Key Points
Question
Does either negative pressure wound therapy or standard wound dressing result in less disability 12 months after sustaining an open fracture of the lower limb?
Findings
In this randomized clinical trial that included 460 adults, there was no statistically significant difference in self-rated disability between negative pressure wound therapy or standard wound dressing at 12 months (45.5 vs 42.4 points of a possible 100).
Meaning
Negative pressure wound therapy did not improve 12-month disability for patients with severe open fracture of the lower limb compared with standard wound dressing.
Abstract
Importance
Open fractures of the lower limb occur when a broken bone penetrates the skin. There can be major complications from these fractures, which can be life-changing.
Objectives
To assess the disability, rate of deep infection, and quality of life in patients with severe open fracture of the lower limb treated with negative pressure wound therapy (NPWT) vs standard wound management after the first surgical debridement of the wound.
Design, Setting, and Participants
Multicenter randomized trial performed in the UK Major Trauma Network, recruiting 460 patients aged 16 years or older with a severe open fracture of the lower limb from July 2012 through December 2015. Final outcome data were collected through November 2016. Exclusions were presentation more than 72 hours after injury and inability to complete questionnaires.
Interventions
NPWT (n = 226) in which an open-cell solid foam or gauze was placed over the surface of the wound and connected to a suction pump, creating a partial vacuum over the dressing, vs standard dressings not involving application of negative pressure (n = 234).
Main Outcomes and Measures
Disability Rating Index score (range, 0 [no disability] to 100 [completely disabled]) at 12 months was the primary outcome measure, with a minimal clinically important difference of 8 points. Secondary outcomes were complications including deep infection and quality of life (score ranged from 1 [best possible] to −0.59 [worst possible]; minimal clinically important difference, 0.08) collected at 3, 6, 9, and 12 months.
Results
Among 460 patients who were randomized (mean age, 45.3 years; 74% men), 88% (374/427) of available study participants completed the trial. There were no statistically significant differences in the patients’ Disability Rating Index score at 12 months (mean score, 45.5 in the NPWT group vs 42.4 in the standard dressing group; mean difference, −3.9 [95% CI, −8.9 to 1.2]; P = .13), in the number of deep surgical site infections (16 [7.1%] in the NPWT group vs 19 [8.1%] in the standard dressing group; difference, 1.0% [95% CI, −4.2% to 6.3%]; P = .64), or in quality of life between groups (difference in EuroQol 5-dimensions questionnaire, 0.02 [95% CI, −0.05 to 0.08]; Short Form–12 Physical Component Score, 0.5 [95% CI, −3.1 to 4.1] and Mental Health Component Score, −0.4 [95% CI, −2.2 to 1.4]).
Conclusions and Relevance
Among patients with severe open fracture of the lower limb, use of NPWT compared with standard wound dressing did not improve self-rated disability at 12 months. The findings do not support this treatment for severe open fractures.
Trial Registration
isrctn.org Identifier: ISRCTN33756652
This randomized clinical trial compares the effects of negative pressure wound therapy vs standard wound management on disability, deep infections, and quality of life in patients with severe open fracture of the lower limb.
Introduction
Fractures of the lower limb are common injuries in civilian and military populations.1,2 Most fractures are closed, meaning that the skin overlying the fracture is intact. Open fractures exist when the broken bone is exposed to contamination, resulting in a greatly increased risk of complications.3 In severe open fractures of the lower limb, infection rates up to 27% are reported, even in specialist trauma centers.4 The costs of treating wound complications is high for both patients and health care systems.5
The initial management of open fractures involves surgical debridement with excision of damaged tissue, removal of contamination, and antibiotic administration.6,7 The fracture is usually immobilized with bone fixation and a dressing is applied to the surface of the wound. Traditionally, a sealed nonadhesive layer is applied to protect the open fracture from further contamination. Reassessment and further debridement of the wound are typically performed 48 to 72 hours later.
Negative pressure wound therapy (NPWT) is an alternative form of dressing. This device creates a partial vacuum using suction, which removes blood and fluid that may collect in the wound. The vacuum may also encourage the formation of granulation (healing) tissue.4,8 However, NPWT dressings and the vacuum machines are considerably more expensive than traditional wound dressings.
Before this study, there has been only 1 randomized clinical trial comparing standard wound dressing with NPWT for patients with open fractures of the lower limb.9 Improved outcomes were suggested in patients treated with NPWT but the study was not definitive because it included only 59 patients treated at a single trauma center. Despite the lack of strong evidence, clinical guidelines around the world have recommended the use of NPWT for open fracture wounds.6,7,10
The aim of this pragmatic, multicenter randomized clinical trial was to compare standard wound dressings with NPWT for adults with an open fracture of the lower limb.
