Abstract
By using a combination of experimental and computational experiments, we demonstrated that a second-generation dendrigraft of poly-l-lysine neutralizes the anticoagulant activity of unfractionated heparin, low-molecular-weight heparin, and fondaparinux more efficiently than protamine does in human plasma, making this synthetic polymer a promising surrogate of this problematic protein in clinical settings.
Keywords: Anticoagulant, antidote, heparin, dendrigraft of poly-l-lysine, protamine
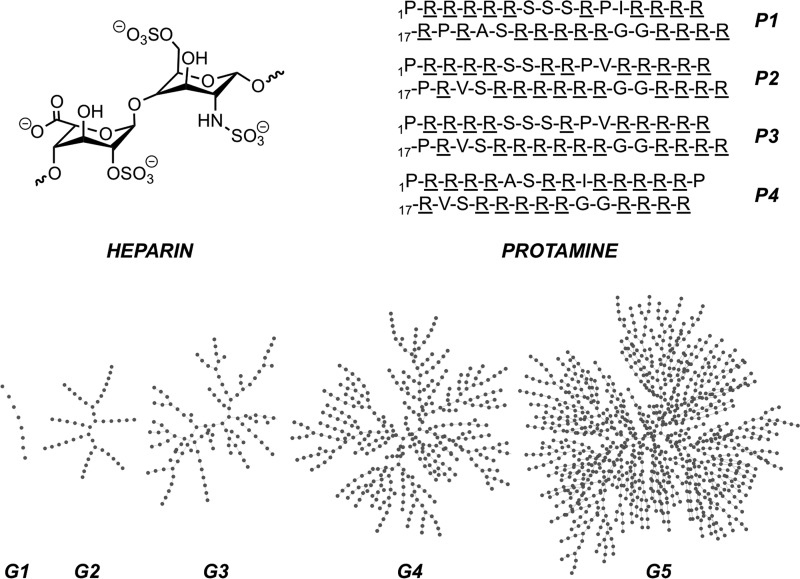
Heparins are acidic polyanionic glycosaminoglycans (GAGs) that are broadly used as intravenous anticoagulants (Figure 1).1−5 Heparins include unfractionated heparin (UFH), low-molecular-weight heparins (LMWHs), and the synthetic pentasaccharide fondaparinux. Their anticoagulant property originates from their binding to antithrombin III (AT III) and the subsequent potentiation of AT III toward the inactivation of factor Xa and other serine proteases.6 UFH are used to treat acute thrombotic events and to maintain blood fluidity throughout the circuits during procedures requiring extracorporeal circulation such as cardio-pulmonary bypass and hemodialysis.7,8 LMWHs and fondaparinux are prescribed for the treatment and/or prevention of deep vein thrombosis and pulmonary embolism.9,10 At the opposite of the electrostatic potential spectrum, protamine is a mixture of small (i.e., 5 kDa) and alkaline polycationic proteins (Figure 1),11 which neutralize heparins by forming—with varying degrees of success—inert complexes. In particular, the protein is systematically administrated for complete UFH neutralization after cardiac bypass surgery to more than 2,000,000 patients yearly.12 Regarding LMWHs, accidental overdosage or massive bleeding that may follow administration of enoxaparin is treated by intravenous infusion of protamine as a partial reversal agent (i.e., it neutralizes 60% at most of enoxaparin’s antifactor Xa activity).13 Finally, protamine is not an antidote for fondaparinux, where the management of hemorrhagic complications is primarily supportive.14
Figure 1.
Top left: major heparin disaccharide repeat unit. Top right: sequences of four major protamine components P1–P4 extracted from salmon sperm with basic amino acids underlined, see ref (15). Bottom: schematic representation of first- to fifth-generation DGLs G1–G5, where each dot represents an l-lysine residue. For a comprehensive study of DGLs’ three-dimensional structural features from experimental and computational experiments, see ref (16).
Four major concerns about the therapeutic use of protamine exist. (1) Protamine has no approved medicinal substitute and is therefore included in the list of essential medicines by the World Health Organization.17 (2) As protamine is extracted from fished salmon milt, its production is limited by the wild and bred livestock availability. For instance, the radioactive pollution in Fukushima resulted in a worldwide supply shortage of protamine in 2012. In addition, active ingredients extracted from animal source carry the risk of contamination.18 (3) As mentioned above, protamine has a limited efficacy spectrum: whereas it potently neutralizes UFH, it is only partially active against LMWHs and nonactive against fondaparinux, which both dominate the heparin product market.19 (4) The therapeutic use of protamine involves medical risks, related to immune responses and to pharmacodynamics issues of this exogenous protein. The incidence of adverse, sometime fatal reactions to protamine has been reported as varying from 0.06% to 10.7%.20 Noncomplexed protamine has also a weak anticoagulant effect at high doses, requiring careful monitoring.
