Abstract
Estrogen decreasing during menopause can create problems in the cardiovascular organs, brain, urogental tract, and bone. Estrogen replacement therapy (ERT) can be used to increase estrogen levels. However, side-effects including breast cancer may limit their use. Tempe and tofu are natural plat-based foods which contain phytoestrogens. The aim of this research was to compare estrogen serum levels in ovariectomized rats given tempe flour and tofu flour.
This research was conducted on female rats, aged 12 months (n = 72 rats). Rats were grouped into 5 groups: tempe flour, tofu flour, estradiol, casein and non-ovariectomized. The intervention was carried out for two months with three observation points; i) in the second week, ii) fifth week and iii) eighth week. Estrogen serum analysis was done by ELISA (Estradiol EIA-2693). The mean and the differences between treatment groups were analysed using one way ANOVA with post hoc polynomial contrasts (LSD).
The highest estrogen serum in the second week intervention was found in the estradiol group followed by the tempe flour, tofu flour, non-ovariectomized and casein groups, respectively. The tempe flour group had the highest mean serum estrogen levels in the fifth week, followed by the estradiol group, non-ovariectomized group, tofu group and casein group. At the eighth week of intervention, the highest estrogen serum level was found in the tempe flour group followed by the estradiol group, tofu flour group, non ovariectomy group and casein group. Interventions in the fifth and eighth weeks showed significant differences between groups (p < 0.05). It was concluded that tempe flour rendered twice the serum estrogen level compared to tofu flour. Further research is needed in the form of clinical trials to prove that effect in humans.
Keywords: Anatomy, Systems biology, Estrogen, Ovariectomized rats, Tempe, tofu
1. Introduction
The estrogen levels decrease seen after menopause is associated with an increase in risk of coronary artery disease, cardiovascular disease, osteoporosis, urinary incontinence, urinary tract infections, weight gain, and a loss of neuroprotective effects [1, 2, 3, 4]. Estrogen replacement therapy (ERT) is intended to increase estrogen hormone levels in postmenopausal women. Estrogen affects and helps regulate bodily functions which include the reproductive system, brain and central nervous system, bone, liver and urinary tract [5,6]. Estrogen replacement therapy (ERT) has shown various benefits in the aging process, but use of hormone therapy also confers risks [7]. Various natural and artificial substances have been found to have estrogen-like activity. Artificial substances that are estrogen-like are called xenoestrogens, while natural substances from plants that have estrogen-like activity are called phytoestrogens [8].
The main phytoestrogen group consists of isoflavones, coumestans and lignans. Isoflavones are polyphenol compounds that have estrogen-like effects. Nuts, especially soybeans, are the richest sources of isoflavones in human food. Isoflavones are the most potent and dominant phytoestrogens in soy. They are structurally similar to estrogens and functionally similar to 17β-estradiol [9]. In soybeans, the main isoflavone aglycones are genistein, daidzein, and glycitein [10]. Soy isoflavones can protect myocardial cells in ovariectomized rats [2]. Isoflavone-rich soy products decrease FSH and LH in premenopausal women and may increase estradiol levels in postmenopausal women [11]. The most widely consumed soy products in Indonesia are tempe and tofu.
Tempe and tofu have similar structural but they have different textural, how to made and nutritional value. Tempe is made directly from cooking and fermenting soybeans, tofu is made from condensed, unfermented soy milk that's been processed into solid white blocks. Tempe of soybean processed products through the fermentation process causes an increase in total isoflavones, especially from aglycones which are much higher isoflavones than tofu [12]. Tofu contains more glucoside, so the total isoflavones are lower than tempe [13].
Tempe and tofu contain isoflavones which can bind to estrogen receptors in the body. Similar to estrogens, isoflavones can reduce psychovasomotor complaints, especially bursts of heat in the chest (hot flushes or flashes) as experienced by women during menopause [14]. The fermentation process makes soybeans in tempe easier to digest. Tempe has an excellent nutritional profile which includes protein, calcium, phosphorus, iron, B vitamins, and minerals, and it can reduce LDL levels and maintain HDL levels [15,16]. Soybean flour and tempe flour were found to increase estrogen hormones in rats aged 12 weeks which had undergone ovariectomy [17]. The difference with previous research was in this study using rats with 12 months of age that had been ovariectomized while in previous studies using rats aged 12 weeks who had been ovariectomized. The use of older rats is expected to approach the menopausal rats model [18]. However, it is unclear whether soy would also affect estrogen levels in older rats, which are also more at risk of chronic morbidity. Similar to estrogens, consumption of soy products has been linked to reduction in incidence or severity of chronic diseases such as cardiovascular morbidity, menopausal symptoms, bone loss, etc. However, unlike estrogens, soy could also reduce breast and prostate cancers. Overall, consuming moderate amounts of traditionally prepared and minimally processed soy foods may offer modest health benefits while minimizing potential for any adverse health effects [19]. The objective of this study was to analyze the effect of tempe and tofu flours on serum estrogen in ovariectomized female rats who were 12 months old.
