Abstract
Background
Numerous studies have explored the anti-tumor effect of berberine (BBR), but little clinical evidence guides the use of BBR in cancer patients.
Objectives
Our aim was to investigate the impact of BBR on various cancers in healthy animals to promote the transformation from bench to bed.
Search methods
PubMed, Embase, Springer, and Cochrane databases were searched from January 2000 to October 2018 for relevant articles.
Selection criteria
Only published studies focusing on the relationship between BBR and various cancers in vivo were qualified. Two review authors independently assessed the risk of bias for each study, and any disagreement was resolved by discussion or by involving a third assessor.
Results
A total of 26 studies from 2000 to 2018, focusing on various cancer types, including breast cancer, liver cancer, colorectal cancer, nasopharyngeal carcinoma, lung cancer, gastric cancer, neuroepithelial cancer, endometrial carcinoma, esophageal cancer, tongue cancer, cholangiocarcinoma, and sarcoma were included. Overall, BBR reduced tumor volume (SMD =3.72, 95% CI: 2.89, 4.56, Z = 8.73, p < 0.00001) and tumor weight (SMD =2.35, 95% CI: 1.51, 3.19, Z = 5.50, p < 0.00001) in a linear The dose–response relationship (Pearson r = − 0.6717, p < 0.0001 in tumor volume analysis; Pearson r = − 0.7704, p < 0.0005 in tumor weight analysis). BBR inhibited angiogenesis in tumor tissues (SMD = 4.29, 95% CI: 2.14, 6.44, Z = 3.92, p < 0.00001), but it had no significant effect on the body weight of experimental animals (SMD = 0.11, 95% CI: − 0.70, 0.92, Z = 0.27, p = 0.78). Publication bias was not detected.
Conclusion
BBR exerted anti-tumor effects in a variety of tumors in vivo, especially breast cancer and lung cancer, and the evidence was still insufficient in colorectal cancer and gastric cancer.
Keywords: Berberine, Cancer, Experimental animals, Meta-analysis
Background
Berberine (BBR) is a natural component purified from the species of the genus Berberis, which has long been used as an anti-diarrheal drug in gastrointestinal disorders in traditional Chinese medicine [1]. At the same time, the anti-tumor effect of BBR has been a hot topic in experimental research in recent years. In the past 3 years, latest studies have shown the anticancer actions of BBR against several high-risk cancers, including lung cancer [2], breast cancer [3], prostate cancer [4], colorectal cancer [5], and gastric cancer [6].
However, little clinical evidence guides the use of BBR in cancer patients. Thus, systematic reviews and meta-analyses of animal studies may help to clarify whether cancer patients could benefit from this approach and promote the transformation of animal studies into humans at the same time [7].
Our aim was to investigate the impact of BBR on cancer growth and its adverse effects in randomized controlled trials in healthy animals.
Methods
Identification of studies
From January 2000 to October 2017, relevant literature from PubMed, Embase, Springer, and Cochrane databases was systematically screened. The following Mesh terms and textwords were used: “Neoplasms”[Mesh], “Neoplasia,” “Neoplasias,” “Neoplasm,” “Tumors,” “Tumor,” “Cancer,” “Cancers,” “Malignant Neoplasms,” “Malignant Neoplasm,” “Neoplasm, Malignant,” “Neoplasms, Malignant,” “Malignancy,” “Malignancies,” “Berberine”[Mesh]," “Berberine,” “Umbellatine,” and “BBR.” The “AND” or “OR” operator was used to combine these terms in varying combinations. At the same time, references in the articles were also included in the screening. We did not set a language limit during the process. Two authors (Jianhao Xu, Yuming Long) independently reviewed the titles and abstracts identified in the search. In this process, we discussed the articles to incorporate the differences. If problems still could not be resolved, a third assessor (Yusong Zhang) was invited to make a decision. Only published articles were included. No protocol was developed for this review.
Selection criteria
The inclusion criteria were as follows: (1) participants: experimental animals including rodent, mouse, rat, rabbit, guinea pig, dog, horse, sheep, and monkey; (2) invention: BBR only; (3) outcomes: the effects of BBR in animal models after tumor implantation, including tumor volume, tumor weight, tumor vessel density, and body weight; and (4) study design: experiments should be prospectively controlled. The exclusion criteria were as follows: (a) literature published as letters, editorials, abstracts, reviews, and expert opinions; (b) non-animal-based studies; (c) articles with missing data information; (d) similar and repeated studies; and (e) outdated articles with little significance and credibility. Cohen’s kappa statistic was used to assess chance-corrected agreement between reviewers (SPSS version 18. 0, SPSS Inc. Chicago, IL) [8].
Study characteristics and data extraction
A detailed form was designed for data extraction: first author, publication year, country, cancer type, animals’ baseline characteristics, intervention, duration, and the data of specific outcomes (tumor volume, tumor weight, tumor vessel density, and body weight). Two review authors extracted the data by using the agreed form.
Quality of evidence and risk of bias
For risk of bias of individual studies, the ARRIVE checklist was used to assess pre-clinical animal studies [9]. For risk of bias among studies, such as publication bias and selective reporting, funnel chart analysis, subgroup analysis, and sensitive analysis were all conducted. Two review authors (Jian−hao Xu, Yuming Long) independently assessed the risk of bias for each study.
Data synthesis and statistical analysis
We carried out statistical analysis by using the Review Manager software (RevMan 5.3) and STATA statistical software package version 12.0 (Stata Corporation, College Station, TX). The primary outcomes were tumor volume, tumor weight, and tumor vessel density of BBR group compared with the control group. The secondary outcome was the change of body weight. Mean value and standard difference (SD) were used as summary statistics. Standard mean difference (SMD) was measured for continuous data. Linear regression and Pearson’s correlation analysis were used to study the The dose–response relationship between BBR and the four outcomes. The heterogeneity among studies was measured by using the I2 test. The latent publication bias was assessed by using a funnel plot. All statistical tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Search results
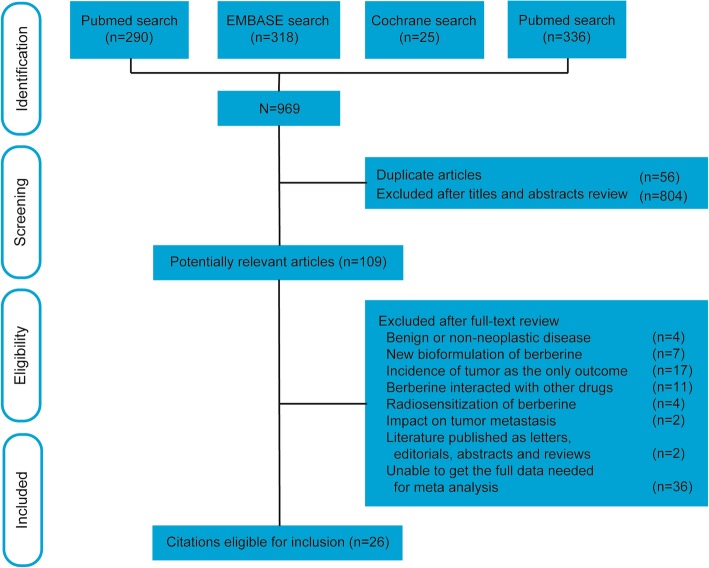
A total of 969 potential articles were identified from the literature search. After selection, 26 studies [10–35] matched the inclusion criteria and were suitable for our meta-analysis. The flow diagram in Fig. 1 showed the selection process. A review of the study selection and data extraction indicated excellent agreement between reviewers (k = 0.820).
Fig. 1.
Flow chart for the selection of records to include
Study characteristics and quality assessment
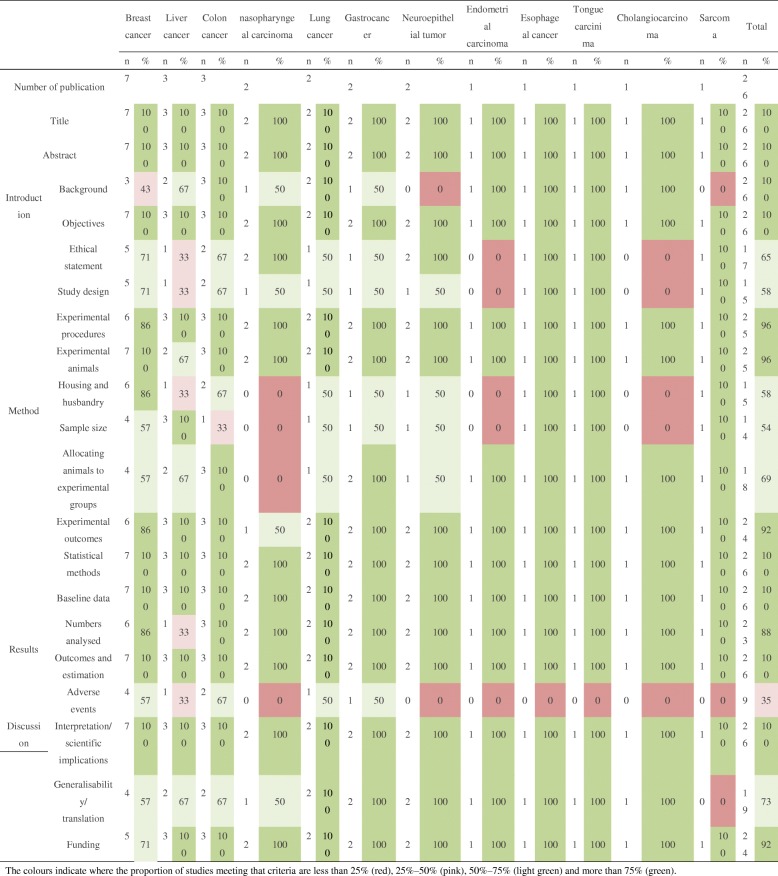
Study characteristics are summarized in Table 1. A total of 26 studies [10–35] from 2000 to 2018, focusing on various cancer types, including breast cancer [10–16], liver cancer [17–19], colorectal cancer [20–22], nasopharyngeal carcinoma [23, 24], lung cancer [25, 26], gastric cancer [27, 28], neuroepithelial tumor [29, 30], endometrial carcinoma [31], esophageal cancer [32], tongue cancer [33], cholangiocarcinoma [34], and sarcoma [35] were included. The studies used rats [14], hamsters [34], and mice [10–13, 15–33, 35] modeled via subcutaneous tumor implantation [10–13, 16–35] or induced tumor formation [14, 15]. BBR was administered in doses ranging between 2.5 mg/kg and 200 mg/kg body weight through intraperitoneal injection [10, 15–17, 19, 21, 23, 24, 30, 33, 35] and gavage [11–14, 18, 20, 22, 25–29, 31, 32, 34] or from 1000 ppm to 5400 ppm in drinking water [13, 20, 25]. The size of the study sample ranged from 6 to 20, while the follow-up ranged from 1 week to 32.5 weeks. Quality assessment based on the ARRIVE guideline is presented in Table 2. Overall, the studies included in our analysis were of moderate quality.
