Abstract
Anti-SOX1 antibodies are associated with diverse neurological syndromes, targeting both the central (paraneoplastic cerebellar degeneration) and peripheral nervous systems (Lambert Eaton myasthenic syndrome, paraneoplastic neuropathy). Although the pathogenic role of these antibodies remains unclear, their strong association with underlying neoplastic disease (mainly small-cell lung cancer) has designated them as onconeural antibodies. Here, we present a case of cerebellar ataxia with marked photophobia, with severe atrophy of the cerebellum and brain stem, associated with anti-SOX1 antibodies without evidence of an underlying malignancy. Although anti-SOX1-associated cerebellar syndrome is infrequent, investigation of these antibodies should be considered as a part of the diagnostic algorithm if more common causes have been ruled out. Extensive brain stem lesions causing disruption of the trigeminal pathway and its connections with the pretectal area might explain the underlying mechanism of the associated photophobia. Early recognition of anti-SOX1 antibodies, exclusion of underlying neoplasm, and prompt initiation of immunotherapy are essential to achieve a better outcome.
Keywords: cerebellar ataxia, paraneoplastic cerebellar degeneration, SOX1 protein
Introduction
Sry-like high mobility group box (SOX) proteins belong to the DNA-binding protein superfamily.1 Those proteins are expressed in neuronal precursor cells in the central nervous system (CNS), mainly during embryogenesis. They are mainly located in the periventricular area of the lateral ventricles and the dentate gyrus of the hippocampus. Radial glial cells, a specialized subtype of glial cells present in the cerebellum, express proteins which remain functional through adulthood (cerebellar Bergmann glia cells).2 The anti-SOX1 antibodies are antiglial nuclear antibodies directed against the SOX proteins and they have been related to different neurological syndromes, including Lambert-Eaton myasthenic syndrome (LEMS), limbic encephalitis, polyneuropathy, and paraneoplastic cerebellar degeneration (PCD).3,4 These antibodies are strongly related to underlying malignancy, and the most frequent of which is small-cell lung cancer (SCLC). Accordingly, anti-SOX1 antibodies have been classified as cancer-related onconeural antibodies.5 However, some patients develop neurological syndromes despite the absence of a demonstrable underlying cancer.3,6 Its pathogenic role is not clear, but it seems to be associated with immune process.4 We present a patient with cerebellar ataxia and marked photophobia with the presence of anti-SOX1 antibodies, in the absence of underlying cancer.
Case
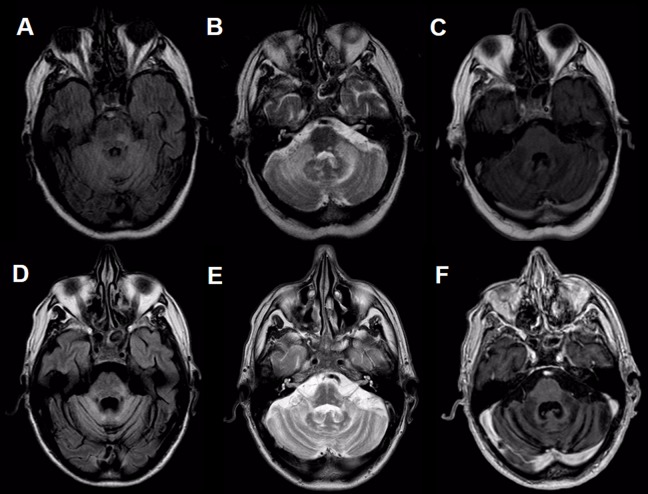
A 63-year-old man, previously healthy, begins with ataxia of the left upper extremity, which then progressively developed dysarthria and generalized ataxia. He was evaluated in another center, without arriving at diagnosis or treatment, so he continued to get worse. After 15 months of onset of the symptoms, he consulted our institution. Physical examination revealed severe dysarthria, horizontal gaze-evoked nystagmus, upbeat nystagmus, saccadic dysmetria and severe limb, and truncal ataxia. In addition, for the prior 3 months, he had noted progressive photophobia, which forced him to use dark sunglasses permanently. Brain magnetic resonance imaging (MRI) performed 6 months after the onset of symptoms showed diffuse hyperintensity in fluid-attenuated inversion recovery and T2 sequences of the cerebellum and brain stem, without gadolinium enhancement (Figure 1A–C). Cerebrospinal fluid (CSF) revealed mild hyperproteinorrachia of 52 mg/dL (normal value < 45 mg/dL) and absence of pleocytosis (<5 cell/mm3). No atypical cells were evidenced in cytology or flow cytometric analysis in the CSF. Intrathecal and serum oligoclonal bands were negative. Serology for human immunodeficiency virus was negative. Assays testing rheumatologic antibody profile, antibodies for celiac disease, and thyroid-related antibodies were unrevealing. Serum levels of vitamins B1 and B12 and serum and urine levels of copper did not yield pathological values. We tested for neural surface autoantibodies (NMDA, GABA-A and -B, AMPA1 and 2, LGI1, CASPR2, and DPPX) and intracellular autoantibodies (amphiphysin, Ma2, CRMP5, Hu, Yo, Ri, Recoverin, SOX1, Titin, Zic4, GAD65, and Tr) were performed in serum and in CSF. Only the anti-SOX1 antibodies were detected in the serum, although negative in CSF. The rest of the autoantibodies were negative. Total body positron emission tomography computed tomography (PET-CT) with fluorodeoxyglucose (FDG), testicular ultrasound, and blood smear were performed and did not demonstrate an occult neoplasm.
Figure 1.
Magnetic resonance imaging of the brain. Hyperintensity on FLAIR (A, D), y T2 (B, E), and hypointensity on T1 (C, F) in both cerebellar hemispheres and peduncles with extension to the pons, predominantly at the level of the left posterolateral sector, without contrast enhancement. Figures A-C correspond to 6 months after initial symptoms, and Figures D-F, after 17 months. FLAIR indicates fluid-attenuated inversion recovery.
On the other hand, due to the marked photophobia, an exhaustive ophthalmological evaluation was carried out, with evaluation of ocular tone, fundus, electroretinogram (ERG), and visual evoked response (VER), which did not show alterations in the visual pathway. The clinical case was interpreted as autoimmune cerebellar ataxia associated with anti-SOX1 antibodies and treatment was started with intravenous (IV) methylprednisolone (1000 mg IV daily for 5 days). Unfortunately, due to the very late diagnosis, the severity of disability at the time of consultation, and the marked atrophy of the brain stem and cerebellum visualized in surveillance MRI performed 2 months into treatment (Figure 1D–F), the patient and his family decided not to advance with a more aggressive immunosuppressive treatment. Therefore, the patient continued only with an oral prednisone for 2 years (40 mg). During the follow-up, the patient did not show any neurological improvement. His symptoms progressed to severe incapacitating ataxia, confining him to a wheelchair and he endorsed marked photophobia with functional blindness when exposed to light without dark sunglasses. Furthermore, during 36 months of follow-up, no evidence of occult neoplastic process could be demonstrated. The oncological screening was performed by a routine blood test, FDG-PET-CT, and testicular ultrasound every 6 months.
Discussion
Autoimmune cerebellar ataxia represents up to 32% of cases of acquired ataxias.7 The most frequent etiologies include gluten ataxia, PCD, and anti-GAD-related ataxia. Paraneoplastic cerebellar degeneration is attributable to a wide variety of autoantibodies, which can target different epitopes located in the cerebellum similar to those of tumor cells. Anti-Yo antibodies (mostly associated with breast and ovarian cancer) and anti-Hu (highly related to SCLC) are the most prevalent.8 Anti-SOX1 antibody-associated PCD is rare, with few isolated cases reported in the literature.3,4 More commonly anti-SOX1 antibodies have been linked with other paraneoplastic syndromes such as LEMS9 and sensory or sensorimotor polyneuropathy.6 Although SCLC is the most frequently involved, there are many types of tumors associated with anti-SOX1 autoantibodies, such as breast, thyroid, Hodgkin’s lymphoma, and non-SCLC.3,4,6 In addition, anti-SOX1 antibodies can be found concomitantly with other onconeural antibodies in the presence of cancer (eg, anti-Hu). These antibodies are markers of an immune response directed against different neoplasms.3 On the other hand, a sizable group of patients develops neurological syndromes associated with anti-SOX1 antibodies without the presence of neoplasm, even after several years of follow-up (up to 15 years).3,6,10 Furthermore, it reaffirms that the presence of anti-SOX1 antibodies only appears in immune-mediated processes and is not observed in healthy controls.3
As mentioned above, during the advanced stages of the disease, our patient developed a marked photophobia and reached functional blindness when exposed to front light. There was no deterioration in visual acuity. Eye tone, fundus, ERG, and VER were normal. We found 2 theoretical models that try to explain the underlying mechanisms by which patients may develop photophobia.11,12 The first explanation evolves connections between neurons of the trigeminal nuclei and the pretectal area. In this pathway, the light afferents that travel along the ophthalmic division of the trigeminal nerve are sensed as a nociceptive signal, which is returned through trigeminal efferents, causing ocular vasodilation and activation of pain sensing neurons in the blood vessels. A second mechanism recently proposed postulate that retinal cells called melanopsins, which constitute an intrinsically photosensitive retinal ganglion cell (IPRGC), works as a transducer, which reacts to light and sends signals to the thalamic pain nuclei. In this way, the IPRGC would work as a protection mechanism, reacting to a very intense light capable of damaging the retina. Both circuits involve a large network of neurons that show multiple synapses at different levels of the CNS and probably both are interconnected.12 Although the physiopathological mechanism of photophobia in this case is uncertain, the extent of the lesions in the brain stem and the subsequent injury of the trigeminal-pretectal pathway could be postulated as a possible mechanism. This explanation could be transposed to neurodegenerative conditions such as progressive supranuclear palsy, in which similar phenomenon could be seen.12,13
Patients with PCD generally have poor outcomes.8 Only 10% of patients respond to a combination of immunotherapies.14 Among the positive prognostic factors, early detection and effective treatment of the underlying neoplasm are essential in preventing oncological progression and in halting the immune-mediated response.15 In addition, early introduction of immunotherapy, prior to Purkinje cell necrosis and cerebellar atrophy, may be associated with better patient outcomes.16
In conclusion, anti-SOX1 antibodies are associated with diverse neurological syndromes that affect both the central and peripheral nervous systems. Although it does not have a pathological role per se, the appearance of them is present in the context of immune-mediated diseases. Brain stem lesions, especially those which damage the trigeminal pathways, might explain the development of photophobia. Finally, despite the intrinsically poor prognosis of anti-SOX1 antibody-associated disease, we must consider that early oncological screening and both onco-specific and immunological treatments are the most important prognostic determinants.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: The patient described in this manuscript provided written consent to its publication.
References
- 1. Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27(6):1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alcock J, Lowe J, England T, Bath P, Sottile V. Expression of Sox1, Sox2 and Sox9 is maintained in adult human cerebellar cortex. Neurosci Lett. 2009;450(2):114–116. [DOI] [PubMed] [Google Scholar]
- 3. Berger B, Dersch R, Ruthardt E, Rasiah C, Rauer S, Stich O. Prevalence of anti-SOX1 reactivity in various neurological disorders. J Neurol Sci. 2016;369:342–346. [DOI] [PubMed] [Google Scholar]
- 4. Stich O, Klages E, Bischler P, et al. SOX1 antibodies in sera from patients with paraneoplastic neurological syndromes. Acta Neurol Scand. 2012;125(5):326–331. [DOI] [PubMed] [Google Scholar]
- 5. Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257(4):509–517. [DOI] [PubMed] [Google Scholar]
- 6. Tschernatsch M, Singh P, Gross O, et al. Anti-SOX1 antibodies in patients with paraneoplastic and non-paraneoplastic neuropathy. J Neuroimmunol. 2010;226(1-2):177–180. [DOI] [PubMed] [Google Scholar]
- 7. Hadjivassiliou M, Boscolo S, Tongiorgi E, et al. Cerebellar ataxia as a possible organ specific autoimmune disease. Movement Disord. 2008;23(10):1270–1377. [DOI] [PubMed] [Google Scholar]
- 8. Mitoma H, Hadjivassiliou M, Honnorat J. Guidelines for treatment of immune-mediated cerebellar ataxias. Cerebellum Aataxias. 2015;2(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabater L, Titulaer M, Saiz A, Verschuuren J, Güre AO, Graus F. SOX1 antibodies are markers of paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology. 2008;70(12):924–928. [DOI] [PubMed] [Google Scholar]
- 10. Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75(8):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol. 2012;32(1):68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Y, Hallett M. Photophobia in neurologic disorders. Transl Neurodegener. 2017;6(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nath U, Ben-Shlomo Y, Thomson RG, Lees AJ, Burn DJ. Clinical features and natural history of progressive supranuclear palsy: a clinical cohort study. Neurology. 2003;60(6):910–916. [DOI] [PubMed] [Google Scholar]
- 14. Dropcho EJ. Principles of paraneoplastic syndromes. Ann NY Acad Sci. 1998;841:246–261. [DOI] [PubMed] [Google Scholar]
- 15. Keime-Guibert F, Graus F, Fleury A, et al. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (Anti-Hu, Anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry. 2000;68(4):479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voltz R. Paraneoplastic neurological syndromes: an update on diagnosis, pathogenesis, and therapy. Lancet Neurol. 2002;1(5):294–305. [DOI] [PubMed] [Google Scholar]