Key Points
Question
Does a single intravesical instillation of gemcitabine reduce risk of recurrence after resection of low-grade non–muscle-invasive urothelial cancer?
Finding
In this randomized clinical trial of 406 patients with suspected low-grade non–muscle-invasive urothelial cancer, intravesical instillation of gemcitabine, compared with saline, significantly reduced the risk of recurrence over a median of 4.0 years (recurrence rate: gemcitabine, 35%; saline, 47%; hazard ratio, 0.66).
Meaning
Intravesical gemcitabine immediately following tumor excision reduced risk of recurrence in patients with suspected low-grade non–muscle-invasive urothelial cancer.
Abstract
Importance
Low-grade non–muscle-invasive urothelial cancer frequently recurs after excision by transurethral resection of bladder tumor (TURBT).
Objective
To determine whether immediate post-TURBT intravesical instillation of gemcitabine reduces recurrence of suspected low-grade non–muscle-invasive urothelial cancer compared with saline.
Design, Setting, and Participants
Randomized double-blind clinical trial conducted at 23 US centers. Patients with suspected low-grade non–muscle-invasive urothelial cancer based on cystoscopic appearance without any high-grade or without more than 2 low-grade urothelial cancer episodes within 18 months before index TURBT were enrolled between January 23, 2008, and August 14, 2012, and followed up every 3 months with cystoscopy and cytology for 2 years and then semiannually for 2 years. Patients were monitored for tumor recurrence, progression to muscle invasion, survival, and toxic effects. The final date of follow-up was August 14, 2016.
Interventions
Participants were randomly assigned to receive intravesical instillation of gemcitabine (2 g in 100 mL of saline) (n = 201) or saline (100 mL) (n = 205) for 1 hour immediately following TURBT.
Main Outcomes and Measures
The primary outcome was time to recurrence of cancer. Secondary end points were time to muscle invasion and death due to any cause.
Results
Among 406 randomized eligible patients (median age, 66 years; 84.7% men), 383 completed the trial. In the intention-to-treat analysis, 67 of 201 patients (4-year estimate, 35%) in the gemcitabine group and 91 of 205 patients (4-year estimate, 47%) in the saline group had cancer recurrence within 4.0 years (hazard ratio, 0.66; 95% CI, 0.48-0.90; P<.001 by 1-sided log-rank test for time to recurrence). Among the 215 patients with low-grade non–muscle-invasive urothelial cancer who underwent TURBT and drug instillation, 34 of 102 patients (4-year estimate, 34%) in the gemcitabine group and 59 of 113 patients (4-year estimate, 54%) in the saline group had cancer recurrence (hazard ratio, 0.53; 95% CI, 0.35-0.81; P = .001 by 1-sided log-rank test for time to recurrence). Fifteen patients had tumors that progressed to muscle invasion (5 in the gemcitabine group and 10 in the saline group; P = .22 by 1-sided log-rank test) and 42 died of any cause (17 in the gemcitabine group and 25 in the saline group; P = .12 by 1-sided log-rank test). There were no grade 4 or 5 adverse events and no significant differences in adverse events of grade 3 or lower.
Conclusions and Relevance
Among patients with suspected low-grade non–muscle-invasive urothelial cancer, immediate postresection intravesical instillation of gemcitabine, compared with instillation of saline, significantly reduced the risk of recurrence over a median of 4.0 years. These findings support using this therapy, but further research is needed to compare gemcitabine with other intravesical agents.
Trial Registration
clinicaltrials.gov Identifier: NCT00445601
This randomized clinical trial compares the effect of immediate postresection intravesical instillation of gemcitabine vs instillation of saline on cancer recurrence in patients with suspected low-grade non–muscle-invasive urothelial cancer.
Introduction
Almost 80 000 patients in the United States were diagnosed as having bladder cancer in 20171; the vast majority of these cancers were urothelial cancer. About 75% of bladder cancers are non–muscle invasive at diagnosis and the majority are histologically low grade.2 Because these tumors often recur after initial resection, monitoring requires frequent cystoscopic examinations, and tumor recurrences usually are treated by repeat transurethral resection of bladder tumor (TURBT). The frequency of these invasive procedures contributes to the cost and morbidity of managing urothelial cancer. Courses of weekly intravesical instillations of chemotherapy or immunotherapy are used to reduce the likelihood of recurrence in patients with frequently recurring, multifocal, or large low-grade or any high-grade non–muscle-invasive urothelial cancer, further increasing morbidity and costs.3,4,5 Thus, urothelial cancer is one of the most costly tumors to treat over a patient’s lifetime.3
Randomized trials have found that a single postoperative intravesical instillation of one of several chemotherapeutic agents, including mitomycin C6,7 and epirubicin,8 reduces the risk of recurrence following TURBT of low-grade non–muscle-invasive urothelial cancer.9 However, despite compelling clinical trial data and guideline recommendations,4,5 these treatments are infrequently used in the United States.10
Gemcitabine (2′,2′-difluorodeoxycytidine) is a chemotherapeutic agent that inhibits DNA synthesis in dividing cells.11 Regimens containing gemcitabine are used systemically to treat muscle-invasive and more advanced urothelial cancer.12,13 Additionally, preliminary evidence suggests that courses of intravesical gemcitabine are safe14 and as effective or more effective than other chemotherapeutic agents for non–muscle-invasive urothelial cancer.14 The SWOG S0337 randomized clinical trial was developed to determine the efficacy of a single intravesical instillation of gemcitabine immediately after TURBT to prevent recurrence of low-grade (grade 1 and grade 2 based on the 1973 World Health Organization classification15), stage Ta or T1 urothelial cancer of the bladder.
