Key Points
Question
How do individual non–vitamin K antagonist oral anticoagulants compare with aspirin with regard to the risk of intracranial hemorrhage?
Findings
This systematic review and meta-analysis of 5 randomized clinical trials that had compared non–vitamin K antagonist oral anticoagulants with aspirin; the use of 15 to 20 mg of rivaroxaban daily was associated with increased risk of intracranial hemorrhage (OR, 3.31 [95% CI, 1.42 to 7.72]) compared with aspirin, while 10 mg of rivaroxaban daily and 5 mg of apixaban twice daily were not.
Meaning
In this study, a rivaroxaban dose of 15 to 20 mg once daily greatly increased the risk of intracranial hemorrhage compared with aspirin, while neither the lower doses of rivaroxaban nor apixaban conferred such comparative risk.
This systematic review and meta-analysis of randomized clinical trials assesses the risk of intracranial hemorrhage with non–vitamin K antagonist oral anticoagulant use compared with aspirin use.
Abstract
Importance
Non–vitamin K antagonist oral anticoagulants (NOACs) might be an attractive choice for stroke prevention in people without atrial fibrillation who may harbor a potential source of cardiac emboli, but not if certain individual NOACs carry risks of intracranial hemorrhage that are heightened relative to aspirin.
Objective
To conduct a systematic review and meta-analysis of randomized clinical trials to assess the risk of intracranial hemorrhage with individual NOACs vs aspirin across all indications.
Data Sources
We searched PubMed, Embase, CENTRAL, and ClinicalTrials.gov from inception to May 28, 2018, with the terms novel oral anticoagulants, non–vitamin K antagonist oral anticoagulants, direct oral anticoagulants, dabigatran, rivaroxaban, apixaban, edoxaban, warfarin, Coumadin, vitamin K antagonist, aspirin, acetylsalicylic acid, or ASA, and major bleeding, fatal bleeding, or intracranial hemorrhage. We restricted our search to clinical trials on humans. There were no language restrictions.
Study Selection
Randomized clinical trials of 3 months or longer that included a comparison of the outcomes of NOAC use vs use of aspirin.
Data Extraction and Synthesis
Two investigators independently abstracted data from eligible studies. We computed a fixed-effect estimate based on the Mantel-Haenszel method.
Main Outcomes and Measures
Odds ratios (ORs) with 95% CI were used as a measure of the association of individual NOAC vs aspirin with the risk of intracranial hemorrhage. The hypothesis that intracranial hemorrhage risk would be higher with NOACs than aspirin was formulated during data collection.
Results
Our principal analysis included 5 randomized clinical trials comparing 1 or more NOACs with aspirin, with 39 398 individuals enrolled. Pooling the results from the fixed-effects model showed that a dose of 15 to 20 mg of rivaroxaban once daily was associated with an increased risk of intracranial hemorrhage (2 trials; OR, 3.31 [95% CI, 1.42 to 7.72]) compared with aspirin, while a 10-mg dose of rivaroxaban once daily or a 5-mg dose twice daily (3 trials; OR, 1.43 [95% CI, 0.93 to 2.21]) and a 5-mg dose of apixaban twice daily (1 trial; OR, 0.84 [95% CI, 0.38 to 1.88]) were not.
Conclusions and Relevance
A 15-mg to 20-mg dose of rivaroxaban once daily is associated with substantially increased risks of intracranial hemorrhage, while smaller daily doses of rivaroxaban and apixaban were not, implying that risk increase is dose dependent. It may be worthwhile to conduct randomized clinical trials comparing specific NOACs in specific doses (eg, apixaban, 5 mg twice daily) and aspirin in patients without atrial fibrillation, but with potential sources of cardiac emboli that could cause stroke.
Introduction
Intracranial hemorrhage is a major concern when an antithrombotic agent is given to a patient, because intracranial hemorrhage is generally associated with a high risk of mortality1 and poorer health over a lifetime,2,3 which could offset the benefits of antithrombotic treatment in reducing major ischemic events. Such a concern may lead some physicians to resort to aspirin in certain situations, such as atrial fibrillation, when an oral anticoagulant is indicated. However, in a large clinical trial of patients with atrial fibrillation,4 a 5-mg dose of apixaban twice daily was shown to not cause more intracranial hemorrhage than aspirin, and the medication substantially reduced stroke risk, thereby making aspirin, with its much smaller stroke protection benefit, no longer a viable therapeutic option in people with atrial fibrillation, even from a standpoint of safety concern.
