Abstract
Background
Global dietary recommendations for and cardiovascular effects of linoleic acid, the major dietary omega-6 fatty acid, and its major metabolite, arachidonic acid, remain controversial. To address this uncertainty and inform international recommendations, we evaluated how in vivo circulating and tissue levels of linoleic acid (LA) and arachidonic acid (AA) relate to incident cardiovascular disease (CVD) across multiple international studies.
Methods
We performed harmonized, de novo, individual-level analyses in a global consortium of 30 prospective observational studies from 13 countries. Multivariable-adjusted associations of circulating and adipose tissue LA and AA biomarkers with incident total CVD and subtypes (coronary heart disease (CHD), ischemic stroke, cardiovascular mortality) were investigated according to a prespecified analytical plan. Levels of LA and AA, measured as % of total fatty acids, were evaluated linearly according to their interquintile range (i.e., the range between the mid-point of the first and fifth quintiles), and categorically by quintiles. Study-specific results were pooled using inverse-variance weighted meta-analysis. Heterogeneity was explored by age, sex, race, diabetes, statin use, aspirin use, omega-3 levels, and fatty acid desaturase 1 genotype (when available).
Results
In 30 prospective studies with medians of follow-up ranging 2.5 to 31.9 years, 15,198 incident cardiovascular events occurred among 68,659 participants. Higher levels of LA were significantly associated with lower risks of total CVD, cardiovascular mortality, and ischemic stroke, with hazard ratios per interquintile range of 0.93 (95% CI: 0.88–0.99), 0.78 (0.70–0.85), and 0.88 (0.79–0.98), respectively, and nonsignificantly with lower CHD risk (0.94; 0.88–1.00). Relationships were similar for LA evaluated across quintiles. AA levels were not associated with higher risk of cardiovascular outcomes; comparing extreme quintiles, higher levels were associated with lower risk of total CVD (0.92; 0.86–0.99). No consistent heterogeneity by population subgroups was identified in the observed relationships.
Conclusions
In pooled global analyses, higher in vivo circulating and tissue levels of LA and possibly AA were associated with lower risk of major cardiovascular events. These results support a favorable role for LA in CVD prevention.
Keywords: Linoleic acid, Arachidonic acid, Pooled analysis, Cardiovascular Disease, Diet and Nutrition, Epidemiology, Primary Prevention, Biomarkers
INTRODUCTION
Recommendations for dietary consumption omega-6 (n-6) polyunsaturated fatty acids (PUFA) for cardiovascular disease (CVD) prevention remain controversial and inconsistent.1 For example, the American Heart Association and the Academy of Nutrition and Dietetics recommend 5–10%,1, 2 the United Nations Food and Agriculture Organization recommends 2.5–9%,3 while the French national guidelines recommend 4%.4 Pooled evidence from clinical trials and cohort studies suggests a moderate benefit of consuming n-6 PUFA, predominantly linoleic acid (LA, 18:2n-6), for coronary heart disease (CHD) risk, whether replacing saturated fat or total carbohydrate.5–7 In contrast, recent secondary analyses of clinical trials of LA-rich corn oil (although not LA-rich soybean oil) conducted in the 1960s-1970s suggest a possible increased risk of overall and CHD mortality.8, 9 The interpretation of these latter trials is hampered by their short duration,8, 9 small numbers of events,8 substantial drop-out,9 and confounding by industrial trans-fats.8, 9 In addition, many of the other prior trials are limited by lack of blinding or randomization, and major dietary pattern shifts; and most are decades old, creating potentially low generalizability to contemporary diets and clinical settings. Cohort studies are limited by the common reliance on self-reported dietary habits, which can be influenced by memory errors and inaccurate nutrient databases. Thus, for many scientists, clinicians, and policy makers, the role of LA in CVD risk remains uncertain.
In addition, concerns have been raised that n-6 PUFA could actually increase CVD risk, due to potential pro-inflammatory effects.9, 10 LA is a precursor of the n-6 PUFA arachidonic acid (AA, 20:4n-6), which gives rise to a range of eicosanoids considered to be pro-inflammatory and pro-thrombotic.10, 11Yet, stable isotope studies suggest very limited conversion of LA to AA in humans,12 and trials show limited effects of increasing dietary LA on plasma and adipose tissue AA levels.12–14 These findings indicate the importance of directly evaluating AA levels instead of inferring them from LA levels or intakes in relation to CVD risk. As LA cannot be produced endogenously (making tissue levels reasonable markers of intake), biomarker (circulating and adipose tissue) levels correlate with dietary consumption.15, 16 Such objective biomarkers allow evaluation of dietary exposure of LA status independent of self-reported food habits and estimated nutrient composition of different foods. Circulating and adipose biomarkers also allow direct evaluation of AA, which is highly metabolically regulated and for which dietary estimates correlate poorly with in vivo levels.
Yet, the relations between in vivo levels of LA and AA and CHD risk have been evaluated in relatively few studies, with different study designs, outcomes, exposures (e.g., lipid compartment), covariates, and statistical methodology. Results from meta-analyses of published studies using circulating or adipose tissue levels of n-6 PUFA have been contradictory.17, 18 Furthermore, associations between in vivo n-6 PUFA levels and other CVD outcomes including stroke, total CVD, and CVD mortality have been studied less frequently19–23 and remain uncertain.
To address these major gaps in knowledge, we conducted a pooled analysis of harmonized, de novo, individual-level data across 30 cohort studies in the Fatty Acid and Outcome Research Consortium (FORCE) to evaluate associations of LA and AA levels with incident total CVD and subtypes (CHD, ischemic stroke, CVD mortality).
METHODS
Data Availability
The institutional review board approvals and data sharing agreements for the participating cohorts allowed us to share cohort results. Individual participant data are owned by individual participating cohorts and are available to researchers consented from participating cohorts. For further queries or requests, please contact force@tufts.edu. Further details are available at the FORCE website: http://force.nutrition.tufts.edu/.
Study setting and population: FORCE Consortium
The study was conducted within FORCE (http://force.nutrition.tufts.edu), a consortium of studies with circulating or adipose tissue fatty acid biomarker measurements and ascertained chronic disease events.24 Studies were identified and invited to participate if assessing biomarker (circulating or adipose tissue) levels of LA and AA, and incident CVD (or subtypes thereof), based on previous FORCE projects,24, 25 expert contacts, and online searches. Studies with adult participants (≥18 y) free of CVD (myocardial infarction, angina, coronary revascularization, stroke) at the time of fatty acid sampling were invited. Retrospective case-control studies were included in a sensitivity analysis if fatty acids were assessed in adipose tissue, which have a long half-life of exposure.26 To minimize potential reverse causation, the main analysis included only prospective studies. Of 38 studies invited by September 2017, 31 participated (Table 1 and Supplemental Tables 1–2 in the online-only Data Supplement), while 7 were ineligible, declined to participate, or failed to respond (Supplemental Table 3 in the online-only Data Supplement). The study was approved by the institutional review boards of the participating cohorts.
Table 1.
Characteristics of 31 studies and baseline characteristics of individual study participants with linoleic acid (LA; 18:2n6) and arachidonic acid (AA; 20:4n6) biomarker measures and follow-up for cardiovascular disease incidence or mortality.*
| Study† | Country | Study design‡ | Age, y (mean) | Sex (% male) | BMI, kg/m2 (mean) | Biomarker compartment§ | Year of biomarker sampling | Outcome assessed|| |
|---|---|---|---|---|---|---|---|---|
| AGES-Reykjavik | Iceland | PC | 77 | 39 | 27.1 | PP | 2002–2006 | All# |
| ARIC | USA | PC | 54 | 52 | 27.0 | PP | 1987–1989 | All |
| CCCC | Taiwan | PC | 61 | 55 | 23.3 | TP | 1992–2000 | All |
| CHS | USA | PC | 73 | 36 | 26.7 | PP | 1992–1993 | All |
| CRS | Costa Rica | RCC | 58 | 73 | 26.2 | AT | 1994–2004 | Non-fatal MI |
| DCH | Denmark | PNC | 57 | 61 | 26.6 | AT** | 1993–1997 | Total CHD |
| EPIC-Norfolk | UK | PCC | 63 | 49 | 26.5 | PP | 1993–1997 | All |
| EPIC-Potsdam | Germany | PC | 50 | 37 | 26.0 | RBC | 1994–1998 | Total CVD |
| FHS | USA | PC | 66 | 43 | 28.2 | RBC | 2005–2008 | All |
| HPFS | USA | PCC | 65 | 100 | 25.8 | RBC, TP | 1993–1995 | Total CVD, CHD, & stroke |
| HS | Japan | PC | 61 | 42 | 23.1 | TP | 2002–2003 | All |
| KIHD | Finland | PC | 52 | 100 | 26.7 | TP | 1984–1989 | All |
| MCCS | Australia | PC | 56 | 46 | 27.2 | PP | 1990–1994 | Fatal CVD, CHD, & ischemic stroke |
| MESA | USA | PC | 62 | 47 | 28.3 | PP | 2000–2002 | All |
| METSIM | Finland | PC | 55 | 100 | 26.5 | CE, PP, RBC | 2006–2010 | Total CVD |
| MORGEN (CHD) | Netherlands | PCC | 52 | 79 | 26.2 | CE | 1993–1997 | Fatal CHD |
| MORGEN (Stroke) | Netherlands | PCC | 50 | 53 | 25.9 | CE | 1993–1997 | Ischemic stroke |
| MPCDRF | Netherlands | PCC | 51 | 70 | 25.9 | CE | 1987–1991 | Fatal CHD |
| NHS | USA | PCC | 60 | 0 | 25.6 | RBC, TP | 1989–1990 | Total CVD, CHD & stroke |
| NSHDS I | Sweden | PCC | 54 | 79 | 26.2 | PP | 1987–1994 | Total CHD |
| NSHDS II | Sweden | PCC | 54 | 76 | 26.4 | PP | 1987–1999 | Total CHD |
| NSHDS III | Sweden | PCC | 55 | 61 | 26.7 | PP | 1987–1995 | Ischemic stroke |
| PHS | USA | PCC | 69 | 100 | 25.7 | RBC | 1995–2001 | Total CHD |
| PIVUS | Sweden | PC | 70 | 47 | 26.9 | CE, PP | 2001–2004 | All |
| SCHS | Singapore | PCC | 66 | 65 | 23.0 | TP | 1994–2005 | Total CHD |
| SHHEC | UK | PC | 49 | 52 | 25.6 | AT | 1985–1986 | All |
| 60YO | Sweden | PC | 60 | 48 | 26.8 | CE | 1997–1998 | All |
| 3C Study | France | PC | 75 | 39 | 26.0 | TP | 1999–2000 | All |
| ULSAM-50†† | Sweden | PC | 50 | 100 | 25.0 | CE | 1970–1973 | All |
| ULSAM-70†† | Sweden | PC | 71 | 100 | 26.4 | AT | 1991–1995 | All |
| WHIMS | USA | PC | 70 | 0 | 28.2 | RBC | 1996 | All |
AA, arachidonic acid; BMI, body mass index; LA, linoleic acid.
