Abstract
Mobile technologies can be leveraged to meet the need for evidence-based psychological depression treatment via primary care. The purpose of the present study was to preliminarily examine the feasibility and efficacy of a self-help Brief Behavioral Activation mobile application (app; “Moodivate”) for depressive symptoms among adults treated via primary care. Participants (N = 52) were recruited from primary care practices between January and December 2017 and were randomized 2:2:1 to receive: 1) Moodivate, 2) an active control Cognitive Behavioral Therapy-based mobile app (“MoodKit”), or 3) Treatment As Usual (TAU; no app). Participants completed assessments of depressive symptoms weekly for eight weeks. App analytics data were captured to examine Moodivate feasibility (analytics unavailable for control app). Moodivate participants on average had 46.76 (SD = 30.10) app sessions throughout the trial duration, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total throughout the trial. Nearly 70% of Moodivate participants continued to use the app one month after trial enrollment and 50% at the end of the eight-week follow-up period. A generalized estimating equation model examining change in depressive symptoms over time by treatment condition indicated a significant interaction between time and treatment condition (χ2 = 42.21, df = 14, p < .001). As compared to TAU, participants in both app conditions evidenced significant decreases in depressive symptoms over time, and these treatment gains were sustained across the trial period. These results preliminarily indicate feasibility of Moodivate as well as efficacy of both Moodivate and MoodKit for the treatment of depression among adults recruited via primary care. Future studies should focus on larger scale examinations of treatment efficacy and effectiveness across primary care settings.
Keywords: depression, primary care, mobile health
Introduction
Fewer than one-third of United States adults who screen positive for depression receive treatment (Olfson, Blanco, & Marcus, 2016). Most adults with depressive symptoms make at least one annual medical visit to a primary care physician (PCP) (Olfson et al., 2016). As such, primary care models for the delivery of evidence-based depression treatment may improve treatment access for those with depressive symptoms. This is consistent with recommendations from the United States Preventive Services Task Force (USPSTF), which note that PCPs should screen, treat, and provide follow-up for all patients who endorse depressive symptoms (Siu et al., 2016).
Psychotherapy and/or antidepressant medications are both evidence-based treatments for depression that can be delivered via primary care. Psychotherapy can be delivered by a behavioral medicine specialist within the practice or via referral to an external mental health treatment provider. Although primary care patients with depressive symptoms tend to prefer psychotherapy for depression treatment (Dwight-Johnson, Sherbourne, Liao, & Wells, 2000; van Schaik et al., 2004), they most often are prescribed antidepressant medications without psychotherapy as an option (Rossom et al., 2016; Shim, Baltrus, Ye, & Rust, 2011; Stafford, Ausiello, Misra, & Saglam, 2000). One key barrier to the delivery of psychotherapy via primary care is limited access to mental health specialty care (Thomas, Ellis, Konrad, Holzer, & Morrissey, 2009). Within one nationally representative survey, 66.8% of PCPs reported that they were unable to get high-quality outpatient mental health services for their patients (Cunningham, 2009).
Mobile technologies, such as mobile applications (apps), could be leveraged to fill this depression treatment gap (Steinhubl, Muse, & Topol, 2013). Smartphone ownership in the U.S. has grown year over year and now 77% of adults own smartphones (Pew Research Center, 2017), with high smartphone ownership rates across age groups (e.g., 92% of Millenials, 85% of Gen Xers, and 67% of Baby Boomers own smartphones; Jiang, 2018). Thus, treatment access barriers could be reduced if evidence-based psychotherapy could be efficaciously delivered via smartphone. Apps delivered directly within primary care settings could provide an immediately actionable, evidence-based treatment for patients in need of services for depressive symptoms.
To address the need for disseminable, evidence-based depression treatment resources via primary care, our team developed a self-help mobile app treatment for depression called “Moodivate” (Dahne et al., 2017). Moodivate development was informed by the Brief Behavioral Activation (BA) Treatment for Depression originally developed by Lejuez and colleagues (2011) as well as our team’s prior research developing a Brief BA mobile app (“Behavioral Apptivation”) intended to be used in conjunction with traditional face-to-face therapy (Dahne, Kustanowitz, & Lejuez, 2018). Brief BA is an evidence-based treatment grounded in behavioral theories, which suggest that depression is caused by a lack of reinforcement in the environment for positive, non-depressed behaviors. The goal of Brief BA, and by extension Moodivate, is to help the user reengage in positive, non-depressed activities by identifying, scheduling, and completing activities. The efficacy of Brief BA for the treatment of depression has been established across more than 10 randomized clinical trials with diverse populations (Collado, Long, MacPherson, & Lejuez, 2014; Daughters et al., 2008; Gawrysiak, Nicholas, & Hopko, 2009; Gros et al., 2012; Hopko et al., 2011; Hopko et al., 2013; Hopko, Lejuez, & Hopko, 2004; Hopko, Lejuez, LePage, Hopko, & McNeil, 2003; Jakupcak, Wagner, Paulson, Varra, & McFall, 2010; MacPherson et al., 2010; Reynolds, Macpherson, Tull, Baruch, & Lejuez, 2011). Effect size estimates for Brief BA are in the medium to large range indicating meaningful clinical impact (Sturmey, 2009). Because Moodivate is directly informed by an evidence-based treatment for depression, it may be an improvement upon the majority of commercially available mental health treatment apps which largely do not clearly reflect evidence-based practice (Bry, Chou, Miguel, & Comer, 2018; Neary & Schueller, 2018).
Although Moodivate is based on an evidence-based treatment for depression and was developed for utilization in primary care, the efficacy of the app as a treatment for depressive symptoms among primary care patients has not yet been examined. The aims of the present study were two-fold: 1) to examine the feasibility and uptake of Moodivate among adults with elevated depressive symptoms recruited from primary care and 2) to examine the preliminary efficacy of Moodivate as compared to both an active control depression treatment mobile app and to treatment as usual (TAU).
Methods
Participants
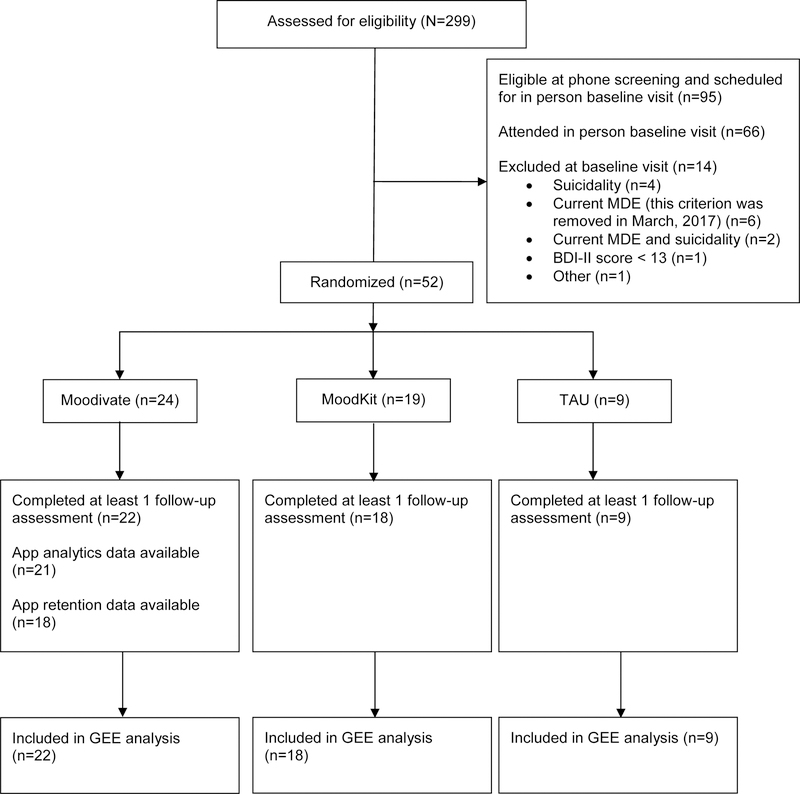
Study participants were recruited between January, 2017 and December, 2017 from family medicine/primary care clinics affiliated with the Medical University of South Carolina (MUSC). Study participants were recruited in one of three ways: 1) by physician referral (n=26), 2) by a research staff member who sat in the waiting room of the clinics and discussed the study with patients (n=6), or 3) via flyers posted in participating clinics (n=6; Note that data on recruitment source were missing for 4 participants). Those interested completed a brief screening via phone to establish preliminary study eligibility. Potentially eligible individuals were scheduled for an in-person baseline visit at which time final study eligibility was confirmed. Study participants had to meet the following criteria: 1) between the ages of 18 and 65, 2) currently own a smartphone, 3) report willingness to utilize a mobile app for the treatment of depressed mood, 4) have a current, valid e-mail address that is checked at least once per day, and 5) score of > 10 on the Patient Health Questionnaire-8 (PHQ-8; Kroenke et al., 2009). Because both apps utilized in this trial were only accessible via iOS, participants who did not own an appropriate iPhone (iOS 8 or higher) were lent one for the duration of the trial. Participants were excluded if, during the baseline screening, they: 1) scored < 13 on the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) or 2) endorsed current suicidal ideation, defined as either a response of “I would like to kill myself” or “I would kill myself if I had the chance” on the BDI-II, or an indication of past month suicidal ideation on the Major Depressive Episode (MDE) module of the Mini International Neuropsychiatric Interview for DSM-5 (Sheehan et al., 2015). For the first three months of trial enrollment, meeting diagnostic criteria for a current MDE was an exclusionary criterion. However, research suggests that those with elevated depressive symptoms who do not meet criteria for a current MDE experience similar levels of functional impairment as those with a current MDE, but are less likely to receive treatment (Cuijpers, de Graaf, & van Dorsselaer, 2004; Judd, Schettler, & Akiskal, 2002). Thus, both to broaden the generalizability of results and to improve the pace of recruitment, this exclusion criterion was removed in March, 2017. See Figure 1 for CONSORT diagram.
Figure 1.
CONSORT Flow Diagram
Procedures
All study procedures were approved by the MUSC IRB. Upon consent, participants completed self-report and interviewer-administered assessments and were subsequently randomized 2:2:1 to receive either the Behavioral Activation mobile app (Moodivate), an active control Cognitive Behavioral Therapy (CBT) mobile app (MoodKit), or Treatment as Usual (i.e., no app). Because clinical data are limited on other available mobile apps that purport to treat depressive symptoms (Chan, Torous, Hinton, & Yellowlees, 2015; Leigh & Flatt, 2015; Neary & Schueller, 2018; Price et al., 2014), we opted to compare Moodivate efficacy to an active control mobile app in addition to TAU to extend the literature on efficacy data for other clinical apps. We selected MoodKit (Erhardt & Dorian, 2013) as the active control app because, at the time of trial onset, it was one of the only available mobile apps that clearly adhered to Cognitive Behavioral Therapy (CBT) principles. Similar to Brief BA, CBT is an evidence-based psychotherapy for depression that may be administered via primary care. Thus, an app-based adaptation of CBT would be a fitting active control for a Brief BA-based app.
Across all groups, providers managed depressive symptoms as they normally did, including possible administration of adjunctive pharmacotherapy (see Table 1 for use of pharmacotherapy by treatment condition). Randomization was stratified by participants’ self-reported use of medication for a mental or emotional problem. In addition, to mimic standard care practices for depression treatment via primary care, participants across all groups were provided with a brief handout about stress management, derived from standard patient education materials within the clinic.