Considering the low risks of intracranial hemorrhage with non–vitamin K antagonist oral anticoagulants (NOACs) use, one may wonder whether NOACs could be applied to clinical scenarios beyond atrial fibrillation that traditionally been treated by aspirin. However, NOACs are not all the same. A network meta-analysis comparing one NOAC with another clearly show they are different in terms of effectiveness and safety.5 Indeed, another large clinical trial showed that a 15-mg dose of rivaroxaban once daily substantially increased the risk of intracranial hemorrhage in patients with embolic stroke of undetermined source.6
Although NOACs and aspirin are in general protective in different vascular beds, NOACs might be an attractive choice for stroke prevention in certain clinical situations, such as in people without recognized atrial fibrillation but with an embolic stroke of undetermined source7,8 or atrial cardiopathy9,10,11 because of their propensity to also cause stroke through cardiac emboli. Nonetheless, the benefit of NOACs compared with aspirin for stroke protection is likely to be less promising in people without atrial fibrillation, compared with people with atrial fibrillation. Therefore it is even more important to clarify whether individual NOACs in various doses harbor comparable risks of intracranial hemorrhage with aspirin based on the evidence currently available before conducting a large clinical trial in such a population. To do this, we conducted a systematic review and meta-analysis of randomized clinical trials for the comparison across all indications of intracranial hemorrhage with individual NOACs vs aspirin.
Methods
This study was performed in accordance with the recommendations of the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) statement.12
Data Sources and Searches
We searched PubMed (from 1966 to May 28, 2018), Excerpta Medica dataBASE (EMBASE; from 1966 to May 28, 2018), the Cochrane Central Register of Controlled Trials (CENTRAL), and the clinical trial registry maintained at ClinicalTrials.gov with the terms novel oral anticoagulants, non–vitamin K antagonist oral anticoagulants, direct oral anticoagulants, dabigatran, rivaroxaban, apixaban, edoxaban, warfarin, Coumadin, vitamin K antagonist, aspirin, acetylsalicylic acid, or ASA, and major bleeding, fatal bleeding, or intracranial hemorrhage. We restricted our search to human and clinical trials. There were no language restrictions. We also reviewed the Introduction and Discussion sections of retrieved trials and relevant review articles to identify additional trials. Two investigators (W.-Y.H. and M.L.) independently conducted the literature search, screen of abstracts, and selection of included trials.
Study Selection
Criteria for inclusion of a study were (1) a randomized clinical trial study design, (2) inclusion of a comparison of NOACs with aspirin, (3) reporting of intracranial hemorrhage as an end point, (4) reporting of the number of participants and the number of intracranial hemorrhage separately in each group, and (5) a treatment duration of at least 3 months. When comparison of individual NOACs (eg, dabigatran and edoxaban) with aspirin were not available, we selected studies comparing dabigatran or edoxaban with warfarin, as well as comparing aspirin with warfarin, to obtain the indirect comparison between dabigatran or edoxaban and aspirin.
Criteria for exclusion of a study were that it (1) was an NOAC trial that did not use at least of 1 of the 4 eligible NOACs (ie, dabigatran, rivaroxaban, apixaban, and edoxaban), because only these NOACs are approved for use in atrial fibrillation in current guidelines; (2) included a combination of 2 or more antithrombotic agents as a treatment strategy in a study arm; (3) used antiplatelet agents other than aspirin; and (4) was published prior to 2000. In addition, we excluded all studies that included a population in which most participants had (5) cancer; (6) a mechanic heart valve (because the use of 1 NOAC, dabigatran, in patients with mechanical heart valves was associated with increased rates of thromboembolic and bleeding complications compared with warfarin, and NOAC use is thus not justified for these patients13); or (7) end-stage renal disease.
Data Abstraction
We abstracted data about baseline characteristics, which include age, sex, duration of follow-up, and the number of patients in each group. We also abstracted data on safety outcomes from each trial, which include numbers with intracranial hemorrhage, major bleeding, and fatal bleeding in each group under NOACs and aspirin treatment. Two investigators (M.L. and W.-Y.H.) independently abstracted data from eligible studies. Any discrepant judgments were resolved by joint discussion.
Quality Assessment
The risk of bias (eg, sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other issues) in each trial was independently assessed in accordance with the Cochrane risk of bias tool. The risk of bias was rated as low, unclear, or high, according to established criteria.14
Statistical Analysis
The primary end point was the association of individual NOACs (compared with aspirin) with risks for intracranial hemorrhage. The secondary end points were risks of major bleeding and fatal bleeding.
The definitions of intracranial hemorrhage and fatal bleeding were consistent across trials. (Intracranial hemorrhage was defined as traumatic and atraumatic intracerebral, subarachnoid, and subdural or epidural hemorrhage. Fatal bleeding was defined as mortality caused by bleeding.) However, major bleeding differed slightly across trials. It was defined as clinically overt bleeding accompanied by 1 or more of the following: a decrease in the hemoglobin level of 2 g/dL or more in a 24-hour period, transfusion of 2 or more units of packed red cells, and bleeding at a critical site4 or any site in the body,6 as per the criteria of the International Society of Thrombosis and Hemostasis.
