 1996). No overall difference was observed between both arms (560 participants, one trial, Analysis 1.9).
Adverse events
One trial reported episodes of hypoglycaemia (Analysis 1.10). Other adverse effects reported were abscess, induration at injection site, leg discomfort, chest infection and gastrointestinal bleeding. However, these trials were insufficiently powered to detect differences in adverse events. The trials had similar definitions of adverse events (included only adverse effects that could not be attributable to malaria).
Publication bias
We constructed a funnel plot for trials that reported death (Figure 3). Visual examination suggested the funnel plot is asymmetric due to the absence of smaller trials at the base. Asymmetry in the funnel plot could result from possible selection bias where smaller studies reporting greater treatment benefit for the experimental group were published (publication bias). The gap in the bottom corner of the graph suggests that smaller studies without statistically significant effects remain unpublished (Higgins 2011).
3.
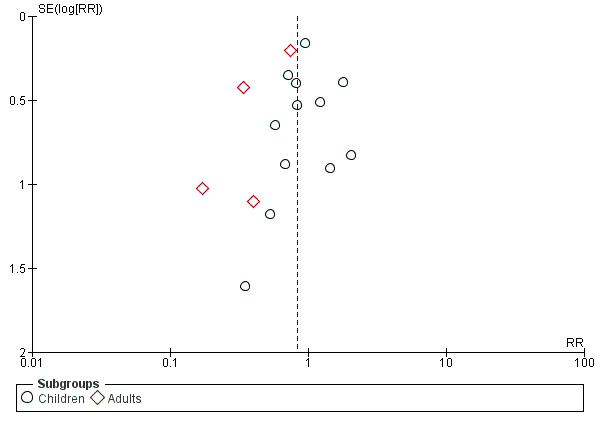
Funnel plot of comparison: 1 Artemether versus quinine, outcome: 1.1 Death.
Artemether versus artesunate
Adults
Two trials, both from Asia, were conducted in adults.
Death
Only two trials directly compared intramuscular artemether and intravenous artesunate. Overall, the risk of all‐cause mortality was higher following treatment with artemether (RR 1.80, 95% CI 1.09 to 2.97; 494 participants, 2 trials, Analysis 2.1). However, both trials were too small to detect or exclude clinically important differences, and the overall meta‐analysis remains significantly underpowered to prove superiority (see Table 4 and Table 5).
2.1. Analysis.
Comparison 2 Artemether versus artesunate, Outcome 1 Death.
Coma resolution time
Two trials reported median coma resolution times. Both trials reported a shorter coma resolution time with artesunate. However, these differences were not statistically significant (Table 13).
10. Additional data: Artemether versus artesunate in adults.
| Pre‐specified outcome | Trial reported outcome | Trial | Number of participants | Artemether | Artesunate IM | Artesunate IV | Comparative results reported in article |
| Coma resolution time (hours) | Median (range) | Phu 2010 | 370 | 72 (2 to 2232) n = 184 | 60 (4 to 2136), n = 186 | ‐ | P = 0.11 |
| Median (95% CI) | Vinh 1997 | 124 | 47 (31 to 63) | 30 (18 to 42) | 24 (4 to 44) | ‐ | |
| Fever clearance (hours) | Median (range) | Phu 2010 | 370 | 108 (0 to 888) n = 184 | 108 (0 to 888), n = 186 | ‐ | P = 0.27 |
| Median (95% CI) | Vinh 1997 | 124 | 48 (38 to 58) | 36 (30 to 42) | 30 (18 to 42) | ‐ | |
| Parasite clearance (hours) | Median (range) | Phu 2010 | 370 | 72 (2 to 204) | 72 (7 to 330) | ‐ | P = 0.97 |
| Median (95% CI) | Vinh 1997 | 124 | 30 (26 to 34) | 24 (15 to 33) | 24 (15 to 33) | Not statistically significant |
Abbreviations: CI: confidence interval; IM: intramuscular; IV: intravenous.
Parasite clearance time
Two trials found no overall difference in median parasite clearance time (Vinh 1997; Phu 2010).
Fever clearance time
Phu 2010 found no statistically significant difference in median fever clearance time between intramuscular artemether and intravenous artesunate. The additional small trial found a benefit in favour of artesunate although this was not statistically significant (Vinh 1997).
Need for blood transfusion
Phu 2010 found no difference between treatments with respect to the need for blood transfusion in adult severe malaria patients (370 participants, one trial, Analysis 2.2).
2.2. Analysis.
Comparison 2 Artemether versus artesunate, Outcome 2 Need for blood transfusion.
Adverse effects
Only Phu 2010 reported adverse events. Overall, the risk of hypoglycaemia was higher in adults treated with intramuscular artemether compared with artesunate (RR 1.70, 95% CI 0.40 to 7.24; 370 participants, 1 trial, Analysis 2.3).
2.3. Analysis.
Comparison 2 Artemether versus artesunate, Outcome 3 Episodes of hypoglycaemia.
Discussion
We present the main results of the review alongside a GRADE appraisal of the certainty of evidence in the ‘Summary of findings' tables (Table 1; Table 2; Table 3).
Summary of main results
We included 19 RCTs, enrolling 2874 children and adults with severe malaria. Twelve trials were conducted in Africa and seven trials were undertaken in Asia.
Artemether versus quinine
For children (trials mostly conducted in Africa), there is probably little or no difference in the risk of death between intramuscular artemether and quinine (moderate‐certainty evidence). Artemether may shorten the coma recovery time by about five hours (low‐certainty evidence), and makes little difference in the number of children with subsequent neurological sequelae (low‐certainty evidence). Artemether probably shortens the parasite clearance time by about nine hours (moderate‐certainty evidence), and may shorten the fever clearance time by about three hours (low‐certainty evidence).
For older children (> 15 years) and adults in Asia, artemether probably reduces deaths compared with quinine (moderate‐certainty evidence), but larger trials are required to have full confidence in this finding.
Artemether versus artesunate
Artemether and artesunate have only been compared in two trials in adults from Asia, and mortality is probably higher with intramuscular artemether (moderate‐certainty evidence) but larger trials are required to have confidence in this finding.
Overall completeness and applicability of evidence
Although 16 trials directly compared artemether versus quinine, none were adequately powered to detect clinically important differences. The total number of participants included in these trials (2375 participants) remains far short of the 7429 participants included in trials of artesunate versus quinine (Sinclair 2012). The majority of data comparing artemether and quinine were from trials conducted in sub‐Saharan Africa where artemether is most widely used.
The two trials directly comparing artemether and artesunate were conducted in adults in Asia, and the results are therefore poorly applicable to children in Africa.
Artemether is prone to erratic and partial absorption and takes longer to achieve peak plasma concentrations, as demonstrated in animal and human studies (Murphy 1997; Hien 2004; Mithwani 2004). These pharmacokinetic attributes make artemether that is injected intramuscularly less readily available in the human body and may explain the difference in outcomes between artesunate and intramuscular artemether we have observed in this review.
Certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach and have presented it in three ‘Summary of findings' tables (Table 1; Table 2; Table 3).
The evidence of equivalence between intramuscular artemether and intravenous quinine in children is of moderate certainty due to the small sample sizes of the included trials and the overall lack of power to fully exclude clinically important differences.
Similarly, we downgraded the certainty of the evidence for reductions in mortality in adults treated with artemether compared with quinine, and artesunate compared with artemether, to moderate certainty due to imprecision. Larger trials are needed to have full confidence in these effects.
Potential biases in the review process
Our search strategy was comprehensive, and was without restrictions based on language, date of publication or publication status. We found several studies which mostly had small sample sizes and thus even the overall meta‐analysis was underpowered for several review outcomes. There was also a lack of direct evidence comparing artemether with artesunate. The findings of the funnel plot for death (Artemether versus quinine: Figure 3) may suggest publication bias. However, asymmetry in the funnel plot may also be due to other reasons, such as poor methodological quality in smaller studies leading to spuriously inflated effects, true heterogeneity in the treatment effect, sampling variation, or chance (Higgins 2011).
Agreements and disagreements with other studies or reviews
Some meta‐analyses and systematic reviews have evaluated the efficacy of artemether compared to quinine in treating severe malaria in children and adults. The earliest study was a meta‐analysis of randomized controlled trials (Pittler 1999).
Pittler 1999 found no difference in deaths between intramuscular artemether and parenteral quinine in adults and children although there were fewer adverse effects associated with the administration of intramuscular artemether. AQMSG 2001 reported significantly fewer deaths and neurological sequelae with artemether compared to quinine. PrayGod 2008 found no difference in deaths, parasite clearance time, fever clearance time and neurological sequelae among children between artemether and quinine. However, the study found coma resolution time in children was faster with artemether compared to quinine.
Another systematic review concluded that artemether was not inferior to quinine for preventing death from cerebral malaria in children (Kyu 2009). After the meta‐analyses and systematic reviews mentioned above, new clinical trials in which artemether has been compared with quinine and artesunate for the treatment of severe malaria in Africa and Asia have been published. However, no studies have compared artemether to artesunate in African children. These new studies are included in this Cochrane Review.
Authors' conclusions
Implications for practice.
Although there is a paucity of direct evidence comparing artemether with artesunate, artemether is probably less effective than artesunate at preventing deaths from severe malaria. In circumstances where artesunate is not available, artemether is an alternative to quinine. Since 2015, the World Health Organization recommends intravenous or intramuscular artesunate over quinine for the treatment of severe malaria in adults and children. Artemether is also recommended in preference to quinine for the treatment of severe malaria where artesunate is not available.
Implications for research.
Larger, adequately powered clinical trials are necessary for conclusive evidence on the relative effects of artemether. However, additional studies are unlikely given the current treatment recommendations.
What's new
| Date | Event | Description |
|---|---|---|
| 13 June 2019 | New citation required but conclusions have not changed | This is an update of a Cochrane Review. The conclusions remain the same as the last published version (Esu 2014) |
| 13 June 2019 | New search has been performed | We updated the literature search to 7 September 2018 and included one new study. |
Acknowledgements
The Academic Editor of this review update was Professor Mical Paul.
We are grateful to David Sinclair for his help with the GRADE assessment in Esu 2014. We also acknowledge Dr Amirhaobu Uwaoma's contributions to Esu 2014.
The protocol for the original review was developed during the Reviews for Africa Fellowship Programme organized by the Nigerian Branch of the South Africa Cochrane Centre in July 2012 (Esu 2013).
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | Embaseb | LILACSb | ISI Web of Science |
| 1 | malaria | Malaria ti, ab, MeSH | Malaria ti, ab, MeSH | Malaria ti, ab, Emtree | malaria | malaria |
| 2 | artemether | Artemether ti, ab | Artemether ti, ab | Artemether ti, ab, Emtree | artemether | artemether |
| 3 | Artemisinin* | Artemisinin* ti, ab | Artemisinin* ti, ab | Artemisinin* ti, ab | Artemisinin* | Artemisinin* |
| 4 | intramuscular | Intramuscular ti, ab | Intramuscular ti, ab | Intramuscular ti, ab | intramuscular | intramuscular |
| 5 | parenteral | Injections, Intramuscular [MeSH] | Injections, Intramuscular [MeSH] | Intramuscular drug administration [Emtree] | parenteral | parenteral |
| 6 | 2 or 3 | Parenteral ti, ab | Parenteral ti, ab | Parenteral drug administration [Emtree] | 2 or 3 | 2 or 3 |
| 7 | 4 or 5 | 2 or 3 | 2 or 3 | 2 or 3 | 4 or 5 | 4 or 5 |
| 8 | 1 and 5 and 7 | 4 or 5 or 6 | 4 or 5 or 6 | 4 or 5 or 6 | 1 and 5 and 7 | 1 and 5 and 7 |
| 9 | ‐ | 1 and 7 and 8 | 1 and 7 and 8 | 1 and 7 and 8 | ‐ | Randomised clinical trial* |
| 10 | ‐ | ‐ | ‐ | ‐ | ‐ | 8 and 9 |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by the Cochrane Collaboration (Lefebvre 2011).
Data and analyses
Comparison 1. Artemether versus quinine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 13 | 1659 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.21] |
| 1.2 Adults | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.83] |
| 2 Death: Time since admission to hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Death within 24 hours | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.10] |
| 3 Coma resolution time (hours) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Children | 6 | 358 | Mean Difference (IV, Fixed, 95% CI) | ‐5.45 [‐7.90, ‐1.00] |
| 4 Neurological sequelae at discharge | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Children | 7 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 4.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.31, 27.86] |
| 5 Neurological sequelae at follow‐up | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.38] |
| 5.1 Day 7 | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.14] |
| 5.2 Day 28 | 1 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.53] |
| 6 Parasite clearance time | 8 | 446 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐11.20, ‐6.45] |
| 6.1 Children | 7 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐9.03 [‐11.43, ‐6.63] |
| 6.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐15.56, 18.96] |
| 7 Proportion with parasite clearance | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.91, 1.00] |
| 7.1 At 72 hours | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.84, 1.02] |
| 7.2 At 7 days | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.95, 1.02] |
| 8 Fever clearance time (hours) | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Children | 8 | 457 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐6.55, ‐0.92] |
| 8.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐29.70 [‐54.14, ‐5.26] |
| 9 Need for blood transfusion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Children | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.62, 2.59] |
| 9.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 10 Episodes of hypoglycaemia | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Children | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 10.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.64] |
| 11 Adverse events | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 QT prolongation | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.33, 7.19] |
| 11.2 Local skin reactions | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.50] |
| 11.3 Abscess | 2 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 11.4 Urticarial rash | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 11.5 Supraventricular tachycardia | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.59] |
| 11.6 Pruritus | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 11.7 Urinary tract infection | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.15, 81.36] |
| 11.8 Induration at injection site | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.44 [0.94, 253.49] |
| 11.9 Leg discomfort | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.16] |
| 11.10 Chest infection | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.53] |
| 11.11 GI bleeding | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.20] |
| 11.12 Haemoglobinuria | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.56] |
Comparison 2. Artemether versus artesunate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Adults | 2 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.09, 2.97] |
| 2 Need for blood transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Adults | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.32] |
| 3 Episodes of hypoglycaemia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Spontaneous bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.4. Analysis.
