Abstract
Purpose.
Growing rates of metabolic syndrome and associated obesity warrant the development of appropriate animal models for better understanding of how those conditions may affect sensitivity to IR exposure.
Materials and Methods.
We subjected male NZO/HlLtJ mice, a strain prone to spontaneous obesity and diabetes, to 0, 5.5, 6.37, 7.4 or 8.5 Gy (137Cs) of total body irradiation (TBI). Mice were monitored for 30 days, after which proximal jejunum and colon tissues were collected for further histological and molecular analysis.
Results.
Obese NZO/HlLtJ male mice are characterized by their lower sensitivity to IR at doses of 6.37Gy and under, compared to other strains. Further escalation of the dose, however, results in a steep survival curve, reaching LD100/30 values at a dose of 8.5Gy. Alterations in the expression of various tight junction-related proteins coupled with activation of inflammatory responses and cell death were the main contributors to the gastrointestinal syndrome.
Conclusions.
We demonstrate that metabolic syndrome with exhibited hyperglycemia but without alterations to the microvasculature is not a pre-requisite of the increased sensitivity to TBI at high doses. Our studies indicate the potential of NZO/HlLtJ mice for the studies on the role of metabolic syndrome in acute radiation toxicity.
Keywords: acute radiation gastrointestinal injury, glucose metabolism, metabolic syndrome, obesity, tight junction-related proteins
Introduction
Among the body’s organ systems, the gastrointestinal (GI) and hematopoietic systems are among the most sensitive to IR exposure (Singh and Seed 2017). In the hematopoietic system, exposure to high doses of IR causes substantial loss of stem and progenitor cell compartments. The effects of IR to the GI are characterized by profound cell death of GI epithelium, which is further exacerbated by the death of mucosal stem cells. Bone marrow transplantation and introduction of granulocyte-colony stimulating factors, such as the FDA-approved drug filgrastim (Neupogen®), substantially decrease hematopoietic toxicity and significantly increase survival in animal models, including non-human primates (Singh and Seed 2017) . Nevertheless, there are no FDA-approved strategies for mitigation of acute radiation-induced GI (RIGI) syndrome.
A number of patient-related factors can affect the severity of RIGI syndrome. For instance, smoking history is a known independent predictor of instances of gut toxicity caused by radiotherapy delivered to the abdominal and pelvic areas (Eifel et al. 2002). Metabolic syndrome is particularly concerning, as are frequently co-occurring instances of obesity and diabetes. Modern societies are experiencing global increases in body weight and the prevalence of type 2 diabetes, with over one third of the U.S. population categorized as obese (ACS 2017). Adiposity and blood glucose levels are recognized factors that can influence the response to IR. For instance, an obese state requires a higher dose in order to reach target tissues during radiotherapy (Ector et al. 2007; Hsi et al. 2013). On the other hand, metabolic syndrome and diabetes are generally considered as comorbidities, with diabetics having a decreased survival rate (Herold et al. 1999; van de Poll-Franse et al. 2007).
Taking this into consideration, development of specific models to investigate the influence of metabolic syndrome – obesity and diabetes particularly – on response to accidental or medicinal exposure to IR are imperative. Our choice fell on the novel NZO/HlLtJ mouse strain that is characterized by its diet-independent subcutaneous and visceral obesity. Furthermore, these mice are characterized by hyperglycemia that inevitably leads to the development of type 2 diabetes in male mice by the age of 18 weeks.
Materials and Methods
Animals and Irradiation Procedures
NZO/HILtJ and CBA/CaJ mice were purchased from Jackson Laboratory (Bar Harbor, ME), housed and bred (NZO/HlLtJ only) at the University of Arkansas for Medical Sciences (UAMS). Animals were housed 4-5 per cage under standard conditions at ambient temperature of 25 ± 1 °C, 12:12 light: dark cycle; NIH-31 chow and water were provided ad libitum.
Male NZO/HlLtJ and CBA/CaJ mice (age 46-82 days) were randomly divided into the following groups: 0, 5.5, 6.37, 7.4 or 8.5 Gy of total body irradiation (TBI) (Shepherd Mark I model 25 137Cs irradiator, J. L. Shepherd & Associates, San Fernando, CA). These doses were chosen based on previous studies (Vacha et al. 1984; Booth et al. 2012). Un-anesthetized mice were placed in well-ventilated cylindrical Plexiglas chambers (J. L. Shepherd & Associates) divided into eight 45° “pie slice” compartments. Two chambers were stacked on top of each other and placed on a turntable rotating at 5 rpm in the position furthest from the radiation source, allowing all mice belonging to the same exposure regimen to be irradiated simultaneously. The average dose rate was 1.21 Gy/min. Animals were observed for any signs of distress; moribund animals and mice with weight loss of greater than 20% of pre-irradiation weight were humanely terminated. Surviving mice were terminated on day 30 post-irradiation via exsanguination under isoflurane anesthesia.
Blood glucose was measured at termination via a Freestyle Precision Neo Blood Glucose Monitoring System (Abbott Diabetes Care Ltd. Alameda, CA). Tissues collected for histology were stored in Carnoy fixative [60% methanol (Pharmco-Aaper, cat# 339000000); 30% chloroform (cat# C-928-4) and 10% glacial acetic acid (cat# A38-212), (Fisher Scientific, Pittsburgh, PA)] for 24 hours and then transferred to 70% ethanol (Pharmco-Aaper, cat#111000200). Blood samples were collected in lithium heparin-coated 1.5 mL microcentrifuge tubes (Fisher Scientific, cat#022379208) and stored on ice. The tubes were then centrifuged for 15 minutes at 2000 × g at 4 °C. The plasma was collected into 1.5 mL microcentrifuge tubes, snap frozen in liquid nitrogen and then stored at −80 °C. All other tissues were collected in 1.5 mL microcentrifuge tubes, snap frozen in liquid nitrogen, and stored at −80 °C until analysis. All experiments were approved by the UAMS Institutional Animal Care and Use Committee.
Histology
Fixed tissues were processed by the Experimental Pathology Core Laboratory at UAMS for paraffin embedding and hematoxylin and eosin (H&E) staining. H&E slides were analyzed by a skilled veterinary pathologist. The following criteria were scored: acute inflammation, mucosal necrosis and crypt mitosis (average of 10 fields at 600X magnification). Grading was performed using the following scale: 0 – no lesions/changes, +0.5 to +1.0 – mild changes, +1.5 to +2.0 – moderate changes, +2.5 to +3.0 – marked changes, +3.5 to +4.0 – severe changes.
Gene Expression
Jejunum mucosa and colon tissue were homogenized using a Bullet Blender (Next Advance, Inc., Troy, NY) and the manufacturer’s recommendation for RINO tubes, speed and duration (pink screw cap tubes for 3 minutes on power 8, PINKR5-RNA; or green screw cap tubes for 3 minutes on power 12, GREENR5-RNA) and buffers from the Qiagen AllPrep DNA/RNA mini kit (Qiagen, cat# 80204, Germantown, MD). DNA and RNA were extracted from the homogenized samples following the manufacturer’s protocol. Total RNA was measured using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA). RNA was then reverse transcribed with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, cat# 4368813, Thermo Fisher) according to the manufacturer’s protocol. We then used custom TaqMan 384-target arrays (targets listed in Table S1; select genes for colon expression listed in Table S2), TaqMan Fast Advanced Gene Expression Master Mix (Applied Biosystems, cat# 4444557) or SYBR Select Master Mix (colon genes, Applied Biosystems, cat# 4472908) and a Viia 7 qRT-PCR System (Applied Biosystems) to amplify cDNA. Expression levels were normalized to Ywhaz using the 2−ΔΔCt method and then expressed as difference from average of control.
Statistical Analyses
Group sizes were 4 animals in the control group and 8-10 in treatment groups. These group sizes were chosen based on previously published literature (Kodell et al. 2010). All data were analyzed using GraphPad Prism (La Jolla, CA). We used a D’Agostino & Pearson omnibus normality tests and Brown-Forsythe F test for homogenous variances to test the assumptions for ANOVA. Comparisons between groups were analyzed with a One-Way ANOVA with a Tukey’s multiple comparisons test for normally distributed datasets or a Kruskal-Wallis test with a Dunn’s multiple comparisons test for non-normally distributed datasets as required based on the results of the assumption tests. Differences between groups were considered significant at p < 0.05. In all figures, single * indicate p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
Results
Steep survival curve and blood glucose changes in response to TBI
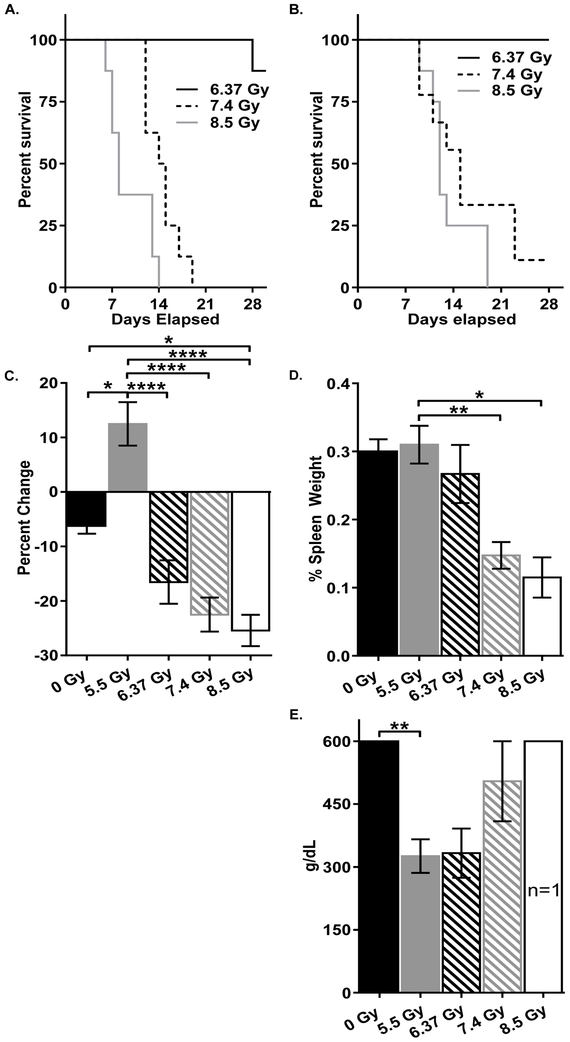
Exposure to 5.5 Gy of TBI, as well as escalation of dose to 6.37 Gy did not result in any mortality in NZO/HlLtJ mice within 30 days after exposure and only one death in the 6.37 Gy CBA/CaJ mouse group. (Fig 1a and b). However, the dose of 7.4 Gy appeared to be LD90/30, and the dose of 8.5 Gy was the LD100/30, indicating a steep survival curve for NZO/H1LtJ male mice. CBA/CaJ male mice exposed to 7.4 and 8.5 Gy succumbed to radiation toxicity at earlier time-points compared to NZO/H1LtJ male mice.
Figure 1.
Male NZO/H1LtJ mice were subjected to 0, 5.5, 6.37, 7.4 or 8.5 Gy of TBI with an n of 4, 9, 10, 9, and 8, respectively. A) 30-day survival of male CBA/CaJ mice exposed to 0, 5.5, 6.37, 7.4, and 8.5 Gy. 0 and 5.5 Gy not shown as all mice survived the 30 days. B) 30-day survival of male NZO/HILtJ mice after exposure to each dose. 0 and 5.5 Gy not shown as all mice survived the experimental period. C) Average change in weight after TBI. D) Spleen weight expressed as percentage of body weight. E) Blood glucose levels at time of death. For C-E, data shown as mean ± SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.0001; and ****: p < 0.0001.
All NZO/HlLtJ mice except for animals at the 5.5 Gy group, including the control mice, exhibited weight loss (Fig 1c). Spleen to body weight ratios in 7.4 Gy and 8.5 Gy groups were significantly decreased compared to other groups (Fig 1d). Significant differences were observed in the weight of liver, heart and kidney tissues, although they were not dose-dependent (Fig S1).
At termination, blood glucose levels in 0, 7.4 and 8.5 Gy groups were near or greater than 600 g/dL, the upper limit for the glucometer. At the same time, animals in 5.5 and 6.37 Gy groups exhibited substantially lower blood glucose levels (near 300 g/dL) (Fig 1e), despite being terminated at Day 30 (in contrast to the mice at 7.4 and 8.5 Gy that were terminated between the days 8 and 17).
Histopathological evaluation
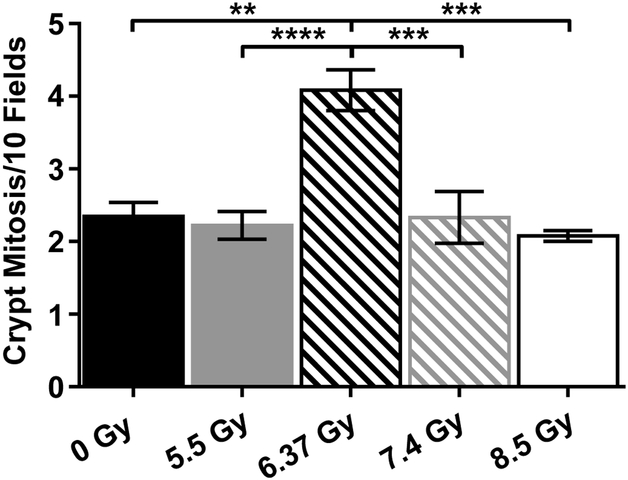
Histologically, no indications of inflammation or necrotic cells in any of the investigated groups were observed (Table S3), and the number of mitotic crypt cells was only increased in the 6.37 Gy group (Fig 2). However, it must be considered that 0, 5.5 and 6.37 Gy animals were scored on Day 30 after irradiation, while animals at 7.4 and 8.5 Gy were scored between Days 8 and 17.
Figure 2.
Number of crypt mitotic figures averaged over 10 fields at 600x. Data shown as mean ± SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.0001; and ****: p < 0.0001.
Irradiation leads to changes in tight junctions in obese NZO/HlLtJ mice
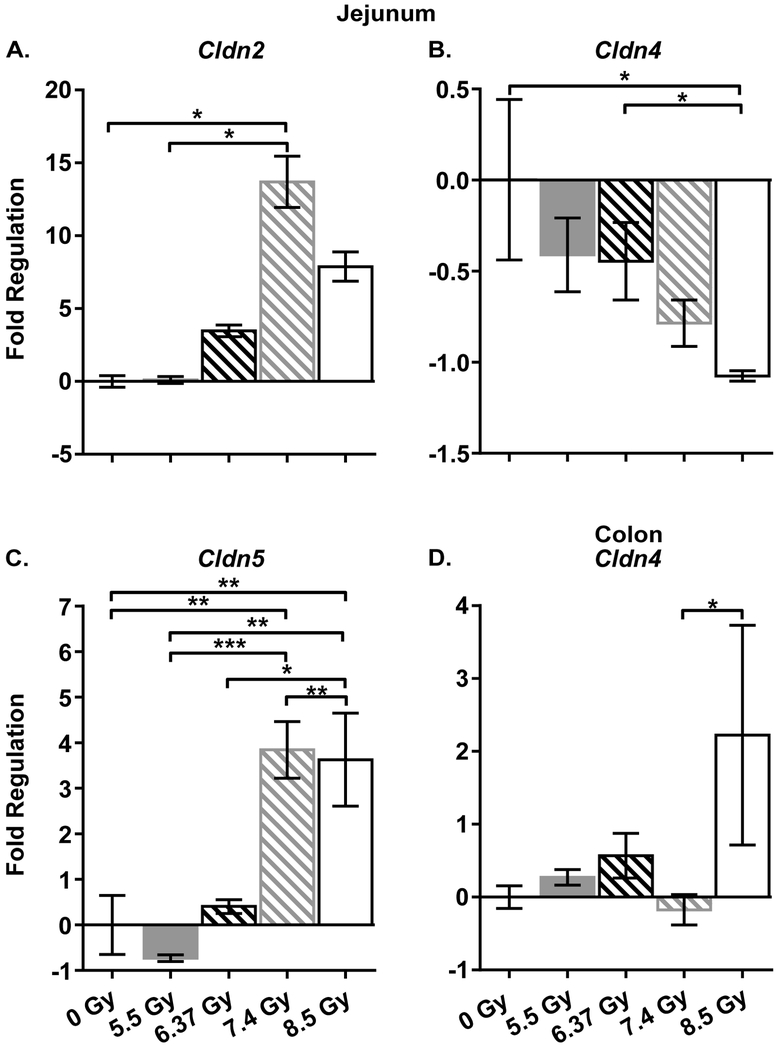
In order to gain insights into the molecular mechanism of response to IR in obese NZO/HlLtJ mice, a custom gene expression array was developed based on the existing literature of IR-induced responses in the gut (Zheng J et al. 2015; Garg et al. 2016). In total, significant changes in the expression of 27 genes were observed in all irradiated groups (Table 1, Fig 3, Fig S2)]. Of particular interest are the changes in the expression of the tight junction-related proteins, Cldn2, Cldn4, Cldn5 and Ocln, in combination with up-regulation of the extracellular matrix remodeling genes Mmp2 and Mmp7.
Table 1.
Jejunum and colon gene expression after TBI.
| Jejunum | ||||
|---|---|---|---|---|
| Gene | 5.5 Gy | 6.37 Gy | 7.4 Gy | 8.5 Gy |
| Adhesion proteins | ||||
| Cldn1 | −0.7075 | 1.332 | −0.7333 | 0.09 |
| Cldn11 | 2.68 | 0.825 | 3.034 | 1.158 |
| Cldn12* | −0.2525 | 0.105 | 0.9483 | 0.1275 |
| Cldn14 | −1.173 | −0.7817 | 1.562 | −0.97 |
| Cldn15 | −0.31 | 0.115 | −0.4883 | −0.5925 |
| Cldn2* | 0.105 | 3.477 | 13.7 | 7.878 |
| Cldn3 | −0.15 | 0.15 | 0.03167 | −0.3925 |
| Cldn4* | −0.41 | −0.445 | −0.785 | −1.075 |
| Cldn5* | −0.7325 | 0.404 | 3.845 | 3.628 |
| Cldn6 | 0.4525 | 0.5833 | 0.8 | −0.6175 |
| Cldn7 | −0.03 | −0.1333 | −0.3283 | −0.605 |
| Cldn8 | −0.29 | −1.062 | 0.076 | 0.2525 |
| Cldn9 | 1.183 | −0.222 | 1.79 | 0.1975 |
| Ctnna1 | −0.1075 | 0.205 | 0.1833 | −0.2475 |
| Esam | −1.023 | −0.5733 | −0.2317 | −0.4875 |
| Jam2* | 0.2775 | 2.985 | 8.367 | 5.263 |
| Jam3* | −0.1075 | 1.448 | 4.967 | 4.038 |
| Magi1 | −0.46 | −0.16 | 0.2783 | −0.235 |
| Magi3 | −0.635 | −0.7717 | −0.59 | −0.915 |
| Ocln | −0.22 | −0.2717 | −0.5467 | −0.635 |
| Rhoa | −0.1725 | 0.4983 | 0.6433 | 0.33 |
| Tjap1 | −0.59 | −0.49 | −0.16 | −0.505 |
| Tjp1 | −0.06 | 0.1333 | 0.4767 | −0.1775 |
| Tjp2 | 0.005 | 0.3 | 0.2633 | −0.1875 |
| Tjp3 | −0.1975 | 0.31 | 0.2483 | −0.195 |
| Extracellular matrix remodeling | ||||
| Mmp2* | −0.29 | 0.165 | 1.605 | 4.165 |
| Mmp7* | 1.88 | 4.002 | 26.88 | 11.52 |
| Mmp9 | 0.2275 | 1.123 | 8.088 | 9.367 |
| Genes involved in apoptosis or cell cycle regulation | ||||
| Aifm1 | −0.255 | 0.3833 | 0.33 | −0.15 |
| Apaf1 | −0.1425 | −0.03 | 0.1617 | −0.2675 |
| Api5* | −0.0575 | 0.8083 | 1.293 | 0.9075 |
| Bad | −0.305 | −0.035 | 0.1533 | −0.3225 |
| Bcl2 | −0.0225 | 0.388 | 1.22 | 0.365 |
| Casp1 | −0.02 | 0.47 | −0.2933 | −0.3425 |
| Casp2 | −0.5275 | −0.2517 | 0.2667 | −0.3725 |
| Casp3* | −0.505 | 0.315 | −0.565 | −0.7 |
| Casp4 | −0.0825 | 0.07333 | 0.8983 | −0.285 |
| Cflar | −0.325 | −0.2433 | −0.4667 | −0.645 |
| Dad1 | −0.4525 | 0.1667 | 0.195 | −0.1975 |
| Dapk1* | −0.82 | 0.5883 | 3.09 | 2.535 |
| Fadd | −0.1475 | 0.1283 | −0.295 | −0.395 |
| Fas | −0.5075 | −0.0783 | −0.2983 | −0.4925 |
| Gadd45a | −0.435 | −0.1033 | −0.125 | −0.6525 |
| Igf1r* | 0.1175 | 3.148 | 12.34 | 16.13 |
| Mapk1 | 0.115 | 0.2117 | 0.7083 | 0.1225 |
| Nfkb1 | −0.2575 | 0.115 | −0.12 | −0.3925 |
| Pcna | −0.045 | 0.79 | 0.4617 | 0.13 |
| Rac1 | −0.27 | −0.4117 | 0.35 | −0.4425 |
| Tgfb1* | −0.6375 | −0.0683 | 0.785 | 0.2475 |
| Tnf | −0.7425 | 0.885 | 2.723 | −0.175 |
| Tnfsf10 | −0.3825 | −0.125 | −0.055 | −0.335 |
| Trp53 | −0.175 | 0.9167 | 0.515 | 0.49 |
| Trp53bp2 | −0.235 | 0.1533 | 0.5467 | −0.1575 |
| Inflammation and immune reactions | ||||
| Anxa5 | −0.19 | −0.015 | −0.2517 | −2.2E-08 |
| C3* | 0.6575 | 25.74 | 73.89 | 80.82 |
| Ccl11* | −0.375 | 3.418 | 19.43 | 9.673 |
| Ccl4* | 0.1125 | 0.6283 | −0.925 | −1.053 |
| Ccl5 | 0.1175 | 1.94 | 0.66 | −0.19 |
| Cd14* | −0.35 | 0.19 | 3.545 | 1.13 |
| Cd40 | −0.5525 | 0.82 | 1.627 | 0.225 |
| Cd40lg | −0.88 | −0.328 | −0.3875 | −0.37 |
| Cebpb* | −0.3525 | 0.01 | 1.442 | 0.195 |
| Cxcl1 | −1.45 | 0.03333 | 1.593 | 2.023 |
| Cxcl9 | −0.535 | 1.958 | 4.987 | −0.5175 |
| Cxcr2 | −0.995 | −0.565 | −0.94 | −0.62 |
| Icam1* | −0.7475 | 0.05833 | 6.493 | 1.265 |
| Icam2* | −0.0975 | 0.44 | 2.415 | 1.94 |
| Il18* | −0.3825 | 0.2983 | 2.023 | 1.155 |
| Il1a* | −0.8775 | −0.72 | 1.888 | −0.16 |
| Il1b* | −0.405 | 0.9233 | 19.79 | 4.37 |
| Il1r1* | −0.58 | −0.4 | 2.392 | 1.438 |
| Ltb | −0.78 | 0.8617 | −0.9967 | −0.365 |
| Myd88 | −0.28 | 0.06667 | 0.495 | −0.0825 |
| Nos2 | −0.5725 | 10.03 | 11.77 | 0.025 |
| Pecam1* | −1.225 | −0.642 | 1.172 | 1.065 |
| Tirap | −0.6 | −0.2083 | −0.2583 | −0.3525 |
| Tlr1 | −0.2825 | 1.078 | 1.453 | 1.81 |
| Tlr2* | −1.723 | 3.183 | 10.83 | 2.538 |
| Tlr3 | −0.2375 | 0.1933 | −0.23 | −0.4625 |
| Tlr4* | −0.325 | 0.4933 | 2.687 | 1.858 |
| Tlr9 | −0.435 | −0.01833 | −0.33 | 0.0825 |
| Colon | ||||
| Gene | 5.5 Gy | 6.37 Gy | 7.4 Gy | 8.5 Gy |
| Adhesion proteins | ||||
| Cldn2* | 0.203 | 0.6258 | 0.02597 | −0.2403 |
| Cldn4* | 0.2707 | 0.5697 | −0.2565 | 3.873 |
| Jam2* | 0.683 | 0.3355 | −0.2137 | −0.1171 |
| Jam3 | 0.6237 | 0.5144 | 0.7014 | 0.05798 |
| Ocln | 0.1033 | 0.6828 | 0.3822 | 0.3847 |
| Genes involved in apoptosis or cell cycle regulation | ||||
| Igf1r | −0.9823 | 0.5043 | 1.196 | 0.4716 |
| Inflammation and immune reactions | ||||
| Ccl11 | 0.7775 | 0.4456 | 1.325 | 12.45 |
| Ccl4 | 0.7262 | 1.916 | 30.71 | 2.048 |
| Icam2* | −0.1521 | −0.1245 | −0.3376 | −0.7724 |
| Tlr2* | 0.4136 | 0.6219 | 3.386 | 2.269 |
| Tlr4* | 1.587 | 1.391 | 2.695 | 1.82 |
Values calculated as sample 2−ΔΔCt – average control 2−ΔΔCt. Negative values are in italics and indicate down-regulation.
indicates a significant ANOVA or Kruskall-Wallis test.
Figure 3.
Select genes in jejunum (A-C) and colon (D) that were differentially expressed. Data represented as fold change in 5.5, 6.37, 7.4, and 8.5 Gy compared to control and calculated as sample 2−ΔΔCt – average control 2−ΔΔCt; mean ± SEM *: p < 0.05; **: p < 0.01; ***: p < 0.0001; and ****: p < 0.0001.
We further extended our gene expression analysis on the colon samples by analyzing the expression of genes whose expression was affected the most in proximal jejunum. Of the 11 genes analyzed, 6 exhibited significant alterations between at least two groups (Table 1). Among them, decreased expression of Jam2 associated with exposure to lethal doses (7.4 and 8.5 Gy) and up-regulation of Tlr2 and Tlr4 were observed.
Discussion
Growing rates of metabolic syndrome and co-occurring obesity warrant the development of appropriate animal models for better understanding of how those conditions may affect the sensitivity to IR exposure. The importance of those studies is further underlined by the absence of the FDA-approved mitigators for the RIGI syndrome. Therefore, the goal of this study was to investigate the potential of the novel NZO/HlLtJ mouse model for the studies on obesity and type II diabetes modulation of the IR-induced gastrointestinal toxicity.
We report that obese NZO/HlLtJ male mice are characterized by lower sensitivity to IR at doses of 6.37 Gy and under, compared to other strains, including those of the agouti phenotype, such as CBA/CaJ mice, (Fig 1a) (Booth et al. 2012). The higher resistance could be potentially explained by the dose distribution via fat tissue and lower internal doses to the gut and bone marrow, however, this hypothesis requires further investigation with lean NZO/HlLtJ mice using the caloric restriction/exercise model. The lower sensitivity in this range of doses could be considered a somewhat positive outcome in regards to TBI. At the same time, radiation oncologists may incur differential toxicity effects in patients with metabolic syndrome. This is because of the radiation absorption by adipose tissue and its associated necessity for dose escalation in order to reach target tissues. With the latter, the risk of developing IR-induced normal tissue injury could be greater in these patients.
These findings also suggest that the metabolic syndrome and associated high blood glucose levels themselves are not the pre-requisites to higher sensitivity to IR. It is most likely that diabetic-associated changes to microvasculature that develop with the progression of the disease are the major factor in radiosensitization as radiation itself is known to target microvasculature (Stansborough et al. 2016). This hypothesis may be further confirmed with the same NZO/HlLtJ model using the mice at advanced stages of diabetes.
Interestingly, at higher doses, NZO/HlLtJ mice were characterized by a very steep survival curve, resulting in 90% lethality at 7.4 Gy and reaching LD100/30 values at a dose of 8.5Gy. These survival rates are higher than those of similar mouse strains, including radiosensitive CBA/J mice (Booth et al. 2012), but lower than other strains (Vacha et al. 1984). Causes of this sharp dose-response are unknown, but the expression array discussed below may bring some insights into its molecular aspects.
The observed IR-associated amelioration of blood glucose levels in mice exposed to 5.5 and 6.37 Gy, although somewhat surprising, is not without precedent. Indeed, amelioration of blood sugar levels after exposure to non-lethal doses of IR has been reported previously (Takahashi et al. 2000). Furthermore, at 5.5 Gy, a decrease in blood glucose levels was paralleled by an increase in body weight, characteristic of mice at this age. Despite lower blood glucose levels, mice exposed to 6.37 Gy still experienced significant decreases in body weight by day 30. This can be explained by the higher extent of GI injury caused by exposure to 6.37 Gy compared to 5.5 Gy. This is further confirmed by the delayed regeneration exhibited by the increased number of mitotic crypt cells observed in mice exposed to 6.37 Gy only. In future studies, it would be prudent to compare histological changes at earlier time points, corresponding to overt toxicity seen at higher IR doses.
Gene expression changes were characterized by alterations in specific adhesion proteins, inflammatory markers and proteins involved in apoptosis and cell cycle regulation. Of particular interest is the increased expression of a number of inflammatory markers in the jejunum, Ccl11, Cd14, Il18, Il1r1, as well as Tlr2 and Tlr4. For instance, Cd14 is associated with Tlr4 and loss of both has been implicated in reduced intestinal barrier function and subsequent bacterial translocation (Basic et al. 2018), while up-regulation of Tlr4 is also associated with increased inflammatory response (Alhasson et al. 2017). Up-regulation of both Il18 and its receptor, Il1r1, is associated with chronic inflammatory diseases, such as colitis and IBD (Shouval et al. 2016; Zhu et al. 2017), and is required for adequate Il-22 production for protection against infectious agents (Chen et al. 2013).
Along with altered expression of inflammatory mediators, substantial dysregulation of various tight junction-related proteins was observed in the jejunum. Of interest is up-regulation of Cldn2, a junction protein responsible primarily for pore formation that allows transcellular transport of ions. Inflammation leads to up-regulation of Cldn2, which in turn results in increased intestinal permeability, as seen in IBD patients (Hu et al. 2015). Exposure to IR also led to down-regulation of the barrier forming genes, such as Cldn4. Cldn4, along with other claudins (Cldn1, Cldn5, Cldn8 and Cldn11), serves primarily to increase trans-epithelial resistance, leading to decreased intestinal permeability (Angelow et al. 2008). In this regard, the observed up-regulation of Cldn5 in NZO/HlLtJ mice may suggest a compensatory mechanism to correct the loss of barrier function. The combination of down-regulated Cldn4 with up-regulated Tlr2 could also indicate a compensatory reaction that was unsuccessful in rescuing barrier function (Cario et al. 2004; Chavarria-Velazquez et al. 2018).
Other gene expression changes were associated either with extracellular matrix remodeling (Mmp2 and Mmp7) or with cell death and cell cycle regulation (Api5, Casp3, Dapk1, Igf1r, and Tgfb1). The most prominent changes were observed in Igf1r and Tgfb1. The former is involved in a multitude of pathways including apoptosis, autophagy and cell proliferation; when stimulated by IGF1, it can also inhibit apoptosis in intestinal crypts (Santoro et al. 2015). Tgfb1 plays a central role in inflammation, cell proliferation, immune responses and extracellular matrix remodeling. IR causes up-regulation of Tgfb1 in the gut, attracting mast cells which then signal over-production of collagen and fibroblasts in the smooth muscle surrounding enterocytes as well as producing inflammatory cytokines (Zheng H et al. 2000).
In summary, we demonstrate that obese NZO/HlLtJ mice in pre-diabetic condition are less sensitive to irradiation at lower doses (6.37 Gy and below), but exhibit higher sensitivity at higher doses (7.4 Gy and above). Alterations in the expression of various tight junction-related proteins coupled with activation of inflammatory responses and cell death seem to be the main contributors to RIGI syndrome. Altogether, these findings indicate that metabolic syndrome with exhibited hyperglycemia but without alterations to the microvasculature is not the pre-requisite of increased sensitivity to TBI at high doses.
Supplementary Material
Figure S1. Percent tissue weights of A) liver, B) kidney, and C) heart at time of death after IR.
Figure S2. Representative H&E stained slides of proximal jejunum segments. Bar is equal to 100 µm.
Table S1. List of genes included on the TaqMan Array card. Primer sequences are proprietary information of the manufacturer (Thermo-Fisher Scientific).
Table S2. List of all genes analyzed in colon tissue to include probe, primers, and/or reporters.
Table S3. Pathology scores of proximal jejunum to include acute inflammation, necrosis, and number of crypt mitotic figures.
Acknowledgements
IK would like to acknowledge the guidance provided during preparation of this manuscript made possible via R25CA203650 (Yale University, PI: Melinda Irwin). We are thankful to Dr. Christine Simecka, Robin Mulkey and Bridget Engi for excellent animal care, and to Christopher Fettes for editing this manuscript.
Funding
This work was supported by National Institutes of Health [1P20GM109005], the Arkansas Biosciences Institute, and National Institute of General Medical Sciences [T32GM106999].
Abbreviations:
- H&E
(hematoxylin and eosin)
- IR
(irradiation)
- TBI
(total body irradiation)
- GI
(gastrointestinal)
- Gy
(Gray)
- LD
(lethal dose)
- 137Cs
(cesium isotope 137)
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
Ethical Statement
All animal experiments were approved by the UAMS IACUC.
References
- ACS. 2017. Cancer Facts and Figures 2017. Atlanta: American Cancer Society. [Google Scholar]
- Alhasson F, Das S, Seth R, Dattaroy D, Chandrashekaran V, Ryan CN, Chan LS, Testerman T, Burch J, Hofseth LJ et al. 2017. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 12(3):e0172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelow S, Ahlstrom R, Yu AS. 2008. Biology of claudins. Am J Physiol Renal Physiol. 295(4):F867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basic M, Buettner M, Keubler LM, Smoczek A, Bruesch I, Buchheister S, Bleich A. 2018. Loss of CD14 leads to disturbed epithelial-B cell crosstalk and impairment of the intestinal barrier after E. coli Nissle monoassociation. Sci Rep. 8(1):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. 2012. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 103(4):383–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E, Gerken G, Podolsky DK. 2004. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 127(1):224–238. [DOI] [PubMed] [Google Scholar]
- Chang J, Feng W, Wang Y, Luo Y, Allen AR, Koturbash I, Turner J, Stewart B, Raber J, Hauer-Jensen M et al. 2015. Whole-body proton irradiation causes long-term damage to hematopoietic stem cells in mice. Radiat Res. 183(2):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Velazquez CO, Torres-Martinez AC, Montano LF, Rendon-Huerta EP. 2018. TLR2 activation induced by H. pylori LPS promotes the differential expression of claudin-4, −6, −7 and −9 via either STAT3 and ERK1/2 in AGS cells. Immunobiology. 223(1):38–48. [DOI] [PubMed] [Google Scholar]
- Chen VL, Surana NK, Duan J, Kasper DL. 2013. Role of murine intestinal interleukin-1 receptor 1-expressing lymphoid tissue inducer-like cells in Salmonella infection. PLoS One. 8(6):e65405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ector J, Dragusin O, Adriaenssens B, Huybrechts W, Willems R, Ector H, Heidbuchel H. 2007. Obesity is a major determinant of radiation dose in patients undergoing pulmonary vein isolation for atrial fibrillation. J Am Coll Cardiol. 50(3):234–242. [DOI] [PubMed] [Google Scholar]
- Eifel PJ, Jhingran A, Bodurka DC, Levenback C, Thames H. 2002. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 20(17):3651–3657. [DOI] [PubMed] [Google Scholar]
- El-Saghire H, Michaux A, Thierens H, Baatout S. 2013. Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes. Int J Mol Med. 32(6):1407–1414. [DOI] [PubMed] [Google Scholar]
- Garg S, Zheng J, Wang J, Authier S, Pouliot M, Hauer-Jensen M. 2016. Segmental Differences in Radiation-Induced Alterations of Tight Junction-Related Proteins in Non-Human Primate Jejunum, Ileum and Colon. Radiat Res. 185(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold DM, Hanlon AL, Hanks GE. 1999. Diabetes mellitus: a predictor for late radiation morbidity. Int J Radiat Oncol Biol Phys. 43(3):475–479. [DOI] [PubMed] [Google Scholar]
- Hsi RS, Zamora DA, Kanal KM, Harper JD. 2013. Severe obesity is associated with 3-fold higher radiation dose rate during ureteroscopy. Urology. 82(4):780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CA, Hou Y, Yi D, Qiu Y, Wu G, Kong X, Yin Y. 2015. Autophagy and tight junction proteins in the intestine and intestinal diseases. Anim Nutr. 1(3):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodell RL, Lensing SY, Landes RD, Kumar KS, Hauer-Jensen M. 2010. Determination of sample sizes for demonstrating efficacy of radiation countermeasures. Biometrics. 66(1):239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. 2007. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 120(9):1986–1992. [DOI] [PubMed] [Google Scholar]
- Santoro MA, Blue RE, Andres SF, Mah AT, Van Landeghem L, Lund PK. 2015. Obesity and intestinal epithelial deletion of the insulin receptor, but not the IGF 1 receptor, affect radiation-induced apoptosis in colon. Am J Physiol Gastrointest Liver Physiol. 309(7):G578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval DS, Biswas A, Kang YH, Griffith AE, Konnikova L, Mascanfroni ID, Redhu NS, Frei SM, Field M, Doty AL et al. 2016. Interleukin 1beta Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology. 151(6):1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Seed TM. 2017. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int J Radiat Biol. 93(9):851–869. [DOI] [PubMed] [Google Scholar]
- Stansborough RL, Al-Dasooqi N, Bateman EH, Keefe DM, Gibson RJ. 2016. Radiotherapy-induced gut toxicity: Involvement of matrix metalloproteinases and the intestinal microvasculature. Int J Radiat Biol. 92(5):241–248. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kojima S, Yamaoka K, Niki E. 2000. Prevention of type I diabetes by low-dose gamma irradiation in NOD mice. Radiat Res. 154(6):680–685. [DOI] [PubMed] [Google Scholar]
- Vacha J, Znojil V, Hola J, Sikulova J. 1984. A comparison of the survival (LD50/30) of a number of inbred mouse strains after X and cobalt-60 gamma irradiation. Z Versuchstierkd. 26(4):157–162. [PubMed] [Google Scholar]
- Zheng H, Wang J, Hauer-Jensen M. 2000. Role of mast cells in early and delayed radiation injury in rat intestine. Radiat Res. 153(5 Pt 1):533–539. [DOI] [PubMed] [Google Scholar]
- Zheng J, Wang J, Pouliot M, Authier S, Zhou D, Loose DS, Hauer-Jensen M. 2015. Gene expression profiling in non-human primate jejunum, ileum and colon after total-body irradiation: a comparative study of segment-specific molecular and cellular responses. BMC Genomics. 16:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Gu L, Li Y, Lin X, Shen H, Cui K, Chen L, Zhou F, Zhao Q, Zhang J et al. 2017. miR-148a inhibits colitis and colitis-associated tumorigenesis in mice. Cell Death Differ. 24(12):2199–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Percent tissue weights of A) liver, B) kidney, and C) heart at time of death after IR.
Figure S2. Representative H&E stained slides of proximal jejunum segments. Bar is equal to 100 µm.
Table S1. List of genes included on the TaqMan Array card. Primer sequences are proprietary information of the manufacturer (Thermo-Fisher Scientific).
Table S2. List of all genes analyzed in colon tissue to include probe, primers, and/or reporters.
Table S3. Pathology scores of proximal jejunum to include acute inflammation, necrosis, and number of crypt mitotic figures.