Abstract
Poor sleep and chronic pain are known to be interrelated, but the influence of negative and positive affect on this relationship are not fully understood. The present study sought to examine whether negative and positive affect mediate the relationship between sleep and pain interference. Secondary data analysis from Midlife in the United States (MIDUS-III) was used to examine 948 individuals with chronic pain (mean age = 64.73). Sleep disturbance was conceptualized as the sum of self-reported difficulty with sleep onset latency, wake after sleep onset, early morning awakening, and daytime sleepiness and total sleep time was assessed via self-reported sleep duration. Pain interference was operationalized as the sum of pain-related interference with general activity, relationships, and enjoyment of life. Finally, items from the Positive and Negative Affect Schedule were used to measure affect. Mediation analyses revealed that sleep disturbance indirectly predicted pain interference via both negative affect (β = .15, CI: .10, .21) and positive affect (β = .18, CI: .12, .25). Similarly, negative (β = −.003, CI: −.01, −.001) and positive affect (β = −.003, CI: −.01, −.001) also mediated the effect between total sleep time and pain interference. This study highlights the unique role of negative and positive affect on pain interference for individuals with chronic pain in mid to late-life. Additionally, findings suggest that holistic treatment approaches, which assess both sleep and affect in the context of chronic pain, may be beneficial.
Keywords: sleep, pain, negative affect, positive affect
Sleep disturbance and chronic pain are among the most common health concerns faced in mid to late-life (Artner et al., 2013). The high comorbidity between sleep disturbance and chronic pain has long fostered research efforts to disentangle the temporal relationship between sleep and pain. Emerging research suggests that impaired sleep is a stronger and more reliable predictor of increased pain and functional disability than vice versa (Finan et al., 2013). This temporal relationship is supported by the presence of population-based (Boardman et al., 2006; Gupta et al., 2007; Mork and Nilsen, 2012), micro-longitudinal,(Tang et al., 2012; Dzierzewski et al., 2010), and experimental studies (Haack and Mullington, 2005; Irwin et al., 2012). As a result of these recent findings, current research efforts have shifted their focus to identifying the mechanisms through which sleep impairments influence pain.
Sleep, Affect, and Pain
Although depression has been identified as one significant factor in the sleep-pain pathway (O’Brien et al., 2010), relatively little research has examined the role of negative affect. Negative affect is considered the extent to which a person experiences subjective distress, unpleasant engagement, and emotional pain (Watson et al., 1988). This gap in the literature is significant given that affect is considered to be a neuropsychological state which naturally occurs in the presence of stimuli but lacks any cognitive component. Affect therefore differs from both emotions (shorter duration, higher intensity) and mood (longer duration, lower intensity) which both include cognitive appraisal as a defining characteristic. Given that adults later in the lifespan are known to experience lower rates of clinical depression compared to their younger counterparts (Kessler et al., 2007), negative affect may represent a more widely applicable mechanism through which sleep influences pain.
In contrast to negative affect, positive affect is a mental state best characterized by pleasurable engagement with the environment and is typically accompanied by either happiness, joy, excitement, or contentment (Pressman and Cohen, 2005). Though positive and negative affect have traditionally been viewed as opposites on a continuum, findings suggest that they often operate independently of each other (Goldstein and Strube, 1994), and may therefore have unique associations with health. While the impact of poor sleep on negative affect has traditionally received greater attention, recent research suggest that poor sleep may decrease positive affect more than it increases negative affect (Finan et al., 2016). Given that positive affect serves as a buffer against adverse pain outcomes (Zautra et al., 2005), greater attention regarding the role of positive affect on the sleep-pain relationship is warranted.
Sleep, Affect, and Pain-Related Outcomes in Mid- to Late-Life
While negative and positive affect have been found to play a role in the relationship between sleep and pain-related outcomes in pediatric pain populations (Evans et al., 2017; Valrie et al., 2008), the influence of sleep and affect, particularly positive affect, among adult chronic pain populations remains largely overlooked. Additionally, even less is known about the influence of these factors on pain interference, or the degree to which pain hinders the ability to complete daily, social, or work-related tasks. These gaps are significant given that the prevalence of pain interference is elevated among chronic pain patients, tied to important functional outcomes, and known to increase significantly across the lifespan (Thomas et al., 2004; Wicksell et al., 2016).