Comparison 2 Artemether versus artesunate, Outcome 4 Adverse events.
Comparison 3. Artemether versus quinine (sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 5 | 898 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.37] |
| 1.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.50, 1.11] |
| 2 Death: Time since admission to hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Death within 24 hours | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.10] |
| 3 Coma resolution time (hours) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Children | 2 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐5.53 [‐12.39, 1.33] |
| 4 Neurological sequelae at discharge | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Children | 3 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.01] |
| 4.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.31, 27.86] |
| 5 Neurological sequelae at follow‐up | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.38] |
| 5.1 Day 7 | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.14] |
| 5.2 Day 28 | 1 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.53] |
| 6 Parasite clearance time | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐7.73 [‐12.65, ‐2.80] |
| 6.1 Children | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐7.73 [‐12.65, ‐2.80] |
| 7 Fever clearance time (hours) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Children | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐4.03 [‐10.05, 1.99] |
| 8 Need for blood transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 9 Episodes of hypoglycaemia | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Children | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 9.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.64] |
| 10 Adverse events | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 QT prolongation | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.33, 7.19] |
| 10.2 Local skin reactions | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.50] |
| 10.3 Abscess | 2 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 10.4 Urticarial rash | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 10.5 Supraventricular tachycardia | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.59] |
| 10.6 Pruritus | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 10.7 Urinary tract infection | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.15, 81.36] |
| 10.8 Induration at injection site | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.44 [0.94, 253.49] |
| 10.9 Leg discomfort | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.16] |
| 10.10 Chest infection | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.53] |
| 10.11 GI bleeding | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.20] |
| 10.12 Haemoglobinuria | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.56] |
3.1. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 1 Death.
3.2. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 2 Death: Time since admission to hospital.
3.4. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 4 Neurological sequelae at discharge.
3.5. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 5 Neurological sequelae at follow‐up.
3.6. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 6 Parasite clearance time.
3.7. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 7 Fever clearance time (hours).
3.8. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 8 Need for blood transfusion.
3.9. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 9 Episodes of hypoglycaemia.
3.10. Analysis.
Comparison 3 Artemether versus quinine (sensitivity analysis), Outcome 10 Adverse events.
Comparison 4. Artemether versus artesunate (sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Adults | 2 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.09, 2.97] |
| 2 Need for blood transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Adults | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.32] |
| 3 Episodes of hypoglycaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Spontaneous bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
Comparison 4 Artemether versus artesunate (sensitivity analysis), Outcome 1 Death.
4.2. Analysis.
Comparison 4 Artemether versus artesunate (sensitivity analysis), Outcome 2 Need for blood transfusion.
4.3. Analysis.
Comparison 4 Artemether versus artesunate (sensitivity analysis), Outcome 3 Episodes of hypoglycaemia.
4.4. Analysis.
Comparison 4 Artemether versus artesunate (sensitivity analysis), Outcome 4 Adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adam 2002.
| Methods | Trial design: open label RCT Trial dates: November 2001 to January 2002 | |
| Participants | Number of participants: 41 children enrolled
Inclusion criteria: children with severe malaria (age range not stated); cerebral malaria; repeated convulsions; severe anaemia with haemoglobin < 5g/dL; hyperparasitaemia (parasite count > 100,000 rings/µL); or combinations of these criteria. Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Outpatient clinic in New Halfa, Eastern Sudan
Transmission: "meso‐endemic" Funding: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each child was randomized". No further details provided. |
| Allocation concealment (selection bias) | Low risk | "Envelopes containing the assigned treatment were opened sequentially at the time when each patient was recruited to the study". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Described as open‐label. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Aguwa 2010.
| Methods | Trial design: RCT Trial dates: July to October 2007 |
|
| Participants | Number of participants: 90 children enrolled Inclusion criteria: children between 6 months and 12 years of age presenting with fever (> 37.5 °C ) and P falciparum infection with 1 or more general danger signs of severe or complicated malaria based on the WHO criteria for severe malaria. Exclusion criteria: serious concomitant illness (for example, sickle cell anaemia, HIV, tuberculosis and other chronic diseases); severe malnutrition; known hypersensitivity to 1 of the trial drugs. |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Federal Medical Centre, Birnin Kudu, Jigawa State of Nigeria Transmission: stable perennial transmission Funding: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were assigned to receive quinine if the last digit of their hospital identification number was odd and to receive artemether if the last digit of their hospital identification number was even or zero". |
| Allocation concealment (selection bias) | High risk | Trial authors did not describe any methods of allocation concealment, and this would not be possible using this randomization method. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | No blinding was described. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No blinding is described, and blinding would not be feasible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up at day 14 were > 10% in both trial arms. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Bobossi‐Serengbe 2015.
| Methods | Trial design: randomized trial Trial dates: June to August 2010 |
|
| Participants | Number of participants: 212 hospitalized children Inclusion criteria: children between 6 and 59 months with clinical signs of severe malaria; P falciparum infection; and informed consent from parents or guardians. Exclusion criteria: children with known hypersensitivity to quinine. |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: none |
|
| Notes | Location: Bangui Pediatric Complex, Bangui, Central African Republic Transmission: stable perennial transmission Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based block randomization used. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Not stated. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No blinding is described, and blinding would not be feasible as the route of administration is different for artemether and quinine. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Hien 1996.
| Methods | Trial design: double‐blind RCT Trial dates: not stated |
|
| Participants | Number of participants: 560 adults aged 15 to 79 years enrolled Inclusion criteria: patients were included in the trial if they:
Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Special Research Ward, Centre for Tropical Diseases, Ho Chi Minh City, Vietnam Transmission: not stated Funding: Wellcome Trust |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation. |
| Allocation concealment (selection bias) | Low risk | "The drugs for each patient were placed in a coded sealed envelope and the envelopes were randomized in blocks of 20. Once a patient was enrolled in the study the envelope was opened". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | "To maintain blinding, a separate team of nurses, who were not otherwise involved with the care of the study patients, drew up and gave the injections. The drugs were kept in an opaque packet in a locked cabinet during the study". Both interventions were administered by intramuscular injection so blinding was feasible. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | Low risk | "To maintain blinding, a separate team of nurses, who were not otherwise involved with the care of the study patients, drew up and gave the injections. The drugs were kept in an opaque packet in a locked cabinet during the study". Both interventions were administered by intramuscular injection so blinding was feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Huda 2003.
| Methods | Trial design: open RCT Trial dates: April 2000 to July 2001 |
|
| Participants | Number: 46 children aged 6 months to 12 years enrolled Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Supportive therapy was given to all patients. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: none |
|
| Notes | Location: Inpatient Unit of Department of Pediatrics, and Parasitology laboratory, Department of Microbiology, Uttar Pradesh, India Transmission: unknown Funding: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Trial authors provided no information on allocation concealment. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | Low risk | "The slides for malarial parasites were transported to the parasitology laboratory where the person examining the slides was unaware of the clinical status of the patient and also the treatment assignment group". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Karbwang 1992.
| Methods | Trial design: RCT Trial dates: May to December 1991 |
|
| Participants | Number of participants: 26 adults aged 15 to 45 years enrolled Inclusion criteria: patients with severe P falciparum malaria (WHO definition) with no history of antimalarials within 24 hours prior to admission, aged between 15 to 45 years and weighed 45 to 60 kg Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Prapokklao Hospital, Chantaburi, Thailand Transmission: not stated Funding: support from United Medical Ltd., Bangkok (provided artemether) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomized to receive either quinine or artemether". |
| Allocation concealment (selection bias) | Unclear risk | Trial authors provided no information on allocation concealment. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Trial authors provided no information on blinding. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | Trial authors provided no information on blinding, however it may not be feasible due to different routes of administration for both interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Karbwang 1995.
| Methods | Trial design: RCT Trial dates:1992 to 1994 |
|
| Participants | Number of participants: 102 adults aged between 15 and 55 years enrolled Inclusion criteria: male and non‐pregnant female patients with severe P falciparum malaria (WHO definition) with no history of antimalarial treatment within 24 hours before admission aged 15 to 65 years and weighing 45 to 75 kg Exclusion criteria: patients with concurrent diseases were excluded |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Prapokklao Hospital, Chantaburi, Thailand Transmission: not stated Funding: UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization at WHO Office. |
| Allocation concealment (selection bias) | Low risk | "Each treatment was enclosed in a sealed envelope, which was opened only after the physician in charge had decided to recruit the patient into the study". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Trial authors provided no information on blinding. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | Trial authors provided no information on blinding; however it might not have been feasible due to different routes of administration for both interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up about 5%. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Minta 2005.
| Methods | Trial design: open RCT Trial dates: June 1993 to February 1994 and June 1994 to December 1994 |
|
| Participants | Number of participants: 67 children aged 3 months to 15 years enrolled Inclusion criteria: fever (core temperature ≥ 38 °C); positive blood smear for P falciparum with ≥ 0.1% of parasitized erythrocytes; 1 major criterion or 2 minor criteria for severe malaria cases (WHO criteria); and parental consent Exclusion criteria: children with infection who had been treated within 24 hours with quinine or intramuscular injection were not eligible. |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Gabriel Touré's Hospital, Mali Transmission: unknown Funding: Rhône‐Poulenc Rorer Doma (France) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization by clinical monitor. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes used to conceal allocation. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. No blinding is described, and blinding would not be feasible. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Murphy 1996.
| Methods | Trial design: open RCT Trial dates: not stated |
|
| Participants | Number: 160 children aged 5 months to 12 years enrolled Inclusion criteria: children were admitted to the trial if they had P falciparum asexual parasitaemia; were comatose; and parental consent was obtained. Exclusion criteria: children were excluded if there was evidence of a pre‐existing neurological deficit; head injury; or history of recent treatment with antimalarial drugs other than chloroquine. |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Kenya Medical Research Institute (KEMRI) Coastal Research Unit, Kilifi district hospital, Kenya. Transmission: unknown Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally‐coded unique trial numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared by the clinical monitor. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 patients (14 from artemether arm and 26 from quinine arm) excluded (mostly for not meeting inclusion criteria). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Ojuawo 1998.
| Methods | Trial design: RCT Trial dates: not stated |
|
| Participants | Number of participants: 37 children enrolled (age range not stated) Inclusion criteria: Children with unrousable coma, asexual forms of P falciparum parasitaemia and no other identifiable cause of coma. Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: none |
|
| Notes | Location: University of Ilorin Teaching Hospital, Nigeria Transmission: unknown Funding: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to either of the two treatment modalities". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information about blinding provided by trial authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up recorded. |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes reported. |
| Other bias | Low risk | No other bias identified. |
Olumese 1999.
| Methods | Trial design: open label RCT Trial dates: not stated |
|
| Participants | Number of participants: 103 children aged 11 months to 5 years enrolled Inclusion criteria: Children aged 6 months to 5 years satisfying the WHO criteria for cerebral malaria, viz. unrousable coma lasting more than 30 minutes (with or without convulsions) with the presence of peripheral P falciparum parasitaemia were included in the trial. Exclusion criteria: none stated |
|
| Interventions |
Loading dose quinine was omitted in patients with a positive history of quinine or mefloquine ingestion in the preceding 24 hours before hospital presentation. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Emergency Paediatric ward, University College Hospital, Ibadan, Nigeria Transmission: unknown Funding: World Bank/UNDP/WHO special fund for Research and Training in Tropical Diseases (TDR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation. |
| Allocation concealment (selection bias) | Unclear risk | Methods not described by trial authors. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Osonuga 2009.
| Methods | Trial design: RCT Trial dates: not stated |
|
| Participants | Number: 32 children aged 1 to 12 years enrolled Inclusion criteria: children aged 1 to 12 years, with fever (temperature > 37.5 °C), presence of convulsion, vomiting, hypoglycaemia, anaemia and headache. Informed consent obtained from the parents and guardians. Assurance that patients will be resident within catchments of trial for follow‐up. Absence of concomitant illness such as bronchopneumonia, typhoid, meningitis, urinary tract infection. Exclusion criteria: history of blood transfusion in the last 2 months, presence of concomitant illness, history of previous allergy to quinine and artemether. |
|
| Interventions |
Treatment with quinine was for a total of 7 days. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: none |
|
| Notes | Location: Overcomers Specialist Clinic Ileshan and General Hospital Ikenne, Nigeria Transmission: unknown Funding: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The children were randomly allocated into 2 treatment groups; treatment Q and A for quinine and artemether respectively". |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided by trial authors. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition about 6% and not likely to affect outcomes. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Authors have published 3 outcomes in 3 different publications from the same trial. |
| Other bias | Low risk | No other bias identified. |
Phu 2010.