Thus, the search for alternatives to protamine has a long history,21 which involves small molecules,22 proteins and peptides,23−25 linear and branched polymers,26−30 and macrocycles.31,32 Still, no potential substitute has reached the market so far, which rapidly grows, driven by demographics of the aging population and increased incidence of cardiovascular diseases.19 Less than a decade ago, dendrigrafts of poly-l-lysine (DGLs) joined the family of polycationic dendritic macromolecules, sharing structural features with both dendrimers and hyperbranched polymers (Figure 1).33 In a biomedical context, the attractiveness of these macromolecules mainly relies on their straightforward, robust, and green synthesis on a multigram scale,34 as well as their biodegradability,35 nonimmunogenicity,36 and low toxicity.37 On the latter point, arginine-rich peptides like protamine interact more strongly with lipidic bilayers than lysine-rich peptides.38 Consequently, polymers of arginine are toxic to the cells at concentrations from 800 nM to 50 μM, whereas polymers of lysine are only cytotoxic at higher concentrations (>50 μM).39 Recently, we demonstrated that DGLs are able to efficiently interact with UFH in a biosensing purpose, allowing—for the first time—the detection and the quantification of the anticoagulant in human blood at clinically relevant levels,40 or its discrimination against other similar GAGs.41 Armed with this success, we decided to explore the ability of DGLs to replace protamine as a universal antidote for heparins in clinical settings.
First, we used a well-established heparin assay to benchmark synthetic (i.e., DGLs) and natural (i.e., protamine) heparin antidotes in buffered water at pH 7.4. UFH was mixed with Azure A—a cationic dye known as heparin binder whose absorption spectrum changes upon complexation, a phenomenon know as metachromasia—and then the potential heparin antidote was added in order to displace the dye and restore its original color (Table 1).42,43 The effective concentration required to displace 50% of Azure A (i.e., EC50 value) was 11.71 μM for protamine and dropped from 128 μM for DGL G1 to 0.23 μM for DGL G5. More interestingly, the CE50 value that is the number of positive charges required per heparin negative charge to achieve 50% displacement of Azure A,44,45 was higher for DGL G1—a short linear poly-l-lysine—than for protamine, while higher generation DGLs displayed lower CE50 values. Since the CE50 reflects the relative ability of binding per cationic charge, it certainly highlights the importance of the antidote’s topology, where more flexible branched architectures in comparison with linear ones possibly allow the polymers to marshal their charges (vide infra). It also turned out that, whereas DGL G3 displayed a slightly lower CE50 value than DGL G2, the use of higher generation DGLs G4 and G5 did not lead to a significant improvement of these CE50 values. It can be related to our previous theoretical structural studies on DGLs, where we showed that all DGLs—independently of their generation—displayed adjacent charges separated by a similar average distance of 8.0 Å.16 Such a uniformity in charges’ density could explain similar binding behavior from branched DGLs G2 to G5. Therefore, we selected the synthetically more accessible and cheaper DGLs G2 and G3 as the most promising heparin antidotes for subsequent biological studies.
Table 1. Mw, Net Charge, EC50, and CE 50 Data for UFH Antidotes from Azure A-Displacement Assays in PBS 1X (pH 7.4).
| antidote | Mw (Da)a | net chargea | EC50 (μM)b | CE50c |
|---|---|---|---|---|
| protamine | 5197 | +22 | 11.7 | 0.79 ± 0.02 |
| DGL G1 | 1950 | +7 | 128 | 2.79 ± 0.01 |
| DGL G2 | 10081 | +41 | 4.63 | 0.61 ± 0.04 |
| DGL G3 | 24480 | +92 | 1.70 | 0.50 ± 0.03 |
| DGL G4 | 70945 | +280 | 0.54 | 0.48 ± 0.02 |
| DGL G5 | 208994 | +727 | 0.23 | 0.51 ± 0.02 |
Accurate Mw and net charges at pH 7.4 for DGLs G1–G5 were extracted from their previously reported potentiometric titrations, see ref (8).
EC50, effective concentration at which 50% of Azure A is displaced from its complex with UFH.
CE50, charge excess of antidote/UFH at which 50% of Azure A is displaced from its complex along with its standard deviation.
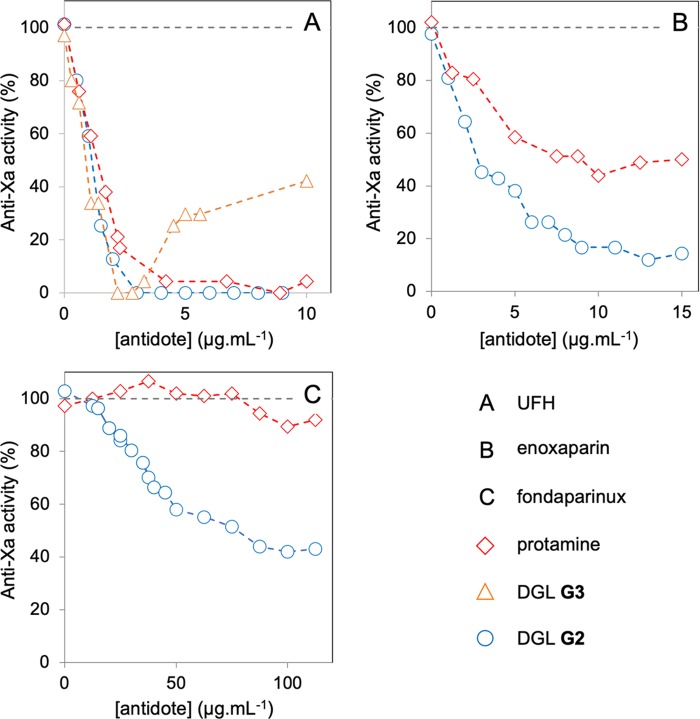
We next investigated the performances of DGL G2, DGL G3, and protamine (as reference) as antidotes for clinically used heparins in human plasma: UFH, the LMWH enoxaparin, and fondaparinux. The reversal activity of the antidotes was assessed in human plasma with the reliable anti-Xa assay (Figure 2), which is based on the AT III-mediated inhibition of activated Xa in the presence of heparins as measured by a chromogenic Xa substrate (STA-Rotachrom assay).46,47 Regarding UFH (Figure 2, A), both DGLs G2 and G3 were more efficient than protamine in neutralizing the anticoagulant activity, which agrees well with their lower CE50 values.48 Contrary to DGL G2, a sharp paradoxical effect (i.e., the restoration of the anti-Xa activity) was observed for DGL G3 after full neutralization of UFH when the antidote was used in excess. Coagulation is a complex process involving parallel positive and inhibitory feedback loops initiated by interactions between the endothelium, plasma proteins, and platelets. According to the literature, one may hypothesize that—as for large cationic dendritic structures such as PAMAMs—DGL G3 may disrupt key platelet functions.49 Whatever the mechanism(s) involved in this observed paradoxical effect with either UFH or enoxaparin, DGL G3 was therefore disqualified as a clinically relevant antidote for heparin-based anticoagulants. Regarding the LMWH enoxaparin, we confirmed that protamine neutralized ca. 50–60% of its anti-Xa activity as reported previously (Figure 2B).13 This contrasts with DGLs G2 that allows to reach ca. 90% of neutralization. Finally, we tested our lead candidate on fondaparinux, for which protamine is not an antidote (Figure 2C).14 Remarkably, DGL G2 was able to neutralize ca. 60% of its anti-Xa activity, therefore qualifying the dendritic polymer as universal reversal agent for heparin-based anticoagulants.
Figure 2.
Neutralization by various antidotes of three different clinically used heparins at therapeutic concentrations (UFH, 0.3 U/mL; enoxaparin, 0.4 U/mL; fondaparinux, 1.1 U/mL) in human plasma from anti-Xa assays.