Methods
Study Design and Eligibility Criteria
The National Research Ethics Service approved the study, the approved trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively. The trial was overseen by independent steering and data and safety monitoring committees.
The trial took place in 24 major trauma hospitals representing the UK Major Trauma Network; in the UK, patients with serious injuries such as open fractures are transported directly to a specialist trauma center with joint orthopedic and plastic surgery facilities. Eligible patients were aged 16 years or older and had a severe open fracture of the lower limb. Although not specified in the trial protocol, the operating surgeon assessed the wound and if it was decided that the wound could not be closed, the patient was considered eligible for study entry. Wounds were then graded as a Gustilo and Anderson II or III wound; type II is an open fracture with a laceration more than 1 cm long without extensive soft-tissue damage, flaps, or avulsions and type III, either an open segmental fracture or an open fracture with extensive soft-tissue damage.11
Because surface NPWT can only be applied to wounds that are left open, the surgeons could only include the most severe injuries, ie, those in which it was not possible to safely suture the wound edges at the end of the first surgical debridement. Patients had to present to the trial hospital within 72 hours of their injury, including those who were transferred from other hospitals.
Patients were excluded if they had known contraindications to anesthesia or were deemed unable to adhere to trial procedures or complete questionnaires, eg, those with a preexisting diagnosis of dementia. For patients who were acutely confused or had temporary impairment of consciousness, a consultee was approached to provide agreement on behalf of the patient, as per the UK Mental Capacity Act 2005. All participants randomized under this provision were subsequently approached for consent once capacity was restored, with the option to continue or discontinue involvement in the trial. For this reason, we anticipated higher levels of postrandomization withdrawal than might be expected in most clinical trials.
Randomization and Masking
A computer-generated randomization algorithm was created by the trial statistician and delivered by an accredited Clinical Trials Unit to ensure that the allocation sequence was concealed. The individual patient was allocated treatment on a 1:1 basis, stratified by trial center and Gustilo and Anderson grade. When a patient entered the trial, nonidentifiable details were logged on the secure, encrypted, web-based system.
Participants were assigned to their treatment allocation intraoperatively at the end of initial surgery, but before any wound dressing was applied. It was not possible to blind trial participants to treatment allocation as wound dressings were clearly visible. In addition, the treating surgeons could not be blind to the intervention, but the surgical and health care teams were not involved in any trial assessments. Wound photographs taken at 6 weeks and standard radiographs were used to look for signs of delayed wound healing and nonunion of the bone, respectively. These were reviewed by independent clinicians who were blind to the treatment allocation.
Interventions
At presentation, all patients were listed for the next available trauma operating list. In the operating theater, all patients received a general or regional anesthetic. The wound associated with the fracture was surgically debrided and the fracture immobilized with either internal (under the skin) or external fixation. At the end of the initial operation, if the wound could not be closed primarily, ie, direct suture of the wound edges was not possible, the patient was randomized to either standard dressings or NPWT. All other elements of postoperative care remained the same for all patients.
Standard Dressing Group
All hospitals used a sterile dressing sealed from external contamination. However, the details of the materials used were left to the discretion of the treating health care team as per routine care at their center. Details of each dressing applied in the trial were recorded and classified according to British National Formulary classification.
NPWT Group
The NPWT dressing used an open-cell solid foam or gauze laid onto the wound followed by an adherent sealed dressing. A sealed tube was connected from the dressing to a suction pump, which created a partial vacuum over the wound. The basic features of the NPWT are universal, but the exact details of the dressing and pressure (mm Hg) were left to the discretion of the treating health care team. Details of the NPWT were recorded in trial documentation. Patients with an open fracture of the lower limb that could not be closed primarily had a second operation at 48 to 72 hours, when a further wound assessment and debridement was performed and the wound closed either primarily with sutures or by soft-tissue reconstruction as necessary.
Data Collection and Outcome Measures
The primary outcome was the patient-reported Disability Rating Index (DRI) at 12 months after randomization.12 The DRI provides a 100-point score, in which zero represents normal function and 100, complete disability, with a minimum clinically important difference of 8 points.
Secondary outcomes were health-related quality of life using EuroQol 5-dimension, 3-level questionnaire (EQ-5D-3L)13,14 and Short Form–12 (SF-12)15,16 deep surgical site infection (SSI) at 30 days as per the Centers for Disease Control and Prevention definition17 and other complications. EQ-5D-3L responses were converted into an overall utility score14 that ranged from 1 (best possible) to −0.59 (worst possible), in which zero represents the quality of life associated with death; the minimum clinically important difference is 0.08 points. Physical and Mental Health Component Scores were computed from SF-12 responses18; these scores range from 0 to 100, in which a zero score indicates the lowest level of health. Infection outcomes and complications were extracted from the patients’ medical records by independent research staff in each trial center. Wound photographs and radiographs were reviewed independently and blind to treatment allocation.