Methods
The study was approved by institutional review boards at each of the 23 enrolling sites in the United States and was conducted according to the Declaration of Helsinki guidelines. All patients provided written informed consent. The study protocol is available in the Supplement.
Eligible patients were those with suspected low-grade papillary urothelial cancer based on tumor appearance on office cystoscopy performed because of symptoms indicative of bladder cancer (eg, hematuria) or during surveillance for previously treated non–muscle-invasive urothelial cancer. Patients with any prior nonurothelial or muscle-invasive bladder cancer were not eligible. Patients were also not eligible if within 18 months before the index TURBT, they had any high-grade or more than 2 low-grade non–muscle-invasive urothelial cancer episodes or had received intravesical therapy within 6 months. Patients with previous or concurrent upper urinary tract or prostatic urethral urothelial cancer, previous pelvic radiotherapy for any malignancy, or prior treatment for any malignancy within 5 years other than nonmelanoma skin cancer or non–muscle-invasive bladder urothelial cancer were not eligible. Entry requirements included a serum creatinine level less than 2.2 mg/dL (194 mmol/L) and serum bilirubin, alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase levels below 2 times the institution’s upper limits of normal; adequate hematologic function (hematocrit >35% and <52%; white blood cell count ≥3000/μL; platelet count >75 000/μL and <500 000/μL); Eastern Cooperative Oncology Group performance status score20 of 1 or lower; uninfected urine; and normal upper urinary tract imaging findings (for malignancy) within 1 year before the index TURBT.
Patients were randomized to receive 2 g of gemcitabine in 100 mL of saline vs 100 mL saline alone within 3 hours after TURBT (Figure 1). Patients and physicians were blinded to treatment assignment. Urethral catheters were unclamped after 1 hour of instillation or sooner if patients experienced significant discomfort. As specified in the protocol, drug instillation could be withheld if a treating surgeon thought that bladder perforation, deep or extensive resection, or postoperative hematuria led to unacceptable risk. To replicate standard care in the United States (both to facilitate accrual and to maximize the study’s relevance), in-office biopsy before TURBT was not permitted, and overnight hospital admission for continuous bladder irrigation after TURBT was neither mandated nor encouraged but was permitted if investigators thought it was medically warranted.
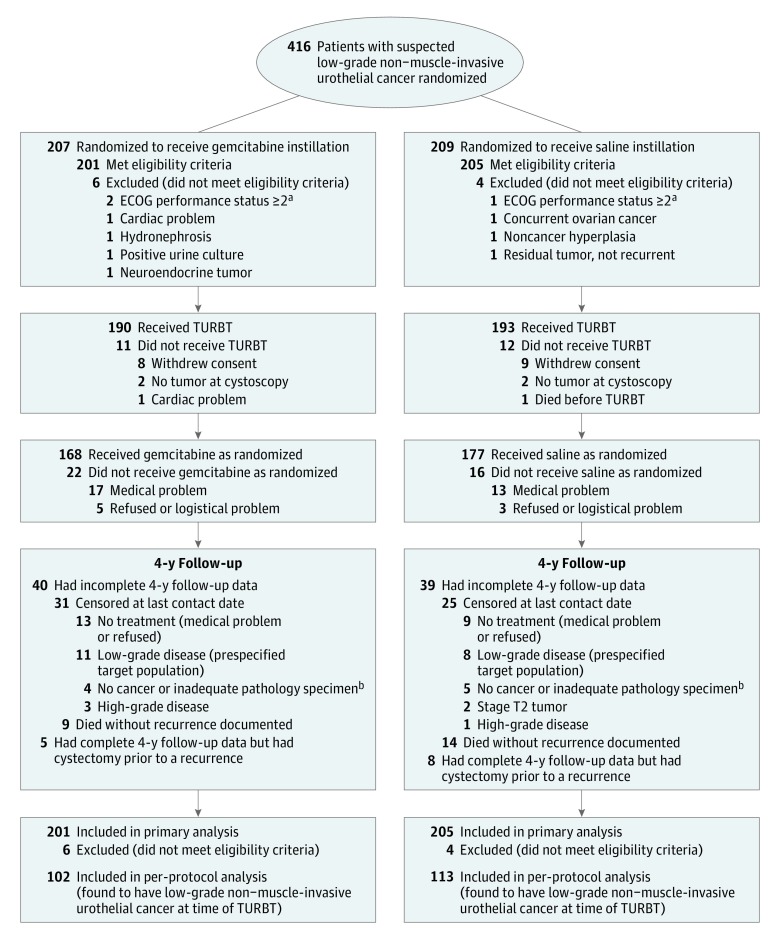
Figure 1. Participant Flow Through the SWOG S0337 Randomized Clinical Trial.
No data are available for number of patients approached to enroll in the trial; 23 sites participated. TURBT indicates transurethral resection of bladder tumor.
aEastern Cooperative Oncology Group (ECOG) performance status scores20: 0 = fully active, able to carry on all predisease performance without restriction; 1 = restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; eg, light housework, office work.
bInadequate pathology specimen indicates that based on scantiness of the specimen, the pathologist was unable to assess to unequivocally diagnose a benign condition or bladder cancer.