2. Material and methods
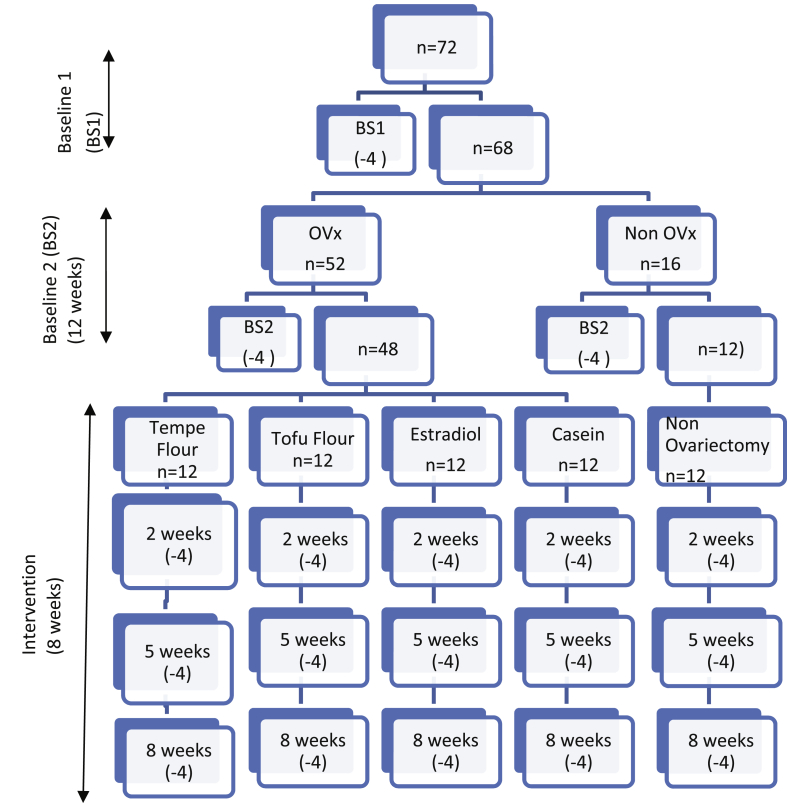
This research was conducted using a completely randomized controlled design approach. This study had been approved and had received ethical clearance number: 07–2012 RSH-IPB from the Animal Care and Use Committee of Veterinary Teaching Hospital of Bogor Agricultural University. The rats used were Spraque Dawley female rats, aged 12 months. Baseline 1 measurements were performed after the adaptation period in four randomly selected rats. The next step was ovariectomy in 52 rats, and 16 non ovariectomized rats were used as controls. Before surgery, the rat was anesthetized using 10% ketamine (10–20 mg/kg BW) and 2% xylazine (2 mg/kg BW). Surgery was carried out by a veterinarian. Three months after ovariectomy, Baseline 2 assessment was done. Ovariectomized rats were given standard feed for 3 months as an experimental animal model of old female rats [18]. The experimental animals were divided into 5 groups: tempe flour, tofu flour, estradiol group, casein group, and non OVx. Each intervention group consisted of 12 rats randomized to treatment. The intervention was carried out for two months with three observation points in the second, fifth, and eighth weeks. At each observation point, surgery was taken to draw blood was assessed within 4 of each group [3]. Chart of implementation of treatment of rats can be seen in Fig. 1.
Fig. 1.
Sample group during research.