AGES-Reykjavik: Age, gene/environment susceptibility – Reykjavik Study; ARIC: Atherosclerosis Risk in Communities; CCCC: Chin-Shan Community Cardiovascular Cohort Study; CHS: Cardiovascular Health Study; CRS: Costa Rica study on adults; DCH: Diet, Cancer, and Health study; EPIC: European Prospective Investigation into Cancer; FHS: Framingham Heart Study; HPFS: Health Professionals Follow-up Study; HS: The Hisayama Study; KIHD: Kuopio Ischaemic Heart Disease Risk Factor Study; MCCS: Melbourne Collaborative Cohort Study; MESA: Multi-Ethnic Study of Atherosclerosis; METSIM: Metabolic syndrome in men study; MORGEN: Monitoring Project on Risk Factors for Chronic Diseases; MPCDRF: Monitoring Project on Cardiovascular Disease Risk Factors; NHS I: Nurses’ Health Study I; NSHDS I-III: Northern Sweden Health and Disease Study; PHS: Physicians’ Health Study; PIVUS: Prospective Investigation of the Vasculature in Uppsala Seniors; SCHS, Singapore Chinese Health Study; SHHEC, Scottish Heart Health Extended Cohort; 60YO, 60-year-old Swedish men and women; 3C Study: Three City Study; ULSAM-50 &−70: Uppsala Longitudinal Study of Adult Men investigations at ages 50 y and 70 y, respectively.
PC, prospective cohort; PCC, prospective nested case-control; PNC, prospective nested case-cohort; RCC, retrospective case-control.
AT, adipose tissue; CE, cholesterol ester; PP, plasma phospholipid; RBC, erythrocyte phospholipid; TP, total plasma.
CVD, cardiovascular disease; CHD, coronary heart disease; MI, myocardial infarction.
All specified outcomes (total CVD, CVD mortality, total CHD, and ischemic stroke) were assessed.
In DCH, the association of adipose tissue arachidonic acid, but not linoleic acid, with total CHD was evaluated.
Fatty acids were measured in cholesterol ester and adipose tissue at the first and third ULSAM investigation, respectively.
Fatty acid measurements
Studies measured fatty acids in differing compartments, including plasma phospholipids, erythrocytes, plasma, serum, cholesterol esters, and adipose tissue. All fatty acid levels were reported as percent of total fatty acids. Detailed information regarding fatty acid measurements in each study is provided in the Supplemental Material.
Outcome assessment
In each cohort, study participants were excluded if they were children (age <18 years) or had prevalent CVD at the time of fatty acid measurement. Among the remaining participants, we evaluated incident CVD (defined as incident CHD or stroke) and its subtypes including CHD (fatal or nonfatal myocardial infarction, CHD death, or sudden cardiac death), ischemic stroke (fatal or nonfatal ischemic stroke), and CVD mortality (the subset of fatal events from these causes). Studies that did not separately assess ischemic stroke used total stroke (n=5 studies). Detailed information on outcomes in each study is provided in the Supplemental Material.
Covariates
To minimize potential confounding, prespecified and harmonized covariates were utilized included age (years), sex (male/female), race (Caucasian/non-Caucasian, or study-specific), field center if applicable (categories), body-mass index (BMI, kg/m2), education (less than high school graduate, high school graduate, some college or vocational school, college graduate), smoking (current, former, never; if history not assessed, then current/not current), physical activity (quintiles of metabolic equivalents (METs)/week), alcohol intake (none, 1–6 drinks/week, 1–2 drinks/day, >2 drinks/day), prevalent diabetes mellitus (defined as treatment with oral antihyperglycemic agents, insulin, or fasting plasma glucose >126 mg/dL), treated hypertension (defined as hypertension drug use; or if unavailable, as diagnosed/history of hypertension), treated hypercholesterolemia (defined as LDL-lowering drug use; if unavailable, as diagnosed/history of hypercholesterolemia), regular aspirin use (defined as ≥2 times/week), levels of α-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3), sum of trans isomers of oleic acid (trans18:1), and sum of trans isomers of LA (trans-18:2) (each expressed as % total FAs). If data did not allow such categorization, study-specific categories were used. Imputation was allowed for linear covariates if previously established in each cohort; missing indicator categories were utilized for missing covariate data in categories.
Statistical analysis and pooling
All participating studies followed a prespecified, harmonized analysis protocol with standardized exclusions, exposures, outcomes, covariates, and analytical methods. In each study, de novo analyses of individual data were performed according to the protocol. Cox and weighted Cox proportional hazards models were used to estimate hazard ratios in cohort and nested unmatched case-cohort studies, respectively, with follow-up from the date of blood or adipose tissue sampling to date of incident event, death, loss to follow-up, or end of follow-up. In matched nested case-control studies, conditional logistic regression was used to estimate odds-ratios for each outcome, considered to approximate hazard ratios. To assess potential nonlinear associations, each cohort also evaluated study-specific quintiles as indicator categories, with the lowest quintile as the reference. Studies assessing fatty acids in multiple compartments conducted separate analyses in each compartment. To investigate potential heterogeneity by other factors, associations in each study were also assessed in prespecified strata by age, sex, race, ALA and EPA levels, prevalent diabetes, drug-treated hypercholesterolemia, and regular aspirin use. Potential interactions by genotype were examined in the 14 studies with available data for rs174547 (single nucleotide polymorphism in the gene for fatty acid desaturase 1, a major genetic determinant of circulating LA and AA).27 Interaction terms were constructed as a cross-product of LA or AA and rs174547 (as an additive effect: 0, 1, or 2 T-alleles) and included with the main effects in the models. Robust variance was used in all analyses.
Results from each study were provided to the lead author in standardized electronic forms and pooled using inverse-variance weighted meta-analysis. The results were pooled overall and within each specific type of fatty acid compartment including phospholipids (erythrocyte phospholipids or plasma phospholipids), total plasma, cholesterol esters, and adipose tissue. To allow comparison and pooling of results across different compartments, LA and AA concentrations were standardized to study-specific interquintile range defined as the range between the midpoint of the first and fifth quintiles (i.e., range between 10th and 90th percentiles). Potential semi-parametric associations were assessed by meta-regression with restricted cubic splines constructed from study-specific quintiles.28
Overall heterogeneity was assessed by the I2-statistic, with values of ~ 25%, 50%, and 75%, considered to indicate low, medium, and high heterogeneity, respectively.29 Heterogeneity between prespecified subgroups was explored by meta-analyzing study-specific effect estimates from each stratum, with statistical differences between subgroups tested by meta-regression. Potential interactions by desaturase genotype were examined by meta-analyzing study-specific interaction terms. For each study, associations of n-6 PUFA with CVD per genotype at rs174547 (i.e, CC, CT, or TT) were calculated from beta coefficients and the variance-covariance matrix of the main and interaction terms.24 The genotype-specific estimates were pooled using pooled using inverse-variance weighted meta-analysis. While subgroups were prespecified, all heterogeneity analyses were considered exploratory and Bonferroni-corrected for multiple comparisons (10 subgroups; corrected α=0.005).
