Abstract
Objective
The molecular mechanism of low bone mass in girls with adolescent idiopathic scoliosis (AIS) has not been ascertained. Runx2 is a critical transcription factor regulating osteoblast differentiation and maturation. The present study aimed to explore the possible relationship between Runx2 expression in osteoblasts and bone mineral density (BMD) in subjects with AIS.
Methods
Twenty‐two girls with AIS scheduled to corrective surgery with iliac crest as donor site of autograft for spinal fusion were recruited. The BMD of lumbar spine and femoral neck were assessed by dual‐energy X‐ray absorptiometry, then patients were divided into two groups with either normal or reduced BMD. Cancellous bone was harvested from their iliac crests for primary culture of osteoblasts. mRNA and protein expression of Runx2 were assayed by reverse transcription‐polymerase chain reaction and western blotting, respectively. Results were compared between the two groups and correlated with BMD.
Results
AIS patients with normal BMD showed comparable maturity and body mass index but significant lower Cobb angle of main curve than those of patients with reduced BMD. The mean BMD of lumbar spine and femoral neck were 0.993 g/m2 and 0.911 g/m2 in patients with normal BMD, and were 0.757 g/m2 and 0.733 g/m2 in those with reduced BMD, respectively. The differences were significant between two groups (P < 0.05). The relative mean mRNA and protein expression of Runx2 were 0.49 ± 0.12 and 0.062 ± 0.020 in AIS with normal BMD, 0.35 ± 0.12 and 0.042 ± 0.006 in AIS with reduced BMD, respectively. Significantly lower Runx2 mRNA and protein expression were found in patients with AIS patients with reduced BMD than in those with normal BMD (P < 0.05). After controlling for age, weight and body mass index, positive correlations were found between Runx2 expression of both mRNA and protein and BMD of lumbar spine and femoral neck.
Conclusion
The abnormal expression of Runx2 in patients with AIS and reduced BMD indicates abnormal regulation of differentiation of their osteoblasts. Runx2 may play an important role in the pathogenesis of reduced BMD in patients with AIS.
Keywords: Bone mineral density, Idiopathic scoliosis, Osteopenia, Osteoblast, Runx
Introduction
Adolescent idiopathic scoliosis (AIS) is a three dimensional deformity of the spine that occurs during pubertal growth. One of its well‐defined characteristics is osteopenia. Reduced bone mass in patients with AIS was first reported by Burner et al. in 19821; subsequent studies confirmed reduced bone mineral density (BMD) in 27% to 38% of patients with AIS2, 3, 4, 5, 6, 7, 8. In a series of studies, Cheng et al. found there is a generalized low bone mass and osteopenia in both the axial and peripheral skeletons of female subjects with AIS5, 6. In additional, longitudinal follow‐up to skeletal maturity has shown that osteopenia persists in over 80% of girls with AIS7, suggesting that osteopenia may be a lifelong systematic abnormality of bone metabolism in girls with AIS. Furthermore, patients with osteopenia reportedly have a greater risk of curve progression, indicating that osteopenia could be an important prognostic factor for curve severity in AIS9. These findings have created growing concern about the factors that cause low bone mass in girls with AIS. It is generally accepted that multiple factors, such as growth disturbance, genetic vulnerability, calcium intake and physical activity, contribute to the etiopathogenesis of osteopenia in AIS3, 10, 11, 12, 13. However, the molecular mechanisms of the reduced bone mass in AIS have not yet been well documented.
The process of accumulation of BMD involves bone formation, mediated by osteoblast differentiation with matrix synthesis, and bone absorption mediated by osteoclasts. Soluble receptor activator of nuclear factor‐κB ligand and osteoprotegerin (RANKL/OPG) synthesized by osteoblasts are the key factors regulating the activity of osteoclasts, which play important roles in bone absorption and BMD accumulation. Increased RANKL and RANKL:OPG ratio have been reported in patients with AIS compared with control14, which indicates possible abnormal biological activity of osteoblasts in patients with AIS. Runx2, a key transcription modulator of osteoblasts differentiation, plays a fundamental role in regulating osteoblasts maturation and homeostasis15, 16, 17. It has been reported that in Runx2‐mutant mice, some skeletons had weakly calcified bone in which no osteoblasts were detected18. Recent studies have demonstrated a positive correlation between Runx2 and BMD19, 20, 21, 22, 23. According to in vitro studies, the role of Runx2 in bone formation is up‐regulation of expression of bone matrix formation, including type I collagen, osteopontin, osteocalcin, bone sialoprotein and fibronectin24, 25. We postulated that Runx2 is involved in the pathogenesis of osteopenia in girls with AIS. The aim of this study was to investigate the expression of Runx2 in osteoblasts from AIS patients both with and without osteopenia and to explore the relationship between Runx2 expression and osteopenia in these patients.
Materials and Methods
Subjects
With the approval of the Institutional Ethical Committee of Nanjing University, a prospective study was performed on patients undergoing surgical treatment in the authors' hospital from January 2008 to December 2009. During this period, girls with AIS undergoing posterior correction and autografting spinal fusion were selected as candidates for the study. Diagnosis of AIS was confirmed clinically and radiologically.