Preliminary research on sleep and pain among adults, employing a within-subject repeated measures design, suggests that poor sleep significantly predicts greater levels of pain, higher levels of negative affect, and poorer physical functioning (Gerhart et al., 2017). Yet, to our knowledge, only one study, which used a similar within-subject repeated measures design, has examined the mediating role of affect on the sleep-pain relationship in adults (Kothari et al., 2015). However, this study was limited to adults with fibromyalgia.
The Present Study
The purpose of the present study was to examine the relationship between sleep, affect, and pain interference among individuals with chronic pain. Specifically, the study sought to extend past research and examine whether positive and negative affect each act as unique mechanisms through which sleep influences pain interference. A better understanding of the unique roles of positive and negative affect on the sleep-pain relationship can inform future interventions on the utility of either increasing positive affect or decreasing negative affect while also treating impaired sleep. Based on the existing literature, we hypothesized that: 1) sleep will predict pain interference and that 2) positive and negative affect will each partially mediate the relationship between sleep and pain interference, with poorer sleep contributing to increased negative affect and greater pain interference and better sleep predicting more positive affect and lower levels of pain interference.
Methods
Participants
The present study included archival data analysis from Project 1 of the Midlife in the United States-III study (MIDUS-III). The primary goal of MIDUS-III was to examine the influence of behavioral, psychological, and social factors on health and well-being in a nationally representative sample of Americans in mid- to late-life (Radler, 2014). Individuals were randomly selected to participate in the larger MIDUS-III sample if they were non-institutionalized, English speaking adults in mid- to late-life. Of the larger MIDUS-III sample, 948 adults who endorsed having chronic pain, that is “pain that persists beyond the time of normal healing and has lasted anywhere from a few months to many years”, were included in the present study. No exclusion criteria were imposed for participation in the current analyses. After obtaining participants’ written informed consent, measures of sleep, affect, and pain were completed cross-sectionally.
Measures
Sleep.
Sleep disturbance was conceptualized as participants’ self-reported difficulty with sleep onset latency, wake after sleep onset, early morning awakening, and daytime sleepiness during the past 30 days. Each of these sleep parameters was rated on a 5-point Likert scale from 1 (never) to 5 (almost always), with sleep disturbance operationalized as the sum of these four items. In addition to sleep disturbance, participants’ self-reported total sleep time (TST) for weekdays and weekends was also measured. Participants’ mean TST for the present study was calculated by averaging weekday and weekend TST.
Affect.
Positive and negative affect were each measured using items from the Positive and Negative Affective Schedule (PANAS; Watson et al., 1988). Specifically, for positive affect, participants were asked to rate the extent to which they felt “enthusiastic”, “attentive”, “proud” and “active” during the past 30 days (α = .86). Similarly, for negative affect, participants rated the degree to which they felt “afraid”, “jittery”, “irritable”, “ashamed”, and “upset” during the past 30 days (α = .80). Positive and negative items were each rated on a 5-point Likert scale from all the time to none of the time. Items were recoded so that higher scores reflect a higher average level of either positive or negative affect.
Pain Interference.
Pain interference was operationalized as the degree to which pain adversely influenced three different domains in the past week: general activity, relationships, and enjoyment in life. Pain interference in each of these domains was rated on an 11-point Likert scale from 0 (not at all) to 10 (completely). Total pain interference was calculated by summing participants’ scores on the three aforementioned domains.
Data Analyses
In order to examine the pathways through which sleep predicts pain interference, mediation analyses were conducted using an asymptotic bootstrapping approach (Preacher and Hayes, 2008). This approach allows for an examination of the indirect effects without requiring a direct effect. Parallel multiple mediator models were conducted using PROCESS macro (Hayes, 2013). Specifically, two parallel mediation models were run using Model 4 in PROCESS with N = 5,000 resamples. Sleep disturbance and TST were included as the respective predictor variables for the two separate mediation models. In both models, negative and positive affect were entered as the mediator variables. Finally, both models included pain interference as the criterion variable and controlled for age, sex, and education level. Results of the regression/path coefficients are presented in an unstandardized form.
Results
Sample Characteristics
Of the 948 participants included in the study, 58.6% identified as female and 89.3% identified as White. Participants’ ages ranged from 39 to 93, with a mean age of 63.37 (SD = 8.58). In terms of education, 25.7% of participants listed a high school degree as their highest level of education, while 19.4% reported having obtained a bachelor’s degree. Participants’ mean TST was 430.50 minutes (SD = 79.83), with 30.2% of the sample reporting fewer than seven hours of sleep which falls below the recommendations set by the National Sleep Foundation (Hirshkowitz et al., 2015). Participants’ average pain interference scores were 9.69 (SD = 8.24). Consistent with past research among adults in mid- to late-life (Charles et al., 2001), participants reported greater levels of positive affect (M = 3.40, SD = .83) than negative affect (M = 1.59, SD = .58). Complete descriptive demographic and clinical information are presented in Table 1.
Table 1.
Participant Descriptive Statistics (N=948)
| Variable | Mean (Std. Deviation) |
|---|---|
| Participant Demographics | |
| Age (years) | 64.73 (11.09) |
| Sex (% male) | 41.4 |
| Sleep Characteristics | |
| Sleep Disturbancea | 11.16 (3.69) |
| Total Sleep Time (in minutes) | 430.38 (80.20) |
| Pain Characteristics | |
| Pain Interferenceb | 9.46 (7.96) |
| Affective Characteristics | |
| Negative Affectc | 1.59 (.57) |
| Positive Affectc | 3.41 (.83) |
Notes.
Sleep disturbance was measured on a scale from 4 to 20, with higher scores indicating greater levels of sleep disturbance,
Pain interference measured on a scale from 0 to 30, with higher scores indicating greater levels of pain interference,
Affect measured on a scale from 1 to 5, with higher scores indicating greater levels of affect.
Sleep Disturbance, Affect, and Pain Interference
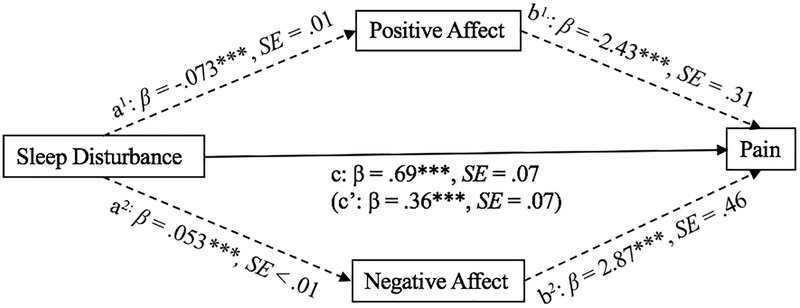
The total effect of sleep disturbance on pain was significant (Path c, β = .69, p < .001). In addition, the direct effect of sleep disturbance on pain when controlling for both negative and positive affect was also significant (Path c’, β = .36, p < .001). However, findings suggest the presence of an indirect of effect of sleep disturbance on pain via both positive affect (Path a1 × b1, β = .18, CI: .12, .25) and negative affect (Path a2 × b2, β = .15, CI: .10, .21). Specifically, results revealed a negative association between sleep disturbance and positive affect (Path a1, β = −.073, p < .001), as well as a negative association between positive affect and pain interference (Path b1, β = −2.43, p < .001). In contrast, results revealed a positive association between sleep disturbance and negative affect (Path a2, β = .053, p < .001) and a positive association between negative affect and pain (Path b2, β = 2.87, p < .001). Calculations of effect size (ab/c) indicate that 22% of the variance in the relationship between sleep disturbance and pain interference is explained by negative affect, while 26% is explained by positive affect. The mediational model for sleep disturbance, affect, and pain is represented in Figure 1.
Figure 1.
Mediational model for sleep disturbance, affect, and pain interference (*** p < .001, ** p <.01)
Total Sleep Time, Affect, and Pain Interference
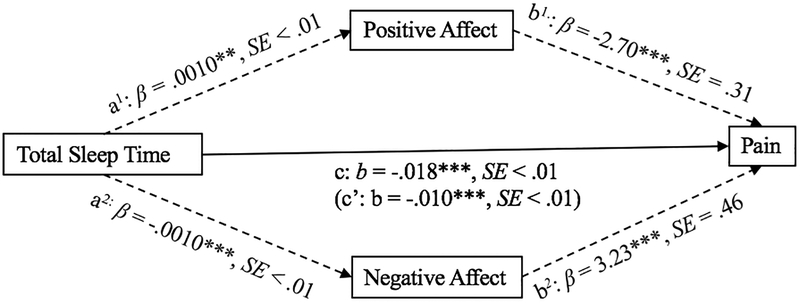
The total effect of TST on pain was significant (Path c, β = −.018, p < .001). In addition, the direct effect of TST on pain, when controlling for positive and negative affect was also significant (Path c’, β = −.010, p < .001). However, results revealed that the relationship between TST and pain were mediated by both positive (Path a1 × b1, β = −.003, CI: −.01, −.001) and negative affect (Path a2 × b2, β = −.003, CI: −.01, −.001). Specifically, greater TST was associated with higher levels of positive affect (Path a1, β = .0010, p = .004) and lower levels of negative affect (Path a2, β = −.0010, p < .001). Finally, positive affect (Path b1, β = −2.70, p < .001) and negative affect (Path b2, β = 3.23, p < .001) each predicted pain interference. Calculations of effect size (ab/c) reveal that 20% of the total effect of TST on pain can be explained by negative affect, while 17% is explained by positive affect. The mediational model for TST, affect, and pain is represented in Figure 2.
Figure 2.
Mediational model for total sleep time, affect, and pain interference (*** p < .001, ** p <.01)
Discussion
Overall, the purpose of the present study was to examine the relationship between sleep, affect, and pain interference. Our first hypothesis, that sleep would predict pain interference, was supported. Sleep disturbance and TST each predicted pain, with greater sleep disturbance predicting greater levels of pain interference, and longer TST predicting lower levels of pain interference. Our second hypothesis, that positive and negative affect would each mediate the relationship between sleep and pain, was also supported. Both positive affect and negative affect mediated the relationship between sleep and pain interference, with poorer sleep leading to greater negative affect and higher pain interference, and better sleep predicting higher levels of positive affect and less pain interference. These findings were consistent whether sleep disturbance or TST was used as the predictor variable.
Extending previous research, results from the present study indicate that sleep not only predicts pain severity, but also predicts pain interference. Given the high prevalence of pain interference among chronic pain patients, as well as its impact on daily functioning (Thomas et al., 2004), the results from the present study suggest that pain interference has been overlooked as a negative consequence of poor sleep. In addition, in light of recent findings suggesting a temporal relationship whereby poorer sleep increases pain, (Dzierzewski et al., 2010; Finan et al., 2013), findings from the present study align with past recommendations in suggesting that routine assessment and treatment of sleep disturbance would benefit from being incorporated into the treatment of chronic pain (Dworkin et al., 2008).
One novel aspect of the present study was the examination of the unique roles of positive and negative affect on the relationship between sleep and pain interference in mid to late-life. Though positive affect was previously shown to mediate the relationship between poor sleep quality and activity interference (Kothari et al., 2015), our study builds on previous findings in several important ways. First, our findings suggest that both positive affect and negative affect influence the relationship between sleep and pain interference. Secondly, given that prior work has focused on individuals with fibromyalgia, results from the present study suggest that affect mediates the sleep-pain relationship among individuals with heterogeneous chronic pain conditions.
These findings also shed more light on our understanding of the importance of affect in the experience of pain. Specifically, previous research has found that the experience of pain is subjective, and varies as a function of individual differences (Ong et al., 2010). Among chronic pain patients, those who displayed greater resiliency and positive emotions, experienced decreased pain catastrophizing (Ong et al., 2010). Additional research has found that positive affect reduces sensitivity to pain (Finan and Garland, 2015). The role of positive affect may be particularly relevant in the context of central sensitization, in which the central nervous system can become more sensitive to lower thresholds of pain over time. In fact, recent research examining the relationship between sleep and pain has found that individuals with more consistent and severe insomnia symptoms show stronger pain reactivity and poor sleep quality than their counterparts (Wei et al., 2018). Thus, as reviewed earlier, positive affect may serve as a buffer against the negative impacts of chronic pain.
Such an interpretation would be in line with the broaden-and-build theory of positive emotions (Fredrickson and Branigan, 2005). The broaden-and-build theory posits that experiencing positive emotions allows for individuals to broaden attentional scope to include a wider range of presently held thoughts and potential behaviors. On the contrary, negative emotions restrict this attentional scope, causing a narrowing of attention onto fewer thoughts and behaviors. This theory may be particularly relevant for chronic pain patients, as research has suggested that inducing positive mood states can decrease pain ratings among chronic pain patients (Tang et al., 2008). Therefore, positive emotions may help to broaden one’s thought repertoire during the experience of chronic pain, and allow the individual to move their attention outward rather than focus on the pain itself. Consequently, a reduction in the experience of pain may lead to a reduction in pain interference throughout one’s day as well.
However, our study’s findings add an additional layer to the role of emotions in pain interference. Namely, that sleep is an important factor in the promotion of positive or negative affect. Previous research has found that poor sleep results in an increased physiological stress response (Minkel et al., 2014), as well as increased negative affect and reduced positive affect (McCrae et al., 2008). In one study assessing affect repeatedly throughout the day for three days, researchers found that poor sleep was significantly predictive of positive affect but was not predictive of negative affect, indicating that positive affect may be particularly influenced by sleep (Bower et al., 2010). Our study’s findings align with such work and indicate that while sleep is predictive of negative affect, it is also predictive of positive affect among chronic pain patients.
Limitations
Though the present study has several strengths, it is not without limitations. First, sleep affect, and pain information were collected cross-sectionally, thus, not permitting an examination of the temporal association between these variables. Secondly, the MIDUS-III dataset lacked a measure of pain intensity, therefore, pain intensity could not be included as a covariate in our statistical models and may correlate with participants’ affective ratings and influence pain interference. Third, while the use of abbreviated scales was beneficial for examining sleep and pain interference in a large sample, administration of commonly used and well validated scales may provide greater information regarding these constructs and their relationships. Finally, the current study focused on examining the relationship between sleep, affect, and pain interference in a community sample of adults in mid to late-life. The degree of sleep disturbance and pain interference endorsed and the nature of the relationship between sleep, affect, and pain interference may differ among specific chronic pain conditions (e.g., rheumatoid arthritis, fibromyalgia).
Future Directions
Future work would benefit from utilizing longitudinal or experimental research designs in order to identify the temporal relationship between sleep, affect, and pain interference. Specifically, the use of experimental designs would assist in establishing evidence of a causative link between sleep and affect or affect and pain interference. In addition, future research would benefit from including both direct and indirect measures of pain to examine the influence of sleep and affect on each type of measure. Incorporating varying methodology, such as neuroimaging, may shed some light on the exact nature of these associations as well. Furthermore, studies may benefit from measuring additional pain constructs such as pain intensity and pain beliefs to derive a more comprehensive understanding of an individual’s pain experience and its relationship with affect. Finally, other subjective or objective sleep measurement tools such as sleep diaries, self-report measures, actigraphy, or polysomnography may be useful for capturing more detailed and comprehensive sleep information.
Conclusions
In conclusion, our results suggest that sleep impacts pain interference through affect, with poorer sleep predicting more negative affect and greater pain interference, and better sleep predicting more positive affect and lower pain interference. Importantly, these results highlight that pain interference in chronic pain patients is significantly affected by sleep. Thus, interventions which simultaneously target the promotion of positive affect as well as the reduction of negative affect may be most beneficial for reduced pain interference. Based on our current findings, the promotion of better sleep may be one such way to concurrently address positive and negative affect. Our results add to our understanding of the mechanisms through which sleep impacts pain interference among chronic pain patients.
Acknowledgments
Disclosure statement: Dr. Dzierzewski was supported by a grant from the National Institute on Aging (K23AG049955). Participant recruitment and data collection was supported by an additional grant from the National Institute on Aging (PO1AG020166). No other authors report commercial or financial conflicts of interest.
References
- Artner J, Cakir B, Spiekermann J-A, Kurz S, Leucht F, Reichel H, & Lattig F (2013). Prevalence of sleep deprivation in patients with chronic neck and back pain: a retrospective evaluation of 1016 patients. J of Pain Res, 6: 1–6. 10.2147/JPR.S36386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman HF, Thomas E, Millson DS, & Croft PR (2006). The natural history of headache: predictors of onset and recovery. Cephalalgia, 26: 1080–1088. [DOI] [PubMed] [Google Scholar]
- Bower B, Bylsma LM, Morris BH, & Rottenberg J (2010). Poor reported sleep quality predicts low positive affect in daily life among healthy and mood-disordered persons. J of Sleep Res, 19: 323–332. 10.1111/j.1365-2869.2009.00816.x [DOI] [PubMed] [Google Scholar]
- Charles ST, Reynolds CA, & Gatz M (2001). Age-related differences and change in positive and negative affect over 23 years. J Pers and Soc Psychol, 80: 136–151. [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, … et al. (2008). Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain, 9: 105–121. [DOI] [PubMed] [Google Scholar]
- Dzierzewski JM, Williams JM, Roditi D, Marsiske M, McCoy K, McNamara J, … McCrae CS (2010). Daily variations in objective nighttime sleep and subjective morning pain in older adults with insomnia: Evidence of covariation over time. J Am Geriatr Soc, 58: 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekkekakis P (2013). The Measurement of Affect, Mood, and Emotion: A Guide for Health-Behavioral Research. Cambridge University Press. [Google Scholar]
- Evans S, Djilas V, Seidman LC, Zeltzer LK, & Tsao JC (2017). Sleep quality, affect, pain and disability in children with chronic pain: Is affect a mediator or moderator? J of Pain, 18: 1087–1095. 10.1016/j.jpain.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, & Garland EL (2015). The Role of Positive Affect in Pain and Its Treatment: The Clin J Pain, 31: 177–187. 10.1097/AJP.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, & Smith MT (2013). The Association of Sleep and Pain: An Update and a Path Forward. J of Pain, 14: 1539–1552. 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Quartana PJ, Remeniuk B, Garland EL, Rhudy JL, Hand M, … Smith MT (2016). Partial sleep deprivation attenuates the positive affective system: Effects across multiple measurement modalities. Sleep, 40:, zsw017. 10.1093/sleep/zsw017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, & Branigan C (2005). Positive emotions broaden the scope of attention and thought-action repertoires. Cogn Emot, 19: 313–332. 10.1080/02699930441000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart JI, Burns JW, Post KM, Smith DA, Porter LS, Burgess HJ, … Keefe FJ (2017). Relationships Between Sleep Quality and Pain-Related Factors for People with Chronic Low Back Pain: Tests of Reciprocal and Time of Day Effects. Ann of Behav Med, 51: 365–375. 10.1007/s12160-016-9860-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MD, & Strube MJ (1994). Independence Revisited: The Relation between Positive and Negative Affect in a Naturalistic Setting. Pers Soc Psychol Bull, 20: 57–64. 10.1177/0146167294201005 [DOI] [Google Scholar]
- Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, MacFarlane GJ, … McBeth J (2007). The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology, 46: 666–671. [DOI] [PubMed] [Google Scholar]
