Key Points
Question
What were the trends for early postprocedural stroke in the first 5 years of transcatheter aortic valve replacement (TAVR) use in the United States?
Findings
In this retrospective cohort study based on a US registry of 101 430 patients who underwent TAVR from November 9, 2011 through May 31, 2017, the rate of 30-day postprocedure stroke was 2.3%. This rate did not change significantly over these years.
Meaning
The rate of 30-day stroke was stable over the first 5 years of TAVR recorded in a US clinical registry.
Abstract
Importance
Reducing postprocedural stroke is important to improve the safety of transcatheter aortic valve replacement (TAVR).
Objective
This study evaluated the trends of stroke occurring within 30 days after the procedure during the first 5 years TAVR was used in the United States, the association of stroke with 30-day mortality, and the association of medical therapy with 30-day stroke risk.
Design, Setting, and Participants
Retrospective cohort study including 101 430 patients who were treated with femoral and nonfemoral TAVR at 521 US hospitals in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry from November 9, 2011, through May 31, 2017. Thirty-day follow-up ended June 30, 2017.
Exposures
TAVR.
Main Outcomes and Measures
The rates of 30-day transient ischemic attack and stroke were assessed. Association of stroke with 30-day mortality and association of antithrombotic medical therapies with postdischarge 30-day stroke were assessed with a Cox proportional hazards model and propensity-score matching, respectively.
Results
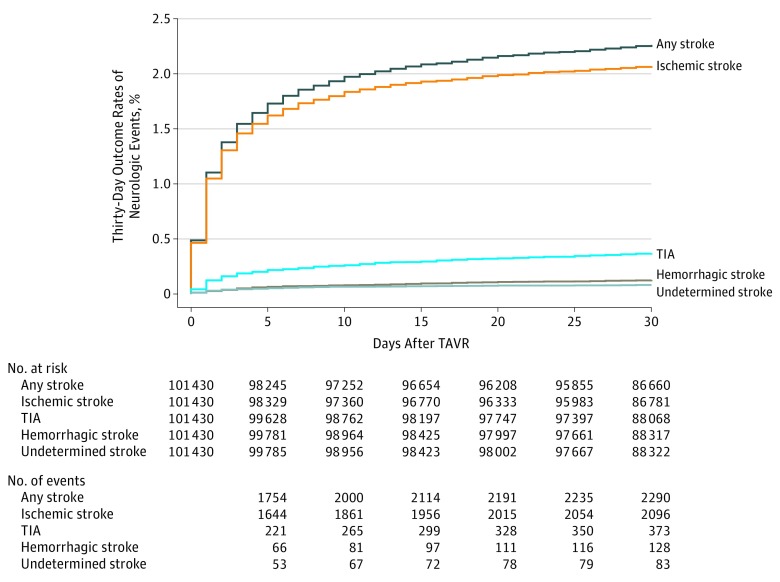
Among 101 430 patients included in the study (median age, 83 years [interquartile range {IQR}, 76-87 years]; 47 797 women [47.1%]; and 85 147 patients [83.9%] treated via femoral access), 30-day postprocedure follow-up data was assessed in all patients. At day 30, there were 2290 patients (2.3%) with a stroke of any kind (95% CI, 2.2%-2.4%), and 373 patients (0.4%) with transient ischemic attacks (95% CI, 0.3%-0.4%) . During the study period, 30-day stroke rates were stable without an increasing or decreasing trend in all patients (P for trend = .22) and in the large femoral access subgroup (P trend = .47). Among cases of stroke within 30 days, 1119 strokes (48.9%) occurred within the first day and 1567 (68.4%) within 3 days following TAVR. The occurrence of stroke was associated with a significant increase in 30-day mortality: 383 patients (16.7%) of 2290 who had a stroke vs 3662 patients (3.7%) of 99 140 who did not have a stroke died (P < .001; risk-adjusted hazard ratio [HR], 6.1 [95% CI, 5.4-6.8]; P < .001). After propensity-score matching, 30-day stroke risk was not associated with whether patients in the femoral cohort were (0.55%) or were not (0.52%) treated with dual antiplatelet therapy at hospital discharge (HR, 1.04; 95% CI, 0.74-1.46) nor was it associated with whether patients in the nonfemoral cohort were (0.71%) or were not (0.69%) treated with dual antiplatelet therapy (HR, 1.02; 95% CI, 0.54-1.95). Similarly, 30-day stroke risk was not associated with whether patients in the femoral cohort were (0.57%) or were not (0.55) treated with oral anticoagulant therapy at hospital discharge (HR, 1.03; 95% CI, 0.73-1.46) nor was it associated with whether patients in the nonfemoral cohort were (0.75%) or were not (0.82%) treated with an oral anticoagulant (HR, 0.93; 95% CI, 0.47-1.83).
Conclusions and Relevance
Between 2011 and 2017, the rate of 30-day stroke following transcatheter aortic valve replacement in a US registry population remained stable.
This cohort study uses registry data to describe trends and incidence of 30-day stroke among patients who had undergone transcatheter aortic valve replacement (TAVR) in the first 5 years the procedure’s use in US clinical practice.
Introduction
Transcatheter aortic valve replacement (TAVR) has become an established and widely adopted approach to treat aortic stenosis in the past decade and is approved by the US Food and Drug Administration (FDA) for patients with severe symptomatic aortic stenosis and at least intermediate surgical risk.1,2,3,4,5 Compared with surgical aortic valve replacement, the risk of stroke following TAVR is similar.6,7 However, post-TAVR stroke remains a serious complication associated with increased mortality.8,9 Despite more than 500% growth in TAVR volume in US clinical practice from 2012 to 2015, it is unknown whether stroke risk after TAVR has declined in clinical practice as device technology and operator experience have improved.10 The purpose of this study was to evaluate the temporal trends and prognosis of early stroke occurring within 30 days of the procedure among unselected patients who underwent TAVR in the first 5 years TAVR was used in US clinical practice, using a broadly based national registry.
Methods
Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry
The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry is a national registry of 524 US hospitals that developed in collaboration with the FDA and the Centers for Medicare & Medicaid Services (CMS). The Chesapeake Research Review Inc is the designated institutional review board for the TVT Registry. The TVT Registry protocol on file has been granted a waiver of informed consent. The registry promotes patient safety and quality of transcatheter valve procedures by surveillance and reporting of procedural outcomes. Participation in the registry is necessary for CMS payment for transcatheter valve procedures; thus, nearly all TAVR procedures performed in the United States are included. Annual data quality checks are implemented at the National Cardiovascular Data Registry data warehouse and the Duke Clinical Research Institute analysis center, including data quality feedback reports, data range, consistency checks, and yearly random external audits of data quality.
Study Population
The population included consecutive cases of TAVR from November 9, 2011, through May 31, 2017, with 30-day follow-up for all patients completed through June 30, 2017. Reasons for exclusion included aborted procedures, procedures performed within 30 days of the last study date, cases with missing data on neurologic events, and other reasons (eFigure 1 in the Supplement). Among patients with multiple admissions for TAVR, only the first admission was included. The population was stratified by femoral vs nonfemoral access types (eFigure 2 in the Supplement). Data pertaining to patients’ race and ethnicity were included in this study because it is unknown whether race or ethnicity may be associated with neurologic outcomes after TAVR. Race and ethnicity determination was made by the patient or family and based on fixed categories.
Outcomes
The rates of 30-day stroke, transient ischemic attack (TIA), and any neurologic event (stroke or TIA) were assessed. Study outcomes were the trends in crude 30-day neurologic event rates over time, the association of post-TAVR 30-day stroke with mortality, and the association of antithrombotic medical therapy at discharge with stroke in the 30 days after discharge adjusted for the competing risk of death.
Neurologic events in the registry are ascertained as part of clinical care. Each site is responsible for voluntary reporting of events. The registry does not mandate routine neurologic assessment or neuroimaging in asymptomatic patients. Outcomes were collected from the TVT Registry, and neurologic outcomes were adjudicated by at least 2 board-certified cardiologists using Valve Academic Research Consortium definitions.11 This process involved review of specific site queries and deidentified source records as needed. Adjudicators were provided with clinical information for each case but were not blinded to procedural data. Neurologic events were defined based on criteria prespecified by the TVT Registry (eTable 1 in the Supplement). All clinical data and outcomes were collected as part of the ongoing function of the TVT Registry and were not collected specifically for the purpose of this study.
Data Analyses
The incidence of neurologic events within 30 days was assessed as both a categorical outcome variable (event, censor, death) in time-to-event analyses and as a binary outcome variable (yes, no) in temporal trend analyses. A Cochran-Armitage test was used to evaluate temporal trends. The 29 cases of TAVR performed from November 9, 2011, through December 31, 2011, were grouped as part of 2012 for temporal trend analysis.
Cox proportional hazards modeling of unadjusted and risk-adjusted 30-day mortality among patients with vs without stroke was performed. To mitigate the problem of immortal time bias, stroke was considered as a time-dependent variable that changes value from “no” to “yes” over the course of a 30-day period. Patients were only considered to have stroke from the date of stroke, which allowed for patients to be at risk of death prior to stroke. The marginal model approach was used to account for within-hospital clustering. The marginal model approach estimates regression parameters under an independent working assumption while using a robust sandwich covariance matrix to account for intracluster correlation. The adjusted model was adjusted for all variables in the original STS/ACC TVT TAVR in-hospital mortality risk model, which was previously validated.12 The proportional hazards model assumption was tested for all adjustment variables by including a covariate interaction with time in the model. For adjustment variables for which the proportional hazards assumption did not hold, we included the time-interaction term in the multivariable model to deal with nonproportionality.
A series of propensity-score matched analyses to study the association of post-TAVR antiplatelet and anticoagulant therapy with 30-day stroke was undertaken. Separate propensity-matched analyses were performed within the femoral and nonfemoral subgroups with matching on whether patients received (1) dual antiplatelet therapy or not, (2) oral anticoagulant or not, or (3) dual antiplatelet therapy vs oral anticoagulant therapy. Variables used for matching and standardized differences of the matched populations are provided in the data supplement (eTables 2 and 7-12 in the Supplement). All except one variable used in propensity models had less than 1.3% missing data (eTable 2 in the Supplement). One variable had less than 5% missing data. Because missing data were less than 5%, simple imputation procedures were used to impute all missing values. Continuous variables were imputed to their median values and categorical variables were imputed to the highest frequency category. For the taking vs not taking dual antiplatelet therapy analysis, patients taking single antiplatelet therapy were included in the non–dual antiplatelet therapy group and patients taking oral anticoagulants were eligible for either group. In the taking or not taking oral anticoagulant analyses, patients taking any antiplatelet regimen were eligible for either group. In the dual antiplatelet therapy vs oral anticoagulant analyses, only patients taking dual antiplatelet therapy without oral anticoagulant were eligible for the dual antiplatelet therapy group and patients taking oral anticoagulants with single antiplatelet therapy but not dual antiplatelet therapy were eligible for the oral anticoagulant group. Patients with vs without therapy were matched 1:1 on the logit of propensity score with a caliper width of 0.2 times the pooled standard deviation of logit propensity scores. Because medication information was only available at time of hospital discharge, only patients discharged alive without in-hospital stroke events were considered in the propensity-score-matched analyses. Patients with neurologic events or death prior to hospital discharge were excluded from propensity matching. After propensity matching, the hazard of 30-day stroke, TIA, and stroke or TIA via time-to-event analysis was assessed. The Fine and Gray proportional subdistribution hazards model was used to account for competing risk of death and the marginal model approach was used to account for hospital clustering as described above. For both femoral and nonfemoral cohorts, the proportional hazards assumption was tested by including a covariate interaction with time in the model. All covariate interactions with time were nonsignificant at the .05 level indicating no violation of the proportionality assumption.
Categorical variables were presented as frequencies and compared with Pearson χ2 tests. Ordinal variables (presented as frequencies) and continuous variables (presented as median [interquartile range] {IQR}) were compared using χ2 rank-based group means score statistic (Wilcoxon equivalent). Two sided P < .05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Patients and Procedural Characteristics
The study population included 101 430 patients treated with TAVR at 521 sites (83.9% femoral access). At day 30 there were 373 patients (0.4%) with TIA (95% CI, 0.3%-0.4%) and 2290 patients (2.3%) with stroke of any kind (95% CI 2.2%-2.4%) (Figure 1 and eFigure 3 in the Supplement). There was no difference in the 30-day stroke rate between sites with fewer than 100 or more than 100 TAVR cases (eTable 3 in the Supplement). Patients with 30-day strokes were older and more commonly women vs patients without strokes (Table 1). Patients with 30-day stroke had a higher proportion of prior stroke, prior TIA, known peripheral arterial disease, hypertension, porcelain aorta, and carotid stenosis. The rate of prior atrial fibrillation was not significantly different between patients with and without 30-day strokes. Patients with stroke within 30 days were less likely to be treated with femoral access and had a higher median STS in-hospital or 30-day mortality risk score. General anesthesia and self-expanding TAVR devices were more prevalent in the 30-day stroke group. In-hospital atrial fibrillation after TAVR occurred more frequently in the 30-day stroke group.
Figure 1. Neurologic Events Within 30 Days of Transcatheter Aortic Valve Replacement.
The cumulative incidence of 30-day neurologic events are shown. TAVR indicates transcatheter aortic valve replacement; TIA, transient ischemic attack.
Table 1. Baseline and Procedural Characteristics.
| 30-Day Stroke, No. (%) | Difference (95% CI) | P Value | ||
|---|---|---|---|---|
| No (N = 99 140) | Yes (N = 2290) | |||
| Demographics | ||||
| Age, median (IQR), y | 82.0 (76.0 to 87.0) | 84.0 (78.0 to 88.0) | 2.0 (1.7 to 2.3) | <.001 |
| Sex | ||||
| Men | 52 581 (53.0) | 1039 (45.4) | −7.7 (−5.6 to −9.7) | <.001 |
| Women | 46 546 (47.0) | 1251 (54.6) | 7.7 (9.7 to 5.6) | |
| Race/ethnicity | ||||
| Native Hawaiian or Pacific Islander | 145 (0.1) | 3 (0.1) | −0 (0.1 to −0.2) | .60 |
| American Indian or Alaskan Native | 242 (0.2) | 3 (0.1) | −0.1 (0.0 to −0.3) | |
| Asian | 1169 (1.2) | 32 (1.4) | 0.2 (0.7 to −0.3) | |
| Black or African American | 3740 (3.8) | 93 (4.1) | 0.3 (1.1 to −0.5) | |
| White | 92 988 (94.6) | 2127 (94.2) | −0.4 (0.6 to −1.4) | |
| Hispanic or Latino ethnicity | 3871 (4.0) | 88 (3.9) | −0.1 (0.8 to −0.9) | .90 |
| Comorbidities | ||||
| Hypertension | 89 216 (90.0) | 2097 (91.6) | 1.5 (2.7 to 0.4) | .02 |
| Heart failure <2 wk | 77 019 (77.8) | 1809 (79.1) | 1.3 (3.0 to −0.4) | .13 |
| Atrial fibrillation or flutter | 39 468 (39.9) | 928 (40.6) | 0.7 (2.7 to −1.3) | .49 |
| Diabetes | 37 236 (37.6) | 871 (38.0) | 0.4 (2.4 to −1.6) | .67 |
| Prior PAD | 29 297 (29.6) | 791 (34.6) | 5.0 (6.9 to 3.0) | <.001 |
| Prior MI | 23 834 (24.1) | 563 (24.6) | 0.5 (2.3 to −1.3) | .56 |
| Stenosis of 1 or both carotid arteriesa | 19 920 (24.6) | 553 (29.1) | 4.5 (6.6 to 2.5) | <.001 |
| Prior stroke | 11 647 (11.8) | 396 (17.3) | 5.5 (7.1 to 4.0) | <.001 |
| Chronic lung disease | 12 521 (12.7) | 271 (11.9) | −0.8 (0.5 to −2.2) | .25 |
| Transient ischemic attack | 8705 (8.8) | 311 (13.6) | 4.8 (6.2 to 3.4) | <.001 |
| Cardiac procedure <30 d | 9116 (9.2) | 226 (9.9) | 0.7 (1.9 to −0.6) | .27 |
| Hostile chestb | 7762 (7.8) | 162 (7.1) | −0.8 (0.3 to −1.8) | .18 |
| Smoking status | 5561 (5.6) | 143 (6.3) | 0.6 (1.6 to −0.4) | .19 |
| Porcelain aortac | 4898 (4.9) | 141 (6.2) | 1.2 (2.2 to 0.2) | .008 |
| Current dialysis | 3966 (4.0) | 83 (3.6) | −0.4 (0.4 to −1.2) | .36 |
| Cardiogenic shock <24 h | 596 (0.6) | 21 (0.9) | 0.3 (0.7 to −0.1) | .06 |
| STS patient predicted mortality for aortic valve replacement only, median (IQR)d | 6.0 (3.9 to 9.3) | 6.7 (4.4 to 10.3) | 0.7 (0.6 to 0.9) | <.001 |
| Clinical data | ||||
| Height, median (IQR), cm | 167.6 (158.0 to 175.0) | 165.0 (157.0 to 173.0) | −2.6 (−2.8 to −2.4) | <.001 |
| Weight, median (IQR), kg | 77.0 (65.0 to 90.7) | 72.9 (61.3 to 86.0) | −4.2 (−4.9 to −3.4) | <.001 |
| Hemoglobin, median (IQR), g/dL | 11.9 (10.6 to 13.2) | 11.9 (10.6 to 13.1) | −0.0 (−0.1 to 0.1) | .39 |
| Platelet count, median (IQR), × 103/L | 193.0 (154.0 to 239.0) | 200.0 (159.0 to 247.0) | 7.0 (4.1 to 9.9) | <.001 |
| INR, median (IQR) | 1.1 (1.0 to 1.2) | 1.1 (1.0 to 1.2) | 0 | .02 |
