Abstract
BACKGROUND:
Chemotherapy sensitivity, defined simply as at least a partial response to chemotherapy, is an important outcome predictor for non-Hodgkin lymphoma (NHL) patients undergoing reduced-intensity allogeneic hematopoietic stem cell transplantation (allo-HCT). The authors hypothesized that further differentiation of chemotherapy sensitivity by specific response, complete remission (CR) versus partial remission (PR) versus stable disease (SD) versus progression of disease (PD), correlates with post-transplant outcomes.
METHODS:
The impact of pretransplant and early (28 days) post-transplant disease response on transplant outcomes was analyzed in 63 NHL patients treated with reduced-intensity allo-HCT.
RESULTS:
The 3-year event-free survival (EFS) and overall survival (OS) (median potential follow-up after reduced-intensity allo-HCT = 58 months) for all patients was 37% and 47%, respectively. The 3-year EFS based on pretransplant response was: CR = 50%; PR = 66%; SD = 18%; no patient with PD pretransplant reached 3-year follow-up. The 3-year OS based on pretransplant response was: CR = 63%; PR = 69%; SD = 45%. The 3-year EFS based on post-transplant response was: CR = 57%; PR = 32%; SD = 33%; no patient with PD post-transplant reached 3-year follow-up. The 3-year OS based on post-transplant response was: CR = 65%; PR = 43%; SD = 50%. In multivariate analyses, pretransplant response was the best predictor of EFS (P < .0001). Pretrans-plant response (P < .0001) and age (P = .0035) were jointly associated with OS.
CONCLUSIONS:
These data suggest that NHL patients with pretransplant SD, generally considered inappropriate candidates, may benefit from reduced-intensity allo-HCT, and patients with pretransplant PD should only receive this therapy in clinical trials.
Keywords: chemotherapy sensitivity, hematopoietic stem cell transplantation, non-Hodgkin lymphoma, nonmyeloablative, reduced-intensity
The option of allogeneic hematopoietic cell transplantation (HCT) for relapsed and refractory non-Hodgkin lymphomas (NHL)1–5 has been extended to patients who are older and/or have previously received high-dose therapy and autologous HCT through the use of less intensive conditioning regimens.6–9 Although both nonmyeloablative and reducedintensity allogeneic HCT can result in long-term survival in patients with advanced NHL,10–14 the use of these conditioning regimens has also increased the reliance on graft-versus-lymphoma effects to achieve durable remissions.15,16
The chemotherapy sensitivity of lymphomas has been identified as an important prognostic factor in the nonmyeloablative and reduced-intensity transplant settings.17–20 Determination of chemotherapy sensitivity is based on the classic definition of at least a partial response to salvage therapy given immediately before consideration for transplant.21 This classification does not make a distinction between pretransplant disease state, specifically complete versus partial remissions, and furthermore does not distinguish stable disease from progressive disease among patients whose disease is classified as being chemotherapy resistant. The latter distinction is clinically relevant, as a lack of chemotherapy sensitivity has been considered as an ineligibility criterion in certain nonmyeloablative or reduced-intensity allogeneic HCT protocols for lymphoma.
We previously reported that pretransplant disease status may influence the subsequent outcomes of patients with relapsed or refractory NHL undergoing reduced-intensity allogeneic HCT.22 This latter analysis suggested that differentiating patients according to pretransplant disease state had prognostic significance. On the basis of these preliminary data, we undertook a further analysis on a larger patient population with longer follow-up to verify these results. To further differentiate the impact of chemotherapy sensitivity, we also assessed the impact of early post-transplant disease status, which is attributable in large part to the transplant conditioning regimen, on outcomes after reduced-intensity allogeneic HCT. It was our hypothesis that transplant outcomes would be directly associated with specific disease states determined pretransplant and early post-transplant.
MATERIALS AND METHODS
Patient Eligibility
The analysis included patients with advanced NHL, enrolled onto sequential National Cancer Institute (NCI) protocols NCT00019851, NCT00055744, and NCT00077480 (http://clinicaltrials.gov/ct2/home), from January 2000 through November 2005. The NCI is only permitted to treat patients participating in clinical trials. As such, all eligible NHL patients were treated sequentially on 1 of these 3 protocols within the time frame described above, and all enrolled patients were included in the analysis. None of the 3 protocols required patients to have chemotherapy-sensitive disease to be eligible for participation; patients were only excluded from trial participation if they did not meet minimal requirements for major organ function and performance status. Diagnosis and histology of NHL was confirmed by the NCI Laboratory of Pathology using the World Health Organization classification.23 All 3 protocols were approved by the NCI Institutional Review Board, and informed written consent was obtained from each patient and his or her respective donor.
Treatment
Patients were treated in a nearly identical manner on all 3 protocols. Before transplant, all patients received a modified version of the EPOCH regimen, which had demonstrated efficacy in relapsed NHL.24 The regimen, EPOCH-F, consisted of etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin, administered with fludarabine at conventional doses.22 Some patients, whose lymphoma expressed CD20, also received rituximab with EPOCH-F. The purpose of including EPOCH-F was to provide tumor control, to induce host immune depletion, and to assess chemotherapy sensitivity in a uniform manner before proceeding to transplant. All patients then received an identical reduced-intensity conditioning regimen consisting fludarabine (30 mg/ m2/d) and cyclophosphamide (1200 mg/m2/d) administered intravenously over 4 days followed by a T-cell– replete, peripheral blood allograft, collected from their human leukocyte antigen-matched siblings after mobilization with filgrastim. All patients received a cyclosporine-based graft-versus-host disease (GVHD) prophylaxis regimen. The 3 protocols differed only by the addition of donor Th2 cells,25 methotrexate, or sirolimus for prevention of GVHD, respectively. Preclinical studies had demonstrated that Th2 cells had the ability to reduce GVHD as compared with unmanipulated T cells in a murine transplant model.26 Clinical Th2 cells were generated ex vivo by CD3/ CD28 costimulation of steady-state CD4+ T cells cultured in medium containing IL-4 and IL-2 and were administered 1 day after HCT.