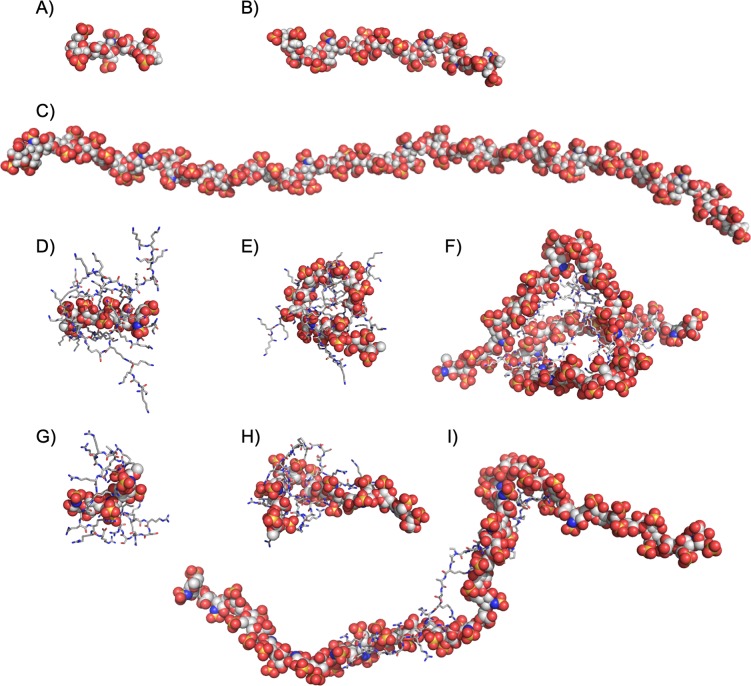
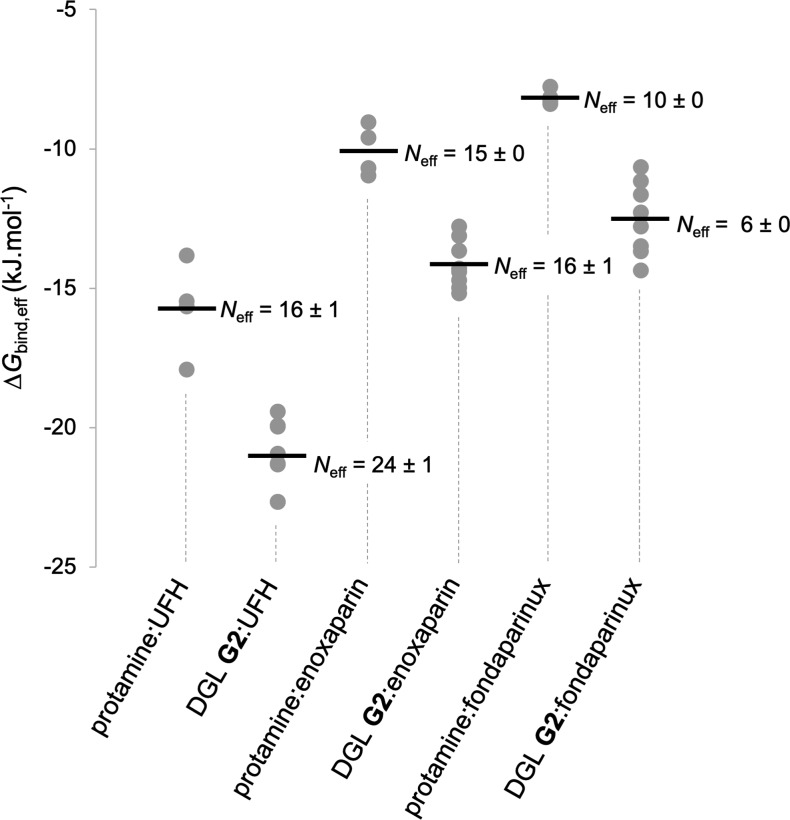
In order to obtain structural and energetic information that could explain the superior neutralization activity of DGL G2 in comparison to protamine, we performed molecular dynamics (MD) simulations on their stoichiometric complexes with UFH (44 saccharide units, Mw = 14.6 kDa), enoxaparin (14 saccharide units, Mw = 4.6 kDa), and fondaparinux (5 saccharide units, Mw = 1.7 kDa). Using the Amber 18 software package,50 long MD trajectories of 2 μs in explicit TIP3P water were collected for the complexes involving the four known sequences of protamine and eight possible regioisomers of DGL G2 previously generated (Figure 3, see the Supporting Information for full computational details).51 From the postprocessing analysis of each simulation, we extracted the corresponding charge-normalized free energy of binding value ΔGbind,eff = ΔGbind/Neff of the complex, where Neff is the average number of electrostatic interactions between the partners along the trajectory. As testified by their relative ΔGbind,eff values, DGL G2 interacts with each anticoagulant in a more effective way in comparison with protamine (Figure 4). In the case of UFH, it was possible to verify that the computed ΔGbind,eff ratio of 1.3 between DGL G2 and protamine (Figure 4) perfectly fits its related experimental CE50 ratio (Table 1), therefore validating the MD simulations a posteriori. Previous microsecond MD simulations revealed that DGL G2 alone is unfolded into a myriad of interconverting conformations.16 This conformational plasticity, which allows to optimize ion pairing at low enthalpic cost, may explain why DGL G2 is a superior binder for heparins. In addition, the experimental decreasing efficacy of both protamine and DGL G2 as the size of the GAGs decreases (i.e., UFH to enoxaparin to fondaparinux) could be correlated with the computed decreasing affinities and average number of electrostatic interactions Neff between the partners (Figure 3), the latter being also observable from the snapshots of the complexes (Figure 4). One could also note that the dendrigraft led to a much larger deformation of the anticoagulants upon complexation compared to protamine. Such a behavior was coined as adaptive multivalency by Smith et al.,52 where the individual charges of both partners are not so well locally optimized for binding, but the overall flexibility allows them to adapt their global shape more easily to maximize the total number and efficiency of contacts between the partners.
