This nonrandomized clinical trial of women with ductal carcinoma in situ examines reasons for conversion to mastectomy after magnetic resonance imaging and adherence to radiotherapy use guided by the 12-gene ductal carcinoma in situ score.
Key Points
Question
What is the association of breast magnetic resonance imaging and a 12-gene expression assay with the treatment of women with ductal carcinoma in situ of the breast who are candidates for breast conservation surgery and radiotherapy?
Findings
In this nonrandomized clinical trial of a prespecified primary outcome among 339 women with pure ductal carcinoma in situ, after magnetic resonance imaging, 19% of patients eligible for wide local excision converted to mastectomy; 38% of conversions were based on magnetic resonance imaging findings and 62% on other reasons. Wide local excision was the final surgical procedure in 96% of women who received it as the first procedure after magnetic resonance imaging, and adherence to radiotherapy use guided by a 12-gene assay exceeded 90%.
Meaning
Breast magnetic resonance imaging and a 12-gene assay may be used to tailor primary surgical treatment and radiotherapy, respectively, and inform patient and physician decision-making to support more targeted therapy.
Abstract
Importance
Advanced diagnostics, such as magnetic resonance imaging (MRI) and gene expression profiles, are potentially useful to guide targeted treatment in patients with ductal carcinoma in situ (DCIS).
Objectives
To examine the proportion of patients who converted to mastectomy after MRI and the reasons for those conversions and to measure patient adherence to radiotherapy guided by the 12-gene DCIS score.
Design, Setting, and Participants
Analysis of a prospective, cohort, nonrandomized clinical trial that enrolled women with DCIS on core biopsy who were candidates for wide local excision (WLE) from 75 institutions from March 25, 2015, to April 27, 2016, through the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network trial E4112.
Interventions
Participants underwent breast MRI before surgery, and subsequent management incorporated MRI findings for choice of surgery. The DCIS score was used to guide radiotherapy recommendations among women with DCIS who had WLE as the final procedure and had tumor-free excision margins of 2 mm or greater.
Main Outcomes and Measures
The primary end point was to estimate the conversion rate to mastectomy and the reason for conversion.
Results
Of 339 evaluable women (mean [SD] age, 59.1 [10.1] years; 262 [77.3%] of European descent) eligible for WLE before MRI, 65 (19.2%; 95% CI, 15.3%-23.7%) converted to mastectomy. Of these 65 patients, conversion was based on MRI findings in 25 (38.5%), patient preference in 25 (38.5%), positive margins after attempted WLE in 10 (15.4%), positive genetic test results in 3 (4.6%), and contraindication to radiotherapy in 2 (3.1%). Among the 285 who had WLE performed after MRI as the first surgical procedure, 274 (96.1%) achieved successful breast conservation. Of 171 women eligible for radiotherapy guided by DCIS score (clear margins, absence of invasive disease, and score obtained), the score was low (<39) in 82 (48.0%; 95% CI, 40.6%-55.4%) and intermediate-high (≥39) in 89 (52.0%; 95% CI, 44.6%-59.4%). Of these 171 patients, 159 (93.0%) were adherent with recommendations.
Conclusions and Relevance
Among women with DCIS who were WLE candidates based on conventional imaging, multiple factors were associated with conversion to mastectomy. This study may provide useful preliminary information required for designing a planned randomized clinical trial to determine the effect of MRI and DCIS score on surgical management, radiotherapy, overall resource use, and clinical outcomes, with the ultimate goal of achieving greater therapeutic precision.
Trial Registration
ClinicalTrials.gov identifier: NCT02352883
Introduction
Ductal carcinoma in situ (DCIS) of the breast is a clonal proliferation of cells within the ductal lumen that does not invade the basement membrane into the adjacent breast stroma but is a nonobligate precursor to invasive carcinoma.1 The incidence of DCIS has increased 7-fold since widespread mammographic screening began2 and accounts for approximately 22% of all new breast carcinomas diagnosed in the United States.3 Although these findings may be viewed as evidence of the benefit of screening because of the low mortality rates associated with DCIS, another view is that DCIS detection may be harmful because of overdiagnosis and aggressive treatment of disease that would not progress.4 Currently, treatment of DCIS is complex and variable. Approximately 70% of women diagnosed with DCIS in the United States are treated with wide local excision (WLE), 28% with mastectomy,5,6,7 and 2% with no surgical intervention.7,8 After WLE is complete, 38% do not receive radiotherapy,6 although randomized clinical trials have found that radiotherapy after WLE reduces local recurrence rates by approximately 50%.9 The 20-year breast cancer mortality risk of approximately 3% is low, however, and not affected by radiotherapy.10 Similarly, Surveillance, Epidemiology, and End Results data from 1991 to 2010 show that the adjusted 10-year disease-specific survival was roughly similar for patients undergoing WLE with radiotherapy (99%), mastectomy (99%), and WLE without radiotherapy (98%).6
In the context of a nonlethal disease for which current standards of care entail significant morbidity and cost, the National Institutes of Health convened a State of the Science Conference in 2009 that recommended studies of advanced diagnostic tests and imaging to improve risk stratification and management.11 Advanced imaging techniques, such as breast magnetic resonance imaging (MRI), may more accurately define the extent of disease, offering the potential to better inform surgical planning. Tests that involve gene expression profiling may better determine recurrence risk so that radiotherapy can be tailored to those more likely to benefit.