In sensitivity analyses, we evaluated compartment-specific associations using absolute percent of total fatty acids as the unit of exposure, instead of study-specific interquintile range. In other sensitivity analyses, we censored events at maximum 10 y of follow-up, to minimize bias by changes in fatty acid levels over time; used alternative blood compartments in the overall pooled analysis for studies having more than one measure; included one retrospective study; and excluded studies assessing only fatal outcomes.
Meta-analyses were performed using Stata 13 (StataCorp, College Station, TX), with two-tailed α=0.05 for the primary analyses.
RESULTS
The pooled analyses included 76,356 fatty acid measurements from 68,659 participants in 30 prospective studies from 13 countries (Table 1). The studies included 18 cohort and 12 nested case-control or case-cohort studies. Most studies assessed fatty acids in blood compartments (plasma phospholipids, n=11 studies; erythrocyte phospholipids, total plasma, or cholesterol esters, n=7 studies each), while adipose tissue was less commonly used (n=3 studies). One retrospective case-control study measuring adipose tissue biomarkers was included in a sensitivity analysis, but not in the primary analyses.
Across studies, mean age at baseline ranged from 49 to 77 years (Table 1 and Supplemental Table 4). Overall proportions of women and men were comparable, although some studies included one sex only (Table 1). Most participants were Caucasian, but several studies included sizable numbers of African Americans, Asians, and Hispanics (Supplemental Table 5). In most studies, up to 30% of the participants smoked, and alcohol intake was generally moderate (<1 drink/d). Education level, diabetes prevalence, and medication use varied across studies. As would be expected, levels of fatty acids varied between different compartments (Figure 1 and Supplemental Tables 4 and 6).
Figure 1. Concentration of A) linoleic acid (LA; 18:2n6) and B) arachidonic acid (AA; 20:4n6) across different biomarker compartments measured in the 31 contributing studies.
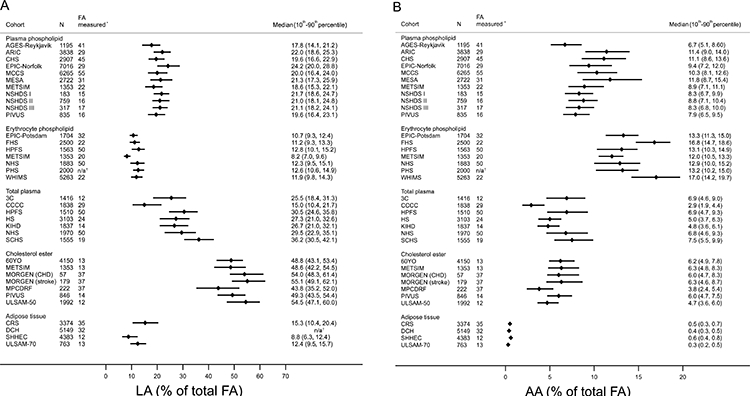
Concentrations of arachidonic acid and linoleic acid concentrations are expressed as % of total fatty acids (FA), and indicated as median (circles) and interquintile range (lines; defined as the range between the midpoint of the bottom quintile [10th percentile] and the top quintile [90th percentile]), respectively. For MPCDRF and the MORGEN, values are only shown for controls.*Total number of individual FA measured in the biomarker compartment. †Not reported.
Median study follow-up durations ranged from 2.5 to 31.9 years. Among the 30 prospective studies, 10,477 total incident CVD events, 4,508 CVD deaths, 11,857 incident CHD events, and 3,705 incident ischemic strokes occurred (Supplemental Table 7).
Per interquintile range, higher LA levels were associated with 7% (95%CI: 1–12%), 22% (15–30%), and 12% (2–21%) lower incidence of total CVD, CVD mortality, and ischemic stroke, respectively (Figures 2–3, Table 2). LA levels were also nonsignificantly (P=0.065) associated with lower incidence of total CHD. Overall heterogeneity was moderate (I2=28–63%). Associations of LA with total CVD, total CHD, and CVD mortality varied by compartment (P-interaction≤0.031), with generally less prominent inverse associations in studies utilizing phospholipids (Figures 2–3).
Figure 2. Associations of linoleic acid (LA; 18:2n6) with total CVD (A) and CVD mortality (B) in pooled analysis of 30 prospective studies.
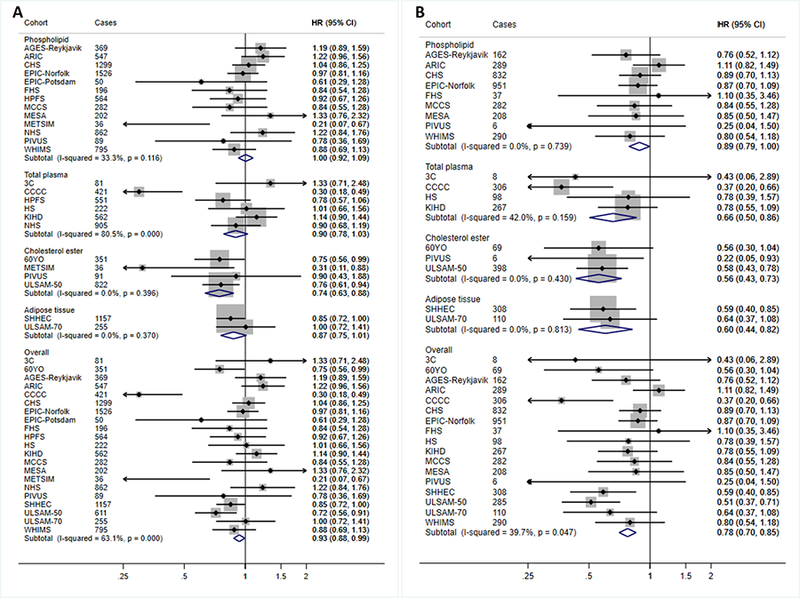
Study-specific estimates for hazard ratio (HR) per interquintile range (i.e., range between the midpoint of the bottom quintile [10th percentile] and the top quintile [90th percentile]) of biomarker linoleic acid were pooled based on the following order: 1) adipose tissue, 2) erythrocyte phospholipid, 3) plasma phospholipid 4) cholesterol ester, and 5) total plasma. Study weights are indicated (grey squares) by individual biomarker compartment and overall. Study-specific analyses were conducted using models that included the following covariates: age (years), sex (male/female), race (Caucasian/non-Caucasian, or study-specific), field center if applicable (categories), body-mass index (BMI, kg/m2), education (less than high school graduate, high school graduate, some college or vocational school, college graduate), smoking (current, former, never; if history not assessed, then current/not current), physical activity (quintiles of metabolic equivalents (METs)/week), alcohol intake (none, 1–6 drinks/week, 1–2 drinks/day, >2 drinks/day), prevalent diabetes mellitus (defined as treatment with oral antihyperglycemic agents, insulin, or fasting plasma glucose >126 mg/dL), treated hypertension (defined as hypertension drug use; or if unavailable, as diagnosed/history of hypertension), treated hypercholesterolemia (defined as LDL-lowering drug use; if unavailable, as diagnosed/history of hypercholesterolemia), regular aspirin use (defined as ≥2 times/week), levels of α-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3), sum of trans isomers of oleic acid (trans18:1), and sum of trans isomers of LA (trans-18:2) (each expressed as % total FAs). If data did not allow such categorization, study-specific categories were used. See Table 1 footnote for abbreviations of cohorts.
Figure 3. Associations of linoleic acid (LA; 18:2n6) with total CHD (A) and ischemic stroke (B) in pooled analysis of 30 prospective studies.
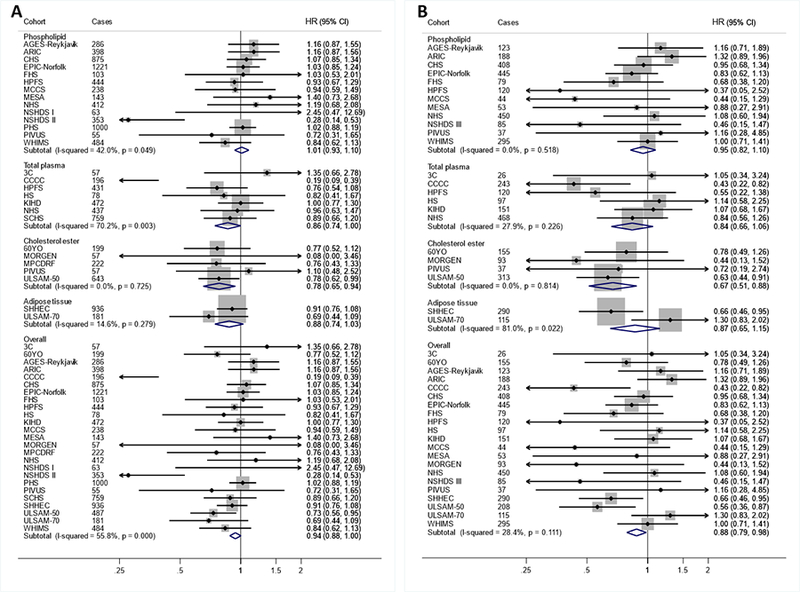
Study-specific estimates for hazard ratio (HR) per interquintile range (i.e., range between the midpoint of the bottom quintile [10th percentile] and the top quintile [90th percentile]) of biomarker linoleic acid were pooled based on the following order: 1) adipose tissue, 2) erythrocyte phospholipid, 3) plasma phospholipid 4) cholesterol ester, and 5) total plasma. Study weights are indicated (grey squares) by individual biomarker compartment and overall. Study-specific analyses were conducted using models that included the following covariates: age (years), sex (male/female), race (Caucasian/non-Caucasian, or study-specific), field center if applicable (categories), body-mass index (BMI, kg/m2), education (less than high school graduate, high school graduate, some college or vocational school, college graduate), smoking (current, former, never; if history not assessed, then current/not current), physical activity (quintiles of metabolic equivalents (METs)/week), alcohol intake (none, 1–6 drinks/week, 1–2 drinks/day, >2 drinks/day), prevalent diabetes mellitus (defined as treatment with oral antihyperglycemic agents, insulin, or fasting plasma glucose >126 mg/dL), treated hypertension (defined as hypertension drug use; or if unavailable, as diagnosed/history of hypertension), treated hypercholesterolemia (defined as LDL-lowering drug use; if unavailable, as diagnosed/history of hypercholesterolemia), regular aspirin use (defined as ≥2 times/week), levels of α-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3), sum of trans isomers of oleic acid (trans18:1), and sum of trans isomers of LA (trans-18:2) (each expressed as % total FAs). If data did not allow such categorization, study-specific categories were used. See Table 1 footnote for abbreviations of cohorts.