Response Criteria and Evaluation
Standard criteria, available at the times these studies were performed,27 were used for response during the conduct of the 3 trials. Complete remission (CR) was defined as regression of all lymph nodes to normal size (≤1.5 cm), resolution of soft tissue masses or palpable organomegaly because of lymphoma, and clearance of bone marrow infiltration if previously present. Partial remission (PR) required a minimum 50% reduction in the sum of the products of the diameter of reference lesions without enlargement of other lesions, including liver and/or spleen. Progression of disease (PD) was defined by appearance of any new lesion or a minimum 50% increase in sum of the products of the diameter of an existing lesion. Patients who did not meet criteria for PD, PR, or CR by these definitions were categorized as having stable disease (SD).
Disease status was assessed by computed tomography (CT) and bone marrow examination immediately before study entry. CT was subsequently performed after the completion of EPOCH-F, at 28 days, 100 days, 6 months, 9 months, and 12 months post-transplantation, and annually thereafter. 18F-Fluorodeoxyglucose positron emission tomography was used to assess questionable abnormalities. Bone marrow examination was repeated in all patients at 28 and 100 days. If bone marrow involvement by lymphoma was present at study entry, repeat examinations were also performed after the completion of EPOCH-F, at 6 and 12 months after transplant, and at other times if clinically warranted.
Statistical Analysis
The primary aim of this analysis was to determine the association of pretransplant and early (defined as 28 days post-transplant) disease status on outcomes, specifically event-free survival (EFS) and overall survival (OS), after transplant. Pretransplant response to chemotherapy was based on a composite of response to pre-enrollment chemotherapy plus response to induction chemotherapy (EPOCH-F) after enrollment into this study.
Outcomes were analyzed in regard to individual disease states (CR vs PR vs SD vs PD), as well as by the classical definition for chemotherapy sensitive (ie, CR + PR) versus chemotherapy resistant (ie, SD + PD).21 Secondary aims were determination of the incidences of treatment-related mortality and disease progression. Clinical outcomes were also compared with several other disease and transplant characteristics, including recipient Follicular Lymphoma International Prognostic Index score at study entry for follicular lymphomas and International Prognostic Index score at study entry for all other histologies (with the exception of chronic lymphocytic leukemia), histologic aggressiveness, age, acute and chronic GVHD, and Hematopoietic Cell Transplantation Comorbidity Index scores (0–2 vs 3+). Hematopoietic Cell Transplantation Comorbidity Index was determined using the method described in Sorror et al,28 based on comorbidities present at study enrollment. Assignment of histologic aggressiveness as either indolent or aggressive was defined by the Physician Data Query Modification of the Revised European American Lymphoma Classification of Lymphoproliferative Diseases (http://www.cancer.gov/cancertopics/pdq/treatment/adult-nonhodgkins/HealthProfessional/page3#Section_31). Acute and chronic GVHD were assessed according to standard criteria.29,30
Survival, time to progression, and treatment-related mortality (TRM) were calculated from the date of transplantation until death, progression, or last follow-up, as appropriate, through January 2008. Patients without progression or death from treatment were censored at the date of last follow-up. The probability of EFS or OS was determined by the Kaplan-Meier method,31 and the significance of the difference between pairs of Kaplan-Meier curves was calculated with the Mantel-Haenszel test.32 The Cox proportional hazards model was used to identify factors that were jointly significant with respect to their association with survival or EFS.33 Cumulative incidence plots of TRM, adjusting for a competing risk of death from disease, as well as progression adjusting for a competing risk of TRM, were created based on a methodology described by Gooley et al, beginning at the peripheral blood stem cell date.34 Because acute GVHD is defined as occurring up to 3 months after transplant, a landmark analysis was used to report the association between acute GVHD and EFS and OS by subtracting 3 months from all follow-up times and thereby excluding a small number of patients with a short follow-up time.35 In addition, the association of acute GVHD, chronic GVHD, and the first of the 2 types of GVHD with EFS and OS was determined by including the individual GVHD parameters as time-varying covariates in a Cox proportional hazards model. All P values are 2-tailed and reported without adjustment for multiple comparisons.
RESULTS
Patient Characteristics at Study Enrollment
The characteristics of the 63 NHL patients included in the analysis are presented in Table 1. The median patient age was 53 years. The most common histologies were diffuse large B-cell lymphoma (DLBCL; n = 20), follicular lymphoma (FL; n = 12), and T-cell lymphoma (n = 11). Fourteen (22%) patients had their disease classified as indolent, and the remaining 49 (78%) were classified as aggressive. The median number of treatments before study enrollment was 4.