Patients were followed up for 4 years, with cystoscopy and cytology quarterly for the first 2 years and semiannually for 2 more years. This time horizon was prespecified in the protocol and was chosen because in several studies published during the time of the SWOG S0337 trial’s development (2003), the major benefit of post-TURBT single-instillation chemotherapy on recurrence occurred before then.6,7,16 Recurrences had to be confirmed histologically. Management following recurrence was at physician discretion. Similarly, if on the index TURBT no cancer was found or if high-grade urothelial cancer, nonurothelial bladder cancer, or muscle-invasive cancer was diagnosed, management was based on physician discretion but follow-up for disease progression to muscle invasion and overall survival continued for 4 years. Patients were accrued from January 23, 2008, through August 14, 2012, with the last follow-up occurring on August 14, 2016.
Statistical Analysis
It was estimated that 10% of enrolled patients would not have the expected histology (low-grade stage Ta or T1 urothelial cancer) because eligibility was based on cystoscopic appearance, not confirmatory histology.17 Also, it was anticipated that another 10% would not receive study drug instillation because of surgeon discretion.18
Patients were randomized in a blinded 1:1 fashion to receive gemcitabine or saline with dynamic balancing for 2 stratification factors: disease status (newly diagnosed vs recurrent) and number of lesions (single vs multiple). The primary end point was time to recurrence, where death or cystectomy without recurrence were managed as competing risks in a cumulative incidence analysis. Censoring occurred at the last contact date. A 2-year 60% recurrence rate was assumed for the saline group.19 Assuming exponential distribution, 2 years of accrual and 2 additional years of follow-up, and a 1-sided α = .025 with a stratified log-rank test, there would be 89% power to detect a hazard ratio (HR) of 0.65, which translates into an absolute decrease of 15% in recurrence rates at 2 years.9 The final analysis was to occur when 226 recurrences had been reported or after 4 years of maximum follow-up for all patients, at the 1-sided α = .02 level to account for interim testing. The primary analysis was based on a modified intention to treat, including all eligible patients randomized. A prespecified subgroup analysis was based on patients who had low-grade non–muscle-invasive urothelial cancer at TURBT and received drug instillation. Prespecified secondary end points included time to muscle invasion and death due to any cause. As prespecified in the protocol, all P values are 1-sided. Hazard ratios adjusted for stratification factors (disease status and number of lesions) as covariates and 95% confidence intervals are reported for descriptive purposes. SAS version 9.4 statistical software (SAS Institute Inc) was used.
These were 2 post hoc analyses. A Wald χ2 analysis was used to evaluate each stratification factor’s interaction with treatment in the Cox model for time to recurrence, and a stratified log-rank test for time to recurrence was applied to the subgroup with high-grade non–muscle-invasive urothelial cancer at index TURBT. Because there were 23 sites and 42 enrolling investigators, center effects are not accounted for in the analyses.
Results
During the 4.5 years of accrual, 416 patients were randomized at 23 participating centers; 10 did not meet entry criteria, resulting in 406 eligible patients (201 in the gemcitabine group and 205 in the saline group) who were well matched in demographics, bladder tumor histories, and index tumor characteristics (Table 1). Transurethral resection of bladder tumor was performed in 383 patients (190 in the gemcitabine group and 193 in the saline group); 168 and 177 patients received randomized drug instillation, respectively (Figure 1). Reasons for not receiving the instillation included depth of TURBT (n = 20), resection extent (n = 9), hematuria (n = 1), and withdrawal of consent and other logistical reasons (n = 8). Two prespecified interim analyses were conducted. In each case, the SWOG data and safety monitoring committee recommended that the trial continue as planned.
Table 1. Baseline Characteristics of All Randomized, Eligible Participants.
| Characteristics | No. (%) of Participantsa | |
|---|---|---|
| Gemcitabine Group (n = 201) | Saline Group (n = 205) | |
| Age, median (IQR), y | 66 (59-74) | 66 (59-75) |
| Male sex | 163 (81) | 181 (88) |
| Race | ||
| White | 186 (93) | 185 (90) |
| Black | 6 (3) | 9 (4) |
| Asian | 4 (2) | 5 (2) |
| American Indian | 0 | 2 (1) |
| Unknown | 5 (2) | 4 (2) |
| ECOG performance status, 0 (vs 1)c | 157 (78) | 165 (80) |
| Smoking history | ||
| Current | 49 (24) | 54 (26) |
| Prior | 98 (49) | 101 (49) |
| Never | 54 (27) | 46 (22) |
| Unknown | 0 | 4 (2) |
| Occurrence, first (vs recurrent) | 128 (64) | 128 (62) |
| No. of tumors at index TURBT, 1 (vs ≥2) | 135 (67) | 140 (68) |
| Pathologic findings among patients who received TURBT and drug instillation | (n = 168) | (n = 177) |
| No cancer or inadequate pathology specimenb | 17 (10) | 14 (8) |
| Stage T2 tumor | 5 (3) | 8 (5) |
| High-grade non–muscle-invasive disease | 44 (26) | 42 (24) |
| Low-grade non–muscle-invasive disease | 102 (61) | 113 (64) |
| Prior intravesical therapy | ||
| All types | 39 (19) | 39 (19) |
| Bacille Calmette-Guérin | 18 | 25 |
| Adriamycin | 1 | 0 |
| Mitomycin C | 1 | 2 |
| Not specified | 19 | 12 |
Abbreviations: IQR, interquartile range; TURBT: transurethral resection of bladder tumor.
Data are expressed as No. (%) of participants unless otherwise indicated.
Inadequate pathology specimen indicates that based on scantiness of the specimen, the pathologist was unable to assess to unequivocally diagnose a benign condition or bladder cancer.
Eastern Cooperative Oncology Group (ECOG) performance status scores20: 0 = fully active, able to carry on all predisease performance without restriction; 1 = restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; eg, light housework, office work.
