Abstract
The present work analyses the chromatographic profile of the peels from fruits of different citrus cultivated in Colombia: sweet orange (Citrus sinensis [L.] Osbeck var. Valencia), mandarins (Citrus reticulata L. var. Arrayana and Oneco), Key lime (Citrus aurantifolia [Christ.] Swingle var. Pajarito), Mandarine lime (Citrus x limonia, a hybrid between Citrus reticulata and Citrus x limon) and Tahitian lime (C. latifolia Tanaka, syn. Persian lime). Coumarins, furanocoumarins and polymethoxylated flavones are the major compounds. Then, six coumarins were isolated and identified from fruits of Tahitian and Key lime corresponding to 5-geranyloxy-7-methoxycoumarin; 5,7-dimethoxycoumarin (syn. limettin); 5,8-dimethoxypsoralen (syn. isopimpinellin); 5-methoxypsoralen (syn. bergaptene); 5-geranoxypsoralen (syn. bergamottin) and 5-(2,3-dihydroxy-3-methylbutoxy) psoralen (syn. oxypeucedanin hydrate). Coumarins and furanocoumarins were quantified by liquid chromatography (HPLC-DAD). Results show that the prenylated compounds were present in high concentrations in Tahitian and Key lime but in very low amounts in mandarins and sweet orange. Subsequently, the antifungal activity (inhibition of mycelial growth and germination of spores) of the coumarins against the fungus causing the anthracnose, Colletotrichum sp. (isolated from aerial parts of Tahitian lime) was determined. The compounds limettin and bergaptene, as well as mixtures of them, showed significant inhibitory effect (radial growth and spore germination) when compared to the control. Finally, the effect of some recognized elicitors to induce the coumarin production in fruits of C. latifolia was evaluated. The results showed that the chemical profiles are dependent on the applied elicitor and the post-induction time. As a result of the induction, a high concentration of some coumarins and furanocoumarins was maintained in the course of time for the Tahitian lime. In conclusion, isolated coumarins could be involved in the defense mechanisms of C. latifolia, C. aurantifolia and C. limonia and their accumulation may be modulated by the application of elicitors.
Keywords: Food science, Citrus fruits, Coumarin, Plant elicitor molecule, Antifungal activity, Rutaceae, Tahitian lime, Limettin, Elicitors, Furanocoumarins, Spore germination inhibition
1. Introduction
The genus Citrus sp. belongs to the family Rutaceae and includes some of the main fruit crops in the world. Global citrus production was estimated at 130 million tons in 2015, becoming one of the most commercialized horticultural products (FAO, 2017). Citrus can be classified into the following four categories: sweet oranges, mandarins (including clementine and tangerine), grapefruit (including pummelo), and lemons/limes (Dugrand et al., 2013). The different cultivated species of citrus are a valuable source of nutrients and bioactive compounds, such as vitamins B and C, carotenoids, flavonoids and their glycosides, essential oils, coumarins, phenylpropanoids, limonoids, minerals, fiber and high water contents that have effects positive for human health (Duan et al., 2017; Zhang et al., 2017; Hung et al., 2017; De Moraes Barros et al., 2012). Additionally, nutritional, medicinal, aromatic and other therapeutic properties of citrus fruits, such as anticancer, cardioprotective, free radical scavenging, and bactericidal and antiviral activities, are well known (Hung et al., 2017; Tundis et al., 2014; Benavente-García and Castillo, 2008). However, the production of oranges, mandarins, grapefruit, and lemons/limes is largely limited by pathogenic microorganisms, which easily proliferate in citrus fruits because of the high nutrient content, humidity, and low pH values during the period between harvest and consumption.
On the other hand, plants have developed a variety of low molecular weight antimicrobial compounds to protect themselves from damage caused by pathogens. These antimicrobial secondary metabolites can be grouped into two categories: constitutive (phytoanticipins) and induced (phytoalexins) compounds (Piasecka et al., 2015; VanEtten et al., 1994). Phytoanticipins are present in plants prior to the attack of a microorganism or can be also produced from pre-existing constituents after infection. These compounds act as the first chemical protection against a pathogen. Meanwhile, the phytoalexins are synthesized and accumulated in plants as a response to diseases or stress. Some chemical compounds of very diverse chemical nature, called elicitors, also induce the synthesis of phytoalexins. The rapid accumulation of phytoalexins has been extensively related to the disease resistance of plants (Ahuja et al., 2012; Dixon, 2001).
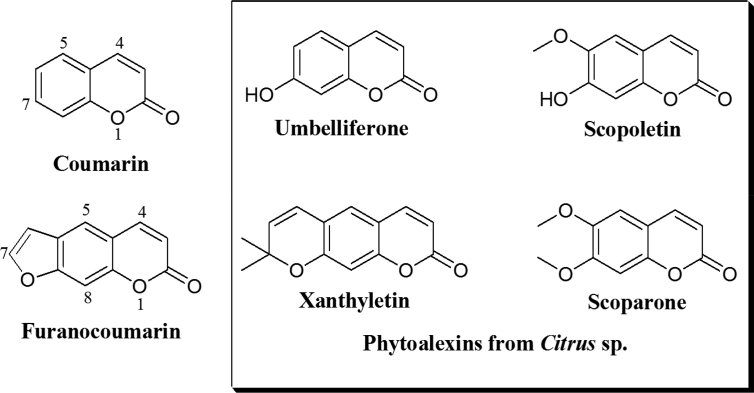
In citrus, coumarins and furanocoumarins (a subclass of coumarins with an additional furan ring) have been associated with the defense against pathogens (Fig. 1) (Bourgaud et al., 2006). Several authors have reported that scoparone (5,6-dimethoxycoumarin) is the main phytoalexin of citrus in response to the plant pathogenic fungi Phytophthora citrophthora (Smith and Smith) Leonian (Afek and Sztejnberg, 1988), Guignardia citricarpa Kiely (De Lange et al., 1976), Penicillium digitatum Sacc. (Rodov et al., 1992), P. italicum (Arras et al., 2006), Diaporthe citri (Wolf) (Aritmo et al., 1986), and Botrytis cinerea (Persoon) (Kuniga et al., 2015). The production of scoparone can also be induced by UV radiation (Kuniga et al., 2015; Kuniga and Nesumi, 2011) and heat treatment (Kim et al., 1991). The coumarins scopoletin (6-methoxy-7-hydroxycoumarin) and xanthyletin (6,7-dimethylpyranocoumarin) were also found in citrus tissues induced by pathogens (like Phytophthora spp) or UV light (Rodov et al., 1994; Khan et al., 1985). In addition, umbelliferone (7-hydroxycoumarin), a biosinthetic precursor of methoxylated coumarins and furanocoumarins, was found in Marsh grapefruit (Citrus paradisi) when challenged by Penicillium digitatum (Afek et al., 1999). Scoparone, scopoletin, xanthyletin and umbelliferone have shown antifungal activity in vitro (Sanzani et al., 2014; Afek et al., 1999; Ortuño et al., 2011; Khan et al., 1985). In the present work, the isolation, identification, quantification and antifungal activity of coumarins from peels of citrus cultivated in Colombia is described.
Fig. 1.
Structure of coumarin, furanocoumarin and known citrus phytoalexins.
2. Materials and methods
2.1. General
Umbelliferone, scopoletin, methyl jasmonate, D-(+)-trehalose dihydrate, salicylic acid, 5-sulfosalicylic acid dihydrate, pectin from citrus peel (galacturonic acid, ≥74%, dried basis), chitosan (from shrimp shells, ≥ 75%, deacetylated), Span® 80 and Tween® 80 were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Scoparone was isolated from Platymiscium gracile L as described elsewhere (Martinez et al., 2017). Xanthyletin was isolated from Brosimum rubescens Taub. The spectroscopic data were in agreement with those of Braz-Filho et al. (1972). β-D-glucans from fruiting bodies of Ganoderma lucidum were acquired from Progal BT S.A.S. Galactomannan polysaccharide (guar gum from Cyamopsis tetragonoloba [L.] Taub.), and polysaccharide from Xanthomonas campestris (xanthan gum) were purchased from Tecnas S.A. (Itaguí, Colombia). Mixture of glycoproteins and polysaccharides (arabic gum from Acacia sp.) was from Protokimica S.A.S. (Medellín, Colombia). Gentamicin sulfate MK® and lincomycin MK® were from Tecnoquimicas S.A. (Cali, Colombia). Enoxaparin sodium Clexane® was from Sanofi-Aventis S.A. (Bogotá, Colombia). Hemicelluloses from corn cobs were obtained by alkaline extraction at room temperature following the methodology described in literature (Da Silva et al., 2015). Mucilage from chia seeds (Salvia hispanica) was obtained at pH 6 and room temperature in accordance to Muñoz et al. (2012). All solvents were analytical or commercial grade (previously purified by bidestilation and drying with anhydrous sodium sulfate).