| Methods | Trial design: double‐blind RCT Trial dates: May 1996 to June 2003 |
|
| Participants | Number of participants: 370 adults aged between 15 and 77 years enrolled Inclusion criteria: peripheral blood smears had asexual forms of P falciparum and had at least 1 of the following severe complications:
Exclusion criteria: patients were not included if they were < 14 years, were pregnant in the first trimester, were known intravenous drug abusers, had received more than 3 g of quinine or 2 doses of any artemisinin derivatives in the previous 48 hours before admission, had a past history of allergy to any artemisinin derivatives, or if known to be HIV positive. |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam Transmission: not stated Funding: Wellcome Trust, UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was generated from random number tables". |
| Allocation concealment (selection bias) | Low risk | "Labels with the name of drug for each patient were put in coded sealed opaque envelopes, and the envelopes were randomized in blocks of 20. Once a patient was enrolled in the study the envelope was opened". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | "An independent team of nurses, not otherwise involved in the study or responsible for the care of these patients, open the envelope, randomized the patient and prepared the injection. Neither the treating physicians, study doctors and nurses, or patients knew which anti‐malarial drugs was administered". |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | Low risk | "An independent team of nurses, not otherwise involved in the study or responsible for the care of these patients, open the envelope, randomized the patient and prepared the injection. Neither the treating physicians, study doctors and nurses, or patients knew which anti‐malarial drugs was administered". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Satti 2002.
| Methods | Trial design: RCT Trial dates: May 1995 to June 1996 |
|
| Participants | Number: 77 children enrolled aged 3 months to 15 years Inclusion criteria: children who satisfied the WHO criteria for diagnosis of cerebral malaria (such as unrousable coma for at least 30 minutes following convulsions, positive blood film for malaria, exclusion of other causes of encephalopathy); and informed consent by parent or guardian. Exclusion criteria: concomitant acute illness such as pneumonia, meningitis or acute renal failure and any contraindication to IM injection. |
|
| Interventions |
Treatment with quinine was for a total of 7 days. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Khartoum Children's Emergency Hospital and Ahmed Gasim Specialist Hospital for Children, Sudan Transmission: unknown Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The cases were randomly allocated into two groups". |
| Allocation concealment (selection bias) | Unclear risk | No information provided by trial authors about allocation concealment. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 10% of participants in each arm dropped out before day 28. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Seaton 1998.
| Methods | Trial design: open‐label RCT Trial dates: June 1992 to May 1995 |
|
| Participants | Number: 33 adults aged above 12 years Inclusion criteria: blood smear showed asexual forms of P falciparum; in addition to fulfilling 1 or more of the WHO criteria for severe or complicated malaria. Exclusion criteria: patients under the age of 12 years, pregnant women, those who had received parenteral antimalarial treatment prior to admission and those with a co‐existent bacterial, viral, fungal or mixed malarial infection. |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: none |
|
| Notes | Location: Port Moresby General Hospital, Papua New Guinea Transmission: seasonal/sporadic Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned to treatment with quinine or artemether. |
| Allocation concealment (selection bias) | Low risk | Envelopes containing the assigned treatment were opened sequentially when a patient was recruited. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential proportion of withdrawals from both arms (25% versus 10%). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Taylor 1998.
| Methods | Trial design: open label RCT Trial dates: January 1992 to June 1994 |
|
| Participants | Number: 183 children enrolled (age range not stated) Inclusion criteria: children with asexual forms of P falciparum detected in a peripheral blood smear, and a Blantyre Coma Score ≤ 2, and if no other cause of fever or altered consciousness could be discovered. Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Paediatric ward at the Queen Elizabeth Central Hospital, Malawi Transmission: unknown Funding: UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization. |
| Allocation concealment (selection bias) | Low risk | "Randomized treatment assignments were prepared in blocks of ten by the sponsoring agency. Following initial stabilization, diagnosis and examination, a sealed envelope containing the treatment group was opened, and the patient was allocated to treatment". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential proportion of withdrawals from both arms (13% versus 8%). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
van Hensbroek 1996.
| Methods | Trial design: open‐label trial Trial dates: 1992 to 1994 |
|
| Participants | Number: 576 children aged 1 to 9 years enrolled Inclusion criteria: unconscious children 1 to 9 years of age with a Blantyre coma score of 2 or less, asexual forms of P falciparum identified on a thick blood film, and a parent or guardian gave informed consent. Exclusion criteria: patients with diseases other than malaria at the time of admission and those who recovered consciousness immediately after correction of hypoglycaemia or within 1 hour if they were convulsing on admission. Patients treated with quinine before admission. |
|
| Interventions |
An oral dose of approximately 1.25 mg/kg pyrimethamine and 25 mg/kg sulfadoxine was given to both arms to reduce recrudescence (in the 2nd and 3rd years of the trial). |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Royal Victoria Hospital and Sibanor Health Centre, Banjul, Gambia Transmission: unknown Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors do not provide details of sequence generation. |
| Allocation concealment (selection bias) | Low risk | "The treatment code for each child was stored in a sealed envelope that was opened after the admission procedure was completed and parental consent had been obtained". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | Low risk | "Each blood film was examined by two independent observers who were unaware of the treatment code". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Vinh 1997.
| Methods | Trial design: open RCT Trial dates: March 1992 to September 1994 |
|
| Participants | Number: 124 adults aged between 16 and 66 years Inclusion criteria: 15 years of age or older, with clinical symptoms and signs of malaria and the presence of asexual forms of P falciparum in their peripheral blood. In addition, having at least 1 of the following signs:
Exclusion criteria: patients were excluded from the trial if prior treatment with more than 3 g of quinine or 2 doses of artemisinin or a derivative had been recorded by the peripheral health care worker. Pregnant patients in the first trimester, and patients with concomitant diseases (such as active tuberculosis, bacterial meningitis), or mixed infections with P vivax were also excluded from the trial. |
|
| Interventions |
All patients received 750 mg mefloquine (Lariam®, Roche) as a single dose after regaining consciousness or at day 4. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Tan Phu Regional Hospital, Vietnam Transmission: endemic Funding: Roche Asian Research Foundation, Hong Kong |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors do not provide details about random sequence generation. |
| Allocation concealment (selection bias) | Low risk | "When a patient fulfilled the enrolment criteria, a sealed envelope containing the code for the treatment regimen was opened to allocate him/her to one of the following 4 treatment groups". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Described as open‐label. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Walker 1993.