Table 1.
Demographics for the Full Sample and by Treatment Group
| Full Sample (N=52) |
Moodivate (n=24) |
MoodKit (n=19) |
TAU (n=9) |
|
|---|---|---|---|---|
| Age (M(SD)) | 43.79 (13.27) | 44.67(13.95) | 43.00(13.63) | 43.11(11.88) |
| Gender (% Female) | 84.6% | 83.3% | 78.9% | 84.6% |
| Race | ||||
| White | 40.4% | 41.7% | 36.8% | 44.4% |
| Black | 55.8% | 54.2% | 57.9% | 55.6% |
| Other | 3.8% | 4.2% | 5.3% | 0.0% |
| Ethnicity (% Hispanic) | 3.8% | 0.0% | 10.5% | 0.0% |
| Relationship Status | ||||
| In Relationship | 36.5% | 33.3% | 36.8% | 44.4% |
| Single | 63.4% | 66.7% | 63.2% | 55.5% |
| Education | ||||
| ≤ High School diploma | 15.3% | 8.3% | 21.1% | 22.2% |
| ≥ High School diploma | 84.6% | 91.6% | 78.9% | 77.8% |
| Annual Household Income | ||||
| < $50k | 61.5% | 54.1% | 68.4% | 66.6% |
| ≥$50k | 28.8% | 37.5% | 21.1% | 22.2% |
| Employment Status | ||||
| Unemployed | 13.5% | 12.5% | 10.5% | 22.2% |
| Employed ≥ part time | 59.6% | 54.2% | 57.9% | 77.8% |
| Other | 27.0% | 33.3% | 31.7% | 0.0% |
| Phone Ownership | ||||
| iPhone | 44.2% | 50.0% | 42.1% | 33.3% |
| Android or Other Smartphone | 55.7% | 50.0% | 57.9% | 66.7% |
| Current MDE (% Yes) | 71.2% | 70.8% | 73.7% | 66.7% |
| Lifetime MDE (% Yes) | 94.3% | 95.8% | 89.5% | 100% |
| Currently Taking Meds for Mental or Emotional Problems (% Yes) | 36.5% | 37.5% | 36.8% | 33.3% |
| Number of Follow-Up Assessments Completed (M(SD)) | 6.25(2.74) | 6.21(2.90) | 5.79(2.90) | 7.33(1.66) |
Note: There were no baseline differences as a function of study group.
Moodivate and MoodKit were provided to study participants at no cost (both currently cost $4.99 on the iTunes App Store). If randomized to either mobile app condition, study staff helped the participant download the app and provided a brief, scripted overview (~10 minutes) on how to use the app. Those provided with an app were instructed to use the app regularly, at least once per day, for the duration of the study. Following the baseline visit, participants were e-mailed assessment measures weekly for eight weeks. All measures were completed via REDCap, a secure, web-based research data capture system (Harris et al., 2009). Participants were compensated up to $120 for completion of all assessments. Participants who borrowed an iPhone as part of the trial were compensated an additional $50 for return of the iPhone in working, non-damaged order within two-weeks of their trial completion date.
Interventions
Behavioral Activation Mobile App (Moodivate).
Details of the Moodivate app have been published previously, including suggested instructions for integrating the app into primary care practice (Dahne et al., 2017). Briefly, Moodivate is a self-help adaptation of Brief BA (Lejuez et al., 2011). The main components of Moodivate include psychoeducation, identification of life areas, values, and associated activities, daily monitoring and activity planning, mood monitoring and social support to facilitate completion of difficult activities. Users are reinforced for continued app utilization and completion of activities via badges that can be earned within the app.
Cognitive Behavioral Therapy (CBT) Mobile App (MoodKit).
MoodKit includes various components of CBT including thought checking, mood tracking, journaling, and activity scheduling. Via the thought checker feature, users identify maladaptive thoughts and alternative, more rationale thoughts. Users track daily mood while using the app and can view a graph of change in mood over time. The app’s journal function allows users to write responses to a variety of templates. Via the activity scheduler, users can select from 200+ suggested activities or create a new custom activity.
The treatment components of Moodivate and MoodKit differ in several ways. First, although Moodivate and MoodKit both incorporate activity scheduling, Moodivate appoaches activity scheduling from a values-driven approach consistent with Brief BA. In contrast, MoodKit prompts users to select from a suggested activities list or create a new activity, but new activities are not generated consistent with values. Second, whereas MoodKit incorporates the cognitive restructuring components of CBT, Moodivate does not include any cognitive treatment components. Third, in general, Moodivate has a somewhat simpler design with fewer treatment components incorporated into the app.
Treatment As Usual.
Participants randomized to the treatment as usual condition received only the patient education materials about stress management and did not receive a mobile app.
Measures
Primary outcomes for this trial include: 1) feasibility of Moodivate as indicated by app utilization analytics (similar analytics data not available for MoodKit) and 2) change in depressive symptoms over time as a function of treatment condition.
Moodivate Analytics Data.
User analytics data for participants randomized to the Moodivate condition were available via Yahoo’s Flurry Analytics system. Specific analytics data captured included: 1) total number of app sessions across the trial duration, 2) average time per app session, 3) total time spent using the app across the trial duration, 4) number of times the participant added a new value within the app, 5) number of times the participant created a value-driven activity within the app, 6) number of activities completed across the trial duration, and 7) weekly retention, defined as the percentage of study participants who utilized Moodivate at least once during each week after study enrollment.
We did not have access to similar data for participants randomized to the MoodKit condition because this app was not developed by our research team.
Depressive Symptoms.
