Abstract
Objective:
Stereotactic body radiation therapy (SBRT) is the preferred treatment modality for patients with inoperable early-stage non-small cell lung cancer. However, comparative outcomes between SBRT and surgery for high-risk patients remain controversial. The primary aim of the present meta-analysis was to assess overall survival in matched and unmatched patient cohorts undergoing SBRT or surgery. Secondary endpoints included cancer-specific survival, diseasefree survival, disease recurrence, and perioperative outcomes.
Methods:
A systematic review of relevant studies was performed through online databases using predefined criteria. The most updated studies were selected for meta-analysis according to unmatched and matched patient cohorts.
Results:
Thirty-two studies were identified in the systematic review, and 23 were selected for quantitative analysis. Surgery was associated with superior overall survival in both unmatched (odds ratio [OR], 2.49; 95% confidence interval [CI], 2.10–2.94; p<0.00001) and matched (OR, 1.71; 95% CI, 1.52–1.93; p<0.00001) cohorts. Subgroup analysis demonstrated superior overall survival for lobectomy and sublobar resection, compared with SBRT. In unmatched and matched cohorts, cancer-specific survival, disease-free survival, and freedom from locoregional recurrence were superior after surgery. However, SBRT was associated with fewer perioperative deaths.
Conclusions:
The current evidence suggests surgery is superior to SBRT in terms of mid- and long-term clinical outcomes; SBRT is associated with lower perioperative mortality. The improved outcomes after surgery, however, may be attributable at least in part to an imbalance of baseline characteristics. Future studies should aim to provide histopathological confirmation of malignancy and compare SBRT with minimally invasive anatomical resections.
INTRODUCTION
Stereotactic body radiation therapy (SBRT) is the preferred treatment modality for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC).1, 2 Compared to conventional radiotherapy, SBRT delivers fewer fractions of high-dose radiation per fraction with increased precision, sparing the surrounding normal tissue to maximize the biologically effective dose whilst minimizing toxicity, resulting in improved local control and overall survival.3, 4 The accumulating clinical experience with SBRT in prospective trials has led to heightened interest among the oncology community about the comparative outcomes of SBRT versus surgical resection for early-stage NSCLC in operable patients.5, 6
Recently, a retrospective pooled analysis of two prematurely terminated randomized controlled trials suggested that SBRT is better tolerated than surgery and may lead to improved overall survival.7 However, several study limitations necessitate caution to avoid overinterpreting these results, and there remains a paucity of robust clinical data to support the above statement, given the heterogeneity of study cohorts.8, 9 To address this issue, a number of studies have used propensity score matching to minimize the risk of selection bias.10 The purpose of the present systematic review and meta-analysis is to assess the clinical outcomes of SBRT versus surgery for patients with early-stage NSCLC. Primary endpoints included overall survival in matched and unmatched cohorts. Secondary endpoints included cancer-specific survival, disease-free survival, freedom from locoregional recurrence, freedom from distant recurrence, and perioperative mortality and morbidity. Each endpoint was assessed using matched and unmatched cohorts to compare relative outcomes, whenever possible. Subgroup analyses of lobectomy and sublobar resection versus SBRT were also performed for overall survival.
METHODS
Literature Search Strategy
A systematic review was performed using EMBASE and Ovid Medline, from their dates of inception to January 2018. To identify all potentially relevant studies, we combined the search terms (“SBRT” or “SABR” or “stereotactic” or “radiosurgery”) and (“NSCLC” or “non-small cell lung” or “carcinoma, non-small cell lung”) and (“surg*” or “resect*” or lobectomy) as either Medical Subject Headings or keywords. All identified articles were then assessed by applying the predefined selection criteria. A summary of search strategies and techniques has been described in detail previously.11
Selection Criteria and Data Appraisal
Eligible studies for selection in the systematic review were those in which comparative overall survival was reported for patients who underwent SBRT or surgical resection for NSCLC. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete or updated reports were included for meta-analysis. Abstracts, case reports, conference presentations, editorials, expert opinions, and publications not written in English were excluded. Data were extracted from article texts, tables, figures, and supplementary material. Two investigators (D.W. and C.D.C.) independently reviewed each retrieved article. Discrepancies between the two reviewers were resolved by discussion and consensus. To assess the quality of the non-randomized studies, the Newcastle-Ottawa scale was used to evaluate the selection, comparability, and outcomes reported in each study, with 0 – 3 stars indicating poor quality, 4 – 6 stars indicating moderate quality, and 7 or more stars indicating high quality.12
Statistical Analysis
When more than four studies provided relevant data on the same predetermined endpoint, meta-analysis was performed by combining the reported clinical outcomes of individual studies using a random effect model. Odds ratio (OR) and standard error were extracted or calculated from each study using methods described by Parma and Tierney.13, 14 When calculations were not possible because of inadequate data, ORs were estimated using Kaplan-Meier graphs. I2 statistic was used to estimate the percentage of total variation across studies attributable to heterogeneity rather than chance. Meta-analysis was performed using Review Manager (version 5.1.2, Cochrane Collaboration, Oxford, United Kingdom). All P values were two-sided, and P ≤ 0.05 was considered to indicate statistical significance.
Individual patient survival data were reconstructed using Guyot’s iterative algorithm to solve the Kaplan-Meier equations originally used to produce the published graphs.15 This algorithm used digitalized Kaplan-Meier curve data to find numerical solutions to the inverted Kaplan-Meier equations, and it assumes a constant, noninformative censoring mechanism. The reconstructed patient survival data were then aggregated to form the combined survival curve. Reconstructed Kaplan-Meier analyses were conducted using R (version 3.2.5, R Core Team, Vienna, Austria).
RESULTS
Quantity and Quality of Trials
Applying the predefined inclusion criteria, we identified a total of 2211 records through the electronic search. After identification of additional records through other sources and removal of duplicate studies, 1744 articles remained for screening. Of these, 1698 were excluded on the basis of title and abstract content. After review of the full text of the remaining 46 articles, 32 were found to meet the selection criteria for the systematic review.7, 16–46 These included one retrospective pooled analysis of two randomized controlled trials and 31 observational studies, of which 24 provided data on propensity matched populations. By selecting the most complete and updated studies from each institution or database, we identified 23 studies for quantitative meta-analysis. Quality assessment using the Newcastle-Ottawa Scale reported scores that ranged from 5 – 8 points, with a median of 6 points, indicating moderate quality overall. A summary of the study selection process is presented in the PRISMA chart in Supplementary Figure 1, and a summary of each study, with detailed characteristics, is presented in Table 1.
Table 1.
Summary of studies comparing overall survival outcomes between stereotactic body radiation therapy (SBRT) and surgical resection for patients with non-small cell lung cancer
| Institution | Author | Study Period | N | Mortality | Morbidity | OS | DFS | CSS | REC | |
|---|---|---|---|---|---|---|---|---|---|---|
| USA | SBRT | Surgery | ||||||||
| SEER | Paul14 | 2007–2012 | 714 | 2253 | ● | ● | ||||
| Paul14 | 2007–2012 | 643 | 643 | ● | ● | |||||
| Smith15 | 2003–2010 | 382 | 1496S 7215L |
○ | ||||||
| Smith15 | 2003–2010 | 300 243 |
300S 243L |
● | ||||||
| Ezer16 | 2002–2009 | 362 | 1881 | ● | ○ | ○ | ||||
| Ezer16 | 2002–2009 | NS | NS | ○ | ○ | |||||
| Yu17 | 2007–2009 | 383 | 3852 | |||||||
| Yu17 | 2007–2009 | 367 | 711 | ○ | ● | ○ | ○ | |||
| Shirvani18 | 2003–2009 | 382 | 8711 | ○ | ● | ● | ||||
| Shirvani18 | 2003–2009 | 251 | 251L | ● | ● | |||||
| Shirvani19 | 2001–2007 | 124 | 6531L 1277S |
○ | ○ | |||||
| Shirvani19 | 2001–2007 | 99 112 |
99L 112S |
○ | ○ | |||||
| NCBD | Yerokun20 | 2008–2011 | 1778 | 4517 | ○ | |||||
| Yerokun20 | 2008–2011 | 1584 | 1584 | ○ | ||||||
| Rosen21 | 2008–2012 | 1781 | 13652 | ● | ● | |||||
| Rosen21 | 2008–2012 | 1781 | 1781 | ● | ● | |||||
| Puri22 | 1998–2010 | 5887 | 111731 | ● | ● | |||||
| Puri22 | 1998–2010 | 5355 | 5355 | ● | ● | |||||
| VA Cancer Registry | Boyer23 | 2001–2010 | 3012 | 8248 | ● | ● | ○ | ○ | ||
| Boyer23 | 2001–2010 | 468 | 468 | ● | ● | |||||
| VA Informatics and Computing Infrastructure | Bryant24 | 2006–2015 | 449 | 4069 | ● | ● | ● | |||
| Washington University | Crabtree25 | 2004–2010 | 151 | 458 | ○ | ○ | ○ | ○ | ||
| Crabtree25 | 2004–2010 | 56 | 56 | ○ | ● | ○ | ||||
| Robinson26 | 2004–2008 | 118 | 260 | ● | ● | ● | ● | ● | ||
| Robinson26 | 2004–2008 | 76 | 76 | ● | ● | ● | ||||
| Puri27 | 2000–2007 | 76 | 462 | |||||||
| Puri27 | 2000–2007 | 57 | 57 | ○ | ○ | ○ | ○ | |||
| Weill Cornell Medical College | Parashar28 | 1993–2012 | 97 | 123W | ● | ● | ● | ○ | ||
| Port29 | 2001–2012 | NR | NR | |||||||
| Port29 | 2001–2012 | 23 | 38W | ● | ● | ○ | ○ | ○ | ||
| Parashar30 | 1999–2010 | 30 | 17 | ○ | ○ | ○ | ||||
| Michael DeBakey VAMC | Cornwell31 | 2009–2014 | 56 | 127 | ||||||
| Cornwell31 | 2009–2014 | 37 | 37 | ● | ● | ● | ● | ● | ○ | |
| Indiana University | Varlotto32 | 1999–2008 | 137 | 132L 48S |
● | ● | ○ | |||
| Varlotto32 | 1999–2008 | 77 | 77 | ● | ● | ● | ||||
| William Beaumont Hospital | Grills33 | 2003–2009 | 55 | 69 | ● | ● | ● | ● | ● | |
| Netherlands | ||||||||||
| St. Antonius Hospital | Kastelijn34 | 2008–2011 | 53 | 175 | ● | ● | ● | ● | ||
| Kastelijn34 | 2008–2011 | 23 | 23 | ○ | ● | ○ | ||||
| VU University Med Center | Verstegen35 | 2003–2007 | 527 | 86 | ||||||
| Verstegen35 | 2003–2007 | 64 | 64 | ● | ● | ● | ● | ● | ||
| VU and Erasmus University | Mokhles36 | 2003–2012 | 481 | 96 | ||||||
| Mokhles36 | 2003–2012 | 73 | 73 | ○ | ○ | ○ | ○ | |||
| Erasmus University | Mokhles37 | 2001–2011 | 209 | 216 | ● | ● | ||||
| University of Groningen | van den Berg38 | 2007–2010 | 197 | 143 | ● | ● | ● | |||
| Amsterdam Cancer Registry | Palma39 | 2005–2007 | 81 | 109 | ||||||
| Palma39 | 2005–2007 | 60 | 60 | ● | ● | |||||
| Japan | ||||||||||
| Nagasaki University Hospital | Miyazaki40 | 2008–2014 | 41 | 57 | ● | ● | ● | ● | ○ | |
| Miyazaki40 | 2008–2014 | 27 | 27 | ● | ● | |||||
| Kyoto University Hospital | Hamaji41 | 2003–2009 | 104 | 413 | ● | ● | ● | ● | ● | |
| Hamaji41 | 2003–2009 | 41 | 41 | ● | ● | ● | ● | ● | ||
| Matsuo42 | 2003–2009 | 115 | 65 | ○ | ○ | ○ | ||||
| Matsuo42 | 2003–2009 | 53 | 53 | ○ | ○ | ○ | ○ | |||
| Tenri and Kurashiki Hospitals | Nakagawa43 | 2001–2011 | 35 | 183 | ● | ● | ● | ○ | ||
| Others | ||||||||||
| PLA General Hospital, China | Wang44 | 2002–2010 | 74 | 106 | ● | ● | ● | ● | ● | ● |
| Wang44 | 2002–2010 | 35 | 35 | ● | ● | ● | ● | |||
| Multi-institutional | Chang7 | 2008–2014 | 31 | 27 | ● | ● | ● | ● | ● | |
CSS, cancer-specific survival; DFS, disease-free survival
, lobectomy; NCDB, National Cancer Database; OS, overall survival; PLA, People’s Liberation Army; REC, locoregional or distant recurrence
, sublobar resection; SEER, Surveillance, Epidemiology, and End Results; VA, Veterans Affairs; VAMC, Veterans Affairs Medical Center; VU, Vrije Universiteit
, wedge. Dots denote presented data. Solid dots denote data selected for quantitative analysis. Shaded studies indicate matching of patients by propensity score analysis or retrospective pooling of randomized data.
Propensity Score Matching
The systematic review identified 24 studies that used propensity score matching by statistically balancing a number of covariables, which can be categorized into patient characteristics, preoperative risk factors, and tumor characteristics. The most commonly used factors included age; gender; Charlson comorbidity index; performance status; pulmonary function test; size, stage, location, and histologic profile of the tumor; and the preprocedural use of positron emission tomography. A summary of all the chosen covariates for propensity matched studies selected for meta-analysis is presented in Table 2. When individual studies used more than one caliper for comparison between treatment groups, data were derived from the most detailed comparison.
Table 2.
Summary of covariates used for propensity score matching in comparative studies on stereotactic body radiation therapy versus surgical resection for early-stage non-small cell lung cancer
| Study | Patient Characteristics | Pre-Operative Risk Factors | Tumor Characteristics | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Race | Education | Income | Insurance | Geography | CCI | ACE | PS | DI | PFT | O2 Use | Home Services | Size | Stage | Location | Histology | PET | |
| Paul14 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||
| Smith15 | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||
| Ezer16 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||
| Yu17 | ● | ● | ● | ● | ● | ● | ● | ||||||||||||
| Shirvani18 | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||
| Rosen21 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||
| Puri22 | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||
| Boyer23 | ● | ● | ● | ● | ● | ● | ● | ||||||||||||
| Crabtree25 | ● | ● | ● | ● | ● | ● | |||||||||||||
| Robinson26 | ● | ||||||||||||||||||
| Port29 | ● | ● | ● | ||||||||||||||||
| Cornwell31 | ● | ● | ● | ||||||||||||||||
| Varlotto32 | ● | ● | ● | ● | ● | ||||||||||||||
| Kastelijn34 | ● | ● | ● | ● | ● | ● | |||||||||||||
| Verstegen35 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||
| Palma39 | ● | ● | ● | ||||||||||||||||
| Miyazaki40 | ● | ● | ● | ● | ● | ● | |||||||||||||
| Hamaji41 | ● | ● | ● | ● | ● | ||||||||||||||
| Wang44 | ● | ● | ● | ● | ● | ● | ● | ||||||||||||
ACE, adult comorbidity evaluation; CCI, Charlson comorbidity index; DI, disability index; PET, pre-treatment position-emission; PFT, pulmonary function tests; PS, performance status; tomography.
Patient Characteristics
A summary of baseline characteristics of matched patients selected for meta-analysis— including age, gender, SBRT regimen, and surgical procedure details—is presented in Table 3. A summary of these details for unmatched patients is presented in Supplementary Table 1. In brief, the interquartile range of ages for matched patients was 71–78 years for those who underwent SBRT and 68–78 years for those who underwent surgery. Gender variations were noted to be significantly different among studies, with four studies, primarily from military institutions or registries, reporting study populations comprising <10% females.25, 26, 33, 46 SBRT regimens varied in dosage and fractions among centers and within each institution, depending on the location, size, and type of the tumor. When resection type was specified, lobectomies accounted for >60% of resections in the studies selected for meta-analysis, with sublobar resections accounting for the majority of the remaining surgical procedures. The use of video-assisted thoracoscopic surgery (VATS) varied among reports, with four studies only reporting on VATS procedures.16, 33, 37, 43 A summary of histopathological details and clinical staging for the matched SBRT and surgical patients is presented in Table 4. A summary of these details for unmatched patients is presented in Supplementary Table 2. In brief, adenocarcinoma and squamous cell carcinoma were the most common types of NSCLC. Up to 70% of patients who underwent SBRT did not have a pretreatment pathological diagnosis of NSCLC.36 However, the proportion of patients who underwent SBRT without histopathological confirmation appeared to differ between European centers and institutions in the United States. Histopathological demonstration of malignancy was confirmed in >90% of surgical patients in all selected studies. In regard to clinical staging, 71% to 84% of matched patients who underwent SBRT had stage IA disease, and 16% to 29% had stage IB disease. For matched patients who underwent surgery, 70% to 82% had stage IA disease, and 18% to 34% had stage IB disease (staged according to the 7th edition of the TNM classification for NSCLC).47
Table 3.
Summary of baseline patient characteristics and treatment details of matched patients who underwent stereotactic body radiation therapy (SBRT) or surgical resection for early-stage non-small cell lung cancer in studies selected for meta-analysis
| Authors | Median Age | Females (%) | Treatment Regimen | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBRT | Sx | SBRT | Sx | SBRT | Resection Type (%) | Technique (%) | ||||||
| Total Gys | Fractions | Lobectomy | Sublobar | Other | VATS | Open | ||||||
| Wedge | Segmentectomy | |||||||||||
| Paul14 | 78M | 78M | 60 | 62 | NR | NR | NR | NR | NR | 0 | 100 | 0 |
| Smith15 | 77L 78S |
77L 78S |
59L 58S |
62L 61S |
NR | NR | 100 | 100 | 0 | 27L 40S |
73L 60S |
|
| Shirvani18 | NS | NS | NS | NS | NR | NR | 100 | NR | NR | NR | NR | NR |
| Rosen21 | 76M | 75M | 57 | 56 | NR | 3–5 | 100 | 0 | 0 | 0 | NR | NR |
| Puri22 | NS | NS | NS | NS | NR | NR | NS | NS | NS | NS | NR | NR |
| Boyer23 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Crabtree25 | 71M | 70M | 48 | 44 | 45–60 | 3–6 | 78 | 9 | 11 | 2B | NR | NR |
| Robinson26 | 76 | 65 | 45 | 51 | 45–54 | 3–5 | 94 | 0 | 0 | 3B 3P | NR | NR |
| Cornwell31 | 66 | 68 | 3 | 3 | 50–56 | 4–5 | 100 | 0 | 0 | 0 | 100 | 0 |
| Varlotto32 | NR | NR | NR | NR | 48–60 | 3–5 | NR | NR | NR | NR | NR | NR |
| Kastelijn34 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Verstegen35 | 71M | 68M | 42 | 44 | 54–60 | 3–12 | 100 | 0 | 0 | 0 | 100 | 0 |
| Palma39 | 79 | 79 | 33 | 33 | 32–60 | 2–8 | 82 | 15 | 3P | NR | NR | |
| Miyazaki40 | 82 | 82 | 33 | 27 | NR | NR | NR | NR | NR | NR | NR | NR |
| Hamaji41 | 73 | 74 | 24 | 22 | 48–60 | 4–8 | 100 | 0 | 0 | 0 | 100 | 0 |
| Wang44 | 77M | 75M | 6 | 6 | NR | NR | NR | NR | NR | NR | NR | NR |
| Chang7 | 67 | 67 | 55 | 59 | 50–54STARS 54–60ROSEL |
3–4STARS 4–5ROSEL |
88 | 4 | 0 | 8* | 23 | 77 |
, bilobectomy
, lobectomy
, mean value; NR, not reported
, pneumonectomy
, sublobar; Sx, surgery; VATS; video-assisted thoracoscopic surgery.
