Abstract
Purpose
To characterize the peripheral fundus autofluoresence (FAF) abnormalities in patients with age-related macular degeneration (AMD), correlate these with clinical findings and identify risk factors associated with these FAF abnormalities.
Design
Clinic-based cross-sectional study.
Participants
119 consecutive patients; 100 with AMD (200 eyes) and 19 normal patients (38 eyes).
Methods
In a prospective study performed at the Doheny Eye Institute, University of Southern California, widefield 200-degree FAF and color images were obtained by the Optos 200Tx Ultra-Widefield device using a standardized imaging protocol. The FAF images were captured centered on the fovea and additional images were captured after steering the field of view inferiorly and superiorly. All FAF and color images were graded independently by two masked ophthalmologists with the respect to the presence, location, extent, and type of peripheral (defined as outside the central 30 degrees) FAF abnormality.
Main outcome measures
Presence and type of peripheral FAF abnormalities.
Results
Peripheral FAF abnormalities were evident in 164 eyes (68.9%), with several distinct FAF patterns identified: Granular (46.2%), Mottled (34.0%), and Nummular (18.1%). A 90% concordance of AF patterns was observed between both eyes. Abnormal FAF occurred more frequently in neovascular compared with non-neovascular AMD or normal eyes (86% vs. 72.8% vs. 18.4%, p<0.001). Significant risk factors for peripheral FAF abnormalities were AMD type (neovascular AMD odds ratio [OR] 12.7 and non-neovascular AMD OR 6.2 compared with normal eyes, p<0.001), older age (OR 6.5, 95% confidence interval [C.I.] 2.4 – 17.8, p<0.001 for the oldest quartile compared with the youngest), and female gender (OR 4.1, 95% C.I. 1.9 – 8.9, p<0.001). Clinical features on colour photography were detected in 174 eyes (73.1%): peripheral drusen (51.7%), retinal pigment epithelium (RPE) depigmentation (34.9%), RPE hyperpigmentation (branching reticular pigmentation) (22.7%), and atrophic patches (16.8%). There was a high correlation between specific FAF and clinical findings: Granular FAF with peripheral drusen (p<0.001), and Mottled FAF with RPE depigmentation (p<0.001).
Conclusions
Several distinct patterns of peripheral FAF abnormalities were observed in 68.9% of patients, with AMd type, female gender and age being independent risk factors. The peripheral FAF patterns correlate strongly with specific clinical features seen in eyes with AMD.
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in developed countries,1-6 and, overall, the third most common cause of blindness worldwide.7 Significant visual loss typically occurs over time in neovascular AMD and advanced non-neovascular AMD, in particular, those who progress to geographic atrophy.
Fundus autofluorescence (FAF) is a non-invasive imaging technique which is often used to assess the health and function of the retinal pigment epithelium (RPE) and the overlying neurosensory retina.8,9 Its appearance reflects the lipofuscin content in these tissues,8,9 with increased FAF in areas with greater concentration of lipofuscin,8,10,11 and decreased FAF in tissues which are no longer viable (e.g., areas of RPE atrophy). Several studies have demonstrated that characteristic FAF abnormalities may be present in variety of diseases including inherited retinal dystrophies12-15 and AMD.8,9,16-18 In AMD, patterns of FAF abnormalities, particularly those in the junctional zone surrounding areas of geographic atrophy, have been shown to be of prognostic importance, in some cases identifying a subset of eyes with a higher risk for progression.8,19-22
Conventional FAF imaging approaches, however, have largely focused on the posterior pole (central 30 to 50 degrees).18,23,24 More recently, technology has become available (Optos Optomap systems, Optos, Dunfermline, Scotland) which permits non-invasive, non-contact widefield imaging of the peripheral retina, extending beyond 150 degrees in some cases, without the need for montaging multiple individual images.25-34 Previous studies using these devices have demonstrated that widefield reflectance imaging or fluorescein angiography may allow for the detection of peripheral pathology that is missed with the reference standard Early Treatment of Diabetic Retinopathy Study (ETDRS) 7-field photography.27 Widefield imaging devices have now been adapted to collect autofluorescence images as well of the retinal periphery.
In this study, we aimed to characterize the frequency and patterns of peripheral FAF and color photographic abnormalities in the eyes of patients with AMD, and study their relationship with the stage of the macular disease.
Methods
Subject Recruitment
One hundred and twenty-four consecutive patients were recruited from the tertiary care clinic of a single retina specialist (SS) at the Doheny Eye Institute (Los Angeles, California) for participation in this prospective study – 105 with age-related macular degeneration and 19 with normal eyes. The research study was approved by the Institutional Review Board of the University of Southern California, USA, and adhered to the tenets of the Declaration of Helsinki. Written, informed consent was obtained from all patients prior to enrollment and imaging. Demographic factors including age, gender, medical history, ophthalmic exam findings, diagnosis (including a simplified classification of the AMD as being neovascular or non-neovascular) were recorded for all patients. Eyes with history of retinal surgery, laser photocoagulation, radiation therapy or other retinal diseases such as hereditary retinal dystrophies were excluded from this study.
Widefield Image Acquisition
Although widefield imaging may be performed using an undilated pupil, all subjects had both eyes dilated to facilitate the capture of high quality images. Imaging was performed under typical mesosopic lighting conditions using the Optos 200Tx Ultrawidefeld retinal imaging device. A standardized acquisition protocol was used for all subjects. The protocol consisted of effectively a “three-field” capture of color (actually “pseudo-color image since the blue channel is absent) and then green-light (532nm) FAF images in each eye. Color images were obtained for subsequent correlation with the FAF findings. The primary field was centered on the fovea. Because of the very large depth of the field of the Optos device, the eyelids may interfere with the visualization of the far inferior and superior field in some eyes. For this reason, two additional color and two additional FAF images were captured after steering the field of view superiorly and inferiorly to the greatest extent possible.
All images were reviewed using the Optos V2 Vantage Pro Review software version 2.6.3.2 for grading of peripheral abnormalities. Assessments were made by two independent, masked, certified Doheny Image Reading Center (DIRC) graders (CT, FH). Graders assessed all images from each eye according to a pre-determined standardized grading protocol. Color and FAF images were graded separately in a masked fashion, to facilitate independent assessment of the two modalities. Color images were graded for the presence of peripheral pigmentary abnormalities, such as peripheral drusen, areas of RPE depigmentation, hyperpigmentation/hyperplasia, and atrophy. Other non-pigmentary abnormalities were also noted if present. Autofluorescence abnormalities on the FAF images were determined by the presence of any areas of “peripheral” increased or decreased autofluorescence relative to the homogenous greyish-white background of the green-light FAF image. A forced “Yes” or “No” choice for the presence of abnormalities was used for this analysis, and questionable grades were not allowed. If the images were deemed ungradable, the patients were excluded from the analysis.
For these color and FAF analyses, “peripheral” was defined to be the zone outside of the central 30 degrees (centered on the fovea – i.e., “Field 2”) out to the maximal extent/peripheral edge of the widefield image. In addition to ‘presence’, the graders assessed the location of the peripheral abnormalities according to four quadrants (each 90 degrees or 3 clock hours): superior, inferior, nasal, and temporal. If more than one quadrant was involved, all involved quadrants were counted. In addition a crude estimate of the extent of the peripheral FAF abnormalities was performed by estimating the number of clock hours or degrees involved in four broad categories (0 to <90 degrees, 90 to <180 degress, 180 to <270 degrees, 270 to 360 degrees). A more detailed quanification of the extent was not attempted in this analysis, as the widefield images my show distortion with increasing eccentricity (with lesions further peripheral measuring larger than more posteriorly oriented abnormalities) and a validated calibration schema for contending with this has not been developed.
Finally, a rudimentary assessment of the pattern of the peripheral FAF abnormality was performed. The candidate patterns (“granular”, “mottled”, and “nummular) to be scrutinized for were defined by an a priori inspection by the authors (CT, FH, SS) of a previous AMD widefield FAF dataset.35 Granular increased FAF was defined as small, discrete areas of bright, increased hyper-autofluorescence (Fig 1). Mottled decreased FAF consisted of areas of generally decreased autofluorescence in an uneven / irregular pattern. Nummular decreased FAF consisted of small to medium areas of discrete, uniformly decreased autofluorescence. Since more than one pattern of peripheral FAF abnormality could be present in the same eye, the primary pattern in a given eye was defined to be the predominant abnormality (based on extent/area involved), although the secondary (or if appropriate tertiary) abnormality was also recorded.
Figure 1.