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Total number of patients | 63 |
| Median age, y (range) | 53 (32–74) |
| Sex | |
| Men | 37 |
| Women | 26 |
| Median ECOG performance statusa (range) | 1 (0–2) |
| Number of prior chemotherapy regimens (range) | 4 (1–9) |
| Prior autologous stem cell transplant | |
| Yes | 14 |
| No | 29 |
| NHL histology | |
| Diffuse large B-cell lymphoma | 20 |
| Follicular lymphoma | 12 |
| T-cell lymphomas | 11 |
| Mantle cell lymphoma | 7 |
| Transformed B-cell lymphomas | 7 |
| CLL | 3 |
| Marginal zone | 2 |
| SLL | 1 |
| PDQ REAL disease classification | |
| Indolent | 14 |
| Aggressive | 49 |
| Chemotherapy sensitivity to last treatment before study enrollment | |
| Chemotherapy sensitive | 33 |
| Chemotherapy resistant | 27 |
| Untested | 3 |
| Median IPI scorea (n=50) | 2 |
| Median FLIPI scorea (n=10) | 2 |
| Median HCT-CI scorea (range) | 2 (0–11) |
ECOG indicates Eastern Cooperative Oncology Group; NHL, non-Hodgkin lymphoma (NHL); CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; PDQ REAL, Physician Data Query Modification of the Revised European American Lymphoma Classification of Lymphoproliferative Diseases; IPI score, International Prognostic Index score at study entry; FLIPI score, Follicular Lymphoma International Prognostic Index score at study entry; HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index.
Determined at study enrollment.
Response to Pretransplant Chemotherapy
Twenty-three (37%) patients were determined to have chemotherapy-sensitive disease to the last treatment before study enrollment, 33 (52%) had chemotherapy-refractory disease, and 7 (11%) were untested. Response to induction chemotherapy correlated well with pre-enrollment treatment response, as 96% of patients were assessed as having maintained or improved on their prior response after treatment with EPOCH-F. Among patients with chemotherapy-resistant disease at enrollment, 27% actually had a response to EPOCH-F. The overall composite response to pretransplant chemotherapy was as follows: CR = 8 (13%), PR = 27 (43%), SD = 11 (17%), and PD = 17 (27%).
Assessment of Day 28 Disease Status
Sixty patients were evaluable for Day 28 post-transplant response. Day 28 evaluation demonstrated CR in 23 (38%) patients, PR in 27 (45%) patients, SD in 6 (10%) patients, and PD in 4 (7%) patients. Thirty-two (52%) patients had an improvement in disease status as compared with their pretransplant response.
Treatment-Related Mortality and Disease Relapse and Progression
The minimum post-transplant follow-up is 2 years; the median potential post-transplant follow-up is 58 months. The cumulative incidence of TRM at 6, 12, and 24 months post-transplant, adjusting for the impact of the competing factor of death because of progression, was 19.0%, 20.6%, and 25.4%, respectively. Similarly, the cumulative incidence of disease relapse and progression at 6, 12, and 24 months, after adjusting for TRM, was 30.2%, 33.3%, and 34.9%, respectively.
EFS
The median EFS for all 63 patients was 6.6 months. The 12-, 24-, and 36-month EFS probabilities were 47.6%, 41.3%, and 37.4%, respectively (Fig. 1A). The 3-year EFS for the most common histologies was as follows: FL = 58.3%; DLBCL = 38.9%; T-cell lymphoma = 40.0% (Fig. 2A). There was a strong trend (P = .051) of improved EFS favoring FL over DLBCL. The 3-year EFS probabilities for patients assessed as having chemotherapy-sensitive and chemotherapy-resistant disease after pretransplant chemotherapy were 62% and 7%, respectively (Fig. 3A). The 3-year EFS probabilities for patients who were assessed as having CR, PR, or SD after pretransplant chemotherapy were 50%, 66%, and 18%, respectively (Fig. 4A). No patient with PD has reached 3-year EFS. The 3-year EFS probabilities for patients who were assessed as having CR, PR, SD, or PD at their Day 28 post-transplant assessment were 57%, 32%, 33%, and 0, respectively (Fig. 4C).
Figure 1.
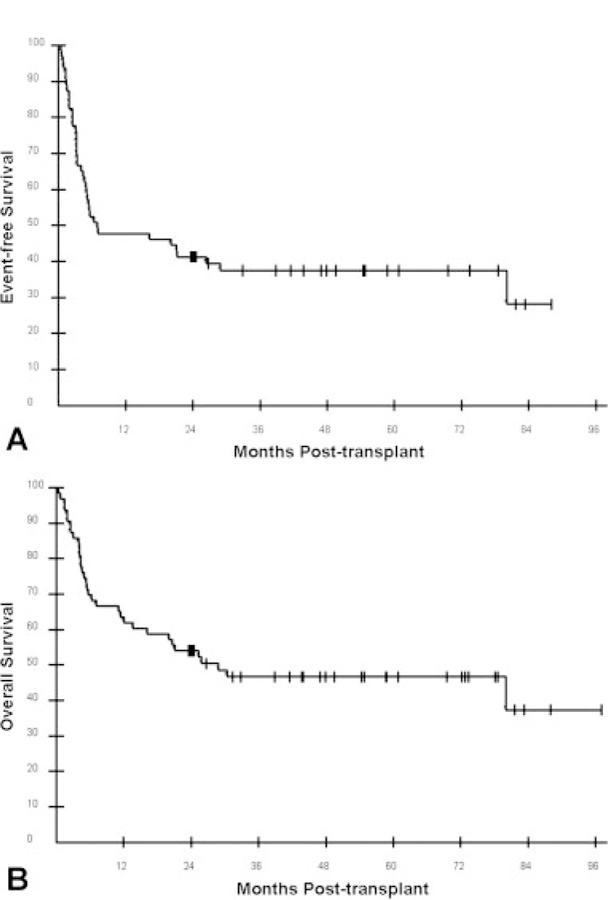
Transplant outcomes are shown for all 63 non-Hodgkin lymphoma patients undergoing reduced-intensity allogeneic hematopoietic cell transplantation: (A) event-free survival; (B) overall survival.
Figure 2.
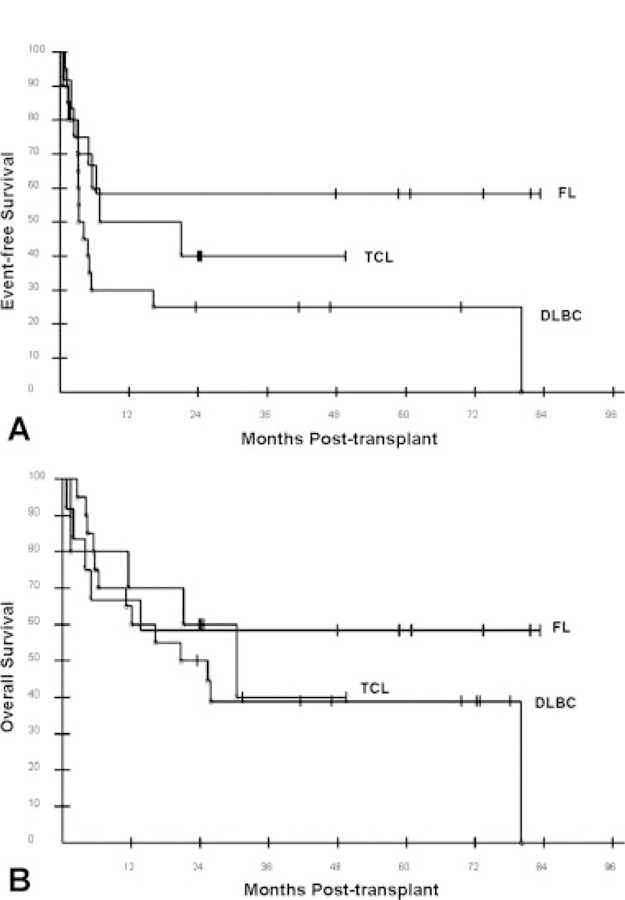
Transplant outcomes of non-Hodgkin lymphoma patients undergoing reduced-intensity allogeneic hematopoietic cell transplantation (HCT) based on histology are shown: (A) event-free survival after reduced-intensity allogeneic HCT according to histology; (B) overall survival after reduced-intensity allogeneic HCT according to histology. FL indicates follicular lymphoma; TCL, T-cell lymphoma; DLBC, diffuse large B-cell lymphoma.
Figure 3.
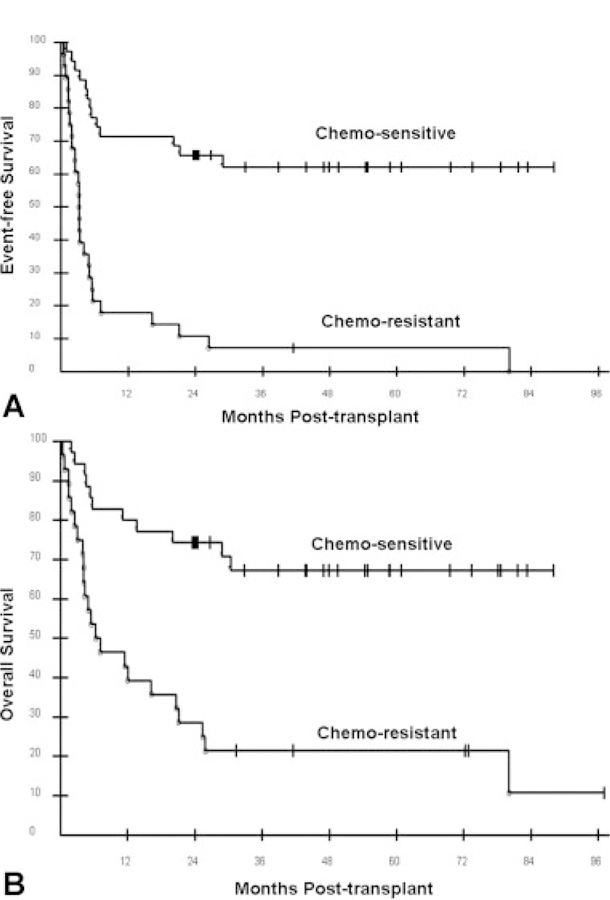
Transplant outcomes based on pretransplant chemotherapy sensitivity are shown: (A) event-free survival; (B) overall survival.
Figure 4.
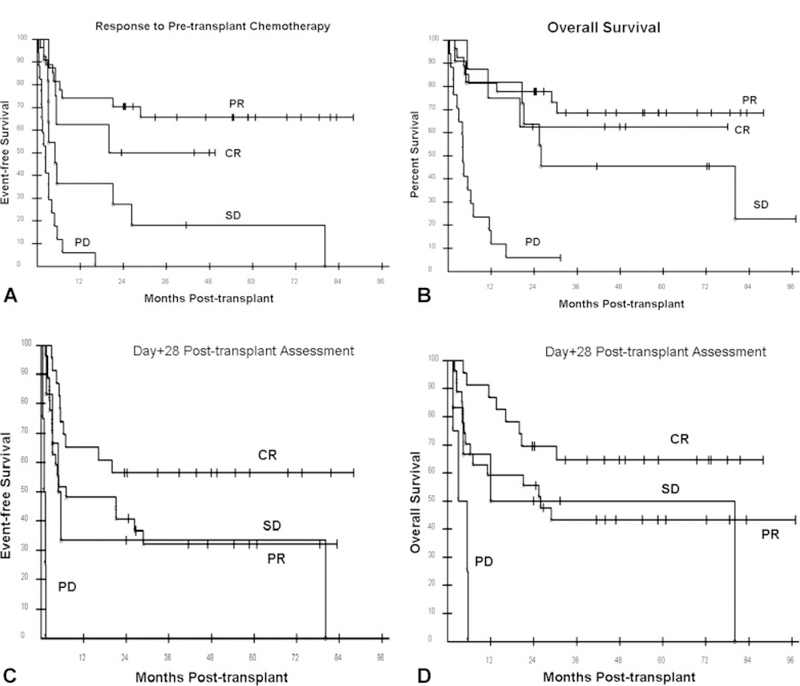
Transplant outcomes based on pretransplant and early post-transplant specific disease responses are shown: (A) event-free survival after reduced-intensity allogeneic hematopoietic cell transplantation (HCT) according to response to pretransplant chemotherapy; (B) overall survival after reduced-intensity allogeneic HCT according to response to pretransplant chemotherapy; (C) event-free survival after reduced-intensity allogeneic HCT according to response to Day 28 post-transplant evaluation; (D) overall survival after reduced-intensity allogeneic stem cell transplantation according to response to Day 28 post-transplant evaluation. CR indicates complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Potential variables found to be favorably associated with EFS in univariate analyses (Table 2) included either CR or PR to pretransplant chemotherapy (P < .0001), disease status (CR or PR) at Day 28 (P = .0096), and lower Hematopoietic Cell Transplantation Comorbidity Index score (P = .05). There was also an association of EFS with specific responses to pretransplant chemotherapy and Day 28 post-transplant evaluation. No other variable was found to be statistically associated with EFS. A multivariate Cox model analysis of these factors determined that the response (CR + PR vs SD + PD) to pretransplant chemotherapy (hazard ratio, 5.77; 95% CI, 2.92–11.43; P < .0001) was the sole predictor for EFS when potential factors were evaluated jointly.
Table 2.
Variables Associated With Event-Free and Overall Survival After Reduced-Intensity Allogeneic Hematopoietic Cell Transplantation for NHL
| Variable | 3-Year EFS | Log Rank P Value for EFS (Adjusted) | 3-Year OS | Log Rank P Value for OS (Adjusted) |
|---|---|---|---|---|
| Pretransplant chemotherapy response | ||||
| CR+PR vs SD+PD | 62% vs 7% | <.0001 | 67% vs 21% | <.0001 |
| CR vs PR | 50% vs 66% | .41 | 63% vs 69% | .73 |
| PR vs SD | 66% vs 18% | .001 | 69% vs 45% | .12 |
| SD vs PD | 18% vs not reached | .014 | 45% vs not reached | .0008 |
| Day 28 disease state | ||||
| CR+PR vs SD+PD | 43% vs 20% | .0032 (.0096) | 53% vs 30% | .011 (.033) |
| CR vs PR | 57% vs 32% | .066 | 65% vs 43% | .10 |
| PR vs SD | 32% vs 33% | .71 | 43% vs 50% | .70 |
| SD vs PD | 32% vs 0 | .001 | 50% vs 0 | .072 |
| HCT-CI | ||||
| 0–2 vs 3+ | 43% vs 24% | .05 | 54% vs 29% | .021 |
| Histologic aggressivenessa | ||||
| Indolent vs aggressive | 57% vs 32% | .08 | 57% vs 44% | .41 |
| Histology | ||||
| FCC vs DLBC | 58% vs 25% | .051 | 58% vs 39% | >.30 |
| FCC vs other | 58% vs 36% | .34 | 58% vs 46% | >.30 |
| Other vs DLBC | 36% vs 25% | .11 | 46% vs 39% | >.30 |
| Age, y | ||||
| 30–40 vs 41–50 | 50% vs 42% | >.25 | 58% vs 59% | >.1 |
| 41–50 vs 51–60 | 42% vs 29% | >.25 | 59% vs 38% | >.1 |
| 51–60 vs 60+ | 29% vs 33% | >.25 | 38% vs 33% | >.1 |
| IPI | ||||
| 0–1 vs 2 | 35% vs 26% | >.25 | 58 vs 31% | .07 |
| 2 vs 3–4 | 26% vs 42% | >.25 | 31% vs 39% | .35 |
| GVHD | ||||
| Acute (yes vs no) | 52% vs 45% | .55b | 48% vs 60% | .10b |
| Chronic (yes vs no) | — | .031c | — | .085c |
| Acute or chronic (yes vs no) | — | .42c | — | .040c |
NHL indicates non-Hodgkin lymphoma; EFS, event-free survival; OS, overall survival; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index; FCC, follicular center cell; DLBC, diffuse large B cell; IPI = International Prognostic Index; GVHD = graft-versus-host disease.
Assessment made by Physician Data Query Modification of the Revised European American Lymphoma Classification of Lymphoproliferative Diseases.
The P values shown are for the association with acute GVHD using a time-varying covariate analysis. By a landmark analysis, P = 0.36 for EFS and P = 0.23 for OS for the association of acute GVHD.
The P values shown were determined by time-varying covariate analysis; the 3-year EFS and OS cannot be determined when the grouping parameter varies over time.
