Key Points
Question
Does use of glyburide compared with subcutaneous insulin result in an increase in perinatal complications among women with gestational diabetes?
Findings
In this noninferiority randomized trial that included 914 pregnant women with gestational diabetes, the rate of the composite criterion (including macrosomia, neonatal hypoglycemia, and hyperbilirubinemia) was 27.6% with glyburide and 23.4% with insulin; the upper confidence limit of the difference was 10.5%, which exceeded the prespecified noninferiority margin of 7%.
Meaning
These findings do not support noninferiority of glyburide for prevention of perinatal complications of gestational diabetes.
Abstract
Importance
Randomized trials have not focused on neonatal complications of glyburide for women with gestational diabetes.
Objective
To compare oral glyburide vs subcutaneous insulin in prevention of perinatal complications in newborns of women with gestational diabetes.
Design, Settings, and Participants
The Insulin Daonil trial (INDAO), a multicenter noninferiority randomized trial conducted between May 2012 and November 2016 (end of participant follow-up) in 13 tertiary care university hospitals in France including 914 women with singleton pregnancies and gestational diabetes diagnosed between 24 and 34 weeks of gestation.
Interventions
Women who required pharmacologic treatment after 10 days of dietary intervention were randomly assigned to receive glyburide (n=460) or insulin (n=454). The starting dosage for glyburide was 2.5 mg orally once per day and could be increased if necessary 4 days later by 2.5 mg and thereafter by 5 mg every 4 days in 2 morning and evening doses, up to a maximum of 20 mg/d. The starting dosage for insulin was 4 IU to 20 IU given subcutaneously 1 to 4 times per day as necessary and increased according to self-measured blood glucose concentrations.
Main Outcomes and Measures
The primary outcome was a composite criterion including macrosomia, neonatal hypoglycemia, and hyperbilirubinemia. The noninferiority margin was set at 7% based on a 1-sided 97.5% confidence interval.
Results
Among the 914 patients who were randomized (mean age, 32.8 [SD, 5.2] years), 98% completed the trial. In a per-protocol analysis, 367 and 442 women and their neonates were analyzed in the glyburide and insulin groups, respectively. The frequency of the primary outcome was 27.6% in the glyburide group and 23.4% in the insulin group, a difference of 4.2% (1-sided 97.5% CI, −∞ to 10.5%; P=.19).
Conclusion and Relevance
This study of women with gestational diabetes failed to show that use of glyburide compared with subcutaneous insulin does not result in a greater frequency of perinatal complications. These findings do not justify the use of glyburide as a first-line treatment.
Trial Registration
clinicaltrials.gov Identifier: NCT01731431
This noninferiority randomized clinical trial compares the effects of glyburide vs subcutaneous insulin on macrosomia, neonatal hypoglycemia, and hyperbilirubinemia in infants born to women with gestational diabetes.
Introduction
Gestational diabetes is a major public health concern, and its rate is increasing worldwide.1,2 Adequate treatment of women with gestational diabetes reduces fetal and maternal morbidity.3 Insulin continues to be the American Diabetes Association–recommended first-line therapy and the only pharmacologic treatment approved by the US Food and Drug Administration.4 The American College of Obstetricians and Gynecologists recommends not using glyburide as a first-choice pharmacologic treatment.5 However, insulin is expensive and inconvenient because it requires several subcutaneous injections a day and careful management of dose adaptation.6 Glyburide is a potential alternative treatment and, as an oral drug, is more acceptable to patients. Since the first randomized trial by Langer et al7 showed that glycemic control is similar for the 2 treatments, glyburide has become a common additional pharmacotherapy for gestational diabetes in the United States,2 while in Europe it is not used routinely. Meta-analyses8,9,10,11,12,13 and recent studies14,15 question use of glyburide, reporting an increased rate of neonatal morbidities compared with insulin, especially macrosomia and hypoglycemia. However, because all randomized trials comparing glyburide with insulin used maternal glycemic control as the primary outcome, they were not optimally designed to investigate neonatal complications.7,8 The aim of the present study was therefore to compare glyburide and insulin for prevention of perinatal complications, especially because the American College of Obstetricians and Gynecologists recommends equivalence or noninferiority trials comparing oral agents with insulin.5 The comparison was planned as a noninferiority test because glyburide is an oral pharmacotherapy for gestational diabetes and is more convenient for patients, with greater ease of administration and reduced costs.
Methods
Study Design and Patients
We performed a multicenter randomized noninferiority trial (the Insulin Daonil trial [INDAO]) in 13 tertiary care university hospitals in France. Recruitment lasted from May 2012 to September 2016, and November 2016 was the end of patient follow-up. The trial protocol is available in Supplement 1 and the statistical analysis plan in Supplement 2. All patients provided written informed consent before enrollment. The trial protocol was approved by the ethics committee of the Poissy St-Germain Hospital. An independent data and safety monitoring committee periodically reviewed study outcomes.
Women with a singleton pregnancy who were diagnosed as having gestational diabetes between 24 and 34 weeks of gestation were eligible. Gestational diabetes was diagnosed if a 75-g oral glucose tolerance test resulted in 1 or more abnormal blood glucose values: greater than 92 mg/dL (5.1 mmol/L), greater than 180 mg/dL (10 mmol/L), or greater than 153 mg/dL (8.5 mmol/L) for fasting, 1-hour postprandial, or 2-hour postprandial blood glucose concentration, respectively.16
Exclusion criteria were diabetes, fasting blood glucose concentration greater than 126 mg/dL (7 mmol/L), glucose screening test performed before 24 weeks of gestation, multiple pregnancy, chronic hypertension, preeclampsia, and known liver or renal disease.
Women diagnosed with gestational diabetes were given individual nutrition education by a dietitian designed to provide 25 kcal/kg per day for overweight and obese women and 35 kcal/kg per day for women at normal weight. Nutritional intake was divided into 3 meals and 2 snacks daily. Women were taught to self-monitor capillary blood glucose levels 4 times daily (fasting and 2 hours after each meal). Glycemic goals were considered not achieved after 10 days of well-managed diet if at least 2 blood glucose values above the targets were observed over a week (fasting: ≥95 mg/dL; 2-hour postprandial: ≥120 mg/dL), with no variations in diet. Women who did not meet glycemic goals were eligible for randomization to 1 of the 2 treatment groups.
Randomization
Eligible women were randomly assigned in a 1:1 ratio to receive glyburide or insulin. An independent, centralized, computer-generated randomization sequence (CleanWeb, Tele-medicine Technologies) was used for this allocation according to a permuted-block method with block sizes randomly chosen from 2 to 8, stratified by center. Clinicians and participants had no access to the list but could not be blinded to group allocation after randomization.
Procedures
For glyburide, the starting dosage for therapy was 2.5 mg orally once per day and could be increased if necessary 4 days later by 2.5 mg and thereafter by 5 mg every 4 days in 2 doses, morning and evening, up to a maximum of 20 mg/d. If the maximum tolerated dosage was reached without achieving the desired glucose values of less than 95 mg/dL (5.3 mmol/L) for fasting measurements and less than 120 mg/dL (6.7 mmol/L) for 2-hour postprandial measurements, treatment was switched to insulin.
For insulin, the starting dosage for rapid analogs was 4 IU given subcutaneously before meals, 1 to 3 times per day as necessary and increased by 2 IU every 2 days according to the postprandial blood glucose value. If necessary, the starting dosage for basal or intermediate insulin was 4 IU to 8 IU given subcutaneously at bedtime and increased by 2 IU every 2 days according to the morning fasting blood glucose value. Women were taught to self-adjust their insulin doses in an effort to reach and maintain glycemic goals throughout pregnancy.
All women had a clinical assessment with a visit to an endocrinologist at days 8 and 21 after randomization and then every 15 days to once per month according to level of glycemic control. In addition to these planned visits, women received prenatal care at their institutions as deemed appropriate by their caregivers (eg, general physicians, obstetricians, midwives). Newborn monitoring was identical to the usual recommendation for newborns of mothers with diabetes, with early and frequent breast or bottle feeding: from birth, at 30 minutes and then every 2 or 3 hours. Neonatal glucose monitoring started immediately after birth and included frequent measurements (8 in the first 24 hours and 2 on day 2). The number of glucose measurements was increased when clinically required.
Outcome Variables
The primary outcome was a composite criterion of perinatal complications associated with gestational diabetes, including macrosomia, neonatal hypoglycemia, and hyperbilirubinemia. Macrosomia was defined as birth weight greater than 4000 g or above the 90th percentile for gestational age according to French curves.17 Neonatal hypoglycemia was defined as blood glucose value less than 36 mg/dL (2 mmol/L) after 2 hours of life.18 Hyperbilirubinemia was defined as the need for phototherapy without another cause of jaundice.
The prespecified secondary outcomes included (1) neonatal outcomes of perinatal death, admission to a neonatal intensive care unit (NICU) and neonatal ward, respiratory distress syndrome, birth injury, ponderal index (calculated as [birth weight in grams divided by height in centimeters cubed] times 100), pH level of less than 7, lactates in 3 categories (<6 mmol/L, 6-9 mmol/L, and >9 mmol/L), and base excess (not recorded); (2) maternal outcomes of glycemic control during pregnancy (see below), hypoglycemia (defined as blood glucose level <60 mg/dL [3.3 mmol/L]) and/or a symptomatic episode of hypoglycemia with clinical symptoms of severity (confusion, poor coordination, double vision, convulsion, or inability to self-treat symptoms), premature delivery, mode of delivery, perineal trauma, percentage switch from glyburide to insulin, and maternal satisfaction; and (3) other outcomes, data for which are not presented here, including number of prenatal visits, number of diabetologist visits, and hospitalization days during pregnancy.
Glycemic control during pregnancy was quantified for each woman by the percentage of blood glucose measurements at 95 mg/dL or greater for fasting measurements and 120 mg/dL or greater for 2-hour postprandial measurements. Three categories were defined (separately for fasting and postprandial measurements): good glycemic control (<20%), moderate or fairly good glycemic control (20%-40%), and poor glycemic control (>40%). A satisfaction questionnaire was given to women in the first postpartum week to assess treatment acceptability.
Additional outcomes considered exploratory were components of the primary composite outcome, characteristics of the newborn (birth weight, Apgar score, severity of hypoglycemia), reasons for admission to the NICU, mean insulin or glyburide dosages received by women, and severe episodes of maternal hypoglycemia (defined as glucose level <40 mg/dL).
Sample Size
We estimated the usual frequency of the primary outcome (macrosomia, hypoglycemia, and hyperbilirubinemia) in newborns of women with gestational diabetes treated with insulin to be 18% based on published randomized trials comparing insulin vs either usual prenatal care3,19 or glyburide treatment7,20,21,22,23 and based on (unpublished) retrospective data from the participating centers. The noninferiority boundary was based on clinical evidence.24 We asked a group of clinicians to consider what increase in neonatal complications they would accept in exchange for the potential benefits offered by glyburide.25 They estimated that a 25% rate of perinatal complications in the glyburide group was acceptable. Glyburide was then considered noninferior to insulin if the upper confidence limit of the difference did not exceed the prespecified noninferiority margin of 7%. With these assumptions and with a statistical power of 80%, a type I error of 5%, and a 2-sided test, 372 women per group were required. With anticipation that approximately 20% of patients in the glyburide group would be switched to the insulin group,26 450 women per group were necessary.
Statistical Analysis
Continuous variables were summarized as means and standard deviations or medians and interquartile ranges if non–normally distributed and qualitative variables as numbers and percentages of participants. To take into account that the trial was multicenter, mixed-effects models were used to compare the 2 groups (logistic for qualitative variables and linear for quantitative variables). These models were used to estimate means and standard deviations or percentages in each group and differences in means or percentages and 95% confidence intervals between groups.
Primary Outcome
The analysis of the primary outcome was performed on the per-protocol population excluding women who were switched from glyburide to insulin treatment comparing the difference (glyburide rate − insulin rate) in the frequency of the primary outcome.27
Noninferiority of glyburide compared with insulin was demonstrated if the upper bound of the 1-sided 97.5% confidence interval of this difference was smaller than the prespecified threshold of 7%.
A prespecified sensitivity analysis for women who switched treatment was performed on the intention-to-treat population. A complementary prespecified analysis was performed by adjusting for baseline characteristics that differed between randomized groups.
Secondary Outcomes
Analyses of secondary outcomes and post hoc analyses were made with superiority tests and 95% CIs for differences and should be considered exploratory because no correction for multiple comparisons was done.
No imputation was made for missing data because there were only 2 missing data for the primary outcome and very few for secondary outcomes. Complete case analyses were done. The threshold for statistical significance was set at P<.05 with a 2-sided test; for the primary outcome, a 1-sided 97.5% confidence interval was considered. Statistical analyses were performed using Stata software, release 14.28
Results
Characteristics of the Women
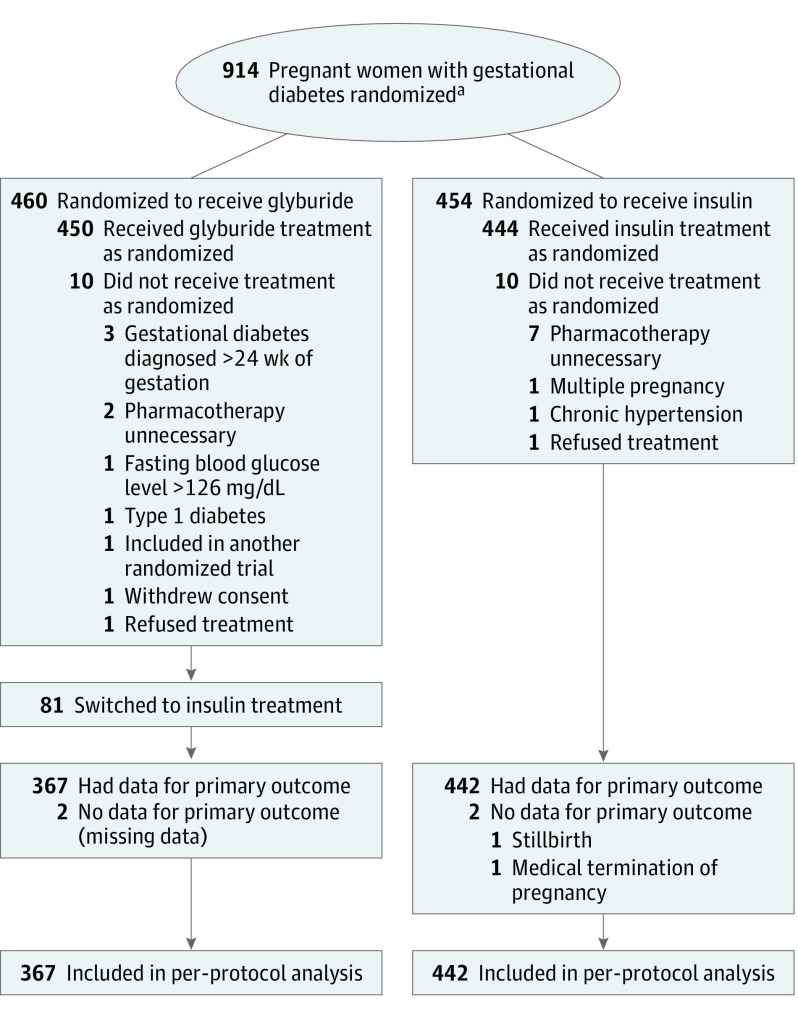
Among 914 women with gestational diabetes (mean age, 32.8 [SD, 5.2] years), 460 were randomized to receive glyburide treatment and 454 to receive insulin treatment. After randomization, 18 patients (2.0%) were excluded from the analysis because they did not meet the inclusion criteria and 6 because they had no data for the primary outcome or refused the treatment (Figure). Among the 448 women remaining in the glyburide group, 81 (18%) were switched to insulin, most often before reaching the maximum glyburide dosage. The per-protocol analysis therefore included 809 women and their neonates, 367 in the glyburide group and 442 in the insulin group (Figure).
Figure. Participant Flow in the Insulin Daonil Trial.
aData on women who were screened for eligibility and reasons why some of them were not randomized were not recorded and are not shown.
The baseline characteristics of the 809 included women are shown in Table 1. Overall, there were no noticeable between-group differences in demographic variables or blood glucose values at inclusion. Multiparity was more frequent in the insulin group (66.3% vs 58.9%), and gestational age at treatment was slightly greater in the glyburide group.
Table 1. Baseline Characteristics of Women Included in the Primary Per-Protocol Analysis.
| Characteristics | Glyburide Group (n = 367) | Insulin Group (n = 442) |
|---|---|---|
| Age, mean (SD), y | 32.5 (5.1) | 32.6 (5.3) |
| Multiparity, No. (%) | 216 (58.9) | 293 (66.3) |
| Prepregnancy BMI, mean (SD)a | 27.3 (5.5) | 27.8 (5.8) |
| BMI at diagnosis, mean (SD)a | 30.7(5.1) | 31.1(5.4) |
| Geographical origin, No. (%) | ||
| Europe | 146 (41.3) | 184 (43.2) |
| North Africa | 124 (34.9) | 136 (31.9) |
| Sub-Saharan Africa | 35 (9.9) | 50 (11.7) |
| Asia | 19 (5.4) | 21 (4.9) |
| Other | 31 (8.7) | 35 (8.2) |
| Previous gestational diabetes, No. (%) | 73 (20.0) | 88 (19.9) |
| Gestational age, median (IQR), wk+d | ||
| At OGTT screening | 26+5 (25+3 to 28+0) | 26+3 (25+1 to 28+0) |
| At treatment | 32+6 (30+6 to 34+3) | 32+3 (30+3 to 34+1) |
| Type of blood glucose measurement with abnormal result at randomization, No. (%) | ||
| Only fasting | 12 (3.3) | 13 (2.9) |
| Only 2-h postprandial | 65 (17.7) | 72 (16.3) |
| Both fasting and 2-h postprandial | 290 (79.0) | 357 (80.8) |
| Proportion of abnormal blood glucose results at randomization, median (IQR)b | ||
| Fasting blood glucose ≥95 mg/dL | 0.3 (0.1-0.6) | 0.3 (0.1-0.5) |
| Postprandial blood glucose ≥120 mg/dL | 0.2 (0.1-0.3) | 0.2 (0.1-0.3) |
Abbreviations: IQR, interquartile range; OGTT, oral glucose tolerance test.
Body mass index (BMI) is calculated as weight in kilograms divided by height in meters squared.
For each woman, the number of abnormal blood glucose assay results over all of her blood glucose assays between inclusion and randomization was computed, then medians and IQRs of these proportions were calculated.
Primary Outcome
The frequency of the primary outcome was 27.6% in the glyburide group and 23.4% in the insulin group (difference, 4.2%; 1-sided 97.5% CI, −∞ to 10.5%; P = .19), and the upper confidence limit exceeded the noninferiority margin of 7% (Table 2). When adjusted for multiparity and gestational age at treatment, the results remained similar (difference, 4.4%; 1-sided 97.5% CI, −∞ to 10.5%; P = .20) (eFigure in Supplement 3).
Table 2. Primary and Secondary Per-Protocol Outcomes Analyses.
| Outcomes | Glyburide Group (n = 367) | Insulin Group (n = 442) | Difference, % (95% CI) | P Valuea |
|---|---|---|---|---|
| Primary Outcome | ||||
| Neonatal composite criterion, No. (%)b | 101 (27.6) | 103 (23.4) | 4.2 (−∞ to 10.5)c | .19d |
| Neonatal Secondary Outcomes | ||||
| Admission to neonatal intensive care unit before 48 h of life, No. (%) | 10 (2.3) | 11 (2.4) | 0.02 (−2.4 to 2.7) | .87 |
| Admission to neonatal ward, No. (%)e | 27 (7.9) | 34 (8.2) | −0.3 (−5.2 to 4.6) | .86 |
| Severe respiratory distress syndrome, No (%) | 8 (1.9) | 11 (2.2) | −0.3 (−2.7 to 2.0) | .75 |
| Birth injury, No. (%) | 6 (1.5) | 9 (1.9) | −0.4 (−2.3 to 1.5) | .66 |
| Shoulder dystocia, No. | 1 | 2 | ||
| Bone fracture, No. | 1 | 6 | ||
| Nerve palsy, No. | 1 | 0 | ||
| Other, No.f | 3 | 1 | ||
| Ponderal index, mean (SD)g | 2.75 (0.04) | 2.74 (0.04) | 0.01 (−0.08 to 0.10) | .52 |
| pH <7, No. (%) | 2 (0.6) | 2 (0.5) | 0.0 (−1.0 to 1.2) | .88 |
| Lactates, mmol/L, No. (%)h | ||||
| <6 | 175 (79.2) | 219 (82.3) | −3.1 (−10.2 to 3.9) | .14 |
| 6-9 | 33 (14.9) | 41 (15.4) | −0.5 (−6.9 to 5.9) | |
| >9 | 13 (5.9) | 6 (2.3) | 3.6 (0.05 to 7.2) | |
| Maternal Secondary Outcomes | ||||
| Glycemic control during pregnancyi | ||||
| Fasting blood glucose ≥95 mg/dL, No. (%) | ||||
| Good (<20%) | 248 (71.7) | 264 (63.2) | 8.5 (1.9 to 15.2) | .003 |
| Moderate or fair (20%-40%) | 68 (19.7) | 83 (19.9) | −0.2 (−5.9 to 5.5) | |
| Poor (>40%) | 30 (8.7) | 71 (17.0) | −8.3 (−13.0 to −3.7) | |
| Postprandial blood glucose ≥120 mg/dL, No. (%) | ||||
| Good (<20%) | 200 (57.8) | 206 (49.3) | 8.5 (1.5 to 15.6) | .051 |
| Moderate or fair (20%-40%) | 109 (31.5) | 165 (39.5) | −8.0 (−14.8 to −1.2) | |
| Poor (>40%) | 37 (10.7) | 47 (11.2) | −0.5 (−5.0 to 3.9) | |
| Maternal hypoglycemia, No. (%)j | 93 (28.8) | 13 (3.5) | 25.3 (16.6 to 34.0) | <.001 |
| Preterm delivery, No. (%) | 25 (6.8) | 18 (4.1) | 2.7 (−1.0 to 6.4) | .09 |
| Mode of delivery, No. (%) | ||||
| Spontaneous vaginal | 205 (55.9) | 251 (56.8) | −0.9 (−7.8 to 5.9) | .08 |
| Assisted vaginal | 63 (17.2) | 67 (15.2) | 2.0 (−3.1 to 7.1) | |
| Elective cesarean | 36 (9.8) | 66 (14.9) | −5.1 (−9.6 to −0.6) | |
| Emergency cesarean | 63 (17.2) | 58 (13.1) | 4.0 (−0.9 to 9.0) | |
| Any perineal trauma | 3 (0.8) | 1 (0.2) | 0.6 (−0.8 to 2.0) | .27 |
| Maternal satisfaction: preferred treatment for a future pregnancy, No. (%)k | ||||
| Glyburide | 207 (78.7) | 125 (43.6) | 35.2 (27.6 to 42.7) | <.001 |
| Insulin | 7 (2.7) | 57 (19.9) | −17.2 (−22.2 to −12.2) | |
| Unknown | 49 (18.6) | 105 (36.6) | −18.0 (−25.3 to −10.7) | |
P value of the test of the coefficient of the mixed-effects model used to account for multiple centers (logistic for qualitative variables, linear for quantitative ones).
Macrosomia, neonatal hypoglycemia, and hyperbilirubinemia.
Confidence interval for the primary outcome is a 1-sided 97.5% confidence interval.
P value of the noninferiority test.
Less sick newborns are hospitalized in this unit; more severely ill newborns are in the neonatal intensive care unit.
Glyburide group: facial hematoma, serosanguine hump, scalp wound; insulin group: serosanguine hump.
Ponderal index (calculated as [birth weight in grams divided by height in centimeters cubed] times 100) may increase when quality maternal glycemic control decreases. The usual 10th, 50th, and 90th percentiles of the ponderal index are 2.2, 2.6, and 3.1.
Lactate levels were known for 60% of newborns in both groups.
Percentage of measurements at or above the blood glucose thresholds of 95 mg/dL for fasting measurements and 120 mg/dL for 2-hour postprandial measurements. Three categories were defined: good glycemic control (<20% of measurements meeting threshold), moderate or fairly good glycemic control (20%-40%), and poor glycemic control (>40%). Data were missing for 21 in the glyburide group and 24 in the insulin group.
At least 1 fasting or postprandial blood glucose measurement <60 mg/dL during pregnancy.
There were 554 responses (68.5%).
Intention-to-treat sensitivity analysis indicated a difference of 4.2% (1-sided 97.5% CI, −∞ to 10.0%; P = .17) (eTable 1 in Supplement 3).
Prespecified Secondary Neonatal Outcomes
No perinatal deaths occurred in the glyburide group, and there were 2 in the insulin group (1 patient with unexplained intrauterine death at 40 weeks of gestation with birth of an infant weighing 3400 g; 1 medical termination of pregnancy performed at 36 weeks of gestation because of severe brain abnormalities).
The rates of admission to an NICU and neonatal nursery did not differ significantly in the 2 groups. There were no significant between-group differences in birth injury, ponderal index, pH level of less than 7, lactate levels, and respiratory distress syndrome (Table 2).
Prespecified Secondary Maternal Outcomes
Blood glucose control was significantly better during pregnancy in the glyburide group, with 71.7% of women maintaining good fasting glycemic control compared with 63.2% in the insulin group (difference, 8.5%; 95% CI, 1.9%-15.2%; P = .003) (Table 2). Good control of postprandial glucose was achieved in 57.8% of women in the glyburide group and 49.3% in the insulin group (difference, 8.5%; 95% CI, 1.5%-15.6%; P = .051).
More women in the glyburide group had at least 1 episode of fasting or postprandial glycemia (blood glucose <60 mg/dL) during pregnancy (glyburide group, 93 [28.8%] vs insulin group, 13 [3.5%]; difference, 25.3%; 95% CI, 16.6%-34.0%; P < .001). There were no significant between-group differences in mode of delivery, preterm delivery, and perineal trauma. Overall, 68.5% of women completed the satisfaction questionnaire during their maternity stay. There was no significant relationship between nonresponse and the composite criterion (P = .82). In terms of maternal satisfaction, 23.6% of patients in the insulin group indicated that therapy is the most difficult part of the treatment, whereas only 5% did in the glyburide group (P < .001). Among women treated with glyburide, 78.7% indicated that they would choose glyburide treatment in a subsequent pregnancy, whereas only 19.9% of women treated with insulin would choose insulin again (P < .001) (Table 2).
Post Hoc Analyses
Per-protocol analysis results for the components of the composite criterion are given in Table 3 (and in eTable 2 in Supplement 3 for the intention-to-treat analysis). Hypoglycemia occurred in 12.2% of the glyburide group and in 7.2% of the insulin group (difference, 5%; 95% CI, 0.5%-9.5%; P = .02). There were no significant between-group differences in macrosomia and hyperbilirubinemia rates.
Table 3. Post Hoc Per-Protocol Outcomes Analyses.
| Outcomes | Glyburide Group (n = 367) | Insulin Group (n = 442) | Difference, % (95% CI) | P Valuea |
|---|---|---|---|---|
| Composite Criterion Components | ||||
| Macrosomia, No. (%) | 59 (16.2) | 65 (14.8) | 1.4 (−3.9 to 6.6) | .59 |
| Hypoglycemia, No. (%) | 45 (12.2) | 32 (7.2) | 5.0 (0.5 to 9.5) | .02 |
| Hyperbilirubinemia, No. (%) | 14 (3.8) | 14 (3.1) | 0.6 (−2.0 to 3.3) | .61 |
| Neonatal Outcomes | ||||
| Birth weight, mean (SD), g | 3341 (513) | 3331 (476) | 10 (−58 to 78) | .77 |
| Birth weight >4000 g, No. (%) | 33 (9.3) | 28 (6.6) | 2.7 (−1.9 to 7.4) | .16 |
| Apgar score ≤7 at 5 min, No. (%) | 3 (0.8) | 11 (2.5) | −1.7 (−3.4 to 0.04) | .08 |
| Reason for admission to neonatal intensive care unit before 48 h of life, No. | ||||
| Severe respiratory distress syndrome | 8 | 11 | ||
| Otherb | 2 | 0 | ||
| Maternal Outcomes | ||||
| Insulin dosage received, mean (SD), units/dc | 19.6 (14.6) | |||
| Glyburide dosage received, mean (SD), mg/dc | 5.4 (3.4) | |||
| Maternal hypoglycemia, No. (%)d | 13 (3.8) | 4 (1.0) | 2.8 (0.2 to 5.5) | .02 |
P value of the test of the coefficient of the mixed-effects model used to account for multiple centers (logistic for qualitative variables, linear for quantitative ones).
One malformation and 1 maternal/fetal infection.
Mean dosages were calculated from diagnosis to delivery.
At least 1 fasting or postprandial blood glucose measurement <40 mg/dL during pregnancy.
Severity of hypoglycemia among newborns did not differ significantly between the groups, with blood glucose levels of 1 mmol/L or lower among 2 newborns in the glyburide group and 3 newborns in the insulin group. No neonate presented with severe clinical signs of hypoglycemia. Among newborns admitted to the NICU, none were admitted because of clinical signs of hypoglycemia or for treatment of hypoglycemia.
More women in the glyburide group had a severe hypoglycemia episode (blood glucose level <40 mg/dL: 13 [3.8%] in the glyburide group vs 4 [1.0%] in the insulin group; difference, 2.8%; 95% CI, 0.2%-5.5%; P = .02). Among them, 2 women in the glyburide group and 1 woman in the insulin group reported symptoms with an inability to self-treat their symptoms.
Discussion
This study of women with gestational diabetes failed to show that use of glyburide compared with subcutaneous insulin does not result in a greater frequency of perinatal complications. These findings do not justify use of glyburide as a first-line treatment.
The higher rate of the primary outcome in the glyburide group was mainly due to an increased rate of neonatal hypoglycemia. This is consistent with results of meta-analyses including observational studies and randomized trials.8,9,10,11,12,13 However, most of these meta-analyses also mention a significantly increased risk of macrosomia8,9,11 in neonates of women treated with glyburide, and 2 studies using large administrative databases have additionally reported an increased risk of respiratory distress syndrome and NICU admissions with glyburide.14,15 These findings are not consistent with the results, albeit exploratory, reported in Table 3, because no significant between-group differences were found in the rates of macrosomia, hyperbilirubinemia, admission to the NICU or neonatal nursery, or respiratory distress syndrome. An isolated increase in neonatal hypoglycemia in the glyburide group is in agreement with data from the latest meta-analysis comparing management of gestational diabetes with glyburide vs insulin.12 The rate of neonatal hypoglycemia in the glyburide group was 12.2%, which is of the same magnitude as the 9% reported by Langer et al7 in their insulin group but much lower than the 33% reported by Bertini et al21 and the 25% reported by Silva et al23 in their glyburide groups. A prospective cohort study involving neonates considered to be at risk of hypoglycemia, including 40% of neonates born to a mother with diabetes, showed that with an on-treatment blood glucose level threshold of 47 mg/dL (2.6 mmol/L), neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes at 2 years compared with neonates with normal glucose levels.29
Because women had gestational diabetes, study physicians knew the infants were at risk of hypoglycemia, and neonatal glucose was monitored frequently after birth, so it is unlikely that there were neonates with unrecognized and untreated hypoglycemia. The 18% glyburide-to-insulin switch rate is consistent with literature reports.26
The strengths of the present study include that it was a multicenter study, which increases its generalizability. In addition, a neonatal criterion was chosen as the primary outcome because, to our knowledge, no previous randomized trials have optimally assessed the potential effect of glyburide on prevention of perinatal complications.7,20,21,22,23
Limitations
This study had several limitations. First, some criteria were not prespecified in the initial protocol, such as the components of the primary outcome or the reason for admission to the NICU, and these must therefore be considered as exploratory or post hoc analyses and are of reduced weight. Second, criteria chosen to assess satisfaction in terms of women’s treatment preferences for a future pregnancy may be questioned, inasmuch as each woman received only 1 of the 2 treatments and had no experiential basis for making such a comparison.
The noninferiority framework provides, on one hand, a binary conclusion (significant or not) and, on the other hand, a more quantitative result with the boundaries of the 95% confidence interval.30
Although the data do not allow a conclusion that glyburide is not inferior to insulin in the prevention of perinatal complications, the results suggest that the increase in complications may be no more than 10.5% compared with insulin. This result should be balanced with the ease of use and better satisfaction with glyburide. In clinical situations in which an oral agent may be necessary, mothers, informed by their physicians, would be appropriate decision makers based on their own weighing of benefits and risks.
Conclusions
This study of women with gestational diabetes failed to show that use of glyburide compared with subcutaneous insulin does not result in a greater frequency of perinatal complications. These findings do not justify the use of glyburide as a first-line treatment.
Trial Protocol
Statistical Analysis Plan
eFigure. Confidence Interval of the Difference of the Composite Criterion Rates and Noninferiority Margin
eTable 1. Primary and Secondary Intention-to-Treat Analysis
eTable 2. Post Hoc Intention-to-Treat Analyses
References
- 1.Farrar D. Hyperglycemia in pregnancy: prevalence, impact, and management challenges. Int J Womens Health. 2016;8:519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Trends in glyburide compared with insulin use for gestational diabetes treatment in the United States, 2000-2011. Obstet Gynecol. 2014;123(6):1177-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women Trial Group . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486. [DOI] [PubMed] [Google Scholar]
- 4.Simmons D, McElduff A, McIntyre HD, Elrishi M. Gestational diabetes mellitus: NICE for the US? a comparison of the American Diabetes Association and the American College of Obstetricians and Gynecologists guidelines with the UK National Institute for Health and Clinical Excellence guidelines. Diabetes Care. 2010;33(1):34-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Committee on Practice Bulletins—Obstetrics ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49-e64. [DOI] [PubMed] [Google Scholar]
- 6.Goetzl L, Wilkins I. Glyburide compared to insulin for the treatment of gestational diabetes mellitus: a cost analysis. J Perinatol. 2002;22(5):403-406. [DOI] [PubMed] [Google Scholar]
- 7.Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138. [DOI] [PubMed] [Google Scholar]
- 8.Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang YF, Chen XY, Ding T, Wang XF, Zhu ZN, Su SW. Comparative efficacy and safety of OADs in management of GDM: network meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2015;100(5):2071-2080. [DOI] [PubMed] [Google Scholar]
- 10.Moretti ME, Rezvani M, Koren G. Safety of glyburide for gestational diabetes: a meta-analysis of pregnancy outcomes. Ann Pharmacother. 2008;42(4):483-490. [DOI] [PubMed] [Google Scholar]
- 11.Poolsup N, Suksomboon N, Amin M. Efficacy and safety of oral antidiabetic drugs in comparison to insulin in treating gestational diabetes mellitus: a meta-analysis. PLoS One. 2014;9(10):e109985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song R, Chen L, Chen Y, et al. Comparison of glyburide and insulin in the management of gestational diabetes: a meta-analysis. PLoS One. 2017;12(8):e0182488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng YC, Li MJ, Chen Y, et al. The use of glyburide in the management of gestational diabetes mellitus: a meta-analysis. Adv Med Sci. 2014;59(1):95-101. [DOI] [PubMed] [Google Scholar]
- 14.Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr. 2015;169(5):452-458. [DOI] [PubMed] [Google Scholar]
- 15.Cheng YW, Chung JH, Block-Kurbisch I, Inturrisi M, Caughey AB. Treatment of gestational diabetes mellitus: glyburide compared to subcutaneous insulin therapy and associated perinatal outcomes. J Matern Fetal Neonatal Med. 2012;25(4):379-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzger BE, Gabbe SG, Persson B, et al. ; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamelle N, Munoz F, Grandjean H. Fetal growth from the AUDIPOG Study, I: establishment of reference curves [in French]. J Gynecol Obstet Biol Reprod (Paris). 1996;25(1):61-70. [PubMed] [Google Scholar]
- 18.Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105(5):1141-1145. [DOI] [PubMed] [Google Scholar]
- 19.Landon MB, Spong CY, Thom E, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network . A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anjalakshi C, Balaji V, Balaji MS, Seshiah V. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Res Clin Pract. 2007;76(3):474-475. [DOI] [PubMed] [Google Scholar]
- 21.Bertini AM, Silva JC, Taborda W, et al. Perinatal outcomes and the use of oral hypoglycemic agents. J Perinat Med. 2005;33(6):519-523. [DOI] [PubMed] [Google Scholar]
- 22.Ogunyemi D, Jesse M, Davidson M. Comparison of glyburide versus insulin in management of gestational diabetes mellitus. Endocr Pract. 2007;13(4):427-428. [DOI] [PubMed] [Google Scholar]
- 23.Silva JC, Bertini AM, Taborda W, et al. Glibenclamide in the treatment for gestational diabetes mellitus in a compared study to insulin [in Portuguese]. Arq Bras Endocrinol Metabol. 2007;51(4):541-546. [DOI] [PubMed] [Google Scholar]
- 24.Lesaffre E. Superiority, equivalence, and non-inferiority trials. Bull NYU Hosp Jt Dis. 2008;66(2):150-154. [PubMed] [Google Scholar]
- 25.Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials. 2011;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buschur E, Brown F, Wyckoff J. Using oral agents to manage gestational diabetes: what have we learned? Curr Diab Rep. 2015;15(2):570. [DOI] [PubMed] [Google Scholar]
- 27.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594-2604. [DOI] [PubMed] [Google Scholar]
- 28.Stata Corp Stata Statistical Software Release 14 College Station, TX: Stata Corp; 2015. [Google Scholar]
- 29.McKinlay CJ, Alsweiler JM, Ansell JM, et al. ; CHYLD Study Group . Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med. 2015;373(16):1507-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaji AH, Lewis RJ. Noninferiority trials: is a new treatment almost as effective as another? JAMA. 2015;313(23):2371-2372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eFigure. Confidence Interval of the Difference of the Composite Criterion Rates and Noninferiority Margin
eTable 1. Primary and Secondary Intention-to-Treat Analysis
eTable 2. Post Hoc Intention-to-Treat Analyses