Table 2.
Risk of incident CVD according to objective biomarker levels of linoleic acid (18:2n6) and arachidonic acid (20:4n6) in 30 pooled prospective cohort studies
| Multivariable-adjusted hazard ratio (95% CI) per interquintile range† |
|||||
|---|---|---|---|---|---|
| Outcome | Biomarker | Studies (n) | Cases (n) | Linoleic acid | Arachidonic acid |
| Total CVD | Phospholipid | 14 | 6 853 | 1.00 (0.92–1.09) | 0.95 (0.87–1.03) |
| Total plasma | 6 | 2 742 | 0.90 (0.78–1.03) | 0.81 (0.70–0.94) | |
| Cholesterol esters | 4 | 1 300 | 0.74 (0.63–0.88) | 1.03 (0.88–1.20) | |
| Adipose tissue | 2 | 1 412 | 0.87 (0.75–1.01) | 0.98 (0.87–1.10) | |
| Overall‡ | 21 | 10 477 | 0.93 (0.88–0.99) | 0.95 (0.90–1.01) | |
| CVD mortality | Phospholipid | 9 | 3 057 | 0.89 (0.79–1.00) | 0.93 (0.83–1.05) |
| Total plasma | 4 | 679 | 0.66 (0.50–0.86) | 0.85 (0.66–1.09) | |
| Cholesterol esters | 3 | 473 | 0.56 (0.43–0.73) | 0.99 (0.76–1.29) | |
| Adipose tissue | 2 | 418 | 0.60 (0.44–0.82) | 1.02 (0.84–1.23) | |
| Overall‡ | 17 | 4 508 | 0.78 (0.70–0.85) | 0.94 (0.86–1.02) | |
| Total CHD | Phospholipid | 14 | 6 075 | 1.01 (0.93–1.10) | 0.96 (0.90–1.03) |
| Total plasma | 7 | 2 430 | 0.86 (0.74–1.00) | 0.86 (0.74–1.01) | |
| Cholesterol esters | 5 | 1 178 | 0.78 (0.65–0.94) | 1.02 (0.85–1.23) | |
| Adipose tissue | 3§ | 3 255 | 0.88 (0.74–1.03) | 1.10 (0.98–1.23) | |
| Overall‡ | 26§ | 11 857 | 0.94 (0.88–1.00) | 0.99 (0.94–1.04) | |
| Ischemic stroke | Phospholipid | 12 | 2 327 | 0.95 (0.82–1.10) | 0.98 (0.85–1.13) |
| Total plasma | 6 | 1 105 | 0.84 (0.66–1.06) | 0.93 (0.73–1.18) | |
| Cholesterol esters | 4 | 598 | 0.67 (0.51–0.88) | 1.13 (0.89–1.43) | |
| Adipose tissue | 2 | 405 | 0.87 (0.65–1.15) | 0.91 (0.74–1.11) | |
| Overall‡ | 21 | 3 705 | 0.88 (0.79–0.98) | 0.99 (0.90–1.10) | |
AA, arachidonic acid; CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; LA, linoleic acid.
Based on harmonized, de novo individual-level analyses in each cohort, pooled using inverse-variance weighted meta-analysis. Risk was assessed according to the interquintile range (i.e., range between the midpoint of the bottom quintile [10th percentile] and the top quintile [90th percentile]) of each fatty acid, corresponding to the difference between the midpoint of the first and fifth quintiless. Study-specific analyses were adjusted for age (years), sex (male/female), race (Caucasian/non-Caucasian, or study-specific), field or clinical center if applicable (study-specific categories), body-mass index (BMI, kg/m2), education (less than high school graduate, high school graduate, some college or vocational school, college graduate), smoking (current, former, or never; if former not assessed, then current or not current), physical activity (quintiles of metabolic equivalents (METs) per week; or if METs unavailable, quintiles of study-specific definitions of physical or leisure activity), alcohol intake (none, 1–6 drinks/week, 1–2 drink/day, >2 drink/day [14 g alcohol=1 standard drink]), diabetes mellitus (yes/no; defined as treatment with oral hypoglycemic agents, insulin, or fasting plasma glucose >126 mg/dL), treated hypertension (yes/no; defined as hypertension drug use; or if unavailable, as diagnosed/history of hypertension according to study-specific definitions), treated hypercholesterolemia (yes or no; defined as lipid-lowering drug use; if unavailable, as diagnosed/history of hypercholesterolemia according to study-specific definitions), regular aspirin use (yes/no), biomarker concentrations of α-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3), sum of trans-18:1 fatty acids, and sum of trans-18:2 fatty acids (each expressed as % total fatty acids).
For studies that assessed LA and AA levels in more than one biomarker compartment, the primary compartment for that study was pre-selected for pooled analyses based on the following order: 1) adipose tissue, 2) erythrocyte phospholipid, 3) plasma phospholipid 4) cholesterol ester, and 5) total plasma.
Because the Diet, Cancer and Health study assessed associations of AA, but not LA, with total CHD (n cases=2138), a total of, 2 studies (n cases= 1117) evaluated adipose tissue LA and 25 studies (n cases=9719) assessed any biomarker level of LA in relation to total CHD.
Compared to the lowest quintile, participants in the highest quintile of LA levels experienced lower risk of CVD mortality (HR=0.77; 95% CI, 0.69–0.86), with nonsignificant trends toward lower risk of total CVD (0.94; 0.87–1.01), CHD (0.92; 0.85–1.00), and ischemic stroke (0.90; 0.79–1.02) (Supplemental Table 8). There was no significant evidence of non-linear associations between LA and each outcome (P-nonlinearity>0.05 each).
AA levels evaluated linearly were not significantly associated with CVD events, with a hazard ratio of 0.95 (0.90–1.01) for total CVD (Table 2, Figures 4–5). When different lipid compartments were assessed, AA levels in total plasma, but not other compartments, were associated with lower risk of total CVD (HR=0.81 (0.70–0.94) (Table 2, Figure 4). Overall heterogeneity was low to moderate (I2≤54%). When AA levels were evaluated in quintiles (Supplemental Table 9), participants in the highest quintile, compared to the lowest, experienced significantly lower incidence of total CVD (0.92; 0.86–0.99). There was evidence for a borderline nonlinear association (P-nonlinearity=0.039) between total plasma AA and ischemic stroke (Supplemental Figure 1).
Figure 4. Associations of arachidonic acid (AA; 20:4n6) with total CVD (A) and CVD mortality (B) in pooled analysis of 30 prospective studies.