The PHQ-8 (Kroenke et al., 2009) was utilized as a study screening measure to determine whether participants were experiencing at least minimal symptoms of depression. At baseline, participants also completed the interviewer-administered Major Depressive Episode (MDE) module of the Mini International Neuropsychiatric Interview for DSM-5 (Sheehan et al., 2015) to assess for current and lifetime MDEs. A participant was given a lifetime MDE diagnosis if they met criteria for either a current or past MDE. Across the study follow-up (including baseline), depressive symptoms were assessed via the Beck Depression Inventory-II (BDI-II; Beck et al., 1996). Scores on the BDI-II are interpreted as follows: 0–13 = minimal depression, 14–19 = mild depression, 20–28 = moderate depression, 29–63 = severe depression.
Statistical Analysis Plan
Chi-square and ANOVA analyses were used to determine baseline differences in participant demographics as a function of treatment condition. Descriptive statistics were utilized to examine Moodivate feasibility and acceptability as indicated by app analytics data. ANOVA was used to examine the association between Moodivate app usage and treatment response, defined as a significant (i.e., 10-point) decrease on the BDI-II at any point during the study. Subsequently, a generalized estimating equation (GEE) model assuming a normal distribution, identity link function, and exchangeable correlation matrix was used to examine the interaction between time and treatment condition on BDI-II depressive symptoms adjusting for the main effects of baseline depressive symptoms, time, and treatment condition. Because GEE accommodates within-subject correlations across repeated measures and utilizes all available data, it provides less biased estimates than traditional regression approaches in analyzing longitudinal data (Hardin & Hilbe, 2002).
Results
Participant Characteristics
As this was a pilot trial, we sought to keep sample size low (i.e., ~20 participants per app group) while still providing rich feasibility and preliminary efficacy data to inform future trials. In total, 52 participants were enrolled in the trial (n=24 Moodivate, n=19 MoodKit, n=9 TAU). See Table 1 for participant demographics for the full sample as well as by treatment group. There were no significant demographic differences as a function of treatment condition. At baseline, participants on average reported experiencing depressive symptoms in the moderate to severe depression range (BDI-II baseline M(SD) = 29.02(10.09); Moodivate = 28.08(7.83), MoodKit = 28.63(11.30), TAU = 32.33(13.03)). Nearly all participants (94.3%) met diagnostic criteria for a lifetime MDE and 71.2% of participants met criteria for a current MDE. Study retention was high, with 76.8% of participants across treatment groups completing at least six of the eight follow-up assessments. There were no significant differences in study retention between treatment groups.
Moodivate Utilization
App Utilization.
Analytics data were available for 21 out of the 24 Moodivate participants. Data for the remaining three participants were lost due to technical issues. All participants used the app at least once during the trial, 71.4% of participants used the app at least 28 times, and 42.9% of participants used the app more than 56 times (i.e., at least once per day on average). Participants on average had 46.76(30.10) app sessions throughout the eight-week trial duration, spent 3.50(2.76) minutes using the app per session, and spent 120.76(101.02) minutes using the app in total throughout the trial. Participants created on average 6.10(3.22) unique values within the app, 14.71(10.22) activities across values, and completed 52.24(89.31) activities (Table 2). Analytics outcomes did not significantly differ as a function of whether study participants used their own iPhone or a study iPhone throughout the trial.
Table 2.
Moodivate Analytics Over Eight Weeks of Study Duration
| Metric | M(SD) | Range |
|---|---|---|
| Total Number of Sessions | 46.76(30.10) | 3.00 – 95.00 |
| Average Time per Session (min) | 3.50(2.76) | 1.27 – 11.18 |
| Total Time Spent Using Moodivate (min) | 120.76(101.02) | 31.00 – 421.00 |
| Add Value Event Occurrences | 6.10(3.22) | 1.00 – 11.00 |
| Add Value-Driven Activity Event Occurrences | 14.71(10.22) | 2.00 – 44.00 |
| Complete Activity Event Occurrences | 52.24(89.31) | 0.00 – 371.00 |
Note: Add value event occurrences = number of values added, Add value-driven activity event occurrences = number of activities added across values, Complete activity event occurrences = number of activities marked as completed.
Retention.
Weekly retention data, defined as any app use within each week following trial enrollment, were available for 18 of the 24 Moodivate participants (retention data for the remaining 6 participants were lost due to system upgrades within the Flurry Analytics platform). No procedures to increase app retention (e.g., contacting participants who were not using the app to remind them to login) were instituted by the study team during the trial. In general, retention was high across the study duration: 88.9% of participants utilized Moodivate at least once during the first week following trial enrollment, 83.3% during the second week, 66.7% during the third week, 66.7% during the fourth week, 66.7 percent during the fifth week, 66.7% during the sixth week, 61.1% during the seventh week, and 50.0% during the eighth week.
Association Between Moodivate Usage and Treatment Response.
There were no significant associations between any of the Moodivate analytics metrics (i.e., total number of Moodivate sessions, average time per session, total time spent using Moodivate, add value event occurrences, add value-driven activity event occurrences, complete activity event occurrences) and treatment response. However, there was a trend toward significance for the association between total number of Moodivate sessions and significant BDI change [F(1,19)=4.15, p=0.056], suggesting that those who utilized Moodivate more frequently were less likely to have a significant BDI change during the course of the study.