On the basis of these considerations, we undertook a multicenter, nonrandomized, prospective cohort clinical trial of breast MRI and the 12-gene DCIS score12,13 in patients with DCIS who were candidates for breast conservation surgery based on conventional imaging to determine their association with management. Our objectives were to examine the proportion who converted to mastectomy after MRI and the reasons for those conversions and to evaluate adherence to radiotherapy use guided by the 12-gene DCIS score.
Methods
Data Source and Study Population
Patients newly diagnosed with pure DCIS were enrolled from 75 institutions from March 25, 2015, to April 27, 2016, through the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network trial E4112. Key eligibility criteria for enrollment included (1) WLE candidate based on clinical examination and mammography findings (and ultrasonography findings if performed), (2) pathologically confirmed diagnosis of unilateral DCIS with no evidence of microinvasive or invasive disease obtained by core needle biopsy within 4 months of registration, (3) diagnostic mammography of the affected breast within 3 months before registration and no prior breast MRI within 6 months, (4) no prior ipsilateral invasive breast cancer or DCIS, (5) no known deleterious mutations in breast cancer genes, (6) no prior antiestrogen therapy for prevention of breast cancer within 3 months of the biopsy documenting DCIS, and (7) no contraindications to contrast-enhanced MRI (eg, claustrophobia, renal dysfunction). Written informed consent was obtained from all patients. The study was approved by the National Cancer Institute, Division of Cancer Prevention, and by the local institutional review board at each participating site. The trial protocol is given in Supplement 1.
Study Registration and Surgical Management
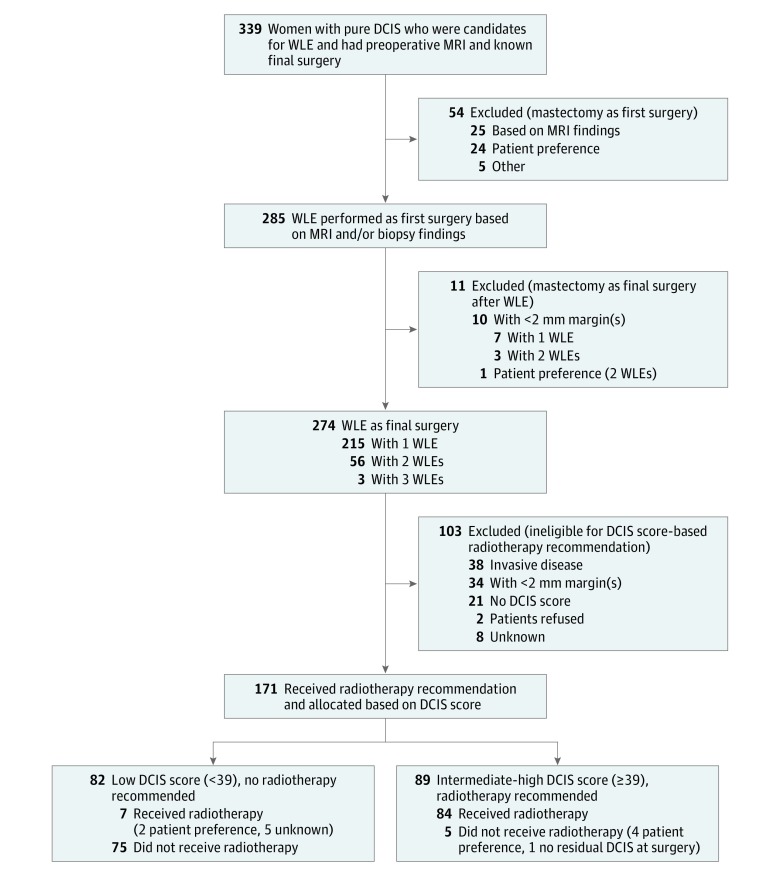
Eligible patients were registered and underwent MRI; additional imaging and/or biopsies based on MRI findings were performed before the first surgery (eFigure in Supplement 2). After completion of post-MRI workup, the surgical plan was recorded, including specific reason for mastectomy vs WLE. For patients who achieved successful WLE as the final procedure (final tumor-free surgical margin of ≥2 mm, no invasive or microinvasive carcinoma identified), tissue samples were submitted for DCIS score analysis (Figure).