Figure 3.
Representative snapshots taken at the end of the MD trajectories for fondaparinux (A), enoxaparin (B), UFH (C), and the stoichiometric complexes DGL G2:fondaparinux (D), DGL G2:enoxaparin (E), DGL G2:UFH (F), protamine:fondaparinux (G), protamine:enoxaparin (H), and protamine:UFH (I). Heparins are represented as balls, and antidotes as sticks. For the full set of computed complexes, see the Supporting Information.
Figure 4.
Charge-normalized free energy of binding values ΔGbind,eff and average number of electrostatic interactions per frame Neff for the 36 complexes studied by MD simulations. For each antidote:anticoagulant pair, circles refer to the individual ΔGbind,eff values, and the black line to the corresponding mean value.
In conclusion, we identified a second-generation dendrigraft of poly-l-lysine to be a broad spectrum antidote for both unfractioned and low-molecular-weight heparins, as well as fondaparinux in human plasma. The superior efficacy of the dendritic polymer in comparison to protamine was demonstrated to be the result of its ability to more effectively use its charges. In addition, its abiotic origin, large-scale availability, nonimmunogenicity, and superior efficacy made this dendrigraft a very promising candidate for the replacement of the prevalent protein in clinical settings. Preclinical studies, including animal testing, are now necessary in order to assess the product’s pharmacodynamic and safety profile. We are currently working in this direction.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique (CNRS), the CHU de Nîmes, the Université de Lorraine, the Université de Lyon, and the Université de Montpellier. GPU resources were allocated by the mésocentre EXPLOR of the Université de Lorraine. DGLs were supplied by the COLCOM company (Montpellier, France).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00090.
Full experimental and computational details (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mackman N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918. 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. W. Prophylaxis for thromboembolism in hospitalized medical patients. N. Engl. J. Med. 2007, 356, 1438–1444. 10.1056/NEJMcp067264. [DOI] [PubMed] [Google Scholar]
- De Caterina R.; Husted S.; Wallentin L.; Agnelli G.; Bachmann F.; Baigent C.; Jespersen J.; Kristensen S. D.; Montalescot G.; Siegbahn A.; Verheugt F. W.; Weitz J. Anticoagulants in heart disease: current status and perspectives. Eur. Heart. J. 2007, 28, 880–913. 10.1093/eurheartj/ehl492. [DOI] [PubMed] [Google Scholar]
- Bussey H.; Francis J. L. Heparin overview and issues. Pharmacotherapy 2004, 24, 103S–107S. 10.1592/phco.24.12.103S.36109. [DOI] [PubMed] [Google Scholar]
- Weitz J. I. Low-molecular-weight heparins. N. Engl. J. Med. 1997, 337, 688–699. 10.1056/NEJM199709043371007. [DOI] [PubMed] [Google Scholar]
- Hirsh J.; Warkentin T. E.; Raschke R.; Granger C.; Ohman E. M.; Dalen J. E. Heparin and low-molecular-weight heparin. Chest 1998, 114, 489S–510S. 10.1378/chest.114.5_Supplement.489S. [DOI] [PubMed] [Google Scholar]
- Suranyi M.; Chow J. S. Review: anticoagulation for haemodialysis. Nephrology 2010, 15, 386–392. 10.1111/j.1440-1797.2010.01298.x. [DOI] [PubMed] [Google Scholar]
- Vincentelli A.; Jude B.; Bélisle S. Antithrombotic therapy in cardiac surgery. Can. J. Anaesth. 2006, 53, S89–S102. 10.1007/BF03022256. [DOI] [PubMed] [Google Scholar]
- Schiele F.; Meneveau N.; Seronde M. F.; Descotes-Genon V.; Dutheil J.; Chopard R.; Ecarnot F.; Bassand J.-P. Routine use of fondaparinux in acute coronary syndromes: a 2-year multicenter experience. Am. Heart J. 2010, 159, 190–198. 10.1016/j.ahj.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Yusuf S.; Mehta S. R.; Chrolavicius S.; Afzal C. B. G.; Pogue J.; Budaj A.; Peters R. J.; Bassand J. P.; Wallentin L.; Fox K. A. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N. Engl. J. Med. 2006, 354, 1464–1476. 10.1056/NEJMoa055443. [DOI] [PubMed] [Google Scholar]