Change in Depressive Symptoms Over Time as Function of Treatment Condition
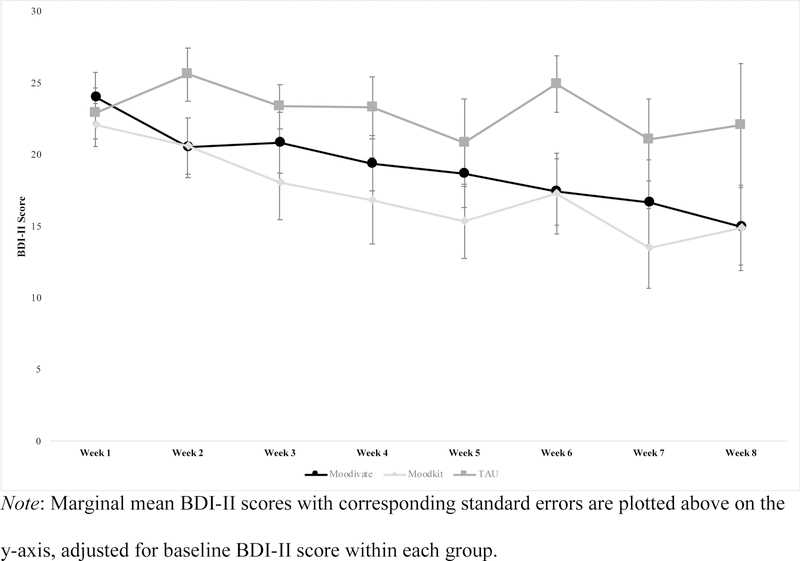
The GEE model indicated a significant main effect of baseline depressive symptoms (χ2 = 19.29, df = 1, p<.001) as well as a significant interaction between time and treatment condition (χ2 = 42.21, df = 14, p<.001). Post-hoc pairwise comparisons were utilized to understand the nature of the significant time by treatment interaction. Adjusting for baseline, depressive symptoms among participants in the Moodivate condition were significantly lower as compared to the Week 1 assessment at Weeks 2, 4, 5, 6, 7, and 8. Depressive symptoms among participants in the MoodKit condition were significantly lower as compared to the Week 1 assessment at Weeks 4, 5, 6, 7, and 8. Depressive symptoms among participants in the TAU condition did not differ significantly from the Week 1 assessment at any other follow-up timepoint. On average, participants in the Moodivate condition had a 12.21(13.15) point decrease on the BDI-II from pre- to post-treatment, participants in the MoodKit condition had a 13.36(11.51) point decrease, and participants in the TAU condition had a 7.75(14.99) point decrease. In addition, 50.0% of participants in the Moodivate condition, 52.6% of participants in the MoodKit condition, and 33.3% of participants in the TAU condition evidenced less than minimal symptoms of depression on the BDI-II (i.e., score ≤ 13) at some point during the study. Among those who completed the Week 8 assessment, 52.6% of Moodivate participants, 50.0% of MoodKit participants, and 25.0% of TAU participants had less than minimal symptoms of depression at that time. Between-group mean differences were not significantly different on average across time points when adjusting for baseline depressive symptoms (Moodivate vs. TAU mean difference (standard error) = −3.94(2.94), p=0.18; Moodivate vs. MoodKit = 1.74(2.89), p=0.55; MoodKit vs TAU = −5.69(3.15), p=0.07). Across time points, the only significant between-group difference in depressive symptoms was observed at the Week 6 follow-up such that those in the Moodivate and MoodKit conditions endorsed significantly lower symptoms of depression than those in the TAU condition (Moodivate vs. TAU mean difference = −7.51(3.14), p=0.02; MoodKit vs. TAU mean difference = −7.68(3.62), p=0.03)). Effect sizes for pre- to post-treatment changes in depressive symptoms for Moodivate vs. TAU (d = 0.33) and MoodKit vs. TAU (d = 0.44) were small. See Figure 2 for a graph of depressive symptoms by time as a function of treatment condition.
Figure 2.
BDI-II Depressive Symptoms by Treatment Condition
Discussion
These results preliminarily indicate feasibility of Moodivate as well as efficacy of both Moodivate and MoodKit for the treatment of depressive symptoms among adults with elevated depressive symptoms recruited via primary care. Across a number of indices, Moodivate was highly feasible. These participants had roughly one app session per day and completed one activity per day, suggesting moderate engagement. Retention was high across the trial duration, with nearly 70% of Moodivate participants continuing to use the app one month after trial enrollment, and 50% at the end of the trial. This is in contrast to prior trials which have suggested that those who download apps for mood management tend to use these tools as intended for no longer than two weeks (Arean et al., 2016; Rosa, Campbell, Miele, Brunner, & Winstanley, 2015) and is also in contrast to utilization of most commercially available apps which retain on average only 30% of users one week after download (Sigg, Lagerspetz, Peltonen, Nurmi, & Tarkoma, 2016).
Although app usage was strong, app usage metrics were not significantly associated with treatment response, which may be due to a lack of power to detect significant associations in light of the small sample within the Moodivate arm. There was a trend toward significance for the association between total number of Moodivate sessions and treatment response such that those who utilized Moodivate more frequently were less likely to have a significant BDI change during the course of the study. This could either indicate that the number of Moodivate sessions negatively impacts change in depression or could indicate that those who were more symptomatic continued to utilize Moodivate with greater frequency throughout the trial. In future studies, it will be important to incorporate a longer follow-up time frame to determine if those who are more symptomatic and continue to utilize Moodivate frequently have subsequent significant decreases in depressive symptoms.
This pilot trial also suggests preliminary efficacy for both Moodivate and MoodKit for the treatment of depressive symptoms among a racially and socioeconomically diverse sample of adults recruited from primary care. As compared to TAU, participants in both app conditions had significant decreases in depressive symptoms over time, and these treatment gains were sustained across the trial period. The lack of efficacy data is a major problem within the field of mobile health research (Neary & Schueller, 2018; Price et al., 2014) and this study provides preliminary data speaking to the efficacy of these two mobile apps. To our knowledge, no other clinical trial has examined the efficacy of mobile apps for the treatment of depression with primary care patients. Future research should focus on the improvement of these app-based treatments for depression for primary care patients and ways to implement these treatments within primary care practices.