| Methods | Trial design: open RCT Trial dates: not stated |
|
| Participants | Number: 54 children aged 1 to 5 years enrolled Inclusion criteria: patients were admitted if they satisfied the strict WHO definition of cerebral malaria. Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: University College Hospital, Ibadan, Nigeria Transmission: unknown Funding: World Bank/UNDP/WHO Special Fund for Research and Training in Tropical Diseases (TDR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers used. |
| Allocation concealment (selection bias) | Low risk | "Each child was then assigned a random number from a list prepared by an independent collaborator and thus allocated at random to receive either intramuscular artemether or intravenous quinine". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | "This was a randomized, open, controlled study in which no attempt was made to ‘blind’ the investigators, as the test drug and the control drug were given by 2 different routes". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 patient excluded from fever clearance time outcome assessment because of urinary tract infection. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aceng 2005 | Intervention was rectal artemisinin. |
| Bhattacharya 1997 | Not an RCT. |
| Bunnag 1992 | Drug‐resistant malaria not severe malaria. |
| Dunyo 2011 | Uncomplicated malaria. |
| Falade 2007 | Comparison not parenteral treatment. |
| Fargier 1999 | Not an RCT. |
| Karbwang 1994 | Comparison not parenteral treatment. |
| Karunajeewa 2006 | Not an RCT. |
| Myint 1987 | Not an RCT. |
| Osonuga 2006 | No desired review outcomes. |
| Reham 2012 | Patients not randomly selected (non‐probability consecutive sampling used). |
| Rehman 2013 | Design is a case‐control prospective study. |
| Shwe 1988 | Not an RCT. |
| Shwe 1992 | Participants in the artemether arm also received single dose of mefloquine. |
| White 1992 | Participants not randomized. |
Abbreviations: RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Danis 1996.
| Methods | Multicentre trial |
| Participants | Information not available |
| Interventions | Information not available |
| Outcomes | Information not available |
| Notes | Information not available |
Faiz 2001.
| Methods | RCT |
| Participants | 105 adults enrolled |
| Interventions | Information not available |
| Outcomes | Information not available |
| Notes | Information not available |
Abbreviations: RCT: randomized controlled trial.
Differences between protocol and review
In the protocol, we specified that we would include only trials in children (aged < 15 years) (Esu 2013). However, we amended the inclusion criteria to include trials in adults and children.
We planned to explore data by drug regimen, type of severe malaria (cerebral versus non‐cerebral malaria), time since admission to hospital, length of follow‐up, and geographical region, but data were insufficient.
Dr Amirhaobu Uwaoma stepped down from the review author team.
Contributions of authors
Ekpereonne Esu (EE) and Emmanuel E Effa (EEE) identified and extracted data from eligible trials for this review. EE entered data into Review Manager 5 (Review Manager 2014). EEE and EE performed ‘Risk of bias' assessments and analysed data. EE prepared the ‘Summary of findings' tables and the first draft of the review. All authors read, gave input to all sections, and approved the final version.
Sources of support
Internal sources
University of Calabar, Nigeria.
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Project number: 300342‐104
Declarations of interest
EE has no known conflicts of interest. EEE has no known conflicts of interest. OO has no known conflicts of interest. MM has no known conflicts of interest.
Unchanged
References
References to studies included in this review
Adam 2002 {published data only}
- Adam I, Idris HM, Mohamed‐Ali AA, Aelbasit IA, Elbashir MI. Comparison of intramuscular artemether and intravenous quinine in the treatment of Sudanese children with severe falciparum malaria. East African Medical Journal 2002;79(12):621‐5. [DOI] [PubMed] [Google Scholar]
Aguwa 2010 {published data only}
- Aguwa CN, Ukwe CV, Adibe MO. A comparative study of quinine and artemether in the treatment of severe malaria in Nigerian children. Tropical Journal of Pharmaceutical Research 2010;9(1):11‐7. [Google Scholar]
Bobossi‐Serengbe 2015 {published data only}
- Bobossi‐Serengbe G, Gody JC, Fioboy R, Elowa JB, Manirakiza A. Comparison of the effectiveness of artemether and quinine for treatment of severe malaria in children, Bangui, Central African Republic. Bulletin de la Societe de Pathologie Exotique (1990) 2015;108(2):107‐11. [DOI] [PubMed] [Google Scholar]
Hien 1996 {published data only}
- Tran TH, Day NP, Nguyen HP, Nguyen TH, Tran TH, Pham PL, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. New England Journal of Medicine 1996;335(2):76‐83. [DOI] [PubMed] [Google Scholar]
Huda 2003 {published data only}
- Huda SN, Shahab T, Ali SM, Afzal K, Khan HM. A comparative clinical trial of artemether and quinine in children with severe malaria. Indian Pediatrics 2003;40(10):939‐45. [PubMed] [Google Scholar]
Karbwang 1992 {published data only}
- Karbwang J, Sukontason K, Rimchala W, Namsiripongpun W, Tin T, Auprayoon P, et al. Preliminary report: a comparative clinical trial of artemether and quinine in severe falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(4):768‐72. [PubMed] [Google Scholar]
Karbwang 1995 {published data only}
- Karbwang J, Tin T, Rimchala W, Sukontason K, Namsiripongpun V, Thanavibul A, et al. Comparison of artemether and quinine in the treatment of severe falciparum malaria in south‐east Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(6):668‐71. [DOI] [PubMed] [Google Scholar]
Minta 2005 {published data only}
- Minta D, Sissoko M, Sidibe I, Dolo A, Poudiougou B, Dembele M, et al. Efficacy and safety of artemether in the treatment of severe and complicated malaria in Mali [Efficacite et tolerance de l'artemether dans le traitement du paludisme grave et complique au Mali]. Mali Médical 2005;20(1‐2):28‐32. [PubMed] [Google Scholar]
Murphy 1996 {published data only}
- Murphy S, English M, Waruiru C, Mwangi I, Amukoye E, Crawley J, et al. An open randomized trial of artemether versus quinine in the treatment of cerebral malaria in African children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1996;90(3):298‐301. [DOI] [PubMed] [Google Scholar]
Ojuawo 1998 {published data only}
- Ojuawo A, Adegboye AR, Oyewalo O. Clinical response and parasite clearance in childhood cerebral malaria: A comparison between intramuscular artemether and intravenous quinine. East African Medical Journal 1998;75:450‐2. [Google Scholar]
Olumese 1999 {published data only}
- Olumese PE, Björkman A, Gbadegesin RA, Adeyemo AA, Walker O. Comparative efficacy of intramuscular artemether and intravenous quinine in Nigerian children with cerebral malaria. Acta Tropica 1999;73(3):231‐6. [DOI] [PubMed] [Google Scholar]
Osonuga 2009 {published data only}