- Sokolowska E.; Kalaska B.; Miklosz J.; Mogielnicki A. The toxicology of heparin reversal with protamine: past, present and future. Expert Opin. Drug Metab. Toxicol. 2016, 12, 897–909. 10.1080/17425255.2016.1194395. [DOI] [PubMed] [Google Scholar]
- Kitchens C. S.; Konkle B. A.; Kessler C. M.. Consultative Hemostasis and Thrombosis, 3rd ed.; Elsevier, 2013; pp 493–525. [Google Scholar]
- Makris M.; Veen J. J. V.; Tait C. R.; Mumford A. D.; Laffan M. Guideline on the management of bleeding in patients on antithrombotic agents. Br. J. Hamaetol. 2013, 160, 35–46. 10.1111/bjh.12107. [DOI] [PubMed] [Google Scholar]
- Giangrande P. Fondaparinux (Arixtra): a new anticoagulant. Int. J. Clin. Pract. Suppl. 2002, 56, 615–617. [PubMed] [Google Scholar]
- Hoffmann J. A.; Chance R. E.; Johnson M. G. Purification and analysis of the major components of chum salmon protamine contained in insulin formulations using highperformance liquid chromatography. Protein Expression Purif. 1990, 1, 127–133. 10.1016/1046-5928(90)90005-J. [DOI] [PubMed] [Google Scholar]
- Francoia J.-P.; Rossi J.-C.; Monard G.; Vial L. Digitizing poly-l-lysine dendrigrafts: from experimental data to molecular dynamics simulations. J. Chem. Inf. Model. 2017, 57, 2173–2180. 10.1021/acs.jcim.7b00258. [DOI] [PubMed] [Google Scholar]
- WHO Model List of Essential Medicines (March 2017): https://apps.who.int/iris/handle/10665/273826.
- European Medicines Agency completes review of protamine-containing medicines (November 2012): https://www.ema.europa.eu/en/news/european-medicines-agency-completes-review-protamine-containing-medicines.
- Melnikova I. The anticoagulants market. Nat. Rev. Drug Discovery 2009, 8, 353–353. 10.1038/nrd2851. [DOI] [PubMed] [Google Scholar]
- Porsche R.; Brenner Z. R. Allergy to protamine sulfate. Heart Lung 1999, 28, 418–428. 10.1016/S0147-9563(99)70031-2. [DOI] [PubMed] [Google Scholar]
- Bromfield S. M.; Wilde E.; Smith D. K. Heparin sensing and binding – taking supramolecular chemistry towards clinical applications. Chem. Soc. Rev. 2013, 42, 9184. 10.1039/c3cs60278h. [DOI] [PubMed] [Google Scholar]
- Schuksz M.; Fuster M. M.; Brown J. R.; Crawford B. E.; Ditto D. P.; Lawrence R.; Glass C. A.; Wang L.; Tor Y.; Esko J. D. Surfen, a small molecule antagonist of heparan sulfate. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 13075–13080. 10.1073/pnas.0805862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godavarti R.; Sasisekharan R. Heparinase I fromFlavobacterium heparinum. J. Biol. Chem. 1998, 273, 248–255. 10.1074/jbc.273.1.248. [DOI] [PubMed] [Google Scholar]
- Ammar T.; Fisher C. F. The effects of heparinase 1 and protamine on platelet reactivity. Anesthesiology 1997, 86, 1382–1386. 10.1097/00000542-199706000-00021. [DOI] [PubMed] [Google Scholar]
- Wu H.; Lundblad R.; Church F. Neutralization of heparin activity by neutrophil lactoferrin. Blood 1995, 85, 421–428. [PubMed] [Google Scholar]
- Kalathottukaren M. T.; Abbina S.; Yu K.; Shenoi R. A.; Creagh A. L.; Haynes C.; Kizhakkedathu J. N. A polymer therapeutic having universal heparin reversal activity: molecular design and functional mechanism. Biomacromolecules 2017, 18, 3343–3358. 10.1021/acs.biomac.7b00994. [DOI] [PubMed] [Google Scholar]
- Wen J.; Weinhart M.; Lai B.; Kizhakkedathu J.; Brooks D. E. Reversible hemostatic properties of sulfabetaine/quaternary ammonium modified hyperbranched polyglycerol. Biomaterials 2016, 86, 42–55. 10.1016/j.biomaterials.2016.01.067. [DOI] [PubMed] [Google Scholar]
- Välimäki S.; Khakalo A.; Ora A.; Johansson L.-S.; Rojas O. J.; Kostiainen M. A. Effect of PEG–PDMAEMA block copolymer architecture on polyelectrolyte complex formation with heparin. Biomacromolecules 2016, 17, 2891–2900. 10.1021/acs.biomac.6b00699. [DOI] [PubMed] [Google Scholar]