Beyond the specific apps studied here, this trial also provides general guidance on the development of mobile treatments for depression for primary care patients. First, although we developed Moodivate for iOS only and MoodKit was similarly available only on iOS, 55.7% of study participants owned an Android smartphone. We opted to provide Android owners with an iPhone to use for the duration of the trial, a practice which has been used in prior studies (Ben-Zeev et al., 2015). However, this approach is likely not sustainable within real-world clinical practice. To reach the majority of primary care patients with depressive symptoms, future mobile-based treatments must be developed for both iOS and Android platforms. Second, although Moodivate utilization rates are promising for a pilot feasibility trial, they still could be increased, whether by the number of activities scheduled per day, or through activities completed per day, either of which may further enhance treatment efficacy. Modifications to Moodivate, such as providing users with a list of suggested activities or directing them to continue to increase their activity level throughout the treatment duration may be useful strategies to consider for future incorporation. Additional strategies such as engaging the primary care provider in ongoing care via the app may be useful for promoting engagement with mobile-based treatments more broadly (i.e., Moodivate, MoodKit, and other treatments). At present, Moodivate is not directly integrated with routine primary care practice, such as via integration with the electronic health record or by having a stand-alone provider portal that PCPs could review to determine patient treatment response. This may be an important future avenue for additional software development and may improve patient treatment engagement. Third, any mobile app-based trial must consider the potential impact of evolving technology on app functionality. For example, during the course of this trial, Apple released iOS 11, which impacted calendar functionality for Moodivate participants who upgraded their operating system. Our team released an app update to resolve the calendar issue, which was then pushed out to all Moodivate participants enrolled in the trial at that time. Similarly, at trial outset, we tested Moodivate on a variety of different iOS versions and noted that app functionality was optimal on iOS 8 or later. Thus, a study inclusion criterion was that participants needed to own an iPhone running iOS 8 or higher or be willing to use a study iPhone for the duration of the trial. Future mHealth trials should consider the impact of technology upgrades on intervention functionality and have systems in place to continuously test the functionality of their intervention following new technology releases (Mohr, Cheung, Schueller, Hendricks Brown, & Duan, 2013). Finally, in the present trial, we selected MoodKit as our active control app given mounting data highlighting that: 1) the majority of mental health apps readily available on the consumer marketplace lack any research support or evidence-based content and 2) mental health apps developed in academic circles lack consumer marketplace penetration (Bry et al., 2018). MoodKit is a commercially popular mental health treatment app (at the time of the writing of this manuscript, it was #68 within the iTunes App Store “Health and Wellness” category) that adheres to CBT principles, yet there are no published trials examining clinical outcomes associated with MoodKit use. To begin to fill this gap between commercially successful mental health apps and treatment outcomes research, within this trial we sought to preliminarily examine clinical outcomes associated with Moodivate and with MoodKit use. Similar trials in the future may consider incorporating commercially popular mental health apps as control treatment conditions in order to expand the literature on mental health outcomes associated with use of these readily available apps.
Results of this study should be interpreted with limitations in mind. This was a small pilot study and results should be interpreted accordingly. Although we found a significant time by treatment effect such that both app conditions outperformed TAU, we may have been underpowered to detect significant effects between the two app conditions and it is possible that within a larger scale trial either MoodKit or Moodivate would be more efficacious. Small sample size may have also contributed to mostly non-significant between-group differences in BDI-II symptoms across follow-up. Although use of psychiatric medications was similar across the three treatment groups, we did not assess duration of medication use (i.e., whether a study participant was stabilized on medication or recently started taking a new medication). As such, we cannot disentangle whether treatment gains were a result of Moodivate/MoodKit use or of possible new medication use. Participants did not complete follow-up assessments beyond eight-weeks. Thus, it is unclear whether treatment gains would be sustained outside of this timeframe. This issue of beyond treatment assessment is particularly complicated within the area of app-based interventions. Unless app use is restricted, study participants can continue to use the app and engage in treatment beyond the initially prescribed period of time. Moodivate analytics data were missing for a small subset of participants and we did not have access to similar analytics data from MoodKit. Thus, we were unable to compare feasibility as indicated by analytics data between the two apps.
Future larger scale efficacy and effectiveness trials within primary care will be needed for both Moodivate and MoodKit before these interventions can be recommended for the treatment of depressive symptoms via primary care. Early evidence from this trial offers promise toward that goal. Furthermore, although one strength of mobile health interventions is that they tend to be low cost and, thus, more disseminable and cost effective than traditional in-person psychological interventions, not all primary care patients with depressive symptoms will have improvements in depressive symptoms solely by using a self-help app such as Moodivate or MoodKit. Future investigations of these mobile treatments should consider adaptive treatment strategies in which the level of treatment can be adjusted based on whether each individual patient is responding to the mHealth treatment (Collins, Murphy, & Strecher, 2007; Murphy, 2005).
Highlights.
Moodivate is a self-help Brief Behavioral Activation mobile app
Moodivate feasibility was examined and efficacy was compared to MoodKit and TAU
70% of Moodivate participants had continued app use one month after trial enrollment
Participants in both app conditions had significant decreases in depression
Results indicate feasibility of Moodivate and preliminary efficacy of both apps
Acknowledgments
The authors would like to thank MountainPass Technology LLC, including Zachary Gavin, Jim Nichols, Bryan Hobbs, Brian Cordyack, and Tamara Wiesen, for their contributions to the development of Moodivate. Funding for this research was provided by the National Institute of Mental Health (R41 MH108219) and by the National Institute on Drug Abuse (T32 DA007288, K23 DA045766). The funding sources had no role in study design, data collection, data analysis, data interpretation, in writing this report, or in the decision to submit this article for publication. The authors (JD, CWL, JK) are co-owners of Behavioral Activation Tech, LLC which owns the rights to Moodivate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no other actual or potential conflicts of interest that may bias the present work.