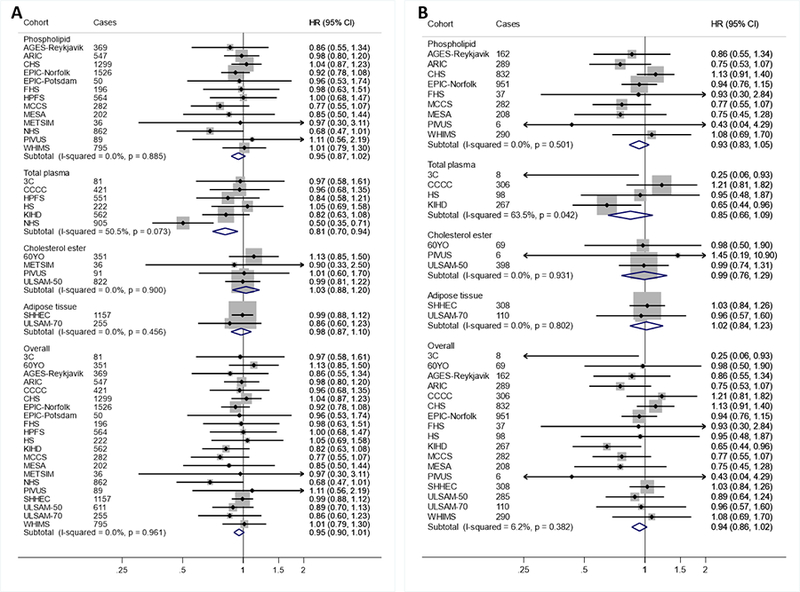
Study-specific estimates for hazard ratio (HR) per interquintile range (i.e., range between the midpoint of the bottom quintile [10th percentile] and the top quintile [90th percentile]) of biomarker linoleic acid were pooled based on the following order: 1) adipose tissue, 2) erythrocyte phospholipid, 3) plasma phospholipid 4) cholesterol ester, and 5) total plasma. Study weights are indicated (grey squares) by individual biomarker compartment and overall. Study-specific analyses were conducted using models that included the following covariates: age (years), sex (male/female), race (Caucasian/non-Caucasian, or study-specific), field center if applicable (categories), body-mass index (BMI, kg/m2), education (less than high school graduate, high school graduate, some college or vocational school, college graduate), smoking (current, former, never; if history not assessed, then current/not current), physical activity (quintiles of metabolic equivalents (METs)/week), alcohol intake (none, 1–6 drinks/week, 1–2 drinks/day, >2 drinks/day), prevalent diabetes mellitus (defined as treatment with oral antihyperglycemic agents, insulin, or fasting plasma glucose >126 mg/dL), treated hypertension (defined as hypertension drug use; or if unavailable, as diagnosed/history of hypertension), treated hypercholesterolemia (defined as LDL-lowering drug use; if unavailable, as diagnosed/history of hypercholesterolemia), regular aspirin use (defined as ≥2 times/week), levels of α-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3), sum of trans isomers of oleic acid (trans18:1), and sum of trans isomers of LA (trans-18:2) (each expressed as % total FAs). If data did not allow such categorization, study-specific categories were used. See Table 1 footnote for abbreviations of cohorts.
Figure 5. Associations of arachidonic acid (AA; 20:4n6) with total CHD (A) and ischemic stroke (B) in pooled analysis of 30 prospective studies.
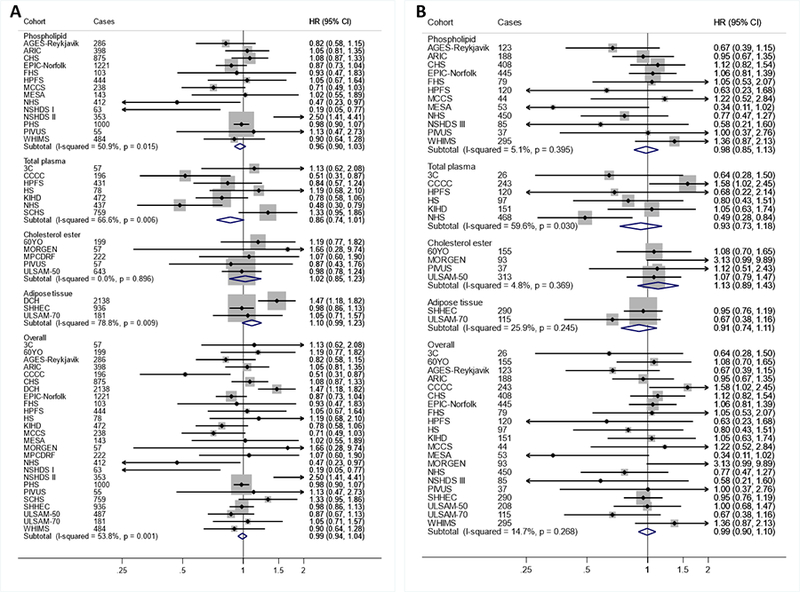
Study-specific estimates for hazard ratio (HR) per interquintile range (i.e., range between the midpoint of the bottom quintile [10th percentile] and the top quintile [90th percentile]) of biomarker linoleic acid were pooled based on the following order: 1) adipose tissue, 2) erythrocyte phospholipid, 3) plasma phospholipid 4) cholesterol ester, and 5) total plasma. Study weights are indicated (grey squares) by individual biomarker compartment and overall. Study-specific analyses were conducted using models that included the following covariates: age (linear), sex (male/female), race (binary: Caucasian/non-Caucasian, or study-specific), field or clinical center if applicable (study-specific categories), body-mass index (BMI, linear), education (less than high school graduate, high school graduate, some college or vocational school, college graduate), smoking (current, former, or never; if former not assessed, then current or not current), physical activity (quintiles of metabolic equivalents (METs) per week; or if METs unavailable, quintiles of study-specific definitions of physical or leisure activity), alcohol intake (none, 1–6 drinks/week, 1–2 drink/day, >2 drink/day [14 g alcohol=1 standard drink]), diabetes mellitus (yes or no; defined as treatment with oral hypoglycemic agents, insulin, or fasting plasma glucose >126 mg/dL), treated hypertension (yes or no; defined as hypertension drug use; or if unavailable, as diagnosed/history of hypertension according to study-specific definitions), treated hypercholesterolemia (yes or no; defined as lipid-lowering drug use; if unavailable, as diagnosed/history of hypercholesterolemia according to study-specific definitions), regular aspirin use (yes or no), biomarker concentrations of α-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3), sum of trans-18:1 fatty acids, and sum of trans-18:2 fatty acids (all linear; expressed as % total fatty acids). If data did not allow such categorization, study-specific categories were used. See Table 1 footnote for abbreviations of cohorts.
Associations of LA and AA with CVD outcomes did not significantly differ according to subgroups defined by age, sex, race, n-3 PUFA levels, diabetes status, statin use, aspirin use, or baseline year of fatty acid measurement (Supplemental Table 10). In 14 studies with genotype data (Supplemental Table 11), a significant interaction (P-interaction=0.002) was observed between LA and rs174547 genotype in relation to risk of ischemic stroke (Supplemental Table 12), with inverse associations appearing stronger in carriers of the major T-allele. The associations of AA with cardiovascular outcomes did not significantly vary by rs174547 genotype.
In sensitivity analyses, results of compartment-specific analysis that utilized units of percent of total fatty acids, rather than study-specific interquintile ranges, were not appreciably different from the main findings (Supplemental Table 13). Results were also similar across all other sensitivity analyses (Supplemental Table 14).
DISCUSSION
In this harmonized, individual-level pooled analysis across 30 prospective studies from 13 countries, higher in vivo levels of the n-6 PUFA LA were associated with lower risk of CVD events, in particular CVD mortality and stroke. AA levels were not associated with higher risk, and were associated with lower CVD risk in some analyses. To our knowledge, this is the largest pooled analysis of fatty acid levels and CVD endpoints, including almost 70,000 individuals and 10,000 total CVD events.
Our findings provide evidence to help inform currently inconsistent global dietary recommendations on n-6 PUFA consumption. LA, an essential fatty acid not synthesized by humans, is the main dietary PUFA, comprising about 85–90% of the total. While circulating and adipose tissue LA levels can be influenced by metabolism,27, 30 they are established and useful markers of diet as they increase in a dose-response manner in response to dietary LA in controlled feeding trials15, 26, 30 and consistently correlate with self-reported dietary estimates in large cohort studies,26 including a considerable number of studies participating in the current analysis (Supplemental Table 15). Several lines of evidence support mechanisms by which dietary LA may reduce CVD. In randomized controlled feeding trials, dietary PUFA (primarily LA) as a replacement for either carbohydrates or saturated fat lowers low density lipoprotein (LDL)-cholesterol, triglycerides, and ApoB levels, and raises high density lipoprotein (HDL)-cholesterol;14, 31 and also lowers hemoglobin A1c and insulin resistance and potentially augments insulin production.32 Other potential cardiometabolic benefits of dietary LA may include favorable effects on inflammation,14 blood pressure,33 and body composition, including prevention and reduction of visceral and liver fat.14, 34 In a pooled analyses of prospective cohort studies, self-reported estimates of LA consumption are associated with lower CHD risk.6 Similarly, in meta-analyses of older, limited clinical trials, increased consumption of LA-rich vegetable oils, especially soybean oil, reduces the risk of CHD.5 Our findings evaluating in vivo levels of LA status across multiple global studies add strong support for cardiovascular benefits of LA.
While AA has long been considered an archetypical pro-inflammatory and pro-thrombotic fatty acid, growing evidence suggests its effects may be more complex.35 In the present investigation, AA levels were not associated with higher risk of CVD, and indeed in some analyses were associated with lower risk. These results do not provide support for adverse cardiovascular effects of AA. While AA is the precursor to potentially pro-inflammatory leukotrienes, it is also the main precursor to key anti-inflammatory metabolites, such as epoxyeicosatrienoic acids and prostaglandin E2, as well as other mediators that actively resolve inflammation, such as lipoxin A4.35 It also gives rise to prostacyclin, a potent anti-aggregatory and vasodilatory molecule.36 These complex biologic effects preclude simplistic inference on health effects of AA metabolites and further support the importance of empiric assessment of relationships with clinical events, such as in our investigation.
Overall, our findings provide little support for the hypothesis that LA or AA, the major n-6 PUFA, may increase CVD risk. We also identified little evidence for any interaction between n-6 and n-3 PUFA levels, consistent with prior reviews of dietary data.1 n-6 PUFA may also have additional metabolic benefits. For example, a recent pooled analysis from FORCE identified a strong inverse association of circulating and adipose tissue LA levels and incidence of type 2 diabetes, with no significant associations for AA.25 Taken together with results of randomized controlled feeding trials of blood lipids, glucose-insulin homeostasis, and other metabolic risk factors; prospective cohort studies of self-reported consumption; and (older, methodologically limited) clinical trials of LA-rich plant oils, our novel findings do not support recommendations of some10 to reduce n-6 PUFA consumption or reduce the n-6:n-3 ratio (as opposed to increasing n-3 intake). Rather, the findings from the present study, together with the prior research summarized above, support independent cardioprotective benefits of LA.
Our results provide important evidence that helps inform clinical and population recommendations. Dietary guidelines from several organizations, including the American Heart Association, recommend increased consumption of n-6 PUFA to prevent CVD.7 However, some researchers9, 10, 37 and other national guidelines4 currently recommend avoidance of n-6 PUFA and reductions from current intake levels. Furthermore, current trends in oil production are leading to increased use of high-oleic, LA-depleted seed oils,38 which can increase the risk of insufficient PUFA consumption in population subgroups. Our findings, combined with prior evidence from metabolic feeding trials, supports cardiovascular benefits of LA and a need to harmonize international guidelines and priorities for oilseed production and use.
A unique strength of our investigation was the ability to assess associations across distinct lipid compartments across which LA (AA) levels intercorrelate to varying degrees (e.g., r=0.4–0.9),26, 39, 40 suggesting that each compartment reflects partly differing metabolic and physiologic influences. Yet, our findings were generally concordant across compartments, providing support for common or similar biologic effects of these n-6 fatty acids across these compartments.
The inverse association of LA levels with ischemic stroke was more pronounced in T-allele carriers of rs174547, a polymorphism in FADS1 associated with higher fatty acid desaturase activities27, 41 and FADS1 expression.42 Although located in FADS1, rs174547 is also in strong linkage disequilibrium with polymorphisms in FADS2 (encoding the LA-desaturating FADS2) and has emerged as the main genetic determinant of circulating LA and AA in a recent genome-wide association study.27 The T-allele has been linked to several metabolic traits including higher cholesterol (total, LDL, and HDL)43 and fasting glucose44, but also lower triglycerides43 and heart rate.45 The pleiotropy of the FADS cluster and the specificity for ischemic stroke rather than all CVD endpoints complicates the interpretation of the observed gene-LA interaction, which should therefore be viewed cautiously. Yet, one could also speculate that carriers of the major T-allele derive greater benefits from the established LDL-lowering effects of dietary LA and thus have accentuated health benefits –a ripe area for further investigation.
Few prior meta-analyses of LA and AA levels in CVD have been performed. In one analysis of 10 published studies with 28,000 participants and 3,800 events, LA was not significantly associated with coronary events, while AA was associated with a 17% reduction in risk.18 In a meta-analysis of published studies acute myocardial infarction and coronary syndromes including many retrospective case-control studies, circulating and adipose tissue LA levels were inversely associated with the risk of CHD events, while overall associations for AA were null.17 Our investigation considerably extends these prior results by focusing on prospective studies, performing new individual-level study-specific analyses using a standardized and harmonized analysis protocol, including a much larger number of participants and events, and evaluating several major CVD outcomes. Importantly, our consortium also greatly minimizes publication bias by incorporating new (unpublished) findings from all available studies, rather than pooling only prior published results.
Other strengths include use of in vivo n-6 PUFA levels, which complement self-reported dietary estimates, reduce errors from memory, and allow assessment of biologically relevant in vivo levels-especially important for AA. Outcomes in nearly all studies were defined by centralized adjudication processes or validated registries rather than from self-report alone, reducing the potential for missed or misclassified endpoints. Inclusion of cohorts from 13 countries across several continents enhances generalizability. The large numbers of participants and events allowed us to explore several potential effect modifiers and the shape of the associations.
Potential limitations deserve attention. For certain compartments, such as adipose tissue, few studies were available. Most individuals were of European descent, lowering statistical power for evaluating other races/ethnicities. Despite extensive efforts to harmonize study-specific methods, some dissimilarities remained between cohorts in outcome definitions (see Expanded Methods in the Supplemental Material) and covariate categorization (Supplemental Table 5). Although such variety and unmeasured background population characteristics may increase generalizability, these may also have contributed to the moderate between-study heterogeneity observed for some exposure-outcome relationships. Fatty acids were measured once at baseline, and changes over time could lead to misclassification, which would attenuate the associations. However, reasonable temporal reproducibility has been reported for LA and AA concentrations over time.46 Since few studies evaluated multiple compartments, and because cholesterol esters were only assessed by studies from Northern Europe, we were hampered in drawing any conclusions of true predictive differences between lipid fractions. Although fatty acid analytical methods were not standardized across studies, the use of a quintile-based statistical approach minimizes this concern. We did not adjust for non-fatty acid dietary factors, but pooling results across multiple cohorts with different population characteristics increases the validity of the findings. While all studies consistently adjusted for other major CVD risk factors, we cannot exclude residual confounding due to unmeasured or imprecisely measured covariates. However, the concordance of the present observed associations with other lines of evidence on cardiovascular benefits of LA1, 5, 6, 32 provide biologic plausibility for our findings. We did not evaluate the associations after exclusion of early cases. However, such sensitivity did not produce results substantially different from the main findings in our previous pooling projects24, 25 and in cohort-specific analyses,23 suggesting that the observed associations are not likely due to reverse causation.
In summary, based on pooled individual-level analyses of prospective studies, circulating and adipose tissue biomarker concentrations of LA were inversely associated with CVD while AA was not associated with higher CVD risk. Together with prior research, these results support CVD benefits of LA.
Supplementary Material
Clinical perspective.
What is new?
We conducted the hitherto largest pooled individual-level analysis using circulating and adipose tissue levels of linoleic acid and arachidonic acid to examine the link between omega-6 fatty acids and cardiovascular outcomes in various populations.
Our approach increases statistical power and generalizability compared to individual studies; lowers the risk of publication bias and heterogeneity compared to meta-analyses of existing literature; and allows evaluation of the associations in key population subgroups.
Strikingly, higher level of linoleic acid was associated with lower risks of total cardiovascular disease, ischemic stroke, and cardiovascular mortality, while arachidonic acid was not associated with cardiovascular risk.
What are the clinical implications?
Our findings support potential benefits of the main dietary omega-6 fatty acid, i.e., linoleic acid, for cardiovascular disease prevention.
Furthermore, our results do not support any theorized cardiovascular harms of omega-6 fatty acids.
Our findings provide evidence to help inform currently inconsistent global dietary recommendations on omega-6 consumption.
SOURCES OF FUNDING
Funding for the Fatty acids & Outcomes Research Consortium (FORCE): Cohort-specific funding is outlined in Supplemental Table 2. Unilever provided Tufts University with a restricted grant (‘Epidemiological research on circulating polyunsaturated fatty acids in relation to cardiometabolic health within the CHARGE-consortium’) to partly support this analysis. Unilever had no role in study design, study conduct, data analysis, manuscript preparation, or decision to submit. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES
Drs. Wu and Micha report research support from Unilever for this work. Dr. Mozaffarian reports research funding from the National Institutes of Health and the Gates Foundation; personal fees from GOED, DSM, Nutrition Impact, Pollock Communications, Bunge, Indigo Agriculture, Amarin, Acasti Pharma, and America’s Test Kitchen; scientific advisory board, Elysium Health (with stock options), Omada Health, and DayTwo; and chapter royalties from UpToDate; all outside the submitted work. Dr. Psaty serves on the DSMB of a clinical trial funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. No other conflicts were reported.