For the purification of the compounds different chromatographic techniques were used (column chromatography, CC; and thin layer chromatography, TLC). For CC, silica gel 60 (0.040–0.063 mm, Merck, Darmstadt, GE) or Sephadex LH-20 (Sigma-Aldrich, St. Louis, MO, USA) were used. The monitoring of the compounds was carried out by means of TLC on aluminum plates with stationary phase of silica gel (Si 60 F254, 20 × 20 cm, 0.25 mm, Merck, Darmstadt, GE). The identification of the compounds was performed by modern spectroscopic methods such as ultraviolet (UV/Vis), infrared (IR) and nuclear magnetic resonance (NMR) mono- (1H and 13C NMR) and two-dimensional spectroscopy. The infrared spectra were taken on a FT-IR Spectrum Two Perkin Elmer equipment (Attenuated Total Reflectance, ATR), while the NMR spectra were determined using CDCl3 on a Bruker AMX 300 spectrometer (300 MHz for 1H and 75 MHz for 13C). The chemical shifts (δ) were expressed in ppm and the coupling constants (J) in Hertz (Hz). Mass spectrometry analysis was carried out using a GCMS-QP2010 Ultra (Shimadzu, Kyoto, Japan) operated in the electron impact mode (ionization energy: 70 eV; scan time: 0.5 sec; mass range of 200–400 amu). The accumulation of compounds in the course of time was evaluated by High Performance Liquid Chromatography (HPLC-DAD) in a Shimadzu Prominense chromatoghaph equipped with Diode Array Detector (DAD, SPD-M20A), software (LabSolutions Lite version 1.22 SP1) and a column RP-C18 (Luna, 150 mm x 4.6 mm- 5 μm, Phenomenex). The antifungal activity assays were carried out aseptically within a laminar flow cabin (or biological safety cabinet CSB 180 A) class II type A; using sterilized materials in an automatic horizontal autoclave (Centricol AUA 80 L brand). Spore count was determined microscopically using a Carl Zeiss Primo Star microscope with Neubauer chamber (Deep 1/10 mm, Boeco).
2.2. Plant material
This study was performed using citrus extracts taken from samples of peels of sweet orange (var. Valencia), mandarins (var. Arrayana and Oneco), Key lime (var. Pajarito), Mandarine lime and Tahitian lime. Key, Mandarine and Tahitian limes were harvested on the extremities of branches between 6:00 and 7:00 a.m. from the Agronomic Research Station Cotové (municipality of Santa Fé de Antioquia, Antioquia, Colombia). On the other hand, sweet orange, mandarins, and some Tahitian limes were purchased from local markets. Some Tahitian limes were harvested between 12:00 and 2:00 p.m. The ripeness degree and post-harvest conditions were in accordance with Colombian Technical Norms (ICONTEC: NTC 4086 and 4087) for commercial fruits. Ripeness degree of citrus fruits was between categories two and three of the table of colors (NTC 4086 and 4087).
2.3. Microorganism
The phytopathogenic fungus Colletotrichum sp. was isolated from Tahitian lime fruits with evident symptoms of the disease (anthracnose) and characterized morphologically. The fungus was preserved in Papa Dextrose Agar medium (PDA, Merck-KGaA, Darmstadt, Germany) at 24 ± 2 °C and subcultured monthly in Petri dishes (9.0 cm in diameter and 15.0 mL of medium) until the realization of the bioassays.
2.4. Extract preparation
Citrus fruits: sweet orange (var. Valencia), mandarins (var. Arrayana and Oneco), Key lime (var. Pajarito), and Mandarine and Tahitian limes, were washed with running water and dipped in a 1% sodium hypochlorite solution for 10 minutes and thereafter, rinsed with distilled water. Then, the peels (flavedo and albedo) of the fruits were removed manually. The thickness of the albedo was different, varying between two (mandarin) and five millimeters (sweet orange). Citrus peels (5 g) were cut into small pieces, weighed, and extraction was performed with HPLC-grade methanol (3 × 15 mL) in the dark in an ultrasonic bath (Ultrasonik 28H) for 30 minutes. The methanol soluble extracts were filtered through Whatman No. 1 filter paper, combined and brought to a final volume of 50 mL with methanol. The samples were kept in amber glass flasks and stored at -20 °C until HPLC-DAD analysis.
2.5. Isolation and identification of compounds
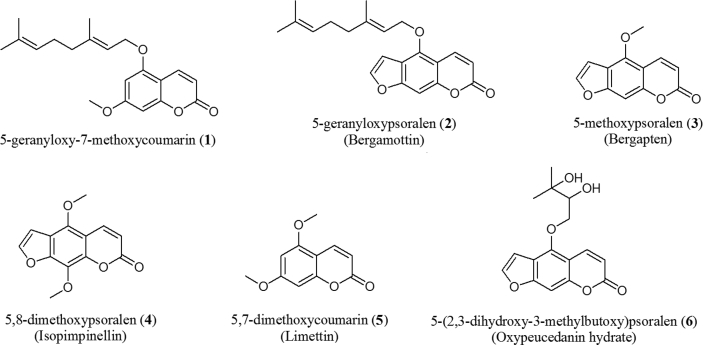
Tahitian lime peels (2500 g) were cut into small pieces and subjected to exhaustive extraction by percolation with methanol (2 L) for 5 days at room temperature. The obtained methanolic extract was concentrated by rotoevaporation under reduced pressure and temperature below 40 °C. The extract was subjected to fractionation in a column of silica gel using mixtures of petroleum ether-dichloromethane and dichloromethane-ethyl acetate, of increasing polarity as the mobile phase. Similar fractions were combined by TLC monitoring and further separated by CC with Sephadex LH-20, using the mixture of petroleum ether-dichloromethane-methanol (2:1:1). In total, six compounds were obtained, which were identified by spectroscopic methods. The spectroscopic data were in agreement with those reported in literature: compounds were identified as 5-geranyloxy-7-methoxycoumarin (1) (Patil et al., 2013), bergamottin (2) (Liu et al., 2017), bergaptene (3) (Famobuwa et al., 2019), isopimpinellin (4) (Wang et al., 2008), limettin (5) (Jerezano et al., 2011) and oxypeucedanin hydrate (6) (Sbai et al., 2016).
2.6. Detection and quantification
The analysis of coumarins and furanocoumarins was performed by HPLC. The compounds were eluted at a flow rate of 0.7 mL/min with a gradient acetonitrile (HPLC grade) and deionized water, as follows: from 5% acetonitrile to 34% in 10 min, then from 34 to 70% in 26 min, 70–87% in 17 min, 87–90% in 5 min, and subsequently by holding for 7 min. Injection volume was 10 μL. The temperature of the separation column was 33 °C. Coumarins and furanocoumarins were monitored at the wavelengths of 254 and 272 nm, respectively, although DAD was used from 200 to 800 nm for peak characterization. Identification was carried out by comparing the retention times (Rt) or by co-elution with the authentic samples. The purity of the standards was calculated by dividing the area of the respective compound between the total areas of the peaks in the chromatogram and multiplying by 100.
Quantification of coumarins and furanocoumarins was performed using standard calibration curves (peak areas vs. compound concentrations). Working solutions of 1, 2, 3, and 5 were prepared, dissolving an amount (4 mg) of each compound in acetonitrile (10 mL). Each solution was subsequently diluted with the mobile phase at five different concentrations between 0.5 and 10 mg/L. The concentration of the compounds in the extracts was calculated by interpolation from the area of the peaks in the chromatograms and the calibration curves. All data were expressed as the mean ± SD. The analysis of each concentration was carried out in triplicate, injecting 10 μL of the solution. The limits of detection (LOD) and quantification (LOQ) for each curve were determined according to ICH Harmonised Tripartite Guideline (2005).
2.7. Elicitation
For the elicitation assays, only Tahitian lime fruits were used. Aqueous solutions of the potential inducing agents (concentration used in units mg/L): guar, xanthan, and arabic gums (1000), pectin from citrus peel (1000), chitosan (700), β-D-glucans from G. lucidum (400 and 50), hemicelluloses from corn cobs (400 and 50), gentamicin (400), enoxaparin (100), lincomycin (1200), mucilage from chia seeds (Salvia hispanica) (1000), methyl jasmonate (1000), D-(+)-trehalose dehydrate (1000), salicylic acid (1000), 5-sulfosalicylic acid dehydrate (1000), Span® 80 (1000) and Tween® 80 (1000) were prepared. Small amounts (<10%) of ethanol or acetic acid were used to improve the solubility of the compounds (salicylic acid and chitosan) in water. Ultraviolet (UV) radiation from a UVP UVGL-58 (6 watt) lamp at 254 nm was used as physical induction agent. Prior to induction, Tahitian lime fruits were washed and disinfected as previously described. Then, fruits were immersed for 1 hour in each solution or placed 20 cm under the UV lamp for 30 min. Tahitian lime fruits submerged into sterile distilled water were used as controls. Then, citrus fruits were placed in a sterile polystyrene box and stored at room temperature in the darkness for 6 days.
In addition, time-course studies were carried out with the following solutions: T1 and T2 hemicelluloses from corn cobs at 50 and 400 mg/L, respectively. T3 and T4, β-D-glucans from G. lucidum at 50 and 400 mg/L, respectively, and T5 gentamicin at 400 mg/L. Finally, T6 ultraviolet radiation at 254 nm for 30 min. All treatments, with the exception of UV radiation, were applied by immersion for 1 hour. After that time, the fruits were removed from the solution, air dried and stored separately in plastic boxes that were protected from light at room temperature for 2, 4, 6, 8, 10, 13 and 16 days. The tests were performed in quadruplicate and each experimental unit consisted of four fruits.
2.8. Antifungal activity
2.8.1. Mycelial growth inhibition
Measurements of the antifungal activity against Colletotrichum sp. of the isolated compounds, mixtures of them and compounds reported in the literature as citrus phytoalexins (scoparone, scopoletin and umbelliferone), were developed using the poisoned food technique (Grover and Moore, 1962) with some modifications (Velasco et al., 2010). Different concentrations of the compounds (0.25, 0.50 and 1.00 mM) were incorporated into the oak agar medium (Oak Agar; 2% oats, 1.8% Agar-Agar, Scharlau) and dissolved at 1% in dimethyl sulfoxide (DMSO). All concentrations were evaluated in triplicate. The results are shown as mean mycelial growth values corresponding to the diameters of the colony. Inhibition percentage of the radial growth was calculated using the formula: Inhibition (%) = [1- (T/C)] x 100; where, C = average colony diameter (mm) of the absolute control and T = average colony diameter (mm) of the treatment. Petri dishes without the compounds were used as the negative control, containing only culture medium (absolute control) and addition of 1% DMSO (solvent control). The common fungicide Carbendazim (methyl benzimidazol-2-yl carbamate) and the antifungal natural agent thymol were used as positive controls at 0.5 mM. Antifungal activity of mixtures in different proportion (0.25–0.75, 0.50–0.50, and 0.75–0.25 mM) of the two most active compounds was also evaluated. All data were expressed as the mean ± SD.
2.8.2. Spore germination inhibition
Compounds that showed the highest mycelial growth inhibitions were evaluated for antifungal activity through the inhibition of spore germination, using the technique described by Cronin et al. (1996) with minor modifications. Spores of Colletotrichum sp. were collected from a Petri dish culture that was 7 days old. 20 mL of sterile water was added to the mycelium of the Petri dish, and a repetitive sweep with sterile cotton swab was performed to solubilize the largest number of spores. The suspension of mycelium and spores was filtered through sterile cotton and diluted with water (Type 1) until reaching a concentration of 3 × 105 spores/mL (hemocytometer). 15 μL of the desired concentration (1 mM) of compounds in DMSO (1%) was mixed with sterile liquid PDA (9.0 g/100 mL water, 0.5 mL) and placed on an Eppendorf microcentrifuge tube (2 mL capacity). After 2 minutes, 1 mL of a suspension of freshly prepared spores of Colletotrichum sp. was placed in the Eppendorf tube, capped, and maintained at 25 °C. The negative control was an Eppendorf tube containing DMSO (1%) mixed with sterile liquid PDA. After 8, 24, and 48 hours, the spore germination was examined under an optical microscope (40X magnification). 200 spores of four microscopic fields in three replicated plates were counted for the determination of the number of germinated spores. It was considered that a spore germinated when the length of the germ tube was greater than twice the radius of the spore. Results were expressed in terms of the percentage of spores germinated as compared to the control from the average of the triplicates. Percentage of spore germination inhibition was calculated according to the following formula: Spore germination inhibition (%) = [1- (T/C)] x 100; where, C = average number of spore germinated in control set and T = average number of spore germinated in treatment set. All data were expressed as the mean ± SD.
2.9. Statistical analysis
Results were analyzed by a one-way ANOVA, and mean values were compared with the Fisher's least significant differences (LSD) at the 0.05 probability level.
3. Results and discussion
3.1. Chromatographic profiles
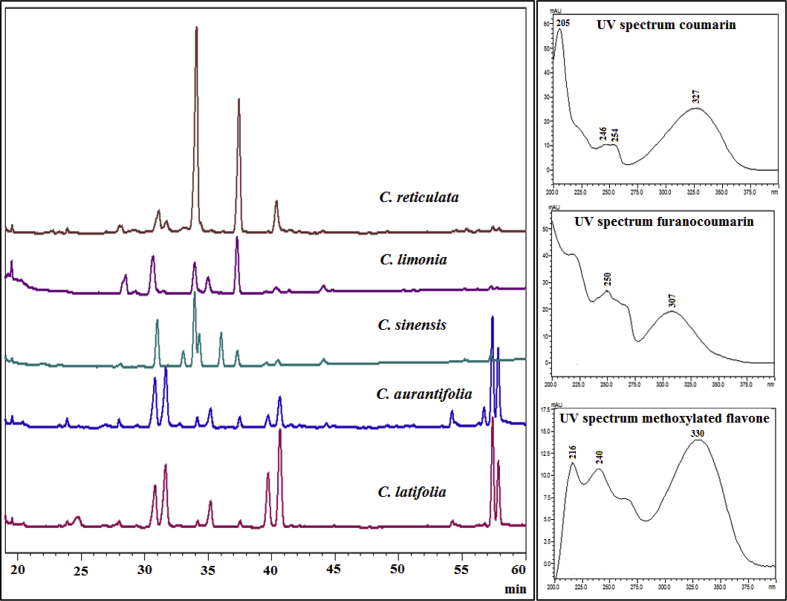
The compounds in methanolic extracts from citrus peels were separated by HPLC and UV/Vis spectra were obtained using a diode-array detector. The chromatographic profiles are presented in Fig. 2. The HPLC profile of mandarin var. Arrayana was omitted because it was identical to that of mandarin var. Oneco. UV/Vis spectra for the peaks gave a preliminary indication of the family of phenolic compounds. Tentative identifications of major peaks in these five citrus extracts can be found in Table 1. Coumarins (tentatively 5,7-dioxygenated form showing UV maxima around 205–240 and 315–330 nm and peaks or shoulders around 240 and 270 nm) were detected at Rt 28.0, 30.7, 31.5, 37.5, and 44.3 min. A 6,7-dioxygenated coumarin, displaying a UV spectrum with two major bands at 268 and 345 nm, was observed at Rt 24.5 min. These data are in agreement with Ibrahim and Barron (1990) for 5,7- and 6,7-dioxygenated coumarins. Typically, linear furanocoumarins show four absorption bands at 205–235, 240–255, 260–270, and 290–316 nm (Ibrahim and Barron, 1990). So, peaks at Rt 23.9, 31.6, 35.0, 54.0, 55.7, and 57.2 min were tentatively assigned to linear furanocoumarins. In addition, UV spectra of citrus polymethoxylated flavones generally present two strong bands at 240–280 (Band II) and 300–380 nm (Band I) (Mabry et al., 1970). Chromatographic peaks at 33.0, 33.9, 34.3 and 36.0 min present these characteristic bands. Therefore, under the conditions used, the main constituents found in citrus peels were coumarins, furanocoumarins and polymethoxylated flavones. As can be seen in Fig. 2, the chemical profile of methanolic extracts from peels varies among citrus species. Composition of sweet orange, mandarin, and Mandarine lime showed marked differences with respect to Key and Tahitian limes.
Fig. 2.
HPLC-DAD chromatograms of methanolic extracts from citrus fruit peels. Tahitian lime (C. latifolia); Key lime (C. aurantifolia var. Pajarito); Sweet orange (C. sinensis var. Valencia); Mandarine lime (Citrus x limonia) and mandarin (C. reticulata var. Oneco), monitored at 254 nm.
Table 1.
Major components in methanolic extracts from citrus peels.
| Retention time (min) | λmax (nm) | Citrus especie | Tentative type of compound§ |
|---|---|---|---|
| 23.9 | 214, 249, 266, 308 | Cla, Ca | Furanocoumarin |
| 24.5 | 268, 348 | Cla | Coumarin |
| 28.0 | 229, 286, 326 | Cr, Ca, Cla | Coumarin |
| 28.4 | 270, 342 | Cs | Coumarin |
| 28.5 | 231, 268, 325 | Cli | Coumarin |
| 30.7 | 205, 246, 326 | Cla, Ca, Cli, Cr | Coumarin† |
| 31.0 | 216, 240, 264, 331 | Cs | Methoxylated flavone |
| ∗31.5 | 222, 249, 267, 311 | Cla, Ca, Cli, Cr | Furanocoumarin† |
| ∗31.6 | 222, 248, 268, 312 | Cla, Ca, Cli, Cr | Furanocoumarin† |
| 32.8 | 209, 245, 302 | Cla, Ca | Furanocoumarin |
| 33.0 | 244, 337 | Cs | Methoxylated flavone |
| 33.9 | 215, 248, 269, 333 | Cr, Cli, Cs, Ca, Cla | Methoxylated flavone |
| 34.3 | 226, 269, 332 | Cs | Methoxylated flavone |
| 35.0 | 216, 249, 268, 307 | Cla, Ca, Cli | Furanocoumarin |
| 36.0 | 253, 338 | Cs | Methoxylated flavone |
| 37.3 | 233, 270, 323 | Cs | Coumarin |
| 37.5 | 232, 270, 324 | Cr, Cli, Cs, Ca, Cla | Coumarin |
| 39.8 | 237, 310 | Cli, Cs, Ca, Cla | Unidentified |
| 40.7 | 238, 312 | Cr, Cli, Cs, Ca, Cla | Unidentified |
| 44.1 | 240, 287, 321 | Cs, Ca | Coumarin |
| 45.3 | 219, 268, 305 | Cli | Furanocoumarin |
| 54.4 | 214, 248, 265, 297 | Ca, Cla | Furanocoumarin |
| 55.7 | 215, 248, 267, 308 | Ca, Cla | Furanocoumarin |
| 57.2 | 219, 250, 267, 307 | Ca, Cla | Furanocoumarin† |
| 57.7 | 206, 246, 325 | Ca, Cla | Coumarin† |
Cla: C. latifolia; Ca: C. aurantifolia; Cs: C. sinensis; Cli: C. limonia; Cr: C. reticulata.