| Creatinine, median (IQR), mg/dL | 1.1 (0.9 to 1.4) | 1.1 (0.9 to 1.4) | 0 | .23 |
| ≥Moderate aortic insufficiency | 19 432 (19.7) | 433 (19.0) | −0.7 (0.9 to −2.4) | .39 |
| Valve morphology | ||||
| Unicuspid, quadracuspid, uncertain | 7660 (7.8) | 165 (7.2) | −0.5 (0.6 to −1.6) | .63 |
| Tricuspid | 88 356 (89.7) | 2050 (90.1) | 0.4 (1.7 to −0.8) | |
| Bicuspid | 2537 (2.6) | 61 (2.7) | 0.1 (0.8 to −0.6) | |
| Annular calcificatione | 77 361 (79.4) | 1811 (80.5) | 1.1 (2.7 to −0.6) | .22 |
| AV peak velocity, median (IQR), m/s | 4.1 (3.7 to 4.5) | 4.1 (3.8 to 4.5) | 0 | .29 |
| AV area, median (IQR), cm2f | 0.7 (0.6 to 0.8) | 0.7 (0.5 to 0.8) | −0 | <.001 |
| Procedural Data | ||||
| Access site | ||||
| Femoral | 83 374 (84.1) | 1773 (77.4) | −6.7 (−4.9 to −8.4) | <.001 |
| Nonfemoral | 15 766 (15.9) | 517 (22.6) | 6.7 (8.4 to 4.9) | |
| Procedure year | ||||
| Up to May 2017 | 17 089 (17.2) | 397 (17.3) | 0.1 (1.7 to −1.5) | .53 |
| 2016 | 33 223 (33.5) | 735 (32.1) | −1.4 (0.5 to −3.3) | |
| 2015 | 22 507 (22.7) | 512 (22.4) | −0.3 (1.4 to −2.1) | |
| 2014 | 14 531 (14.7) | 364 (15.9) | 1.2 (2.8 to −0.3) | |
| 2013 | 7855 (7.9) | 187 (8.2) | 0.2 (1.4 to −0.9) | |
| 2012 | 3935 (4.0) | 95 (4.1) | 0.2 (1.0 to −0.6) | |
| Procedure location | ||||
| Hybrid OR suite | 60 924 (61.5) | 1367 (59.7) | −1.8 (0.3 to −3.8) | .23 |
| Hybrid catheter laboratory suite | 26 639 (26.9) | 660 (28.8) | 1.9 (3.8 to 0.1) | |
| Catheter laboratory | 11 180 (11.3) | 254 (11.1) | −0.2 (1.1 to −1.5) | |
| Other | 299 (0.3) | 7 (0.3) | 0.0 (0.2 to −0.2) | |
| Procedure status | ||||
| Elective | 90 396 (91.2) | 2052 (89.6) | −1.6 (−0.4 to −2.9) | .01 |
| Urgent | 8448 (8.5) | 228 (10.0) | 1.4 (2.7 to 0.2) | |
| Emergency | 197 (0.2) | 8 (0.3) | 0.2 (0.4 to −0.1) | |
| Salvage | 31 (0.0) | 2 (0.1) | 0.1 (0.2 to −0.1) | |
| Valve-in-valve procedureg | 6004 (6.1) | 143 (6.3) | 0.2 (1.2 to −0.8) | .72 |
| Heart team reason for procedure | ||||
| Inoperable or extreme risk (technical, comorbid, or deconditioned) | 28 940 (29.2) | 711 (31.1) | 1.8 (3.7 to −0.1) | |
| Risk level of 30-d mortality | ||||
| High (>8%) | 57 652 (58.3) | 1354 (59.2) | 0.9 (2.9 to −1.1) | .001 |
| Intermediate (4%-7%) | 10 934 (11.1) | 192 (8.4) | −2.7 (−1.5 to −3.8) | |
| Low (<4%) | 937 (0.9) | 19 (0.8) | −0.1 (0.3 to −0.5) | |
| Patient preference or other | 483 (0.5) | 12 (0.5) | 0.0 (0.3 to −0.3) | |
| Conversion to open heart surgery | 643 (0.6) | 49 (2.1) | 1.5 (2.1 to 0.9) | <.001 |
| General anesthesiah | 75 305 (76.1) | 1818 (79.5) | 3.4 (5.0 to 1.7) | <.001 |
| Device successi | 94 397 (96.0) | 2128 (93.8) | −2.1 (−1.1 to −3.1) | <.001 |
| Valve type | ||||
| Balloon expandable valve | 73 535 (74.3) | 1532 (67.0) | −7.3 (−5.3 to −9.2) | <.001 |
| Self-expanding valve | 25 494 (25.7) | 755 (33.0) | 7.3 (9.2 to 5.3) | |
| Fluoroscopy time, median (IQR), min | 17.4 (12.7 to 24.0) | 19.7 (13.4 to 27.3) | 2.3 (1.8 to 2.8) | <.001 |
Abbreviations: AV, aortic valve; INR, international normalized ratio; IQR, interquartile range; MI, myocardial infarction; OR, operating room; PAD, peripheral arterial disease; STS, Society of Thoracic Surgeons.
SI conversion factor: To convert creatinine from mg/dL to μmol/L, multiply by 88.4.
Carotid stenosis was assessed in 82 907 patients (81.7%) prior to TAVR, while 17 247 (17%) were not assessed for carotid stenosis, and 1276 (1.3%) had missing data. Patients with carotid stenosis not assessed and missing were imputed to no carotid stenosis.
Hostile chest defined as a medical condition that precludes an open chest procedure including but not limited to abnormal chest wall anatomy, severe kyphoscoliosis or other skeletal abnormality, complications from prior surgery, prior radiation involving the mediastinum or thorax, evidence of severe radiation damage, history of multiple recurrent pleural effusions with internal adhesions, chronic and ongoing skin defects or extremely severe soft tissue atrophy, and complete absence of reconstructive options based on plastic surgery consult.
Porcelain aorta defined as severe atherosclerosis of the aorta, calcification may be severe and diffuse, causing an eggshell appearance seen on chest x-ray or computed tomography.
STS-predicted risk of 30-day patient mortality for AV replacement; higher values indicate higher predicted 30-day mortality. Values of less than 4% are considered low risk; 4% to 8%, intermediate risk; and more than 8%, high risk.
Annular calcification defined as present if echo reports document calcification in the AV leaflets, aorta adjacent to the AV leaflets, or the left ventricular outflow tract, or if the echo documents AV calcific degeneration.
Aortic valve area was assessed only among patients with aortic stenosis.
Valve-in-valve procedure is defined as a TAVR performed within a prior bioprosthetic AV.
All other patients received procedural sedation, an epidural, or both.
Device success is defined as by the Standardized Endpoint Definitions for Transcatheter Aortic Valve Implantation Clinical Trials: (1) successful vascular access, delivery, and deployment of the device and retrieval of the delivery system, (2) correct position of the device in the proper anatomic location, (3) intended performance of the prosthetic heart valve (AV area >1.2 cm2 and mean AV gradient <20 mm Hg or peak velocity <3 m/s without moderate or severe aortic regurgitation), and (4) only 1 valve implanted in the proper anatomical location.
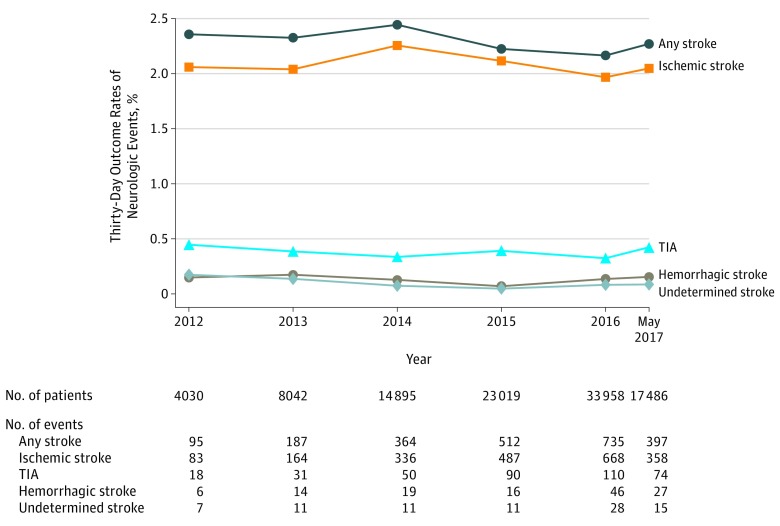
Temporal Trends in Neurologic Events
The annual rates of 30-day TIA varied between 0.3% and 0.5% and the annual rate for any type of stroke from between 2.2% and 2.4%, but both 30-day TIA and stroke rates were stable over time without a significant increasing or decreasing trend (P for trend = .22; Figure 2; eTable 4 in the Supplement). When considering the large proportion of the population treated only with femoral access TAVR, the 30-day stroke rate was also stable over time between 2.0% and 2.5% without a significant increasing or decreasing trend (P for trend = .47; eTable 5 in the Supplement).
Figure 2. Temporal Trends in Neurologic Events After Transcatheter Aortic Valve Replacement.
Temporal trends in annual 30-day neurologic event rates in the overall study population are shown. The 29 cases of transcatheter aortic valve replacement performed between November 9 through December 31, 2011, were included in the 2012 group for these analyses. TIA indicates transient ischemic attack.
The median time to stroke events was 2.0 days (IQR, 1.0-5.0) days after TAVR. Of all patients with 30-day stroke events, 1119 patients (48.9%) had a stroke within 1 day and 1567 (68.4%) within 3 days. Of all those with strokes, 2096 patients (91.5%) had ischemic strokes; 128 (5.6%) had hemorrhagic strokes; and 83 (3.6%) had strokes of an undetermined subtype. Neuroimaging was performed for 1876 patients (98.7%) with strokes and neurologist or neurosurgeon confirmation of the diagnosis was obtained in 1782 patients (93.8%) with strokes. Nine hundred twenty-four patients (48.6%) with stroke had substantial impairment of social or recreational activities, 655 (34.5%) had impairment of neurocognitive functions, and 784 (41.2%) required new aids or assistance at the time of event adjudication (eTable 6 in the Supplement).
Association of Post-TAVR Stroke With 30-Day Mortality
The rate of 30-day mortality was significantly higher among patients with vs without 30-day strokes (16.7% [383 of 2290] vs 3.7% [3662 of 99 140], P < .001). Compared with patients without 30-day strokes, the occurrence of stroke within 30 days after TAVR was associated with increased unadjusted 30-day mortality (HR, 6.7 [95% CI, 6.0-7.5], P < .001) and risk-adjusted 30-day mortality (HR 6.1 [95% CI, 5.4-6.8], P < .001). Additionally, among patients surviving to hospital discharge, those with 30-day stroke were significantly less likely to be discharged directly home following TAVR vs those without strokes (36.1% [721 of 2290] vs 78.9% [76 024 of 99 140], P < .001).
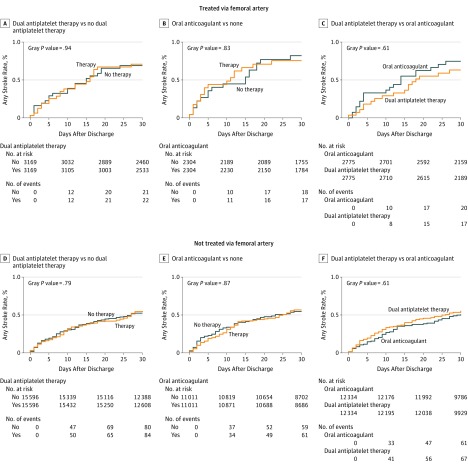
Association of Medical Therapy With Risk of Postdischarge 30-Day Neurologic Events
Baseline characteristics of patients propensity-score matched on dual antiplatelet therapy vs no dual antiplatelet therapy, oral anticoagulant vs no oral anticoagulant, and dual antiplatelet therapy vs oral anticoagulant at hospital discharge in femoral and nonfemoral cohorts are provided in eTables 7 through 12 in the Supplement. After matching, standardized differences for all baseline variables were <0.10, consistent with well-balanced populations. In the femoral and nonfemoral cohorts, we observed no association between dual antiplatelet therapy or oral anticoagulant and the risk of postdischarge 30-day stroke, TIA, or any neurologic event (Table 2, Figure 3, and eTables 13 and 14 in the Supplement). When comparing dual antiplatelet therapy vs oral anticoagulant after propensity matching, we again observed no significant difference in the risk of postdischarge 30-day stroke, TIA, or any neurologic event between treatment strategies in either cohort.
Table 2. Association of Medical Therapy With Postdischarge 30-Day Neurologic Events After Propensity Score Matchinga.
| Outcome | Dual Antiplatelet Therapyb | Oral Anticoagulantc | Dual Antiplatelet Therapy vs Oral Anticoagulantd | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | Absolute Risk, % | P Value | HR (95% CI) | Absolute Risk, % | P Value | HR (95% CI) | Absolute Risk, % | P Value | ||||
| Yes | No | Yes | No | Dual Antiplatelet Therapy | Oral Anticoagulant | |||||||
| Femoral Cohort | ||||||||||||
| No. | 31 192 | 22 022 | 24 668 | |||||||||
| 30-d stroke | 1.04 (0.74-1.46) | 0.55 | 0.52 | .81 | 1.03 (0.73-1.46) | 0.57 | 0.55 | .87 | 1.09 (0.79-1.52) | 0.55 | 0.51 | .59 |
| 30-d TIA | 0.87 (0.51-1.49) | 0.18 | 0.21 | .61 | 0.84 (0.46-1.52) | 0.19 | 0.24 | .56 | 0.83 (0.49-1.40) | 0.21 | 0.25 | .48 |
| 30-d stroke or TIA | 0.99 (0.74-1.33) | 0.73 | 0.73 | .96 | 0.98 (0.73-1.33) | 0.76 | 0.77 | .92 | 0.99 (0.75-1.30) | 0.74 | 0.76 | .92 |
| Nonfemoral Cohort | ||||||||||||
| No. | 6338 | 4608 | 5550 | |||||||||
| 30-d stroke | 1.02 (0.54-1.95) | 0.71 | 0.69 | .94 | 0.93 (0.47-1.83) | 0.75 | 0.82 | .83 | 0.84 (0.44-1.64) | 0.63 | 0.75 | .62 |
| 30-d TIA | 0.61 (0.22-1.72) | 0.17 | 0.28 | .35 | 1.31 (0.29-5.84) | 0.18 | 0.15 | .72 | 0.62 (0.20-1.90) | 0.19 | 0.32 | .40 |
| 30-d stroke or TIA | 0.87 (0.49-1.57) | 0.84 | 0.97 | .65 | 0.98 (0.52-1.87) | 0.94 | 0.97 | .96 | 0.74 (0.41-1.34) | 0.78 | 1.06 | .33 |
Abbreviations: HR, hazard ratio; TIA, transient ischemic attack.
Characteristics of patients in the propensity-matched analyses are provided in eTables 7 through 12 in the Supplement. All medications for the propensity-matched analyses were based on discharge medications.
Patients taking single-antiplatelet therapy were eligible to be included in the no dual antiplatelet therapy group. Patients taking oral anticoagulants were eligible for either group.
Patients taking any antiplatelet regimen were eligible for either group.
Only patients taking dual antiplatelet therapy without oral anticoagulants were eligible for the dual antiplatelet therapy group and patients taking oral anticoagulants with single antiplatelet therapy but no dual antiplatelet therapy were eligible for the oral anticoagulant group.
Figure 3. Association of Medical Therapy With Postdischarge 30-Day Strokes After Transcatheter Aortic Valve Replacement.
Medications were assessed at the time of hospital discharge, and the association between discharge medical therapy and postdischarge stroke within 30 days is shown. Cumulative incidence of 30-day stroke in propensity-matched patients is shown.
Discussion
This study of early post-TAVR stroke in the first 5 years of TAVR in US clinical practice yielded the following principal findings. First, despite improvements in TAVR technology and operator experience, the rate of post-TAVR 30-day stroke was stable over time. Second, the majority of early post-TAVR strokes occurred within the first 3 days following TAVR. Third, early stroke was independently associated with a significant increase in 30-day mortality. Fourth, in propensity-matched analyses, neither dual antiplatelet therapy nor oral anticoagulant therapy was associated with the risk of 30-day neurologic events. These findings offer insight into population trends in the safety of TAVR in the United States as a framework for future efforts to reduce post-TAVR stroke rates.
The lack of decline in post-TAVR 30-day stroke rates in the first 5 years of TAVR use in US clinical practice is notable given the favorable improvements in device technology and operator experience over that time.13 The findings of this analysis confirm that these advances have not translated into lower early stroke rates in clinical practice. The results of this study are consistent with those of Carroll et al14 that increasing TAVR site volume in the TVT Registry had no association with stroke events although increasing site volume was linked to reduced rates of other major TAVR complications including bleeding, vascular injury, and mortality. Additional strategies are needed to improve the neurologic safety of TAVR in the United States.
Strokes that occur intraprocedurally are potentially modifiable by the use of cerebral embolic protection devices. In a randomized trial involving 363 patients treated with TAVR, a cerebral embolic protection device captured embolic debris in 99% of cases, although the rate of 30-day major adverse cardiac and cerebrovascular events was not significantly different between the device and control groups (7.3% vs 9.9%, P = .41).15 In a meta-analysis involving 625 patients across 5 trials, the association of embolic protection during TAVR with the risk of death or stroke did not reach statistical significance (6.1% with vs 9.6% without protection, P = .08).16 Cerebral embolic protection is now FDA approved for use during TAVR, and future studies are needed to characterize the adoption of cerebral embolic protection during TAVR in US clinical practice and its effect on clinical outcomes.
There is uncertainty regarding the optimal antithrombotic regimen after TAVR. Standard therapy in many centers includes indefinite aspirin (75 to 100 mg daily) and dual antiplatelet therapy with clopidogrel (75 mg daily) for 3 to 6 months immediately after TAVR, although there is variability from center to center.17,18 Use of dual antiplatelet therapy was not associated with reduced 30-day stroke risk in femoral and nonfemoral cohorts in this study. The results of this analysis are similar to recent studies that have suggested that single antiplatelet therapy after TAVR may be as effective as dual antiplatelet therapy for prevention of ischemic events with lower risk of bleeding.18,19,20 Similarly, this study identified that oral anticoagulant therapy was not associated with lower neurologic risk within the first 30 days, and there was no significant difference between dual antiplatelet therapy and oral anticoagulant therapy in terms of 30-day neurologic events. This analysis is limited because in-hospital strokes could not be included in the propensity-matched analyses due to lack of medication information prior to hospital discharge. However, the present findings call into question the efficacy of intensive antithrombotic therapy after TAVR, a hypothesis that warrants randomized trial investigation. Careful attention to choice of antithrombotic therapies is needed to balance ischemic and bleeding risks in this elderly postoperative population.21
Postoperative atrial fibrillation is associated with postprocedural stroke after open cardiac surgery,22,23 and a recent analysis from the TVT Registry has established a similar association between postoperative atrial fibrillation and post-TAVR stroke.24 Other studies have identified cardioembolic events from the left atrial appendage as a potential mechanism of post-TAVR stroke.25,26 Strategies to mitigate post-TAVR stroke in patients with atrial fibrillation are needed given that atrial fibrillation is a frequent comorbidity in TAVR candidates.24 Although this study did not identify a protective association with oral anticoagulant and 30-day neurologic risk, this analysis included both patients with and without atrial fibrillation with indications for oral anticoagulant therapy, so the association with oral anticoagulant therapy and 30-day post-TAVR stroke in patients with atrial fibrillation was not specifically examined. The WATCH-TAVR Trial (NCT03173534) is currently testing a strategy of TAVR with concomitant percutaneous left atrial appendage closure in patients with atrial fibrillation, which is a novel nonpharmacologic strategy to mitigate stroke risk after TAVR.
Limitations
This study has several limitations. First, this is a registry-based study with use of voluntary site-reported neurologic events. Second, the TVT Registry does not mandate routine neurologic assessment or neuroimaging of asymptomatic patients after TAVR. Therefore the TVT Registry may not capture clinically minor or asymptomatic cerebral embolic events. Given the voluntary nature of event reporting in the TVT registry, it is likely that neurologic event rates in this study underestimate the true post-TAVR event rates. Third, it is possible that the proportion of missing data on 30-day neurologic outcomes may have changed over time in the TVT registry, potentially confounding the trend analysis described above. Fourth, the propensity-matched analyses of the association of antithrombotic medical therapies with 30-day neurologic event rates focused only on patients with events after hospital discharge. Therefore, the association of these therapies with in-hospital strokes, which comprise a majority of 30-day strokes, was not assessed in this study. Fifth, this study focused on early neurologic events after TAVR, and the association of medical therapy with long-term neurologic risk after TAVR is beyond the scope of this analysis.
Conclusions
Between 2011 and 2017, the rate of 30-day stroke after transcatheter aortic valve replacement in a US registry population remained stable.
eFigure 1. Study Population
eFigure 2. TAVR Access Type by Year
eFigure 3. 30-Day Neurologic Events Stratified by Access Type
eFigure 4. 30-Day Stroke by Year Stratified by Access Type
eTable 1. TVT Registry Neurologic Endpoint Definitions
eTable 2. Variable List for Analysis Purpose
eTable 3. 30-Day Stroke Rates in Key Subgroups
eTable 4. 30-Day Neurologic Event Rates by Year
eTable 5. 30-Day Stroke Rates by Year Stratified by Access Type
eTable 6. Adjudication of Neurologic Events and Relation to Health Status
eTable 7. Characteristics of Propensity Score Matched Patients on Dual Antiplatelet Therapy (Femoral Cohort)
eTable 8. Characteristics of Propensity Score Matched Patients on Oral Anticoagulant Therapy (Femoral Cohort)
eTable 9. Characteristics of Propensity Score Matched Patients on Dual Antiplatelet Therapy vs. Oral Anticoagulant Therapy (Femoral Cohort)
eTable 10. Characteristics of Propensity Score Matched Patients on Dual Antiplatelet Therapy (Non-femoral Cohort)
eTable 11. Characteristics of Propensity Score Matched Patients on Oral Anticoagulant Therapy (Non-femoral Cohort)
eTable 12. Characteristics of Propensity Score Matched Patients on Dual Antiplatelet Therapy vs. Oral Anticoagulant Therapy (Non-femoral Cohort)
eTable 13. Propensity Matched Neurologic Outcomes (Femoral Cohort)
eTable 14. Propensity Matched Neurologic Outcomes (Non-femoral Cohort)
References
- 1.Leon MB, Smith CR, Mack M, et al. ; PARTNER Trial Investigators . Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. ; PARTNER Trial Investigators . Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 3.Adams DH, Popma JJ, Reardon MJ, et al. ; U.S. CoreValve Clinical Investigators . Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790-1798. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack MJ, et al. ; PARTNER 2 Investigators . Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609-1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 5.Reardon MJ, Van Mieghem NM, Popma JJ, et al. ; SURTAVI Investigators . Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321-1331. doi: 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 6.Kapadia SR, Huded CP, Kodali SK, et al. ; PARTNER Trial Investigators . Stroke after surgical versus transfemoral transcatheter aortic valve replacement in the PARTNER Trial. J Am Coll Cardiol. 2018;72(20):2415-2426. doi: 10.1016/j.jacc.2018.08.2172 [DOI] [PubMed] [Google Scholar]
- 7.Durko AP, Reardon MJ, Kleiman NS, et al. Neurological complications after transcatheter versus surgical aortic valve replacement in intermediate-risk patients. J Am Coll Cardiol. 2018;72(18):2109-2119. doi: 10.1016/j.jacc.2018.07.093 [DOI] [PubMed] [Google Scholar]
- 8.Kapadia S, Agarwal S, Miller DC, et al. Insights into timing, risk factors, and outcomes of stroke and transient ischemic attack after transcatheter aortic valve replacement in the PARTNER Trial (Placement of Aortic Transcatheter Valves) [published online September 6, 2016]. Circ Cardiovasc Interv. 2016;9(9):e002981. doi: 10.1161/CIRCINTERVENTIONS.115.002981 [DOI] [PubMed] [Google Scholar]
- 9.Kleiman NS, Maini BJ, Reardon MJ, et al. ; CoreValve Investigators . Neurological events following transcatheter aortic valve replacement and their predictors: a report from the CoreValve Trials [published online September 6, 2016]. Circ Cardiovasc Interv. 2016;9(9):e003551. doi: 10.1161/CIRCINTERVENTIONS.115.003551 [DOI] [PubMed] [Google Scholar]
- 10.Grover FL, Vemulapalli S, Carroll JD, et al. ; STS/ACC TVT Registry . 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69(10):1215-1230. doi: 10.1016/j.jacc.2016.11.033 [DOI] [PubMed] [Google Scholar]
- 11.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60(15):1438-1454. doi: 10.1016/j.jacc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 12.Edwards FH, Cohen DJ, O’Brien SM, et al. ; Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry . Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1(1):46-52. doi: 10.1001/jamacardio.2015.0326 [DOI] [PubMed] [Google Scholar]
- 13.Jones BM, Krishnaswamy A, Tuzcu EM, et al. Matching patients with the ever-expanding range of TAVI devices. Nat Rev Cardiol. 2017;14(10):615-626. doi: 10.1038/nrcardio.2017.82 [DOI] [PubMed] [Google Scholar]
- 14.Carroll JD, Vemulapalli S, Dai D, et al. Procedural experience for transcatheter aortic valve replacement and relation to outcomes: the STS/ACC TVT Registry. J Am Coll Cardiol. 2017;70(1):29-41. doi: 10.1016/j.jacc.2017.04.056 [DOI] [PubMed] [Google Scholar]
- 15.Kapadia SR, Kodali S, Makkar R, et al. ; SENTINEL Trial Investigators . Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 2017;69(4):367-377. doi: 10.1016/j.jacc.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 16.Giustino G, Sorrentino S, Mehran R, Faggioni M, Dangas G. Cerebral embolic protection during TAVR: a clinical event meta-analysis. J Am Coll Cardiol. 2017;69(4):465-466. doi: 10.1016/j.jacc.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 17.Otto CM, Kumbhani DJ, Alexander KP, et al. 2017 ACC expert consensus decision pathway for transcatheter aortic valve replacement in the management of adults with aortic stenosis: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2017;69(10):1313-1346. doi: 10.1016/j.jacc.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 18.Ahmad Y, Demir O, Rajkumar C, et al. Optimal antiplatelet strategy after transcatheter aortic valve implantation: a meta-analysis. Open Heart. 2018;5(1):e000748. doi: 10.1136/openhrt-2017-000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hioki H, Watanabe Y, Kozuma K, et al. ; OCEAN-TAVI investigators . Pre-procedural dual antiplatelet therapy in patients undergoing transcatheter aortic valve implantation increases risk of bleeding. Heart. 2017;103(5):361-367. doi: 10.1136/heartjnl-2016-309735 [DOI] [PubMed] [Google Scholar]
- 20.Rodés-Cabau J, Masson JB, Welsh RC, et al. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon-expandable valve: the ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) randomized clinical trial. JACC Cardiovasc Interv. 2017;10(13):1357-1365. doi: 10.1016/j.jcin.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 21.Windecker S, Tijssen J, Giustino G, et al. Trial design: rivaroxaban for the prevention of major cardiovascular events after transcatheter aortic valve replacement: rationale and design of the GALILEO study. Am Heart J. 2017;184:81-87. doi: 10.1016/j.ahj.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 22.Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43(5):742-748. doi: 10.1016/j.jacc.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 23.Burgess DC, Kilborn MJ, Keech AC. Interventions for prevention of post-operative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. Eur Heart J. 2006;27(23):2846-2857. doi: 10.1093/eurheartj/ehl272 [DOI] [PubMed] [Google Scholar]
- 24.Vora AN, Dai D, Matsuoka R, et al. Incidence, management, and associated clinical outcomes of new-onset atrial fibrillation following transcatheter aortic valve replacement: an analysis from the STS/ACC TVT Registry. JACC Cardiovasc Interv. 2018;11(17):1746-1756. doi: 10.1016/j.jcin.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 25.Palmer S, Child N, de Belder MA, Muir DF, Williams P. Left atrial appendage thrombus in transcatheter aortic valve replacement: incidence, clinical impact, and the role of cardiac computed tomography. JACC Cardiovasc Interv. 2017;10(2):176-184. doi: 10.1016/j.jcin.2016.10.043 [DOI] [PubMed] [Google Scholar]
- 26.Williams PD, de Belder MA, Maredia N, Muir DF. Embolization of left atrial appendage thrombus during transcatheter aortic valve replacement: a potential mechanism of periprocedural stroke. JACC Cardiovasc Interv. 2015;8(13):1770-1771. doi: 10.1016/j.jcin.2015.07.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study Population
eFigure 2. TAVR Access Type by Year
eFigure 3. 30-Day Neurologic Events Stratified by Access Type
eFigure 4. 30-Day Stroke by Year Stratified by Access Type
eTable 1. TVT Registry Neurologic Endpoint Definitions
eTable 2. Variable List for Analysis Purpose
eTable 3. 30-Day Stroke Rates in Key Subgroups
eTable 4. 30-Day Neurologic Event Rates by Year
eTable 5. 30-Day Stroke Rates by Year Stratified by Access Type
eTable 6. Adjudication of Neurologic Events and Relation to Health Status
eTable 7. Characteristics of Propensity Score Matched Patients on Dual Antiplatelet Therapy (Femoral Cohort)
eTable 8. Characteristics of Propensity Score Matched Patients on Oral Anticoagulant Therapy (Femoral Cohort)
eTable 9. Characteristics of Propensity Score Matched Patients on Dual Antiplatelet Therapy vs. Oral Anticoagulant Therapy (Femoral Cohort)
eTable 10. Characteristics of Propensity Score Matched Patients on Dual Antiplatelet Therapy (Non-femoral Cohort)
eTable 11. Characteristics of Propensity Score Matched Patients on Oral Anticoagulant Therapy (Non-femoral Cohort)
eTable 12. Characteristics of Propensity Score Matched Patients on Dual Antiplatelet Therapy vs. Oral Anticoagulant Therapy (Non-femoral Cohort)
eTable 13. Propensity Matched Neurologic Outcomes (Femoral Cohort)
eTable 14. Propensity Matched Neurologic Outcomes (Non-femoral Cohort)