REFERENCES
- 1.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB and Sacks F. Omega-6 Fatty Acids and Risk for Cardiovascular Disease: A Science Advisory From the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. [DOI] [PubMed] [Google Scholar]
- 2.Vannice G and Rasmussen H. Position of the Academy of Nutrition and Dietetics: Dietary Fatty Acids for Healthy Adults. J Acad Nutr Diet. 2014;114:136–153. [DOI] [PubMed] [Google Scholar]
- 3.Food and Agriculture Organization of the United Nations. Fats and fatty acids in human nutrition : report of an expert consultation : 10-14 November 2008, Geneva. Rome: Food and Agriculture Organization of the United Nations; 2010. [Google Scholar]
- 4.Legrand P, Morise A and Kalonji E. Update of French nutritional recommendations for fatty acids. World Rev Nutr Diet. 2011;102:137–143. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Micha R and Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC and Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, Stone NJ and Van Horn LV. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation. 2017;136:e1–e23. [DOI] [PubMed] [Google Scholar]
- 8.Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM and Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013;346:e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, Davis JM, Ringel A, Suchindran CM and Hibbeln JR. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ. 2016;353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simopoulos AP, Leaf A and Salem N Jr. Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab. 1999;43:127–130. [DOI] [PubMed] [Google Scholar]
- 11.Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie. 2009;91:791–795. [DOI] [PubMed] [Google Scholar]
- 12.Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA and Millward DJ. Long-chain conversion of [13C]linoleic acid and α-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res. 2005;46:269–280. [DOI] [PubMed] [Google Scholar]
- 13.Sarkkinen ES, Agren JJ, Ahola I, Ovaskainen ML and Uusitupa MI. Fatty acid composition of serum cholesterol esters, and erythrocyte and platelet membranes as indicators of long-term adherence to fat-modified diets. Am J Clin Nutr. 1994;59:364–370. [DOI] [PubMed] [Google Scholar]
- 14.Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M, Rudling M, Arner P, Cederholm T, Ahlstrom H and Riserus U. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003–1012. [DOI] [PubMed] [Google Scholar]
- 15.Skeaff CM, Hodson L and McKenzie JE. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr. 2006;136:565–569. [DOI] [PubMed] [Google Scholar]
- 16.Hodson L, Eyles HC, McLachlan KJ, Bell ML, Green TJ and Skeaff CM. Plasma and erythrocyte fatty acids reflect intakes of saturated and n-6 PUFA within a similar time frame. J Nutr. 2014;144:33–41. [DOI] [PubMed] [Google Scholar]
- 17.Harris WS, Poston WC and Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw KT, Mozaffarian D, Danesh J and Di Angelantonio E. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 19.Simon JA, Fong J, Bernert JT Jr. and Browner WS. Serum fatty acids and the risk of stroke. Stroke. 1995;26:778–782. [DOI] [PubMed] [Google Scholar]
- 20.Wiberg B, Sundstrom J, Arnlov J, Terent A, Vessby B, Zethelius B and Lind L. Metabolic risk factors for stroke and transient ischemic attacks in middle-aged men: a community-based study with long-term follow-up. Stroke. 2006;37:2898–2903. [DOI] [PubMed] [Google Scholar]
- 21.Yamagishi K, Folsom AR and Steffen LM. Plasma fatty acid composition and incident ischemic stroke in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis. 2013;36:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Goede J, Verschuren WM, Boer JM, Kromhout D and Geleijnse JM. N-6 and n-3 fatty acid cholesteryl esters in relation to incident stroke in a Dutch adult population: a nested case-control study. Nutr Metab Cardiovasc Dis. 2013;23:737–743. [DOI] [PubMed] [Google Scholar]
- 23.Wu JH, Lemaitre RN, King IB, Song X, Psaty BM, Siscovick DS and Mozaffarian D. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: the Cardiovascular Health Study. Circulation. 2014;130:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC, Fretts AM, Guallar E, Matsumoto C, Prem K, Tanaka T, Wu JH, Zhou X, Helmer C, Ingelsson E, Yuan JM, Barberger-Gateau P, Campos H, Chaves PH, Djousse L, Giles GG, Gomez-Aracena J, Hodge AM, Hu FB, Jansson JH, Johansson I, Khaw KT, Koh WP, Lemaitre RN, Lind L, Luben RN, Rimm EB, Riserus U, Samieri C, Franks PW, Siscovick DS, Stampfer M, Steffen LM, Steffen BT, Tsai MY, van Dam RM, Voutilainen S, Willett WC, Woodward M and Mozaffarian D. omega-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Intern Med. 2016;176:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, Zhou X, Yang WS, de Oliveira Otto MC, Kroger J, Qureshi W, Virtanen JK, Bassett JK, Frazier-Wood AC, Lankinen M, Murphy RA, Rajaobelina K, Del Gobbo LC, Forouhi NG, Luben R, Khaw KT, Wareham N, Kalsbeek A, Veenstra J, Luo J, Hu FB, Lin HJ, Siscovick DS, Boeing H, Chen TA, Steffen B, Steffen LM, Hodge A, Eriksdottir G, Smith AV, Gudnason V, Harris TB, Brouwer IA, Berr C, Helmer C, Samieri C, Laakso M, Tsai MY, Giles GG, Nurmi T, Wagenknecht L, Schulze MB, Lemaitre RN, Chien KL, Soedamah-Muthu SS, Geleijnse JM, Sun Q, Harris WS, Lind L, Arnlov J, Riserus U, Micha R, Mozaffarian D, Cohorts for H, Aging Research in Genomic Epidemiology Fatty A and Outcomes Research C. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodson L, Skeaff CM and Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. [DOI] [PubMed] [Google Scholar]
- 27.Guan W, Steffen BT, Lemaitre RN, Wu JH, Tanaka T, Manichaikul A, Foy M, Rich SS, Wang L, Nettleton JA, Tang W, Gu X, Bandinelli S, King IB, McKnight B, Psaty BM, Siscovick D, Djousse L, Ida Chen YD, Ferrucci L, Fornage M, Mozafarrian D, Tsai MY and Steffen LM. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ Cardiovasc Genet. 2014;7:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsini N, Li R, Wolk A, Khudyakov P and Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F and Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. [DOI] [PubMed] [Google Scholar]
- 30.Baylin A and Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol. 2006;17:22–27. [DOI] [PubMed] [Google Scholar]
- 31.Katan MB, Zock PL and Mensink RP. Effects of fats and fatty acids on blood lipids in humans: an overview. Am J Clin Nutr. 1994;60:1017S–1022S. [DOI] [PubMed] [Google Scholar]
- 32.Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO, Abioye AI and Mozaffarian D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016;13:e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura K, Stamler J, Nakagawa H, Elliott P, Ueshima H, Chan Q, Brown IJ, Tzoulaki I, Saitoh S, Dyer AR, Daviglus ML, Kesteloot H, Okayama A, Curb JD, Rodriguez BL, Elmer PJ, Steffen LM, Robertson C, Zhao L, Macro-Micronutrients ftISo BPR. Relationship of Dietary Linoleic Acid to Blood Pressure: The International Study of Macro-Micronutrients and Blood Pressure Study. Hypertension. 2008;52:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson H-E, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I and Risérus U. Overfeeding Polyunsaturated and Saturated Fat Causes Distinct Effects on Liver and Visceral Fat Accumulation in Humans. Diabetes. 2014;63:2356–2368. [DOI] [PubMed] [Google Scholar]
- 35.Shearer GC and Walker RE. An overview of the biologic effects of omega-6 oxylipins in humans. Prostaglandins Leukot Essent Fatty Acids. 2018;137:26–38. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JA and Kirkby NS. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br J Pharmacol. 2018, doi: 10.1111/bph.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamazaki T and Okuyama H. The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid. A review and critique of the scientific evidence. World Rev Nutr Diet. 2003;92:109–132. [DOI] [PubMed] [Google Scholar]
- 38.Raatz SK, Conrad Z, Jahns L, Belury MA and Picklo MJ. Modeled replacement of traditional soybean and canola oil with high-oleic varieties increases monounsaturated fatty acid and reduces both saturated fatty acid and polyunsaturated fatty acid intake in the US adult population. Am J Clin Nutr. 2018;108:594–602. [DOI] [PubMed] [Google Scholar]
- 39.Iggman D, Arnlov J, Cederholm T and Riserus U. Association of Adipose Tissue Fatty Acids With Cardiovascular and All-Cause Mortality in Elderly Men. JAMA cardiology. 2016;1:745–753. [DOI] [PubMed] [Google Scholar]