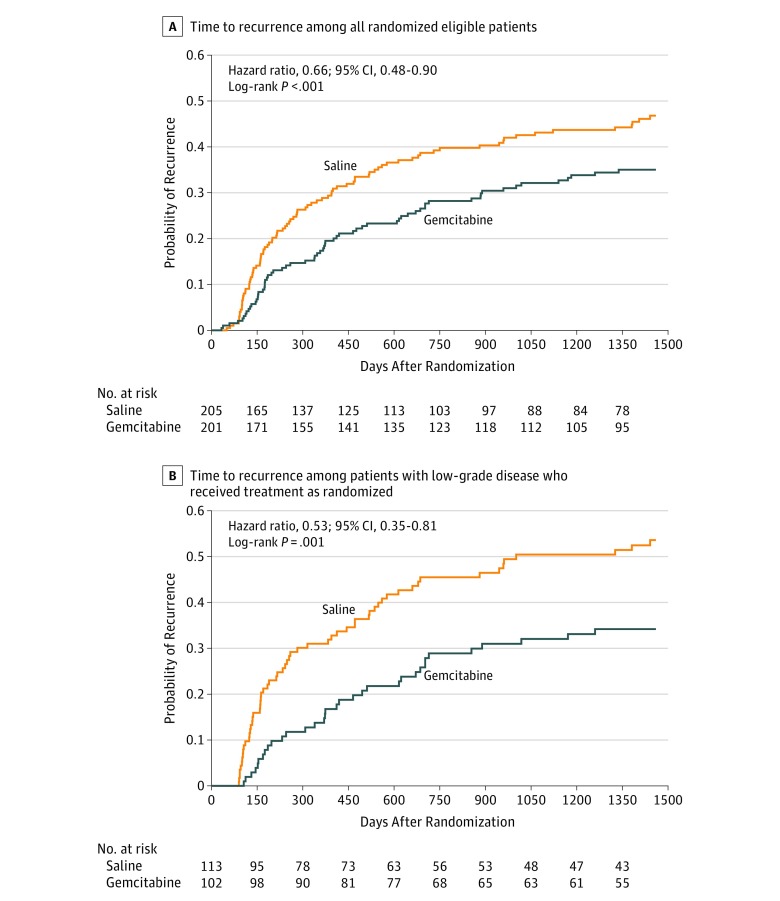
Of the 201 patients randomized to receive gemcitabine and 205 to receive saline in the intention-to-treat analysis, 67 patients in the gemcitabine group (4-year estimate, 35%) and 91 patients in the saline group (4-year estimate, 47%) experienced a recurrence by 4-year median follow-up (HR, 0.66; 95% CI, 0.48-0.90; P<.001 by 1-sided stratified log-rank test for time to recurrence) (Table 2 and Figure 2A). In the protocol’s prespecified target population (patients found to have low-grade non–muscle-invasive urothelial cancer), 34 of 102 patients receiving gemcitabine (4-year estimate, 34%) and 59 of 113 patients receiving saline (4-year estimate, 54%) had recurrences (HR, 0.53; 95% CI, 0.35-0.81; P = .001 by 1-sided stratified log-rank test for time to recurrence) (Table 2 and Figure 2B). First recurrences were high grade in 6 (5.9%) target population patients treated with gemcitabine and in 10 (8.8%) target population patients treated with saline, and the remaining recurrences were low grade. Gemcitabine’s efficacy did not vary by stratification factor (2-sided test of interaction with treatment: newly diagnosed vs recurrent, P = .25; solitary vs multiple, P = .14). Fifteen patients in the intention-to-treat cohort (n = 406) had progression to muscle-invasive urothelial cancer (5 in the gemcitabine group and 10 in the saline group; HR, 0.51; 95% CI, 0.17-1.49; P = .11 by 1-sided log-rank test). Among the 5 patients receiving gemcitabine in whom disease progressed, 3 tumors were high grade and 2 were low grade, while among the 10 patients receiving saline in whom disease progressed, 6 tumors were high grade, 3 were low grade, and 1 was not cancer. Forty-two participants died of any cause (17 in the gemcitabine group and 25 in the saline group; HR, 0.68; 95% CI, 0.37-1.27; P = .12 by 1-sided log-rank test) over the 4 years of follow-up (Table 2).
Table 2. Primary and Secondary Analysis Comparisons by Treatment Group.
| Outcomes and Populations | Gemcitabine Group | Saline Group | Hazard Ratio (95% CI)c | P Value by 1-Sided Log-Rank Test | ||
|---|---|---|---|---|---|---|
| No. With Outcome/Total No.a | 4-y Recurrence Rate, % (95% CI)b | No. With Outcome/Total No.a | 4-y Recurrence Rate, % (95% CI)b | |||
| Primary Outcome and Primary Population | ||||||
| Recurrence among all randomized, eligible patients (intention-to-treat population) | 67/201 | 35 (29-42) | 91/205 | 47 (41-54) | 0.66 (0.48-0.90) | <.001d |
| Secondary Populations | ||||||
| Recurrence among all patients who received instillation and had low-grade non–muscle-invasive disease | 34/102 | 34 (26-44) | 59/113 | 54 (45-65) | 0.53 (0.35-0.81) | .001d |
| Recurrence among all patients who received instillation and had high-grade non–muscle-invasive disease | 17/44 | 40 (27-58) | 19/42 | 45 (32-63) | 0.84 (0.45-1.60) | .38d |
| Secondary Outcomes | ||||||
| Muscle invasion in intention-to-treat population | 5/201 | 10/205 | 0.51 (0.17-1.49) | .11 | ||
| Death due to any cause in intention-to-treat population | 17/201 | 25/205 | 0.68 (0.37-1.27) | .12 | ||
Only a first recurrence for a given patient was counted; number of recurrences represents the number of individuals with a first recurrence.
Four-year event rates were estimated from cumulative incidence curves in which either cystectomy or death prior to recurrence was managed as a competing risk.
Hazard ratios are adjusted for stratification factors as covariates except for time to muscle-invasive disease and survival, which had no adjustment because of low event rates.
Stratified by primary vs recurrent tumor and 1 vs 2 or more tumors.
Figure 2. Time to Recurrence of Bladder Cancer.
A, Cumulative incidence of recurrence in the primary analysis by randomized treatment group. All randomized patients who met eligibility criteria were included. B, Cumulative incidence of recurrence by randomized treatment group among patients who were found to have low-grade non–muscle-invasive urothelial cancer at transurethral resection of bladder tumor and received instillation as randomized.
In a post hoc analysis, of the 86 patients with high-grade non–muscle-invasive urothelial cancer or carcinoma in situ who received post-TURBT study drug instillation, 17 of 44 (4-year estimate, 40%) receiving gemcitabine and 19 of 42 (4-year estimate, 45%) receiving saline experienced recurrence (HR, 0.84; 95% CI, 0.45-1.60; P = .38 by 1-sided log-rank test for time to recurrence) (Table 2). Many of these patients underwent additional treatments after the index TURBT, including repeat TURBT within 6 weeks (n = 18), an induction course of intravesical bacille Calmette-Guérin within 4 months (n = 52 [the number is probably more than reported because site investigators were not required to submit this information]), and cystectomy within 6 months (n = 4). These reported secondary treatments were similarly distributed in the treatment groups.
Intravesical gemcitabine was well tolerated, with no grade 4 or 5 toxic effects in either group and similar grade 3 adverse events (4 [2.4%] in the gemcitabine group and 6 [3.4%] in the saline group; P = .29 by 1-sided χ2 test). Grade 1 and 2 adverse events also were similarly distributed in both groups (Table 3).
Table 3. Adverse Events Reported Among Patients Who Underwent TURBT and Received Instillationa.
| Adverse Events | No. of Events | |||
|---|---|---|---|---|
| Gemcitabine Group (n = 165) | Saline Group (n = 175) | |||
| Grade 1-2 | Grade 3 | Grade 1-2 | Grade 3 | |
| Voiding dysfunction | 31 | 0 | 32 | 3 |
| Voiding pain/sexual pain | 26 | 0 | 23 | 2 |
| Hematuria | 12 | 3 | 14 | 1 |
| Gastrointestinal | 8 | 0 | 4 | 0 |
| Hematologic | 5 | 0 | 5 | 0 |
| Flu-like/other syndromes | 3 | 0 | 4 | 0 |
| Pain (not urologic or gastrointestinal) | 5 | 0 | 1 | 0 |
| Allergy/dermatologic | 4 | 0 | 2 | 0 |
| Genitourinary infection/perforation | 1 | 0 | 4 | 0 |
| Metabolic/mood alteration | 2 | 0 | 1 | 0 |
| Infection/pulmonary | 0 | 1 | 1 | 0 |
| Maximum grade, No. of patients | 53 | 4 | 47 | 6 |
Abbreviation: TURBT: transurethral resection of bladder tumor.
Patients could have had more than 1 adverse event. There were no grade 4 or grade 5 adverse events in patients in either group who had adverse event data reported. Three patients in the gemcitabine group and 2 patients in the saline group did not have adverse events evaluated and reported. Grade 1 indicates mild (such as minimal hematuria or urinary frequency that resolved either without treatment or with temporary changes of behavior such as drinking more fluid or reducing activity); grade 2, moderate (such as symptomatic bacteruria treated with an oral antibiotic); grade 3, severe (such as hematuria which required hospitalization for catheter irrigation); grade 4, life threatening/disabling; and grade 5, death.21
Discussion
In this randomized clinical trial of patients with suspected low-grade non–muscle-invasive urothelial cancer, immediate postresection instillation of intravesical gemcitabine, compared with intravesical saline, significantly reduced the risk of cancer recurrence over a median of 4.0 years among the intention-to-treat population (HR, 0.66 [absolute reduction in recurrence of 12% at 4 years]) and among the target population of patients found to have low-grade non–muscle-invasive urothelial cancer (HR, 0.53 [absolute reduction in recurrence of 20% at 4 years]). To our knowledge, this phase 3 trial is the first to demonstrate a significant benefit for a single postoperative instillation of a new agent in more than 2 decades.6,8,16 These findings support the European Association of Urology and American Urological Association recommendations4,5 and provide a new, readily available drug for patients with bladder cancer.
Although patients with these cancers rarely experience progression to muscle invasion or die of urothelial cancer, the frequent disease recurrences9,19 cause significant morbidity and result in a high cost of care.3 Full anesthesia is usually required for TURBT, in patients often burdened by other comorbidities.22 Following TURBT, an indwelling urethral catheter may remain for several days, and patients can experience irritable voiding symptoms, hematuria, and, occasionally, more serious complications. Additional courses of intravesical therapy are administered to patients with large, multifocal, or frequently recurring low-grade or any high-grade non–muscle-invasive urothelial cancer.4,5
Despite compelling evidence supporting immediate postoperative intravesical chemotherapy, US urologists infrequently use this therapy.10 Examination of a national database and questionnaires given to a national sample of urologists shows that as little as 1% to 16% of appropriate patients receive this treatment.10,23 Although some patients do not undergo the treatment because of medical contraindications, even with physician education and no logistical barriers to administration, less than 35% of patients similar to the SWOG S0337 target population receive this treatment.24 Largely explaining this pattern, particularly for use of mitomycin C, are problems with drug availability,24 expense,25 and toxic effects, including prolonged chemical cystitis, dystrophic bladder calcifications and eschars, and perivesical inflammation and fibrosis.18 It was for these reasons (and the much larger sample size required) that a direct comparison with mitomycin in a 2- or 3-group trial was not conducted. With establishment of efficacy of a readily available, less costly, and less toxic agent, greater adoption of this treatment is anticipated.
A previous study tested immediate postoperative intravesical gemcitabine in a similar patient population.26 Although the drug was found to be safe and well tolerated, efficacy was not demonstrated.26 Major differences between the previous and current trials include the longer 60-minute retention of gemcitabine in SWOG S0337 compared with a 35-minute dwell time and that both groups in the previous trial received 20 hours of continuous saline bladder irrigations after study drug instillation. Although prolonged irrigation of saline has been reported to be beneficial in nonrandomized comparisons,9 the one randomized study assessing its benefits was published only in abstract form and reported a modest 6% improvement in recurrence-free rate at 2 years for saline irrigations, a difference that did not reach statistical significance (HR, 0.83; 95% CI, 0.69-1.0; P = .05).27 Moreover, 20 hours of saline irrigations may have washed out any remnants of gemcitabine that persisted after the initial instillation, possibly eliminating some of gemcitabine’s benefits.28 Gemcitabine is a pyrimidine analog that interferes with DNA synthesis and is believed to act only during the S phase of the cell cycle26; as such, longer residual drug exposure may be necessary to affect all cells during DNA replication.26 In support of this possibility are observations that the effects of gemcitabine on depleting deoxynucleotide triphosphate pools in vivo begin during the first 30 minutes but reach their maximal effect in 2 hours.26 Another difference between the 2 studies that could have mitigated gemcitabine’s effect was that 25% of participants in the earlier trial underwent immediate repeat TURBT26 compared with 4.4% in SWOG S0337.
Immediate post-TURBT intravesical chemotherapy prevents tumor cell implantation that is facilitated by traumatic pertubations of the TURBT.29 This treatment may also eliminate unrecognized small cancers or precancerous cells present in the urothelium at the time of TURBT.30 That such poorly visualized cancers exist is well documented, particularly with improvement in detection rate and recurrence-free survival reported for methods that make them more apparent than they are with standard white light cystoscopy, such as fluorescence cystoscopy30,31 or narrow band imaging.32,33
A higher proportion of patients in SWOG S0337 had high-grade urothelial cancer (21.2%) than was originally predicted (approximately 10%). This may be due in part to the 2003 reclassification from grade 1, 2, and 3 urothelial cancer15 to papillary urothelial neoplasm of low malignant potential (PUNLMP), low-grade urothelial cancer, and high-grade urothelial cancer.34 In SWOG S0337, PUNLMPs were grouped together with low-grade cancers (in the roughly 50% of patients graded by the 2003 classification, only 5 PUNLMPs occurred in each group), but the new grading system was adopted at participating institutions during the conduct of SWOG S0337, resulting in some cancers that would have been grade 2 lesions in the 1973 system being classified as high grade.
In an underpowered post hoc subgroup analysis, there was no evidence of a benefit of immediate post-TURBT gemcitabine in patients with high-grade non–muscle-invasive urothelial cancer. Likely contributing to this result was the administration of subsequent courses of intravesical therapies (and at times other treatments), which most SWOG S0337 patients with high-grade non–muscle-invasive urothelial cancer in both treatment groups received, possibly diminishing the effect of a single instillation of gemcitabine.35,36 A lack of benefit for patients with high-grade tumors is consistent with findings of other clinical trials of immediate post-TURBT intravesical chemotherapy.9,26
This study demonstrated that an immediate postoperative instillation of gemcitabine significantly reduced recurrence in patients with newly diagnosed or occasionally recurrent low-grade non–muscle-invasive urothelial cancer. Gemcitabine is well tolerated and readily available. Conversely, there have been significant drug shortages and large increases in the cost of mitomycin C,24,25 the agent most commonly used for this indication in the United States.5,6,7 Compared with mitomycin, gemcitabine is considerably less expensive (average sales price for 2 g of gemcitabine is $55.70 and for 40 mg of mitomycin is $1062.72).37 Additionally, mitomycin appears to have greater toxicity than gemcitabine when administered intravesically.14 In SWOG S0337, almost all toxic effects in each group seemed to be due to TURBT alone (Table 3).
Although it is difficult to compare treatment efficacies between different studies, there is no evidence to our knowledge that mitomycin C is more effective than gemcitabine was in SWOG S0337.6,7 A recently published study comparing a single instillation of mitomycin within 24 hours of TURBT with a single instillation 2 weeks later failed to find a significant improvement in recurrence-free survival with immediate post-TURBT mitomycin in the prespecified group of participants with low-risk disease (n = 510; similar to the target population of SWOG S0337); there was a 43% rate of 5-year recurrence with immediate instillation vs 46% with delayed instillation (P = .11).38
Limitations
This study had several limitations. First, less than 60% of enrolled patients had the expected low-grade non–muscle-invasive urothelial cancer histology. We had anticipated a lower rate of inaccurate grade assessments based on cystoscopic appearance of the index tumor,17 possibly explained by some patients with grade 2 cancers being classified as high grade, and an additional 13% had either no cancer or muscle-invasive cancers on their index TURBT (similar to the proportion seen in other trials).6,26,39 Although it is possible that had a biopsy been performed during the diagnostic cystoscopy preceding the index TURBT, the proportion of patients with high-grade cancers would have been less, this was not done in SWOG S0337 because it is not part of standard care, particularly for newly diagnosed tumors.5 Moreover, since cautery is often needed to control bleeding, this might confound interpretation of histology of index TURBT specimens and results of treatment. Additionally, 10% of patients did not receive study drug instillation (generally because of medical factors), a proportion similar to previous reports.7,24,26
Second, information about tumor size could not be reliably obtained from pathology or operative reports and was not required at entry. Also, treatment at recurrence and subsequently was not reliably captured. These factors can affect subsequent tumor progression4,5 and are not known, although arguably the 2 most important outcomes, progression to muscle invasion and death, were monitored for.
Third, mirroring standard practice, we did not have central pathology review. Institutional pathologists of varying levels of experience and training reviewed cases, although most came from sizable institutions where consensus conferences were held to render all diagnoses.
Fourth, we anticipated a higher rate of recurrence in the saline group based on a study conducted at the time of protocol development that randomized patients with grade 1 or 2 non–muscle-invasive urothelial cancer to difluoromethylornithine or placebo starting weeks after index TURBT.19 In that study, pathologic status was known before entry so that patients with high-grade, muscle-invasive, or no cancers were not included, and these groups represented more than 34% of patients accrued to the SWOG S0337 trial. However, for patients with low-grade urothelial cancers who received saline in SWOG S0337, the recurrence rate approached that seen in the study of difluoromethylornithine.19
Conclusions
Among patients with suspected low-grade non–muscle-invasive urothelial cancer, immediate postresection intravesical instillation of gemcitabine, compared with instillation of saline, significantly reduced the risk of recurrence over a median of 4.0 years. These findings support using this therapy, but further research is needed to compare gemcitabine with other intravesical agents.
Trial Protocol
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Messing EM, Madeb R, Young T, et al. . Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006;107(9):2173-2179. [DOI] [PubMed] [Google Scholar]
- 3.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315-1330. [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M, Böhle A, Burger M, et al. . EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447-461. [DOI] [PubMed] [Google Scholar]
- 5.Chang SS, Boorjian SA, Chou R, et al. . Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196(4):1021-1029. [DOI] [PubMed] [Google Scholar]
- 6.Tolley DA, Parmar MK, Grigor KM, et al. . The effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: a further report with 7 years of follow up. J Urol. 1996;155(4):1233-1238. [PubMed] [Google Scholar]
- 7.Solsona E, Iborra I, Ricós JV, Monrós JL, Casanova J, Dumont R. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term followup. J Urol. 1999;161(4):1120-1123. [PubMed] [Google Scholar]
- 8.Oosterlinck W, Kurth KH, Schröder F, Bultinck J, Hammond B, Sylvester R. A prospective European Organization for Research and Treatment of Cancer Genitourinary Group randomized trial comparing transurethral resection followed by a single intravesical instillation of epirubicin or water in single stage Ta, T1 papillary carcinoma of the bladder. J Urol. 1993;149(4):749-752. [DOI] [PubMed] [Google Scholar]
- 9.Sylvester R, Oosterlinck W, Holmang S, et al. . Systematic review and individual patient data meta-analysis of randomized trials comparing a single immediate instillation of chemotherapy after transurethral resection with transurethral resection alone in patients with stage pTa-pT1 urothelial carcinoma of the bladder: which patients benefit from the instillation? Eur Urol. 2016;69(2):231-244. [DOI] [PubMed] [Google Scholar]
- 10.Cookson MS, Chang SS, Oefelein MG, Gallagher JR, Schwartz B, Heap K. National practice patterns for immediate postoperative instillation of chemotherapy in nonmuscle invasive bladder cancer. J Urol. 2012;187(5):1571-1576. [DOI] [PubMed] [Google Scholar]
- 11.von der Maase H. Current and future perspectives in advanced bladder cancer: is there a new standard? Semin Oncol. 2002;29(1)(suppl 3):3-14. [DOI] [PubMed] [Google Scholar]
- 12.von der Maase H, Hansen SW, Roberts JT, et al. . Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068-3077. [DOI] [PubMed] [Google Scholar]
- 13.Scosyrev E, Messing EM, van Wijngaarden E, et al. . Neoadjuvant gemcitabine and cisplatin chemotherapy for locally advanced urothelial cancer of the bladder. Cancer. 2012;118(1):72-81. [DOI] [PubMed] [Google Scholar]
- 14.Addeo R, Caraglia M, Bellini S, et al. . Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol. 2010;28(4):543-548. [DOI] [PubMed] [Google Scholar]
- 15.Mostofi FK, Sobin LH, Torloni H, et al. . Histological Typing of Urinary Bladder Tumours. Geneva, Switzerland: World Health Organization; 1973. [Google Scholar]
- 16.Burnand KG, Boyd PJ, Mayo ME, Shuttleworth KE, Lloyd-Davies RW. Single dose intravesical thiotepa as an adjuvant to cystodiathermy in the treatment of transitional cell bladder carcinoma. Br J Urol. 1976;48(1):55-59. [DOI] [PubMed] [Google Scholar]
- 17.Cina SJ, Epstein JI, Endrizzi JM, Harmon WJ, Seay TM, Schoenberg MP. Correlation of cystoscopic impression with histologic diagnosis of biopsy specimens of the bladder. Hum Pathol. 2001;32(6):630-637. [DOI] [PubMed] [Google Scholar]
- 18.Filson CP, Montgomery JS, Dailey SM, et al. . Complications associated with single-dose, perioperative mitomycin-C for patients undergoing bladder tumor resection. Urol Oncol. 2014;32(1):40.e1-40.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messing E, Kim KM, Sharkey F, et al. . Randomized prospective phase III trial of difluoromethylornithine vs placebo in preventing recurrence of completely resected low risk superficial bladder cancer. J Urol. 2006;176(2):500-504. [DOI] [PubMed] [Google Scholar]
- 20.Oken MM, Creech RH, Tormey DC, et al. . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. [PubMed] [Google Scholar]
- 21.Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events v3.0 (CTCAE) August 9, 2006. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 13, 2018.