References
- Arean PA, Hallgren KA, Jordan JT, Gazzaley A, Atkins DC, Heagerty PJ, & Anguera JA (2016). The Use and Effectiveness of Mobile Apps for Depression: Results From a Fully Remote Clinical Trial. Journal of Medical Internet Research, 18(12), e330. doi: 10.2196/jmir.6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck Depression Inventory-II (BDI-II) San Antonio, TX: Psychological Corporation. [Google Scholar]
- Ben-Zeev D, Schueller SM, Begale M, Duffecy J, Kane JM, & Mohr DC (2015). Strategies for mHealth research: lessons from 3 mobile intervention studies. Administration and Policy in Mental Health, 42(2), 157–167. doi: 10.1007/s10488-014-0556-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bry LJ, Chou T, Miguel E, & Comer JS (2018). Consumer Smartphone Apps Marketed for Child and Adolescent Anxiety: A Systematic Review and Content Analysis. Behavior Therapy, 49(2), 249–261. doi: 10.1016/j.beth.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Torous J, Hinton L, & Yellowlees P (2015). Towards a Framework for Evaluating Mobile Mental Health Apps. Telemedicine Journal and e-Health, 21(12), 1038–1041. doi: 10.1089/tmj.2015.0002 [DOI] [PubMed] [Google Scholar]
- Collado A, Long KE, MacPherson L, & Lejuez CW (2014). The efficacy of a behavioral activation intervention among depressed US Latinos with limited English language proficiency: study protocol for a randomized controlled trial. Trials, 15(7), 231. doi: 10.1186/1745-6215-15-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, & Strecher V (2007). The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. American Journal of Preventive Medicine, 32(5 Suppl), S112–118. doi: 10.1016/j.amepre.2007.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, de Graaf R, & van Dorsselaer S (2004). Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. Journal of Affective Disorders, 79(1–3), 71–79. doi: 10.1016/S0165-0327(02)00348-8 [DOI] [PubMed] [Google Scholar]
- Cunningham PJ (2009). Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Affairs, 28(3), w490–501. doi: 10.1377/hlthaff.28.3.w490 [DOI] [PubMed] [Google Scholar]
- Dahne J, Lejuez CW, Kustanowitz J, Felton JW, Diaz VA, Player MS, & Carpenter MJ (2017). Moodivate: A self-help behavioral activation mobile app for utilization in primary care-Development and clinical considerations. International Journal of Psychiatry in Medicine, 52(2), 160–175. doi: 10.1177/0091217417720899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Braun AR, Sargeant MN, Reynolds EK, Hopko DR, Blanco C, & Lejuez C (2008). Effectiveness of a brief behavioral treatment for inner-city illicit drug users with elevated depressive symptoms: The Life Enhancement Treatment for Substance Use (LETS Act!). Journal of Clinical Psychiatry, 69(1), 122–129. doi: 10.4088/JCP.v69n0116 [DOI] [PubMed] [Google Scholar]
- Dwight-Johnson M, Sherbourne CD, Liao D, & Wells KB (2000). Treatment preferences among depressed primary care patients. Journal of General Internal Medicine, 15(8), 527–534. doi: 10.1046/j.1525-1497.2000.08035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt D, & Dorian E (2013). Going Mobile: A Case Vignette Illustrating the Integration of Mobile Technology in Psychotherapy. Independent Practitioner, 33(1), 15–21. [Google Scholar]
- Gawrysiak M, Nicholas C, & Hopko DR (2009). Behavioral activation for moderately depressed university students: Randomized controlled trial. Journal of Counseling Psychology, 56(3), 468–475. doi: 10.1037/a0016383 [DOI] [Google Scholar]
- Gros DF, Price M, Strachan M, Yuen EK, Milanak ME, & Acierno R (2012). Behavioral activation and therapeutic exposure: an investigation of relative symptom changes in PTSD and depression during the course of integrated behavioral activation, situational exposure, and imaginal exposure techniques. Behavior Modification, 36(4), 580–599. doi: 10.1177/0145445512448097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW, & Hilbe JM (2002). Generalized estimating equations: Chapman and Hall/CRC. [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopko DR, Armento ME, Robertson SM, Ryba MM, Carvalho JP, Colman LK, … Lejuez CW (2011). Brief behavioral activation and problem-solving therapy for depressed breast cancer patients: randomized trial. Journal of Consulting and Clinical Psychology, 79(6), 834–849. doi: 10.1037/a0025450 [DOI] [PubMed] [Google Scholar]
- Hopko DR, Funderburk JS, Shorey RC, McIndoo CC, Ryba MM, File AA, … Vitulano M (2013). Behavioral activation and problem-solving therapy for depressed breast cancer patients: preliminary support for decreased suicidal ideation. Behavior Modification, 37(6), 747–767. doi: 10.1177/0145445513501512 [DOI] [PubMed] [Google Scholar]
- Hopko DR, Lejuez C, & Hopko SD (2004). Behavioral activation as an intervention for coexistent depressive and anxiety symptoms. Clinical Case Studies, 3(1), 37–48. doi: 10.1177/1534650103258969 [DOI] [Google Scholar]
- Hopko DR, Lejuez CW, LePage JP, Hopko SD, & McNeil DW (2003). A brief behavioral activation treatment for depression. A randomized pilot trial within an inpatient psychiatric hospital. Behavior Modification, 27(4), 458–469. doi: 10.1177/0145445503255489 [DOI] [PubMed] [Google Scholar]
- Jakupcak M, Wagner A, Paulson A, Varra A, & McFall M (2010). Behavioral activation as a primary care-based treatment for PTSD and depression among returning veterans. Journal of Traumatic Stress, 23(4), 491–495. doi: 10.1002/jts.20543 [DOI] [PubMed] [Google Scholar]