Overall Survival
The median OS for all 63 patients is 26.5 months. The 12-, 24-, and 36-month OS probabilities were 63.5%, 54.0%, and 46.6%, respectively (Fig. 1B). The 3-year EFS for the most common histologies was as follows: FL = 58.3%; DLBCL = 38.9%; T-cell lymphoma = 40.0% (Fig. 2B). There was no statistical difference in EFS among these 3 histologies. The 3-year OS probabilities for patients assessed as having chemotherapy-sensitive and chemotherapy-resistant disease after pretransplant chemotherapy were 67% and 21%, respectively (Fig. 3B). The 3-year OS rates for patients who were assessed as having CR, PR, or SD after pretransplant chemotherapy were 63%, 69%, and 45%, respectively (Fig. 4B). No patient with PD reached 3-year survival, but 2 patients with PD remain alive at 24 and 31 months. The 3-year OS probabilities for patients who were assessed as having CR, PR, SD, or PD at their Day 28 post-transplant assessment were 65%, 43%, 50%, and 0, respectively (Fig. 4D).
Potential variables found to be favorably associated with OS in the univariate analysis (Table 2) included response to pretransplant chemotherapy (P < .0001), disease status (CR or PR) at Day 28 (P = .033), and lower Hematopoietic Cell Transplantation Comorbidity Index score (P = .021). However, there were no statistical differences in OS between patients in CR, PR, or SD after pretransplant chemotherapy or at their Day 28 assessment. The determination of PD either after pretransplant chemotherapy (P = .0008) or at Day 28 assessment (P = .07) was associated with worse OS than patients with SD. No other variable was found to be statistically associated with OS. A multivariate Cox model analysis of these factors determined that the response (CR + PR vs SD + PD) to pretransplant chemotherapy (hazard ratio, 5.58; 95% CI, 2.62–11.87; P < .0001) and age <50 versus >50 years (hazard ratio, 2.94; 95% CI, 1.43–6.07; P = .0035) were the best predictors for OS when factors were considered jointly.
DISCUSSION
Chemotherapy sensitivity has been previously identified in other retrospective analyses as an important clinical prognostic factor for outcomes of patients with NHL undergoing myeloablative, nonmyeloablative, or reduced-intensity allogeneic HCT.5,14,17,18 Our analysis is consistent with these prior observations and extends the understanding of chemotherapy sensitivity as an important outcome determinant. We recognize the heterogeneity in histology present in our study; however, the overwhelming majority (78%) of cases was classified as aggressive according to PDQ Modification of the REAL Classification of Lymphoproliferative Diseases, which has been used in similar analyses.36
Regardless of histology, the most important patient characteristic associated with outcomes that emerged from this analysis was chemotherapy sensitivity. As previously mentioned, chemotherapy sensitivity of lymphomas has been identified as an important prognostic factor for patients undergoing reduced-intensity allogeneic HCT.18–20 The Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation reported the outcomes of 188 lymphoma patients who underwent reduced-intensity HCT. Twenty-one percent of patients had chemotherapy-resistant disease using the classic definition, and 8% had untested relapse. Chemotherapy sensitivity was the most important factor in progression-free survival and OS, although chemotherapy resistance was associated with disease progression in multivariate analysis. Chemotherapy sensitivity has been reported to be an important prognostic factor regardless of histology among patients undergoing reduced-intensity allogeneic HCT.20,37 In contrast, the Fred Hutchinson Cancer Research Center compared the outcomes of 218 patients with either lymphoma or chronic lymphocytic leukemia who underwent either nonmyeloablative (n = 152) or myeloablative (n = 68) allogeneic HCT at their institution. In multivariate analysis, which included all patients regardless of conditioning type, disease status at transplant was not found to have a statistically significant effect upon overall survival.36 However, in other analyses looking at specific histologies by this group and their collaborators, chemotherapy sensitivity has emerged as a significant factor relative to survival in patients receiving a nonmyeloablative conditioning regimen.38
The further differentiation of chemotherapy sensitivity by specific responses to pretransplant chemotherapy allowed us to make several observations that may be relevant to NHL patients being considered for reduced-intensity allogeneic HCT. The current analysis provides further support to our prior observation that NHL patients with SD after salvage chemotherapy, who would be generally classified as having chemotherapy-resistant disease, can derive benefit from reduced-intensity allogeneic HCT. The 3-year EFS and OS rates for this group of patients were 18% and 45%, respectively. This is of clinical significance, as these patients are often considered inappropriate transplant candidates or are ineligible for transplant trials, including those being conducted by the Blood and Marrow Transplant Clinical Trials Network.
It had been hypothesized that patients who achieve a greater magnitude of response to pretransplant chemotherapy would have improved post-transplant outcomes. Our data do not necessarily support this contention, as patients who were determined to have a PR in response to pretransplant chemotherapy did just as well as those who achieved a CR. In fact, there was no statistical difference in OS between patients who had a CR, a PR, or SD after pretransplant chemotherapy. Furthermore, we did not observe a direct association between early post-transplant response and subsequent transplant outcomes. However, improved post-transplant outcomes appeared to be associated with the achievement of a CR at Day 28 post-transplant, whereas patients who had achieved a PR or whose disease remained stable had similar outcomes after transplant. In stark contrast, there were no long-term survivors among patients who were identified as having PD either before transplant or early post-transplant. Reduced-intensity allogeneic HCT therefore cannot be generally recommended for this latter patient population, and such patients should be referred for investigational treatments.
There are several potential explanations for these observations. First, the results may highlight the importance of conditioning-regimen intensity relative to providing an antitumor effect. More than half of the patients in this analysis experienced a measurable improvement in their disease status after the reduced-intensity conditioning regimen, as determined by their Day 28 evaluation. There now have been several analyses comparing outcomes of patients receiving myeloablative regimens with those receiving nonmyeloablative or reduced-intensity conditioning regimens that consistently demonstrate higher relapse rates with less intense conditioning regimens.19,36,39,40 These data suggest that although a graft-versus-lymphoma effect contributes to a decreased relapse risk after transplant, the more important aspect may be the direct effect of the conditioning regimen.
A second possible explanation is that stabilization and/or reduction in tumor mass is essential until a clinically relevant graft-versus-lymphoma effect can occur. It has long been recognized both in animal models and in the clinic that adoptive immunotherapy is more effective when applied to minimal residual disease states.41–45 Of note, in our study, we were not able to show an association between response and tumor bulk, as only a small percentage of patients had masses in excess of 5 cm. The transient disease stability provided by the conditioning regimen may provide adequate time for a graft-versus-lymphoma effect to occur. This latter hypothesis is further supported by the finding that if relapse did occur in our patient population, it occurred very early (<6 months) after the transplantation process, rarely occurring beyond 1 year after transplantation.
An additional possibility is that tumor susceptibility to a graft-versus-lymphoma effect and to chemotherapy is pathophysiologically related. Resistance to cytotoxic agents and to graft-versus-lymphoma effects may be mediated through common pathways, such as apoptotic signals, which are integral to a graft-versus-lymphoma effect. Conversely, chemotherapy sensitivity may more simply reflect the ability to achieve a minimal disease state. These facets may be interrelated, as chemotherapy-induced tumor cell lysis may itself promote graft-versus-lymphoma activity via increased antigen presentation to allogeneic T cells.46 In contrast, the growth kinetics of rapidly progressive lymphoma may outstrip the pace at which graft-versus-lymphoma– mediated tumor cell death occurs.
If any or all of these explanations are accurate, it provides opportunities to improve outcomes after allogeneic HCT for NHL. One obvious approach would be to increase the intensity of the conditioning regimen; however, this would negate the aforementioned advantages gained through the use of nonmyeloablative and reduced-intensity conditioning regimens. One alternative is to use high-dose therapy and autologous HCT for cytoreduction before nonmyeloablative allogeneic HCT.47–49 Another tactic is to use peritransplant therapies that are either cytotoxic or cytostatic and either do not affect or enhance T-cell function.50,51 Finally, it raises the option of post-transplantation maintenance therapy with readily available agents such as rituximab, which has been demonstrated to be of clinical benefit in patients with follicular lymphomas undergoing reduced-intensity allogeneic HCT.51
In conclusion, the results of this analysis provide further support to previous observations that chemotherapy sensitivity represents an important prognostic factor for patients with NHL undergoing reduced-intensity allogeneic HCT. The further differentiation of chemotherapy sensitivity by specific response to pretransplant chemotherapy provides additional prognostic information in patients being considered for reduced-intensity allogeneic HCT and identifies an additional group (ie, patients with stable disease) for whom this therapy may be of benefit.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
This work was supported by the Center for Cancer Research, National Cancer Institute, Intramural Research Program. The authors declare no competing financial interests.
REFERENCES
- 1.Jones RJ, Ambinder RF, Piantadosi S, Santos GW. Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood 1991;77:649–653. [PubMed] [Google Scholar]
- 2.Chopra R, Goldstone AH, Pearce R, et al. Autologous versus allogeneic bone marrow transplantation for non-Hodgkin lymphoma: a case-controlled analysis of the European Bone Marrow Transplant Group Registry data. J Clin Oncol 1992;10:1690–1695. [DOI] [PubMed] [Google Scholar]
- 3.Ratanatharathorn V, Uberti J, Karanes C, et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin lymphoma. Blood 1994;84:1050–1055. [PubMed] [Google Scholar]
- 4.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. European Bone Marrow Transplantation (EBMT) Lymphoma Registry. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant 2003;31:667–678. [DOI] [PubMed] [Google Scholar]
- 5.Bierman PJ, Sweetenham JW, Loberiza FR, et al. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin lymphoma: a comparison with allogeneic and autologous transplantation. J Clin Oncol 2003;21:3744–3753. [DOI] [PubMed] [Google Scholar]
- 6.Khouri IF, Keating M, Korbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 1998;16:2817–2824. [DOI] [PubMed] [Google Scholar]
- 7.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001;97:3390–3400. [DOI] [PubMed] [Google Scholar]
- 8.Branson K, Chopra R, Kottaridis PD, et al. Role of nonmyeloablative allogeneic stem-cell transplantation after failure of autologous transplantation in patients with lymphoproliferative malignancies. J Clin Oncol 2002;20:4022–4031. [DOI] [PubMed] [Google Scholar]
- 9.Faulkner RD, Craddock C, Byrne JL, et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood 2004;103:428–434. [DOI] [PubMed] [Google Scholar]
- 10.Khouri IF, Saliba RM, Giralt SA, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood 2001;98:3595–3599. [DOI] [PubMed] [Google Scholar]
- 11.Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol 2003;21:4407–4412. [DOI] [PubMed] [Google Scholar]
- 12.Morris E, Thomson K, Craddock C, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood 2004;104:3865–3871. [DOI] [PubMed] [Google Scholar]
- 13.Escalon MP, Champlin RE, Saliba RM, et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin’s lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol 2004;22:2419–2423. [DOI] [PubMed] [Google Scholar]
- 14.Rezvani AR, Storer B, Maris M, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin’s lymphoma. J Clin Oncol 2008;26:211–217. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz N, Dreger P, Glass B, Sureda A. Allogeneic transplantation in lymphoma: current status. Haematologica 2007;92:1533–1548. [DOI] [PubMed] [Google Scholar]
- 16.Bishop MR. The graft-versus-lymphoma effect: fact, fiction, or opportunity? J Clin Oncol 2003;21:3713–3715. [DOI] [PubMed] [Google Scholar]
- 17.van Besien K, Sobocinski KA, Rowlings PA, et al. Allogeneic bone marrow transplantation for low-grade lymphoma. Blood 1998;92:1832–1836. [PubMed] [Google Scholar]
- 18.Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood 2002;100:4310–4316. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez R, Nademanee A, Ruel N, et al. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant 2006;12:1326–1334. [DOI] [PubMed] [Google Scholar]
- 20.Vigouroux S, Michallet M, Porcher R, et al. French Society of Bone Marrow Graft Transplantation and Cellular Therapy (SFGM-TC). Long-term outcomes after reduced-intensity conditioning allogeneic stem cell transplantation for low-grade lymphoma: a survey by the French Society of Bone Marrow Graft Transplantation and Cellular Therapy (SFGM-TC). Haematologica 2007; 92:627–634. [DOI] [PubMed] [Google Scholar]
- 21.Philip T, Armitage JO, Spitzer G, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. N Engl J Med 1987;316:1493–1498. [DOI] [PubMed] [Google Scholar]
- 22.Dean RM, Fowler DH, Wilson WH, et al. Efficacy of reduced-intensity allogeneic stem cell transplantation in chemotherapy-refractory non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2005;11:593–599. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization Classification of Tumours. Vol 3 Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues Lyon, France: IARC Press; 2001. [Google Scholar]
- 24.Wilson WH, Bryant G, Bates S, et al. EPOCH chemotherapy toxicity and efficacy in relapsed and refractory non-Hodgkin’s lymphoma. J Clin Oncol 1993;11:1573–1582. [DOI] [PubMed] [Google Scholar]
- 25.Fowler D, Hou J, Foley J, et al. Phase I clinical trial of donor T-helper type-2 cells after immunoablative, reduced intensity allogeneic PBSC transplant. Cytotherapy 2002;4: 429–430. [DOI] [PubMed] [Google Scholar]
- 26.Fowler DH, Kurasawa K, Smith R, et al. Donor CD4-enriched cells of Th2 cytokine phenotype regulate graft-versus-host disease without impairing allogeneic engraftment in sublethally irradiated mice. Blood 1994;84:3540–3549. [PubMed] [Google Scholar]
- 27.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17:1244–1253. [DOI] [PubMed] [Google Scholar]
- 28.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15:825–828. [PubMed] [Google Scholar]
- 30.Ratanatharathorn V, Ayash L, Lazarus HM, et al. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant 2001;28:121–129. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 32.Mantel N Evaluation of survival data and 2 new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50:163–170. [PubMed] [Google Scholar]
- 33.Cox D Regression models and life tables. J R Stat Soc B 1972;34:187–202. [Google Scholar]
- 34.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JR, Cain KC, Gerber RD, Gelman GS Cancer Treatment Reports 1985;69:1139. [PubMed] [Google Scholar]
- 36.Sorror ML, Storer BE, Maloney DG, et al. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood 2008;111:446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson KJ, Morris EC, Bloor A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol 2009;27:426–432. [DOI] [PubMed] [Google Scholar]
- 38.Rezvani AR, Norasetthada L, Gooley T, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol 2008;143:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant 2008;14:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2008;26:455–462. [DOI] [PubMed] [Google Scholar]
- 41.Salup RR, Wiltrout RH. Treatment of adenocarcinoma in the peritoneum of mice: chemoimmunotherapy with IL-2 -stimulated cytotoxic lymphocytes as a model for treatment of minimal residual disease. Cancer Immunol Immunother 1986;22:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathe G Passive, adoptive, and active immunotherapy: a review of clinical trials in cancer. Cancer Detect Prev Suppl 1987;1:279–290. [PubMed] [Google Scholar]
- 43.Bubenik J, Simova J. Minimal residual disease as the target for immunotherapy and gene therapy of cancer [review]. Oncol Rep 2005;14:1377–1380. [PubMed] [Google Scholar]
- 44.Lizee G, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin Cancer Res 2007;13:5250–5255. [DOI] [PubMed] [Google Scholar]
- 45.Or R, Ackerstein A, Nagler A, et al. Allogeneic cell-mediated and cytokine-activated immunotherapy for malignant lymphoma at the stage of minimal residual disease after autologous stem cell transplantation. J Immunother 1998;21: 447–453. [DOI] [PubMed] [Google Scholar]
- 46.Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumorspecific CD8 T cells. J Immunol 2003;170:4905–4913. [DOI] [PubMed] [Google Scholar]
- 47.Carella AM, Cavaliere M, Lerma E, et al. Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin’s disease and non-Hodgkin’s lymphoma. J Clin Oncol 2000;18: 3918–3924. [DOI] [PubMed] [Google Scholar]
- 48.Maloney DG, Sandmaier BM, Mackinnon S, Shizuru JA. Non-myeloablative transplantation. Hematology Am Soc Hematol Educ Program 2002:392–421. [DOI] [PubMed]
- 49.Slavin S Allogeneic cell-mediated immunotherapy at the stage of minimal residual disease following high-dose chemotherapy supported by autologous stem cell transplantation. Acta Haematol 2005;114:214–220. [DOI] [PubMed] [Google Scholar]
- 50.Atkins MB, Carbone D, Coukos G, et al. Report on the ISBTC mini-symposium on biologic effects of targeted therapeutics. J Immunother 2007;30:577–590. [DOI] [PubMed] [Google Scholar]
- 51.Khouri IF, McLaughlin P, Saliba RM, et al. 8-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide and rituximab. Blood 2008;111:5530–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]