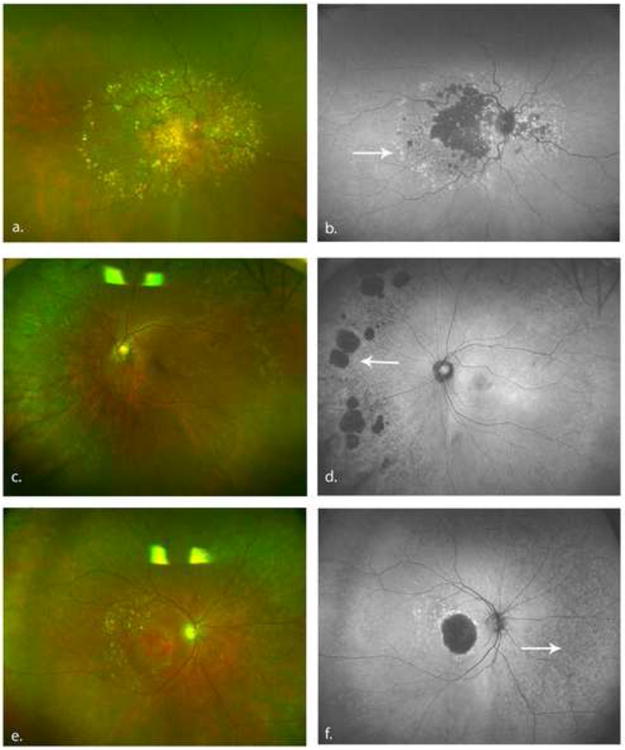
Color and autofluorescence photographs illustrating FAF abnormalities and the associated clinical features. 1a. Color fundus photography illustrating peripheral drusen. 1b. FAF image showing granular increased autofluorescence corresponding to the drusen (white arrow). 1c. Areas of RPE atrophy in the nasal periphery. 1d. FAF image showing areas of nummular decreased autofluorescence (white arrow) corresponding to the RPE atrophy. 1e. Areas of RPE depigmentation in the periphery of the fundus. Small peripheral drusen are seen temporally 1f. FAF image demonstrating large areas of mottled decreased autofluorescence (white arrow) corresponding to the area of RPE depigmentation. Fine granular FAF are also seen temporally corresponding to peripheral drusen. (FAF = Fundus autofluorescence, RPE = retinal pigment epithelium)
Following completion of independent grading of widefield color and FAF images by the two graders, the graders met in open adjudication and re-reviewed and discussed discrepant cases to yield a single consensus grade for each case. In cases where the two initial graders could not agree, a third senior DIRC-certified grader (SS) provided the “tie-breaking” vote.
FAF abnormalities in each quadrant were compared with the findings on the pseudocolor images in these same quadrants.
Statistical analysis was performed using SPSS for windows version 16.0 (SPSS Inc, Chicago, USA). The Chi square test was used to compare proportions, while the t-test was used to compare means. Factors which were significant on univariate analysis were then entered into the multivariate model.
Results
The widefield images of a total of 124 patients were reviewed – 105 with AMD and 19 with normal eyes. Of these, 5 patients had images that were deemed ungradable, most commonly due to significant media opacity, and both eyes from these individuals were excluded from the analysis. The results presented, therefore, are of 238 eyes from 100 patients (200 eyes) with AMD and 19 patients with normal eyes (38 eyes). The mean age of the study population was 79.2 years (range, 60 to 98, SD ± 8.6). Of 238 eyes, 114 (47.9%) had non-neovascular AMD, 86 (36.1%) had neovascular AMD, and 38 (16.0%) were normal. There were no significant differences between the three groups in terms of gender or co-existing systemic diseases (diabetes mellitus, hypertension or ischemic heart disease). However, patients with normal eyes were significantly younger than those with either noneovascular or neovascular AMD (mean age 68.8 years vs. 80.3 for both AMD groups, ANOVA p<0.001).
There was a high level of agreement between graders in the assessment of FAF abnormalities (kappa = 0.95 for type of abnormality and 0.91 for extent of FAF abnormality).
Of 238 eyes, 164 (68.9%) had some evidence of peripheral abnormal FAF, whereas the remaining 74 (31.1%) had normal FAF (Table 1). Several distinct FAF patterns were observed with high frequency, with some eyes having more than one pattern in different regions. The FAF abnormalities were classified into 3 distinct groups (Fig 1). Fine granular increased FAF was observed in 110 (46.2 %) eyes, nummular decreased FAF was evident in 43 (18.1%) eyes, and mottled decreased FAF was observed in 81 (34.0%) eyes. The FAF abnormality classification for the eyes is shown in Table 1. A secondary abnormality was observed in 71 eyes (29.8%), with granular increased FAF being the most frequent secondary abnormality (28 eyes). No eye was noted to have all three patterns of abnormality.
Table 1. Comparison of fundus autofluorescence patterns and color photographic abnormalities in normal eyes and those with non-neovascular and neovascular age-related macular degeneration.
| Normal eyes Number (%) (n=38 eyes) | Non-neovascular AMD Number (%) (n=114 eyes) | Neovascular AMD Number (%) (n=86 eyes) | P value | |
|---|---|---|---|---|
| Fundus autoflourescence pattern* | ||||
| Normal FAF | 31 (81.6) | 31 (27.2) | 12 (14.0) | <0.001 |
| Granular increased FAF | 6 (15.8) | 59 (51.8) | 45 (52.3) | <0.001 |
| Nummular decreased FAF | 2 (5.3) | 20 (17.5) | 21 (24.4) | 0.037 |
| Mottled decreased FAF | 0 (0) | 39 (34.2) | 42 (48.8) | <0.001 |
| Color photographic abnormality* | ||||
| Normal | 28 (73.7) | 25 (21.9) | 11 (12.8) | <0.001 |
| Peripheral drusen | 7 (18.4) | 69 (60.5) | 47 (54.7) | <0.001 |
| Reticular hyperpigmentation | 1 (2.6) | 28 (24.6) | 25 (29.1) | 0.004 |
| Cobblestone degeneration/atrophy | 4 (10.5) | 16 (14.0) | 20 (23.3) | 0.119 |
| Retinal pigment | 0 (0) | 40 (35.1) | 43 (50.0) | <0.001 |
| epithelium depigmentation | ||||
AMD = Age-related macular degeneration, FAF = fundus autofluorescence
More than one pattern or finding could be present in each eye.
Eyes with neovascular AMD had a higher frequency of peripheral FAF abnormalities compared with non-neovascular AMD or normal eyes (86.0% vs. 72.8 vs. 18.4%, p<0.001). For all 3 type of FAF abnormalities, the highest frequency occurred in eyes with neovascular AMD, compared to eyes with non-neovascular AMD and normal eyes respectively (all p<0.05) (Table 1). Females had a higher frequency of abnormal peripheral FAF compared with males (77.3% vs. 59.1%, univariate OR 2.36, 95% C.I. 1.35 – 4.15, p=0.003).
Also, on average, those with peripheral FAF abnormalities of any type were older compared with those with normal FAF in the periphery (80.8 vs. 69.4 years, p<0.001). Dividing the cohort by quartiles, the frequency of peripheral abnormality was 36.4% in those aged 75 years or less, then increased to over 80% in those aged 76 – 70 years and increased gradually after that (Table 2).
Table 2. Multivariate analysis of risk factors for abnormal peripheral FAF.
| Factor | Frequency of peripheral FAF abnormality (%) | Odds ratio | 95% CI | P value |
|---|---|---|---|---|
|
| ||||
| Gender | fc | |||
| Male | 59.1 | 1 | - | <0.001 |
| Female | 77.3 | 4.1 | 1.9 - 8.9 | |
|
| ||||
| AMD type | ||||
| None | 18.4 | 1 | - | - |
| Non-neovascular | 72.8 | 6.2 | 2.1 – 17.8 | 0.001 |
| Neovascular | 86.0 | 12.7 | 3.8 – 41.9 | <0.001 |
|
| ||||
| Age range | ||||
| <75 | 36.4 | - | - | - |
| 76 – 79 | 81.6 | 4.9 | 1.8 – 13.5 | 0.002 |
| 80 – 85 | 86.8 | 11.3 | 3.8 – 33.3 | <0.001 |
| >85 | 86.2 | 6.5 | 2.4 – 17.8 | <0.001 |
The variables in this multivariate model were gender, type of AMD and age grouped into quartiles.
AMD = Age-related macular degeneration, FAF = fundus autofluorescence, CI = confidence interval
The nasal periphery was most commonly involved (54.5%), followed by the temporal (38.2%) and superior (32.5%) periphery, and less frequently, inferior (24.0%). Of the 164 eyes with FAF abnormalities, the abnormality involved <90 degrees of the posterior pole in 67 eyes (40.9%), 90 to <180 degrees in 39 eyes (23.8%), 180 to <270 degrees in 34 eyes (20.7%) and 270 to 360 degrees in 24 eyes (14.6%).
A high concordance of FAF patterns was observed between eyes, with 89.6% of patients having the same pattern or patterns in both eyes. Of the patients with dissimilar FAF patterns, 2 (1.6%) had two different patterns of FAF abnormalities in the two eyes, while in the remainder (8.8%), the FAF abnormalities were only present unilaterally. In patients that had either neovascular or non-neovascular AMD in both eyes, the rates of concordance of FAF patterns were 90.9% and 89.3% respectively.
Using multiple logistic regression, eyes with neovasular AMD had a higher risk for abnormal FAF compared to non-neovascular AMD and normal eyes (Table 2). Female gender remained a significant risk factor (multivariate OR 4.1, 95% C.I. 1.9 – 8.9, p<0.001). Older age was also a statistically significant risk factor for the presence of any peripheral FAF abnormality. Compared with the youngest quartiles, the remaining age-groups had a significantly higher risk of peripheral FAF abnormality, with the multivariate ORs ranging from 4.9 to 11.3 (all p<0.01) (Table 2). Analyzing separately for the specific presence of mottled peripheral FAF, age was also a statistically significant risk factor (OR 6.6, 95% C.I. 1.7 – 24.7, p=0.005 for those aged >85 years compared with the youngest quartile and OR 4.2, 95% C.I. 1.1 – 16.0, p=0.039 for those aged 80 - 85). There was, however, no significant association between age and granular and nummular FAF on multivariate analysis.
Sub-analysis of age-matched groups
In order to determine whether age alone might be a determinant of the higher frequency of peripheral FAF abnormalities in eyes with AMD, an age-matched subset of 56 eyes with neovascular AMD, 68 eyes with non-neovascular AMD and 20 normal eyes was compared. In this subgroup, the ages ranged from 65 to 84 years, and there was no significant difference in age between the subgroups (mean ages 77.8 vs. 77.8 vs. 78.1 years respectively, ANOVA p=0.970).
Analysis of this subgroup showed the same results as described above: the frequency of peripheral FAF abnormalities was highest in neovascular AMD (82.1%), followed by non-neovascular AMD (69.1%) and lowest in normal eyes (20.0%). Multivariate analysis revealed that the type of AMD was a risk factor for abnormal peripheral FAF (OR 22.3 for neovascular AMD and 11.9 for non-neovascular AMD compared to normal eyes, p=0.002 and 0.007 respectively). In addition, female gender (OR 4.7, p=and age groups remained significant risk factors.
Assessment of widefield color photographs revealed that 174 eyes (73.1%) had some abnormality in the periphery. The abnormalities detected included peripheral drusen (123 eyes, 51.7%), retinal pigment epithelium (RPE) depigmentation (83 eyes, 34.9%), RPE hyperpigmentation (54 eyes, 22.7%; typically in a reticular pattern), and atrophic patches (40 eyes, 16.8%; in some cases consistent with cobblestone degeneration). No peripheral disciform lesions were observed in this series. Of the 164 eyes with abnormal peripheral FAF, 158 (96.3%) also had abnormal clinical findings on widefield color photography (p<0.001).
Of the 110 eyes with granular FAF, 91 (82.7%) had peripheral drusen, while drusen were present in only 32 of 128 eyes (25.0%) without granular FAF (p<0.001). Of those with nummular FAF, 69.8% had atrophic patches on color photography which could be classified as cobblestone degeneration (p<0.001). However, an additional 13 eyes with nummular FAF did not demonstrate evidence of cobblestone degeneration on color photography. Mottled FAF was most commonly associated with RPE depigmentation (76.5% vs. 13.4%, p<0.001).
Discussion
In this study, we describe the peripheral FAF abnormalities in a consecutive series of 238 eyes (119 patients) and report that 68.9% of such individuals have peripheral FAF abnormalities. Of those eyes with neovascular AMD, abnormal FAF patterns were observed in 86%, compared with 72.8% of those with non-neovascular AMD and 18.4% in normal eyes. These FAF abnormalities were highly concordant between eyes in 90% of individuals, and manifest in several distinct patterns, for which we have proposed a classification system. The observed peripheral FAF changes also appeared to correlate with the type of macular AMD. To the best of our knowledge, these findings have not previously been described in detail, although peripheral FAF abnormalities in retinal diseases have been discussed (Wolf-Schnurrbusch et al. Invest Ophthalmol Vis Sci 2011;52:E-Abstract 3531, Puliafito et al. Invest Ophthalmol Vis Sci 2011;52:E-Abstract 4796; Lammersdorf et al. Invest Ophthalmol Vis Sci 2010;51:E-Abstract 264; Zenger et al. Invest Ophthalmol Vis Sci 2012;53:E-Abstract 6534).
An earlier study by Reznicek et al36 described an increase in peripheral FAF intensity and irregularity (defined as the standard deviation of the FAF intensity) in eyes with treated and untreated AMD compared with normal eyes. However, that study did not report the number of eyes with FAF abnormalities and whether these proportions differed between eyes with differing classifications/types of AMD.36 In addition, Reznicek et al did not describe the specific features or patterns of the FAF abnormalities, and their location or extent.36
Abnormalities in FAF are caused by differences in the level of lipofuscin, in particular A2-E, in the retinal pigment epithelial cells.8,37,38 In diseased cells, lipofuscin accumulates, causing increased FAF.8,10,11 When the cells eventually die, these areas appear dark on FAF imaging. It has been demonstrated that the area of abnormal FAF may be more extensive than the clinical appearance on fundoscopy, and the changes seen on FAF imaging may precede the development of ophthalmoscopically visible lesions.
Earlier studies on the FAF features of AMD focused on the central 30 degrees,18,19,23,24 partly due to limitations of the equipment and the inability to image the periphery in a convenient non-contact manner. A variety of central FAF patterns, particularly in the junctional zones surrounding atrophic lesions, have been described in detail,8,18,19,23 and some patterns (e.g., banded or diffuse junctional FAF) were identified to be of potential prognositic value. In the longitudinal natural history arm of the Fundus Autofluorescence in age-related Macular degeneration (FAM) study,8 classification of eyes according to FAF pattern at baseline appeared to have stronger predictive value on progression of atrophy over time compared with other risk factors, including baseline size of geographic atrophy (GA). In this study, we did not correlate the peripheral FAF patterns with those in the central 30 degrees because the FAF images were obtained using the green wavelength, whereas those in the FAM study used a blue (488nm) wavelength.8,9 In addition, as the widefield images are not averaged, this may have some effect on the comparisons (Lammersdorf et al. Invest Ophthalmol Vis Sci 2010;51:E-Abstract 264).
Our study has characterized the FAF patterns in the peripheral retinae of individuals with AMD, and identified three distinct patterns. We observed differences in the frequency of FAF abnormalities between eyes with neovascular and non-neovascular AMD and normal eyes. All types of abnormal FAF were more frequent in eyes with neovascular AMD and least frequent in normal eyes. On multivariate analysis, eyes with both neovascular and non-neovascular AMD had significantly higher risk of having abnormal FAF compared to normal eyes. Just as some FAF patterns in the central 30 degrees were found to carry increased risk of GA progression, one wonders whether non-neovascular AMD eyes with these peripheral FAF patterns may be at higher risk for converting to neovascular AMD. However, a prospective longitudinal study will be required to establish this association. Hopefully, the widefield FAF imaging data that is being collected in a sub-study of the Age-Related Eye Diseases Study 2 (AREDS2), will be able to more fully evaluate this question.
We found a high level of concordance (90%) in the peripheral FAF findings in our patients. Similarly, Bindewald et al. described 89.1% concordance in central FAF patterns.9 It was suggested that, in view of the high degree of intra-individual symmetry,39 genetic determination rather than nonspecific aging changes may have been involved. This hypothesis is further supported by our finding that in eyes with bilateral neovascular or non-neovascular AMD, the concordance of peripheral FAF patterns was equally high, regardless of whether the individual had neovascular or non-neovascular AMD. Refinements in phenotyping by peripheral FAF patterns may shed light on the genetic risk factors in a complex, multifactorial disease like AMD.
In this study, we also observed that patients with any type of peripheral FAF abnormality were significantly older compared with those with normal peripheral FAF (80.8 vs. 69.4 years, p<0.001). Older age was also found to be a significant risk factor for the presence of any type of FAF abnormality (OR 4.9 to 11.3 for the older quartiles compared with the younger quartiles (Table 2) and, in particular, mottled FAF on multivariate analysis. These findings may be consistent with Reznicek's study which described that the FAF intensity increased with age.36 We also observed that females had a higher risk of abnormal peripheral FAF compared to males, and this warrants further investigation.
On comparing FAF abnormalities with findings in the retinal periphery on color photography, a high correlation was found between abnormal FAF lesions and color abnormalities, with 96.3% of eyes with abnormal peripheral FAF also demonstrating abnormalities on the color photographs. The different peripheral FAF patterns correlated with specific abnormalities on color photography: granular FAF with peripheral drusen, nummular FAF with cobblestone degeneration, and mottled FAF with RPE depigmentation. However, it is important to note discrepancies (between color and FAF) were noted in a few cases, and the high contrast of FAF imaging facilitated an easier recognition of these findings.
Our study is not without limitations. First, our study, though a prospective collection according to a standardized protocol, was only a single point assessment. Thus, we do not have data regarding that stability or progression of these findings, and their true predictive power for progression to advanced disease. Second, we only evaluated green-light FAF, which is currently available with the Optos widefield instrument. FAF imaging with blue or near-infrared light could potentially reveal other more informative abnormalities, and should be the subject of future studies. Although there have not been any studies comparing FAF images using widefield and conventional imaging, an earlier study of color fundus photographs in patients with AMD reported good agreement between conventional and widefield images.40 This gives clinicians greater confidence that images seen on widefield imaging are comparable with conventional images. Thirdly, the normal controls in this study were not age-matched with the patients with AMD, and were significantly younger compared to those with AMD. As there was a small group of normal individuals being seen in the clinic, we thought that it would be interesting to observe the frequency of FAF abnormalities in this group. However, the primary objective of this study was not to compare eyes with AMD and normal controls. Rather, we aimed to determine the differences in FAF patterns among eyes with neovascular and non-neovascular AMD. In addition, there may be a variety of other confounders that might affect peripheral FAF findings (e.g. systemic hypertension), so matching for age only would not fully address this issue. The effect of age would have been accounted for in the multivariate analysis, which demonstrated that the phenotype (type of AMD) affected the frequency of peripheral FAF abnormalities independent of age. In addition, a sub-analysis comparing a smaller group of normal eyes with age-matched AMD eyes showed the same overall results, hence we believe that the differences in frequency of peripheral FAF abnormalities are not due only to age, but may reflect the effect of the AMD type.
In summary, several distinct patterns of peripheral FAF abnormalities were observed in 68.9% of individuals. Among eyes with AMD, mottled decreased FAF was more common in neovascular compared with non-neovascular AMD or normal eyes. These peripheral FAF abnormalities also correlated well with specific clinical findings on color photography. The prognostic significance of these abnormalities requires further elucidation in ongoing prospective trials.
Acknowledgments
Financial support: Supported in part by NIH Grant EY03040, NEI Grant R01 EY014375, and DFG Grant He 6094/1-1 (Dr Sadda), and the National Healthcare Group Clinician Leadership in Research Grant (Dr Tan)
Dr. Sadda previously shared in royalties from intellectual property licensed to Topcon Medical Systems by the Doheny Eye Institute. Dr. Sadda also previously served on the scientific advisory board for Heidelberg Engineering and receives research support from Carl Zeiss Meditec, Optovue Inc. and Optos.
Footnotes
AAO Meeting paper: This study was presented in part at the annual meeting of the American Academy of Ophthalmology, October 2011, Orlando, FL.
Drs Tan and Heussen have no financial interests to declare.
Conflict of interest: No conflict of interest exists for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prokofyeva E, Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res. 2012;47:171–88. doi: 10.1159/000329603. [DOI] [PubMed] [Google Scholar]
- 2.Wong TY, Loon SC, Saw SM. The epidemiology of age related eye diseases in Asia. Br J Ophthalmol. 2006;90:506–11. doi: 10.1136/bjo.2005.083733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothe Nissen K, Sjolie AK, Jensen H, et al. The prevalence and incidence of visual impairment in people of age 20-59 years in industrialized countries: a review. Ophthalmic Epidemiol. 2003;10:279–91. doi: 10.1076/opep.10.4.279.15909. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Wang Y, Li Y, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006;113:1134–41. doi: 10.1016/j.ophtha.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 6.Maberley DA, Hollands H, Chuo J, et al. The prevalence of low vision and blindness in Canada. Eye (Lond) 2006;20:341–6. doi: 10.1038/sj.eye.6701879. [DOI] [PubMed] [Google Scholar]
- 7.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 8.Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. FAM-Study Group. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–72. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Bindewald A, Schmitz-Valckenberg S, Jorzik JJ, et al. Classification of abnormal fundus autofluorescence patterns in the junctional zone of geographic atrophy in patients with age related macular degeneration. Br J Ophthalmol. 2005;89:874–8. doi: 10.1136/bjo.2004.057794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Opthalmol Vis Sci. 1995;36:718–29. [PubMed] [Google Scholar]
- 11.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–66. [PubMed] [Google Scholar]
- 12.Fujinami K, Tsunoda K, Hanazono G, et al. Fundus autofluorescence in autosomal dominant occult macular dystrophy. Arch Ophthalmol. 2011;129:597–602. doi: 10.1001/archophthalmol.2011.96. [DOI] [PubMed] [Google Scholar]
- 13.Gomes NL, Greenstein VC, Carlson JN, et al. A comparison of fundus autofluorescence and retinal structure in patients with Stargardt disease. Invest Ophthalmol Vis Sci. 2009;50:3953–9. doi: 10.1167/iovs.08-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara T, Imamura Y, Giovinazzo VJ, Spaide RF. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina. 2010;30:1206–16. doi: 10.1097/IAE.0b013e3181e097f0. [DOI] [PubMed] [Google Scholar]
- 15.Chen RW, Greenberg JP, Lazow MA, et al. Autofluorescence imaging and spectral-domain optical coherence tomography in incomplete congenital stationary night blindness and comparison with retinitis pigmentosa. Am J Ophthalmol. 2012;153:143–54. doi: 10.1016/j.ajo.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holz FG, Bellman C, Staudt S, et al. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1051–6. [PubMed] [Google Scholar]
- 17.Bindewald A, Bird AC, Dandekar SS, et al. Classification of fundus autofluorescence patterns in early age-related macular disease. Invest Ophthalmol Vis Sci. 2005;46:3309–14. doi: 10.1167/iovs.04-0430. [DOI] [PubMed] [Google Scholar]
- 18.von Ruckmann A, Fitzke FW, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 1997;38:478–86. [PubMed] [Google Scholar]
- 19.Holz FG, Bellmann C, Margaritidis M, et al. Patterns of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1999;237:145–52. doi: 10.1007/s004170050209. [DOI] [PubMed] [Google Scholar]
- 20.Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106:1768–79. doi: 10.1016/S0161-6420(99)90340-8. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz-Valckenberg S, Bindewald-Wittich A, Dolar-Szczasny J, et al. Fundus Autofluorescence in Age-Related Macular Degeneration Study Group. Correlation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with AMD. Invest Ophthalmol Vis Sci. 2006;47:2648–54. doi: 10.1167/iovs.05-0892. [DOI] [PubMed] [Google Scholar]
- 22.Lois N, Owens SL, Coco R, et al. Fundus autofluorescence in patients with age-related macular degeneration and high risk of visual loss. Am J Ophthalmol. 2002;133:341–9. doi: 10.1016/s0002-9394(01)01404-0. [DOI] [PubMed] [Google Scholar]
- 23.Bindewald A, Jorzik JJ, Loesch A, et al. Visualization of retinal pigment epithelial cells in vivo using digital high-resolution confocal scanning laser ophthalmoscopy. Am J Ophthalmol. 2004;137:556–8. doi: 10.1016/j.ajo.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Jorzik JJ, Bindewald A, Dithmar S, Holz FG. Digital simultaneous fluorescein and indocyanine green angiography, autofluorescence, and red-free imaging with a solid-state laser-based confocal scanning laser ophthalmoscope. Retina. 2005;25:405–16. doi: 10.1097/00006982-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Wessel MM, Aaker GD, Parlitsis G, et al. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32:785–91. doi: 10.1097/IAE.0b013e3182278b64. [DOI] [PubMed] [Google Scholar]
- 26.Wessel MM, Nair N, Aaker GD, et al. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96:694–8. doi: 10.1136/bjophthalmol-2011-300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho M, Kiss S. Detection and monitoring of sickle cell retinopathy using ultra wide-field color photography and fluorescein angiography. Retina. 2011;31:738–47. doi: 10.1097/IAE.0b013e3181f049ec. [DOI] [PubMed] [Google Scholar]
- 28.Spaide RF. Peripheral areas of nonperfusion in treated central retinal vein occlusion as imaged by wide-field fluorescein angiography. Retina. 2011;31:829–37. doi: 10.1097/IAE.0b013e31820c841e. [DOI] [PubMed] [Google Scholar]
- 29.Shah SP, Jain A, Coffee RE, McCannel TA. Optos Panoramic 200MA ultrawide-field imaging of peripheral RPE adenoma. Semin Ophthalmol. 2009;24:37–9. doi: 10.1080/08820530802520210. [DOI] [PubMed] [Google Scholar]
- 30.Heussen FM, Vasconcelos-Santos DV, Pappuru RR, et al. Ultra-wide-field green-light (532-nm) autofluorescence imaging in chronic Vogt-Koyanagi-Harada disease. Ophthalmic Surg Lasers Imaging. 2011;42:272–7. doi: 10.3928/15428877-20110505-01. [DOI] [PubMed] [Google Scholar]
- 31.Slotnick S, Sherman J. Panoramic autofluorescence: highlighting retinal pathology. Optom Vis Sci. 2012;89:E575–84. doi: 10.1097/OPX.0b013e318250835d. [DOI] [PubMed] [Google Scholar]
- 32.Manivannan A, Plskova J, Farrow A, et al. Ultra-wide-field fluorescein angiography of the ocular fundus. Am J Ophthalmol. 2005;140:525–7. doi: 10.1016/j.ajo.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 33.Prasad PS, Oliver SC, Coffee RE, et al. Ultra wide-field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology. 2010;117:780–4. doi: 10.1016/j.ophtha.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Neubauer AS, Kernt M, Haritoglou C, et al. Nonmydriatic screening for diabetic retinopathy by ultra-widefield scanning laser ophthalmoscopy (Optomap) Graefes Arch Clin Exp Ophthalmol. 2008;246:229–35. doi: 10.1007/s00417-007-0631-4. [DOI] [PubMed] [Google Scholar]
- 35.Heussen FM, Tan CS, Sadda SR. Prevalence of peripheral abnormalities on ultra-widefield greenlight (532 nm) autofluorescence imaging at a tertiary care center. Invest Ophthalmol Vis Sci. 2012;53:6526–31. doi: 10.1167/iovs.12-9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reznicek L, Wasfy T, Stumpf C, et al. Peripheral fundus autofluorescence is increased in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:2193–8. doi: 10.1167/iovs.11-8483. [DOI] [PubMed] [Google Scholar]
- 37.Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye (Lond) 2001;15:384–9. doi: 10.1038/eye.2001.141. [DOI] [PubMed] [Google Scholar]
- 38.Schutt F, Davies S, Kopitz J, et al. Photodamage to human RPE cells by A2-E, a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 2000;41:2303–8. [PubMed] [Google Scholar]
- 39.Bellmann C, Jorzik J, Spital G, et al. Symmetry of bilateral lesions in geographic atrophy in patients with age-related macular degeneration. Arch Ophthalmol. 2002;120:579–84. doi: 10.1001/archopht.120.5.579. [DOI] [PubMed] [Google Scholar]
- 40.Csutak A, Lengyel I, Jonasson F, Leung I, Geirsdottir A, Xing W, Peto T. Agreement between image grading of conventional (45°) and ultra wide-angle (200°) digital images in the macula in the Reykjavik eye study. Eye (Lond) 2010;24:1568–75. doi: 10.1038/eye.2010.85. [DOI] [PubMed] [Google Scholar]