- 22.Scosyrev E, Golijanin D, Wu G, Messing E. The burden of bladder cancer in men and women: analysis of the years of life lost. BJU Int. 2012;109(1):57-62. [DOI] [PubMed] [Google Scholar]
- 23.Madeb R, Golijanin D, Noyes K, et al. . Treatment of nonmuscle invading bladder cancer: do physicians in the United States practice evidence based medicine? the use and economic implications of intravesical chemotherapy after transurethral resection of bladder tumors. Cancer. 2009;115(12):2660-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barocas DA, Liu A, Burks FN, et al. . Practice based collaboration to improve the use of immediate intravesical therapy after resection of nonmuscle invasive bladder cancer. J Urol. 2013;190(6):2011-2016. [DOI] [PubMed] [Google Scholar]
- 25.Davies BJ, Hwang TJ, Kesselheim AS. Ensuring access to injectable generic drugs—the case of intravesical BCG for bladder cancer. N Engl J Med. 2017;376(15):1401-1403. [DOI] [PubMed] [Google Scholar]
- 26.Böhle A, Leyh H, Frei C, et al. ; S274 Study Group . Single postoperative instillation of gemcitabine in patients with non-muscle-invasive transitional cell carcinoma of the bladder: a randomised, double-blind, placebo-controlled phase III multicentre study. Eur Urol. 2009;56(3):495-503. [DOI] [PubMed] [Google Scholar]
- 27.Whelan P, Griffiths G, Stower M, et al. . Preliminary results of a MRC randomized controlled trial of post-operative irrigation of superficial bladder cancer. Proc Am Soc Clin Oncol. 2001;20:708. [Google Scholar]
- 28.Heinemann V, Hertel LW, Grindey GB, Plunkett W. Comparison of the cellular pharmacokinetics and toxicity of 2′,2′-difluorodeoxycytidine and 1-β-D-arabinofuranosylcytosine. Cancer Res. 1988;48(14):4024-4031. [PubMed] [Google Scholar]
- 29.See WA, Rohlf DP, Crist SA. In vitro particulate adherence to fibronectin: correlation with in vivo particulate adherence to sites of bladder injury. J Urol. 1992;147(5):1416-1423. [DOI] [PubMed] [Google Scholar]
- 30.Grossman HB, Stenzl A, Fradet Y, et al. . Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. J Urol. 2012;188(1):58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rink M, Babjuk M, Catto JW, et al. . Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: a critical review of the current literature. Eur Urol. 2013;64(4):624-638. [DOI] [PubMed] [Google Scholar]
- 32.Naito S, Algaba F, Babjuk M, et al. ; CROES Narrow Band Imaging Global Study Group . The Clinical Research Office of the Endourological Society (CROES) multicentre randomised trial of narrow band imaging-assisted transurethral resection of bladder tumour (TURBT) versus conventional white light imaging-assisted TURBT in primary non-muscle-invasive bladder cancer patients: trial protocol and 1-year results. Eur Urol. 2016;70(3):506-515. [DOI] [PubMed] [Google Scholar]
- 33.Xiong Y, Li J, Ma S, et al. . A meta-analysis of narrow band imaging for the diagnosis and therapeutic outcome of non-muscle invasive bladder cancer. PLoS One. 2017;12(2):e0170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montironi R, Lopez-Beltran A, Mazzucchelli R, Bostwick DG. Classification and grading of the non-invasive urothelial neoplasms: recent advances and controversies. J Clin Pathol. 2003;56(2):91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guérin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169(1):90-95. [DOI] [PubMed] [Google Scholar]
- 36.Cai T, Nesi G, Tinacci G, et al. . Can early single dose instillation of epirubicin improve bacillus Calmette-Guérin efficacy in patients with nonmuscle invasive high risk bladder cancer? results from a prospective, randomized, double-blind controlled study. J Urol. 2008;180(1):110-115. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Medicare & Medicaid Services 2018 ASP Drug Pricing Files. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2018ASPFiles.html. Accessed April 17, 2018.
- 38.Bosschieter J, Nieuwenhuijzen JA, van Ginkel T, et al. . Value of an immediate intravesical instillation of mitomycin C in patients with non-muscle invasive bladder cancer: a prospective multicenter randomized study in 2243 patients. Eur Urol. 2018;73(2):226-232. [DOI] [PubMed] [Google Scholar]
- 39.Rajala P, Liukkonen T, Raitanen M, et al. . Transurethral resection with perioperative instillation on interferon-alpha or epirubicin for the prophylaxis of recurrent primary superficial bladder cancer: a prospective randomized multicenter study—Finnbladder III. J Urol. 1999;161(4):1133-1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol