Key Points
Question
What are the health status outcomes after transcatheter edge-to-edge mitral valve repair (TMVR) in routine clinical practice?
Findings
Analysis of a large, registry-based US cohort showed that health status was poor prior to TMVR, improved significantly within 30 days (with a mean Kansas City Cardiomyopathy Questionnaire score increase from 42 to 67 points), and remained stable through 1 year among surviving patients. Arrhythmias, lung disease, and poor baseline health status were independently associated with less health status recovery; high rates of missing health status data are a concern for this registry-based study.
Meaning
While long-term mortality remains high among surviving patients with available data, the health status benefits of edge-to-edge TMVR appear to be robust and consistent across patients.
This cohort study uses data from the Transcatheter Valve Therapy Registry to examine health outcomes among patients undergoing transcatheter mitral valve repair for mitral regurgitation.
Abstract
Importance
Improvements in symptoms, functional capacity, and quality of life are among the key goals of edge-to-edge transcatheter mitral valve repair (TMVR) for mitral regurgitation.
Objective
To examine health status outcomes among patients undergoing TMVR in clinical practice and the factors associated with improvement.
Design, Setting, and Participants
This cohort study used the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, which contains data on patients with severe mitral regurgitation treated with TMVR from 2013 through 2017 in 217 US hospitals.
Main Outcomes and Measures
Change in disease-specific health status (Kansas City Cardiomyopathy Questionnaire–Overall Summary score [KCCQ-OS]; range 0-100 points, with higher scores indicating better health status) at 30 days and 1 year after TMVR. We also examined factors associated with health status at 30 days after TMVR, by means of multivariable linear regression using a generalized estimating equations approach to account for clustering of patients within sites.
Results
The KCCQ data were available in 81.2% at baseline, 69.3% of survivors at 30 days, and 47.4% of survivors at 1 year. Among 4226 patients who underwent TMVR, survived 30 days, and completed the KCCQ at baseline and follow-up, the KCCQ-OS increased from 41.9 before TMVR to 66.7 at 30 days (mean change 24.8 [95% CI, 24.0-25.6] points; P < .001), representing a large clinical improvement. The KCCQ scores remained stable from 30 days to 1 year after TMVR, with no further significant increase or decline. On multivariable analysis, atrial fibrillation (−2.2 [95% CI, −3.7 to −0.6] points; P = .01), permanent pacemaker (−2.1 [95% CI, −3.7 to −0.4] points; P = .01), severe lung disease (−3.9 [95% CI, −6.2 to −1.5] points; P = .001), home oxygen (−2.7 [95% CI, −4.9 to −0.4] points; P = .02), and lower KCCQ scores at baseline (3.9 points for each 10-point increase [95% CI, 3.6-4.2]; P < .001) were independently associated with lower 30-day KCCQ-OS scores. In-hospital renal failure was uncommon but was also associated with significant reductions in 30-day KCCQ-OS scores (−7.3 [95% CI −13.3 to −1.2] points). In estimates calculated with inverse probability weighting, after 1 year after TMVR, 54.2% (95% CI 52.2%-56.1%) of patients were alive and well; 23.0% had died, 21.9% had persistently poor health status (KCCQ-OS <60 points), 5.5% had a health status decline from baseline, and 4.6% had both poor health status and health status decline.
Conclusions and Relevance
In a national cohort of US patients undergoing edge-to-edge TMVR in clinical practice, health status was impaired prior to the procedure, improved within 30 days, and remained stable through 1 year among surviving patients with available data. While long-term mortality remains high, most surviving patients demonstrate improvements in symptoms, functional status, and quality of life, with only modest differences by patient-level factors.
Introduction
Percutaneous edge-to-edge mitral valve repair has been shown to substantially reduce mitral regurgitation with low rates of periprocedural complications in patients with severe mitral regurgitation who are not eligible for surgery.1,2 Most patients who undergo transcatheter edge-to-edge mitral valve repair (TMVR) have clinically significant improvements in functional capacity, which has been most often assessed with the physician-reported New York Heart Association (NYHA) class.1 Patient-reported health status has also been shown to improve after TMVR,3,4,5,6,7 but the sample sizes of these studies have been small, thus precluding more detailed investigation as to the factors that may affect recovery after TMVR.
Improvements in patient health status (ie, heart failure symptoms, functional capacity, and quality of life) are among the key goals of TMVR, and thus an exploration of the health status outcomes of TMVR and the factors driving these outcomes is critical to understand the value of this procedure. Established in 2011, the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry is a US national registry of patients who undergo transcatheter valve procedures that is designed to explore and monitor patient outcomes, including health status, as procedures move outside of clinical trials. After the US Food and Drug Administration approval in 2013, data collection in the TVT Registry was extended to TMVR, and in this analysis, we report for the first time (to our knowledge) the health status outcomes of patients who underwent TMVR in routine clinical practice and the factors associated with the magnitude of benefit achieved.
Methods
Study Sample and Protocol
Details of the design, structure, and data elements for the TVT Registry have been published previously.8,9 Facilities are required to participate in the registry to obtain Medicare reimbursement; as such, the TVT Registry collects data on nearly all edge-to edge TMVR procedures performed outside of clinical trials in the United States. Sites collect data on patient demographics, comorbidities, hemodynamics, functional status, patient-reported health status, and outcomes. The TVT Registry has been linked by the Centers for Medicare and Medicaid Services to Medicare administrative claims data via direct patient identifiers to evaluate long-term clinical outcomes, including hospitalizations and survival.10 Data quality checks are implemented at the National Cardiovascular Data Registry data warehouse and Duke Clinical Research Institute to optimize data completeness and accuracy.
Registry activities have been approved by a central institutional review board. The Duke University School of Medicine institutional review board granted a waiver of informed consent for this study.
Health Status Assessment and Outcome Definition
The principal health status instrument for the TVT Registry is the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12),11 a patient-reported disease-specific health status survey used to describe symptoms, functional status, and quality of life in patients with heart failure.10,12 The KCCQ-12 is collected by sites as part of the TVT Registry at baseline and at 30 days and 1 year after TMVR. The KCCQ-12 assesses 4 domains of health status associated with valvular heart disease (ie, physical limitation, symptom frequency, quality of life, social limitation), which are combined into an overall summary (KCCQ-OS) score. Domain scores and the KCCQ-OS score range from 0 to 100 points, with higher scores indicating less symptom burden, better functional status, and better quality of life. Linguistically and culturally validated translations are available for non-English speakers.
The KCCQ-OS score was categorized as very poor (<25 points), poor (25-49 points), fair (50-74 points), and good health status (≥75 points),13,14 and changes of 5, 10, and 20 points correspond to small, moderate, and large clinical improvements, respectively.15 To integrate health status outcomes with survival, the status of being alive and well at 1 year after TMVR was defined as survival with good health status (defined as a KCCQ-OS score ≥60 points, which is roughly equivalent to New York Heart Association class I and II symptoms15,16) without any meaningful worsening (defined as a decrease of ≥10 points in the KCCQ-OS score from baseline to 1 year).17 We also examined categorical outcomes at 30 days and 1 year according to clinically meaningful changes in KCCQ-OS score. Since survival status is a key part of the definition of these outcomes, only patients who were able to be linked with Medicare administrative claims were included in these categorical analyses.
Statistical Analysis
Baseline characteristics for the analytic cohort are presented as percentages for categorical variables and medians with interquartile ranges (IQR) for continuous variables. Patient mortality risk was estimated using the Society of Thoracic Surgeons Predicted Risk of Operative Mortality score, which has been validated for predicting 30-day mortality after surgical mitral valve replacement.18 Changes in KCCQ scores from baseline were evaluated at 30 days and 1 year using paired t tests. Among patients with KCCQ scores at all 3 points, we also evaluated changes in KCCQ scores from 30 days to 1 year using paired t tests. Mean KCCQ-OS scores at 1 year were compared among key subgroups using analysis of covariance. These comparisons were adjusted for baseline KCCQ-OS scores, except for the comparison across categories of baseline KCCQ-OS scores. Rates of patients who were alive and well at 1 year were estimated for each subgroup and compared using χ2 tests.
To explore factors associated with health status at 30 days after TMVR, we used multivariable linear regression with a generalized estimating equations approach to account for clustering of patients within sites. Nonlinearity (through the use of linear splines) and 2-way interactions were explored and retained in the model at a level of statistical significance of .05 or for clinical importance. After exploring the patient factors associated with 30-day health status, we added to the model the procedural complications of stroke, major or life-threatening bleeding, renal failure (defined by a new serum creatinine level of ≥3 mg/dL or the need for dialysis), and post-TMVR moderate or severe mitral regurgitation (categorized as 0-1+ vs 2+ vs 3+-4+). A similar analysis was not performed using 1-year outcomes owing to higher rates of missing data.
Missing Data
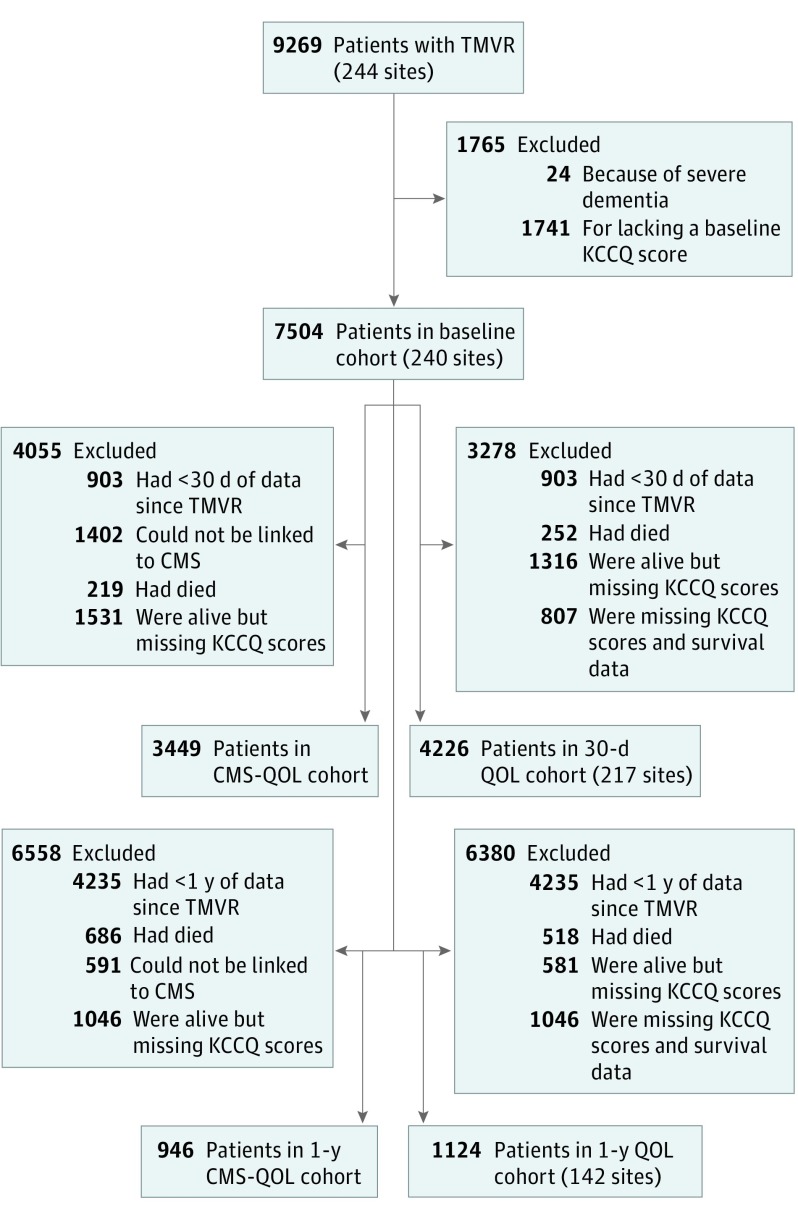
Although efforts to encourage collection of health status data have improved collection rates, missing data are an important consideration in any registry-based health status analysis.9,19 Overall, KCCQ data were available in 7528 of 9269 patients (81.2%) at baseline, 4226 of 6601 survivors at 30 days (69.3% of survivors), and 1124 of 3269 survivors at 1 year (47.4% of survivors) (Figure). To understand factors associated with missingness, we examined differences in baseline characteristics between surviving patients with and without 1-year KCCQ score data. Then, for the analyses examining factors associated with follow-up health status (health status outcomes among subgroups at 1 year and factors associated with 30-day health status), we used the inverse probability weighting approach to increase the weight of patients who were most like those with missing follow-up data, so as to minimize any bias because of missing data.20 This was done by constructing a nonparsimonious multivariable logistic regression model among patients eligible for follow-up to determine the probability of having missing follow-up KCCQ data scores. We then weighted each of the patients in the analytic cohort by the inverse of the likelihood of having follow-up KCCQ data to better reflect the overall population of patients who had had TMVR. The rates of missing data on patient-level factors and in-hospital major complications were all less than 4%, except for mitral valve gradient (1754/4226 [41.5%]) and 6-minute walk distance (1382/4226 [32.7%]), and these 2 variables were not included in the multivariable models. Otherwise, missing data were imputed to the median for continuous variables and to the mode for categorical variables. All analyses were performed with SAS version 9.4 (SAS Institute), and statistical significance was defined as a 2-sided P value <.05.
Figure. Flowchart of Patients.
CMS indicates Centers for Medicare and Medicaid Services; KCCQ, Kansas City Cardiomyopathy Questionnaire; QOL, quality of life; TMVR, transcatheter mitral valve repair.
Results
Study Sample
Between November 2013 and March 2017, 7504 patients at 240 sites underwent edge-to-edge TMVR and provided baseline health status data as part of the TVT Registry. The 30-day cohort included 4226 patients from 217 sites who survived 30 days and completed the KCCQ at baseline and follow-up (Figure). The 1-year cohort included 1124 patients from 142 sites who survived 1 year and completed the KCCQ instrument at both baseline and follow-up. Among 2583 patients who were eligible for 1-year follow-up and were linked to Medicare administrative claims (thereby allowing for evaluation of survival status), 595 patients (23.0%) died, and 1046 patients of the 1988 surviving patients (52.6%) were missing 1-year KCCQ data. There were few differences in baseline characteristics between surviving patients with and without 1-year KCCQ data, although patients with missing data were more likely to have higher Society of Thoracic Surgeons mortality risk scores (those with data, 5.7% [3.8%-8.8%] vs those missing data, 6.2% [3.9%-9.5%]; P = .03) and shorter 6-minute walk distances (those with data, 213.4 [122.8-300.5] m vs those missing data: 198.1 [109.7-280.1] m; P = .02), and were less likely to have TMVR performed electively (those with data, 873 [92.8%] vs those missing data, 932 [89.3%]; P = .01; eTable in the Supplement).
The median age for the 30-day analytic cohort (n = 4226; the primary analysis) was 81 (IQR 74-86) years, and 1979 patients (46.8%) were female, 1196 patients (28.4%) had undergone prior bypass graft surgery, 1104 patients (26.2%) had diabetes, and 596 patients (14.1%) were on home oxygen (Table 1). The median (IQR) Society of Thoracic Surgeons mortality risk score was 5.7% (3.6%-9.1%), the median (IQR) ejection fraction was 55% (40%-60%), 3761 patients (89.0%) had degenerative mitral regurgitation, and 3826 TMVR procedures (90.6%) were done on an elective basis.
Table 1. Baseline Characteristics of the Analytic Cohorts.
| Characteristic | Cohort, No. (%) | |
|---|---|---|
| 30 d (n = 4226) | 1 y (n = 1124) | |
| Age, median (IQR), y | 81 (74-86) | 82 (75-86) |
| Female | 1979 (46.8) | 526 (46.8) |
| White | 3832 (90.7) | 1030 (91.6) |
| Body surface area, median (IQR), m2 | 1.8 (1.6-2.0) | 1.8 (1.6-2.0) |
| Prior diagnoses | ||
| Myocardial infarction | 1094 (26.0) | 284 (25.4) |
| Percutaneous coronary intervention | 1286 (30.5) | 343 (30.6) |
| Coronary artery bypass surgery | 1196 (28.4) | 329 (29.4) |
| Stroke | 470 (11.1) | 119 (10.6) |
| Comorbidities | ||
| Peripheral artery disease | 754 (17.9) | 183 (16.3) |
| Atrial fibrillation/flutter | 2627 (62.3) | 696 (61.9) |
| Permanent pacemaker | 840 (19.9) | 215 (19.1) |
| Diabetes mellitus | 1104 (26.2) | 268 (23.8) |
| Treated with insulin | 368 (33.3) | 70 (26.1) |
| Severe lung disease | 506 (12.1) | 105 (9.4) |
| Home oxygen | 596 (14.1) | 157 (14.0) |
| Current smoker | 236 (5.6) | 54 (4.8) |
| Ejection fraction, median (IQR), % | 55 (40-60) | 55 (43-63) |
| Hemoglobin, median (IQR), g/dL | 12.1 (10.9-13.4) |
12.2 (11.1-13.4) |
| Glomerular filtration rate, median (IQR), mL/min/1.73 m2 | 55.5 (41.2-72.5) |
56.8 (42.5-72.6) |
| Current dialysis | 130 (3.1) | 27 (2.4) |
| STS mortality risk score, median (IQR), % | 5.7 (3.6-9.1) | 5.5 (3.5-8.5) |
| Mitral valve mean gradient, median (IQR), mm Hg | 2.0 (2.0-4.0) | 2.0 (2.0-4.0) |
| Moderate or severe aortic insufficiency | 371 (8.9) | 90 (8.1) |
| Functional mitral regurgitation | 715 (16.9) | 179 (15.9) |
| Degenerative mitral regurgitation | 3761 (89.0) | 999 (88.9) |
| Acuity of procedure | ||
| Elective | 3826 (90.6) | 1031 (91.9) |
| Urgent | 204 (4.8) | 46 (4.1) |
| Shock, inotropes, or assist device | 181 (4.3) | 38 (3.4) |
| Emergency, salvage , or cardiac arrest | 11 (0.3) | 7 (0.6) |
| Baseline KCCQ-OS score, median (IQR) | 40.1 (22.9-58.3) |
40.6 (22.9-59.4) |
| 6-min Walk test, median (IQR), m | 203.1 (120.1-298.2) |
214.3 (128.0-304.8) |
Abbreviations: IQR, interquartile range; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire–Overall Summary score; STS, Society for Thoracic Surgeons.
Health Status Outcomes
At baseline, the mean (SD) KCCQ-OS score was 41.9 (24.0) points (Table 2), indicating substantial impairment in health status prior to TMVR. At 30 days after TMVR, the KCCQ-OS scores had improved by a mean of 24.8 points to a mean (SD) score of 66.7 (24.5) points in the surviving patients. The largest improvement was observed in the quality of life domain (mean improvement, 33.5 [95% CI, 32.5-34.5] points), whereas the physical limitations domain improved the least (mean improvement, 18.1 [95% CI, 17.1-19.1] points). Integrating survival and health status into an estimate calculated with inverse probability weighting, 74.1% of patients were alive with a clinically relevant improvement in health status (increase of ≥5 points in KCCQ-OS score; eFigure 1 in the Supplement).
Table 2. Mean Kansas City Cardiomyopathy Questionnaire Scores and Unadjusted Changes From Baselinea.
| Outcome | Patients, No. | Baseline, Mean (SD) | Outcome, Mean (SD) | Change From Baseline (95% CI) |
|---|---|---|---|---|
| 30 d After TMVR | ||||
| Overall summary score | 4226 | 41.9 (24.0) | 66.7 (24.5) | 24.8 (24.0-25.6) |
| Physical limitation | 3819 | 45.3 (28.1) | 63.9 (28.5) | 18.1 (17.1-19.1) |
| Symptom frequency | 4225 | 50.2 (26.4) | 70.2 (24.1) | 20.0 (19.2-20.8) |
| Quality of life | 4198 | 32.1 (26.9) | 65.6 (29.0) | 33.5 (32.5-34.5) |
| Social limitation | 3820 | 39.4 (30.1) | 66.1 (30.7) | 26.5 (25.4-27.6) |
| 1 y After TMVR | ||||
| Overall summary score | 1124 | 42.3 (23.9) | 71.4 (24.0) | 29.1 (27.5-30.8) |
| Physical limitation | 1024 | 46.4 (27.8) | 67.4 (28.2) | 20.7 (18.7-22.7) |
| Symptom frequency | 1122 | 50.7 (26.7) | 73.9 (23.6) | 23.3 (21.6-25.0) |
| Quality of life | 1118 | 32.4 (27.0) | 71.7 (27.8) | 39.4 (37.4-41.4) |
| Social limitation | 1007 | 38.9 (29.6) | 71.6 (29.6) | 32.2 (30.0-34.5) |
Abbreviation: TMVR, transcatheter mitral valve repair.
Comparisons based on paired t tests. P values for all comparisons of baseline values to outcome values are <.001.
One year after TMVR, surviving patients continued to demonstrate substantial improvement in health status compared with baseline, with a mean (SD) KCCQ-OS score of 71.4 (24.0) points and a mean improvement from baseline of 29.1 points, with similar patterns of large improvements across all KCCQ domains (Table 2). Similar to the 30-day results, the lowest domain score at 1 year was physical limitations (mean [SD] score of 67.4 [28.2] points), with the remaining domain scores ranging from a mean (SD) of 71.6 (29.6) points to 73.9 (23.6) points. Among the 917 surviving patients with health status assessment at all 3 time points, mean KCCQ-OS scores increased significantly from baseline to 30 days (from 42.5 to 71.1 points; P < .001) with no further change from 30 days to 1 year (from 71.1 to 71.2 points; P = .94; eFigure 2 in the Supplement). Integrating survival and health status in estimates calculated with inverse probability weighting, we found that 63.0% of patients were alive with a clinically relevant improvement in health status, and 54.2% (95% CI 52.2%-56.1%) of patients were alive and well at 1 year after TMVR. Among patients with a poor outcome, this was because of death in 23.0%, persistently poor health status (KCCQ-OS scores <60 points) in 21.9%, and health status decline in 5.5%; 4.6% had both poor health status and health status decline.
Subgroup Comparisons
Among the surviving patients, there were modest differences in mean 1-year KCCQ-OS scores across subgroups of patients and the proportion of patients who were alive and well (Table 3). Atrial fibrillation or flutter was associated with worse health status after TMVR (median [IQR] KCCQ-OS score in patients with atrial fibrillation or flutter, 69.6 [67.3-72.0] points vs patients without atrial fibrillation or flutter, 73.3 [70.7-75.9] points; P = .01) and lower rates of alive and well status calculated with inverse probability weighting (patients with atrial fibrillation or flutter, 51.3%; patients without atrial fibrillation or flutter, 59.7%; P < .001). Lower baseline health status was also associated with worse health status at 1 year (median [IQR] KCCQ-OS scores at 1 year: patients who scored 0-25 points at baseline, 61.2 [57.3-65.1] points; 25-49 points, 69.9 [67.2-72.6] points; 50-74 points, 76.7 [73.7-79.6] points; 75-100 points, 83.4 [80.1-86.6] points; P < .001) and lower rates of alive and well status (patients who had KCCQ-OS scores of 0-25 points at baseline, 39.8%; 25-49 points, 53.3%; 50-74 points, 66.2%; 75-100 points, 69.9%; P < .001).
Table 3. Health Status at 1 Year After Transcatheter Mitral Valve Repair in Key Subgroupsa.
| Characteristic | Patients, No. | 1-y KCCQ-OS Score (95% CI) | P Value | Patients Alive and Well, % (95% CI)b | P Value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 598 | 70.8 (68.0-73.6) | .75 | 53.0 (50.4-55.6) | .20 |
| Female | 526 | 71.3 (69.1-73.4) | 55.5 (52.7-58.4) | ||
| Age, y | |||||
| <80 | 435 | 71.4 (68.4-74.4) | .74 | 52.8 (49.5-56.0) | .45 |
| 80-90 | 585 | 70.5 (68.2-72.8) | 55.3 (52.7-57.9) | ||
| ≥90 | 104 | 71.7 (66.9-76.4) | 52.7 (46.8-58.6) | ||
| Ejection fraction, % | |||||
| <30 | 101 | 70.5 (65.8-75.3) | .91 | 46.6 (40.2-53.1) | <.001 |
| 30-45 | 186 | 70.5 (66.7-74.3) | 46.0 (41.4-50.6) | ||
| ≥45 | 813 | 71.2 (69.1-73.4) | 57.0 (54.8-59.3) | ||
| Chronic lung disease | |||||
| None or mild | 886 | 71.7 (69.6-73.7) | .34 | 56.3 (54.1-58.5) | <.001 |
| Moderate | 128 | 68.8 (64.2-73.4) | 50.5 (44.9-56.1) | ||
| Severe | 105 | 68.9 (63.7-74.0) | 42.8 (37.2-48.4) | ||
| Renal function | |||||
| Creatinine level <2 mg/dL, no dialysis | 997 | 71.9 (69.8-73.9) | .26 | 56.5 (54.5-58.6) | <.001 |
| Creatinine level ≥2 mg/dL, no dialysis | 96 | 67.7 (62.3-73.0) | 41.3 (35.4-47.2) | ||
| Dialysis | 27 | 66.2 (52.4-80.0) | 29.2 (17.9-40.6) | ||
| Atrial fibrillation/flutter | |||||
| Yes | 696 | 69.6 (67.3-72.0) | .01 | 51.3 (48.9-53.7) | <.001 |
| No | 428 | 73.3 (70.7-75.9) | 59.7 (56.4-62.9) | ||
| Functional mitral regurgitation | |||||
| Yes | 179 | 70.9 (66.6-75.2) | .96 | 45.5 (40.5-50.6) | <.001 |
| No | 945 | 71.0 (68.9-73.2) | 55.7 (53.6-57.8) | ||
| Degenerative mitral regurgitation | |||||
| Yes | 999 | 71.3 (69.3-73.4) | .25 | 55.6 (53.6-57.7) | <.001 |
| No | 125 | 68.0 (62.6-73.4) | 41.4 (35.8-47.1) | ||
| Mitral valve mean gradient, mm Hg | |||||
| <4 | 492 | 70.8 (67.6-74.0) | .21 | 54.1 (51.0-57.2) | .75 |
| ≥4 | 187 | 74.1 (70.4-77.8) | 53.2 (48.4-57.9) | ||
| Device successc | |||||
| Yes | 1058 | 70.9 (68.8-73.0) | .87 | 55.0 (53.0-57.1) | .01 |
| No | 58 | 70.4 (64.6-76.3) | 46.6 (40.7-52.5) | ||
| Baseline KCCQ-OS score | |||||
| 0-24 | 303 | 61.2 (57.3-65.1) | <.001 | 39.8 (36.4-43.3) | <.001 |
| 25-49 | 403 | 69.9 (67.2-72.6) | 53.3 (50.1-56.4) | ||
| 50-74 | 283 | 76.7 (73.7-79.6) | 66.2 (62.4-70.0) | ||
| 75-100 | 135 | 83.4 (80.1-86.6) | 69.9 (64.2-75.6) |
Abbreviation: KCCQ-OS, Kansas City Cardiomyopathy Questionnaire–Overall Summary score.
Analyses reflect use of inverse probability weighting to account for missing follow-up data.
Defined as alive, with a KCCQ-OS scores of 60 points or more, without significant worsening of KCCQ-OS scores from baseline (decline of ≥10 points).
Defined as moderate or less severe postprocedural mitral regurgitation with 1 or more grades of reduction in severity from baseline.
Lower rates of alive and well status were found in patients with lower left ventricular ejection fraction (patients with <30% ejection fraction, 46.6%; those with 30-45% ejection fraction, 46.0%; those with ejection fraction ≥45%, 57.0%; P < .001), severe lung disease (patients with no or mild lung disease, 56.3%; those with moderate lung disease, 50.5%; those with severe lung disease, 42.8%; P < .001), renal dysfunction (those with creatinine levels <2 mg/dL without dialysis, 56.5%; those with creatinine levels >2 mg/dL without dialysis, 41.3%; those on dialysis, 29.2%; P < .001), functional mitral regurgitation (those with functional mitral regurgitation, 45.5%; those without functional mitral regurgitation, 55.7%; P < .001), and a lack of device success (defined as post-TMVR mitral regurgitation ≤2+ with at least 1 grade improvement from baseline; in patients with device success, 55.0% vs patients without device success, 46.6%; P = .01), driven mainly by lower survival rates among these subgroups (since 1-year KCCQ-OS scores in these subgroups were similar to those in the overall population). There were no statistically significant differences in 1-year KCCQ-OS scores or rates of alive and well status according to sex or age. The lowest rates of alive and well status were among patients with very poor baseline health status (patients with KCCQ-OS scores of 0-25 points, 39.8%) and those with renal dysfunction (29.2% of patients requiring dialysis and 41.3% of patients with advanced chronic kidney disease [creatinine ≥2 mg/dL], vs 56.5% of those with less advanced or no renal dysfunction).
Factors Associated With Health Status Improvement After TMVR
In the multivariable model, patients who had better health status at baseline were more likely to have better health status at 30 days after TMVR, with every 10-point increase in baseline KCCQ-OS score associated with 3.9-point higher 30-day KCCQ-OS score (95% CI, 3.6-4.2; P < .001; Table 4). Both severe lung disease and home oxygen were independently associated with worse health status recovery, with associated changes of −3.9 points (95% CI, −6.2 to −1.5; P = .001) and −2.7 points (95% CI, −4.9 to −0.4; P = .02) in 30-day KCCQ-OS scores, respectively. Rhythm issues were also associated with worse health status recovery. Specifically, history of atrial fibrillation was associated with a −2.2-point change in KCCQ-OS score (95% CI, −3.7 to −0.6; P = .01) and presence of permanent pacemaker was associated with a −2.1-point difference in KCCQ-OS (95% CI, −3.7 to −0.4; P = .01) at 30 days after TMVR. Older age (−0.5 points per 5-year increase [95% CI, −1.0 to −0.0]; P = .03), lower hemoglobin (0.6 point per 1-g/dL increase, [95% CI, 0.1-1.1; P = .02), and not having had prior coronary artery bypass graft surgery (2.0 points [95% CI, 0.3-3.7; P = .02) were also independently associated with lower 30-day KCCQ-OS scores among surviving patients.
Table 4. Factors Independently Associated With 30-Day Kansas City Cardiomyopathy Questionnaire–Overall Summary Scores After Transcatheter Mitral Valve Repaira,b.
| Factor | Estimate (95% CI) | P Value |
|---|---|---|
| Baseline KCCQ-OS score, per 10-point increase | 3.9 (3.6 to 4.2) | <.001 |
| Age, per 5-y increase | −0.5 (−1.0 to −0.0) | .03 |
| Hemoglobin, per 1–g/dL increase | 0.6 (0.1 to 1.1) | .02 |
| Atrial fibrillation or flutter | −2.2 (−3.7 to −0.6) | .01 |
| Severe chronic lung disease | −3.9 (−6.2 to −1.5) | .001 |
| Home oxygen use | −2.7 (−4.9 to −0.4) | .02 |
| Permanent pacemaker | −2.1 (−3.7 to −0.4) | .01 |
| Prior coronary artery bypass graft | 2.0 (0.3 to 3.7) | .02 |
Abbreviation: KCCQ-OS, Kansas City Cardiomyopathy Questionnaire–overall summary score.
Factors in the model that were not significantly associated with quality of life: sex, race/ethnicity, body surface area, prior myocardial infarction, percutaneous coronary intervention, peripheral artery disease, left ventricular ejection fraction, prior stroke, current smoker status, diabetes, glomerular filtration rate, current dialysis, moderate or severe aortic insufficiency, moderate or severe tricuspid insufficiency, and acuity of case.
Analyses reflect use of inverse probability weighting to account for missing follow-up data.
Several procedural complications were associated with impaired 30-day quality of life as well. After adjusting for patient factors, in-hospital renal failure (n = 206; −7.3 [95% CI −13.3 to −1.2] points), and residual 2+ mitral regurgitation (n = 1221; −3.1 [95% CI −4.6 to −1.6] points) and residual 3+ or 4+ mitral regurgitation (n = 216; −7.9 [95% CI, −11.1 to −4.7] points) were all associated with worse 30-day health status among surviving patients. In-hospital stroke (n = 11) and in-hospital bleeding (n = 103) were not significantly associated with 30-day health status.
Discussion
In a large US cohort of patients who underwent edge-to-edge TMVR in routine clinical practice, we found that health status was markedly impaired prior to the procedure, improved quickly after TMVR, and remained stable through 1 year among the surviving patients. The mortality rate after TMVR remains high (23.0% at 1 year), owing to the age and comorbidities of the patients selected for TMVR, and this is particularly true for patients with advanced kidney or lung disease and those with very poor health status prior to TMVR. However, among patients who survive, the health status benefits of TMVR are fairly consistent across groups. While patients with history of atrial fibrillation, permanent pacemaker, lung disease, home oxygen, and anemia had somewhat less health status improvement after TMVR, these effects were modest (<5-point differences in KCCQ-OS scores) compared with the overall benefit of the procedure. In-hospital bleeding, renal failure, and stroke were all uncommon (occurring in ≤5% of patients), further supporting the safety of the procedure. While residual mitral regurgitation occurred more often, it was associated with only a modest reduction in 30-day KCCQ-OS scores. Because one of the main goals of TMVR is health status improvement, this study helps clarify the benefit of TMVR and the factors that may modify the magnitude of benefit.
Comparison With Prior Studies
Similar to our results, a previous trial of surgical mitral valve repair vs replacement for ischemic mitral regurgitation showed a 22-point decrease (improvement) in the Minnesota Living with Heart Failure Questionnaire score at 1-year after valve repair,21 which is roughly equivalent to a 25-point increase (improvement) in the KCCQ-OS score.22 In contrast, 2 studies that examined change in patient-reported health status after TMVR both found mean reductions (improvements) of approximately 13 points in Minnesota Living with Heart Failure Questionnaire score,3,5 which is roughly equivalent to a 14-point increase (improvement) in the KCCQ-OS score.22 Importantly, however, these prior TMVR studies included a much higher prevalence of severe left ventricular dysfunction and functional mitral regurgitation than among patients currently treated with TMVR in the TVT Registry, which may explain some of the attenuation in health status recovery observed in these studies. For this reason, it will be important to explore the extent of health status improvement in the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial and other randomized clinical trials examining the outcomes of TMVR in patients with functional mitral regurgitation. Understanding the clinical and anatomic or echocardiographic factors that identify patients who derive greater and lesser degrees of health status benefit from TMVR will help to define the optimal patient population for this therapy.
Implications
This study provides several novel insights. First, patients with residual mitral regurgitation after edge-to edge TMVR achieved less health status improvement compared with those whose mitral regurgitation was reduced 0 to 1+; however, this difference was only approximately 3 points for moderate regurgitation. While more investigation is needed to understand this association, these results suggest that aggressive efforts to reduce mitral regurgitation to mild may provide diminishing returns. Second, although no individual patient factors were associated with a large health status decrement, we identified a number of factors that, in combination, appear to characterize a cohort with less chance to benefit from TMVR (eg, a patient with severe lung disease on home oxygen with atrial fibrillation and a pacemaker). Furthermore, fewer than half of patients with certain characteristics (eg, advanced kidney disease or very poor baseline health status) were alive and well at 1 year. If these findings are replicated in future studies, these insights could help inform the shared decision-making process prior to TMVR, helping patients understand their potential for recovery beyond just providing results for so-called average patients.
Finally, although monitoring health status outcomes is an important part of the mission of the TVT Registry and has been supported and encouraged by leaders in the field, the logistics of collecting these data in a predominantly elderly population as a part of routine clinical care has proven challenging. While we have used analytic strategies to handle these missing data such that we have reasonable confidence in the results, the degree of missing health status data at follow-up in the TVT Registry has been disappointing. As such, it will be important to develop strategies to improve collection of these data to improve the understanding of these critical outcomes. Potential approaches could include linking reimbursement of TMVR to a minimum threshold of health status ascertainment or providing direct financial support for collection of these data.
Limitations
First, health status data were missing in a relatively high proportion of surviving patients, particularly at the 1-year follow-up. Although patients with missing data were generally similar to those in the analytic cohort, we used inverse probability weighting to adjust for differences between groups to minimize any potential bias. In addition, given the high degree of missing data at 1 year, our exploration of factors associated with health status recovery was limited to the 30-day follow-up assessment. However, since we found that most (if not all) of the health status benefit of TMVR is evident within the first 30 days, it is likely that the same factors would be associated with long-term health status. Nonetheless, this concern remains relevant for residual mitral regurgitation, which may have a greater effect on long-term health status. Second, the long-term health status results are reported for surviving patients only, which is an important consideration given the high mortality rate among patients treated with TMVR. While we also report a composite endpoint that integrates survival with health status, it is possible that long-term health status benefits of TMVR in various subgroups could have been underestimated owing to differential attrition of the sickest patients in the comparator subgroup. Third, although the health status effects of TMVR appear to be durable over 1 year, it is important to note that all patients in this study received TMVR, and therefore we cannot exclude the possibility that some of the observed health status effects reflect a placebo effect.
Conclusions
In this national cohort study of US patients undergoing edge-to-edge TMVR in routine clinical practice, we found that disease-specific health status was substantially impaired prior to the procedure, improved rapidly thereafter, and remained stable through 1 year in surviving patients with available health status data. Although some patient factors (eg, arrhythmias and lung disease) were associated with less robust health status recovery, most of these effects were rather modest in isolation. Nonetheless, long-term mortality remains high among US patients who undergo TMVR (owing to the age and multiple comorbidities present in the TMVR population), such that only slightly more than half of patients treated were alive and well at 1 year after TMVR. Using these data to help inform the decision process prior to TMVR may help improve patient selection as well as patient expectations for recovery, particularly if a number of risk factors for worse health status are present in an individual patient. For a procedure that is currently reserved for patients who are poor candidates for valve surgery, is mainly performed to improve quality of life, and has low periprocedural risk, however, the health status outcomes of surviving patients are encouraging and support the continued use of edge-to-edge TMVR in selected patients who are poor candidates for cardiac surgery.
eTable. Baseline characteristics of CMS linked patients surviving 1 year1, according to presence of 1-year KCCQ data
eFigure 1. Categorical outcomes at 30 days and 1 year after TMVR.
eFigure 2. Mean KCCQ-OS scores over time among 917 patients with complete data
References
- 1.Vakil K, Roukoz H, Sarraf M, et al. . Safety and efficacy of the MitraClip® system for severe mitral regurgitation: a systematic review. Catheter Cardiovasc Interv. 2014;84(1):129-136. doi: 10.1002/ccd.25347 [DOI] [PubMed] [Google Scholar]
- 2.Deuschl F, Schofer N, Lubos E, Blankenberg S, Schäfer U. Critical evaluation of the MitraClip system in the management of mitral regurgitation. Vasc Health Risk Manag. 2016;12:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisano F, Franzen O, Baldus S, et al. . Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol. 2013;62(12):1052-1061. doi: 10.1016/j.jacc.2013.02.094 [DOI] [PubMed] [Google Scholar]
- 4.Glower DD, Kar S, Trento A, et al. . Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol. 2014;64(2):172-181. doi: 10.1016/j.jacc.2013.12.062 [DOI] [PubMed] [Google Scholar]
- 5.Metze C, Matzik AS, Scherner M, et al. . Impact of frailty on outcomes in patients undergoing percutaneous mitral valve repair. JACC Cardiovasc Interv. 2017;10(19):1920-1929. doi: 10.1016/j.jcin.2017.07.042 [DOI] [PubMed] [Google Scholar]
- 6.Iliadis C, Lee S, Kuhr K, et al. . Functional status and quality of life after transcatheter mitral valve repair: a prospective cohort study and systematic review. Clin Res Cardiol. 2017;106(12):1005-1017. doi: 10.1007/s00392-017-1150-x [DOI] [PubMed] [Google Scholar]
- 7.Lim DS, Reynolds MR, Feldman T, et al. . Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation after transcatheter mitral valve repair. J Am Coll Cardiol. 2014;64(2):182-192. doi: 10.1016/j.jacc.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 8.Carroll JD, Edwards FH, Marinac-Dabic D, et al. . The STS-ACC transcatheter valve therapy national registry: a new partnership and infrastructure for the introduction and surveillance of medical devices and therapies. J Am Coll Cardiol. 2013;62(11):1026-1034. doi: 10.1016/j.jacc.2013.03.060 [DOI] [PubMed] [Google Scholar]
- 9.Mack MJ, Brennan JM, Brindis R, et al. ; STS/ACC TVT Registry . Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310(19):2069-2077. doi: 10.1001/jama.2013.282043 [DOI] [PubMed] [Google Scholar]
- 10.Holmes DR Jr, Brennan JM, Rumsfeld JS, et al. ; STS/ACC TVT Registry . Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313(10):1019-1028. doi: 10.1001/jama.2015.1474 [DOI] [PubMed] [Google Scholar]
- 11.Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8(5):469-476. doi: 10.1161/CIRCOUTCOMES.115.001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. doi: 10.1016/S0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 13.Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235-242. doi: 10.1016/j.ejheart.2004.05.012 [DOI] [PubMed] [Google Scholar]
- 14.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110(5):546-551. doi: 10.1161/01.CIR.0000136991.85540.A9 [DOI] [PubMed] [Google Scholar]
- 15.Arnold SV, Spertus JA, Lei Y, et al. . Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61-67. doi: 10.1161/CIRCHEARTFAILURE.112.970053 [DOI] [PubMed] [Google Scholar]
- 16.Spertus J, Peterson E, Conard MW, et al. ; Cardiovascular Outcomes Research Consortium . Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707-715. doi: 10.1016/j.ahj.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 17.Arnold SV, Spertus JA, Lei Y, et al. . How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes. 2013;6(5):591-597. doi: 10.1161/CIRCOUTCOMES.113.000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien SM, Shahian DM, Filardo G, et al. ; Society of Thoracic Surgeons Quality Measurement Task Force . The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg. 2009;88(1)(suppl):S23-S42. doi: 10.1016/j.athoracsur.2009.05.056 [DOI] [PubMed] [Google Scholar]
- 19.Arnold SV, Spertus JA, Vemulapalli S, et al. . Quality-of-life outcomes after transcatheter aortic valve replacement in an unselected population: a report From the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017;2(4):409-416. doi: 10.1001/jamacardio.2016.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 21.Acker MA, Parides MK, Perrault LP, et al. ; CTSN . Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370(1):23-32. doi: 10.1056/NEJMoa1312808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassif ME, Tang Y, Cleland JG, et al. . Precision medicine for cardiac resynchronization: predicting quality of life benefits for individual patients—an analysis from 5 clinical trials. Circ Heart Fail. 2017;10(10):e004111. doi: 10.1161/CIRCHEARTFAILURE.117.004111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Baseline characteristics of CMS linked patients surviving 1 year1, according to presence of 1-year KCCQ data
eFigure 1. Categorical outcomes at 30 days and 1 year after TMVR.
eFigure 2. Mean KCCQ-OS scores over time among 917 patients with complete data