- 40.Marklund M, Pingel R, Rosqvist F, Lindroos AK, Eriksson JW, Vessby B, Oscarsson J, Lind L and Risérus U. Fatty Acid Proportions in Plasma Cholesterol Esters and Phospholipids Are Positively Correlated in Various Swedish Populations. J Nutr. 2017;147:2118–2125. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Vaarhorst A, Merry AHH, Dollé MET, Hovenier R, Imholz S, Schouten LJ, Heijmans BT, Müller M, Slagboom PE, van den Brandt PA, Gorgels APM, Boer JMA and Feskens EJM. Markers of Endogenous Desaturase Activity and Risk of Coronary Heart Disease in the CAREMA Cohort Study. PLoS One. 2012;7:e41681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, Kaplan L, Bennett D, Li Y, Tanaka T, Voight BF, Bonnycastle LL, Jackson AU, Crawford G, Surti A, Guiducci C, Burtt NP, Parish S, Clarke R, Zelenika D, Kubalanza KA, Morken MA, Scott LJ, Stringham HM, Galan P, Swift AJ, Kuusisto J, Bergman RN, Sundvall J, Laakso M, Ferrucci L, Scheet P, Sanna S, Uda M, Yang Q, Lunetta KL, Dupuis J, de Bakker PIW, O’Donnell CJ, Chambers JC, Kooner JS, Hercberg S, Meneton P, Lakatta EG, Scuteri A, Schlessinger D, Tuomilehto J, Collins FS, Groop L, Altshuler D, Collins R, Lathrop GM, Melander O, Salomaa V, Peltonen L, Orho-Melander M, Ordovas JM, Boehnke M, Abecasis GR, Mohlke KL and Cupples LA. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkila K, Hypponen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikainen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Muller-Nurasyid M, Nolte IM, O’Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Doring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimaki T, Lin SY, Lindstrom J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Muller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stancakova A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrieres J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Jarvelin MR, Jula A, Kahonen M, Kaprio J, Kesaniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, Marz W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njolstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E and Abecasis GR. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, Magi R, Strawbridge RJ, Rehnberg E, Gustafsson S, Kanoni S, Rasmussen-Torvik LJ, Yengo L, Lecoeur C, Shungin D, Sanna S, Sidore C, Johnson PC, Jukema JW, Johnson T, Mahajan A, Verweij N, Thorleifsson G, Hottenga JJ, Shah S, Smith AV, Sennblad B, Gieger C, Salo P, Perola M, Timpson NJ, Evans DM, Pourcain BS, Wu Y, Andrews JS, Hui J, Bielak LF, Zhao W, Horikoshi M, Navarro P, Isaacs A, O’Connell JR, Stirrups K, Vitart V, Hayward C, Esko T, Mihailov E, Fraser RM, Fall T, Voight BF, Raychaudhuri S, Chen H, Lindgren CM, Morris AP, Rayner NW, Robertson N, Rybin D, Liu CT, Beckmann JS, Willems SM, Chines PS, Jackson AU, Kang HM, Stringham HM, Song K, Tanaka T, Peden JF, Goel A, Hicks AA, An P, Muller-Nurasyid M, Franco-Cereceda A, Folkersen L, Marullo L, Jansen H, Oldehinkel AJ, Bruinenberg M, Pankow JS, North KE, Forouhi NG, Loos RJ, Edkins S, Varga TV, Hallmans G, Oksa H, Antonella M, Nagaraja R, Trompet S, Ford I, Bakker SJ, Kong A, Kumari M, Gigante B, Herder C, Munroe PB, Caulfield M, Antti J, Mangino M, Small K, Miljkovic I, Liu Y, Atalay M, Kiess W, James AL, Rivadeneira F, Uitterlinden AG, Palmer CN, Doney AS, Willemsen G, Smit JH, Campbell S, Polasek O, Bonnycastle LL, Hercberg S, Dimitriou M, Bolton JL, Fowkes GR, Kovacs P, Lindstrom J, Zemunik T, Bandinelli S, Wild SH, Basart HV, Rathmann W, Grallert H, Maerz W, Kleber ME, Boehm BO, Peters A, Pramstaller PP, Province MA, Borecki IB, Hastie ND, Rudan I, Campbell H, Watkins H, Farrall M, Stumvoll M, Ferrucci L, Waterworth DM, Bergman RN, Collins FS, Tuomilehto J, Watanabe RM, de Geus EJ, Penninx BW, Hofman A, Oostra BA, Psaty BM, Vollenweider P, Wilson JF, Wright AF, Hovingh GK, Metspalu A, Uusitupa M, Magnusson PK, Kyvik KO, Kaprio J, Price JF, Dedoussis GV, Deloukas P, Meneton P, Lind L, Boehnke M, Shuldiner AR, van Duijn CM, Morris AD, Toenjes A, Peyser PA, Beilby JP, Korner A, Kuusisto J, Laakso M, Bornstein SR, Schwarz PE, Lakka TA, Rauramaa R, Adair LS, Smith GD, Spector TD, Illig T, de Faire U, Hamsten A, Gudnason V, Kivimaki M, Hingorani A, Keinanen-Kiukaanniemi SM, Saaristo TE, Boomsma DI, Stefansson K, van der Harst P, Dupuis J, Pedersen NL, Sattar N, Harris TB, Cucca F, Ripatti S, Salomaa V, Mohlke KL, Balkau B, Froguel P, Pouta A, Jarvelin MR, Wareham NJ, Bouatia-Naji N, McCarthy MI, Franks PW, Meigs JB, Teslovich TM, Florez JC, Langenberg C, Ingelsson E, Prokopenko I and Barroso I. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.den Hoed M, Eijgelsheim M, Esko T, Brundel B, Peal DS, Evans DM, Nolte IM, Segre AV, Holm H, Handsaker RE, Westra HJ, Johnson T, Isaacs A, Yang J, Lundby A, Zhao JH, Kim YJ, Go MJ, Almgren P, Bochud M, Boucher G, Cornelis MC, Gudbjartsson D, Hadley D, van der Harst P, Hayward C, den Heijer M, Igl W, Jackson AU, Kutalik Z, Luan J, Kemp JP, Kristiansson K, Ladenvall C, Lorentzon M, Montasser ME, Njajou OT, O’Reilly PF, Padmanabhan S, Pourcain BS, Rankinen T, Salo P, Tanaka T, Timpson NJ, Vitart V, Waite L, Wheeler W, Zhang WH, Draisma HHM, Feitosa MF, Kerr KF, Lind PA, Mihailov E, Onland-Moret NC, Song C, Weedon MN, Xie WJ, Yengo L, Absher D, Albert CM, Alonso A, Arking DE, de Bakker PIW, Balkau B, Barlassina C, Benaglio P, Bis JC, Bouatia-Naji N, Brage S, Chanock SJ, Chines PS, Chung M, Darbar D, Dina C, Dorr M, Elliott P, Felix SB, Fischer K, Fuchsberger C, de Geus EJC, Goyette P, Gudnason V, Harris TB, Hartikainen AL, Havulinna AS, Heckbert SR, Hicks AA, Hofman A, Holewijn S, Hoogstra-Berends F, Hottenga JJ, Jensen MK, Johansson A, Junttila J, Kaab S, Kanon B, Ketkar S, Khaw KT, Knowles JW, Kooner AS, Kors JA, Kumari M, Milani L, Laiho P, Lakatta EG, Langenberg C, Leusink M, Liu YM, Luben RN, Lunetta KL, Lynch SN, Markus MRP, Marques-Vidal P, Leach IM, McArdle WL, McCarroll SA, Medland SE, Miller KA, Montgomery GW, Morrison AC, Muller-Nurasyid M, Navarro P, Nelis M, O’Connell JR, O’Donnell CJ, Ong KK, Newman AB, Peters A, Polasek O, Pouta A, Pramstaller PP, Psaty BM, Rao DC, Ring SM, Rossin EJ, Rudan D, Sanna S, Scott RA, Sehmi JS, Sharp S, Shin JT, Singleton AB, Smith AV, Soranzo N, Spector TD, Stewart C, Stringham HM, Tarasov KV, Uitterlinden AG, Vandenput L, Hwang SJ, Whitfield JB, Wijmenga C, Wild SH, Willemsen G, Wilson JF, Witteman JCM, Wong A, Wong QN, Jamshidi Y, Zitting P, Boer JMA, Boomsma DI, Borecki IB, van Duijn CM, Ekelund U, Forouhi NG, Froguel P, Hingorani A, Ingelsson E, Kivimaki M, Kronmal RA, Kuh D, Lind L, Martin NG, Oostra BA, Pedersen NL, Quertermous T, Rotter JI, van der Schouw YT, Verschuren WMM, Walker M, Albanes D, Arnar DO, Assimes TL, Bandinelli S, Boehnke M, de Boer RA, Bouchard C, Caulfield WLM, Chambers JC, Curhan G, Cusi D, Eriksson J, Ferrucci L, van Gilst WH, Glorioso N, de Graaf J, Groop L, Gyllensten U, Hsueh WC, Hu FB, Huikuri HV, Hunter DJ, Iribarren C, Isomaa B, Jarvelin MR, Jula A, Kahonen M, Kiemeney LA, van der Klauw MM, Kooner JS, Kraft P, Iacoviello L, Lehtimaki T, Lokki MLL, Mitchell BD, Navis G, Nieminen MS, Ohlsson C, Poulter NR, Qi L, Raitakari OT, Rimm EB, Rioux JD, Rizzi F, Rudan I, Salomaa V, Sever PS, Shields DC, Shuldiner AR, Sinisalo J, Stanton AV, Stolk RP, Strachan DP, Tardif JC, Thorsteinsdottir U, Tuomilehto J, van Veldhuisen DJ, Virtamo J, Viikari J, Vollenweider P, Waeber G, Widen E, Cho YS, Olsen JV, Visscher PM, Willer C, Franke L, Erdmann J, Thompson JR, Pfeufer A, Sotoodehnia N, Newton-Cheh C, Ellinor PT, Stricker BHC, Metspalu A, Perola M, Beckmann JS, Smith GD, Stefansson K, Wareham NJ, Munroe PB, Sibon OCM, Milan DJ, Snieder H, Samani NJ, Loos RJF, Global BC, Consortium CA, Consortium PG, Consortium QG, Consortium Q-I and Consortium C-A. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat Genet. 2013;45:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE and Eliassen AH. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The institutional review board approvals and data sharing agreements for the participating cohorts allowed us to share cohort results. Individual participant data are owned by individual participating cohorts and are available to researchers consented from participating cohorts. For further queries or requests, please contact force@tufts.edu. Further details are available at the FORCE website: http://force.nutrition.tufts.edu/.