VATS biopsy and abortion, 4% each.
Table 4.
Summary of histopathological and clinical staging details of matched patients who underwent stereotactic body radiation therapy (SBRT) or surgical resection for early-stage non-small cell lung cancer in studies selected for meta-analysis
| Author | Histopathology – SBRT (%) | Histopathology – Surgery (%) | Clinical Stage – SBRT (%) | Clinical Stage – Surgery (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | S | O | U | A | S | O | U | IA | IB | IIA | IIB/IIIA | IA | IB | IIA | IIB/IIIA | |
| Paul14 | 49 | 43 | 8 | 0 | 47 | 43 | 10 | 0 | 70 | NR | NR | NR | 70 | NR | NR | NR |
| Smith15 | NR | NR | NR | NR | NR | NR | NR | NR | 82 | 18 | 0 | 0 | 82 | 18 | 0 | 0 |
| Shirvani18 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Rosen21 | 48 | 33 | 19 | 0 | 50 | 36 | 14 | 0 | 77 | 23 | 0 | 0 | 77 | 23 | 0 | 0 |
| Puri22 | NR | NR | NR | NR | NR | NR | NR | NR | 76 | 24 | 0 | 0 | 72 | 28 | 0 | 0 |
| Boyer23 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Crabtree25 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 29 | NR | NR | NR | 43 | NR | NR |
| Robinson26 | 45 | 33 | 21 | 1 | 60 | 33 | 3 | 4 | 74 | 22 | 4 | 0 | 77 | 20 | 3 | 0 |
| Cornwell31 | 46 | 41 | 13 | 0 | 41 | 43 | 16 | 0 | 76 | 24 | 0 | 0 | 81 | 19 | 0 | 0 |
| Varlotto32 | NR | NR | NR | NR | NR | NR | NR | NR | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Kastelijn34 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Verstegen35 | 23 | 14 | 16 | 47 | 30 | 11 | 9 | 50 | 61 | 39 | 0 | 0 | 61 | 38 | 0 | 1 |
| Palma39 | NR | NR | NR | NR | NR | NR | NR | NR | 65 | 35 | 0 | 0 | 65 | 35 | 0 | 0 |
| Miyazaki40 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Hamaji41 | 54 | 24 | 22 | 0 | 56 | 27 | 17 | 0 | 71 | 29 | 0 | 0 | 66 | 34 | 0 | 0 |
| Wang 44 | 48 | 46 | 0 | 6 | 51 | 43 | 6 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Chang7 | 52 | 16 | 6 | 26 | 48 | 26 | 4 | 22 | 87 | 13 | 0 | 0 | 96 | 4 | 0 | 0 |
A, adenocarcinoma; L, lobectomy; NR, not reported; O, other type of non-small cell lung cancer; S, squamous cell carcinoma; U, undefined.
Overall Survival
Sixteen studies provided comparative overall survival outcomes on 10,333 patients who underwent SBRT and 142,293 unmatched patients who underwent surgical resection. Fourteen studies reported overall survival for 8946 patients who underwent SBRT and 8942 matched patients who underwent surgery. The unmatched studies demonstrated a significantly superior survival outcome after surgery, compared with SBRT (OR, 2.49; 95% confidence interval [CI], 2.10–2.94; p<0.00001; I2=86%; Figure 1A). When the matched cohorts were compared, overall survival remained superior for surgery, compared with SBRT (OR, 1.71; 95% CI, 1.52–1.93; p<0.00001; I2=63%; Figure 1B). Six studies in which resection type was specified reported unmatched patients who underwent SBRT or lobectomy, demonstrating superior survival outcomes after lobectomy (OR, 2.68; 95% CI, 2.04–3.53; p<0.00001; I2=84%; Supplementary Figure 2). The superiority of lobectomy for overall survival persisted when matched patients from eight studies were compared (OR, 1.61; 95% CI, 1.23–2.12; p=0.0006; I2=77%; Supplementary Figure 3). Six studies compared unmatched patients who underwent SBRT or sublobar resection and found superior outcomes after sublobar resection (OR, 1.54; 95% CI, 1.36–1.75; p<0.00001; I2=32%; Supplementary Figure 4). There was an insufficient number of studies comparing matched patients who underwent SBRT or sublobar resection to conduct a meta-analysis. A reconstructed Kaplan-Meier graph of overall survival, using aggregated data on matched patients who underwent SBRT versus surgery, is shown in Figure 2.
Figure 1.
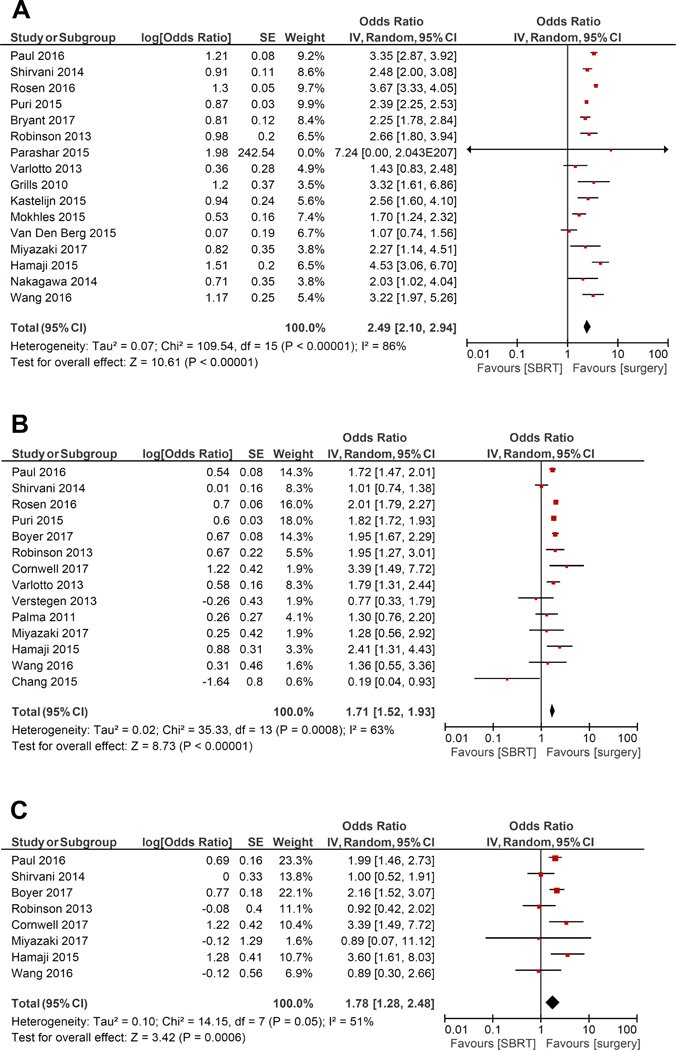
Forest plot of the odds ratio (OR) of overall survival in unmatched patients (A), overall survival in matched patients (B), and cancer-specific survival in matched patients (C) after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Figure 2.