- Kalaska B.; Kaminski K.; Sokolowska E.; Czaplicki D.; Kujdowicz M.; Stalinska K.; Bereta J.; Szczubialka K.; Pawlak D.; Nowakowska M.; Mogielnicki A. Nonclinical evaluation of novel cationically modified polysaccharide antidotes for unfractionated heparin. PLoS One 2015, 10, e0119486 10.1371/journal.pone.0119486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoi R. A.; Kalathottukaren M. T.; Travers R. J.; Lai B. F. L.; Creagh A. L.; Lange D.; Yu K.; Weinhart M.; Chew B. H.; Du C.; Brooks D. E.; Carter C. J.; Morrissey J. H.; Haynes C. A.; Kizhakkedathu J. N. Affinity-based design of a synthetic universal reversal agent for heparin anticoagulants. Sci. Transl. Med. 2014, 6, 260ra150–260ra150. 10.1126/scitranslmed.3009427. [DOI] [PubMed] [Google Scholar]
- Mecca T.; Consoli G. M. L.; Geraci C.; Spina R. L.; Cunsolo F. Polycationic calix[8]arenes able to recognize and neutralize heparin. Org. Biomol. Chem. 2006, 4, 3763. 10.1039/b608887b. [DOI] [PubMed] [Google Scholar]
- Välimäki S.; Beyeh N. K.; Linko V.; Ras R. H.; Kostiainen M. A. A supramolecular host-guest complex for heparin binding and sensing. Nanoscale 2018, 10, 14022–14030. 10.1039/C8NR03132K. [DOI] [PubMed] [Google Scholar]
- Francoia J.-P.; Vial L. Everything you always wanted to know about poly-l -lysine dendrigrafts (but were afraid to ask). Chem. - Eur. J. 2018, 24, 2806–2814. 10.1002/chem.201704147. [DOI] [PubMed] [Google Scholar]
- Collet H.; Souaid E.; Cottet H.; Deratani A.; Boiteau L.; Dessalces G.; Rossi J.-C.; Commeyras A.; Pascal R. An expeditious multigram-scale synthesis of lysine dendrigraft (DGL) polymers by aqueous N-carboxyanhydride polycondensation. Chem. - Eur. J. 2010, 16, 2309–2316. 10.1002/chem.200901734. [DOI] [PubMed] [Google Scholar]
- Chamieh J.; Biron J. P.; Cipelletti L.; Cottet H. Monitoring Biopolymer Degradation by Taylor Dispersion Analysis. Biomacromolecules 2015, 16, 3945–3951. 10.1021/acs.biomac.5b01260. [DOI] [PubMed] [Google Scholar]
- Only the third-generation DGL was reported to be nonimmunogenic, see:; Romestand B.; Rolland J.-L.; Commeyras A.; Coussot G.; Desvignes I.; Pascal R.; Vandenabeele-Trambouze O. Dendrigraft Poly-L-Lysine: A Non-Immunogenic Synthetic Carrier for Antibody Production. Biomacromolecules 2010, 11, 1169–1173. 10.1021/bm9012056. [DOI] [PubMed] [Google Scholar]; As a general trend, unmodified amino dendrimers lack of immunogenicity, but when present, seems to only increase with increasing generation, see:; Boas U.; Christensen J. B.. Dendrimers in Medicine and Biotechnology; Royal Chemical Society Publishing: Cambridge, U.K., 2006. [Google Scholar]
- Hofman J.; Buncek M.; Haluza R.; Streinz L.; Ledvina M.; Cigler P. In vitro transfection mediated by dendrigraft poly(L-lysines): the effect of structure and molecule size. Macromol. Biosci. 2013, 13, 167–176. 10.1002/mabi.201200303. [DOI] [PubMed] [Google Scholar]
- Robison A. D.; Sun S.; Poyton M. F.; Johnson G. A.; Pellois J. P.; Jungwirth P.; Vazdar M.; Cremer P. S. Polyarginine Interacts More Strongly and Cooperatively than Polylysine with Phospholipid Bilayers. J. Phys. Chem. B 2016, 120, 9287–9296. 10.1021/acs.jpcb.6b05604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. J.; Steinman L.; Kim D. T.; Fathman C. G.; Rothbard J. B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Francoia J.-P.; Pascal R.; Vial L. Monitoring clinical levels of heparin in human blood samples with an indicator-displacement assay. Chem. Commun. 2015, 51, 1953–1956. 10.1039/C4CC08563A. [DOI] [PubMed] [Google Scholar]
- Francoia J.-P.; Vial L. A KISS (keep it simple, sensor) array for glycosaminoglycans. Chem. Commun. 2015, 51, 17544–17547. 10.1039/C5CC07628E. [DOI] [PubMed] [Google Scholar]
- The Azure A:UFH complex is purple, whereas the free dye is blue.
- Gundry S. R.; Klein M. D.; Drongowski R. A.; Kirsh M. M. Clinical evaluation of a new rapid heparin assay using the dye azure A. Am. J. Surg. 1984, 148, 191–194. 10.1016/0002-9610(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Bromfield S. M.; Posocco P.; Fermeglia M.; Pricl S.; Rodríguez-López J.; Smith D. K. A simple new competition assay for heparin binding in serum applied to multivalent PAMAM dendrimers. Chem. Commun. 2013, 49, 4830. 10.1039/c3cc41251b. [DOI] [PubMed] [Google Scholar]
- CE50 values were calculated using the following equation: CE50 = [EC50 × number of antidote’s positive charges]/[concentration of anticoagulant’s negative charges].
- Vandiver J. W.; Vondracek T. G. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy 2012, 32, 546–558. 10.1002/j.1875-9114.2011.01049.x. [DOI] [PubMed] [Google Scholar]
- Gehrie E.; Laposata M. Test of the month: the chromogenic antifactor Xa assay. Am. J. Hematol. 2012, 87, 194–196. 10.1002/ajh.22222. [DOI] [PubMed] [Google Scholar]
- Within the same concentration range, DGL G1 did not fully neutralize UFH’s anti-Xa activity (data not shown), which was also in agreement with the data from Azure A-displacement assays.
- Jones C. F.; Campbell R. A.; Franks Z.; Gibson C. C.; Thiagarajan G.; de Abreu A. V.; Sukavaneshvar S.; Mohammad S. F.; Li D. Y.; Ghandehari H.; Weyrich A. S.; Brooks B. D.; Grainger D. W. Cationic PAMAM dendrimers disrupt key platelet functions. Mol. Pharmaceutics 2012, 9, 1599–1611. 10.1021/mp2006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case D.; Ben-Shalom I.; Brozell S.; Cerutti D.; Cheatham T. III; Cruzeiro V.; Darden T.; Duke R.; Ghoreishi D.; Gilson M.; Gohlke H.; Goetz A.; Greene D.; Harris R.; Homeyer N.; Izadi S.; Kovalenko A.; Kurtzman T.; Lee T.; LeGrand S.; Li P.; Lin C.; Liu J.; Luchko T.; Luo R.; Mermelstein D.; Merz K.; Miao Y.; Monard G.; Nguyen C.; Nguyen H.; Omelyan I.; Onufriev A.; Pan F.; Qi R.; Roe D.; Roitberg A.; Sagui C.; Schott-Verdugo S.; Shen J.; Simmerling C.; Smith J.; Salomon-Ferrer R.; Swails J.; Walker R.; Wang J.; Wei H.; Wolf R.; Wu X.; Xiao L.; York D.; Kollman P.. AMBER 18; University of California, San Francisco, 2018.
- For each generation of dendrigraft, a large number of possible regioisomers exists. Using a homemade encoding algorithm, we previously generated eight three-dimensional structures for each generation of DGL that respect their experimental characterizations (i.e., composition, degrees of polymerization, branching ratios, charges). For more details, see ref (16).
- Smith D. K. From fundamental supramolecular chemistry to self-assembled nanomaterials and medicines and back again-how Sam inspired SAMul. Chem. Commun. 2018, 54, 4743–4760. 10.1039/C8CC01753K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.