Table 1.
Characteristics of prospective studies on BBR
| Author, year, country | Species, strain, gender, age | Model cell line | Experiment | Control | Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dosage | Frequency | Adnimistration | Duration | type | mean0 | Sd0 | n0 | mean1 | Sd1 | n1 | p value | ||||
| Breast cancer | |||||||||||||||
| Elisa Pierpaoli, 2015, Italy [10] | mice, FVB/N, F, 4w | SK-BR-3 | 2.5 mg/kg | biw | ip | 32.5w | DMSO | VD | 16.81 | 7.24 | 10 | 12.09 | 1.98 | 10 | 0.07 |
| Yuwan Zhao, 2017, China [11] | mice, BALB/c, F, 6w | MDA-MB-231 | 100 mg/kg | tiw | po | 3w | DMSO | TV | 2.70 | 0.18 | 7 | 0.68 | 0.08 | 7 | < 0.01 |
| BW | 24.40 | 0.69 | 7 | 22.48 | 0.81 | 7 | < 0.01 | ||||||||
| Alaa Refaat, 2013, Japan [12] | mice, BALB/c, F, 6w | 4 T1 | 100 mg/kg | qd | po | 4.3w | CMC | TW | 0.21 | 0.01 | 6 | 0.15 | 0.01 | 6 | < 0.01 |
| Sangmin Kim, 2018, Korea [13] | mice, Balb/c, F, 6-8w | MDA-MB-231 | 0.1% BBR in the drinking water | Daily free intake | po | 6.6w | – | TV | 0.42 | 0.18 | 5 | 0.21 | 0.08 | 5 | 0.05 |
| Kalyani Chowdary Karnam, 2017, India [14] | rats, SD, F, 6.4–8.3w | Induced by DMBA | 50 mg/kg [pretreatment] | tiw | po | 4w | Corn oil | TV | 3.79 | 0.90 | 6 | 0.63 | 0.30 | 6 | < 0.01 |
| TW | 9.64 | 0.90 | 6 | 3.80 | 0.99 | 6 | < 0.01 | ||||||||
| 50 mg/kg [posttreatment] | TV | 3.79 | 0.90 | 6 | 1.31 | 0.60 | 6 | < 0.01 | |||||||
| TW | 9.64 | 0.90 | 6 | 5.71 | 1.32 | 6 | < 0.01 | ||||||||
| Elisa Damiani, 2015, Italy [15] | miceFVB/NF4w | HER2/neu transgenic mice | 2.5 mg/kg | biw | ip | NR | Sterile saline | VD | 16.77 | 5.31 | 7 | 11.07 | 1.75 | 9 | 0.03 |
| Ke Su, 2016, China [16] | mice, Balb/c, F, 6w | MDA-MB-231 | 10 mg/kg | q4d | ip | 3w | DMSO | TV | 0.59 | 0.27 | 6 | 0.27 | 0.12 | 6 | 0.02 |
| TW | 0.50 | 0.11 | 6 | 0.29 | 0.06 | 6 | < 0.01 | ||||||||
| BW | 22.59 | 7.31 | 6 | 19.10 | 3.71 | 6 | 0.32 | ||||||||
| Liver cancer | |||||||||||||||
| Guan-Yu Wang, 2009, China [17] | mice, Balb/c, M, 6w | HEPG2 | 40 mg/kg | qd | ip | 1.4w | Saline | TV | 3.31 | 0.38 | 5 | 2.21 | 0.22 | 6 | < 0.01 |
| BW | 3.13 | 0.43 | 5 | 4.62 | 0.41 | 6 | < 0.01 | ||||||||
| 80 mg/kg | TV | 3.31 | 0.38 | 5 | 1.43 | 0.13 | 5 | < 0.01 | |||||||
| BW | 3.13 | 0.43 | 5 | 3.74 | 0.36 | 5 | 0.04 | ||||||||
| Jing Li, 2015, Canada [18] | mice, Balb/c, NR, 6-8w | H22 | 50 mg/kg | qd | po | 2w | Water | TV | 4.24 | 0.56 | 10 | 0.33 | 0.35 | 10 | < 0.01 |
| Chi Man Tsang, 2015, China [19] | mice, NR, NR, NR | MHCC-97 L-luciferase | 10 mg/kg | qod | ip | 5w | Saline | TV | 1.00 | 0.05 | 7 | 0.21 | 0.03 | 7 | < 0.01 |
| VD | 12.58 | 2.94 | 7 | 2.18 | 1.29 | 7 | < 0.01 | ||||||||
| Colon cancer | |||||||||||||||
| Norio Iizuka, 2002, Japan [20] | mice, Balb/c, M, 6w | Colon26/clone 20 | 0.1% BBR in the driNRing water | Daily free intake | po | 2w | – | TW | 0.22 | 0.15 | 9 | 0.25 | 0.12 | 9 | 0.65 |
| BW | 18.20 | 1.50 | 9 | 18.40 | 1.80 | 9 | 0.80 | ||||||||
| 0.2% BBR in the driNRing water | TW | 0.22 | 0.15 | 9 | 0.25 | 0.15 | 9 | 0.68 | |||||||
| BW | 18.20 | 1.50 | 9 | 22.20 | 1.50 | 9 | < 0.01 | ||||||||
| 0.4% BBR in the driNRing water | TW | 0.22 | 0.15 | 9 | 0.24 | 0.18 | 9 | 0.80 | |||||||
| BW | 18.20 | 1.50 | 9 | 20.90 | 4.20 | 9 | 0.10 | ||||||||
| H Ruan, 2017, China [21] | mice, Balb/c, NR, 6-7w | KM12C/shCtrl | 10 mg/kg | qd | ip | 2w | DMSO | TV | 1.26 | 0.97 | 6 | 0.79 | 0.53 | 6 | 0.32 |
| KM12C/shRXRα | 1.54 | 0.92 | 6 | 1.40 | 0.46 | 6 | 0.76 | ||||||||
| Yuchen Cai, 2013, Japan [22] | mice, Balb/c, NR, 5w | HT-29 | 10 mg/kg | qd | po | 2w | Sterile water | TV | 6.11 | 3.01 | 10 | 4.33 | 2.42 | 10 | 0.16 |
| BW | 6.60 | 3.60 | 10 | 4.90 | 3.20 | 10 | 0.28 | ||||||||
| 30 mg/kg | TV | 6.11 | 3.01 | 10 | 4.09 | 1.76 | 10 | 0.08 | |||||||
| BW | 6.60 | 3.60 | 10 | 3.90 | 2.70 | 10 | 0.07 | ||||||||
| 50 mg/kg | TV | 6.11 | 3.01 | 10 | 3.34 | 1.31 | 11 | 0.01 | |||||||
| BW | 6.60 | 3.60 | 10 | 3.60 | 2.50 | 11 | 0.04 | ||||||||
| nasopharyngeal carcinoma | |||||||||||||||
| Chao Wang, 2017, China [23] | mice, NOD/SCID, F, 8w | HONE-1 | 10 mg/kg | tiw | ip | 3w | DMSO | TV | 0.58 | 0.06 | 5 | 0.10 | 0.03 | 5 | < 0.01 |
| TW | 0.15 | 0.01 | 5 | 0.02 | 0.01 | 5 | < 0.01 | ||||||||
| Chi Man Tsang, 2013, China [24] | mice, NR, M, 6-8w | C666–1 | 5 mg/kg | qod | ip | 6w | DMSO | TV | 0.15 | 0.05 | 5 | 0.04 | 0.03 | 5 | < 0.01 |
| 10 mg/kg | 0.15 | 0.05 | 5 | 0.02 | 0.02 | 4 | < 0.01 | ||||||||
| Lung cancer | |||||||||||||||
| Michael A. James, 2011, Missouri [25] | mice, Balb/c, M, 4-6w | A549 | 1800 ppm | Daily free intake | po | 4w | DMSO | TV | 0.06 | 0.02 | 3 | 0.02 | 0.02 | 4 | 0.05 |
| 5400 ppm | 0.06 | 0.02 | 3 | 0.01 | 0.01 | 2 | 0.04 | ||||||||
| Santosh K. Katiyar, 2009, Alabama [26] | mice, Balb/c, F, 6-7w | A549 | 50 mg/kg | qd | po | 7w | PBS | TV | 1.40 | 0.07 | 10 | 0.99 | 0.04 | 10 | < 0.01 |
| TW | 2.32 | 0.27 | 10 | 2.02 | 0.30 | 10 | 0.03 | ||||||||
| 100 mg/kg | TV | 1.40 | 0.07 | 10 | 0.60 | 0.03 | 10 | < 0.01 | |||||||
| TW | 2.32 | 0.27 | 10 | 1.16 | 0.21 | 10 | < 0.01 | ||||||||
| 200 mg/kg | TV | 1.40 | 0.07 | 10 | 0.30 | 0.06 | 10 | < 0.01 | |||||||
| TW | 2.32 | 0.27 | 10 | 0.62 | 0.09 | 10 | < 0.01 | ||||||||
| H1299 | 50 mg/kg | TV | 1.59 | 0.10 | 10 | 1.36 | 0.05 | 10 | < 0.01 | ||||||
| TW | 2.71 | 0.31 | 10 | 2.36 | 0.29 | 10 | 0.02 | ||||||||
| 100 mg/kg | TV | 1.59 | 0.10 | 10 | 1.05 | 0.05 | 10 | < 0.01 | |||||||
| TW | 2.71 | 0.31 | 10 | 1.82 | 0.29 | 10 | < 0.01 | ||||||||
| 200 mg/kg | TV | 1.59 | 0.10 | 10 | 0.61 | 0.02 | 10 | < 0.01 | |||||||
| TW | 2.71 | 0.31 | 10 | 1.15 | 0.10 | 10 | < 0.01 | ||||||||
| Gastric cancer | |||||||||||||||
| Junxiong Wang, 2016, China [27] | mice, Balb/c, F, 5w | BGC823 | 50 mg/kg | qd | po | 4w | NR | TV | 2.28 | 0.24 | 3 | 0.73 | 0.13 | 3 | < 0.01 |
| TW | 1.37 | 0.37 | 3 | 0.32 | 0.08 | 3 | < 0.01 | ||||||||
| BW | 2.51 | 0.69 | 3 | 0.10 | 0.46 | 3 | < 0.01 | ||||||||
| Hongli Li, 2016, China [28] | mice, Balb/c, M, 4w | MGC803 | 15 mg/kg | qd | po | 3.3w | NR | TV | 0.85 | 0.29 | 6 | 0.44 | 0.09 | 6 | 0.02 |
| TW | 0.68 | 0.18 | 6 | 0.42 | 0.07 | 6 | < 0.01 | ||||||||
| Neuroepithelial tumor | |||||||||||||||
| Juan Wang, 2015, China [29] | miceBalb/cNN | – | 100 mg/kg | qd | po | 3w | NR | TV | 0.04 | 0.02 | 8 | 0.02 | 0.00 | 8 | 0.05 |
| Yuxue Sun, 2018, China [30] | miceBalb/cN6-8w | C6 | 10 mg/kg | qd | ip | 1w | DMSO | TV | 0.77 | 0.22 | 7 | 0.35 | 0.06 | 7 | < 0.01 |
| Endometrial carcinoma | |||||||||||||||
| Yu Wang, 2018, China [31] | mice, Balb/c, NR, 6w | HEC-1-A | 50 mg/kg | qd | po | 4w | DMSO | TV | 1.01 | 0.13 | 6 | 0.65 | 0.06 | 6 | < 0.01 |
| 100 mg/kg | 1.01 | 0.13 | 6 | 0.34 | 0.04 | 6 | < 0.01 | ||||||||
| Esophageal cancer | |||||||||||||||
| Kewei Ren, 2016, China [32] | mice, Balb/c, M, 6-8w | Eca9706 | 20 mg/kg | qd | po | 7w | DMSO | TV | 6.37 | 0.25 | 5 | 5.05 | 0.60 | 5 | < 0.01 |
| TW | 2.66 | 0.29 | 5 | 1.82 | 0.21 | 5 | < 0.01 | ||||||||
| Tongue squamous cell carcinima | |||||||||||||||
| Yung-Tsuan Ho, 2009, China [33] | mice, Balb/c, F, 6w | SCC-4 | 10 mg/kg | q4d | ip | 4w | DMSO | TV | 0.18 | 0.06 | 6 | 0.03 | 0.02 | 6 | < 0.01 |
| TW | 0.26 | 0.16 | 6 | 0.12 | 0.09 | 6 | 0.11 | ||||||||
| Cholangiocarcinoma | |||||||||||||||
| Nattapong Puthdee, 2013, Japan [34] | hamster, Syrian, M, 4-5w | Ham-1 | 10 mg/kg | qd | po | 3w | sterile water | TW | 0.70 | 0.18 | 5 | 0.67 | 0.11 | 5 | 0.79 |
| Sarcoma | |||||||||||||||
| Lei Zhang, 2012, China [35] | mice, Kunming, NR, 6w | S180 | 30 mg/kg | qd | ip | NR | NR | TW | 2.20 | 0.93 | 10 | 1.26 | 0.54 | 9 | 0.02 |
| BW | 2.71 | 2.20 | 10 | −2.54 | 3.24 | 9 | < 0.01 | ||||||||
Mean0: mean value in control group (cm3 for tumor volume, g for tumor weight and body weight, mm/mm2 for vessel density); Sd0: standard difference in control group; N0: sample size in control group; Mean1: mean in berberine group; Sd1: standard difference in berberine group; N1: sample size in berberine group; M: male; F: femle; NR: non reported; TV: tumor volume; TW: tumor weight; VD: vessel density; BW: body weight
Table 2.
Quality assessment of eligible studies with ARRIVE checklist
The colours indicate where the proportion of studies meeting that criteria are less than 25% (red), 25%–50% (pink), 50%–75% (light green) and more than 75% (green)
Tumor volume
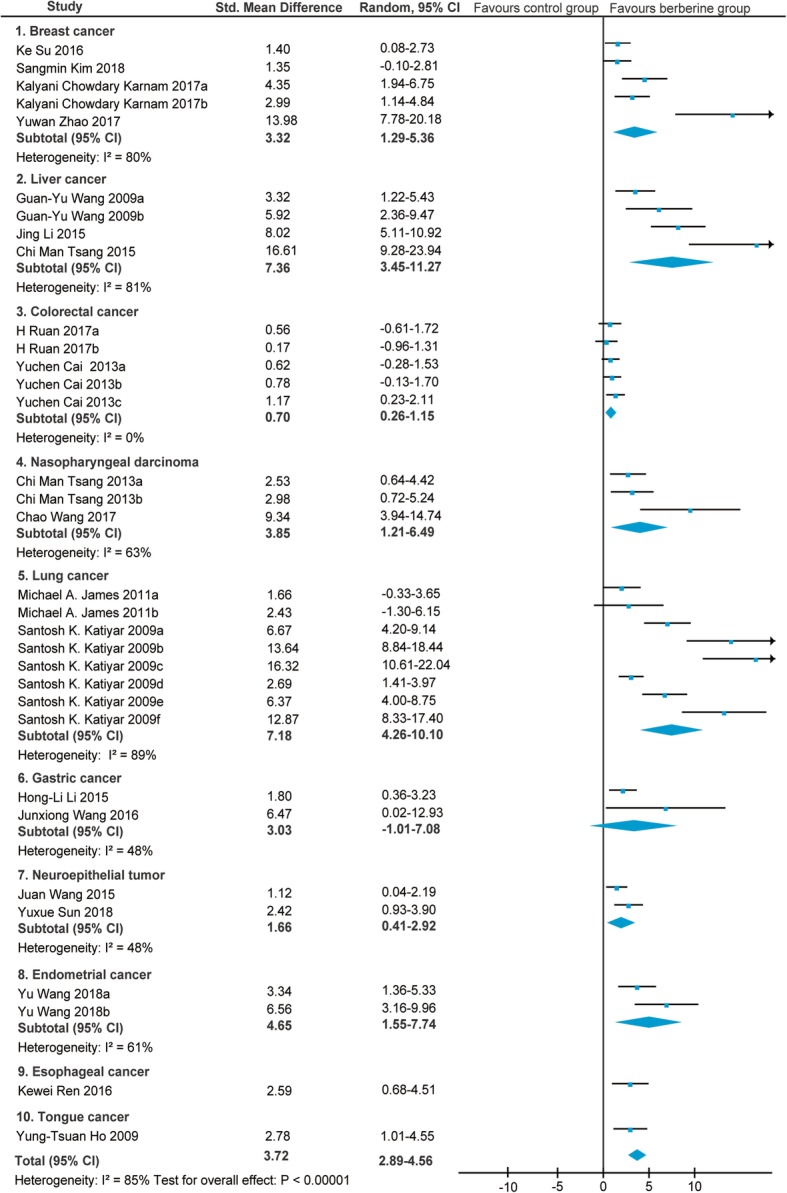
Of the 26 screened articles [10–35], 20 [11, 13, 14, 16–19, 21–33] reported the relationship between BBR and tumor volume in animals with breast cancer [11, 13, 14, 16], liver cancer [17–19], colorectal cancer [21, 22], nasopharyngeal carcinoma [23, 24], lung cancer [25, 26], gastric cancer [27, 28], neuroepithelial cancer [29, 30], endometrial carcinoma [31], esophageal cancer [32], and tongue cancer [33]. The SMD and the 95%CI in each study are shown in Fig. 2. The pooled SMD remained statistically significant in breast cancer (SMD = 3.32, 95% CI: 1.29, 5.36; Z = 3.2, p = 0.001), liver cancer (SMD = 7.36, 95% CI: 3.45, 11.27; Z = 3.69, p = 0.0002), colorectal cancer (SMD = 0.70, 95% CI: 0.26, 1.15; Z = 3.10, p = 0.002), nasopharyngeal carcinoma (SMD = 3.85, 95% CI: 1.21, 6.49; Z = 2.86, p = 0.004), lung cancer (SMD = 7.18, 95% CI: 4.26, 10.10; Z = 4.82, p < 0.00001), neuroepithelial tumor (SMD = 1.66, 95% CI:0.41, 2.92; Z = 2.59, p = 0.010), and endometrial cancer (SMD = 4.65, 95% CI: 1.55, 7.74; Z = 2.94, P = 0.003). The pooled SMD remained statistically insignificant in gastric cancer (SMD = 1.47, 95% CI: − 1.01, 7.08; Z = 1.47, P = 0.14). For total studies, the pooled result suggested that the SMD was 3.72 (95% CI: 2.89, 4.56) with statistical significance (Z = 8.73, p < 0.00001).
Fig. 2.
Forest plot of the tumor volume
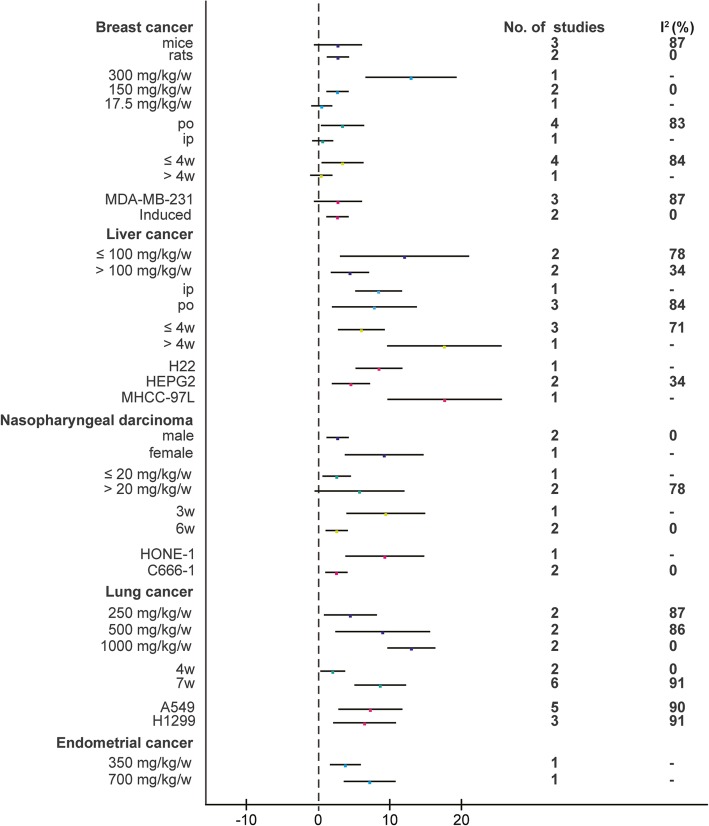
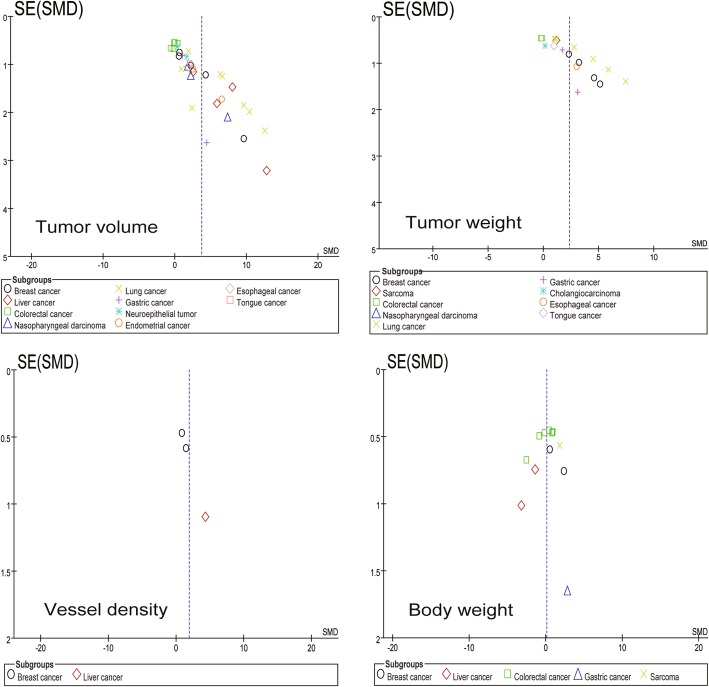
In view of the obvious heterogeneity (I2 = 80% for breast cancer; I2 = 81% for liver cancer; I2 = 63% for nasopharyngeal carcinoma; I2 = 89% for lung cancer; I2 = 61% for endometrial cancer), we conducted a subgroup analysis of different characteristics mainly on the following aspects: gender, animals, BBR dose, administration, duration, and cell lines (Fig. 3). In breast cancer, the BBR dose was a potential influencing factor (I2 decreased to 0% in one subgroup. Another two I2 were missing due to the limited study). In liver cancer, the cell line was a potential influencing factor (I2 decreased to 34% in one subgroup. Another two I2 were missing due to the limited study). In nasopharyngeal carcinoma, gender, duration, and cell line were potential influencing factors (I2 decreased to 0% in one subgroup. Another I2 was missing due to the limited study). In lung cancer, the BBR dose was a potential influencing factor (I2 decreased to 87, 86, and 0% in three subgroups respectively). In endometrial cancer, no potential influencing factor was filtered.
Fig. 3.
Subgroup analyses of the tumor volume
The dose–response relationship of different cancer types on the relationship between BBR and tumor volume of animals is shown in Fig. 4. For single cancer types, a statistically significant linear relationship in colorectal cancer (Pearson r = − 0.8785, p = 0.0499) and lung cancer (Pearson r = − 0.6718, p = 0.0459) was observed. For total studies, the SMD values of all studies showed a statistically significant decreasing trend with increasing concentration of BBR (Pearson r = − 0.6717, p < 0.0001).
Fig. 4.
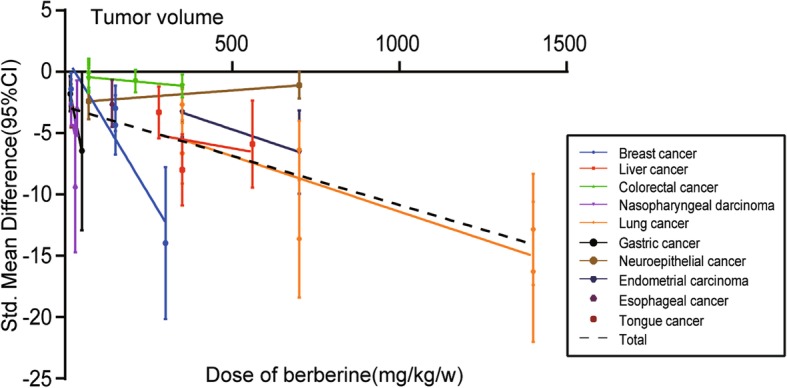
The dose–response relationship of BBR and tumor volume
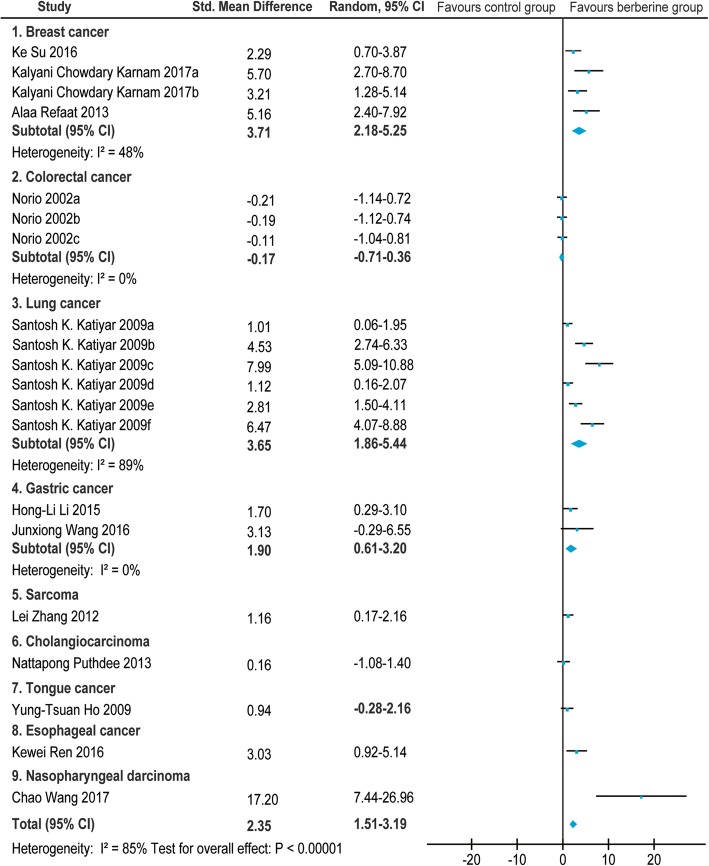
Tumor weight
Of the 26 screened articles [10–35], 12 [12, 14, 16, 20, 23, 26–28, 32–35] reported the relationship between BBR and tumor weight in animals with breast cancer [12, 14, 16], colorectal cancer [20], nasopharyngeal carcinoma [23], lung cancer [26], gastric cancer [27, 28], esophageal cancer [32], and tongue cancer [33]. The SMD and the 95%CI in each study are shown in Fig. 5. The pooled SMD remained statistically significant in breast cancer(SMD = 3.71, 95% CI: 2.18, 5.25; Z = 4.74, p < 0.00001), lung cancer(SMD = 3.65, 95% CI: 1.86, 5.44; Z = 4.00, p < 0.0001), and gastric cancer(SMD = 1.90, 95% CI: 0.61, 3.20; Z = 2.88, p = 0.004). The pooled SMD remained statistically insignificant in colorectal cancer(SMD = − 0.17, 95% CI: − 0.71, 0.36; Z = 0.63, p = 0.53). For total studies, the pooled result suggested that the SMD was 2.35(95% CI: 1.51, 3.19) with statistical significance (Z = 5.50, p < 0.00001).
Fig. 5.
Forest plot of the tumor weight
In view of the obvious heterogeneity(I2 = 89% for lung cancer), we conducted a subgroup analysis of different characteristics mainly on the following aspects: dose of BBR and cell lines(Fig. 6). In lung cancer, the dose of BBR was a potential influencing factor (I2 decreased to 0, 57, and 0% in three subgroups respectively).
Fig. 6.
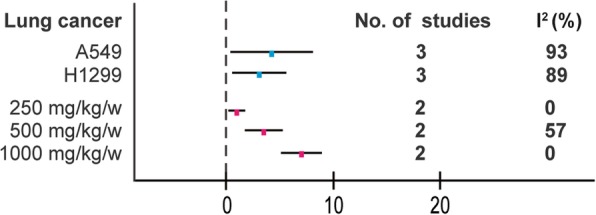
Subgroup analyses of the tumor weight
The dose–response relationship of different cancer types on the relationship between BBR and tumor weight of animals is shown in Fig. 7. For single cancer types, a statistically significant linear relationship in lung cancer (Pearson r = − 0.9623, p = 0.0021) was observed. For total studies, the SMD values of all studies showed a statistically significant decreasing trend with increasing concentration of BBR (Pearson r = − 0.7704, p < 0.0005).
Fig. 7.
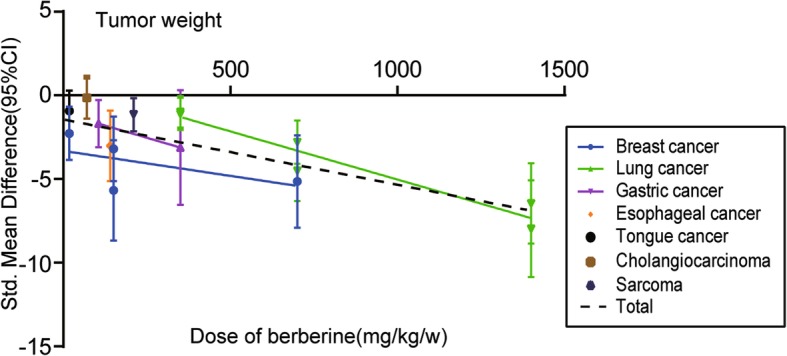
The dose–response relationship of BBR and tumor weight
Tumor vessel density
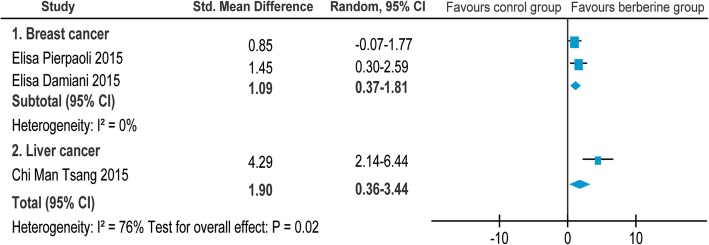
Of the 26 screened articles [10–35], 3 [10, 15, 19] reported the relationship between BBR and tumor vessel density in animals with breast cancer [10, 15] and liver cancer [19]. The SMD and the 95%CI in each study are shown in Fig. 8. The pooled SMD remained statistically significant in breast cancer(SMD = 1.09, 95% CI: 0.37, 1.81; Z = 2.96, p = 0.003). For total studies, the pooled result suggested that the SMD was 4.29(95% CI: 2.14, 6.44) with statistical significance(Z = 3.92, p < 0.00001).
Fig. 8.
Forest plot of the tumor vessel density
No statistical heterogeneity was observed (I2 = 0% for breast cancer).
The dose–response relationship of different cancer types on the relationship between BBR and tumor weight of animals is shown in Fig. 9. For single cancer types, no linear relationship was concluded because of the limited studies. For total studies, the SMD values of all studies showed no statistically significant trend(Pearson r = − 0.9866, p = 0.1044).
Fig. 9.
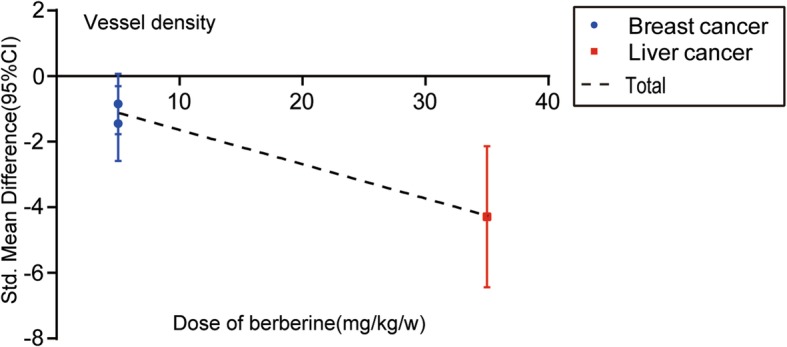
The dose–response relationship of BBR and tumor vessel density
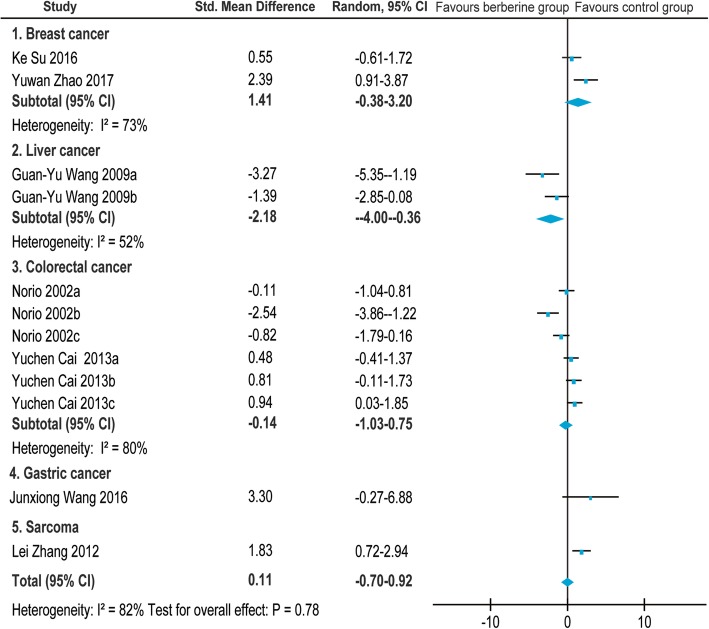
Body weight
Of the 26 screened articles [10–35], 7 [11, 16, 17, 20, 22, 27, 35] reported the relationship between BBR and body weight in animals with breast cancer [11, 16], liver cancer [17], colorectal cancer [20, 22], gastric cancer [27], and sarcoma [35]. The SMD and the 95%CI in each study are shown in Fig. 10. The pooled SMD remained statistically significant in liver cancer(SMD = − 2.18, 95% CI: − 4.00, − 0.36; Z = 2.35, p = 0.02). The pooled SMD remained statistically insignificant in breast cancer(SMD = 1.41, 95% CI: − 0.38, 3.20; Z = 1.54, p = 0.12) and colorectal cancer(SMD = − 0.14, 95% CI: − 1.03, 0.75; Z = 0.30, p = 0.76). For total studies, the pooled result suggested that the SMD was 0.11(95% CI: − 0.70, 0.92) with statistical significance(Z = 0.27, p = 0.78).
Fig. 10.
Forest plot of the body weight
In view of the obvious heterogeneity(I2 = 73% for breast cancer; I2 = 80% for colorectal cancer; I2 = 52% for liver cancer), we conducted a subgroup analysis of different characteristics mainly on the following aspects: dose of BBR, administration, and cell lines(Fig. 11). I2 were missing in breast cancer group and liver cancer group due to limited studies. No potential influencing factor was found in colorectal cancer group.
Fig. 11.
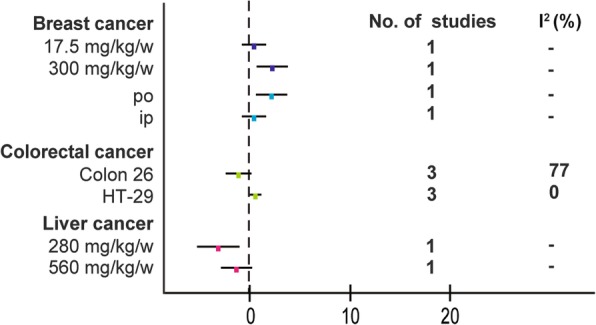
Subgroup analyses of the body weight
The dose–response relationship of different cancer types on the relationship between BBR and body weight of animals is shown in Fig. 12. For single cancer types, no statistically significant linear relationship was found. For total studies, the SMD values of all studies showed no statistically significant trend(Pearson r = − 0.1440, p = 0.7116).
Fig. 12.
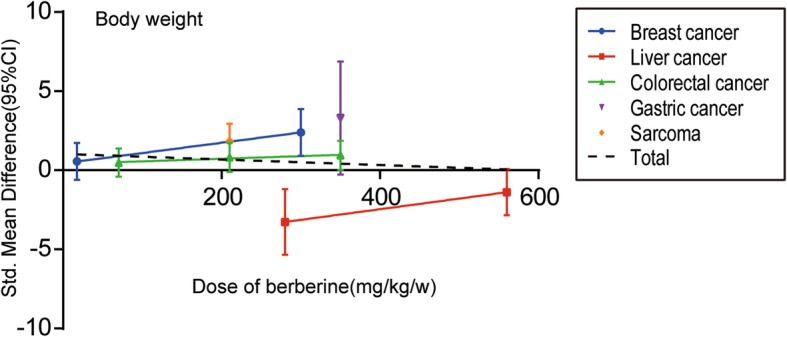
The dose–response relationship of BBR and body weight
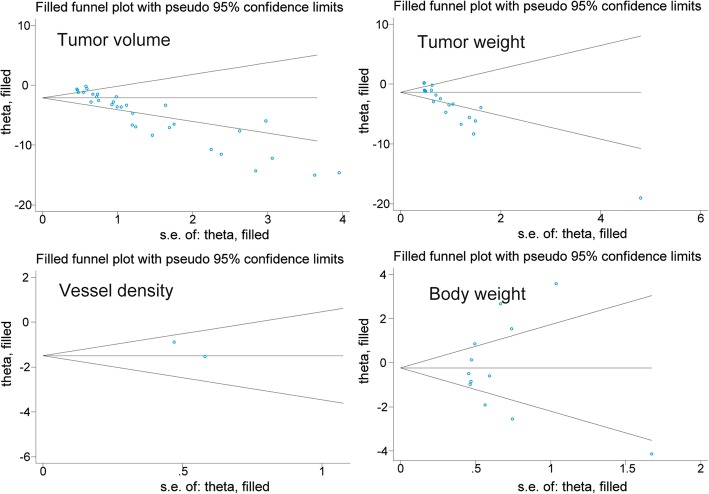
Publication bias and sensitivity analysis
The publication bias evaluation for the meta-analysis of tumor volume, tumor weight, tumor vessel density, and body weight is shown in Fig. 13. These funnel plots showed that most of the studies are in the upper part of the inverted funnel and approximately symmetrical, suggesting that the publication bias was unobvious.
Fig. 13.
Funnel plot of tumor volume studies, tumor weight studies, tumor vessel density studies, and body weight studies
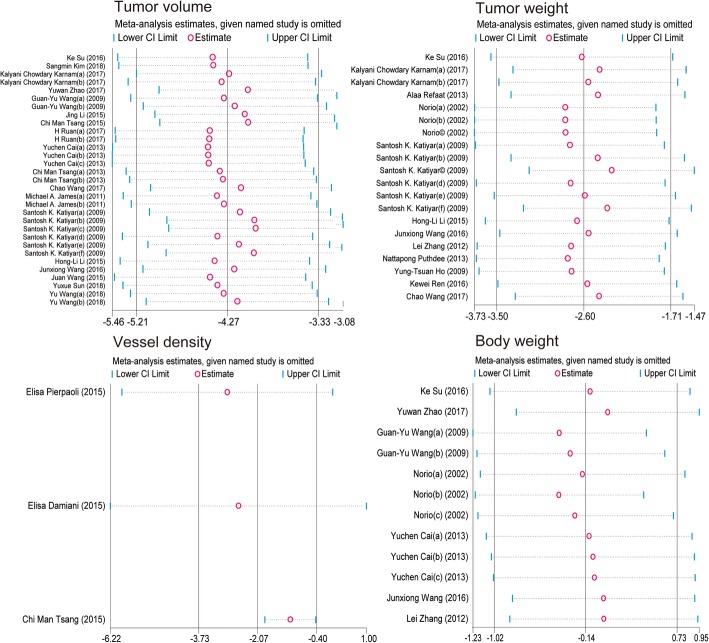
A sensitivity analysis was performed to assess the stability of our results in terms of tumor volume, tumor weight, tumor vessel density, and body weight. The trim method was used, and the results did not show considerable changes between the previous and new SMDs (Fig. 14). Next, we deleted one individual study at a time, and the results of the rest of the studies were checked for any reversal. The statistical outcomes showed that the pooled SMDs were all still significant although one study was excluded (Fig. 15).
Fig. 14.
Metatrim plot of tumor volume studies, tumor weight studies, tumor vessel density studies, and body weight studies
Fig. 15.
Metaninf plot of tumor volume studies, tumor weight studies, tumor vessel density studies, and body weight studies
Molecular pathways and proteins
Among these included studies, a wide range of molecular targets, which are essential for the anti-cancer effect of BBR, was revealed. Except for three articles [15, 33, 35] that did not involve the discussion of molecular mechanisms, the remaining 23 articles [10–14, 16–32, 34] analyzed the anti-tumor mechanism of BBR. The pharmacological effects of BBR was summarized into five aspects: proliferation(including apoptosis, autophagy, cell cycle arrest, and others), intracellular oxidative stress, inflammation, angiogenesis, and migration. Table 3 shows how BBR works in different scenarios of multiple types of cancers. In addition, in order to understand the anticancer mechanism more clearly and deeply, Table 4 shows the clustering analysis of the common molecular pathways and target proteins between studies.
Table 3.
Molecular pathways and proteins in different cancers
| Molecular Pathway | Proteins | Functional clustering |
|---|---|---|
| Breast cancer | ||
|
↑ caspase-9/cytochrome c-mediated apoptosis [11]; TRAIL(TNF-related apoptosis-inducing ligand)-mediated apoptosis [12] ↓ cell proliferation [14] |
↑ caspase-3 [11, 12]; caspase-9, ClvC-3, Bax, Ligase4 [11]; PARP, P53 [12] |
Proliferation(including apoptosis) |
| ↑ intracellular reactive oxygen species (ROS) levels [14] |
↑ MDA [14] ↓ SOD, CAT, GSH, Vit-C [14] |
Intracellular oxidative stress |
| ↓ inflammation [14] | ↓ IL-1β, IL-6, TNF-α, NF-kB [14] | Inflammation |
| ↓ TGF-β1-induced cell migration [13]; vasodilator-stimulated phosphoprotein (VASP)-induced cell migration [16] |
↓TGF-β1, MMP-2, MMP-9 [13] No effect: VASP [16] |
Migration |
| Liver cancer | ||
|
↑ Fas-mediated apoptosis [17] ↓ arachidonic acid metabolic pathway [18]; Id-1-induced cell proliferation [19] |
↑Fas, P53, caspase-3, caspase-8, caspase-9 [17] ↓ PGE2, cPLA2, COX-2 [18]; Id-1 [19] No effect: caspase-3, caspase-9 [18] |
Proliferation(including apoptosis) |
| ↓ Id-1-induced angiogenesis [19] | ↓ Id-1, VEGF, HIF-1α [19] | Angiogenesis |
| ↓ Id-1-induced migration [19] | ↓Id-1 [19] | Migration |
| Colon cancer | ||
| ↓ β-catenin - induced proliferation by binding RXR [21]; cell proliferation by inducing the G2/M phase arrest and down-regulated the expression of the related cyclins [22] |
↑ c-Cbl, p21WAF1/CIP1 [21] ↓ Cdc2 [21, 22]; PCNA, β-catenin, Ki-67, c-myc, RXRα [21]; cyclin B1, cdc25c [22] |
Proliferation(including cell cycle arrest) |
| Nasopharyngeal carcinoma | ||
| ↓cell proliferation via an Epstein-Barr virus nuclear antigen 1(EBNA1)-dependent mechanism [23]; cell proliferation by inhibiting STAT3 activation [24] |
↑ Cleaved PARP [24] |
Proliferation |
| Lung cancer | ||
|
↑ G1 cell cycle arrest [25]; P53-Induced growth inhibition and apoptosis [26] ↓cell proliferation via MAPK pathways [25] |
Proliferation(including apoptosis and cell cycle arrest) | |
| Gastric cancer | ||
|
↑ apoptosis and cell cycle arrest via inhibiting EGFR signaling [27] ↓ cell proliferation via MAPK pathways [28] |
↓pERK [27, 28]; pAKT, pSTAT3, pNFκB, NFκB, Bcl-xL, cyclin D1 [27]; p-P38 MAPK, p-JNK, IL-8 [28] | Proliferation(including apoptosis and cell cycle arrest) |
| Neuroepithelial cancer | ||
|
↑ ERK1/2-mediated impairment of mitochondrial aerobic respiration and autophagy [30] ↓cancer growth by suppressing Hedgehog signaling pathway [29] |
↑ C-parp-1, LC3II [30] |
Proliferation(including autophagy) |
| Endometrial carcinoma | ||
| ↓ cell growth via miR-101/COX-2 [31] | ↓ COX-2, PGE2 [31] | Proliferation |
| ↓ cell metastasis via miR-101/COX-2 [31] | ↓ COX-2, PGE2 [31] | Migration |
| Esophageal cancer | ||
| ↑ cell growth inhibition, apoptosis and cell cycle arrest at G2/M phase [32] |
↑ P21, P27, P53, cleaved-PARP, caspase-3, Bax [32] ↓ PI3K, Rac, p-JAK2, p-STAT3, Wnt3a, β-catenin, Bcl-2, Mcl-1, XIAP, Ki-67, cyclin B, cyclin D, cyclin E, CDK1, CDK2, CDK4, CDK6 [32] |
Proliferation(including apoptosis and cell cycle arrest) |
| Cholangiocarcinoma | ||
|
↑ G1 cell cycle arrest [34] ↓ cell proliferation [34] |
↓ PCNA, cyclin D1, cyclin E [34] | Proliferation(including cell cycle arrest) |
Table 4.
Cluster analysis of molecular pathways and proteins in different cancers
| Functional clustering | Molecular Pathway | Proteins |
|---|---|---|
| Proliferation(apoptosis) |
Breast cancer: ↑ caspase-9/cytochrome c-mediated apoptosis [11]; TRAIL(TNF-related apoptosis-inducing ligand)-mediated apoptosis [12] Liver cancer: ↑ Fas-mediated apoptosis [17]; ↓ arachidonic acid metabolic pathway [18] Lung cancer: ↑ P53-Induced growth inhibition and apoptosis [26] Gastric cancer: ↑ apoptosis via inhibiting EGFR signaling [27] Esophageal cancer: ↑ cell growth inhibition and apoptosis [32] |
↑ caspase-3 [11, 12, 17, 26, 32]; P53 [12, 17, 25, 26, 32]; Bax [11, 26, 32]; caspase-9 [11, 17]; PARP [12, 32]; ClvC-3, Ligase4 [11]; Fas [17]; caspase-8 [17]; Bak [26]; P21, P27 [32] ↓ Bcl-2 [11, 26, 32]; Mcl-1 [12, 32]; Bcl-xl [26, 28]; pERK [27, 28]; pSTAT3 [28, 32]; P65 [12]; PGE2, cPLA2, COX-2 [18]; pAKT, pNFκB, NFκB [28]; PI3K, Rac, p-JAK2, Wnt3a, β-catenin, XIAP, Ki-67 [32] No effect: caspase-3, caspase-9 [18] |
| Proliferation(autophagy) | Neuroepithelial cancer: ↑ ERK1/2-mediated impairment of mitochondrial aerobic respiration and autophagy [30] |
↑ C-parp-1, LC3II [30] ↓ Ki-67, p-ERK1/2 [30] |
| Proliferation(cell cycle arrest) |
Colon cancer: ↓ cell proliferation by inducing the G2/M phase arrest and down-regulated the expression of the related cyclins [22] Lung cancer: ↑ G1 cell cycle arrest [25] Gastric cancer: ↑ cell cycle arrest via inhibiting EGFR signaling [27] Esophageal cancer: ↑ cell cycle arrest at G2/M phase [32] Cholangiocarcinoma: ↑ G1 cell cycle arrest [34] |
↓ cyclin B1 [22, 25, 32]; cyclin D1 [27, 32, 34]; cyclin E [32, 34]; Cdc2 [22]; cdc25c [22]; CDK1, CDK2, CDK4, CDK6 [32] |
| Proliferation(others) |
Breast cancer: ↓ cell proliferation [14] Liver cancer: ↓ Id-1-induced cell proliferation [19] Colon cancer: ↓ β-catenin - induced proliferation by binding RXR [21] Nasopharyngeal carcinoma: ↓ cell proliferation via an Epstein-Barr virus nuclear antigen 1(EBNA1)-dependent mechanism [23]; ↓ cell proliferation by inhibiting STAT3 activation [24] Lung cancer: ↓cell proliferation via MAPK pathways [25] Gastric cancer: ↓ cell proliferation via MAPK pathways [28] Neuroepithelial cancer: ↓cancer growth by suppressing Hedgehog signaling pathway [29] Endometrial carcinoma: ↓ cell growth via miR-101/COX-2 [31] Cholangiocarcinoma: ↓ cell proliferation [34] |
↑ c-Cbl, p21WAF1/CIP1 [21]; Cleaved PARP [24] ↓ PCNA [14, 21, 34]; Mcl-1, p-STAT3 [23, 24]; p-MAPK [25, 28]; Id-1 [19]; β-catenin, Ki-67, c-myc, RXRα [21]; EBNA1 [23]; p-Akt, p-CREB [25]; p-JNK, IL-8 [28]; Gli1, PTCH1 [29]; COX-2, PGE2 [31] |
| Intracellular oxidative stress | Breast cancer: ↑ intracellular reactive oxygen species (ROS) levels [14] |
↑ MDA [14] ↓ SOD, CAT, GSH, Vit-C [14] |
| Inflammation | Breast cancer: ↓ inflammation [14] | ↓ IL-1β, IL-6, TNF-α, NF-kB [14] |
| Angiogenesis | Liver cancer: ↓ Id-1-induced angiogenesis [19] | ↓ Id-1, VEGF, HIF-1α [19] |
| Migration |
Breast cancer: ↓ TGF-β1-induced cell migration [13]; vasodilator-stimulated phosphoprotein (VASP)-induced cell migration [16] Liver cancer: ↓ Id-1-induced migration [19] Endometrial carcinoma: ↓ cell metastasis via miR-101/COX-2 [31] |
↓TGF-β1, MMP-2, MMP-9 [13]; Id-1 [19]; COX-2, PGE2 [31] No effect: VASP [16] |
The most frequently studied pathways were on cell proliferation and 19 articles focused on this mechanism. Seven of these studies involved tumor cell apoptosis pathways (breast cancer [9, 12], liver cancer [17, 18], lung cancer [26], gastric cancer [27], esophageal cancer [32]), one involved autophagy pathways (neuroepithelial cancer [30]), and five involved cell cycle arrest pathways (colon cancer [22], lung cance [25], gastric cancer [27], esophageal cancer [32], cholangiocarcinoma [34]). The second most common frequently studied pathways were on cell migration. Four articles in three cancers studied the relationship between BBR and tumor cell migration (breast cancer [13, 16], liver cancer [19], endometrial carcinoma [31]). There was only one study reported the relationship between BBR and intracellular oxidative stress (breast cancer [14]), inflammation (breast cancer [14]), and angiogenesis (liver cancer [19]) respectively.
Discussion
We performed a systematic review and meta-analysis to systematically evaluate the efficacy and adverse effect of BBR on various cancers. The results showed that BBR could inhibit the growth of a variety of cancers in vivo, especially in breast cancer (SMD = 3.32, 95% CI: 1.29, 5.36 in tumor volume; SMD = 3.71, 95% CI: 2.18, 5.25 in tumor weight; SMD = 1.09, 95% CI: 0.37, 1.81 in tumor vessel density) and lung cancer (SMD = 7.36, 95% CI: 3.45, 11.27 in tumor volume; SMD = 3.65, 95% CI: 1.86, 5.44 in tumor weight). Evidence for the benefit of BBR was not sufficient for gastric cancer (SMD = 1.47, 95% CI: − 1.01, 7.08 in tumor volume) and colorectal cancer (SMD = − 0.17, 95% CI: − 0.71, 0.36 in tumor weight). BBR showed a dose–response relationship in tumor volume and weight (Pearson r = − 0.6717 and − 0.7704, with p < 0.0001 and p < 0.0005, respectively). At the same time, dose was an important influencing factor for heterogeneity from the subgroup analysis. The change in body weight of experimental animals was used as an indicator of the adverse effects of BBR. The above results indicated that no statistically significant difference was observed in terms of body weight under the effect of BBR (SMD =0.11, 95% CI: − 0.70, 0.92).
In the past 3 years, numerous studies have attempted to elucidate the relationship between BBR and breast/lung cancer. By using molecular modeling and in vitro studies, BBR significantly reduced EGFR and AKT phosphorylation and may be a useful alternative to lapatinib, an EGFR inhibitor which can cause acquired drug resistance in breast cancer patients [36]. BBR lowers blood sugar, increases insulin sensitivity, and corrects lipid metabolism disorders; it may reduce the incidence of breast cancer [37]. Single-drug BBR has an obvious inhibitory effect on lung cancer cells; BBR can inhibit doxorubicin (DOX)-mediated STAT3 activation and sensitize lung cancer cells to the cytotoxic effects of DOX treatment. Given the widespread clinical application of BBR and its low toxicity, our findings are important for the development of a new combination of BBR and DOX for the treatment of lung cancer [38]. In addition to medical treatment, BBR has protective effects on radiation-induced lung injury via intercellular adhesion molecular-1 and transforming growth factor-beta-1 in patients with lung cancer [39].
Although, in the present study, the therapeutic effect of BBR in colorectal and gastric cancer required more evidence, numerous studies have confirmed the gain effect of BBR combined with chemotherapy in recent years. Latest research shows that the combination of the second generation Hsp90 inhibitor NVP-AUY922 and BBR therapy could inhibit a variety of oncogenic signaling pathways of colorectal cancer [40]. Another study showed that BBR as an adjunctive therapeutic agent could attenuate chemical resistance during gastric cancer treatment. The combination of 5-FU and BBR showed synergistic inhibition of survivin and STAT3 levels, thereby enhancing the death of gastric cancer cells [41]. In addition to the 5-FU-based chemotherapy regimen, BBR treatment reduced cisplatin resistance in gastric cancer cells by modulating the miR-203/Bcl-w apoptotic axis [42].
In the present study, body weight index was used to evaluate the growth of experimental animals to indirectly evaluate the adverse effects of BBR. However, studies have shown that BBR could induce weight loss in rodents [43, 44] and humans [45, 46]. In recent years, research has reported that BBR affected body weight by upregulating AMPK and UCP3 expression to control energy expenditure [47]. Therefore, the toxic side effects of BBR cannot be objectively and accurately evaluated by the change of body weight alone.
Limitations
There were some limitations to our analysis that deserve discussion. First, we observed considerable heterogeneity between the studies when analyzing tumor volume, tumor weight, and body weight. Although subgroup analysis (Figs. 3, 5, and 11) was performed, some I2 were missing because of the limited studies. Secondly, generally speaking, obviously significant publication bias was not found based on the funnel plot (Fig. 13). However, poor symmetry of the funnel plot on tumor volume suggested more high-quality researches should be included. Thirdly, although PubMed, Embase, Springer, and Cochrane databases had been carefully and comprehensively searched, articles selected for each cancer type were still small which could lead to bias. Fourthly, the anticancer effects of berberine in humans were not identified clearly and further studies in humans were needed to develop it as an anticancer agent.
Conclusion
BBR exerted anti-tumor effects in a variety of tumors in vivo, especially for breast cancer and lung cancer. However, evidence was still insufficient in colorectal cancer and gastric cancer. One of its anti-tumor mechanisms was anti-angiogenesis. There was a dose-response relationship in the anti-tumor effects.
Acknowledgements
The current study was funded by the Kunshan first people s hospital affiliated to Jiangsu University, and the second affiliated hospital of Soochow University.
Abbreviations
- BBR
Berberine
- BW
Body Weight
- CI
Confidence Interval
- DMSO
Dimethyl Sulfoxide
- EBNA1
Epstein-Barr virus nuclear antigen 1
- ROS
Reactive Oxygen Species
- SD
Standard Difference
- SMD
Standard Mean Difference
- TRAIL
TNF-related apoptosis-inducing ligand
- TV
Tumor Volume
- TW
Tumor Weight
- VASP
Vasodilator-Stimulated Phosphoprotein
- VD
Vessel Density
Authors’ contributions
XJH and ZYS designed this study. XJH, LYM, NLW and YXY collected and analyzed the data. XJH, WRH and YN drafted the manuscript. XJH, TJL and ZYS interpreted the data and revised the manuscript. All authors have read and approved the manuscript.
Funding
The current study was supported by grants from the second affiliated hospital of Soochow university pre-research project (SDFEYGJ1609), the second affiliated hospital of Soochow university clinical discipline group project funding (XKQ 2015008), the international team of gastrointestinal tumor project funding (SZYJTD201804) and the project from national key laboratory of radiation medicine (GZK1201820). The funder had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianhao Xu and Yuming Long contributed equally to this work.
Yuming Long is the co-first author.
References
- 1.Chen C, Yu Z, Li Y, Fichna J, Storr M. Effects of Berberine in the gastrointestinal tract u2014 a review of actions and therapeutic implications. Am J Chinese Med. 2014;42:1450066. doi: 10.1142/S0192415X14500669. [DOI] [PubMed] [Google Scholar]
- 2.Kim S, Song YL, Cho HJ. Berberine and zinc oxide-based nanoparticles for the chemo-photothermal therapy of lung adenocarcinoma. Biochemical & Biophysical Research Communications. 2018;501:765–770. doi: 10.1016/j.bbrc.2018.05.063. [DOI] [PubMed] [Google Scholar]
- 3.CAZZANIGA MASSIMILIANO, BONANNI BERNARDO. Relationship Between Metabolic Disorders and Breast Cancer Incidence and Outcomes. Is There a Preventive and Therapeutic Role for Berberine? Anticancer Research. 2018;38(8):4393–4402. doi: 10.21873/anticanres.12741. [DOI] [PubMed] [Google Scholar]
- 4.Yuantong T, Lijing Z, Wang H, Zhang X, Zhao J. Berberine inhibits androgen synthesis by interaction with aldo-keto reductase 1C3 in 22Rv1 prostate cancer cells. Asian J Androl. 2016;18:607–612. doi: 10.4103/1008-682X.169997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Huang C, Wu L, Wen B. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. Oncotargets & Therapy. 2016;9:4121–4127. doi: 10.2147/OTT.S104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Q, Li L, Zou X, Xu L, Yi P. Berberine attenuated proliferation, invasion and migration by targeting the AMPK/HNF4α/WNT5A pathway in gastric carcinoma. Front Pharmacol. 2018;9:1150. [DOI] [PMC free article] [PubMed]
- 7.Hui KK, Yu JL. Effects of protein kinase inhibitor, 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine, on beta-2 adrenergic receptor activation and desensitization in intact human lymphocytes. Journal of Pharmacology & Experimental Therapeutics. 1989;249:492. [PubMed] [Google Scholar]
- 8.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 9.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments--the ARRIVE guidelines. J Cereb Blood Flow Metab. 2011;31:991–993. doi: 10.1038/jcbfm.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierpaoli E, Damiani E, Orlando F, Lucarini G, Bartozzi B, Lombardi P, Salvatore C, Geroni C, Donati A, Provinciali M. Antiangiogenic and antitumor activities of berberine derivative NAX014 compound in a transgenic murine model of HER2/neu-positive mammary carcinoma. Carcinogenesis. 2015;36:1169. doi: 10.1093/carcin/bgv103. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Jing Z, Lv J, Zhang Z, Lin J, Cao X, Zhao Z, Liu P, Mao W. Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed Pharmacother. 2017;95:18–24. doi: 10.1016/j.biopha.2017.08.045. [DOI] [PubMed] [Google Scholar]
- 12.Refaat A, Abdelhamed S, Yagita H, Inoue H, Yokoyama S, Hayakawa Y, Saiki I. Berberine enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast cancer. Oncol Lett. 2013;6:840–844. doi: 10.3892/ol.2013.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Lee J, You D, Jeong Y, Jeon M, Yu J, Kim SW, Nam SJ, Lee JE. Berberine suppresses cell motility through downregulation of TGF-beta1 in TripleNegative breast Cancer cells. Cell Physiol Biochem. 2018;45:795–807. doi: 10.1159/000487171. [DOI] [PubMed] [Google Scholar]
- 14.Karnam KC, Ellutla M, Bodduluru LN, Kasala ER, Uppulapu SK, Kalyankumarraju M, Lahkar M. Preventive effect of berberine against DMBA-induced breast cancer in female Sprague Dawley rats. Biomed Pharmacother. 2017;92:207–214. doi: 10.1016/j.biopha.2017.05.069. [DOI] [PubMed] [Google Scholar]
- 15.Elisa D, Elisa P, Fiorenza O, Abele D, Mauro P. Sidestream dark field videomicroscopy for in vivo evaluation of vascularization and perfusion of mammary tumours in HER2/neu transgenic mice. Clinical & Experimental Pharmacology & Physiology. 2015;42:225–229. doi: 10.1111/1440-1681.12343. [DOI] [PubMed] [Google Scholar]
- 16.Su K, Hu P, Wang X, Kuang C, Xiang Q, Yang F, Xiang J, Zhu S, Wei L, Zhang J. Tumor suppressor berberine binds VASp to inhibit cell migration in basal-like breast cancer. Oncotarget. 2016;7:45849–45862. doi: 10.18632/oncotarget.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan-Yu W, Qing-Hua L, Qi D, Rong-Zhen X, Qing-Hua D. Berbamine induces Fas-mediated apoptosis in human hepatocellular carcinoma HepG2 cells and inhibits its tumor growth in nude mice. J Asian Nat Prod Res. 2009;11:219–228. doi: 10.1080/10286020802675076. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Li O, Kan M, Zhang M, Shao D, Pan Y, Zheng H, Zhang X, Chen L, Liu S. Berberine induces apoptosis by suppressing the arachidonic acid metabolic pathway in hepatocellular carcinoma. Mol Med Rep. 2015;12:4572. doi: 10.3892/mmr.2015.3926. [DOI] [PubMed] [Google Scholar]
- 19.Chi MT, Cheung KCP, Cheung YC, Man K, Lui WY, Tsao SW, Feng Y. Berberine suppresses Id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. BBA - Molecular Basis of Disease. 2015;1852:541–551. doi: 10.1016/j.bbadis.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Norio I, Shoichi H, Kiyoshi Y, Shigefumi Y, Akira T, Koji M, Kiwamu O, Masaaki O. Anticachectic effects of the natural herb Coptidis rhizoma and berberine on mice bearing colon 26/clone 20 adenocarcinoma. Int J Cancer. 2010;99:286–291. doi: 10.1002/ijc.10338. [DOI] [PubMed] [Google Scholar]
- 21.Ruan H, Zhan YY, Hou J, Xu B, Chen B, Tian Y, Wu D, Zhao Y, Zhang Y, Chen X. Berberine binds RXRα to suppress Î2-catenin signaling in colon cancer cells. Oncogene. 2017;36:6906. doi: 10.1038/onc.2017.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Y, Xia Q, Luo R, Huang P, Sun Y, Shi Y, Jiang W. Berberine inhibits the growth of human colorectal adenocarcinoma in vitro and in vivo. J Nat Med-Tokyo. 2014;68:53–62. doi: 10.1007/s11418-013-0766-z. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Wang H, Zhang Y, Guo W, Long C, Wang J, Liu L, Sun X. Berberine inhibits the proliferation of human nasopharyngeal carcinoma cells via an Epstein-Barr virus nuclear antigen 1-dependent mechanism. Oncol Rep. 2017;37(4):2109–2120. doi: 10.3892/or.2017.5489. [DOI] [PubMed] [Google Scholar]
- 24.Chi MT, Cheung YC, Lui WY, Yip YL, Zhang G, Lin VW, Cheung CP, Feng Y, Tsao SW. Berberine suppresses tumorigenicity and growth of nasopharyngeal carcinoma cells by inhibiting STAT3 activation induced by tumor associated fibroblasts. BMC Cancer. 2013;13:619. doi: 10.1186/1471-2407-13-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James MA, Fu H, Liu Y, Chen DR, You M. Dietary administration of berberine or Phellodendron amurense extract inhibits cell cycle progression and lung tumorigenesis. Mol Carcinog. 2011;50:1–7. doi: 10.1002/mc.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katiyar SK, Meeran SM, Katiyar N, Akhtar S. p53 cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol Carcinogen. 2010;48:24–37. doi: 10.1002/mc.20453. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Yang S, Cai X, Dong J, Chen Z, Wang R, Zhang S, Cao H, Lu D, Jin T. Berberine inhibits EGFR signaling and enhances the antitumor effects of EGFR inhibitors in gastric cancer. Oncotarget. 2016;7:76076–76086. doi: 10.18632/oncotarget.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li HL, Wu H, Zhang BB, Shi HL, Wu XJ. MAPK pathways are involved in the inhibitory effect of berberine hydrochloride on gastric cancer MGC 803 cell proliferation and IL-8 secretion in vitro and in vivo. Mol Med Rep. 2016;14:1430. doi: 10.3892/mmr.2016.5361. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Peng Y, Liu Y, Yang J, Ding N, Tan W. Berberine, a natural compound, suppresses hedgehog signaling pathway activity and cancer growth. BMC Cancer. 2015;15:595. doi: 10.1186/s12885-015-1596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Yu J, Liu X, Zhang C, Cao J, Li G, Liu X, Chen Y, Huang H. Oncosis-like cell death is induced by berberine through ERK1/2-mediated impairment of mitochondrial aerobic respiration in gliomas. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;102:699. doi: 10.1016/j.biopha.2018.03.132. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Zhang S. Berberine suppresses growth and metastasis of endometrial cancer cells via miR-101/COX-2. Biomed Pharmacother. 2018;103:1287–1293. doi: 10.1016/j.biopha.2018.04.161. [DOI] [PubMed] [Google Scholar]
- 32.Ren K, Zhang W, Wu G, Ren J, Lu H, Li Z, Han X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed Pharmacother. 2016;84:1748–1759. doi: 10.1016/j.biopha.2016.10.111. [DOI] [PubMed] [Google Scholar]
- 33.Ho YT, Yang JS, Lu CC, Chiang JH, Li TC, Lin JJ, Lai KC, Liao CL, Lin JG, Chung JG. Berberine inhibits human tongue squamous carcinoma cancer tumor growth in a murine xenograft model. Phytomedicine. 2009;16:887–890. doi: 10.1016/j.phymed.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Nattapong P, Kulthida V, Wunchana S, Orasa W, Worasak K, Amornrat J, Somchai P, Chawalit P, Chaisiri W, Seiji O. Establishment of an Allo-transplantable hamster cholangiocarcinoma cell line and its application for in vivo screening of anti-cancer drugs. Korean J Parasitol. 2013;51:711–717. doi: 10.3347/kjp.2013.51.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei Z, Jingjing L, Fei M, Shining Y, Naisan L, Jing W, Yongbin W, Xiuzhen W, Qizheng Y. Synthesis and cytotoxicity evaluation of 13-n-alkyl berberine and palmatine analogues as anticancer agents. Molecules. 2012;17:11294–11302. doi: 10.3390/molecules171011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jabbarzadeh KP, Leong MP, Ismail P, Ling KH. Antitumor effects of berberine against EGFR, ERK1/2, P38 and AKT in MDA-MB231 and MCF-7 breast cancer cells using molecular modelling and in vitro study. Pharmacol Rep. 2018;71:13–23. doi: 10.1016/j.pharep.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Cazzaniga M, Bonanni B. Relationship Between Metabolic Disorders and Breast Cancer Incidence and Outcomes. Is There a Preventive and Therapeutic Role for Berberine? Anticancer Res. 2018;38:4393–4402. doi: 10.21873/anticanres.12741. [DOI] [PubMed] [Google Scholar]
- 38.Zhu T, Li LL, Xiao GF, Luo QZ, Liu QZ, Yao KT, Xiao GH. Berberine increases doxorubicin sensitivity by suppressing STAT3 in lung Cancer. Am J Chinese Med. 2015;43:1550084. doi: 10.1142/S0192415X15500846. [DOI] [PubMed] [Google Scholar]
- 39.Yunfang L, Huiming Y, Cheng Z, Yufeng C, Likuan H, Xiaohui M, Yuxia Z. Protective effects of berberine on radiation-induced lung injury via intercellular adhesion molecular-1 and transforming growth factor-beta-1 in patients with lung cancer. Eur J Cancer. 2008;44:2425–2432. doi: 10.1016/j.ejca.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 40.Su YH, Tang WC, Cheng YW, Sia P, Huang CC, Lee YC, Jiang HY, Wu MH, Lai IL, Lee JW. Targeting of multiple oncogenic signaling pathways by Hsp90 inhibitor alone or in combination with Berberine for treatment of colorectal Cancer. BBA - Molecular Cell Research. 2015;1853:2261–2272. doi: 10.1016/j.bbamcr.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Pandey A, Vishnoi K, Mahata S, Tripathi SC, Misra SP, Misra V, Mehrotra R, Dwivedi M, Bharti AC. Berberine and curcumin target Survivin and STAT3 in gastric Cancer cells and synergize actions of standard chemotherapeutic 5-fluorouracil. Nutrition & Cancer-an International Journal. 2015;67:1293–1304. doi: 10.1080/01635581.2015.1085581. [DOI] [PubMed] [Google Scholar]
- 42.You HY, Xie XM, Zhang WJ, Zhu HL, Jiang FZ. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cellular & Developmental Biology - Animal. 2016;52:1–7. doi: 10.1007/s11626-016-0044-y. [DOI] [PubMed] [Google Scholar]
- 43.Hu Y, Davies GE. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia. 2010;81:358–366. doi: 10.1016/j.fitote.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Xu Z, Yufeng Z, Menghui Z, Xiaoyan P, Jia X, Chaoying K, Meng L, Chenhong Z, Zhiguo Z, Yifei Z. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7:e42529. doi: 10.1371/annotation/82b96c01-6435-4856-80a6-0176b1986e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 46.Hu Y, Ehli EA, Kittelsrud J, Ronan PJ, Munger K, Downey T, Bohlen K, Callahan L, Munson V, et al. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine. 2012;19:861–867. doi: 10.1016/j.phymed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Hu Y, Young AJ, Ehli EA, Nowotny D, Davies PS, Droke EA, Soundy TJ, Davies GE. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9:e93310. doi: 10.1371/journal.pone.0093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.