The dosage of each NOAC was taken into consideration. Rivaroxaban was further categorized into 15 to 20 mg once daily and 10 mg once daily or 5 mg twice daily, because this difference of dosage was substantial.
Odds ratios (OR) with 95% CIs were used as a measure of the association of individual NOACs vs aspirin with the risk of intracranial hemorrhage, fatal bleeding, or major bleeding. We computed a fixed-effect estimate based on the Mantel-Haenszel method when 2 or more studies provided sufficient data for a given outcome. The principal analysis was pairwise comparisons between individual NOACs and aspirin.
In situations where pairwise comparisons were not available between certain NOACs (ie, dabigatran and edoxaban) and aspirin, we conducted accessory analyses using dabigatran or edoxaban vs warfarin and aspirin vs warfarin to obtain indirect comparisons of dabigatran or edoxaban vs aspirin. Results from these indirect comparisons should be regarded as suggestive rather than conclusive. Studies that compared rivaroxaban or apixaban with warfarin were excluded from the study; because direct comparison between rivaroxaban or apixaban vs aspirin has been available in randomized clinical trials, it was not necessary to obtain results from indirect comparison.
Subgroup analyses for the primary end point, intracranial hemorrhage, were conducted for different trial characteristics: patients with a history of ischemic stroke, atrial fibrillation, or venous thromboembolism at entry. This subgroup analysis was driven by the fact that the risk of intracranial hemorrhage within 1 year after ischemic stroke is about 15 times higher than in the reference population,15 and patients with atrial fibrillation have a propensity to have more incidents of bleeding compared with patients without atrial fibrillation.16 Therefore it was important to clarify whether individual NOACs were causing additional risks of intracranial hemorrhage compared with aspirin in these situations.
Heterogeneity was assessed by the P value of χ2 and I2 statistics, which describes the percentage of variability in the effect estimates that is because of heterogeneity rather than chance.17,18 We considered study-level estimates to be heterogeneous if the χ2 test was significant (P < .05) or the I2 statistic was greater than 50%. We report absolute risks in terms of the difference in the number of events per 1000 patients and the respective 95% CI. All analyses were based on the intention-to-treat principle. We evaluated the quality of evidence for primary and secondary outcomes reported in the meta-analysis.19 For all analyses, P < .05 was considered statistically significant. The Review Manager Software Package (RevMan 5; Cochrane Collaboration) and Stata version 13 (Stata Corp) were used for this meta-analysis.
Results
The literature review identified 56 full articles for detailed assessment, of which 31 were excluded for using placebo or no treatment in the control group (k = 4), using another antiplatelet drug rather than aspirin in the comparator group (k = 2), continuing treatment for less than 3 months (k = 19), or comparing rivaroxaban or apixaban with warfarin (k = 6). Twenty trials regarding dabigatran or edoxaban vs warfarin and aspirin vs warfarin were included in accessory analyses for the purpose of obtaining indirect comparisons of dabigatran or edoxaban vs aspirin.
Our final principal analysis included 5 randomized clinical trials comparing NOAC and aspirin, with 39 398 individuals enrolled (eFigure in the Supplement).4,6,20,21,22 One trial4 compared a 5-mg dose of apixaban twice daily with aspirin in patients with atrial fibrillation. One trial6 compared 15 mg of rivaroxaban once daily with aspirin in patients with embolic stroke of undetermined source. One trial22 compared rivaroxaban delivered in 20-mg or 10-mg doses once daily with aspirin in patients with venous thromboembolism. Two trials compared a 5-mg dose of rivaroxaban twice daily with aspirin in patients with stable cardiovascular disease20 and stable peripheral or carotid artery disease.21
The characteristics of these 5 randomized clinical trials are shown in Table 1. The overall quality of included trials was considered good, because all trials showed low risk of bias based on the Cochrane risk of bias assessment (eTable 1 in the Supplement).
Table 1. Characteristics of Included Trials (Non–Vitamin K Antagonist Oral Anticoagulants vs Aspirin).
| Study Acronym | Qualifying Condition | Mean Age, y | Total Patients, No. | Female, No./Total No. (%) | Active Group, No. | Active Treatment | Control Group | Control Treatment | Follow-Up, y | Intracranial Hemorrhage | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events in Active Arm, No. | Events in Control Arm, No. | ||||||||||
| Apixabana | |||||||||||
| AVERROES, 201423 | Atrial fibrillation unsuitable for vitamin K antagonist | 70 | 5599 | 2322 (41.5) |
2808 | Apixaban, 5 mg twice daily | 2791 | Aspirin, 81 to 324 mg once daily | 1.1 | 11 | 13 |
| Rivaroxabanb | |||||||||||
| EINSTEIN CHOICE, 201722 | Venous thromboembolism | 58.5 | 3365 | 1500 (44.6) |
2234 | Rivaroxaban, 20 mg once daily (1107 participants) or 10 mg once daily (1127 participants) | 1131 | Aspirin, 100 mg once daily | 1 | 3 (20 mg); 1 (10 mg) |
2 |
| COMPASS, 201720 | Stable cardiovascular disease | 68.2 | 18 243 | 3961 (21.7) |
9117 | Rivaroxaban, 5 mg twice daily | 9126 | Aspirin, 100 mg once daily | 1.9 | 43 | 24 |
| COMPASS, 201721 | Stable peripheral or carotid artery disease | 67.8 | 4978 | 1391 (27.9) |
2474 | Rivaroxaban, 5 mg twice daily | 2504 | Aspirin, 100 mg once daily | 1.8 | 6 | 9 |
| NAVIGATE ESUS, 20186 | Embolic strokes of undetermined source | 67 | 7213 | 2777 (38.5) |
3609 | Rivaroxaban, 15 mg once daily | 3604 | Aspirin, 100 mg once daily | 0.92 | 20 | 5 |
Abbreviations: AVERROES, Apixaban vs Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; EINSTEIN CHOICE, Reduced-dosed Rivaroxaban in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism; NAVIGATE ESUS, Rivaroxaban vs Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source.
This study used apixaban in the active study arm.
These studies used rivaroxaban in the active study arm.
Primary End Point: Intracranial Hemorrhage
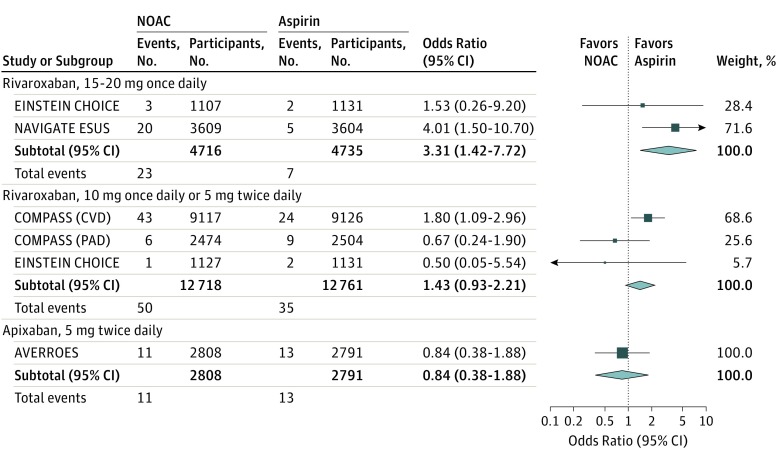
Pooling the results from the fixed-effects model showed that 15-mg to 20-mg of rivaroxaban once daily was associated with increased risk of intracranial hemorrhage (2 trials6,22 ; OR, 3.31 [95% CI, 1.42 to 7.72]; P for heterogeneity = .36) compared with aspirin. A 10-mg dose of rivaroxaban once daily or a 5-mg dose twice daily were not associated with increased risk (3 trials20,21,22; OR, 1.43 [95% CI, 0.93 to 2.21]; P for heterogeneity = 0.17); nor was a 5-mg dose of apixaban twice daily (1 trial4; OR, 0.84 [95% CI, 0.38 to 1.88]; Figure 1).
Figure 1. Odds Ratios of Intracranial Hemorrhage With Use of Individual Non–Vitamin K Antagonist Oral Anticoagulants (NOACs) vs Aspirin, by Trial and Pooled.
This figure presents odds ratios of intracranial hemorrhage of individual NOACs vs aspirin, by trial and pooled, calculated via the Mantel-Haenszel method. AVERROES indicates Apixaban Vs Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; EINSTEIN CHOICE, Reduced-dosed Rivaroxaban in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism; NAVIGATE ESUS, Rivaroxaban Vs Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source.
A 15-mg to 20-mg dose of rivaroxaban once daily, compared with aspirin, would cause an additional 3 intracranial hemorrhage per 1000 patients who receive it. The quality of a body of evidence was moderate (Table 2).
Table 2. Summary of Quality Assessments and Findings for Primary and Secondary Outcomes.
| Outcomes | Quality Assessment | Summary of Findings | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events, No./Total No. (%) | Odds Ratio (95% CI) | Control Risk, Events per 1000 Persons | Risk Difference, Increase per 1000 (95% CI) | Quality | ||||||||||
| Studies, No. | Serious Limitations | Serious Inconsistency | Serious Indirectness | Serious Imprecision | Publication Bias Detected | NOAC | Aspirin | |||||||
| Primary Outcome | ||||||||||||||
| Intracranial hemorrhage | ||||||||||||||
| Rivaroxaban vs aspirin | ||||||||||||||
| 15-20 mg daily | 2 | No | No | No | Yes | No | 23/4716 (0.5) |
7/4735 (0.1) |
3.31 (1.42-7.72) |
1 | 3 (1-10) | Moderate | ||
| 10 mg daily | 3 | No | No | No | Yes | No | 50/12 718 (0.4) |
35/12 761 (0.3) |
1.43 (0.93-2.21) |
3 | NS | Moderate | ||
| Apixaban vs Aspirin | 1 | No | No | No | Yes | No | 11/2808 (0.4) |
13/2791 (0.5) |
0.84 (0.38-1.88) |
5 | NS | Moderate | ||
| Secondary Outcomes | ||||||||||||||
| Fatal bleeding | ||||||||||||||
| Rivaroxaban vs aspirin | ||||||||||||||
| 15-20 mg Daily | 2 | No | No | No | Yes | No | 36/4716 (0.8) |
15/4735 (0.3) |
2.37 (1.3-4.29) |
3 | 4 (1-10) | Moderate | ||
| 10 mg Daily | 3 | No | No | No | Yes | No | 19/12 718 (0.1) |
13/12 761 (0.1) |
1.47 (0.72-2.97) |
1 | NS | Moderate | ||
| Apixaban vs aspirin | 1 | No | No | No | Yes | No | 4/2808 (0.1) |
6/2791 (0.2) |
0.66 (0.19-2.35) |
2 | NS | Moderate | ||
| Major bleeding | ||||||||||||||
| Rivaroxaban vs aspirin | ||||||||||||||
| 15-20 mg Daily | 2 | No | No | No | Yes | No | 68/4716 (1.4) |
26/4735 (0.5) |
2.64 (1.68-4.16) |
5 | 9 (4-17) | Moderate | ||
| 10 mg Daily | 3 | No | No | No | No | No | 221/12 761 (1.7) |
339/12 718 (2.7) |
1.56 (1.31-1.85) |
17 | 9 (5-14) | High | ||
| Apixaban vs aspirin | 1 | No | No | No | Yes | No | 44/2808 (1.6) |
39/2791 (1.4) |
1.12 (0.73-1.73) |
14 | NS | Moderate | ||
Abbreviations: NOACs, non–vitamin K antagonist oral anticoagulants; NS, not significant.
Secondary End Points: Fatal Bleeding and Major Bleeding
Pooling the results from the fixed-effects model showed that a 15 mg to 20 mg dose of rivaroxaban once daily was associated with increased risk of fatal bleeding (2 trials; OR, 2.37 [95% CI, 1.30 to 4.29]; P for heterogeneity = .87),6,22 while a 10-mg dose of rivaroxaban once daily or a 5-mg dose twice daily (3 trials20,21,22; OR, 1.47 [95% CI, 0.72 to 2.97]; P for heterogeneity = .82) and a 5-mg dose of apixaban twice daily (1 trial4; OR, 0.66 [95% CI, 0.19 to 2.35]) were not associated with increased risk. A 15-mg to 20-mg dose of rivaroxaban once daily, compared with aspirin, would cause an additional 4 fatal bleeding events in 1000 patients. The quality of a body of evidence was found to be moderate (Table 2).
Pooling the results from the fixed-effects model showed that both a 15-mg to 20-mg dose of rivaroxaban once daily (2 trials6,22; OR, 2.64 [95% CI, 1.68 to 4.16]; P for heterogeneity = .70) and a 10-mg dose of rivaroxaban once daily or a 5-mg dose twice daily (3 trials20,21,22; OR, 1.56 [95% CI, 1.31 to 1.85]; P for heterogeneity = .87) were associated with an increased risk of major bleeding compared with aspirin, while a 5-mg dose of apixaban twice daily (1 trial4; OR, 1.12 [95% CI, 0.73 to 1.73]) was not associated with increased risks. A 15-mg to 20-mg dose of rivaroxaban once daily and a 10-mg dose once daily or a 5-mg dose twice daily would cause an additional 9 major bleeding events in 1000 patients compared with aspirin use alone. The quality of a body of evidence was found to be moderate to high (Table 2).
Subgroup Analysis
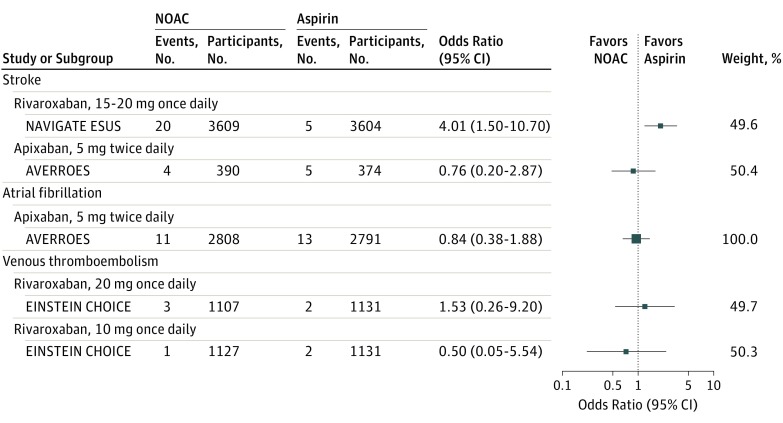
Subgroup analysis for intracranial hemorrhage was presented in Figure 2. For patients with stroke at study entry, a 15-mg dose of rivaroxaban once daily vs aspirin was associated with increased risks of intracranial hemorrhage (OR, 4.01 [95% CI, 1.50 to 10.70]),6 while a 5-mg dose of apixaban twice daily (in a subgroup of stroke patients from the Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment [AVERROES] trial)23 was not associated with increased risks (OR, 0.76 [95% CI, 0.20 to 2.87]). For patients with atrial fibrillation at entry, a 5-mg dose of apixaban twice daily (1 trial4; OR, 0.84 [95% CI, 0.38 to 1.88]) was not associated with increased risk. For patients with venous thromboembolism, both a 20-mg, once-daily dose of rivaroxaban (OR, 1.53 [95% CI, 0.26 to 9.20]) and a 10-mg, once-daily dose (OR, 0.50 [95% CI, 0.05 to 5.54]) were not significantly associated with increased risks of intracranial hemorrhage.22
Figure 2. Odds Ratios of Intracranial Hemorrhage With Use of Individual Non–Vitamin K Antagonist Oral Anticoagulants (NOACs) vs Aspirin, Stratified by Condition at Study Entry.
Results are stratified by A, stroke, B, atrial fibrillation, and C, venous thromboembolism. AVERROES indicates Apixaban Vs Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; EINSTEIN CHOICE, Reduced-dosed Rivaroxaban in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism; NAVIGATE ESUS, Rivaroxaban Vs Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source.
Accessory Analysis
There was no direct comparison of dabigatran or edoxaban vs aspirin. Therefore, 20 trials regarding dabigatran vs warfarin, edoxaban vs warfarin, and aspirin vs warfarin were included for the purpose of obtaining the indirect comparison of dabigatran or edoxaban vs aspirin (eTable 2 in the Supplement). Indirect comparison implied both dabigatran at a dose of 110 mg to 150 mg twice daily and edoxaban at a dose of 30 to 60 mg once daily might be not associated with increased risks of intracranial hemorrhage or fatal bleeding compared with aspirin but might be associated with increased risks of major bleeding (eTable 3 in the Supplement).
Discussion
In this meta-analysis of 5 randomized clinical trials across all indication of high-quality data from almost 40 000 people, we found that a 15-mg to 20-mg dose of rivaroxaban once daily was associated with substantially higher odds of intracranial hemorrhage than aspirin, while a 5-mg dose of apixaban twice daily and a 10-mg dose of rivaroxaban once daily or a 5-mg dose twice daily were not associated with increased risks. The quality of a body of evidence for outcome was moderate. Although the results were derived from a few (even single) large randomized clinical trials, it might provide evidence to inform the potential design and conduct of future NOAC trials beyond atrial fibrillation. Also, indirect comparisons implied that dabigatran in doses of 110 mg to 150 mg twice daily and edoxaban in doses of 30 mg to 60 mg once daily might have comparable risks of intracranial hemorrhage with aspirin, although such results should be interpreted with caution because of the lack of head-to-head comparisons in any randomized clinical trial.
The novelty of this meta-analysis lies in the comparison across all indications of intracranial hemorrhage with individual NOAC vs aspirin. The efficacy and safety of NOACs as a class in patients with atrial fibrillation has been well established.24 However, the risk of intracranial hemorrhage possessed by each individual NOAC is crucial when that NOAC is to be considered in a clinical scenario other than treatment of a patient with atrial fibrillation. For example, several large prospective cohorts have shown that atrial abnormality was associated with increased risks of stroke in patients without recognized atrial fibrillation.9,10,11 Patients with ischemic stroke who do not have atrial fibrillation but do have moderate to severe left atrial enlargement (vs normal left atrial size) also show a trend toward a greater risk of recurrent cardioembolic or cryptogenic stroke.25 Although cardiac emboli are likely to be the cause for stroke, those patients would be treated by aspirin unless atrial fibrillation is recognized. Since a 5-mg dose of apixaban twice daily had comparable risks of intracranial hemorrhage with aspirin, it could be an attractive alternative agent for use on this population. On the other hand, a 15-mg to 20-mg dose of rivaroxaban once daily possessed substantial higher risks of intracranial hemorrhage than aspirin, its use in people without atrial fibrillation could not be encouraged. Although 10 mg of rivaroxaban once daily or 5 mg twice daily did not show higher risks of intracranial hemorrhage than aspirin in this meta-analysis, it is worthwhile to mention that such low-dose rivaroxaban did not reduce major cardiovascular events or stroke compared with aspirin in patients with vascular disease,20,21 and even a 15-mg dose of rivaroxaban was not better than aspirin for recurrent ischemic stroke prevention among embolic stroke patients without recognized atrial fibrillation.6
Limitations
This study has several limitations. First, a meta-analysis may be biased when the literature search fails to identify all relevant trials or the selection criteria for including a trial are applied in a subjective manner. To minimize these risks, we performed thorough searches across multiple literature and trial databases and used explicit criteria for study selection, data abstraction, and data analysis.
Second, the results were derived from a few large clinical trials, and in some cases from single trials, and the quality of a body of evidence was moderate. Also, there is to our knowledge no pairwise comparison in dabigatran or edoxaban vs aspirin in a published randomized clinical trial to date. Ongoing trials with apixaban vs aspirin8 and dabigatran vs aspirin7 in patients with embolic stroke with undetermined source may help to resolve these concerns, at least partially.
Third, intracerebral hemorrhage is known to account for a larger proportion of strokes in Asian vs white populations,26,27 and thus it is important to clarify the risks of intracranial hemorrhage between individual NOACs and aspirin in Asian individuals. However, no trials included in this analysis provided ethnic subgroup analysis, and for this reason, further analysis was not possible.
Finally, in this study-level meta-analysis, we were only able to use data on major bleeding provided by the original trials, which used a variety of differing definitions. Because major bleeding was the secondary end point of this study and the differences in definitions was not substantial, this likely made little difference in the overall results of this meta-analysis.
Conclusion
In conclusion, in this meta-analysis of randomized clinical trials across all indications comparing individual NOACs and aspirin, we found that patients receiving 15 to 20 mg of rivaroxaban once daily had substantially increased risks of intracranial hemorrhage, while patients who took 10 mg of rivaroxaban once daily, 5 mg of rivaroxaban twice daily, or 5 mg of apixaban twice daily had no increased risks. There was no pairwise comparison between dabigatran or edoxaban vs aspirin, but an indirect comparison suggests that taking 110 to 150 mg of dabigatran twice daily or 30 to 60 mg of edoxaban once daily might be not associated with increased risks of intracranial hemorrhage, although such results should be interpreted with caution. Based on these findings, only patients with atrial fibrillation should be considered for administration of 15 to 20 mg of rivaroxaban once daily. In addition, it may be worthwhile to conduct randomized clinical trials comparing certain NOAC dosages (eg, 5 mg of apixaban twice daily) and aspirin in patients who do not have atrial fibrillation but do have potential sources of cardiac emboli that can cause stroke.
eFigure. Study selection
eTable 1. Risk of Bias of Included Trials (New Oral Anticoagulants vs Aspirin)
eTable 2. Characteristics of Included Trials for Accessory Analysis (Dabigatran or Edoxaban vs Warfarin and Aspirin vs Warfarin)
eTable 3. Indirect Comparison of Dabigatran, Edoxaban versus Aspirin in Primary and Secondary Endpoints
References
- 1.Kim HC, Choi DP, Ahn SV, Nam CM, Suh I. Six-year survival and causes of death among stroke patients in Korea. Neuroepidemiology. 2009;32(2):94-100. doi: 10.1159/000177034 [DOI] [PubMed] [Google Scholar]
- 2.Cadilhac DA, Dewey HM, Vos T, Carter R, Thrift AG. The health loss from ischemic stroke and intracerebral hemorrhage: evidence from the North East Melbourne Stroke Incidence Study (NEMESIS). Health Qual Life Outcomes. 2010;8:49. doi: 10.1186/1477-7525-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee HY, Hwang JS, Jeng JS, Wang JD. Quality-adjusted life expectancy (QALE) and loss of QALE for patients with ischemic stroke and intracerebral hemorrhage: a 13-year follow-up. Stroke. 2010;41(4):739-744. doi: 10.1161/STROKEAHA.109.573543 [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Eikelboom J, Joyner C, et al. ; AVERROES Steering Committee and Investigators . Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806-817. doi: 10.1056/NEJMoa1007432 [DOI] [PubMed] [Google Scholar]
- 5.López-López JA, Sterne JAC, Thom HHZ, et al. . Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058. doi: 10.1136/bmj.j5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hart RG, Sharma M, Mundl H, et al. ; NAVIGATE ESUS Investigators . Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191-2201. doi: 10.1056/NEJMoa1802686 [DOI] [PubMed] [Google Scholar]
- 7.Diener HC, Easton JD, Granger CB, et al. ; RE-SPECT ESUS Investigators . Design of randomized, double-blind, evaluation in secondary stroke prevention comparing the efficacy and safety of the oral thrombin inhibitor dabigatran etexilate vs acetylsalicylic acid in patients with embolic stroke of undetermined source (RE-SPECT ESUS). Int J Stroke. 2015;10(8):1309-1312. doi: 10.1111/ijs.12630 [DOI] [PubMed] [Google Scholar]
- 8.Geisler T, Poli S, Meisner C, et al. . Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): rationale and study design. Int J Stroke. 2017;12(9):985-990. doi: 10.1177/1747493016681019 [DOI] [PubMed] [Google Scholar]
- 9.Kamel H, Bartz TM, Elkind MSV, et al. . Atrial cardiopathy and the risk of ischemic stroke in the CHS (cardiovascular health study). Stroke. 2018;49(4):980-986. doi: 10.1161/STROKEAHA.117.020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamel H, Hunter M, Moon YP, et al. . Electrocardiographic left atrial abnormality and risk of stroke: Northern Manhattan study. Stroke. 2015;46(11):3208-3212. doi: 10.1161/STROKEAHA.115.009989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamel H, O’Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the atherosclerosis risk in communities study. Ann Neurol. 2015;78(5):670-678. doi: 10.1002/ana.24482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eikelboom JW, Connolly SJ, Brueckmann M, et al. ; RE-ALIGN Investigators . Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206-1214. doi: 10.1056/NEJMoa1300615 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT; Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Washington, DC: John P Wiley and Sons; 2011. [Google Scholar]
- 15.Ögren J, Irewall AL, Bergström L, Mooe T. Intracranial hemorrhage after ischemic stroke: incidence, time trends, and predictors in a Swedish nationwide cohort of 196 765 patients. Circ Cardiovasc Qual Outcomes. 2015;8(4):413-420. doi: 10.1161/CIRCOUTCOMES.114.001606 [DOI] [PubMed] [Google Scholar]
- 16.Lopes LC, Spencer FA, Neumann I, et al. . Systematic review of observational studies assessing bleeding risk in patients with atrial fibrillation not using anticoagulants. PLoS One. 2014;9(2):e88131. doi: 10.1371/journal.pone.0088131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 21.Anand SS, Bosch J, Eikelboom JW, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 22.Weitz JI, Lensing AWA, Prins MH, et al. ; EINSTEIN CHOICE Investigators . Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211-1222. doi: 10.1056/NEJMoa1700518 [DOI] [PubMed] [Google Scholar]
- 23.Diener HC, Eikelboom J, Connolly SJ, et al. ; AVERROES Steering Committee and Investigators . Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol. 2012;11(3):225-231. doi: 10.1016/S1474-4422(12)70017-0 [DOI] [PubMed] [Google Scholar]
- 24.Cameron C, Coyle D, Richter T, et al. . Systematic review and network meta-analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open. 2014;4(6):e004301. doi: 10.1136/bmjopen-2013-004301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaghi S, Moon YP, Mora-McLaughlin C, et al. . Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015;46(6):1488-1493. doi: 10.1161/STROKEAHA.115.008711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81(3):264-272. doi: 10.1212/WNL.0b013e31829bfde3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feigin V, Carter K, Hackett M, et al. ; Auckland Regional Community Stroke Study Group . Ethnic disparities in incidence of stroke subtypes: Auckland Regional Community Stroke Study, 2002-2003. Lancet Neurol. 2006;5(2):130-139. doi: 10.1016/S1474-4422(05)70325-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Study selection
eTable 1. Risk of Bias of Included Trials (New Oral Anticoagulants vs Aspirin)
eTable 2. Characteristics of Included Trials for Accessory Analysis (Dabigatran or Edoxaban vs Warfarin and Aspirin vs Warfarin)
eTable 3. Indirect Comparison of Dabigatran, Edoxaban versus Aspirin in Primary and Secondary Endpoints