Compounds were characterized by UV spectra only, and therefore, the identification can only be considered tentative.
Both compounds were co-eluted and analyzed as a two-component mixture without further purification.
Compounds were isolated and identified by NMR, UV, MS and IR and corresponding to 5-geranyloxy-7-methoxycoumarin (Rt = 57.7), bergamottin (Rt = 57.2), bergapten (Rt = 31.5), isopimpinellin (Rt = 31.6), limettin (Rt = 30.7).
Sweet orange and mandarin displayed the peak of highest intensity at Rt 33.9 min, whose UV spectrum was consistent with a methoxylated flavone. This peak exhibited a low intensity for Key and Tahitian lime. In addition, peaks at Rt 31.0, 33.0, 34.3, and 36.0 min, corresponding to methoxylated flavones (according to their UV spectra), were only found in sweet orange. It was also clear that Key and Tahitian lime showed several intense peaks between 44.0 and 57.7 min (less polar compounds), which were absent or present in very low quantity in sweet orange, mandarin, and Mandarine lime. These compounds correspond to furanocoumarins and coumarins.
According to UV spectra, peaks at Rt 30.7 and 31.5 min (more polarity compounds) were tentatively assigned to a coumarin and a furanocoumarin, respectively. These compounds were present in Key, Tahitian, Mandarine limes, and mandarins but absent in sweet orange. Similarly, two peaks at 39.8 and 40.7 min, which could not be identified, exhibited a great intensity in Key and Tahitian lime, and mandarins, but low intensity in sweet orange and Mandarine lime. The coumarin, methoxylated flavone and unidentified compound with Rt 37.5, 33.9 and 40.7 min respectively, were found in all citrus species. In general, composition of mandarins was less broad and varied, and only a few compounds were detected. In contrast, a high diversity of compounds (especially coumarins and furanocoumarins) were found in Key lime. The predominant presence of methoxylated flavones in sweet orange, as well as coumarins and furanocoumarins in lime is in agreement with that reported by Fan et al. (2015) and Dugrand-Judek et al. (2015).
3.2. Isolation, identification and quantification of major compounds
Given that an extensive work on polymethoxylated flavones from citrus grown in Colombia has already been carried out (Londoño-Londoño et al., 2010), the present work has been focused on the composition of coumarins and furanocoumarins. Six compounds were isolated from the methanolic extract of citrus peels, particularly C. latifolia and C. aurantifolia. The structures of the isolated compounds 1–6 (Fig. 3) were identified as 5-geranyloxy-7-methoxycoumarin, 1; bergamottin, 2; bergapten, 3; isopimpinellin, 4; limettin, 5; and oxypeucedanin hydrate, 6.
Fig. 3.
Structures of isolated compounds from Citrus peels.
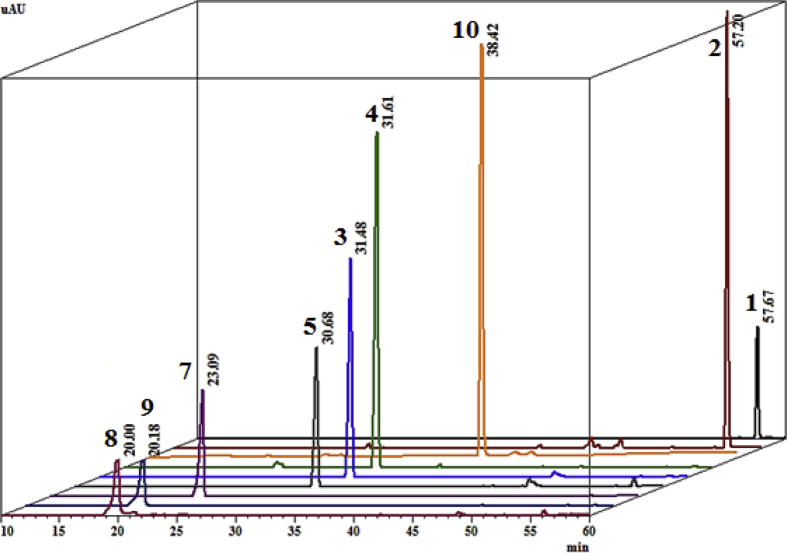
All the compounds had a purity greater than 95% (Fig. 4). However, compound 6 was not included in the chromatographic profiles and the quantification, due to the scarce amount available for this compound. The linear regression equations, correlation coefficients (R2), limits of detection (LOD) and quantification (LOQ) are presented in Table 2. For all the calibration curves, the R2 values were greater than 0.99.
Fig. 4.
HPLC-DAD chromatogram (monitored at 254 nm) for isolated compounds and some phytoalexins reported in Citrus: 5-geranyloxy-7-methoxycoumarin, 1; bergamottin, 2; isopimpinellin, 3; bergapten, 4; limettin, 5; scoparone, 7; scopoletin, 8; umbelliferone, 9 and xanthyletin, 10.
Table 2.
Linear regression equations, correlation coefficients and limit of detection (LOD) and quantification (LOQ) of five coumarins.
| Compound (Rt, min) | Regression equation | R2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|
| 1 (57.7) | y = 87889x + 7004.8 | 0.997 | 0.25 | 0.75 |
| 2 (57.2) | y = 148322x – 5122.9 | 0.998 | 0.11 | 0.33 |
| 3 + 4 (31.5) | y = 414827x - 60250 | 0.997 | 0.13 | 0.41 |
| 5 (30.7) | y = 207993x - 33770 | 0.999 | 0.22 | 0.67 |
The conditions described were used to quantify coumarins and furanocoumarins in peels of different citrus fruit and the results are presented in Table 3.
Table 3.
Amount of coumarins and furanocoumarins in citrus fruit peels.
| Specie | Contents (μg/g f.w.) (mean ± S.D.) |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 + 4 | 5 | Other coumarins∗ | Other furanocoumarins§ | |
| Tahitian lime (C. latifolia) | 392 ± 19 | 349 ± 17 | 168 ± 8 | 183 ± 9 | 357 ± 19 | 217 ± 11 |
| Key lime (C. aurantifolia) | 352 ± 18 | 302 ± 15 | 128 ± 6 | 145 ± 7 | 256 ± 13 | 292 ± 15 |
| Sweet orange (C. sinensis) | traces | traces | n.d. | n.d. | 93 ± 5 | n.d. |
| Mandarine lime (C. limonia) | traces | traces | 44 ± 2 | 100 ± 5 | 478 ± 24 | 41 ± 2 |
| Mandarin (C. reticulata var. Oneco) | traces | traces | 48 ± 2 | n.d. | 569 ± 28 | n.d. |
| Mandarin (C. reticulata var. Arrayana) | traces | traces | 52 ± 3 | n.d. | 570 ± 29 | n.d. |
Coumarins were quantified using the calibration curve obtained for compound 5.
Furanocoumarins were quantified using the calibration curve obtained for compound 3. “Traces” means that the compound was detected but not quantified (concentration under LOQ). n.d. means not detected (concentration under LOD).
Qualitative and quantitative differences were observed among citrus species. According to Table 3, it was found that mandarins and sweet orange peels present low amounts of coumarins and furanocoumarins, whereas Tahitian and Key lime peels contain high levels of these metabolites. Total coumarins were observed in the following concentration order: Tahitian lime (932 ± 47 μg/g) > Key lime (753 ± 38 μg/g) > Mandarine lime (578 ± 29 μg/g) > mandarin var. Arrayana (570 ± 29 μg/g) > mandarin var. Oneco (569 ± 28 μg/g) > sweet orange (93 ± 5 μg/g). Similarly, total furanocoumarins were observed in the following concentration order: Tahitian lime (734 ± 36 μg/g) > Key lime (722 ± 36 μg/g) > Mandarine lime (85 ± 4 μg/g) > mandarin var. Arrayana (52 ± 3 μg/g) > mandarin var. Oneco (48 ± 2 μg/g) > sweet orange (n.d.). Compounds 1 and 2 were predominant in Tahitian and Key lime peels with amounts of 392 and 349 μg/g, and 352 and 302 μg/g, respectively. Both metabolites were detected in sweet orange and mandarins at very low concentrations (detected but not quantified). Except for sweet orange, psoralens 3 and/or 4 were present in all citrus species analyzed. The highest concentration of these compounds was found in Tahitian (168 μg/g) and Key (128 μg/g) limes. Coumarin 5 was only found in Tahitian, Key, and Mandarine limes.
Compounds with Rt 39.8 and 40.7 min, which presented high intensity peaks for Tahitian lime, Key lime, and mandarins, and low intensity peaks for sweet orange and Mandarine lime, could not be isolated and identified. These compounds exhibited a limited stability and seemed to be degraded during the processes of chromatographic separation. Under the conditions used, umbelliferone, scoparone, scopoletin and xanthyletin could not be detected in any of the six Citrus species. This findings could be explained by higher detection limits of the presented method but also by low amounts or absence of these compounds in the extracts. Dugrand et al. (2013) could detect umbelliferone at the trace level in lemon, grapefruit, and bergamot peels using Ultraperformance Liquid Chromatography Coupled with Mass Spectrometry (UPLC-MS).
3.3. Effect of harvest time
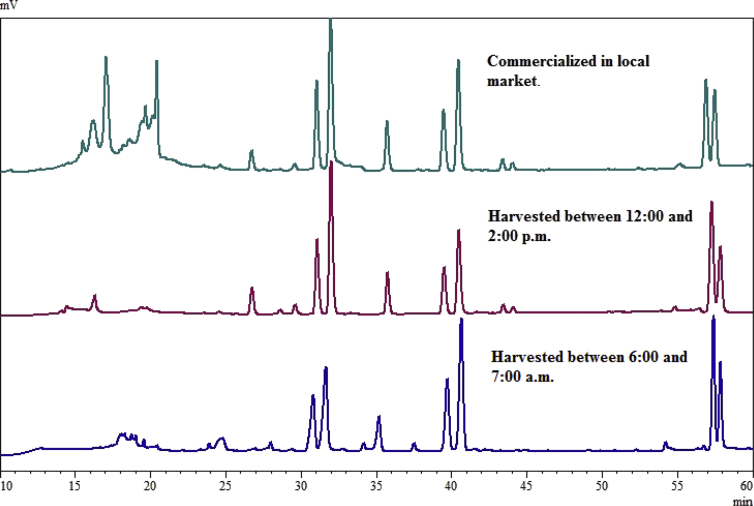
A comparison of the chromatographic profiles of extracts from peels of Tahitian lime fruits harvested between 6:00 and 7:00 a.m., 12:00 and 2:00 p.m., and those commercialized in local market is presented in Fig. 5. The extract of the fruits harvested in the afternoon showed peaks at 27.0, 44.1, and 45.3 min but were not detected or in very low amounts for the fruits collected in the morning hours. Peaks at 34.3, 37.5, and 54.5 min were only observed in fruits harvested in the morning. Interestingly, with the advance of the hours, peaks at 39.8 and 40.7 min were decreasing while those at 30.7 and 31.5 min were increasing. The chromatographic profile in the range 25–60 min of the peels of Tahitian lime fruits commercialized in local market was very similar to that of the fruits harvested between 12:00 and 2:00 p.m. However, a series of peaks corresponding to compounds of higher polarity, in the range 15.0–20.0 min, was evident in the commercialized fruits. According to UV spectra, these peaks showed two strong absorption bands (between 270 and 283 nm and between 326 and 352 nm), which are characteristic of coumarins and flavonoid glucosides. These peaks could be a consequence of long storage periods. These compounds may have a photoprotective function in citrus as a natural sunlight filter. It has been reported that the synthesis of flavonoids and coumarins is enhanced under strong UV and visible light conditions (Takahashi and Badger, 2010).
Fig. 5.
HPLC-DAD chromatograms of methanolic extracts from Tahitian lime fruit peels harvested at different times (6:00 to 7:00 a.m. and 12:00 to 2:00 p.m.) during the day and commercialized in local market.
3.4. Elicitation
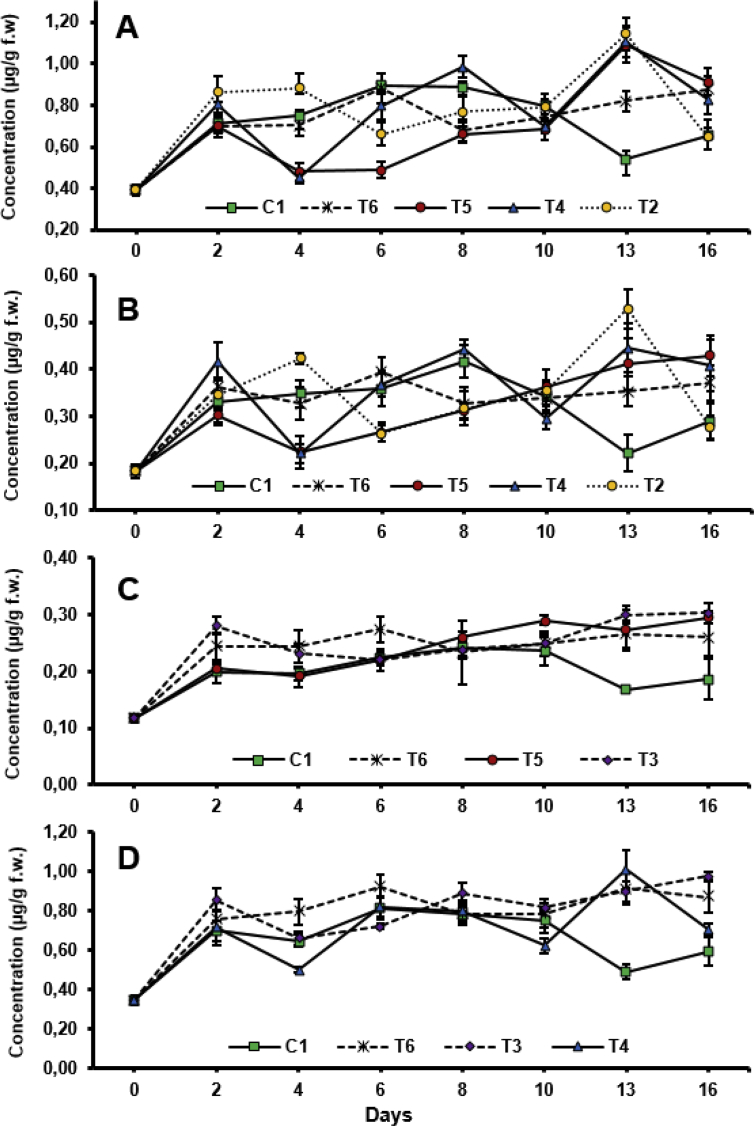
In order to evaluate the effect caused by seventeen different elicitors applied to the fruits of Tahitian lime, a study was carried out in the course of time. For this, the chemical composition of peels from Tahitian lime fruits treated with seventeen elicitors and water (control) was compared at different times after induction. The accumulation in the course of time of coumarins and furanocoumarins in peels of Tahitian lime fruits treated with some elicitors is shown in Fig. 6. In general, the concentration of coumarins and furanocoumarins was dependent on the time post-elicitation and the elicitor. For the same treatment (even for the control experiment), significant differences were observed in the concentration of the compounds over time. The greatest increase in the concentration of coumarins and furanocoumarins occurred during the first two days. The above may be the result of a process of adaptation of plant tissues to induction conditions.
Fig. 6.
Time-course accumulation of coumarins and furanocoumarins (A: compound 1; B: compound 5; C: compounds 3 + 4; D: compound 2) in Tahitian lime fruit peels treated with some different elicitors: C1: water (control); T2: hemicelluloses from corn cobs at 400 mg/L; T3 and T4: β-D-glucans from G. lucidum at 50 and 400 mg/L respectively; T5: gentamicin at 400 mg/L; T6: UV radiation (λ = 254 nm) during 30 min.
During the first ten days, concentration of coumarins and furanocoumarins, in general, showed no significant differences between Tahitian lime fruits treated with the different elicitors and water (control experiments), particularly for compounds 2, and 3 and 4. Subsequently (days 13 and 16), the concentration of coumarins and furanocoumarins showed significant differences between the control experiment and treatments for some elicitors. According to Fig. 6, the amount of coumarins and furanocoumarins in elicited fruits of Tahitian lime was greater than in fruits treated with water. In the control experiment, compounds 1 and 2 reached a maximum concentration of 885 ± 44 and 818 ± 41 μg/g, respectively, after 6 days. Then, the amount of both compounds decreased quickly to reach 541 ± 32 μg/g for 1 and 491 ± 29 μg/g for 2, on day 13. On the other hand, both compounds showed transient increases on different days, depending on the elicitor. For most of the elicitors evaluated, the maximum concentration of 1 (1024 ± 122 μg/g) and 2 (955 ± 56 μg/g) was reached on days 13 and 16 (except for the UV radiation that was on the sixth day). It is noteworthy the substantial increase of 1 and 2 in the peel of Tahitian lime fruits as a result of the treatment with hemicelluloses from corn cobs, β-D-glucans from G. lucidum and gentamycin at 400 mg/L, which reached twice the concentration of the control experiment on day 13. Compound 5 presented maximum amounts ranging from 395 and 437 μg/g (day 8) and from 406 and 528 μg/g (day 13) for Tahitian lime fruits treated with water and elicitors, respectively. The highest concentration of 5 (528 ± 26 μg/g) was elicited by hemicelluloses from corn cobs at 400 mg/L on day 13. Similarly, maximum concentration of compounds 3 + 4 was reached for the control experiment (242 ± 12 μg/g) and treatments (286 ± 17 μg/g) on days 8 and 16, respectively. Overall, a gradual increase in concentration was observed over the time interval; the highest levels recorded for the treatments were reached on day 16, except for fruits treated with UV radiation, whose highest concentration was reached on day 6 and remained almost constant until day 16. The highest concentration of 3 + 4 was reached by the fruits treated with β-D-glucans from G. lucidum at 50 mg/L. It is noteworthy that on days 13 and 16, the concentration of the compounds decreased for the fruits treated with water, while it increased or remained almost stable for the fruits treated with the different elicitors. This suggests that the application of elicitors could prolong higher levels of coumarins and furanocoumarins in the fruits of Tahitian lime. Future studies on induction of coumarins and furanocoumarins in citrus fruits should include longer times. Surprisingly, the exposure of the fruits to the treatments did not elicit the biosynthesis of known citrus phytoalexins, such as scoparone, scopoletin, umbelliferone or xanthyletin or they were present at a very low level. This contrasts with Ben-Yehoshua et al. (1992) who reported the presence of scoparone (23 μg/g f.w.) in the peel of Tahitian lime fruits on the tenth day of being treated with ultraviolet radiation.
The phytoalexins scoparone, scopoletin, umbelliferone, and xanthyletin have been reported after the inoculation of citrus fruits with some fungi. The concentration of scoparone in the resistant species increased rapidly, while in the susceptible ones, it did not increase (Kuniga and Matsumoto, 2006; Afek and Sztejnberg, 1988). It has also been found that the concentration of scoparone in non-inoculated control was very low (about 12–18 μg/g f.w.) (Afek and Sztejnberg, 1994) and that some treatments (i.e. heat alone) do not elicit its formation (Rodov et al., 1994). Thus, the nondetection of scoparone, scopoletin, umbelliferone, and xanthyletin could be due to the higher detection limits of the current method but also by low amounts or the absence of these compounds in the extracts as a result of the citrus species or the non-activity of the elicitor.
3.5. Antifungal activity
3.5.1. Mycelial growth inhibition
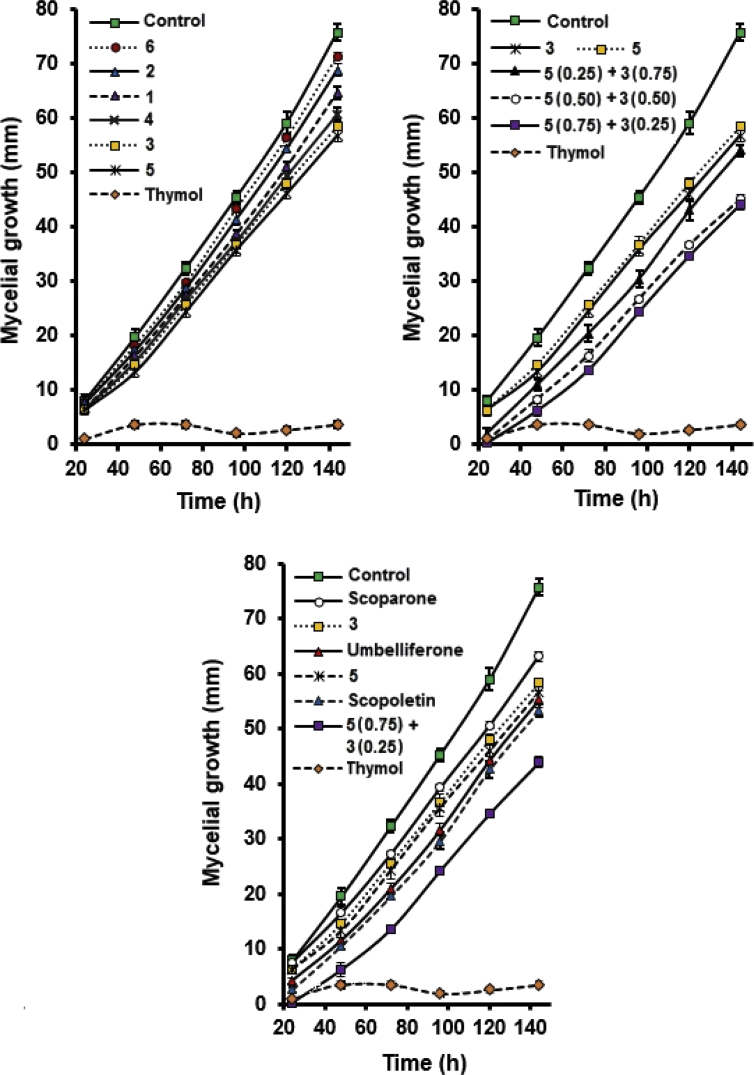
The antifungal activity (inhibition of mycelial growth and spore germination) of nine compounds (1 to 6, umbelliferone, scoparone and scopoletin) was evaluated against Colletotrichum sp. Compounds 1 to 5 were evaluated at 0.25, 0.50, and 1.00 mM, while compound 6 was evaluated at 1.00 mM. As positive controls, Carbendazin and thymol were used at 0.5 mM; the first showed complete inhibition in both radial growth and spore germination, while the second showed percentages of inhibition greater than 85%. Results of mycelial growth are shown in Fig. 7. According to Fig. 7A, compounds 1 to 6 showed significant inhibition on mycelial growth of Colletotrichum sp. when compared to control. For compounds 1 and 2, no significant differences were observed between the concentrations used, and for compounds 3, 4, and 5, at least two concentrations were significantly different (usually 0.25 and 1.0 mM). Overall, as the concentration of these compounds increased, the antifungal activity increased. The 1.0 mM concentration showed the highest antifungal activity, being low to moderate. Compounds 3 and 5 exhibited the highest percentages of inhibition with 32 and 25%, respectively.
Fig. 7.
Mycelial growth of Colletotrichum sp. in the presence of coumarins and furanocoumarins from peels of Tahitian lime fruit (1 to 6 at 1.0 mM) (A), mixtures of the compounds 5 and 3 (B) (at 1.0 mM), and known citrus phytoalexins (scoparone, scopoletin, and umbelliferone) (C) (at 1.0 mM).
Taking into account the greater inhibitory activity of compounds 3 and 5, mixtures of both were prepared in different proportions and their fungistatic effects against Colletotrichum sp. were evaluated. All the mixtures showed significant differences with respect to the control (Fig. 7B). Remarkably, a significantly enhanced antifungal activity of each mixture with respect to the individual compounds was found. Radial growth of Colletotrichum sp. was inhibited by mixtures of 3 and 5 for almost 30 mm after 140 hours. The percentages of inhibition at 24 hours were 95, 91, and 75% for the mixtures of 0.25 mM (3):0.75 mM (5), 0.50 mM (3):0.50 mM (5), and 0.75 mM (3):0.25 mM (5), respectively. No significant differences were found between the mixtures 0.75 mM (5):0.25 mM (3) and 0.50 mM (5):0.50 mM (3).
In addition, antifungal activity against Colletotrichum sp. of the known citrus phytoalexins scoparone, scopoletin and umbelliferone was compared with the isolated compounds from Tahitian lime, 5 and 3, and a mixture of them: 5 (0.75 mM) and 3 (0.25 mM). Results are presented in Fig. 7C. Phytoalexins scopoletin and umbelliferone exhibited slightly higher antifungal activity than the individual compounds 5 and 3, although significant differences were not observed. Once again, the mixture of 5 (0.75 mM) and 3 (0.25 mM) displayed the highest fungistatic effect against Colletotrichum sp., being significantly higher than that of citrus phytoalexins. The significantly greater activity of the mixture of 5 and 3 against Colletotrichum sp. compared with the scoparone, a phytoalexin known for its strong toxicity against several fungi and its involvement in the resistance of citrus fruits against fungal diseases (Afek and Sztejnberg, 1988), is surprising.
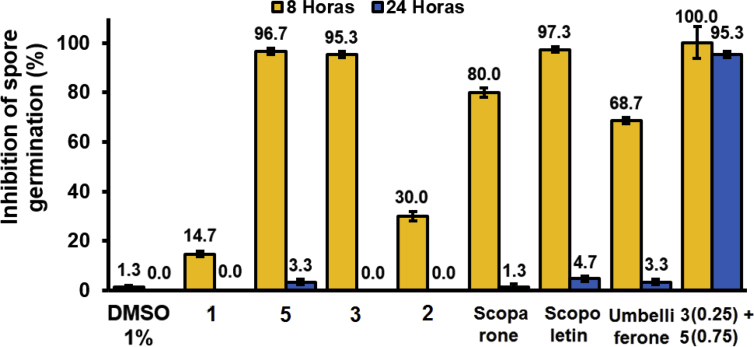
3.5.2. Inhibition of spore germination
The inhibition of spore germination of Colletotrichum sp. after 8 and 24 hours was evaluated using the isolated compounds from Tahitian lime (1, 5, 3) and the mixture of 5 (0.75 mM) and 3 (0.25 mM), the known citrus phytoalexins (scopoletin, scoparone, and umbelliferone), and the solvent control (DMSO). Fig. 8 shows that the highest inhibitory effect after 24 hours was presented by the mixture of compounds 5 (0.75 mM) and 3 (0.25 mM), which completely inhibits germination of the spores.
Fig. 8.
Inhibition of spore germination of Colletotrichum sp. caused by the individual compounds from Tahitian lime (1, 2, 3 and 5), the mixture of 5 (0.75 mM) and 3 (0.25 mM) and the known phytoalexins (scoparone, scopoletin and umbelliferone) from citrus.
Furthermore, under the same conditions, scopoletin and the isolated compounds 5 and 3 inhibited spore germination 97.3, 96.7, and 95.3%, respectively, although significant differences were not found. Among the known phytoalexins of citrus fruits, the decreasing order of antifungal activity was scopoletin (97.3%) > scoparone (80.0%) and umbelliferone (68.7%). However, after 24 hours of incubation, the inhibitory effect of spore germination decreased rapidly for all individual compounds, being less than 5%. Interestingly, the mixture of compounds 5 and 3, which showed the highest inhibitory activity of mycelial growth, prolonged its potent effect against the germination of spores of Colletotrichum sp. From this result, it can be determined that the high antifungal activity of the mixture of compounds 5 and 3 is the result of an additive or synergistic effect. Therefore, it is possible to think that these compounds could be involved with the defense of citrus to pathogenic microorganisms.
It is noteworthy that, although the structural difference between 1 and 5 is only seen in the presence of the O-geranyl substituent attached to carbon 5, the coumpound 5 exhibited higher antifungal activity (mycelial growth and spore germination). Similarly, although compounds 3 and 2 are quite structurally similar, the former is much more active against Colletotrichum sp. The geranyl substituent has been described to be related to the relative lipophilicity of the compounds, which favors its more efficient permeation through the lipid layer of the fungi (Montagner et al., 2008). However, in the present work, the antifungal activity of 1 and 2 was significantly lower than 5 and 3. The latter compounds may have the proper balance of hydrophilicity-lipophilicity, allowing them to cross both the hydrophilic fungal cell wall and the lipophilic membrane. The weak antifungal activity of the geranyloxy substituted coumarin and furanocoumarin is in line with previous work (Penta, 2015; Araújo et al., 2013). Biosynthetically, this prenylation process (insertion of the geranyl group) occurs after the formation of the coumarin or furanocoumarin core (Hung et al., 2017). Two perspectives can be opened from this information for the control of citrus diseases: on the one hand, it is possible to hypothesize that blocking the prenylation stage could increase the defense in citrus. On the other hand, it would be possible to design new antifungal agents based on the coumarin or furanocoumarin core taking into account an adequate hydrophilic-lipophilic balance (Yu et al., 2017). New studies in this regard could be carried out.
4. Conclusions
Chromatographic profiles of peel extracts from citrus fruits grown in Colombia showed qualitative and quantitative differences. Coumarins, furanocoumarins and polymethoxylated flavones were tentatively elucidated as the main compounds found in citrus fruits. Peel composition of Tahitian lime, Key lime, Mandarin lime and mandarins is rich in coumarins and furanocoumarins while polymethoxylated flavones are dominant in sweet orange. Six compounds, including coumarins and furanocoumarins, were isolated from Tahitian and Key lime and identified as 5-geranyloxy-7-methoxycoumarin, limettin, isopimpinellin, bergaptene, bergamottin and oxypeucedanin hydrate. The geranoxy derivatives were the major compounds found in Tahitian and Key lime. Concentration of coumarins and furanocoumarins in Tahitian lime fruits treated with seventeen potential elicitors did not present significant differences when compared to control (fruits treated with water) during the first ten days. On days 13 and 16, the amount of coumarins and furanocoumarins in fruits treated with water was decreased, while in the fruits treated with the potential elicitors, it was maintained or increased. Antifungal activity (mycelial growth and spore germination) of the isolated compounds showed that furanocoumarins were more actives than coumarins. Bergaptene and limettin exhibited the highest inhibitory effects against Colletotrichum sp. being even greater than those of known phytoalexins scoparone and umbelliferone. Also, the mixture of bergaptene and limettin displayed even greater antifungal effect than the individual compounds. Isolated compounds could be involved in the defense mechanisms of C. latifolia, C. aurantifolia and C. limonia.
Declarations
Author contribution statement
Cesar Ramírez-Pelayo, Janio Martínez-Quiñones: Performed the experiments; Analyzed and interpreted the data.
Jesús Gil: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Diego Durango: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Colciencias (grant number 1118-745-58342, CT FP44842-057-2017) and Universidad Nacional de Colombia (grant number 34609).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to Michael James Stablein (University of Illinois, Urbana-Champaign) for the critical review of the manuscript. The authors thank Dr. Quiñones (Universidad de Antioquia) for the NMR spectra.
References
- Afek U., Sztejnberg A. Scoparone (6,7- dimethoxycoumarin), a citrus phytoalexin involved in resistance to pathogens. In: Dniel M., Purkayastha R.P., editors. Handbook of Phytoalexin Metabolismand Action. Marcel Dekker Inc.; New York, Basel, Hong Kong: 1994. pp. 262–286. [Google Scholar]
- Afek U., Sztejnberg A. Accumulation of scoparone, a phytoalexin associated with resistance of citrus to Phytophthora citrophthora. Phytopathology. 1988;78:1678–1682. [Google Scholar]
- Afek U., Orenstein J., Carmeli S., Rodov V., Joseph M.B. Umbelliferone: a phytoalexin associated with resistance of immature Marsh grapefruit to Penicillium digitatum. Phytochemistry. 1999;50:1129–1132. [Google Scholar]
- Ahuja I., Kissen R., Bones A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Araújo R.S.A., Guerra F.Q.S., Lima E.O., de Simone C.A., Tavares J.F., Scotti L., Scotti M.T., de Aquino T.M., de Moura R.O., Mendonça F.J.B., Barbosa-Filho J.M. Synthesis, structure-activity relationships (SAR) and in silico studies of coumarin derivatives with antifungal activity. Int. J. Mol. Sci. 2013;14:1293–1309. doi: 10.3390/ijms14011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aritmo Y., Homma Y., Ohsawa T. Studies on citrus melanose and citrus stem-end rot by Diaporthe citri (Faw.) Wolf. Part. 5 Identification of phytoalexin in melanose spot. Annu. Phytopathol. Soc. Japan. 1986;52:620–625. [Google Scholar]
- Arras G., D'Hallewin G., Molinu M.G., Dore A., Venditti T., Fois M., Lima G., Agabbio M. Induction of phytoalexins biosynthesis in orange fruit by the biocontrol yeast Rhodotorula glutinis. Commun. Agric. Appl. Biol. Sci. 2006;71:915–921. [PubMed] [Google Scholar]
- Ben-Yehoshua S., Rodov V., Kim J.J., Canneli S. Prefonned and induced antifungal materials of citrus fruits in relation to the enhancement of decay resistance by heat and ultraviolet treatments. J. Agric. Food Chem. 1992;40:1217–1221. [Google Scholar]
- Benavente-García O., Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- Bourgaud F., Hehn A., Larbat R., Doerper S., Gontier E., Kellner S., Matern U. Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes. Phytochemistry Rev. 2006;5:293–308. [Google Scholar]
- Braz-Filho R., Magalhães F., Gottlieb O.R. Coumarins from Brosimum rubescens. Phytochemistry. 1972;11:3307–3310. [Google Scholar]
- Cronin M.J., Yohalem D.S., Harris R.F., Andrews J.H. Putative mechanism and dynamics of inhibition of the apple scab pathogen Venturia inaequalis by compost extracts. Soil Biol. Biochem. 1996;28:1241–1249. [Google Scholar]
- Da Silva J.C., de Oliveira R.C., Neto A.S., Pimentel V.C., dos Santos A.A. Extraction, addition and characterization of hemicelluloses from corn cobs to development of paper properties. Procedia Mater Sci. 2015;8:793–801. [Google Scholar]
- De Lange J.H., Vincent A.P., Du Plessis L.M., Van Wyk P.J., Ackerman L.G.J. Scoparone (6,7-dimethoxycoumarin) induced in Citrus peel by black spot, Guignardia citricarpa Kiely. Phytophylactica. 1976;8:83–84. [Google Scholar]
- De Moraes Barros H.R., de Castro Ferreira T.A.P., Genovese M.I. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012;134:1892–1898. doi: 10.1016/j.foodchem.2012.03.090. [DOI] [PubMed] [Google Scholar]
- Dixon R.A. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Duan L., Dou L.L., Yu K.Y., Guo L., Bai-Zhong C., Li P., Liu E.H. Polymethoxyflavones in peel of Citrus reticulata ‘Chachi’ and their biological activities. Food Chem. 2017;234:254–261. doi: 10.1016/j.foodchem.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Dugrand A., Olry A., Duval T., Hehn A., Froelicher Y., Bourgaud F. Coumarin and furanocoumarin quantitation in citrus peel via ultraperformance liquid chromatography coupled with mass spectrometry (UPLC-MS) J. Agric. Food Chem. 2013;61:10677–10684. doi: 10.1021/jf402763t. [DOI] [PubMed] [Google Scholar]
- Dugrand-Judek A., Olry A., Hehn A., Costantino G., Ollitrault P., Froelicher Y., Bourgaud F. The distribution of coumarins and furanocoumarins in Citrus species closely matches citrus phylogeny and reflects the organization of biosynthetic pathways. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famobuwa O.E., Agbowuro A.A., Adekunbi E.A., Akinwale M.A. Isolation and characterization of bergapten from the root bark of Ficus exasperata (Vahl) Int. J. Biochem. Res. Rev. 2019;25:1–5. [Google Scholar]
- Fan H., Wu Q., Simon J.E., Lou S.N., Ho C.T. Authenticity analysis of citrus essential oils by HPLC-UV-MS on oxygenated heterocyclic components. J. Food Drug Anal. 2015;23:30–39. doi: 10.1016/j.jfda.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . 2017. Citrus Fruit - Fresh and processed. Annual Statistics. Rome. [Google Scholar]
- Grover R.K., Moore J.D. Toximetric studies of fungicides against brown rot organism Sclerotina fruticola. Phytopathology. 1962;52:876–880. [Google Scholar]
- Hung W.L., Suh J.H., Wang Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2017;25:71–83. doi: 10.1016/j.jfda.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim R., Barron D. Phenylpropanoids. pp. 75-112. In: Dey P.M., Harborne J.B., editors. Methods in Plant Biochemistry, Vol. 1. Plant phenolics. Academic Press; London, San Diego: 1990. [Google Scholar]
- ICH harmonised tripartite guideline . International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Chicago, USA, 2005. 2005. Validation of analytical procedures: text and methodology Q2(R1) [Google Scholar]
- Jerezano A., Jiménez F., Cruz M.C., Montiel L.E., Delgado G., Tamariz J. New approach for the construction of the coumarin frame and application in the total synthesis of natural products. Helv. Chim. Acta. 2011;94:185–198. [Google Scholar]
- Khan A.J., Kunesch G., Chuilo S., Ravise A. Structure and biological activity of xanthyletin, a new phytoalexin of Citrus. Fruits. 1985;40:807–811. [Google Scholar]
- Kim J.J., Ben-Yehoshua S., Shapiro B., Henis Y., Carmeli S. Accumulation of scoparone in heat-treated lemon fruit inoculated with Penicillium digitatum Sacc. Plant Physiol. 1991;97:880–885. doi: 10.1104/pp.97.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniga T., Matsumoto R. Comparative study of scoparone accumulation in various citrus species after inoculation with gray mold. J. Jpn. Soc. Hortic. Sci. 2006;75:379–384. [Google Scholar]
- Kuniga T., Nesumi H. UV-C irradiation affects accumulation of scoparone in Citrus. Acta Hortic. 2011;907:81–85. [Google Scholar]
- Kuniga T., Nakajima N., Nesumi H., Takishita F. UV-C irradiation reduces gray mold decay and enhances the accumulation of scoparone in some Citrus species. Trop. Agr. Develop. 2015;59:41–49. [Google Scholar]
- Liu Y., Ren C., Cao Y., Wang Y., Duan W., Xie L., Sun C., Li X. Characterization and purification of bergamottin from Citrus grandis (L.) Osbeck cv. Yongjiazaoxiangyou and its antiproliferative activity and effect on glucose consumption in HepG2 cells. Molecules. 2017;22:1227–1239. doi: 10.3390/molecules22071227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño-Londoño J., Rodrigues de Lima V., Lara O., Gil A., Pasa T.C., Arango G.J., Ramirez-Pineda J. Clean recovery of antioxidant flavonoids from citrus peel: optimizing an aqueous ultrasound-assisted extraction method. Food Chem. 2010;119:81–87. [Google Scholar]
- Mabry T.J., Markham K.R., Thomas M.B. The Systematic Identification of Flavonoids. Spinger-Verlag; Berlin: 1970. The ultraviolet spectra of flavones and flavonols. [Google Scholar]
- Martinez J., García C., Durango D. Antifungal activity against Colletotrichum acutatum and Colletotrichum gloeosporioides of the major constituents from wood sawdust of Platymiscium gracile Benth. Bol. Latinoam. Caribe Plant. Med. Aromat. 2017;16:14–25. [Google Scholar]
- Montagner C., de Souza S.M., Groposo C., Delle Monache F., Smânia E.F.A., Smânia A. Antifungal activity of coumarins. Z. Naturforsch. 2008;63c:21–28. doi: 10.1515/znc-2008-1-205. [DOI] [PubMed] [Google Scholar]
- Muñoz L.A., Cobos A., Diaz O., Aguilera J.M. Chia seeds: microstructure, mucilage extraction and hydration. J. Food Eng. 2012;108:216–224. [Google Scholar]
- Ortuño A., Díaz L., Alvarez N., Porras I., García-Lidón A., Del Rio J.A. Comparative study of flavonoid and scoparone accumulation in different Citrus species and their susceptibility to Penicillium digitatum. Food Chem. 2011;125:232–239. [Google Scholar]
- Patil J.R., Jayaprakasha G.K., Kim J., Murthy K.N.C., Chetti M.B., Nam S.Y., Patil B.S. 5-Geranyloxy-7-methoxycoumarin inhibits colon cancer (SW480) cells growth by inducing apoptosis. Planta Med. 2013;79:219–226. doi: 10.1055/s-0032-1328130. [DOI] [PubMed] [Google Scholar]
- Penta S. Antimicrobial agents. In: Penta S., editor. Advances in Structure and Activity Relationship of Coumarin Derivatives. Academic Press; London, San Diego: 2015. [Google Scholar]
- Piasecka A., Jedrzejczak-Rey N., Bednarek P. Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol. 2015;206:948–964. doi: 10.1111/nph.13325. [DOI] [PubMed] [Google Scholar]
- Rodov V., Ben-Yehoshua S., Kim J.J., Shapiro B., Ittah Y. Ultraviolet illumination induces scoparone production in kumquat and orange fruit and improves decay resistance. J. Am. Soc. Hortic. Sci. 1992;117:778–792. [Google Scholar]
- Rodov V., Ben-Yehoshua S., Fang D., D'hallewin G., Castia T. Accumulation of phytoalexins scoparone and scopoletin in citrus fruits subjected to various postharvest treatments. Acta Hortic. 1994;381:517–525. [Google Scholar]
- Sanzani S.M., Schena L., Ippolito A. Effectiveness of phenolic compounds against citrus green mould. Molecules. 2014;19:12500–12508. doi: 10.3390/molecules190812500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbai H., Saad I., Ghezal N., Greca M.D., Haouala R. Bioactive compounds isolated from Petroselinum crispum L. leaves using bioguided fractionation. Ind. Crops Prod. 2016;89:207–214. [Google Scholar]
- Takahashi S., Badger M.R. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 2010;16:53–60. doi: 10.1016/j.tplants.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Tundis R., Loizzo M.R., Menichini F. An overview on chemical aspects and potential health beneficts of limonoids and their derivatives. Crit. Rev. Food Sci. Nutr. 2014;54:225–250. doi: 10.1080/10408398.2011.581400. [DOI] [PubMed] [Google Scholar]
- VanEtten H.D., Mansfield J.W., Bailey J.A., Farmer E.E. Two classes of plant antibiotics: phytoalexins versus "phytoanticipins. Plant Cell. 1994;6:1191–1192. doi: 10.1105/tpc.6.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco R., Gil J.H., García C.M., Durango D.L. Production of 2-phenylethanol in the biotransformation of cinnamyl alcohol by the plant pathogenic fungus Colletotrichum acutatum. Vitae. 2010;17:272–280. [Google Scholar]
- Wang G., Zhou Z., Cheng C., Yao J., Yang Z. Osthol and isopimpinellin from Fructus cnidii for the control of Dactylogyrus intermedius in Carassius auratus. Vet. Parasitol. 2008;158:144–151. doi: 10.1016/j.vetpar.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Yu X., Wen Y., Liang C.G., Liu J., Ding Y.B., Zhang W.H. Design, synthesis and antifungal activity of psoralen derivatives. Molecules. 2017;22:1672. doi: 10.3390/molecules22101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xie Y., Liu C., Chen S., Hu S., Xie Z., Deng X., Xu J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017;230:316–326. doi: 10.1016/j.foodchem.2017.03.040. [DOI] [PubMed] [Google Scholar]