Figure. Flow Diagram of a Nonrandomized Clinical Trial.
DCIS indicates ductal carcinoma in situ; MRI, magnetic resonance imaging; and WLE, wide local excision.
Breast MRI Technique and Interpretation
All participating sites underwent prequalification of MRI scanners and met standards for image quality as directed by the American College of Radiology (ACR) requirements for accreditation.14 Bilateral breast MRI was performed with a dedicated breast coil and included T1-weighted precontrast and postcontrast (early and delayed) and T2-weighted series. The ACR Breast Imaging Reporting and Data System (BI-RADS)15 was used to report findings by the original interpreting radiologists at the site.
DCIS Score and Assignment to Radiotherapy
Patients who had WLE as their final surgical procedure, with negative (≥2 mm) final margins and absence of invasive disease, had their index tumor specimens submitted for DCIS score evaluation (Genomic Health Inc). Patients with a low DCIS score (<39) were recommended to receive no breast radiotherapy, and those with an intermediate-high DCIS score (≥39) were recommended to receive radiotherapy.
Patient Surgical Preference Before MRI and Reason for Mastectomy After MRI
Patient preference for surgery was collected before and after MRI using a patient-completed questionnaire with 3 categorical responses: lumpectomy, mastectomy, and “I don’t know.” After MRI, documentation of surgery planned was recorded by the surgeon, as well as the reason for mastectomy as first or subsequent surgery using the following categories: MRI findings, patient preference, positive margins, and other (eg, positive genetic test results, contraindications to radiotherapy).
Study Objectives and Statistical Analysis Plan
Our objectives were to estimate the proportion of patients eligible for WLE based on conventional assessment who converted to mastectomy and the reasons for conversion and to assess adherence with radiotherapy assignment based on DCIS score. Proportions were estimated, with 95% Wilson CIs. A sample size of 350 patients ensured that the expected length of the CI for the mastectomy conversion proportion was no larger than 0.08, assuming the proportion of conversion at the final surgical procedure would be as high as 0.16 and that up to 5% of cases would not provide complete information for the primary end point assessment. We used χ2 tests to compare binomial or multinomial outcomes and Wilcoxon rank sum tests to compare continuous outcomes across groups defined by final surgery status and DCIS score.
Results
Patient Characteristics
A total of 368 patients were enrolled, of whom 339 (92.1%) (mean [SD] age, 59.1 [10.1] years; 262 [77.3%] of European descent) were evaluable for the primary analysis (Figure). Reasons for exclusion included ineligibility (n = 5), patient withdrawal (n = 8), MRI intolerance (n = 2), MRI completed more than 30 days after registration (n = 2), no documented surgical plan after MRI (n = 6), or no documented surgery (n = 6).
Demographic, radiographic, and pathologic characteristics are given in the eTable in Supplement 2. Among the 339 evaluable patients, nuclear grade of the index DCIS lesion was classified as low (54 [15.9%]), intermediate (136 [40.1%]), high (137 [40.4%]), or unknown or not assessable (12 [3.5%]). Estrogen receptor status was reported as positive in 258 (76.1%), negative in 47 (13.9%), and unknown in 34 (10.0%). Progesterone receptor status was positive in 202 (59.6%), negative in 79 (23.3%), and unknown in 58 (17.1%). The median mammographic lesion size was 11 mm (interquartile range, 6-19.5 mm); 281 (79.4%) manifested as microcalcifications, 24 (6.8%) as masses, 19 (5.4%) as asymmetry or focal asymmetry, 6 (1.7%) as distortion, and 24 (6.8%) as other or unknown. Fifty-five patients (16.2%; 95% CI, 12.7%-20.5%) had invasive breast cancer diagnosed after enrolling: 49 (89.1%) from surgery of the index lesion and 6 (10.9%) from biopsy of the lesion identified on the MRI examination.