Deep infection following an open fracture is a key driver of subsequent disability. However, a deep infection that is treated early and definitively may resolve completely with no disability. Similarly, wounds that are not infected may still heal with excess scar tissue or require extensive tissue grafts, which can lead to reduced mobility and chronic pain. Therefore, the DRI was considered to be more important as a primary outcome measure than the rate of deep infection or size of the wound per se. Patient-reported outcomes (DRI, EQ-5D-3L, SF-12) and self-reported complications were collected by questionnaire. Preinjury baseline scores were collected retrospectively at the time of consent and again by postal questionnaire at 3, 6, 9, and 12 months.
Statistical Analysis
A minimum clinically important difference for the DRI of 8 points was selected to power the study19; for an individual patient, this represents the difference between the ability to climb stairs or run with “some difficulty” vs “great difficulty” and at the population level, 8 points represents the difference between a “healthy patient” (score = 1 point) and a “patient with a minor disability” (score = 9 points). The SD of the DRI used in the sample size calculation was 25 points. Allowing a margin of 10% loss during follow-up, including the small number of patients who die in the first year following their injury, gave a total sample size of 460 patients. Therefore, 230 patients consenting to each intervention group would provide 90% power to detect a difference of 8 points in DRI at 12 months at the 5% significance level.
When calculating summary statistics for assessing treatment efficacy, NPWT data were subtracted from control group data, such that a positive difference indicated that a score or outcome measure was larger in the control group. We investigated differences in the primary outcome measure, the DRI score at 1 year after injury, between the 2 treatment groups on an intention-to-treat basis. Early and midterm disability was assessed and reported at 3, 6, and 9 months. A secondary per-treatment analysis was also performed. Mixed-effects regression analysis, with recruiting center as a random effect, and fixed terms to adjust for age group, sex, baseline preinjury score, and Gustilo and Anderson grade was used to test for treatment group differences using complete case data. Secondary end points were not adjusted for multiple comparisons and should be interpreted as exploratory. In a post hoc sensitivity analysis for the primary outcome, missing data were imputed using the chained equation method20 and models fitted to give a pooled estimate of the treatment effect.
All tests were 2-sided and significance was assessed at the 5% level. Analyses of primary and secondary outcomes used complete-case data and all analyses were implemented in R version 3.3.021 using packages base, graphics, mice, lme4, and nlme (https://cran.r-project.org/).
Results
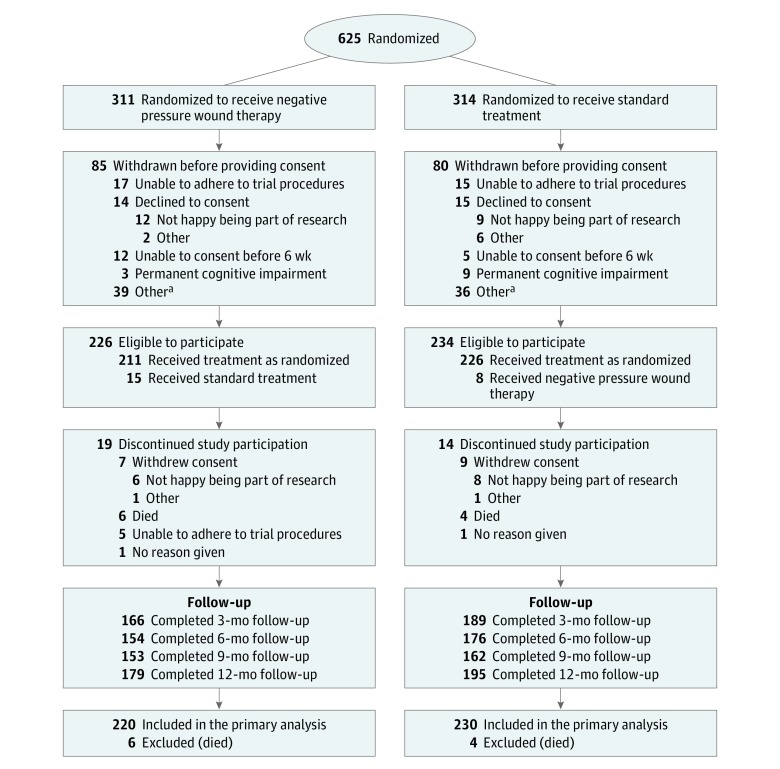
A total of 625 patients were randomized between July 2012 and December 2015. Some patients were cognitively impaired before surgery and were unable or not willing to provide informed consent after randomization. Most of the 165 patients who did not provide consent were found to be ineligible after randomization, eg, due to primary closure of the wound or permanent cognitive impairment that could not be predicted before surgery/randomization. Only 29 potentially eligible patients declined to participate in the trial; 14 in the NPWT group and 15 in the standard dressing group (Figure 1). A total of 460 patients consented to take part in the Wound Management of Open Lower Limb Fractures (WOLLF) Trial: 85% were grade III injuries and 82% involved the tibia. The characteristics of the 2 groups were well balanced after randomization (Table 1).