- Osonuga O, Osonuga AA, Osonuga IO, Osonuga A. Temperature changes in the course of treatment of severe malaria patients with artemether and quinine. Asian Journal of Medical Sciences 2011;2(1):41‐5. [Google Scholar]
- Osonuga OA, Osonuga AA, Osonuga IO, Osonuga A. Comparison of Coma Resolution Time in the Course of Treating Childrenwith Severe Falciparum Malaria with Quinine and Artemether. World Journal of Medical Sciences 2011;6(2):42‐5. [Google Scholar]
- Osonuga OA, Osonuga IO. Parasitaemia changes in the course of treatment of severe malaria patients with artemether and quinine (A preliminary study). Macedonian Journal of Medical Sciences 2009;2(4):319‐23. [Google Scholar]
Phu 2010 {published data only}
- Phu NH, Tuan PQ, Day N, Mai NT, Chau TT, Chuong LV, et al. Randomized controlled trial of artesunate or artemether in Vietnamese adults with severe falciparum malaria. Malaria Journal 2010;9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Satti 2002 {published data only}
- Satti GM, Elhassan SH, Ibrahim SA. The efficacy of artemether versus quinine in the treatment of cerebral malaria. Journal of the Egyptian Society of Parasitology 2002;32(2):611‐23. [PubMed] [Google Scholar]
Seaton 1998 {published data only}
- Seaton RA, Trevett AJ, Wembri JP, Nwokolo N, Naraqi S, Black J, et al. Randomized comparison of intramuscular artemether and intravenous quinine in adult, Melanesian patients with severe or complicated, Plasmodium falciparum malaria in Papua New Guinea. Annals of Tropical Medicine and Parasitology 1998;92(2):133‐9. [PubMed] [Google Scholar]
Taylor 1998 {published data only}
- Taylor TE, Wills BA, Courval JM, Molyneux ME. Intramuscular artemether vs intravenous quinine: an open, randomized trial in Malawian children with cerebral malaria. Tropical Medicine and International Health 1998;3(1):3‐8. [DOI] [PubMed] [Google Scholar]
van Hensbroek 1996 {published data only}
- Hensbroek MB, Onyiorah E, Jaffar S, Schneider G, Palmer A, Frenkel J, et al. A trial of artemether or quinine in children with cerebral malaria. New England Journal of Medicine 1996;335(2):69‐75. [DOI] [PubMed] [Google Scholar]
Vinh 1997 {published data only}
- Ha V, Nguyen NH, Tran TB, Bui MC, Nguyen HP, Tran TH, et al. Severe and complicated malaria treated with artemisinin, artesunate or artemether in Viet Nam. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(4):465‐7. [DOI] [PubMed] [Google Scholar]
Walker 1993 {published data only}
- Walker O, Salako LA, Omukhodion SI, Sowunmi A. An open randomized comparative study of intramuscular artemether and intravenous quinine in cerebral malaria in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(5):564‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aceng 2005 {published data only}
- Aceng JR, Byarugaba JS, Tumwine JK. Rectal artemether versus intravenous quinine for the treatment of cerebral malaria in children in Uganda: randomised clinical trial. British Medical Journal 2005;330(7487):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharya 1997 {published data only}
- Bhattacharya PC, Pai‐dhungat AJ, Patel K. Artemether in moderate to severe malaria: a multicenter trial in India. Southeast Asian Journal of Tropical Medicine and Public Health 1997;28(4):736‐40. [PubMed] [Google Scholar]
Bunnag 1992 {published data only}
- Bunnag D, Karbwang J, Harinasuta T. Artemether in the treatment of multiple drug resistant falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(4):762‐7. [PubMed] [Google Scholar]
Dunyo 2011 {published data only}
- Dunyo S, Sirugo G, Sesay S, Bisseye C, Njie F, Adiamoh M, et al. Randomized trial of safety and effectiveness of chlorproguanil‐dapsone and lumefantrine‐artemether for uncomplicated malaria in children in the Gambia. PLoS One 2011;6(6):e17371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Falade 2007 {published data only}
- Falade CO, Fadero FF, Happi CT, Dada‐Adegbola HO, Gbotosho GO, Ayede I, et al. Dihydroartemisinin suppository in moderately severe malaria: comparative efficacy of dihydroartemisinin suppository versus intramuscular artemether followed by oral sulfadoxine‐pyrimethamine in the management of moderately severe malaria in Nigerian children. American Journal of Tropical Medicine and Hygiene 2007;76(1):1‐6. [PubMed] [Google Scholar]
Fargier 1999 {published data only}
- Fargier JJ, Louis FJ, Duparc S, Hounsinou C, Ringwald P, Danis M. Comparative study of artemether and quinine in severe Plasmodium falciparum malaria in adults and older children in Cameroon. Médicine Tropicale 1999;59(2):151‐6. [PubMed] [Google Scholar]
Karbwang 1994 {published data only}
- Karbwang J, Na‐Bangchang K, Wattanakoon Y, Thanavibul A, Harinasuta T. Artemether 5 versus 7 day regimen for severe falciparum malaria. Southeast Asian Journal ofTropical Medicine and Public Health 1994;25(4):702‐6. [PubMed] [Google Scholar]
Karunajeewa 2006 {published data only}
- Karunajeewa HA, Reeder J, Lorry K, Dabod E, Hamzah J, Page‐Sharp M, et al. Artesunate suppositories versus intramuscular artemether for treatment of severe malaria in children in Papua New Guinea. Antimicrobial Agents and Chemotherapy 2006;50(3):968‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myint 1987 {published data only}
- Myint PT, Shwe T. A controlled clinical trial of artemether (qinghaosu derivative) versus quinine in complicated and severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(4):559‐61. [DOI] [PubMed] [Google Scholar]
Osonuga 2006 {published data only}
- Osonuga OA, Osonuga IO, Akinsomisoye OS, Raji Y, Ademowo OG. Packed cell volume changes in the treatment of severe malarial patients with artemether and quinine (a preliminary study). Journal of Medical Sciences 2006;6(5):853‐7. [Google Scholar]
Reham 2012 {published data only}
- Rehman HU, Bilal H, Ali Q. Comparative study of intramuscular artemether and intravenous quinine in the treatment of cerebral malaria in children. Pakistan Paediatric Journal 2012;36(2):75‐80. [Google Scholar]
Rehman 2013 {published data only}
- Rehman MU, Shrestha B, Zehri T, Thapa S. Efficacy of quinine versus artemether in the treatment of severe malaria. Journal of the Nepal Health Research Council 2013;11(23):17‐21. [PubMed] [Google Scholar]
Shwe 1988 {published data only}
- Tin Shwe, Pe Than Myint, Ye Htut, Win Myint, Lin Soe. The effect of mefloquine artemether compared with quinine on patients with complicated falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988;82(5):665‐6. [DOI] [PubMed] [Google Scholar]
Shwe 1992 {published data only}
- Shwe T, Hla KK. The effect of artemether plus mefloquine on Myanmar patients with complicated falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(Suppl 4):117‐21. [PubMed] [Google Scholar]
White 1992 {published data only}
- White NJ, Waller D, Crawley J, Nosten F, Chapman D, Brewster D, et al. Comparison of artemether and chloroquine for severe malaria in Gambian children. Lancet 1992;339(8789):317‐21. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Danis 1996 {published data only}
- Danis M, Chandenier J, Doumbo O, Kombila M, Kouame J, Louis F, et al. Results obtained with i.m. artemether versus i.v. quinine in the treatment of severe malaria in a multi‐centre study in Africa. Japan Journal of Tropical Medicine and Hygiene 1996;24(Suppl 1):93‐6. [Google Scholar]
Faiz 2001 {published data only}
- Faiz MA, Rahman E, Hossain MA, Rahman MR, Yunus EB, Samad R, et al. A randomized controlled trial comparing artemether and quinine in the treatment of cerebral malaria in Bangladesh. Indian Journal of Malariology 2001;38(1‐2):9‐18. [PubMed] [Google Scholar]
Additional references
Adjuik 2004
- Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, WhiteN. International Artemisinin Study Group. Artesunate combinations for treatment of malaria: meta‐analysis. Lancet 2004;363(9402):9–17. [DOI] [PubMed] [Google Scholar]
AQMSG 2001
- Artemether‐Quinine Meta‐analysis Study Group. A meta‐analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(6):637‐50. [DOI] [PubMed] [Google Scholar]
Ashley 2014
- Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine 2014;371(5):411‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Cui 2015
- Cui L, Mharakurwa S, Ndiaye D, Rathod PK, Rosenthal PJ. Antimalarial drug resistance: literature review and activities and findings of the ICEMR network. American Journal of Tropical Medicine and Hygiene 2015;93(3 Suppl):57‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dondorp 2005
- Dondorp A, Nosten F, Stepniewska K, Day N, White N, South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005;366(9487):717‐25. [DOI] [PubMed] [Google Scholar]
Dondorp 2010
- Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open‐label, randomised trial. Lancet 2010;376(9753):1647‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doolan 2009
- Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clinical Microbiology Reviews 2009;22(1):13‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkes 2014
- Hawkes M, Conroy AL, Kain KC. Spread of artemisinin resistance in malaria. New England Journal of Medicine 2014;371(20):1944–5. [DOI] [PubMed] [Google Scholar]
Hien 2004
- Hien TT, Davis TM, Chuong LV, Ilett KF, Sinh DX, Phu NH, et al. Comparative pharmacokinetics of intramuscular artesunate and artemether in patients with severe falciparum malaria. Antimicrobial Agents and Chemotherapy 2004;48(2):871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Illet 2002
- Ilett KF, Batty KT, Powell SM, Binh TQ, Thu le TA, Phuong HL, et al. The pharmacokinetic properties of intramuscular artesunate and rectal dihydroartemisinin in uncomplicated falciparum malaria. British Journal of Clinical Pharmacology 2002;53(1):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaffar 1997
- Jaffar S, Hensbroek M, Palmer A, Schneider G, Greenwood B. Predictors of a fatal outcome following childhood cerebral malaria. American Journal of Tropical Medicine and Hygiene 1997;57(1):20–4. [DOI] [PubMed] [Google Scholar]
Karbwang 1997
- Karbwang J, Na‐Bangchang K, Congpuong K, Molunto P, Thanavibul A. Pharmacokinetics and bioavailability of oral and intramuscular artemether. European Journal of Clinical Pharmacology 1997;52(4):307–10. [DOI] [PubMed] [Google Scholar]
Kyu 2009
- Kyu HH, Fernández E. Artemisinin derivatives versus quinine for cerebral malaria in African children: a systematic review. Bulletin of the World Health Organization 2009;87(12):896‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
McIntosh 2000
- McIntosh HM, Olliaro P. Artemisinin derivatives for treating severe malaria. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menard 2016
- Menard D, Khim N, Beghain J, Adegnika AA, Shafiul‐Alam M, Amodu O, et al. KARMA Consortium. A worldwide map of Plasmodium falciparum K13‐Propeller polymorphisms. New England Journal of Medicine 2016;374(25):2453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mithwani 2004
- Mithwani S, Aarons L, Kokwaro GO, Majid O, Muchohi S, Edwards G, et al. Population pharmacokinetics of artemether and dihydroartemisinin following single intramuscular dosing of artemether in African children with severe falciparum malaria. British Journal of Clinical Pharmacology 2004;57(2):146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 1997
- Murphy SA, Mberu E, Muhia D, English M, Crawley J, Waruiru C, et al. The disposition of intramuscular artemether in children with cerebral malaria; a preliminary study. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(3):331‐4. [DOI] [PubMed] [Google Scholar]
Navaratnam 2000
- Navaratnam V, Mansor SM, Sit NW, Grace J, Li Q, Olliaro P. Pharmacokinetics of artemisinin‐type compounds. Clinical Pharmacokinetics 2000;39(4):255‐70. [DOI] [PubMed] [Google Scholar]
Nealon 2002
- Nealon C, Dzeing A, Müller‐Römer U, Planche T, Sinou V, Kombila M, et al. Intramuscular bioavailability and clinical efficacy of artesunate in Gabonese children with severe malaria. Antimicrobial Agents and Chemotherapy 2002;46(12):3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pittler 1999
- Pittler MH, Ernst E. Artemether for severe malaria: a meta‐analysis of randomized clinical trials. Clinical Infectious Diseases 1999;28(3):597‐601. [DOI] [PubMed] [Google Scholar]
PrayGod 2008
- PrayGod G, Frey A, Eisenhut M. Artemisinin derivatives versus quinine in treating severe malaria in children: a systematic review. Malaria Journal 2008;7:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sinclair 2012
- Sinclair D, Donegan S, Lalloo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD005967.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
ter Kuile 1993
- ter Kuile F, White NJ, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Experimental Parasitology 1993;76(1):85–95. [DOI] [PubMed] [Google Scholar]
Tyagi 2018
- Tyagi RK, Gleeson PJ, Arnold L, Tahar R, Prieur E, Decosterd L, Pérignon JL, Olliaro P, Druilhe P. High‐level artemisinin‐resistance with quinine co‐resistance emerges in P. falciparum malaria under in vivo artesunate pressure. BMC Medicine 2018;16(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
WHO 2008
- World Health Organization. The global burden of disease: 2004 update. Geneva: World Health Organization, 2008. [Google Scholar]
WHO 2010
- World Health Organization. Roll Back Malaria Department. Guidelines for the treatment of malaria. Second Edition. Geneva: World Health Organization, 2010. [Google Scholar]
WHO 2015
- World Health Organization. Roll Back Malaria Department. Guidelines for the Treatment of Malaria. Third Edition. Geneva: World Health Organization, 2015. [Google Scholar]
Wongsrichanalai 2002
- Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug‐resistant malaria. The Lancet infectious diseases 2002;2(4):209‐18. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Esu 2013
- Esu E, Effa EE, Opie ON, Uwaoma A, Meremikwu M. Artemether for severe malaria. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010678] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esu 2014
- Esu E, Effa EE, Opie ON, Uwaoma A, Meremikwu MM. Artemether for severe malaria. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD010678.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