- Jiang J (2018). Millennials stand out for their technology use, but older generations also embrace digital life. Fact Tank News in the Numbers Retrieved from http://www.pewresearch.org/fact-tank/2018/05/02/millennials-stand-out-for-their-technology-use-but-older-generations-also-embrace-digital-life/
- Judd LL, Schettler PJ, & Akiskal HS (2002). The prevalence, clinical relevance, and public health significance of subthreshold depressions. Psychiatric Clinics of North America, 25(4), 685–698. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, & Mokdad AH (2009). The PHQ-8 as a measure of current depression in the general population. Journal of Affective Disorders, 114(1–3), 163–173. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- Leigh S, & Flatt S (2015). App-based psychological interventions: friend or foe? Evidence-Based Mental Health, 18(4), 97–99. doi: 10.1136/eb-2015-102203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Hopko DR, Acierno R, Daughters SB, & Pagoto SL (2011). Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behavior Modification, 35(2), 111–161. doi: 10.1177/0145445510390929 [DOI] [PubMed] [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, … Lejuez CW (2010). Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. Journal of Consulting and Clinical Psychology, 78(1), 55–61. doi: 10.1037/a0017939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Cheung K, Schueller SM, Hendricks Brown C, & Duan N (2013). Continuous evaluation of evolving behavioral intervention technologies. American Journal of Preventive Medicine, 45(4), 517–523. doi: 10.1016/j.amepre.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SA (2005). An experimental design for the development of adaptive treatment strategies. Statistics in Medicine, 24(10), 1455–1481. doi: 10.1002/sim.2022 [DOI] [PubMed] [Google Scholar]
- Neary M, & Schueller SM (2018). State of the field of mental health apps. Cognitive and Behavioral Practice, 25(4), 531–537. doi: 10.1016/j.cbpra.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Blanco C, & Marcus SC (2016). Treatment of Adult Depression in the United States. JAMA internal medicine, 176(10), 1482–1491. doi: 10.1001/jamainternmed.2016.5057 [DOI] [PubMed] [Google Scholar]
- Pew Research Center. (2017). Mobile Fact Sheet. Pew Internet & American Life Project Retrieved from http://www.pewinternet.org/fact-sheet/mobile/#
- Price M, Yuen EK, Goetter EM, Herbert JD, Forman EM, Acierno R, & Ruggiero KJ (2014). mHealth: a mechanism to deliver more accessible, more effective mental health care. Clinical Psychology & Psychotherapy, 21(5), 427–436. doi: 10.1002/cpp.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds EK, Macpherson L, Tull MT, Baruch DE, & Lejuez CW (2011). Integration of the brief behavioral activation treatment for depression (BATD) into a college orientation program: depression and alcohol outcomes. Journal of Counseling Psychology, 58(4), 555–564. doi: 10.1037/a0024634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa C, Campbell AN, Miele GM, Brunner M, & Winstanley EL (2015). Using e-technologies in clinical trials. Contemporary Clinical Trials, 45(Pt A), 41–54. doi: 10.1016/j.cct.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossom RC, Solberg LI, Vazquez-Benitez G, Whitebird RR, Crain AL, Beck A, & Unutzer J (2016). Predictors of Poor Response to Depression Treatment in Primary Care. Psychiatric Services, 67(12), 1362–1367. doi: 10.1176/appi.ps.201400285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Janavs J, Baker R, Sheehan K, Knapp E, & Sheehan M (2015). Mini international neuropsychiatric interview–version 7.0.0 DSM-5
- Shim RS, Baltrus P, Ye J, & Rust G (2011). Prevalence, treatment, and control of depressive symptoms in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2005–2008. Journal of the American Board of Family Medicine, 24(1), 33–38. doi: 10.3122/jabfm.2011.01.100121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigg S, Lagerspetz E, Peltonen E, Nurmi P, & Tarkoma S (2016). Sovereignty of the Apps: There’s more to Relevance than Downloads. arXiv preprint arXiv:1611.10161
- Siu AL, Force USPST, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, … Pignone MP (2016). Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. JAMA, 315(4), 380–387. doi: 10.1001/jama.2015.18392 [DOI] [PubMed] [Google Scholar]
- Stafford RS, Ausiello JC, Misra B, & Saglam D (2000). National Patterns of Depression Treatment in Primary Care. Primary Care Companion to the Journal of Clinical Psychiatry, 2(6), 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhubl SR, Muse ED, & Topol EJ (2013). Can mobile health technologies transform health care? JAMA, 310(22), 2395–2396. doi: 10.1001/jama.2013.281078 [DOI] [PubMed] [Google Scholar]
- Sturmey P (2009). Behavioral activation is an evidence-based treatment for depression. Behavior Modification, 33(6), 818–829. doi: 10.1177/0145445509350094 [DOI] [PubMed] [Google Scholar]
- Thomas KC, Ellis AR, Konrad TR, Holzer CE, & Morrissey JP (2009). County-level estimates of mental health professional shortage in the United States. Psychiatric Services, 60(10), 1323–1328. doi: 10.1176/ps.2009.60.10.1323 [DOI] [PubMed] [Google Scholar]
- van Schaik DJ, Klijn AF, van Hout HP, van Marwijk HW, Beekman AT, de Haan M, & van Dyck R (2004). Patients’ preferences in the treatment of depressive disorder in primary care. General Hospital Psychiatry, 26(3), 184–189. doi: 10.1016/j.genhosppsych.2003.12.001 [DOI] [PubMed] [Google Scholar]