Patient Surgical Preference Before MRI and Reason for Mastectomy After MRI
Before MRI, 315 evaluable women completed the baseline questionnaire asking the patient’s surgical preference: 254 (80.6%) preferred WLE, 11 (3.5%) preferred mastectomy, and 50 (15.9%) were undecided. After MRI, 275 evaluable women answered the same question: 239 (86.9%) preferred WLE, 22 (8.0%) preferred mastectomy, and 14 (5.1%) were undecided.
A total of 65 of 339 evaluable patients (19.2%; 95% CI, 15.3%-23.7%) underwent mastectomy, with reasons reported by the surgeon as MRI findings in 25 (38.5%), patient preference in 25 (38.5%), positive margins after attempted WLE in 10 (15.4%), positive genetic test results in 3 (4.6%), and contraindication to radiotherapy in 2 (3.1%) (Table 1).
Table 1. Reasons for Mastectomy as First or Final Surgery.
| Reason | Patients Receiving Mastectomy, No. (%) (n = 65) |
|---|---|
| Mastectomy as first surgery | 54 (83.1) |
| Based on MRI findings | 25 (38.5) |
| Lesion size too large for breast conservation | 15 (23.1) |
| Multicentricity | 7 (10.8) |
| Contralateral findings | 3 (4.6) |
| Patient preference | 24 (36.9) |
| Genetic history | 3 (4.6) |
| Contraindications to radiotherapy | 2 (3.1) |
| Mastectomy after attempted WLE | 11 (16.9) |
| <2-mm margin(s) | 10 (15.4) |
| Patient preference | 1 (1.5) |
Abbreviations: MRI, magnetic resonance imaging; WLE, wide local excision.
MRI and Additional Biopsy Results
Both MRI BI-RADS assessments and recommendations as well as additional biopsies after MRI are detailed in Table 2. Of 339 evaluable patients with MRI, 260 (76.7%) were assessed as having BI-RADS scores of 1, 2 (negative or benign), or 6 (known biopsy-proven malignant tumor); 63 (18.6%) as having BI-RADS scores of 4 or 5 (suspicious or highly suspicious); 12 (3.5%) as having BI-RADS score of 0 (needs additional imaging); 1 (0.3%) as having a BI-RADS score of 3 (probably benign); and 3 (0.9%) having scores that were not reported or unknown. Of 333 patients with information regarding subsequent biopsies, 66 (19.8%) had an additional biopsy after MRI and before first surgery: 39 (59.1%) in the ipsilateral breast, 16 (24.2%) in the contralateral breast, and 11 (16.7%) in both breasts. All biopsies were needle biopsies (63 [95.5%] by core needle and 3 [4.5%] by fine needle aspiration); 37 (56.1%) were performed under MRI guidance, 21 (31.8%) under ultrasonography, and 8 (12.1%) under mammography guidance. The most severe histologic findings were benign in 28 (42.4%), high-risk lesions in 17 (25.8%), and malignant in 21 (31.8%). Thus, 21 of 333 women (6.3%) had additional malignant tumors detected by MRI; 15 (71.4%) were DCIS, and 6 (28.6%) were invasive cancers. The location of these additional malignant tumors was ipsilateral to the index DCIS in 14 patients (66.7%), contralateral in 4 (19.0%), and bilateral in 3 (14.3%) (Table 2).
Table 2. MRI BI-RADS Assessments and Recommendations and Post-MRI Biopsies Performed .
| Variable | No. (%) of Patients |
|---|---|
| Pure DCIS Preoperative MRI Final Surgery Known (n = 339) | |
| MRI BI-RADS assessment category | |
| 0 | 12 (3.5) |
| 1 or 2 | 6 (1.8) |
| 3 | 1 (0.3) |
| 4 or 5 | 63 (18.6) |
| 6 | 254 (74.9) |
| Not reported or unknown | 3 (0.9) |
| MRI BI-RADS recommendation | |
| Surgical excision or routine follow-up | 222 (65.5) |
| Tissue diagnosis | 77 (22.7) |
| Targeted ultrasonography | 27 (8.0) |
| Short-interval (6-mo) follow-up | 7 (2.1) |
| Not reported or unknown | 6 (1.8) |
| Additional Biopsy Performed After MRI (n = 66) | |
| No. of lesions biopsied | |
| 1 | 48 (72.7) |
| 2 | 16 (24.2) |
| 3 | 2 (3.0) |
| Biopsy side | |
| Ipsilateral | 39 (59.1) |
| Contralateral | 16 (24.2) |
| Both | 11 (16.7) |
| Post-MRI biopsy guidancea | |
| MRI | 37 (56.1) |
| Ultrasonography | 21 (31.8) |
| Mammography | 8 (12.1) |
| Most severe histologic findingsb | |
| Benign | 28 (42.4) |
| High risk | 17 (25.8) |
| Malignant | 21 (31.8) |
| DCIS | 15 (22.7) |
| Invasive | 6 (9.1) |
Abbreviations: BI-RADS, Breast Imaging Reporting and Data System; DCIS, ductal carcinoma in situ; MRI, magnetic resonance imaging.
For instances of multiple lesions biopsied, guidance is reported at the patient level using the following highest-order ranking: MRI greater than ultrasonography greater than mammography.
For instances of multiple lesions biopsied, histologic findings are reported at the patient level using the following highest-order ranking: invasive greater than DCIS greater than high risk greater than benign.
Initial Surgical Management After MRI
The treatment of the 339 evaluable patients is summarized in the Figure and Table 1. Fifty-four patients (15.9%) proceeded directly to mastectomy, of whom 25 (46.3%) did so because of MRI findings, including lesion size too large for breast conservation in 15 patients (60.0%), multicentric disease in 7 patients (28.0%), and contralateral breast cancer in 3 patients (12.0%). Twenty-four patients (44.4%) proceeded directly to mastectomy based on personal preference and 5 (9.3%) for other reasons (contraindication to radiotherapy, genetic mutation).
Surgical Management After First WLE
Of the 339 evaluable patients, 285 (84.1%) had WLE as the first surgical procedure after MRI; 274 of these women (96.1%) had successful WLE as their final surgical procedure, and 11 (3.9%) had mastectomy as their final surgical procedure, largely because of positive surgical margins. Of the 285 patients who had initial WLE, 63 (22.1%) required 1 (n = 60) or 2 (n = 3) additional excisions (Figure).
DCIS Score and Radiotherapy After WLE
Of the 274 patients who had WLE as the final surgical procedure, 72 patients (26.3%) did not have radiotherapy recommendations based on DCIS score because they did not meet the criteria (Figure), including 38 patients (52.8%) with invasive cancer and 34 patients (47.2%) with tumors less than 2 mm from the final surgical margin. Of the remaining 202 patients eligible for DCIS score to guide radiotherapy, the test was performed for 171 patients (84.7%). The DCIS score was low (<39) in 82 patients (48.0%; 95% CI, 40.6%-55.4%) and intermediate-high (≥39) in 89 patients (52.0%; 95% CI, 44.6%-59.4%). Patients with an intermediate-high DCIS score were significantly more likely to have high nuclear grade and estrogen receptor– and progesterone receptor–negative disease by immunohistochemical analysis (Table 3). A total of 84 of the 89 patients (94.4%) with intermediate-high DCIS scores accepted recommended radiotherapy, and 75 of 82 patients (91.5%) with low DCIS scores accepted a recommendation for no radiotherapy.
Table 3. Clinical Characteristics, DCIS Lesion Features, and Therapy Received Among Patients With Radiotherapy Based on the DCIS Scorea.
| Variable | Radiotherapy Recommendation Based on DCIS Score (n = 171) | Low DCIS Score (<39) (n = 82) | Intermediate-High DCIS Score (≥39) (n = 89) | P Valueb |
|---|---|---|---|---|
| Age, median (range), y | 60 (36-87) | 59 (40-80) | 61 (36-87) | .98 |
| Longest diameter, median (IQR), mm | 11 (6-19) | 10 (6-15.5) | 11 (6-20) | .29 |
| DCIS gradec | ||||
| Low nuclear | 16 (9.4) | 14 (17.1) | 2 (2.2) | <.001 |
| Intermediate nuclear | 65 (38.0) | 46 (56.1) | 19 (21.3) | |
| High nuclear | 89 (52.0) | 21 (25.6) | 68 (76.4) | |
| Not reported or unknown | 1 (0.6) | 1 (1.2) | 0 | |
| ER or PR status | ||||
| ER positive or PR positive | 138 (80.7) | 72 (87.8) | 66 (74.2) | .006 |
| ER negative and PR negative | 17 (9.9) | 2 (2.4) | 15 (16.9) | |
| Unknown | 16 (9.4) | 8 (9.8) | 8 (9.0) | |
| Radiotherapy course based on DCIS score | ||||
| Yes | 91 (53.2) | 7 (8.5) | 84 (94.4) | <.001 |
| No | 80 (46.8) | 75 (91.5) | 5 (5.6) | |
| Endocrine treatment | ||||
| Yes | 110 (64.3) | 61 (74.4) | 49 (55.1) | .02 |
| No | 46 (26.9) | 16 (19.5) | 30 (33.7) | |
| Not reported | 15 (8.8) | 5 (6.1) | 10 (11.2) |
Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; IQR, interquartile range; PR, progesterone receptor.
Data are presented as number (percentage) of patients unless otherwise indicated.
P values are from the nonparametric Wilcoxon rank sum test for continuous variables and the exact Pearson χ2 test for categorical variables. For the comparison of DCIS grade, the category not reported or unknown is excluded; for the comparison of endocrine treatment, the category not reported is excluded.
Reported DCIS grade corresponds to the highest-order DCIS grade identified at the patient level from the initial diagnostic core needle biopsy through the final surgical procedure.
Discussion
We report results from, to our knowledge, the first prospective, multicenter trial examining the association of preoperative breast MRI with surgical treatment and measuring patient acceptance of radiotherapy guided by DCIS score in women diagnosed with pure DCIS by core needle biopsy who were candidates for breast conservation surgery. We found that approximately 6 in 7 women underwent initial WLE after MRI, and of those, only 3.9% required mastectomy as the final procedure. Of importance, 78.5% achieved successful breast conservation with a single operation. Mastectomy was the first surgery in 15.9% of women and was prompted equally by MRI findings and patient preference. In women with pure DCIS who achieved WLE with 2-mm margins or larger and who had available DCIS scores, nearly half had low scores and were advised that radiotherapy could be avoided. Among patients who had radiotherapy recommendations guided by DCIS score, 93.0% agreed to the recommendation.
Although breast MRI use has increased during the past 2 decades, its use in the preoperative context for patients with DCIS remains controversial. Historically, MRI was considered to be suboptimal for DCIS evaluation because it could not detect calcifications visible on mammography. However, a multiple subsequent study16 found that, compared with mammography, MRI is more sensitive for detection and more accurate for determination of the extent of disease. These benefits notwithstanding, several studies17,18 have suggested that MRI has disadvantages. Some hypothesize that MRI use is not only correlated with increasing mastectomy rates but may be causal.18 Although retrospective studies7,8 reporting higher rates of mastectomy in women undergoing preoperative breast MRI have acknowledged that decisions for mastectomy are at least partially driven by patient preference, the authors were unable to quantify this component of a complex treatment decision-making process. Our study is the first, to our knowledge, to prospectively quantify the association of patient preferences with surgeons’ opinions on the decision for surgery. Our findings that more than half of decisions to undergo mastectomy were attributable to factors other than MRI findings highlight the complexity in the shared decision-making process between patients and physicians in this context.
We found that 19.2% of women eligible for breast conservation based on clinical and conventional imaging criteria underwent mastectomy as the final surgical procedure after preoperative MRI and 15.9% as the first surgical procedure. In contrast, among unselected populations of women with DCIS reported from large national data sets from 1998 to 2011, rates of mastectomy as the final surgical procedure ranged from 27% to 32%.5,18 Magnetic resonance imaging was associated with additional biopsies in 19.8% of patients in our study. Prior retrospective studies19,20,21 have reported higher additional biopsy rates ranging from 25% to 40%. This finding suggests that MRI accuracy may have improved since inception, likely because of improved techniques and increasing radiologist experience. The cancer yield in this study was in line with prior preoperative breast MRI studies.20,21,22 Of the 66 women who underwent additional biopsies based on MRI findings, 21 patients (31.8%) were found to have DCIS (n = 15) or invasive cancer (n = 6), and MRI identified otherwise occult cancers in 6.2% of patients presenting with unifocal DCIS.
One goal of preoperative MRI is to improve breast conservation success rate and decrease the number of reexcisions through better delineation of DCIS extent. However, a meta-analysis22 of 9 retrospective studies of MRI in women with DCIS found no significant differences in positive margin or reexcision rates in women undergoing WLE as their first surgery. In our study, WLE was performed as the first surgical procedure in 84.1% of patients, 96.1% of whom ultimately had successful breast conservation. Furthermore, 78.5% of patients in the study who underwent successful WLE achieved breast conservation with a single surgery, whereas 21.5% of patients required at least 1 reexcision. This reexcision rate is substantially lower than prior US reports of reexcision rates, which range from 48% to 59% in patients with DCIS without preoperative MRI.23,24,25,26
Radiotherapy is typically recommended after WLE for DCIS to reduce the risk of local recurrence of invasive and in situ cancer.3 Even in patients selected to receive WLE without radiotherapy because of favorable characteristics, such as smaller tumor size or low-intermediate grade disease, ipsilateral recurrence rates are approximately 1% to 1.5% annually,4,5 resulting in 10-year recurrence rates of 15% or higher.5 Two prospective retrospective validation studies found that the 12-gene DCIS score may be used to risk stratify women with DCIS treated with WLE alone and tumor-free margins, including a clinical trial of 327 women with low-risk disease6 and a population-based cohort study of 571 women.7 Combining results from both reports, approximately 60% to 70% had low-risk DCIS scores associated with a 10-year recurrence risk of 11% to 13% compared with recurrence rates of 25% to 30% in the 30% to 40% with intermediate-high risk DCIS scores. Although we currently do not have long-term outcome data, our prospective study showed at least 90% acceptance between DCIS score and tailored use of radiotherapy based on the DCIS score. Another study8 found similar results with regard to distribution of DCIS score and potential association with radiotherapy.
Our study was the first, to our knowledge, to prospectively recommend radiotherapy based on DCIS score without restricting eligibility by lesion size, nuclear grade, patient age, or other prognostic factors. For the DCIS score to be applied, patients needed to have pure DCIS verified on final surgical excision with 2 mm or greater of clear surgical margins; as a result, a small subpopulation of the study (n = 171) had radiotherapy recommendations based on DCIS score. Of these 171 patients, 82 (48.0%) were at low risk, of whom 75 (91.5%) were adherent with recommendations. These findings suggest that DCIS score can identify a significant fraction of patients who may avoid radiotherapy, with high patient acceptance, which could help address concerns related to overtreatment.
Strengths and Limitations
This trial has several strengths and limitations. Strengths included the prospective nature of the trial and the inclusion of multiple sites, including community practices and academic centers, with the only MRI requirement being that centers meet national accreditation standards as defined by the ACR. Unlike other studies9,12,13 designed to reduce overtreatment, this study did not restrict entry by DCIS features, such as nuclear grade, comedonecrosis, or estrogen receptor status. Another unique strength was the evaluation of patient and surgeon values when assessing reasons for selecting mastectomy or breast conservation.
Limitations include the nonrandomized nature of the trial and the lack of information on recurrence rates at the time of this analysis. The former limitation is relevant to the precise association of MRI with surgical management, and the latter is relevant to assumptions that the recommended radiotherapy based on the DCIS score was the correct choice. In addition, our patient population did not include all patients with DCIS but rather patients with DCIS eligible for WLE. At entry, 80.6% of patients enrolled listed WLE as their preference for treatment and represent a patient group motivated to pursue WLE.
Conclusions
Among women with DCIS who were WLE candidates based on conventional imaging, multiple factors were associated with conversion to mastectomy. This trial may provide useful preliminary information required for designing a planned randomized clinical trial to determine the effect of MRI and DCIS score on surgical management, radiotherapy, overall resource utilization, and clinical outcomes, with the ultimate goal of achieving greater therapeutic precision.
Trial Protocol
eFigure. MRI Findings Management Flowchart
eTable. Baseline Demographics and Diagnostic Core Needle Biopsy and Mammography Characteristics
References
- 1.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430-1441. doi: 10.1056/NEJMra031301 [DOI] [PubMed] [Google Scholar]
- 2.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275(12):913-918. doi: 10.1001/jama.1996.03530360023033 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 4.Kumar AS, Bhatia V, Henderson IC. Overdiagnosis and overtreatment of breast cancer: rates of ductal carcinoma in situ: a US perspective. Breast Cancer Res. 2005;7(6):271-275. doi: 10.1186/bcr1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society Breast cancer facts & figures 2015. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2015-2016.pdf. Accessed August 3, 2018.
- 6.Worni M, Akushevich I, Greenup R, et al. . Trends in treatment patterns and outcomes for ductal carcinoma in situ. J Natl Cancer Inst. 2015;107(12):djv263. doi: 10.1093/jnci/djv263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feigelson HS, Carroll NM, Weinmann S, et al. . Treatment patterns for ductal carcinoma in situ from 2000-2010 across six integrated health plans. Springerplus. 2015;4:24. doi: 10.1186/s40064-014-0776-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zujewski JA, Harlan LC, Morrell DM, Stevens JL. Ductal carcinoma in situ: trends in treatment over time in the US. Breast Cancer Res Treat. 2011;127(1):251-257. doi: 10.1007/s10549-010-1198-z [DOI] [PubMed] [Google Scholar]
- 9.Correa C, McGale P, Taylor C, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010(41):162-177. doi: 10.1093/jncimonographs/lgq039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888-896. doi: 10.1001/jamaoncol.2015.2510 [DOI] [PubMed] [Google Scholar]
- 11.Allegra CJ, Aberle DR, Ganschow P, et al. . NIH state-of-the-science conference statement: diagnosis and management of ductal carcinoma in situ (DCIS). NIH Consens State Sci Statements. 2009;26(2):1-27. [PubMed] [Google Scholar]
- 12.Solin LJ, Gray R, Baehner FL, et al. . A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701-710. doi: 10.1093/jnci/djt067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakovitch E, Nofech-Mozes S, Hanna W, et al. . A population-based validation study of the DCIS score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat. 2015;152(2):389-398. doi: 10.1007/s10549-015-3464-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Radiology Breast MRI Accreditation Program Modalities, breast MRI. http://www.acraccreditation.org/Modalities/Breast-MRI. Accessed August 3, 2018.
- 15.D’Orsi CJ, Sickles EA, Mendelson EB, et al. . ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 16.Lehman CD. Magnetic resonance imaging in the evaluation of ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010(41):150-151. doi: 10.1093/jncimonographs/lgq030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102(3):170-178. doi: 10.1093/jnci/djp482 [DOI] [PubMed] [Google Scholar]
- 18.Shiyanbola OO, Sprague BL, Hampton JM, et al. . Emerging trends in surgical and adjuvant radiation therapies among women diagnosed with ductal carcinoma in situ. Cancer. 2016;122(18):2810-2818. doi: 10.1002/cncr.30105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elder EA, Ferlin A, Vallow LA, et al. . The influence of breast density on preoperative MRI findings and outcome in patients with a known diagnosis of breast cancer. Ann Surg Oncol. 2017;24(10):2898-2906. doi: 10.1245/s10434-017-5981-5 [DOI] [PubMed] [Google Scholar]
- 20.Debald M, Abramian A, Nemes L, et al. . Who may benefit from preoperative breast MRI? a single-center analysis of 1102 consecutive patients with primary breast cancer. Breast Cancer Res Treat. 2015;153(3):531-537. doi: 10.1007/s10549-015-3556-3 [DOI] [PubMed] [Google Scholar]
- 21.Pilewskie M, Kennedy C, Shappell C, et al. . Effect of MRI on the management of ductal carcinoma in situ of the breast. Ann Surg Oncol. 2013;20(5):1522-1529. doi: 10.1245/s10434-012-2771-y [DOI] [PubMed] [Google Scholar]
- 22.Fancellu A, Turner RM, Dixon JM, Pinna A, Cottu P, Houssami N. Meta-analysis of the effect of preoperative breast MRI on the surgical management of ductal carcinoma in situ. Br J Surg. 2015;102(8):883-893. doi: 10.1002/bjs.9797 [DOI] [PubMed] [Google Scholar]
- 23.Caughran JL, Vicini FA, Kestin LL, Dekhne NS, Benitez PR, Goldstein NS. Optimal use of re-excision in patients diagnosed with early-stage breast cancer by excisional biopsy treated with breast-conserving therapy. Ann Surg Oncol. 2009;16(11):3020-3027. doi: 10.1245/s10434-009-0628-9 [DOI] [PubMed] [Google Scholar]
- 24.Tartter PI, Kaplan J, Bleiweiss I, et al. . Lumpectomy margins, reexcision, and local recurrence of breast cancer. Am J Surg. 2000;179(2):81-85. doi: 10.1016/S0002-9610(00)00272-5 [DOI] [PubMed] [Google Scholar]
- 25.Shaikh T, Li T, Murphy CT, et al. . Importance of surgical margin status in ductal carcinoma in situ. Clin Breast Cancer. 2016;16(4):312-318. doi: 10.1016/j.clbc.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 26.Halasz LM, Sreedhara M, Chen YH, et al. . Improved outcomes of breast-conserving therapy for patients with ductal carcinoma in situ. Int J Radiat Oncol Biol Phys. 2012;82(4):e581-e586. doi: 10.1016/j.ijrobp.2011.08.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. MRI Findings Management Flowchart
eTable. Baseline Demographics and Diagnostic Core Needle Biopsy and Mammography Characteristics