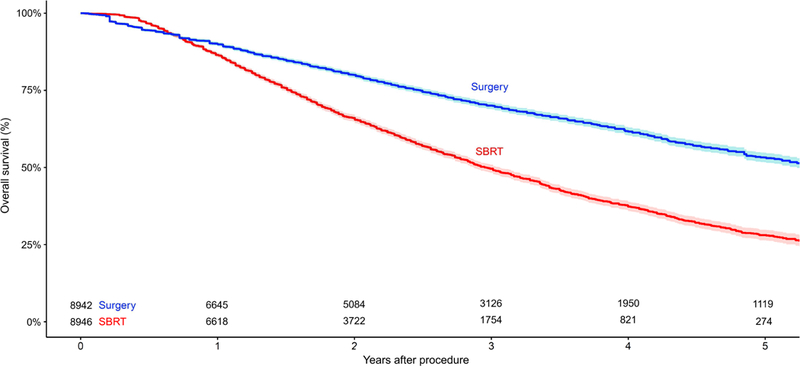
Reconstructed Kaplan-Meier graph of overall survival using aggregated data from matched patients with early-stage non-small cell lung cancer who underwent stereotactic body radiation therapy (SBRT) versus surgery. Shading represents the 95% confidence limits around the central estimate.
Cancer-Specific Survival
Eight studies provided comparative data on cancer-specific survival for unmatched patients who underwent SBRT or surgery, demonstrating significantly superior outcomes after surgery (OR, 2.44; 95% CI, 1.86–3.19; p<0.00001; I2=58%; Supplementary Figure 5). Eight studies also presented cancer-specific survival data on matched patients, showing superior outcomes after surgery (OR, 1.78; 95% CI, 1.28–2.48; p=0.0006; I2=51%; Figure 1C). A reconstructed Kaplan-Meier graph of cancer-specific survival, using aggregated data on matched patients who underwent SBRT versus surgery, is shown in Figure 3.
Figure 3.
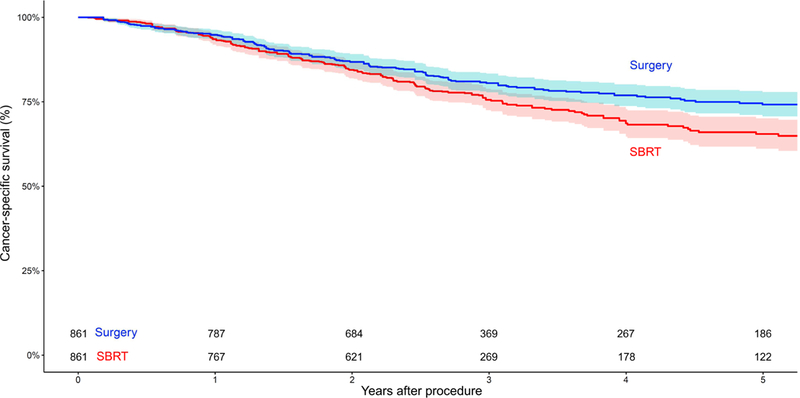
Reconstructed Kaplan-Meier graph of cancer-specific survival using aggregated data from matched patients with early-stage non-small cell lung cancer who underwent stereotactic body radiation therapy (SBRT) versus surgery. Shading represents the 95% confidence limits around the central estimate.
Disease-Free Survival
Five studies provided comparative data on disease-free survival for unmatched patients who underwent SBRT or surgery, demonstrating significantly superior outcomes after surgery (OR, 2.13; 95% CI, 1.65–2.75; p<0.00001; I2=0%; Supplementary Figure 6). When the analysis was limited to matched patients, seven studies demonstrated superior disease-free survival in the surgical cohort (OR, 1.83; 95% CI, 1.06–3.16; p=0.03; I2=82%; Supplementary Figure 7).
Freedom from Disease Recurrence
Six studies provided comparative data on locoregional recurrence for unmatched patients who underwent SBRT or surgery, demonstrating significantly superior outcomes after surgery (OR, 5.44; 95% CI, 1.68–17.56; p<0.005; I2=87%; Supplementary Figure 8). When the analysis was limited to matched patients, six studies demonstrated superior locoregional recurrence rates in the surgical cohort (OR, 2.91; 95% CI, 1.49–5.71; p=0.002; I2=0%; Supplementary Figure 9).
Five studies reported distant recurrence for unmatched patients, showing a nonsignificant trend favoring surgery over SBRT (OR, 1.50; 95% CI, 0.96–2.34; p=0.07; I2=60%). There was an insufficient number of studies comparing matched patients who underwent SBRT versus surgery to conduct a meta-analysis.
Periprocedural Morbidity and Mortality
Periprocedural mortality was defined as death within the same admission or within 30 days of SBRT or surgery. For matched patients, the reported periprocedural mortality was 0% for SBRT and 0% to 8% (interquartile range 0% to 3.25%) for surgery. Periprocedural morbidities varied in nature and frequency after the two treatment modalities. The most commonly reported morbidities after SBRT were fatigue, radiation pneumonitis, chest pain, and rib fractures. The most commonly reported morbidities after surgery were prolonged air leak, pneumonia, pulmonary embolism, cardiac arrhythmia, and myocardial infarction. Summaries of the reported periprocedural mortality and morbidity outcomes for matched and unmatched patients are presented in Supplementary Tables 3 and 4, respectively.
DISCUSSION
Encouraging outcomes of SBRT compared to conventional radiotherapy has led to a paradigm shift in the management of patients with early-stage NSCLC who are considered inoperable surgical candidates.3, 48, 49 Although there is currently no class I evidence to compare SBRT with surgical resection, recent guidelines from the American Society of Radiation Oncology, endorsed by the American Society of Clinical Oncology, recommend that SBRT should be considered for all patients with stage I NSCLC who are considered high risk for surgery.50, 51 With the increasing prevalence of lung cancer screening programs and an aging population with increased co-morbidities, there is a growing number of ‘high risk’ patients diagnosed with resectable NSCLC.52 There is an urgent need to clearly delineate the periprocedural and long-term clinical outcomes of these two modalities to help refine the treatment selection process for this group of patients.
The present systematic review identified 32 comparative studies with overall survival outcomes for SBRT versus surgical resection, and patients from the most updated and complete studies were divided into unmatched and matched cohorts for meta-analysis. Key findings included statistically superior outcomes for surgery for overall survival, cancer-specific survival, disease-free survival, and freedom from locoregional disease recurrence, in both unmatched and matched cohorts. There was a trend favoring surgery for freedom from distant disease recurrence, but this finding was not statistically significant. After matching was performed, ORs were reduced relative to the unmatched comparisons but remained in favor of surgery. This reduction in the magnitude of benefits after matching suggests that some of the long-term clinical outcomes favoring surgery may result from an imbalance in baseline patient characteristics, preoperative comorbidities, or tumor characteristics, rather than treatment efficacy. Nonetheless, it should be noted that the present study identified the most comparable cohorts in the current literature and demonstrated that surgery remained superior to SBRT for mid- and long-term outcomes when analysis was limited to only matched patients. Subgroup analysis of lobectomy versus SBRT demonstrated superior overall survival outcomes for lobectomy for both unmatched and matched cohorts. Sublobar resection was also superior to SBRT for overall survival, although there was a limited number of studies with matched data. Reporting of perioperative mortality and morbidity outcomes varied widely across studies, with slightly higher perioperative mortality for surgery than for SBRT in both the matched and unmatched cohorts. This is consistent with recent findings of higher mortality at 30 and 90 days for surgery than SBRT.53 In addition, it should be acknowledged that clinical benefits in overall and cancer-specific survival associated with surgery were not apparent until 2 to 4 years after the operation, an important consideration for patients with limited life expectancies. Other important findings from the systematic review include significant variations in patient and tumor characteristics among studies, especially between institutions in Europe and the United States. Histopathological confirmation of NSCLC in the SBRT arm varied widely, between 30% and 100%, with five studies reporting <75% of patients with a confirmed histopathological diagnosis.7, 36, 37, 39, 42 It should be noted that two of these studies were the only publications that showed a trend of longer disease-free survival for SBRT than surgery.7, 37
The present study has several limitations. The most important limitation is the lack of level I clinical evidence in the form of randomized controlled trials and the intrinsic patient selection bias present in observational studies. Despite a strong international effort to enroll patients, only 68 of the combined target of 2410 patients (2.8%) were ever successfully enrolled in three planned randomized controlled trials.54, 55 Slow accrual of patients may be at least partially attributable to a lack of equipoise for surgeons who still favor surgical resections with well-established long-term clinical data.47 Patients allocated to the SBRT arm were often those considered inoperable or high risk, with increased comorbidities that prohibited a surgical resection. The Sublobar Resection Versus Stereotactic Ablative Radiotherapy for Lung Cancer (STABLE-MATES) trial (NCT02468024 on ClinicalTrials.gov) is currently recruiting high-risk patients with peripherally located stage I NSCLC, who are randomized to either SBRT or sublobar resection, with the primary endpoint defined as overall survival and secondary endpoints of progression-free survival and toxicity. In randomized trials that experienced difficulties accruing patients, one method of minimizing potential bias was to compare the two treatment arms using propensity scores. Although this statistical technique can balance selected observed covariates, it does not replace the robustness of randomized trials, owing to a wide range of unobserved covariates.10, 56 The closeness of matching, also known as the caliper, differed among studies, depending on the reservoir of potential matches and the number of measured covariates between treatment groups.57 Additional statistical limitations of the present meta-analysis included relatively high heterogeneity identified among studies, potential overlapping of individual patients between institutions and databases, and the intrinsic limitations of the Guyot’s method such as assumptions on constant censoring at each time interval. This assumption affects the relative weights of different portions of the curve, particularly as follow-up durations increase and the levels of information is reduced, potentially underestimating the uncertainty in the reconstructed hazard ratios.15 Other limitations of the current literature included variations in treatment regimens among institutions. Radiation dosages, doses per fraction, and treatment techniques for SBRT differed among centers, and this may have influenced the biological effective dose, treatment delivery precision, and oncologic efficacy. Surgical procedures also differed among studies, with variable portions of patients who underwent lobectomies versus sublobar resections and open thoracotomies versus VATS procedures. Future studies should compare SBRT with the current standard of care for eligible surgical candidates, which is VATS anatomical resection including lobectomy or segmentectomy, with systematic mediastinal lymph node sampling or dissection.58 Finally, it should be noted that the follow-up duration for patients who underwent SBRT was relatively short, with only one study with a specified imaging protocol reporting a median follow-up beyond 5 years. Unfortunately, no data for histopathological diagnosis were provided in this study.40 Although cancer-specific survival and disease-free survival have been considered to be more appropriate endpoints than overall survival for comparisons of SBRT and surgery in the context of patients with significant medical comorbidities, the inconsistent reporting of histopathological diagnosis, the variations in follow-up imaging, and the relative short-term follow-up duration make these endpoints difficult to interpret.
In conclusion, the present systematic review and meta-analysis of propensity-matched observational studies found surgical resection to be associated with superior overall, cancer-specific, and disease-free survival, compared with SBRT. Locoregional recurrence was also found to be significantly less frequent after surgery than SBRT. However, despite propensity matching, caution should be applied when interpreting these findings, given the potential for unrecognized selection bias inherent in observational studies comparing patients with different baseline characteristics. Indeed, differences in clinical outcomes were significant, although to a smaller degree, when analyses were limited to patient cohorts matched by propensity score or retrospective pooling of randomized trials. Nonetheless, it should be recognized that the present systematic review and meta-analysis represents the best evidence in the current literature, and the key analyses performed demonstrated results that were mostly consistent in both direction and magnitude. Perioperative mortality was higher after surgery than SBRT, and the incidences and types of morbidities varied between the two treatment modalities. To strengthen the existing clinical evidence, future studies on SBRT should aim to confirm histopathological diagnosis before treatment whenever possible and should provide long-term follow-up data with clearly defined imaging protocols. Surgical patients in comparative studies should undergo the current standard of care, which is VATS anatomical resection with systematic lymph node sampling or dissection. Comparing modern techniques of SBRT with the current practice of surgical resection will help refine the patient selection process and help define the optimal treatment modality for patients with early-stage NSCLC.
Supplementary Material
Supplementary Figure 1. PRISMA flow chart summarizing the literature search strategy in the systematic review of stereotactic body radiation therapy versus surgical resection for patients with early-stage non-small cell lung cancer.
Supplementary Figure 2. Forest plot of the odds ratio (OR) of overall survival in unmatched patients after stereotactic body radiation therapy (SBRT) versus lobectomy in patients with earlystage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 3. Forest plot of the odds ratio (OR) of overall survival in matched patients after stereotactic body radiation therapy (SBRT) versus lobectomy in patients with earlystage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 4. Forest plot of the odds ratio (OR) of overall survival in unmatched patients after stereotactic body radiation therapy (SBRT) versus sublobar resection in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 5. Forest plot of the odds ratio (OR) of cancer-specific survival in unmatched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 6. Forest plot of the odds ratio (OR) of disease-free survival in unmatched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 7. Forest plot of the odds ratio (OR) of disease-free survival in matched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 8. Forest plot of the odds ratio (OR) of freedom from locoregional recurrence in unmatched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 9. Forest plot of the odds ratio (OR) of freedom from locoregional recurrence in matched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Acknowledgments
Financial Support: This work was supported, in part, by NIH Cancer Center Support Grant P30 CA008748.
Glossary of Abbreviations:
- CI
confidence interval
- NSCLC
non-small cell lung cancer
- OR
odds ratio
- SBRT
stereotactic body radiation therapy
- VATS
video-assisted thoracoscopic surgery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI Statement:
A.R. has received funding from Varian Medical Systems, Boehringer Ingelheim, Pfizer, and Astra Zeneca. All other authors have no potential conflicts of interest.
IRB approval/clinical registry: NA
Central Picture:
Kaplan-Meier graph of overall survival using data from matched patients with NSCLC.
Central Message:
In matched patients with early-stage NSCLC, surgery was superior to SBRT in overall survival, cancer-specific survival, disease-free survival, and freedom from disease recurrence.
Perspective Statement:
With a paucity of randomized data, observational studies have utilized propensity score matching to minimize the risk of selection bias to compare surgery versus SBRT in patients with NSCLC. This systematic review and meta-analysis identified superior mid- and long-term clinical outcomes for surgery in both matched and unmatched patient cohorts. However, periprocedural mortality was lower for SBRT.
REFERENCES
- 1.Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290–6. [DOI] [PubMed] [Google Scholar]
- 2.Baba F, Shibamoto Y, Ogino H, Murata R, Sugie C, Iwata H, et al. Clinical outcomes of stereotactic body radiotherapy for stage I non-small cell lung cancer using different doses depending on tumor size. Radiat Oncol 2010;5:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol 2013;52:1552–8. [DOI] [PubMed] [Google Scholar]
- 4.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153–9. [DOI] [PubMed] [Google Scholar]
- 5.Woody NM, Stephans KL, Marwaha G, Djemil T, Videtic GM. Stereotactic Body Radiation Therapy for Non-Small Cell Lung Cancer Tumors Greater Than 5 cm: Safety and Efficacy. Int J Radiat Oncol Biol Phys 2015;92:325–31. [DOI] [PubMed] [Google Scholar]
- 6.Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989–96. [DOI] [PubMed] [Google Scholar]
- 7.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao C, D’Amico T, Demmy T, Dunning J, Gossot D, Hansen H, et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e370–1. [DOI] [PubMed] [Google Scholar]
- 9.Meyers BF, Puri V, Broderick SR, Samson P, Keogan K, Crabtree TD. Lobectomy versus stereotactic body radiotherapy for stage I non-small cell lung cancer: Post hoc analysis dressed up as level-1 evidence? J Thorac Cardiovasc Surg 2015;150:468–71. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum PR,DB. The central role of propensity score in observational studies for casual effects. Biometrika 1983:41–55. [Google Scholar]
- 11.Phan K, Tian DH, Cao C, Black D, Yan TD. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015;4:112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells GS, O’Connell B, Peterson D, Welch J, Losos V, Tugwell M, P. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses
- 13.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- 15.Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC medical research methodology 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul S, Lee PC, Mao J, Isaacs AJ, Sedrakyan A. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. Bmj 2016;354:i3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith BD, Jiang J, Chang JY, Welsh J, Likhacheva A, Buchholz TA, et al. Costeffectiveness of stereotactic radiation, sublobar resection, and lobectomy for early nonsmall cell lung cancers in older adults. J Geriatr Oncol 2015;6:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezer N, Veluswamy RR, Mhango G, Rosenzweig KE, Powell CA, Wisnivesky JP. Outcomes after Stereotactic Body Radiotherapy versus Limited Resection in Older Patients with Early-Stage Lung Cancer. J Thorac Oncol 2015;10:1201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu JB, Soulos PR, Cramer LD, Decker RH, Kim AW, Gross CP. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer 2015;121:2341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirvani SM, Jiang J, Chang JY, Welsh J, Likhacheva A, Buchholz TA, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage nonsmall cell lung cancers in the elderly. JAMA Surg 2014;149:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirvani SM, Jiang J, Chang JY, Welsh JW, Gomez DR, Swisher S, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yerokun BA, Yang CJ, Gulack BC, Li X, Mulvihill MS, Gu L, et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:675–86.e4. [DOI] [PubMed] [Google Scholar]
- 23.Rosen JE, Salazar MC, Wang Z, Yu JB, Decker RH, Kim AW, et al. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44–54.e9. [DOI] [PubMed] [Google Scholar]
- 24.Puri V, Crabtree TD, Bell JM, Broderick SR, Morgensztern D, Colditz GA, et al. Treatment Outcomes in Stage I Lung Cancer: A Comparison of Surgery and Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyer MJ, Williams CD, Harpole DH, Onaitis MW, Kelley MJ, Salama JK. Improved Survival of Stage I Non-Small Cell Lung Cancer: A VA Central Cancer Registry Analysis. J Thorac Oncol 2017;12:1814–23. [DOI] [PubMed] [Google Scholar]
- 26.Bryant AK, Mundt RC, Sandhu AP, Urbanic JJ, Sharabi AB, Gupta S, et al. Stereotactic Body Radiation Therapy Versus Surgery for Early Lung Cancer Among US Veterans. Ann Thorac Surg 2018;105:425–31. [DOI] [PubMed] [Google Scholar]
- 27.Crabtree TD, Puri V, Robinson C, Bradley J, Broderick S, Patterson GA, et al. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014;147:1183–91; discussion 91–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson CG, DeWees TA, El Naqa IM, Creach KM, Olsen JR, Crabtree TD, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:192–201. [DOI] [PubMed] [Google Scholar]
- 29.Puri V, Crabtree TD, Kymes S, Gregory M, Bell J, Bradley JD, et al. A comparison of surgical intervention and stereotactic body radiation therapy for stage I lung cancer in high-risk patients: a decision analysis. J Thorac Cardiovasc Surg 2012;143:428–36. [DOI] [PubMed] [Google Scholar]
- 30.Parashar B, Port J, Arora S, Christos P, Trichter S, Nori D, et al. Analysis of stereotactic radiation vs. wedge resection vs. wedge resection plus Cesium-131 brachytherapy in early stage lung cancer. Brachytherapy 2015;14:648–54. [DOI] [PubMed] [Google Scholar]
- 31.Port JL, Parashar B, Osakwe N, Nasar A, Lee PC, Paul S, et al. A propensity-matched analysis of wedge resection and stereotactic body radiotherapy for early stage lung cancer. Ann Thorac Surg 2014;98:1152–9. [DOI] [PubMed] [Google Scholar]
- 32.Parashar B, Patel P, Singh P, Monni S, Trichter S, Sabbas A, et al. Management of single malignant lung nodules in elderly patients (70 years or older) who are not candidates for lobectomy. Am J Clin Oncol 2012;35:480–5. [DOI] [PubMed] [Google Scholar]
- 33.Cornwell LD, Echeverria AE, Samuelian J, Mayor J, Casal RF, Bakaeen FG, et al. Video-assisted thoracoscopic lobectomy is associated with greater recurrence-free survival than stereotactic body radiotherapy for clinical stage I lung cancer. J Thorac Cardiovasc Surg 2018;155:395–402. [DOI] [PubMed] [Google Scholar]
- 34.Varlotto J, Fakiris A, Flickinger J, Medford-Davis L, Liss A, Shelkey J, et al. Matchedpair and propensity score comparisons of outcomes of patients with clinical stage I nonsmall cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683–91. [DOI] [PubMed] [Google Scholar]
- 35.Grills IS, Mangona VS, Welsh R, Chmielewski G, McInerney E, Martin S, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-smallcell lung cancer. J Clin Oncol 2010;28:928–35. [DOI] [PubMed] [Google Scholar]
- 36.Kastelijn EA, El Sharouni SY, Hofman FN, Van Putte BP, Monninkhof EM, Van Vulpen M, et al. Clinical Outcomes in Early-stage NSCLC Treated with Stereotactic Body Radiotherapy Versus Surgical Resection. Anticancer Res 2015;35:5607–14. [PubMed] [Google Scholar]
- 37.Verstegen NE, Oosterhuis JW, Palma DA, Rodrigues G, Lagerwaard FJ, van der Elst A, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543–8. [DOI] [PubMed] [Google Scholar]
- 38.Mokhles S, Verstegen N, Maat AP, Birim O, Bogers AJ, Mokhles MM, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: results from propensity score analysis. Lung Cancer 2015;87:283–9. [DOI] [PubMed] [Google Scholar]
- 39.Mokhles S, Nuyttens JJ, Maat AP, Birim O, Aerts JG, Bogers AJ, et al. Survival and treatment of non-small cell lung cancer stage I-II treated surgically or with stereotactic body radiotherapy: patient and tumor-specific factors affect the prognosis. Ann Surg Oncol 2015;22:316–23. [DOI] [PubMed] [Google Scholar]
- 40.van den Berg LL, Klinkenberg TJ, Groen HJ, Widder J. Patterns of Recurrence and Survival after Surgery or Stereotactic Radiotherapy for Early Stage NSCLC. J Thorac Oncol 2015;10:826–31. [DOI] [PubMed] [Google Scholar]
- 41.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240–4. [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki T, Yamazaki T, Nakamura D, Sato S, Yamasaki N, Tsuchiya T, et al. Surgery or stereotactic body radiotherapy for elderly stage I lung cancer? A propensity score matching analysis. Surg Today 2017;47:1476–83. [DOI] [PubMed] [Google Scholar]
- 43.Hamaji M, Chen F, Matsuo Y, Kawaguchi A, Morita S, Ueki N, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015;99:1122–9. [DOI] [PubMed] [Google Scholar]
- 44.Matsuo Y, Chen F, Hamaji M, Kawaguchi A, Ueki N, Nagata Y, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. Eur J Cancer 2014;50:2932–8. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawa T, Negoro Y, Matsuoka T, Okumura N, Dodo Y. Comparison of the outcomes of stereotactic body radiotherapy and surgery in elderly patients with cT1–2N0M0 nonsmall cell lung cancer. Respir Investig 2014;52:221–6. [DOI] [PubMed] [Google Scholar]
- 46.Wang P, Zhang D, Guo XG, Li XM, Du LH, Sun BJ, et al. A propensity-matched analysis of surgery and stereotactic body radiotherapy for early stage non-small cell lung cancer in the elderly. Medicine (Baltimore) 2016;95:e5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 48.Simone CB 2nd, Wildt B, Haas AR, Pope G, Rengan R, Hahn SM. Stereotactic body radiation therapy for lung cancer. Chest 2013;143:1784–90. [DOI] [PubMed] [Google Scholar]
- 49.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama 2010;303:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider BJ, Daly ME, Kennedy EB, Antonoff MB, Broderick S, Feldman J, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2017:Jco2017749671. [DOI] [PubMed] [Google Scholar]
- 51.Videtic GMM, Donington J, Giuliani M, Heinzerling J, Karas TZ, Kelsey CR, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295–301. [DOI] [PubMed] [Google Scholar]
- 52.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247–54. [DOI] [PubMed] [Google Scholar]
- 53.Stokes WA, Bronsert MR, Meguid RA, Blum MG, Jones BL, Koshy M, et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J Clin Oncol 2018:Jco2017756536. [DOI] [PubMed] [Google Scholar]
- 54.Moghanaki D, Karas T. Surgery versus SABR for NSCLC. Lancet Oncol 2013;14:e490–1. [DOI] [PubMed] [Google Scholar]
- 55.Louie AV, Senthi S, Palma DA. Surgery versus SABR for NSCLC. Lancet Oncol 2013;14:e491. [DOI] [PubMed] [Google Scholar]
- 56.Rosenbaum P, Rubin D. Reducing bias in observational studies using subclassification on the propensity score. Journal of the American Statistical Association 1984;79:516–24. [Google Scholar]
- 57.Lunt M Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol 2014;179:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan TD, Cao C, D’Amico TA, Demmy TL, He J, Hansen H, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. PRISMA flow chart summarizing the literature search strategy in the systematic review of stereotactic body radiation therapy versus surgical resection for patients with early-stage non-small cell lung cancer.
Supplementary Figure 2. Forest plot of the odds ratio (OR) of overall survival in unmatched patients after stereotactic body radiation therapy (SBRT) versus lobectomy in patients with earlystage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 3. Forest plot of the odds ratio (OR) of overall survival in matched patients after stereotactic body radiation therapy (SBRT) versus lobectomy in patients with earlystage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 4. Forest plot of the odds ratio (OR) of overall survival in unmatched patients after stereotactic body radiation therapy (SBRT) versus sublobar resection in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 5. Forest plot of the odds ratio (OR) of cancer-specific survival in unmatched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 6. Forest plot of the odds ratio (OR) of disease-free survival in unmatched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 7. Forest plot of the odds ratio (OR) of disease-free survival in matched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 8. Forest plot of the odds ratio (OR) of freedom from locoregional recurrence in unmatched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.
Supplementary Figure 9. Forest plot of the odds ratio (OR) of freedom from locoregional recurrence in matched patients after stereotactic body radiation therapy (SBRT) versus surgery in patients with early-stage non-small cell lung cancer. The estimate of the OR of each study corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary OR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics. SE, standard error.