Independent variables included consumption of tempe flour and tofu flour. Estradiol consumption was given as a positive control and casein as a negative control. The dependent variable was serum estrogen levels. Treatment in rats was carried out at the Animal Inpatient Installation. The enclosure environment was dry, had adequate ventilation and sufficient lighting where the light was on for 14 hours and 10 hours were dark. Intervention was carried out for 2 months, feed in the form of flour. The composition of feed according to AIN-93M consisted of 14% protein, 5% mineral, 4% fat, 1% vitamin, 5% fiber, 5% water and 66% starch. The amount of feed consumption was determined by fair feeding (15 gram/day/rat). The estradiol group was given ethinylestradiol (synthetic estrogen) 9 × 10-3 mg/day/200 g of body weight. The administration of estradiol was carried out by using a sonde. Female rats were from the Faculty of Veterinary Medicine, Bogor Agricultural University. Rats were placed in a plastic cage and drinking water was given ad libitum [3].
Serum estrogen analysis was carried out at the Hormone Laboratory, Rehabilitation and Reproduction Unit of the Faculty of Veterinary Medicine, Bogor Agricultural University. Analysis of serum estrogen was done by Elisa method with Reagent Estradiol EIA-2693. Data analysis for mean and difference in differences between treatment groups was done using one way ANOVA with post hoc LSD polynomial contrasts with SPSS 16.0.
3. Results
Based on Table 1, estrogen levels in baseline 1 and three months after ovariectomy for the ovariectomized group (baseline 2) showed no difference (P > 0.05), but were significantly lower than in the non ovariectomized group (P < 0.05).
Table 1.
Average ± SD (95%CI) serum estrogen level in (non) ovarietomized rats.
| Variabel | Baseline 1 |
Baseline 2 |
|
|---|---|---|---|
| Non Ovariectomized | Ovariectomized | ||
| Estrogen serum (pg/ml) | 12.75 ± 4.12a | 24.05 ± 2.52b | 9.99 ± 1.24a |
*Note. Letter in the same line followed by same letter means no significant difference.
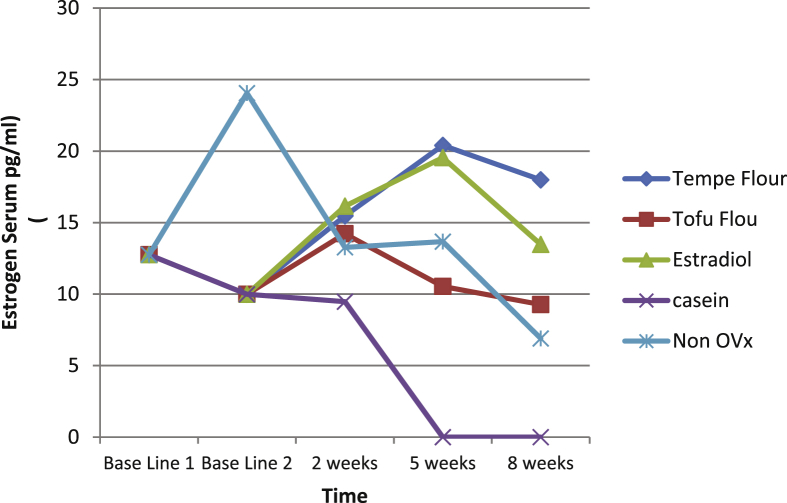
Table 2 shows that the estrogen content in the group with the tempe flour, tofu flour, and estradiol increased until the second week. In the fifth week the tempe and estradiol flour groups were still increasing and in the eighth week decreased. The tofu group in the fifth week showed estrogen levels began to fall until the eighth week. In the group with the casein intervention estrogen levels decreased from ovariectomy to intervention at 8 weeks. Whereas in the non ovariectomy group, estrogen levels were almost the same as the tofu flour intervention group, but the decrease in the eighth week was higher than in the tofu group (Table 2 and Fig. 2).
Table 2.
Averages±SD (95%CI) serum estrogen (pg/ml) after Tempe Flour, Tofu flour, Estradiol, Casein, non Ovariectomized group.
| Duration of treatment | Intervention Group |
P Value | ||||
|---|---|---|---|---|---|---|
| Tempe Flour | Tofu Flour | Estradiol | Casein | Non Ovariect. | ||
| Intervention 2 weeks | 15.47 ± 4.75 | 14.22 ± 1.59 | 16.15 ± 4.84 | 9.47 ± 1.99 | 13.27 ± 4.09 | 0.150 |
| Intervention 5 weeks | 20.4 ± 4.71 | 10.54 ± 1.57 | 19.54 ± 11.44 | 0.0 ± 0.0 | 13.67 ± 4.09 | 0.001 |
| Intervention8 weeks | 17.99 ± 3.44 | 9.27 ± 2.53 | 13.47 ± 2.49 | 0.0 ± 0.0 | 6.9 ± 1.45 | 0.0001 |
Fig. 2.
Serum estrogen in the group given tempe flour, tofu flour, estradiol, or which were non ovariectomized according to time.
The ANOVA test showed that after 2 weeks of intervention, there was no significant difference in serum esterogen between groups. In intervention week 5 and 8, results showed significant differences (P < 0.05). The group of rats that consumed tempe flour had the highest serum estrogen content of 17,987 pg/ml, but the group of rats that consumed tofu only had 9.275 pg/ml after 8 weeks intervention. The increase in serum estrogen in rats that consumed tempe flour was twice as high as those which consumed tofu flour (Table 2).
The results of further testing using LSD Post Hoc comparisons can be seen in Table 3 below which shows that after a two-weeks intervention, the tempe flour group was only significantly different from the casein group. In the fifth week, the tempe flour group was significantly different from the tofu and casein groups. After the eighth week of intervention, the tempe flour group was significantly different from all other intervention groups and had the highest estrogen content.
Table 3.
Differences in average ± SD (95%CI) estrogen serum levels in pg/ml between tempe flour and tofu flour, tempe flour and Estradiol, tempe flour and casein, tempe flour and non ovariectomized group over time.
| Duration of treatment | Tempe flour and tofu flour | Tempe flour and estradiol | Tempe flour and casein | Tempe flour and non ovariectomized |
|---|---|---|---|---|
| 2 Weeks | 1.25 ± 2.63 | -0.67 ± 2.63 | 6 ± 2.63a | 2.2 ± 2.63 |
| 5 Weeks | 9.85±4b | 0.86 ± 4 | 20.40±4c | 6.72 ± 4 |
| 8 Weeks | 8.71 ± 1.63d | 4.51 ± 1.63e | 17.99 ± 1.63f | 11.09 ± 1.63g |
Note: The Alphabet followed P values: a = 0.038; b = 0.026; c = 0.00; d = 0.00; e = 0.014; f = 0.0001; g = 0.00001. Tempe flour, Tofu flour, estradiol, casein, non ovariectomy.
The tofu group at the second week at intervention was not significantly different from all other intervention groups. However, at the fifth and eighth week of interventions, tofu flour was significantly different from the intervention group of estradiol and casein and non ovariectomized controls (Table 4).
Table 4.
Average ± SD (95%CI) Serum Estrogen content difference (pg/ml) between Tofu flour with controls.
| Duration of giving | Tofu flour and Estradiol | Tofu flour and Casein | Tofu flour and non ovariectomized |
|---|---|---|---|
| 2 Weeks | -1.92 ± 2.63 | 4.75 ± 2.63 | 0.95 ± 2.63 |
| 5 Weeks | -8.99±4a | 10.55±4b | -3.13 ± 4 |
| 8 Weeks | -4.2 ± 1.63c | 9.28 ± 1.63d | 2.37 ± 1.6 |
*Note: The Alphabet followed P Values: a = 0.04; b = 0.019; c = 0.021; d = 0.001.
The results of the analysis of different tests between the time of intervention and baseline 2 showed that the tempe flour group was significantly different from baseline 2 at fifth and eighth weeks of intervention. The tofu flour group was significantly different from baseline 2 in the second week intervention. The estradiol group was significantly different from baseline 2 in the fifth week of intervention. At the fifth and eighth week interventions, the casein group was significantly different from baseline 2 (Table 5).
Table 5.
Average ± SD (95%CI) Serum Estrogen level difference (pg/ml) between duration of treatment per group with baseline 2.
| Intervention 2 weeks -baseline 2 |
Intervention 5 weeks -baseline 2 |
Intervention 8 weeks -baseline 2 |
|
|---|---|---|---|
| Tempe Flour | 5.49 ± 2.74 | 10.41 ± 2.74a | 8 ± 2.74b |
| Tofu Flour | 4.24 ± 1.73c | 0.56 ± 1.73 | -0.71 ± 1.73 |
| Estradiol | 6.16 ± 4.23 | 9.55 ± 4.23d | 3.49 ± 4.23 |
| Casein | -0.51 ± 1.49 | -9.99 ± 1.49e | -9.99 ± 1.49f |
| Non Ovariektomy | -10.77 ± 2.16g | -10.37 ± 2.16h | -17.15 ± 2.16i |
Note: Alphabet followed P Values: a = 0.002; b = 0.01; c = 0.027; d = 0.039; e = 0.001; f = 0.0001; g = 0.0001; h = 0.001; i = 0.001.
4. Discussion
Tempe flour may be used as an alternative to hormone replacement because it has a high content of isoflavones which have beneficial health effects [3]. This research also shows that the intervention group with tempe flour induces the highest estrogen in serum compared to tofu flour, estradiol, and casein treatments.
The results showed that tempe flour has an isoflavone content (genistein) of 50.56 mg/100g, which is two times higher than tofu flour at 19,923mg/100g [3]. This result is similar to the results of Rahardjo et al. (2010) [20] which showed that the content of isoflavones (genistein) was 55,409 mg/100 g in tempe flour and 26.68 mg/100 g in tofu flour. The results of Aryani's research (2009) [21] showed that isoflavone (genistein) content of tempe was 38.9 mg/100 g and tofu was 20.8 mg/100 g. In Utari's research (2011) [15], the genistein content of tempe was 30.8 mg. Other studies showed that isoflavones (genistein) in tempe flour were 25,065 mg/100 g bk [17]. Hence, wide variation is found when investigating isoflavone content in tempe, up to a factor 2 difference which may be associated with methods of assessment and/or different soybean strains and methods of fermentation.
The isoflavone content of tofu is lower than that of tempe because in the process of making tofu, some isoflavones are bound in water. This is evidenced in vinegar acid water, which is water from the separation of tofu which shows clumps containing isoflavone compounds. Isoflavones in soybean milk extract in making tofu are mostly bound (glycons) and more soluble in water [22,23]. The dominant isoflavone tempe is aglycone with absorption rates of 20%–55% [12].
The results of the study on the average estrogen serum level in ovariectomized rats decreased when compared to the initial conditions (baseline 1), although not significant. Ovariectomy performed in this study used a postmenopausal rats model. Ovariectomy causes ovarian loss and estrogen levels to be low so that proliferation and cornification of vaginal epithelial cells are disrupted and cause no estrus phase in the mice. Estrogen serum levels at baseline (baseline 2), after a period of adaptation, were higher, although not significantly different from the group that had ovariectomy surgery and a three-month grace period. But the measurement of serum estrogen in the non ovariectomy group was significantly higher compared to the initial group and the ovariectomy group.
If the estrogen content in the body is sufficient, adding phytoestrogens derived from tempe and tofu can be antiestrogens. The main bioactive isoflavones are genistein and daidzein, which are reduced to precursors of biochanin A and formonetin sequentially [24]. Genistein can actively excite estrogen receptors and can inhibit estrogen or antagonists depending on tissue, type of receptor and availability of endogenous estrogen components [25].
The physiological effects of isoflavones such as estrogen depend on the response that occurs, and can be agonist (stimulate) or antagonistic (inhibit) to the receptor in the target cell. The body has two receptors namely beta estrogen receptor and alpha estrogen receptor. These two receptors have a distribution depending on the tissue, and different binding affinity with different ligands. Beta estrogen receptors are distributed in brain, bone, bladder and vascular epithelial tissues. Alpha receptors are distributed in uterine, breast, liver and kidney tissues [26].
The results of this study indicate that the most effective isoflavones found in tempe flour and estradiol will provide adequate estrogen balance until the fifth week. In tofu this is only the case until the second week of treatment. The eighth week of serum estrogen has begun to fall and will continue to decline even though given intervention. This is probably the case because in the fifth week estrogen reserves in the body have begun to run out and the addition of isoflavones in tempe does not provide an adequate effect to increase estrogen levels in the body.
The rats were given 4.4 grams of tempe flour for this research (containing 22.25 mg of isoflavones) and 5.1 grams of tofu flour (containing 10.16 isoflavones). Provision of tempe flour and tofu flour according to Whitten research states that the dose of administration of phytoestrogens that will have biological effects is (10–100 mg/kg body weight/day) [27]. Tempe flour and tofu flour contain genistein, an amino acid isoflavone which functions as the most potent phytoestrogen. The genistein content in tempe flour is 505.58 mg/100g bk, more than tofu flour 199.23 mg/100g bk, so tempe flour has double the content compared to tofu flour. If all receptors are blocked by genistein, then endogenous estrogen has no chance of attaching to the receptor [28]. The similarity in the structure of genistein isoflavones with endogenous estrogen shows their ability to bind to estrogen receptors [29].
Giving tempe flour and tofu flour to female rats of different ages showed different levels of estrogen in serum. Female rats ovariectomized at 3 months of age showed a higher serum estrogen increase with tempe flour compared to female rats aged 6 months [21]. This study was carried out in 12-month-old rats that were ovariectomized which might have different results if carried out on rats aged (3 years) equivalent to the age of 60 years in human. Clinical trials in women need to be conducted in older women to find optimum conditions for consuming tempe and tofu on estrogen levels and health benefits.
5. Conclusion
Serum estrogen analysis showed that tempe flour had the highest increase in serum estrogen compared to other intervention groups (tofu flour, estradiol, casein and non ovariectomized). The increase in serum estrogen in the tempe flour group was significantly different from the intervention group receiving tofu flour. Tempe flour group had twice the serum estrogen level compared to the tofu flour group. The tempe flour group showed a significant increase compared to baseline 2, but the increase in serum estrogen in the flour tofu group was not significant. Tempe flour and estradiol will provide the optimal balance of estrogen until the fifth week. Whereas in tofu flour until the second week.
Declarations
Author contribution statement
Atik Kridawati: Performed the experiments; Wrote the paper.
Tri Budi W. Rahardjo, Rizal Damanik: Conceived and designed the experiments.
Hardinsyah Hardinsyah: Analyzed and interpreted the data.
Eef Hogervorst: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Ministry of Education, Republic of Indonesia by the Directorate General of Higher Education in 2013 and Respati Foundation 2012–2013.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors gratefully acknowledge the technical and scientific influence of Professor Hardinsyah, Professor Ahmad Sulaeman, Dr. Adi Winarto, Professor Ekowati, Professor Barry Sharp,Dr. Helen Griffiths, and Dr. Brenden Theaker, Dr. Rimbawan. Professor Made Astawan, Dr. Nastiti Kusumorini, Dr. Saptawati Bardosono, Professor Widodo Suparno, Tiwi Nurhastuti, Tinon Ambarini, Toni Sugiarso.
References
- 1.Vitale D.C., Piazza C., Melilli B., Drago F., Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013;38(1):15–25. doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- 2.Tang Yan, Li Shuangyue, Zhang Ping, Zhu Jinbiao, Meng Guoliang, Xie Liping, Yu Ying, Ji Yong, Han Yi. Soy isoflavone protects myocardial ischemia/reperfusion injury through increasing endothelial nitric oxide synthase and decreasing oxidative stress in ovariectomized rats. Oxidative Med. Cell. Longev. 2016;2016:1–14. doi: 10.1155/2016/5057405. Article ID 5057405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kridawati Atik, Hardinsyah, Sulaeman A., Damanik Rizal, Winarto A., Rahardjo T.B., Hogervorst E. Tempe reversed effects of ovariectomy on brain function in rats: effects of age and type of soy product. J. Steroid Biochem. Mol. Biol. 2016;160:37–42. doi: 10.1016/j.jsbmb.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Soni Mira, Rahardjo Tri Budi W., Soekardi Rodiyah, Sulistyowati Yenny, Lestariningsih, Yesufu-Udechuku Amina, Irsan Atik, Hogervorst Eef. Phytoestrogens and cognitive function: a review. J. Maturitas. 2014;77:209–220. doi: 10.1016/j.maturitas.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 5.A.D.A.M. Medical Encyssclopedia [Internet] A.D.A.M., Inc.; Atlanta: 2011. http://www.nml.nih.gov/medlineplus/encylopedia.html [cited 2013 Feb 7]. Available from: [Google Scholar]
- 6.Ruggiero R.J., Pharm D., Frances E.L. Estrogen: physiology, Pharmacology, and formulations of replacement therapy. J. Midwifery Womens Health. 2002;47(3):130–138. doi: 10.1016/S1526-9523(02)00233-7. [DOI] [PubMed] [Google Scholar]
- 7.Guyton A. seventh ed. EGC; Jakarta: 1994. Medical Physiology. [Google Scholar]
- 8.Leon Speroff, Fritz Marc A. seventh ed. Lippincot Williams and wilkins; 2005. Clinical Gynecologic Endocrinology and Infertility. [Google Scholar]
- 9.Patisaul Heather B., Jefferson Wendy. The pros and cons of phytoestrogens. NIH Public Access. Front. Neuroendocrinol. 2010 October;31(4):400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wren B.G. The benefits of oestrogen following menopause: why hormone replacement therapy should be offered to postmenopausal women. Med. J. Aust. 2009;190(6):321–325. doi: 10.5694/j.1326-5377.2009.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 11.Hooper L., Ryder J.J., Kurzer M.S., Lampe J.W., Messina M.J., Phipps W.R., Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum. Reprod. Update. 2009;15(4):423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H.-J., Murphy P.A. Isoflavone composition of American and Japanese soybeans in Iowa: effects of variety, Crop year, and location. J. Agric. Food Chem. 1994;42:1674–1677. [Google Scholar]
- 13.Biben A. Padjadjaran University; Bandung (ID): 2001. Effect of Dietary Supplementation on tempe Formula on Pharmaceuticals and Bone Resorption in Pre and post Menopausal Women. [Dissertation] [Google Scholar]
- 14.Villares A., Rostagno M.A., Lafuente A.G., Guillamon E., Martinez J.A. Content and profile of isoflavones in soy- based foods as afunction of the production process. Food Bioprocess Technol. 2011;4(1):27–38. [serial on the Internet]. January [cited 2013 Feb 26]; [Google Scholar]
- 15.Utari D.M. IPB; 2011. Effects of tempe Intervention on Lipid Profiles, Superoxide Dismutase, Oxidized LDL and Malondialdehyde in Menopausal Women. Desertation. [Google Scholar]
- 16.Bavia A.C.F., Silva C.E., Ferreira P., Feriarra M.P., Leite R.S., Mandarino J.M.G., Carrao-Panizzi M.C. Chemical composition of tempeh from soybean cultivars specially developed for human consumption. Ciênc. Tecnol. Aliment., Campinas. 2012;32(3):613–620. [Google Scholar]
- 17.Safrida . Bogor Agricultural University; Bogor: 2008. Changes in Estrogen Hormone Levels in Rats Given Soybean Flour and Tempe Flour. (Thesis) [Google Scholar]
- 18.Safrida . Bogor Agricultural University; Bogor: 2013. Potential of Tempe Extract as Anti Aging in Female Rats as Model Animals. [Desertation] [Google Scholar]
- 19.Zaheer K., Humayoun Akhtar M. An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017;57(6):1280–1293. doi: 10.1080/10408398.2014.989958. [DOI] [PubMed] [Google Scholar]
- 20.Rahardjo T.B., Atik K., Hardinsyah Hogervorst, Yudarini Fatmah. Laporan Penelitian UI; 2010. The effect of tempeh and tofu flours on delaying cognitive function of elderly rats. [Google Scholar]
- 21.Aryani M.F. Universitas Indonesia; 2009. Relationship between Tempe and Tofu Consumption with Elderly Cognitive Function. [Desertation] [Google Scholar]
- 22.Petterson H., Kiessling K.H. Liquid chromatographic determination of the plant estrogens coumesterol and isoflavones in animal feed. J. Assoc. Off. Anal. Chem. 1984;67:503–506. [PubMed] [Google Scholar]
- 23.Taher A. Bogor Agricultural University; 2003. The Role of Soybean Phytoestrogens as Antioxidants in Overcoming Atherosclerosis. [Thesis] [Google Scholar]
- 24.Bhanthena, Velasquez Manual T. Beneficial role of dietary phytoestrogen in obesity and diabetes. Am. J. Clin. Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 25.Barnes S., Peterson T.G. Biochemical targets of the isoflavone genistein in tumor cell illess. Proc. Soc. Exp. Biol. Med. 1995;208:103–108. doi: 10.3181/00379727-208-43840. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper G.G., Shughrue P.J., Merchenthaler I., gustafsson J.A. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendrocrine systems. Front. Neuroendocrinol. 1998;19:253–286. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- 27.Whitten P.L., Patisaul H.B. Cross-species and interassay comparisons of phytoestrogenaction. environ. Health Perspect. 2001;109(l1):5–20. doi: 10.1289/ehp.01109s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahyadi W. Bumi Aksara; Jakarta: 2007. Soybean Benefits and Technology. [Google Scholar]
- 29.Mahmoud A.M., Al-alem U., Ali M.M., Bosland M.C. Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation. J. Steroid Biochem. Mol. Biol. 2015 August;152:62–75. doi: 10.1016/j.jsbmb.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]