Figure 1. Participant Flow Through the WOLLF Study.
aOther reasons for withdrawal before consent included the following: 1 amputation, 8 deaths, 20 primary closures, 5 randomized in error, 2 transferred, and 3 with no reason given in the negative pressure wound therapy group; and 1 amputation, 6 deaths, 18 primary closures, 8 randomized in error, 2 transferred, and 1 with no reason given in the standard treatment group.
Table 1. Characteristics of the Study Participants.
| Characteristic | Negative Pressure Wound Therapy Group (n = 226) | Standard Treatment Group (n = 234) |
|---|---|---|
| Age, y | ||
| Mean (SD) | 46.1 (19.9) | 44.5 (19.0) |
| Median (IQR) | 42 (29-61) | 44 (26-57) |
| Height, mean (SD), m | 1.74 (0.12) | 1.72 (0.16) |
| Weight, mean (SD), kg | 80.9 (16.8) | 80.9 (19.4) |
| Male, No. (%) | 178 (78.8) | 164 (70.1) |
| Diabetes, No. (%) | 14 (6.2) | 13 (5.6) |
| Smoker, No. (%) | 70 (31.0) | 79 (33.8) |
| No. of cigarettes/d, mean (SD) | 15.4 (10.9) | 15.3 (10.4) |
| No. of years smoking, mean (SD) | 17.6 (12.4) | 17.4 (12.0) |
| Employment, No. (%) | ||
| Employed | 147 (65.0) | 160 (68.4) |
| Retired or inactive | 44 (19.5) | 44 (18.8) |
| Unemployed | 28 (12.4) | 25 (10.7) |
| Unknown | 7 (3.1) | 5 (2.1) |
| Mechanism of injury, No. (%) | ||
| Road traffic crash | 125 (55.3) | 139 (59.4) |
| Low-energy fall | 34 (15.0) | 39 (16.7) |
| High-energy fall | 34 (15.0) | 25 (10.7) |
| Crush injury | 17 (7.5) | 19 (8.1) |
| Other | 13 (5.8) | 9 (3.8) |
| Contact sports injury | 3 (1.3) | 1 (0.4) |
| Unknown | 0 (0) | 2 (0.9) |
| Injuries associated with the open fracture, No. (%)a | 58 (25.7) | 76 (32.5) |
| Upper limb | 17 (7.5) | 32 (13.7) |
| Chest | 24 (10.6) | 22 (9.4) |
| Spine | 21 (9.3) | 22 (9.4) |
| Head | 14 (6.2) | 11 (4.7) |
| Pelvis | 8 (3.5) | 15 (6.4) |
| Ipsilateral lower limb | 6 (2.7) | 16 (6.8) |
| Contralateral lower limb | 4 (1.8) | 14 (6.0) |
| Abdomen | 3 (1.3) | 12 (5.1) |
| Gustilo and Anderson grade, No. (%)b | ||
| Grade II | 34 (15.0) | 30 (12.8) |
| Grade III | 171 (75.7) | 180 (76.9) |
| Grade III + IV | 21 (9.3) | 24 (10.3) |
| Fracture fixation, No. (%) | ||
| External fixator-half-pin | 107 (47.3) | 111 (47.4) |
| Nail | 49 (21.7) | 56 (23.9) |
| Plate and screws | 38 (16.8) | 32 (13.7) |
| Other | 21 (9.3) | 21 (9.0) |
| External fixator-fine-wire | 3 (1.3) | 11 (4.7) |
| Wires or tension band wires | 7 (3.1) | 3 (1.3) |
| Unknown | 1 (0.4) | 0 (0) |
Abbreviation: IQR, interquartile range.
Some study participants had multiple injuries with the open fracture.
Type II is an open fracture with a laceration more than 1 cm long without extensive soft-tissue damage, flaps, or avulsions; type III, either an open segmental fracture or an open fracture with extensive soft-tissue damage; and type VI, type III with a vascular injury requiring surgical repair.
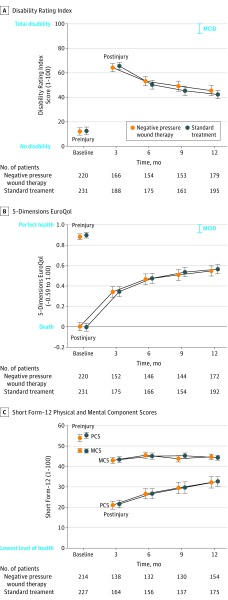
On an intention-to-treat basis, there was no significant difference in the DRI score at 12 months between those patients treated with NPWT vs those treated with standard wound dressings (Figure 2). The mean DRI in the NPWT group was 45.5 vs 42.4 in the standard dressing group, resulting in a difference of −3.9 (95% CI, −8.9 to 1.2) in favor of standard dressings (P = .13; from adjusted mixed-effect regression analysis) (Table 2). Therefore, the results of this trial are consistent with a −8.9 worse disability rating attributable to the use of NPWT, which, based on the minimal clinically important difference, would be clinically important but also ranging to a nonclinically important benefit of these dressings of 1.2 points on the DRI scale. Similarly, there was no significant difference in disability rating at 3, 6, or 9 months (Figure 2).
Figure 2. Temporal Trends in Main Study Outcomes.
Shown are means with 95% CIs at basline and each follow-up. Preinjury assessments were made retrospectively by all study participants and immediately postinjury for EuroQol 5-dimensions questionnaire. MCID indicates minimum clinically important differences.
Table 2. Primary and Secondary Outcomes.
| Outcome | NPWT Group | Standard Treatment Group | Difference (95% CI) | P Value for Adjusted Analysis | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | No. | Mean (SD) | No. | Rawa | Adjustedb | ||
| Primary Outcome | |||||||
| DRI score at 12 mo | 45.5 (28.0) | 179 | 42.4 (24.2) | 195 | −3.1 (−8.5 to 2.2) | −3.9 (−8.9 to 1.2) | .13 |
| Secondary Outcomes | |||||||
| DRI scores over timec | |||||||
| At 3 mo | 64.3 (22.3) | 166 | 65.6 (20.1) | 188 | 1.3 (−3.1 to 5.8) | 0.7 (−3.7 to 5.0) | .76 |
| At 6 mo | 53.2 (23.8) | 154 | 50.3 (24.1) | 175 | −2.8 (−8.0 to 2.4) | −3.5 (−8.4 to 1.5) | .17 |
| At 9 mo | 49.2 (25.9) | 153 | 45.4 (25.2) | 161 | −3.8 (−9.5 to 1.9) | −4.4 (−10.0 to 1.3) | .13 |
| Quality of life at 12 mo | |||||||
| EQ-5D-3Ld | 0.55 (0.33) | 172 | 0.56 (0.32) | 192 | 0.02 (−0.05 to 0.08) | 0.01 (−0.06 to 0.07) | .82 |
| SF-12 PCSe | 32.2 (17.4) | 154 | 32.7 (15.5) | 175 | 0.5 (−3.1 to 4.1) | 0.4 (−3.0 to 3.8) | .82 |
| SF-12 MCSe | 44.7 (8.4) | 154 | 44.3 (8.2) | 175 | −0.4 (−2.2 to 1.4) | −0.2 (−2.1 to 1.6) | .80 |
Abbreviations: DRI, Disability Rating Index; EQ-5D-3L, EuroQol 5-dimension, 3-level questionnaire; MCS, Mental Health Component Score; NPWT, negative pressure wound therapy; PCS, Physical Component Score; SF-12, Short Form–12.
Mean of standard group minus mean of NPWT group; for DRI, a negative value is in favor of the standard treatment, as a lower score indicates less disability.
Mixed-effects regression based on a complete case analysis with treatment group, age group, sex, baseline preinjury score, and wound grade as covariates (fixed effects) and recruiting center as a random effect; P values are from analysis of variance F test.
DRI is assessed on a 100-point score scale, in which zero represents normal function and 100, complete disability, with a minimum clinically important difference of 8 points.
EQ-5D-3L is a measure of health-related quality of life, in the range of −0.59 (worst possible state) to 1 (perfect health), anchored at 0 (death), with a minimum clinically important difference of 0.08 points.
SF-12 PCS and MCS are computed from the Short Form health survey and range from 0 to 100, in which a zero score indicates the lowest level of health.
The secondary per-protocol (per-treatment) analysis of the DRI did not significantly differ from the primary intention-to-treat analysis; the difference between groups being −4.0 (95% CI, −9.1 to 1.0) in favor of the standard dressings (P = .12).
Secondary exploratory analysis showed that there was no significant difference in the health-related quality-of-life scores between the treatment groups at any point in the 12 months following the injury. The mean (SD) SF-12 Physical Component Score at 12 months in the NPWT group was 32.2 (17.4) vs 32.7 (15.5) in the standard dressing group, resulting in an adjusted difference of 0.4 (95% CI, −3.0 to 3.8) in favor of standard dressings (P = .82; from adjusted mixed-effect regression analysis). The mean (SD) EQ-5D-3L score in the NPWT group was 0.55 (0.33) vs 0.56 (0.32) in the standard dressing group, resulting in a difference of 0.01 (95% CI, −0.06 to 0.07) in favor of standard dressing (P = .82) (Table 2).
There was no significant difference in the number of deep surgical site infections between the treatment groups (Table 3). In total, 35 of the 460 participants (7.6%) had a deep surgical site infection at 30 days; 16 (7.1%) in the NPWT treatment group and 19 (8.1%) in the standard dressing group, resulting in an estimated odds ratio of 0.85 (95% CI, 0.42 to 1.70) and a percentage difference in rates of 1.0% (95% CI, −4.2% to 6.3%) in favor of NPWT (P = .64 from adjusted mixed-effect logistic regression analysis). There was no significant difference in the proportion of wounds found to be fully healed on the 6-week photographs; 52.0% (91/175) in the NPWT group and 51.7% (93/180) in the standard dressing group, resulting in an odds ratio of 1.0 (95% CI, 0.6 to 1.6; P = .99) and difference in rates of −0.3% (95% CI, −11.1% to 10.4%). There was no significant difference in the proportion of patients with complete bone union on the radiographs at 12 months: 69.6% (112/161) in the NPWT group and 71.9% (110/153) in the standard dressing group, resulting in an odds ratio of 1.1 (95% CI, 0.7 to 1.9; P = .68) and difference in rates of 2.3% (95% CI, −8.4% to 13.0%).
Table 3. Postoperative Complications Reported as Secondary Outcomes During 12-Month Follow-up.
| Complication | No. With Complication (%) | Difference (95% CI), % |
Odds Ratio (95% CI)a | P Valuea | |
|---|---|---|---|---|---|
| NPWT Group (n = 226) |
Standard Treatment Group (n = 234) |
||||
| Wound complications at 30 d | |||||
| Red and inflamed | 13 (5.8) | 19 (8.1) | 2.4 (−2.7 to 7.4) | 0.64 (0.28 to 1.42) | .27 |
| Swollen | 38 (16.8) | 49 (20.9) | 4.1 (−3.4 to 11.7) | 0.70 (0.42 to 1.16) | .15 |
| Painful or tender | 35 (15.5) | 33 (14.1) | −1.4 (−8.3 to 5.5) | 1.01 (0.58 to 1.77) | .99 |
| Fluid leaking | 28 (12.4) | 27 (11.5) | −0.9 (−7.2 to 5.5) | 1.01 (0.55 to 1.86) | .99 |
| Fluid (pus) cloudy | 11 (4.9) | 10 (4.3) | −0.6 (−4.8 to 3.7) | 1.21 (0.43 to 3.46) | .81 |
| Gaping open | 6 (2.7) | 4 (1.7) | −0.9 (−4.1 to 2.2) | 1.48 (0.34 to 7.22) | .75 |
| Surgeon opened | 2 (0.9) | 0 (0) | NP/S | NP/S | NP/S |
| Fever >38°C | 0 (0) | 0 (0) | NP/S | NP/S | NP/S |
| Abscess or infection | 3 (1.3) | 5 (2.1) | 0.8 (−2.0 to 3.6) | 0.57 (0.09 to 2.95) | .49 |
| Deep surgical site infection at 30 db | 16 (7.1) | 19 (8.1) | 1.0 (−4.2 to 6.3) | 0.85 (0.42 to 1.70) | .64 |
| Other postoperative complications related to the index wound or injury reported during follow-up | |||||
| Soft tissuec | 20 (8.8) | 17 (7.3) | −1.6 (−7.0 to 3.8) | 1.24 (0.60 to 2.59) | .61 |
| Neurovascular | 5 (2.2) | 8 (3.4) | 1.2 (−2.2 to 4.7) | 0.64 (0.16 to 2.26) | .58 |
| Persistent pain | 8 (3.5) | 11 (4.7) | 1.2 (−2.9 to 5.2) | 0.74 (0.25 to 2.08) | .64 |
| DVT or PE | 6 (2.7) | 4 (1.7) | −0.9 (−4.1 to 2.2) | 1.57 (0.37 to 7.65) | .54 |
| Further surgery related to the open fracture reported during follow-up | |||||
| Revision fixation | 18 (8.0) | 15 (6.4) | −1.6 (−6.7 to 3.6) | 1.26 (0.58 to 2.77) | .59 |
| Wound management | 19 (8.4) | 21 (9.0) | 0.6 (−5.0 to 6.1) | 0.93 (0.46 to 1.88) | .87 |
| Bone graft | 10 (4.4) | 18 (7.7) | 3.3 (−1.5 to 8.0) | 0.56 (0.22 to 1.31) | .17 |
| Amputation | 4 (1.8) | 6 (2.6) | 0.8 (−2.3 to 3.9) | 0.69 (0.14 to 2.93) | .75 |
Abbreviations: DVT, deep vein thrombosis; NP/S, testing not possible or sensible; NPWT, negative pressure wound therapy; PE, pulmonary embolism.
Unless stated otherwise, odds ratio, 95% CI, and P value from Fisher exact test; a value greater than 1 indicates a greater risk in the NPWT group.
Deep surgical site infection was recorded according to Centers for Disease Control and Prevention criteria: involvement of deep tissues with purulent drainage from the incision or spontaneous dehiscence or incision deliberately opened by a surgeon and there was fever or localized pain or tenderness, confirmation of abscess, or deep surgical site infection diagnosed by a surgeon/attending physician.
Complications that are not related to the bone and not included under wound infection, eg, problems caused by scar tissue or tendon irritation.
The primary outcome data were 88% complete (374 of 427 available study participants provided final outcome data) and there was no evidence for nonrandom patterns of missingness. Imputing missing data gave pooled estimates of the treatment effect for DRI at 12 months as −4.5 (95% CI, −9.3 to 0.4), with the percentage of the variability attributable to the uncertainty caused by the missing data estimated at 12.8%.
Discussion
This multicenter trial of patients with severe open fractures of the lower limb found no significant difference in the DRI score between those patients treated with NPWT vs those treated with standard wound dressings at 12 months after injury. There was no significant difference in the rate of deep surgical site infection or other healing complications, nor was there a significant difference in health-related quality of life at any point in the first 12 months after the injury.
Before performing this trial, a review of the literature22 revealed only 1 randomized clinical trial comparing standard wound dressing with NPWT for the initial management of patients with severe open fractures of the lower limb. Stannard et al9 demonstrated a difference in health-related quality of life and a reduction in the rate of deep wound infection in patients treated with NPWT compared with control (5.4% vs 20%; relative risk, 0.20 [95% CI, 0.05 to 0.87]). However, this was a small trial (59 patients, 63 fractures), and there were only 7 deep infections in the control group and 2 in the NPWT group. It is possible that this difference in the rate of deep infection was due to systematic differences in the patients and/or treatment pathway in a single center in the United States compared with the WOLLF trial, which took place in the much broader setting of 24 major trauma centers. However, given the relatively small number of cases in the Stannard et al9 trial, it is possible that the result represents a lack of precision in the estimate of the incidence of deep infection.
A trial published in 2016 also compared NPWT with standard dressings for the management of open fractures. This study was performed in Pakistan and used negative pressure dressings over a prolonged period (weeks) to reduce the size of the wound.23 This is a very different use of NPWT than advocated by current guidance for the management of open fractures, in which early definitive wound closure—within 72 hours—is recommended.6,7 Therefore, it is not clear whether the results of that trial are pertinent to other health care systems.
Limitations
This study has several limitations. First, patients with an open fracture of the lower limb have usually experienced severe trauma and present to hospital with variable states of consciousness and cognition. For emergency interventions, it was anticipated that some patients who were randomized would subsequently be found not eligible or not able to provide informed consent, eg, patients who had significant head injury or who died of their injuries in the early postoperative period. Some patients were also found to be ineligible after randomization due to the surgeon deciding that the wound could be closed by direct suturing, which may reflect the difficulties of classifying these injuries at the time of the initial debridement of the wound; only patients whom the surgeon thought that the wound had to be left open were included in the trial.24 However, 460 of the 485 patients (95%) who were randomized and eligible for the trial agreed to participate, suggesting that participants were representative of the overall population with severe open fractures of the lower limb.
Second, after randomization, some patients crossed over from one treatment group to another. However, 95% of patients received the treatment to which they were allocated.
Third, there was loss to follow-up, with study completion by only 88% of the original participants. However, multiple imputation analysis resulted in consistent findings.
Fourth, although patients were only eligible to enter the study if they presented to the treating hospital within 72 hours of their injury, adjustment could not be made for the exact time of the open fracture, which is a possible confounder in the analysis.
Conclusions
Among patients with severe open fracture of the lower limb, use of NPWT compared with standard wound dressing did not improve self-rated disability at 12 months. The findings do not support this treatment for severe open fractures.
Trial Protocol
Statistical Analysis Plan
References
- 1.Court-Brown CM, Rimmer S, Prakash U, McQueen MM. The epidemiology of open long bone fractures. Injury. 1998;29(7):529-534. doi: 10.1016/S0020-1383(98)00125-9 [DOI] [PubMed] [Google Scholar]
- 2.Mody RM, Zapor M, Hartzell JD, et al. . Infectious complications of damage control orthopedics in war trauma. J Trauma. 2009;67(4):758-761. doi: 10.1097/TA.0b013e3181af6aa6 [DOI] [PubMed] [Google Scholar]
- 3.Louie KW. Management of open fractures of the lower limb. BMJ. 2009;339:b5092. doi: 10.1136/bmj.b5092 [DOI] [PubMed] [Google Scholar]
- 4.Pollak AN, Jones AL, Castillo RC, Bosse MJ, MacKenzie EJ; LEAP Study Group . The relationship between time to surgical debridement and incidence of infection after open high-energy lower extremity trauma. J Bone Joint Surg Am. 2010;92(1):7-15. doi: 10.2106/JBJS.H.00984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacKenzie EJ, Jones AS, Bosse MJ, et al. . Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am. 2007;89(8):1685-1692. doi: 10.2106/JBJS.F.01350 [DOI] [PubMed] [Google Scholar]
- 6.British Orthopaedic Association; British Association of Plastic, Reconstructive, and Aesthetic Surgeons . Standard for Trauma: BOAST 4: The Management of Severe Open Lower Limb Fractures. London, United Kingdom: British Orthopaedic Association; 2009. [Google Scholar]
- 7.National Institute for Health and Care Excellence Fractures (Complex): Assessment and Management. London, United Kingdom: National Institute for Health and Care Excellence; 2016. [Google Scholar]
- 8.Labler L, Rancan M, Mica L, Härter L, Mihic-Probst D, Keel M. Vacuum-assisted closure therapy increases local interleukin-8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma. 2009;66(3):749-757. doi: 10.1097/TA.0b013e318171971a [DOI] [PubMed] [Google Scholar]
- 9.Stannard JP, Volgas DA, Stewart R, McGwin G Jr, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma. 2009;23(8):552-557. doi: 10.1097/BOT.0b013e3181a2e2b6 [DOI] [PubMed] [Google Scholar]
- 10.Krug E, Berg L, Lee C, et al. ; International Expert Panel on Negative Pressure Wound Therapy [NPWT-EP] . Evidence-based recommendations for the use of negative pressure wound therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury. 2011;42(suppl 1):S1-S12. doi: 10.1016/S0020-1383(11)00041-6 [DOI] [PubMed] [Google Scholar]
- 11.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458. doi: 10.2106/00004623-197658040-00004 [DOI] [PubMed] [Google Scholar]
- 12.Salén BA, Spangfort EV, Nygren AL, Nordemar R. The Disability Rating Index: an instrument for the assessment of disability in clinical settings. J Clin Epidemiol. 1994;47(12):1423-1435. doi: 10.1016/0895-4356(94)90086-8 [DOI] [PubMed] [Google Scholar]
- 13.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53-72. doi: 10.1016/0168-8510(96)00822-6 [DOI] [PubMed] [Google Scholar]
- 14.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095-1108. doi: 10.1097/00005650-199711000-00002 [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson C, Stewart-Brown S, Petersen S, Paice C. Assessment of the SF-36 version 2 in the United Kingdom. J Epidemiol Community Health. 1999;53(1):46-50. doi: 10.1136/jech.53.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271-292. doi: 10.1016/S0167-6296(01)00130-8 [DOI] [PubMed] [Google Scholar]
- 17.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332. doi: 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 18.Ware JE Jr, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Assessment Lab, New England Medical Center; 1994. [Google Scholar]
- 19.Achten J, Parsons NR, Bruce J, et al. . Protocol for a randomised controlled trial of standard wound management versus negative pressure wound therapy in the treatment of adult patients with an open fracture of the lower limb: UK Wound Management of Lower Limb Fractures (UK WOLLF). BMJ Open. 2015;5(9):e009087. doi: 10.1136/bmjopen-2015-009087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 21.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/. Accessed October 1, 2016.
- 22.Masters JP, Nanchahal J, Costa ML. Negative pressure wound therapy and orthopaedic trauma: where are we now? Bone Joint J. 2016;98-B(8):1011-1013. doi: 10.1302/0301-620X.98B8.BJJ-2016-0373 [DOI] [PubMed] [Google Scholar]
- 23.Arti H, Khorami M, Ebrahimi-Nejad V. Comparison of negative pressure wound therapy (NPWT) & conventional wound dressings in the open fracture wounds. Pak J Med Sci. 2016;32(1):65-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghoshal A, Enninghorst N, Sisak K, Balogh ZJ. An interobserver reliability comparison between the Orthopaedic Trauma Association’s open fracture classification and the Gustilo and Anderson classification. Bone Joint J. 2018;100-B(2):242-246. doi: 10.1302/0301-620X.100B2.BJJ-2017-0367.R1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan