Key Points
Question
What is the benefit of the addition of hyperbaric oxygen therapy (HBOT) vs medical therapy alone for sudden sensorineural hearing loss (SSNHL)?
Findings
Results of this meta-analysis including 2401 patients with SSNHL significantly favored HBOT plus standard medical therapy (MT) over MT alone for complete hearing recovery and any hearing recovery, as well as for absolute hearing gain. The benefit of HBOT was greater in groups with severe hearing loss at baseline, HBOT as a salvage treatment, and a total HBOT duration of at least 1200 minutes.
Meaning
The benefit of HBOT for SSNHL may be greater for those who had severe hearing loss at baseline or who failed to recover after MT; optimal criteria for patient selection and a standardized regimen for HBOT should be established in future trials.
This systematic review and meta-analysis of 19 studies evaluates the benefit of the addition of hyperbaric oxygen therapy for patients with sudden sensorineural hearing loss.
Abstract
Importance
Sudden sensorineural hearing loss (SSNHL) causes substantial disease burden for both individuals and socioeconomic aspects. The benefit of hyperbaric oxygen therapy (HBOT) in addition to standard medical therapy (MT) for idiopathic SSNHL has been unclear.
Objective
To perform a systematic review and meta-analysis to compare HBOT + MT with MT alone as a treatment for patients with SSNHL.
Data Sources
PubMed, Embase, and the Cochrane Database of Systematic Reviews were systematically searched up to February 2018.
Study Selection
Randomized clinical trials and nonrandomized studies comparing HBOT + MT with MT alone for SSNHL treatment.
Data Extraction and Synthesis
Two investigators independently screened the eligible studies, established data, and assessed quality and risk of bias. A systematic review and meta-analysis using random-effects models was conducted.
Main Outcomes and Measures
The primary outcome was complete hearing recovery, and secondary outcomes were any hearing recovery and absolute hearing gain.
Results
Three randomized clinical trials and 16 nonrandomized studies comparing outcomes after HBOT + MT vs MT alone in 2401 patients with SSNHL (mean age, 45.4 years; 55.3% female) were included. Pooled odds ratios (ORs) for complete hearing recovery and any hearing recovery were significantly higher in the HBOT + MT group than in the MT alone group (complete hearing recovery OR, 1.61; 95% CI, 1.05-2.44 and any hearing recovery OR, 1.43; 95% CI, 1.20-1.67). Absolute hearing gain was also significantly greater in the HBOT + MT group than in the MT alone group. The benefit of HBOT was greater in groups with severe to profound hearing loss at baseline, HBOT as a salvage treatment, and a total HBOT duration of at least 1200 minutes.
Conclusions and Relevance
The addition of HBOT to standard MT is a reasonable treatment option for SSNHL, particularly for those patients with severe to profound hearing loss at baseline and those who undergo HBOT as a salvage treatment with a prolonged duration. Optimal criteria for patient selection and a standardized regimen for HBOT should be applied in routine practice, with future trials to investigate maximal treatment benefit.
Introduction
Sudden sensorineural hearing loss (SSNHL) is a subset of sudden hearing loss that occurs within 72 hours and is defined as a hearing loss of at least 30 dB identified at 3 or more consecutive frequencies.1 Such hearing loss is not uncommon, with an incidence of 5 to 20 cases per 100 000 in the general population.1 It causes severe discomfort because of sudden deafness on 1 or both sides and increases the risk of accidents caused by decreased spatial perception.2 This risk further results in substantial disease burden not only among individuals but also socioeconomically.3 However, SSNHL is a disease entity from which recovery by prompt and appropriate treatment could dramatically improve patient quality of life and reduce the need for hearing aids.3
Corticosteroids, antiviral agents, vasodilators, and hyperbaric oxygen therapy (HBOT) are the currently available treatment options for SSNHL, but their comparative efficacy is unclear.1 To date, the most widely used treatment for SSNHL is systemic and/or intratympanic corticosteroids.1 As another option, HBOT is a treatment that may relieve edema and ischemia by administering high-pressure oxygen into the inner ear to restore hearing.4 Since the first case report5 of HBOT for SSNHL treatment in the 1960s, a number of randomized clinical trials (RCTs)6,7,8 and nonrandomized studies9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24 investigating the benefit of HBOT have been reported. As an adjunctive treatment to standard medical therapy (MT) that includes systemic corticosteroids, HBOT has been found to promote hearing gain.25,26 However, there is limited evidence that HBOT definitively improves the outcome of SSNHL.25 Therefore, we performed a systematic review and meta-analysis of 19 studies to compare HBOT + MT with MT alone as a treatment for patients with SSNHL.
Methods
Detailed study methods are available in the eMethods in the Supplement; the search strategy in PubMed, Embase, and Cochrane Database of Systematic Reviews is described, as well as the characteristics of the excluded studies.
Data Sources and Searches
PubMed, Embase, and the Cochrane Database of Systematic Reviews were systematically searched up to February 2018 for published or unpublished studies. This electronic search strategy was augmented by a manual examination of references cited in articles, recent reviews, editorials, and meta-analyses. No restrictions were imposed on the language, study period, or sample size. The search strategy is described in detail in the eMethods in the Supplement.
Study Selection and Outcome Definition
Studies meeting each of the following criteria were eligible for the meta-analysis: (1) performed before February 2018; (2) definitively reported outcomes, including complete hearing recovery and any hearing recovery or absolute hearing gain; (3) compared outcomes after HBOT + MT vs MT alone; (4) included systemic and/or intratympanic corticosteroids in the MT protocol; and (5) used a clear definition of SSNHL. Studies reporting SSNHL treatment outcomes without comparator or control groups were excluded. Two investigators (T.-M.R. and D.H.) independently screened titles and abstracts, identified duplicates, reviewed full articles, and determined their eligibility. Disagreements were resolved by discussion. The last search was performed in February 2018. The primary outcome was complete hearing recovery, and secondary outcomes were any hearing recovery and absolute hearing gain.
Data Extraction and Quality Assessment
Data were compiled for the meta-analysis using a standardized form to extract the following characteristics of studies: study design, number of patients, treatment protocol (HBOT + MT or MT alone), outcome definitions, treatment protocols, and patient demographics. The quality of the eligible studies was assessed using the Cochrane Collaboration’s tool for assessing risk of bias for RCTs. The Newcastle-Ottawa Scale (NOS) and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist were used to assess the quality of nonrandomized prospective studies. However, the thresholds of the NOS or STROBE checklist scores were not grounds for individual study exclusion.
Data Synthesis and Statistical Analysis
Random-effects models were applied for primary and secondary outcome analyses, and odds ratios (ORs) are presented with 95% CIs in the statistical summaries. Because the selected studies were heterogeneous in terms of study population and protocol, fixed-effects models were used for sensitivity analyses, and the results yielded were checked for similarity. The pooled ORs and 95% CIs of the random-effects and fixed-effects models were calculated using the restricted maximum likelihood and Mantel-Haenszel methods, respectively.27 The pooled results for the continuous variables are presented as the weighted mean difference with a 95% CI between the HBOT + MT group and the MT alone group.
Statistical heterogeneity was quantified using I2 statistics. Publication bias, a known threat to meta-analysis validity created by the preferential publishing of studies with statistically significant or clinically favorable results,28 was assessed through funnel plot asymmetry using Egger test and Begg test. If visible asymmetry of funnel plots was observed, the trim-and-fill method was used to estimate the number of missing studies and calculate the corrected risk ratio as if such studies were present. Exploratory meta-regressions were performed to assess the association between effect size (log ORs) and the mean age, proportion of men, and initial hearing level.
Hearing recovery rates were separately analyzed according to the severity of the initial hearing loss. Subgroup analyses were used to assess differential associations with the following methods: (1) the statistical model (fixed effects vs random effects), (2) the HBOT strategy (salvage treatment vs adjunctive treatment), (3) the total HBOT duration (≥1200 vs <1200 minutes), (4) the maximal pressure during HBOT (≥2.5 vs <2.5 atmospheric absolute pressure [ATA]), and (5) the response assessment point (≥3 vs <3 months after treatment). Two-sided P < .05 was considered statistically significant. Statistical computations were performed with a standard software program (Stata/SE, version 12.0; StataCorp LP). The present study complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (eTable 1 in the Supplement) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.
Results
Search Findings
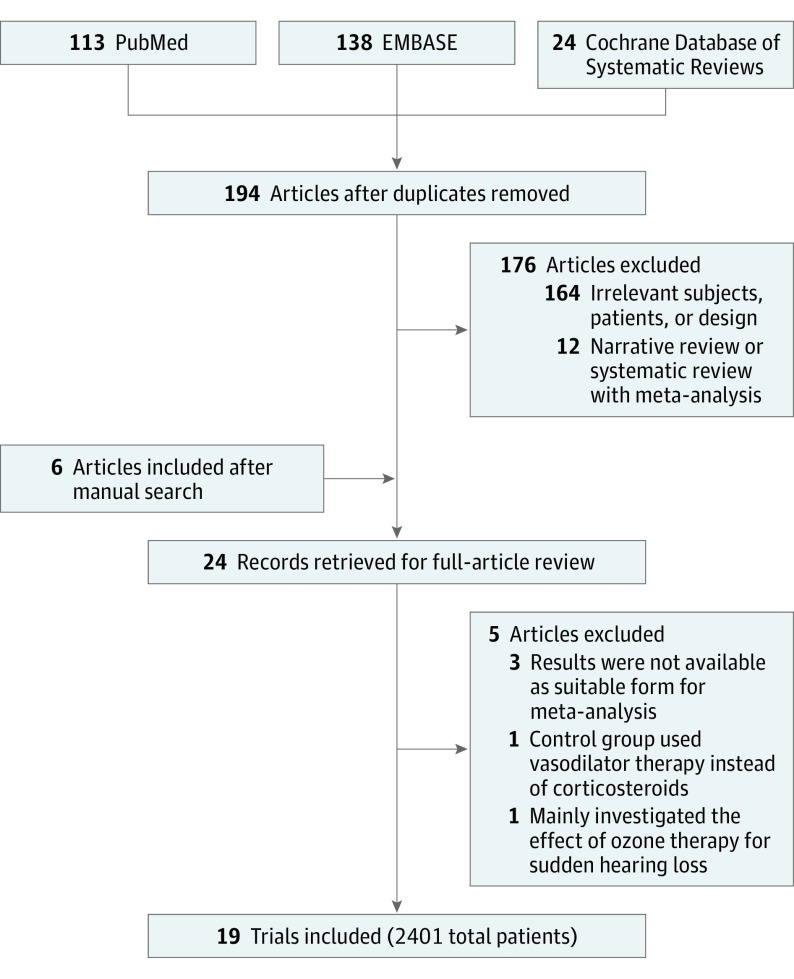
A total of 194 citations were identified. Among these, 24 articles were retrieved for a full review, and 19 met the inclusion criteria (Figure 1). The characteristics of the 5 excluded studies after a full article review are detailed in the eMethods in the Supplement. The final 19 studies included 2401 patients with SSNHL (mean age, 45.4 years; 55.3% female) grouped by treatment protocol as HBOT + MT (1055 of 2401 [43.9%]) or MT alone (1346 of 2401 [56.1%]). Among the 19 target studies, 14 provided the rates of complete hearing recovery and any hearing recovery, and 11 provided absolute hearing gain.
Figure 1. Flow Diagram of Study Selection.
The flow diagram is shown according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Study Characteristics and Risk of Bias Within Studies
The main characteristics of the individual studies are summarized in the Table. Most studies were retrospective observational investigations, while 2 were prospective studies and 3 were RCTs. All studies exclusively enrolled patients with SSNHL who were undergoing treatment. Follow-up duration varied from weeks to months, with 8 studies yielding outcomes immediately after treatment. The mean onset to HBOT time ranged from 3 to 48.2 days, and HBOT was used as a salvage treatment in 7 studies. Demographic features, including age and sex, were evenly distributed in all studies. The initial hearing level ranged from 43.6 to 86.8 dB.
Table. Characteristics of Studies Selected for Meta-analysisa.
| Source | Study Period | Study Design | No. of Patients | Complete Hearing Recovery | Any Hearing Recovery | Protocol | Follow-up Duration | Onset to HBOT Time | HBOT as a Salvage Treatment | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HBOT + MT | MT Alone | MT | HBOT | ||||||||
| Cavallazzi et al,22 1996 | NR | Retrospective | 34 | 30 | Recovery >50% | Recovery >25% | Betamethasone, dose NR | 2.5 ATA for 60 min, 15 sessions | After treatment | NR | No |
| Aslan et al,23 2002 | 1995-1999 | Retrospective | 25 | 25 | NA | NA | Prednisone at an initial dose of 1 mg/kg/d for 2 wk | 2.4 ATA for 100 min, 20 sessions | 8 wk | 5.8 d | No |
| Narozny et al,9 2006 | 1980-2000 | Retrospective | 52 | 81 | NA | NA | Prednisone 30 mg by mouth per day in decreasing dose for up to 14 d | 2.5 ATA for 60 min, 16 ± 6 sessions | After treatment | NR | No |
| Topuz et al,6 2004 | 1998-2002 | RCT | 34 | 21 | NA | NA | Prednisone at an initial dose of 1 mg/kg per day for 2 wk | 2.5 ATA for 90 min, 25 sessions | 4 wk | <2.0 wk | No |
| Desloovere et al,10 2006 | NR | Retrospective | 56 | 160 | AHG>20 dB | AHG>10 dB | Tapered dose of hydrocortisone starting at 250 mg IV | 1.5 ATA for 60 min, 14 sessions | 13.4 mo | 48.2 d | Yes |
| Satar et al,11 2006 | 1996-2003 | Retrospective | 37 | 17 | MHL<25 dB | AHG>10 dB | Dexamethasone 4 mg IV twice a day for 7 d | 2.5 ATA for 90 min, 18 sessions | After treatment | 5.0 d | No |
| Dundar et al,12 2007 | 2002-2004 | Prospective | 55 | 25 | MHL<25 dB | AHG>15 dB | Dexamethasone 4 mg IV twice a day for 7 d | 2.8 ATA for 90 min, 10-28 sessions | After treatment | <1.0 wk | No |
| Fujimura et al,13 2007 | 1979-2005 | Retrospective | 67 | 63 | MHL<20 dB | AHG>10 dB | Dexamethasone 8 mg IV, followed by tapered doses for 12 d | 2.5 ATA for 60 min, 10 sessions | 1 mo | 6.4 d | No |
| Cekin et al,7 2009 | 1994-2006 | RCT | 36 | 21 | AHG>50 dB | AHG>10 dB | Prednisolone 1 mg/kg starting dose, tapering in 3 wk | 2.5 ATA for 90 min, 10 sessions | After treatment | 3.0 d | No |
| Ohno et al,14 2010 | 2001-2008 | Retrospective | 48 | 44 | AHG>30 dB | AHG>10 dB | Betamethasone 10 mg/d tapered for 10 d or prednisolone 30 mg/d tapered for 12 d | 2.0 ATA for 60 min, 10 sessions | 23 wk | 7.4 wk | Yes |
| Alimoglu et al,15 2011 | 2004-2010 | Retrospective | 61 | 58 | MHL<25 dB | AHG>15 dB | Prednisolone 1 mg/kg or equivalent, 10-mg taper every 3 d | 2.5 ATA for 120 min, 20 sessions | After treatment | <1.0 mo | No |
| Liu et al,16 2011 | 1999-2009 | Retrospective | 112 | 353 | <15 dB within unaffected ear | AHG>10 dB | Betamethasone 12 mg tapered for 6 d, followed by oral prednisolone 20 mg/d for 8 d | 2.5 ATA for 60 min, 10-20 sessions | 180 d | <2.0 wk | No |
| Cvorovic et al,8 2013 | 2005-2011 | RCT | 25 | 25 | NA | NA | Dexamethasone 40 mg IV for 3 d, followed by 10 mg/d for 3 d | 2.0 ATA for 60 min, 20 sessions | After treatment | <4.0 wk | Yes |
| Yang et al,17 2013 | NR | Retrospective | 22 | 27 | AHG>15 dB | AHG>10 dB | Dexamethasone 20 mg IV for 2 d, tapered for 5 d, followed by oral prednisolone 30 mg/d for 5 d | 2.5 ATA for 120 min, 10 sessions | 2 mo | 4.2 d | Yes |
| Capuano et al,18 2015 | 2010-2013 | Retrospective | 100 | 100 | <15 dB within unaffected ear | AHG>10 dB | Methylprednisolone 40 mg IV for 7 d, 20 mg for another 3 d | 2.5 ATA for 90 min, 16 sessions | 180 d | <90.0 d | No |
| Pezzoli et al,19 2015 | 2011-2013 | Prospective | 23 | 21 | <15 dB within unaffected ear | AHG>10 dB | Betamethasone 4 mg/d for 6 d, salvage with dexamethasone 25 mg by mouth for 7 d | 2.5 ATA for 30 min, 15 sessions | 3 mo | 9.9 d | Yes |
| Psillas et al,20 2015 | 2013-2015 | Retrospective | 15 | 30 | <15 dB within unaffected ear | AHG>10 dB | Dexamethasone 24 mg/d IV, tapered for 1 wk | 2.2 ATA for 90 min, 15 sessions | 3 mo | 24.0 d | Yes |
| Hosokawa et al,21 2017 | 2011-2015 | Retrospective | 167 | 160 | MHL<20 dB | AHG>10 dB | Prednisolone 80 mg or hydrocortisone 400 mg tapered over 10 d | 1.5 ATA for 60 min, 10 sessions | 3 mo | <1.0 mo | Yes |
| Ricciardiello et al,24 2017 | 2009-2016 | Retrospective | 86 | 85 | MHL<25 dB | AHG>15 dB | Dexamethasone 8 mg/d or methylprednisolone 1 mg/kg tapered for 15 d | 2.5 ATA for 90 min, 15-21 sessions | After treatment | 7.0 d | No |
Abbreviations: AHG, absolute hearing gain; ATA, atmospheric absolute pressure; HBOT, hyperbaric oxygen therapy; IV, intravenously; MHL, mean hearing level; MT, medical therapy; NA, not applicable; RCT, randomized clinical trial.
Demographics of the overall population are available in eTable 4 in the Supplement.
eTable 2 and eTable 3 in the Supplement summarize the assessed risk of bias by study design. Two RCTs did not report any random sequence generation method. Although no masking attempt was observed, all studies objectively defined clinical end points, with a complete follow-up. Therefore, the lack of masking had a low probability of influencing outcomes. All nonrandomized studies met at least 17 variables of the STROBE checklist and fulfilled the adequacy criteria of the NOS for nonrandomized studies.
Addition of HBOT and Hearing Recovery and Absolute Hearing Gain
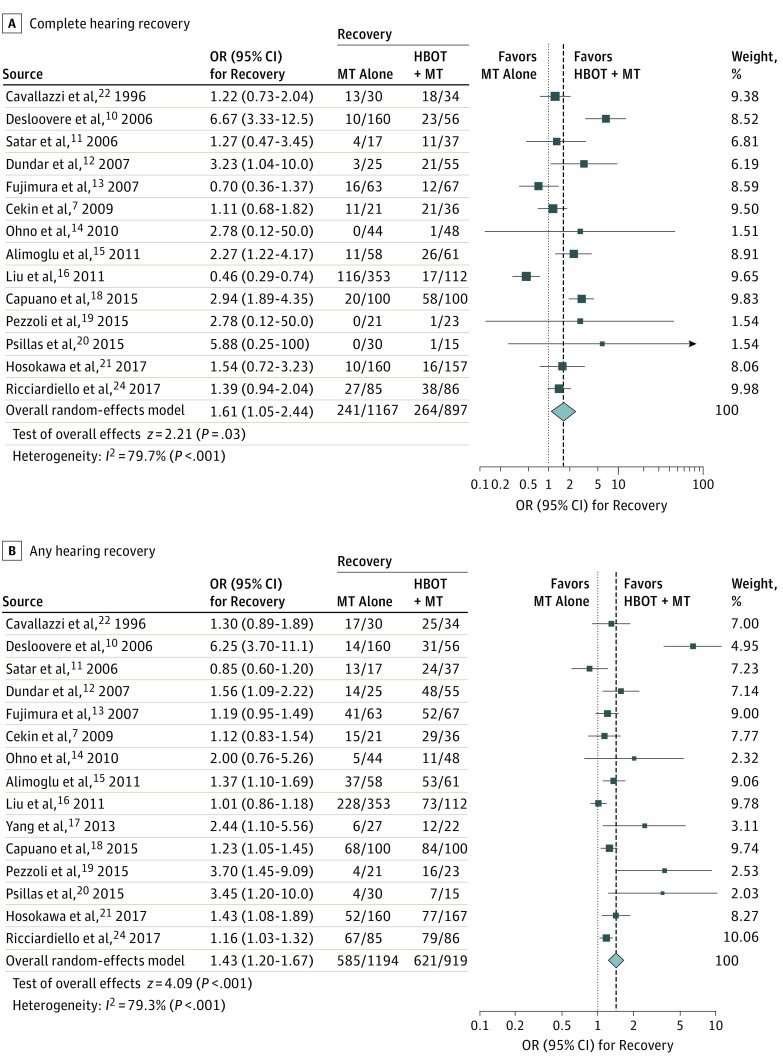
The rates of complete hearing recovery in the HBOT + MT and MT alone groups were 264 of 897 (29.4%) and 241 of 1167 (20.7%), respectively, and the rates of any hearing recovery were 621 of 919 (67.6%) and 585 of 1194 (49.0%), respectively. The pooled results from random-effects models significantly favored the HBOT + MT group over the MT alone group for complete hearing recovery (pooled OR, 1.61; 95% CI, 1.05-2.44) and for any hearing recovery (pooled OR, 1.43; 95% CI, 1.20-1.67) (Figure 2). Significant heterogeneity was observed for both outcomes. Funnel plots, along with Egger test and Begg test results, demonstrated no significant publication bias for complete hearing recovery and any hearing recovery, and there was no need for trim-and-fill adjustment because of the absence of asymmetry (eFigure 1 in the Supplement).
Figure 2. Benefit of the Addition of Hyperbaric Oxygen Therapy (HBOT) on Hearing Recovery.
Odds ratios (OR) with 95% CIs are shown by individual studies describing pooled overall benefits for complete hearing recovery7,10,11,12,13,14,15,16,18,19,20,21,22,24 (A) and for any hearing recovery7,10,11,12,13,14,15,16,17,18,19,20,21,22,24 (B). MT indicates medical therapy.
The weighted mean differences of absolute hearing gain are presented as the mean value for the overall frequencies, as well as for each frequency level (eFigure 2 in the Supplement). Absolute hearing gain was significantly greater in the HBOT + MT group than in the MT alone group for the overall frequencies (weighted mean difference, 8.74; 95% CI, 5.05-12.43 dB). Similar trends were observed for each frequency level, although none were statistically significant except at 500 Hz.
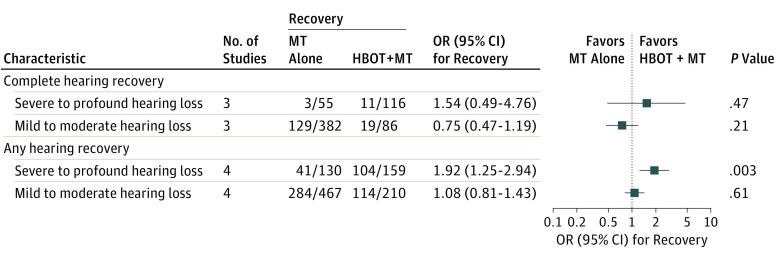
Severity of the Initial Hearing Loss and Hearing Recovery
To assess the differential association of HBOT according to the severity of the initial hearing loss, the outcomes were compared separately by groups with severe to profound hearing loss (≥70 dB) or mild to moderate hearing loss (<70 dB) (Figure 3). For complete hearing recovery and any hearing recovery, HBOT + MT was more beneficial than MT alone in the group with severe to profound hearing loss than in the group with mild to moderate hearing loss.
Figure 3. Hearing Recovery Rate According to the Severity of the Initial Hearing Loss.
Benefit of hyperbaric oxygen therapy (HBOT) on complete hearing recovery and on any hearing recovery according to the severity of the initial hearing loss is shown. MT indicates medical therapy; OR, odds ratio.
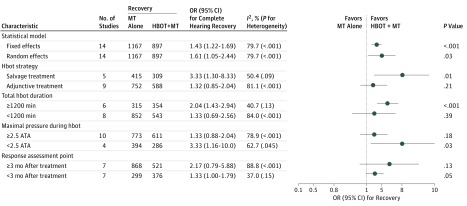
Subgroup Analyses
In a subgroup analysis for complete hearing recovery, the benefit of the strategy and protocols of HBOT on the outcome was apparent (Figure 4). The OR for complete hearing recovery significantly favored HBOT + MT over MT alone, especially for those who underwent HBOT as a salvage treatment and had a total HBOT duration of at least 1200 minutes. The higher maximal pressure during HBOT of at least 2.5 ATA was not beneficial for complete hearing recovery. Among other subgroups of the statistical model and the time point of response assessment, the benefit of HBOT on complete hearing recovery was similar. For any hearing recovery, the favorable results of the HBOT + MT group compared with the MT alone group were consistent across all subgroups (eFigure 3 in the Supplement).
Figure 4. Subgroup Analyses for Complete Hearing Recovery.
Benefit of hyperbaric oxygen therapy (HBOT) on complete hearing recovery according to the various subgroups is shown. ATA indicates atmospheric absolute pressure; MT, medical therapy; OR, odds ratio.
Demographic Characteristics and Baseline Hearing Level and Hearing Recovery
To evaluate the association of age and sex with hearing recovery, exploratory meta-regression analyses were conducted. The mean patient age in each study was not significantly associated with a benefit of HBOT on hearing recovery (eFigure 4 in the Supplement). A significant association between sex and the OR for any hearing recovery was observed, demonstrating that a lower proportion of men was associated with a greater benefit of HBOT (eFigure 5 in the Supplement).
Discussion
We performed a meta-analysis comprising 2401 patients with SSNHL in 19 studies that compared the hearing recovery rate and absolute hearing gain between HBOT + MT and MT alone groups. The principal findings were as follows: (1) The pooled ORs for complete hearing recovery and any hearing recovery were significantly higher in the HBOT + MT group. (2) Absolute hearing gain was also significantly greater in the HBOT + MT group than in the MT alone group, showing similar trends at all individual frequencies. (3) The HBOT appeared to be more beneficial in patients with severe to profound hearing loss at baseline. (4) The results were consistent across various subgroups, and the benefit of HBOT was prominent in groups with HBOT as a salvage treatment and a total HBOT duration of at least 1200 minutes.
Prior Evidence on HBOT for SSNHL
The treatment of SSNHL to date has focused mainly on the improvement of blood flow and increased oxygen supply in the inner ear. Although there are several treatment options, including systemic and/or intratympanic corticosteroids, antiviral agents, vasodilators, anticoagulants, and plasma expanders, no definitive treatment for SSNHL has been proved through qualified trials or meta-analysis.25 Because corticosteroids are thought to reduce the inflammation and edema associated with SSNHL,29 experts recommend systemic and/or intratympanic corticosteroids first unless specific contraindications are present.3
The efficacy of HBOT was updated in the Cochrane Database of Systematic Reviews in 2012 by Bennett et al.25 In their review, a 25% chance of hearing recovery was significantly higher in the HBOT group (risk ratio, 1.39; 95% CI, 1.05-1.84), while there was no difference in a 50% chance of hearing recovery (risk ratio, 1.53; 95% CI, 0.85-2.78). A greater improvement of the mean threshold in the HBOT group than in the control group was also observed (mean difference, 15.6; 95% CI, 1.5-29.8 dB). The use of systemic and/or intratympanic corticosteroids plus HBOT has been considered the most effective SSNHL treatment for several reasons.3 In the study by Bennett et al,25 only 392 patients in 7 studies were analyzed; because the outcome was divided into absolute gains and hearing recovery rates, the statistical power in their review was insufficient, and the results are likely unreliable. In addition, the included studies provided no detailed treatment strategy or protocol information, making it impossible to perform subgroup analyses according to the severity of the initial hearing loss or HBOT protocols.
Since that review, several studies have been published presenting controversial results regarding the benefit of HBOT. Saesen et al26 reported in a recent narrative review that HBOT has a positive benefit on hearing gain as an adjunctive therapy to standard MT. Their study also indicated that HBOT could have a positive role as a salvage treatment after the failure of initial corticosteroid treatment. Therefore, a comprehensive meta-analysis covering recent studies was necessary.
HBOT in Patients With SSNHL
In our meta-analysis, HBOT was found to provide a significant benefit as an additional treatment option, along with systemic and/or intratympanic corticosteroids. In particular, this study demonstrates for the first time to date that HBOT + MT is associated with a significant improvement in complete hearing recovery and in any hearing recovery compared with MT alone. This finding is important evidence for the clinical implications of HBOT in SSNHL treatment.
To date, hypotheses about the benefit of HBOT have been suggested based on various theoretical backgrounds. The structures in the cochlea are vulnerable to a decrease in tissue oxygen supply.3 In addition, the supply to the cochlea depends on oxygen diffusion through the capillaries rather than direct vascular oxygenation.30 Therefore, it is thought that the reduction of blood flow to the inner ear and resultant ischemia are the most important mechanisms for the occurrence of idiopathic SSNHL.31 Using HBOT, it is possible to maximize the oxygen partial pressure supplied to the inner ear.25 This process can minimize ischemic damage after SSNHL and aid vascular recovery.32 Furthermore, it can provide antibacterial effects through oxygen radicals and promote angiogenesis with tissue regeneration.4 The present study supports these theories by incorporating updated clinical data, providing valuable evidence that HBOT + MT is the most beneficial treatment option for SSNHL.
In terms of safety, serious complications from HBOT are uncommon.25 Although middle ear, sinus, and pulmonary barotrauma can occur, their incidence is low. Only ear fullness, which is completely recoverable, is known to be a common adverse effect.33 The use of HBOT is limited in that it is only available in special facilities, and patients with claustrophobia cannot undergo HBOT.32 However, patients with a history of pneumothorax or those with claustrophobia can be excluded from HBOT treatment at the initial patient interview. Therefore, the benefit of hearing gain through HBOT may exceed any possible harm, particularly if there has been no response to standard treatment with corticosteroids or if there is severe initial hearing loss.
In South Korea, HBOT treatment costs US $100 per session, but the actual patient contribution is US $40 based on nationwide medical insurance coverage. In the United States, it costs approximately US $300 per session. However, considering the adverse effects of idiopathic SSNHL on patient quality of life and the risk of accidents caused by decreased spatial perception, HBOT as a salvage treatment may be considered at least for refractory cases after medical treatment with corticosteroids. To achieve better cost-effectiveness, well-defined indications for HBOT and standardized HBOT regimens should be established and applied.
Clinical Implications From Subgroup Analyses
The results of earlier studies6,16 have indicated that the benefit of HBOT may be greater in more severely affected patients. Experts have also suggested that HBOT may be more effective at ages younger than 60 years, with early HBOT application within 3 months, and in a group with greater initial hearing loss of more than 60 dB, although the level of evidence is weak.1 However, the Cochrane Database of Systematic Reviews in 2012 concluded that the influence of the severity of the initial hearing loss could not be confirmed.25 In the present meta-analysis, we found that the benefit of the addition of HBOT was greater in the group with severe to profound hearing loss of at least 70 dB. However, because there were limited studies that provided separate outcomes based on initial severity, the statistical power was low, and follow-up studies are required.
Furthermore, the group with HBOT as a salvage treatment received greater benefit than the group with HBOT as an adjunctive treatment, which is also controversial and warrants further trials that control for the HBOT strategy. Our meta-regression results did not show any evidence that age influences the benefit of HBOT. Furthermore, because HBOT was performed within 3 months in all enrolled participants, the differential association according to the onset to HBOT time could not be assessed.
It is generally recommended that 100% oxygen at 2.0 to 2.5 ATA should be administered for 10 to 20 days, with a 90-minute session each day,3 but there are no universal HBOT protocols that have been proved to be effective. For the first time to date, we have analyzed the associations of the duration and maximal pressure of HBOT with a treatment benefit. Particularly for complete hearing recovery, HBOT + MT was favored in the group with a total HBOT duration of at least 1200 minutes. However, the maximal pressure during HBOT was not found to have a significant association with any benefit of HBOT. These results are expected to have an important role in the standardization of clinical indications for and protocols of HBOT. Sudden sensorineural hearing loss with severe to profound hearing loss (≥70 dB) at baseline can be considered an appropriate indication for applying HBOT. Given the greater benefit when HBOT is used as a salvage treatment, HBOT may be applied to refractory cases that do not respond to medical treatment 2 to 4 weeks after the onset of hearing loss. In terms of HBOT strategy, a 2-week protocol with a 90-minute session per day may be recommended or alternatively a 20-day protocol with a 60-minute session per day to achieve a total HBOT duration of at least 1200 minutes. However, because increasing maximal pressure during HBOT to 2.5 or higher ATA did not provide any benefit for hearing recovery, the air pressure may be kept at 2.0 to 2.5 ATA as currently recommended.
Future trials should use a standardized HBOT regimen to investigate the efficacy of HBOT as a salvage treatment for patients refractory to MT with corticosteroids. In particular, the appropriate intervals should be clarified between onset, assessment of initial treatment failure, and HBOT as a salvage treatment.
Limitations
This meta-analysis has several limitations. First, the selected studies varied in their clinical and methodological characteristics. Second, although there was no evidence of publication bias, the main results showed considerable heterogeneity. Third, considering that most studies except RCTs did not provide adjusted results, measured or unmeasured confounder effects could exist because of different baseline characteristics between study and control groups. Fourth, because a substantial proportion of patients with SSNHL experience spontaneous recovery, the benefit of HBOT or medical treatment may not have been accurately evaluated. There may be a bias effect caused by spontaneous recovery during HBOT as a salvage treatment or prolonged HBOT. However, in the case of HBOT as a salvage treatment, any benefit would not be significant because of the extended period between onset and therapy. For prolonged HBOT, spontaneous recovery during therapy cannot be distinguished from treatment benefits. Therefore, given a sufficient pooled sample size, it is reasonable to assume that the likelihood of such benefit would be evenly distributed in both groups.
Conclusions
For SSNHL, HBOT + MT was shown to be a more advantageous treatment option than MT alone. A benefit of HBOT was observed in patients with SSNHL who had severe to profound hearing loss at baseline and who underwent HBOT as a salvage treatment with a prolonged total HBOT duration. Further trials using well-defined indications and standardized protocols of HBOT are warranted.
eMethods. Supplementary Methods
eTable 1. Checklist of Items to Include When Reporting a Systematic Review or Meta-analysis (PRISMA Guidelines)
eTable 2. The Cochrane Collaboration’s Tool for Assessing Risk of Bias of 3 Randomized Clinical Trials in Meta-analysis
eTable 3. The Newcastle-Ottawa Scale for Assessing the Quality of 16 Non-randomized Studies in Meta-analysis
eTable 4. Demographics of the Overall Population
eFigure 1. Funnel Plots for Evaluation of Publication Bias
eFigure 2. Effect of Additional Hyperbaric Oxygen Therapy on Absolute Hearing Gain
eFigure 3. Subgroup Analysis for Any Hearing Recovery
eFigure 4. Association Between Age and Hearing Recovery
eFigure 5. Association Between Sex and Hearing Recovery
References
- 1.Stachler RJ, Chandrasekhar SS, Archer SM, et al. ; American Academy of Otolaryngology–Head and Neck Surgery . Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3)(suppl):S1-S35. doi: 10.1177/0194599812436449 [DOI] [PubMed] [Google Scholar]
- 2.Wie OB, Pripp AH, Tvete O. Unilateral deafness in adults: effects on communication and social interaction. Ann Otol Rhinol Laryngol. 2010;119(11):772-781. [PubMed] [Google Scholar]
- 3.Murphy-Lavoie H, Piper S, Moon RE, Legros T. Hyperbaric oxygen therapy for idiopathic sudden sensorineural hearing loss. Undersea Hyperb Med. 2012;39(3):777-792. [PubMed] [Google Scholar]
- 4.Gill AL, Bell CN. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97(7):385-395. doi: 10.1093/qjmed/hch074 [DOI] [PubMed] [Google Scholar]
- 5.Appaix A, Pech A, Demard F. [The use of hyperbaric oxygen in oto-rhino-laryngology] [in French]. Ann Otolaryngol Chir Cervicofac. 1970;87(12):735-750. [PubMed] [Google Scholar]
- 6.Topuz E, Yigit O, Cinar U, Seven H. Should hyperbaric oxygen be added to treatment in idiopathic sudden sensorineural hearing loss? Eur Arch Otorhinolaryngol. 2004;261(7):393-396. doi: 10.1007/s00405-003-0688-6 [DOI] [PubMed] [Google Scholar]
- 7.Cekin E, Cincik H, Ulubil SA, Gungor A. Effectiveness of hyperbaric oxygen therapy in management of sudden hearing loss. J Laryngol Otol. 2009;123(6):609-612. doi: 10.1017/S0022215109004277 [DOI] [PubMed] [Google Scholar]
- 8.Cvorovic L, Jovanovic MB, Milutinovic Z, Arsovic N, Djeric D. Randomized prospective trial of hyperbaric oxygen therapy and intratympanic steroid injection as salvage treatment of sudden sensorineural hearing loss. Otol Neurotol. 2013;34(6):1021-1026. doi: 10.1097/MAO.0b013e318297638a [DOI] [PubMed] [Google Scholar]
- 9.Narozny W, Kuczkowski J, Kot J, Stankiewicz C, Sicko Z, Mikaszewski B. Prognostic factors in sudden sensorineural hearing loss: our experience and a review of the literature. Ann Otol Rhinol Laryngol. 2006;115(7):553-558. doi: 10.1177/000348940611500710 [DOI] [PubMed] [Google Scholar]
- 10.Desloovere C, Knecht R, Germonpré P. Hyperbaric oxygen therapy after failure of conventional therapy for sudden deafness. B-ENT. 2006;2(2):69-73. [PubMed] [Google Scholar]
- 11.Satar B, Hidir Y, Yetiser S. Effectiveness of hyperbaric oxygen therapy in idiopathic sudden hearing loss. J Laryngol Otol. 2006;120(8):665-669. doi: 10.1017/S0022215106001769 [DOI] [PubMed] [Google Scholar]
- 12.Dundar K, Gumus T, Ay H, Yetiser S, Ertugrul E. Effectiveness of hyperbaric oxygen on sudden sensorineural hearing loss: prospective clinical research. J Otolaryngol. 2007;36(1):32-37. doi: 10.2310/7070.2006.0061 [DOI] [PubMed] [Google Scholar]
- 13.Fujimura T, Suzuki H, Shiomori T, Udaka T, Mori T. Hyperbaric oxygen and steroid therapy for idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2007;264(8):861-866. doi: 10.1007/s00405-007-0272-6 [DOI] [PubMed] [Google Scholar]
- 14.Ohno K, Noguchi Y, Kawashima Y, Yagishita K, Kitamura K. Secondary hyperbaric oxygen therapy for idiopathic sudden sensorineural hearing loss in the subacute and chronic phases. J Med Dent Sci. 2010;57(2):127-132. [PubMed] [Google Scholar]
- 15.Alimoglu Y, Inci E, Edizer DT, Ozdilek A, Aslan M. Efficacy comparison of oral steroid, intratympanic steroid, hyperbaric oxygen and oral steroid + hyperbaric oxygen treatments in idiopathic sudden sensorineural hearing loss cases. Eur Arch Otorhinolaryngol. 2011;268(12):1735-1741. doi: 10.1007/s00405-011-1563-5 [DOI] [PubMed] [Google Scholar]
- 16.Liu SC, Kang BH, Lee JC, et al. Comparison of therapeutic results in sudden sensorineural hearing loss with/without additional hyperbaric oxygen therapy: a retrospective review of 465 audiologically controlled cases. Clin Otolaryngol. 2011;36(2):121-128. doi: 10.1111/j.1749-4486.2011.02303.x [DOI] [PubMed] [Google Scholar]
- 17.Yang CH, Wu RW, Hwang CF. Comparison of intratympanic steroid injection, hyperbaric oxygen and combination therapy in refractory sudden sensorineural hearing loss. Otol Neurotol. 2013;34(8):1411-1416. doi: 10.1097/MAO.0b013e3182a1eb83 [DOI] [PubMed] [Google Scholar]
- 18.Capuano L, Cavaliere M, Parente G, et al. Hyperbaric oxygen for idiopathic sudden hearing loss: is the routine application helpful? Acta Otolaryngol. 2015;135(7):692-697. doi: 10.3109/00016489.2015.1023355 [DOI] [PubMed] [Google Scholar]
- 19.Pezzoli M, Magnano M, Maffi L, et al. Hyperbaric oxygen therapy as salvage treatment for sudden sensorineural hearing loss: a prospective controlled study. Eur Arch Otorhinolaryngol. 2015;272(7):1659-1666. doi: 10.1007/s00405-014-2948-z [DOI] [PubMed] [Google Scholar]
- 20.Psillas G, Ouzounidou S, Stefanidou S, et al. Hyperbaric oxygen as salvage treatment for idiopathic sudden sensorineural hearing loss. B-ENT. 2015;11(1):39-44. [PubMed] [Google Scholar]
- 21.Hosokawa S, Sugiyama KI, Takahashi G, et al. Hyperbaric oxygen therapy as adjuvant treatment for idiopathic sudden sensorineural hearing loss after failure of systemic steroids. Audiol Neurootol. 2017;22(1):9-14. doi: 10.1159/000464096 [DOI] [PubMed] [Google Scholar]
- 22.Cavallazzi G, Pignataro L, Capaccio P Italian experience in hyperbaric oxygen therapy for idiopathic sudden sensorineural hearing loss. In: Proceedings of the International Joint Meeting on Hyperbaric and Underwater Medicine. Bologna, Italy: Grafica Victoria; 1996:647-649. [Google Scholar]
- 23.Aslan I, Oysu C, Veyseller B, Baserer N. Does the addition of hyperbaric oxygen therapy to the conventional treatment modalities influence the outcome of sudden deafness? Otolaryngol Head Neck Surg. 2002;126(2):121-126. doi: 10.1067/mhn.2002.121915 [DOI] [PubMed] [Google Scholar]
- 24.Ricciardiello F, Abate T, Pianese A, et al. Sudden sensorineural hearing loss: role of hyperbaric oxygen therapy. Translational Med Rep. 2017;1:6497. [Google Scholar]
- 25.Bennett MH, Kertesz T, Perleth M, Yeung P, Lehm JP. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database Syst Rev. 2012;10:CD004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saesen K, Loos E, Montagna C, Vanbrabant T, Goedhuys R, Lemkens N. Hyperbaric oxygen therapy in idiopathic sudden sensorineural hearing loss. B-ENT. 2017;13(2):105-112. https://www.b-ent.be/articles/hyperbaric-oxygen-therapy-in-idiopathic-sudden-sensorineural-hearing-loss. Accessed August 14, 2018. [Google Scholar]
- 27.Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18(3):321-359. doi: [DOI] [PubMed] [Google Scholar]
- 28.Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ. 2012;344:d7762. doi: 10.1136/bmj.d7762 [DOI] [PubMed] [Google Scholar]
- 29.Wei BP, Stathopoulos D, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2013;(7):CD003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagahara K, Fisch U, Yagi N. Perilymph oxygenation in sudden and progressive sensorineural hearing loss. Acta Otolaryngol. 1983;96(1-2):57-68. doi: 10.3109/00016488309132875 [DOI] [PubMed] [Google Scholar]
- 31.Kim SA, Ahn JH. Clinical application of hyperbaric oxygen in treatment of idiopathic sudden sensorineural hearing loss. Korean J Otorhinolaryngol Head Neck Surg. 2016;59(7):490-494. doi: 10.3342/kjorl-hns.2016.59.7.490 [DOI] [Google Scholar]
- 32.Lawrence R, Thevasagayam R. Controversies in the management of sudden sensorineural hearing loss: an evidence-based review. Clin Otolaryngol. 2015;40(3):176-182. doi: 10.1111/coa.12363 [DOI] [PubMed] [Google Scholar]
- 33.Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med. 2000;71(2):119-124. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary Methods
eTable 1. Checklist of Items to Include When Reporting a Systematic Review or Meta-analysis (PRISMA Guidelines)
eTable 2. The Cochrane Collaboration’s Tool for Assessing Risk of Bias of 3 Randomized Clinical Trials in Meta-analysis
eTable 3. The Newcastle-Ottawa Scale for Assessing the Quality of 16 Non-randomized Studies in Meta-analysis
eTable 4. Demographics of the Overall Population
eFigure 1. Funnel Plots for Evaluation of Publication Bias
eFigure 2. Effect of Additional Hyperbaric Oxygen Therapy on Absolute Hearing Gain
eFigure 3. Subgroup Analysis for Any Hearing Recovery
eFigure 4. Association Between Age and Hearing Recovery
eFigure 5. Association Between Sex and Hearing Recovery