- Haack M, & Mullington JM (2005). Sustained sleep restriction reduces emotional and physical well-being. Pain, 119: 56–64. 10.1016/j.pain.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press, New York. [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, … Ware JC (2015). National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health, 1: 233–243. 10.1016/j.sleh.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, FitzGerald JD, Ranganath VK, & Nicassio PM (2012). Sleep Loss Exacerbates Fatigue, Depression, and Pain in Rheumatoid Arthritis. Sleep, 35: 537–543. 10.5665/sleep.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, & Ustun TB (2007). Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry, 20: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari DJ, Davis MC, Yeung EW, & Tennen HA (2015). Positive affect and pain: mediators of the within-day relation linking sleep quality to activity interference in fibromyalgia. Pain, 156: 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae CS, McNamara JPH, Rowe MA, Dzierzewski JM, Dirk J, Marsiske M, & Craggs JG (2008). Sleep and affect in older adults: Using multilevel modeling to examine daily associations. J Sleep Res, 17: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel J, Moreta M, Muto J, Htaik O, Jones C, Basner M, & Dinges D (2014). Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychol, 33: 1430–1434. 10.1037/a0034219 [DOI] [PubMed] [Google Scholar]
- Mork PJ, & Nilsen TI (2012). Sleep problems and risk of fibromyalgia: longitudinal data on an adult female population in Norway. Arthritis & Rheumtol, 64: 281–284. [DOI] [PubMed] [Google Scholar]
- O’Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, & Robinson ME (2010). Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain, 26: 310–319. 10.1097/AJP.0b013e3181c328e9 [DOI] [PubMed] [Google Scholar]
- O’Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, & Robinson ME (2011). Intraindividual variability in daily sleep and pain ratings among chronic pain patients: bidirectional association and the role of negative mood. The Clin J of Pain, 27: 425–433. [DOI] [PubMed] [Google Scholar]
- Ong AD, Zautra AJ, & Reid MC (2010). Psychological Resilience Predicts Decreases in Pain Catastrophizing Through Positive Emotions. Psychol and Aging, 25: 516–523. 10.1037/a0019384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods, 40: 879–891. [DOI] [PubMed] [Google Scholar]
- Pressman SD, & Cohen S (2005). Does positive affect influence health? Psychol Bull, 131: 925–971. [DOI] [PubMed] [Google Scholar]
- Radler BT, (2014). The Midlife in the United States (MIDUS) Series: A National Longitudinal Study of Health and Well-being. Open Health Data, 2: 1–7. 10.5334/ohd.ai [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NK, Goodchild CE, Sanborn AN, Howard J, & Salkovskis PM (2012). Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep,35: 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NKY, Salkovskis PM, Hodges A, Wright KJ, Hanna M, & Hester J (2008). Effects of mood on pain responses and pain tolerance: An experimental study in chronic back pain patients, Pain, 138: 392–401. 10.1016/j.pain.2008.01.018 [DOI] [PubMed] [Google Scholar]
- Thomas E, Peat G, Harris L, Wilkie R, & Croft PR (2004). The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain, 110: 361–368. 10.1016/j.pain.2004.04.017 [DOI] [PubMed] [Google Scholar]
- Valrie CR, Gil KM, Redding-Lallinger R, & Daeschner C (2008). Daily mood as a mediator or moderator of the pain-sleep relationship in children with sickle cell disease. J Pediatr Psychol, 33: 317–322. 10.1093/jpepsy/jsm058 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol, 54: 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Wei Y, Blanken TF, & Van Someren EJ (2018). Insomnia really hurts: effect of a bad night’s sleep on pain increases with insomnia severity. Front in Psychiatry, 9, 1–8. 10.3389/fpsyt.2018.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicksell RK, Kanstrup M, Kemani MK, & Holmström L (2016). Pain Interference Mediates the Relationship between Pain and Functioning in Pediatric Chronic Pain. Front Psychol, 7 10.3389/fpsyg.2016.01978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Johnson LM, & Davis MC (2005). Positive Affect as a Source of Resilience for Women in Chronic Pain. J Consult Clin Psychol 73: 212–220. 10.1037/0022-006X.73.2.212 [DOI] [PMC free article] [PubMed] [Google Scholar]