Inclusion criteria were as follows: female; age 12–18 years; standing Cobb angle ≥40°. Exclusion criteria were as follows: conditions or medications known to impact bone remodeling and calcium metabolism; any form of prior treatment for scoliosis; history of congenital deformities, skeletal dysplasia, neuromuscular diseases, endocrine diseases or connective tissue abnormalities; any intra‐canal cord abnormalities; and other types of scoliosis. In all, twenty‐two girls with AIS were included in this study. Written informed consent was given by each patient and her parents.
Anthropometric Measurements
Anthropometric measurements including body weight and standing height were carried out by standard methods26. Corrected height was derived from the Bjure formula (Log y = 0.011x + 0.177, where y is the loss in trunk height (cm) attributable to the deformed spine and x is the greatest Cobb angle of the primary curve)7. Body mass index (BMI) was calculated by dividing weight (kg) by corrected height squared (m2)26.
Bone Mineral Density Measurement and Subgrouping of Subjects
Lumbar spine BMD (LS‐BMD) and femoral neck BMD (FN‐BMD) of the non‐dominant proximal femur were measured in all AIS patients using dual‐energy X‐ray absorptiometry (Lunar DPX; Lunar Radiation, Madison, WI, USA). The in vivo precision deviations in all subjects for BMD measurements in the two assessed skeletal regions had a mean coefficient of variation (CV) for both sites of 0.62% ± 0.42% (range, 0.34%–0.92%). A control spine phantom scan performed every day for >7 years had a CV of 0.33%–0.40%26.
Based on the BMD values of healthy Han Chinese adolescent girls26, the AIS patients were allocated to two groups: patients with normal BMD (BMD more than −1 SD of the mean BMD values of healthy controls) and patients with reduced BMD (BMD less than −1 SD of the mean BMD values of healthy controls).
Primary Osteoblast Culture
During spinal fusion, a small amount of cancellous bone was taken from the posterior superior iliac crest of each patient, cut into pieces of size 1 mm × 1 mm × 1 mm and then rinsed with phosphate buffered saline three times. The specimens were incubated in 25 cm2 culture dishes at 37 °C in 5% CO2 in an air humidified incubator in a mixture of Dulbecco's modified eagle medium and nutrient mixture F‐12 medium containing 10% fetal bovine serum (Invitrogen, Burlington, ON, Canada), 100 U/ml penicillin and streptomycin (Sigma, St Louis, MO, USA). The culture medium was renewed at the end of the first week, and then once every two to three days for 21 days. After 28 days of culture, osteoblasts emerging from the bone pieces were sub‐cultured in a ratio of 1:3 by trypsinization (Invitrogen) until the cells reached 80% confluence.
Osteoblast Identification
The primary cultured osteoblasts were verified by alkaline phosphatase and mineralization nodule staining27, 28.
Alkaline Phosphatase Staining
Osteoblasts were inoculated in 6‐well culture plates at a density of 1 × 104 cells/cm2. When the cells had reached confluence, the cell layers were fixed in 70% ice cold ethanol for 10 min and incubated in incubation solution (3% β‐sodium glycerophosphate, 2% barbital sodium, 2% calcium chloride and 2% magnesium sulfate, Sigma) at 37 °C for 5 hours, then treated with 2% ammonium sulfide (Sigma) for 4 min followed by 2% cobaltous nitrate (Sigma) for 1 min at room temperature. After counterstaining with hematoxylin, the cell layers were dehydrated with 70% ethanol for 5 min and observed under a light microscope.
Mineralized Nodule Staining
1 × 105 P1 generation osteoblasts were inoculated in 6‐well culture plates. After being cultivated for 28 days, the cell layers were fixed in cold acetone for 10 min, and then stained with 0.1% Alizarin red S (pH = 7.2, Sigma) for 10 min. After washing with diluted water three times, the cell layers were dehydrated with 70% ethanol and observed under a light microscope.
Reverse Transcription Polymerase Chain Reaction (PCR)
To ascertain total RNA, 1 × 106–2 × 106 fresh P2 generation osteoblasts were isolated using TRIzol reagent (Invitrogen). The concentration of extracted RNA was determined from absorption at 260 nm. Then 4 μL of total RNA was reversibly converted to cDNA and 1 μL of cDNA used as a template for PCR. These reactions were carried out using 25 mM MgCl2, 10 × buffer, 2.5 mM deoxyribonucleoside triphosphate, 0.5 mM each primer, and 1 unit of Taq polymerase in a final reaction volume of 25 μL. Runx2 cDNA was amplified using 5′‐TAGATGGACCTCGGGAACCCAGA‐3′ for the forward primer and 5′‐TGGAAGACAGCGGGGTGGTAGA‐3′ for the reverse primer, yielding amplified products of 309 bp. The PCR reaction was performed at 94 °C for 45 seconds, 60 °C for 45 seconds, and 72 °C for 12 min for 30 cycles. PCR conditions for the amplification of β‐actin cDNA using the primer set 5′‐GGCATCCTCACCCTG‐AAGTA‐3′ and 5′‐GGGGTGTTGAAGGTCTCAAA‐3′ were 30 cycles of denaturation for 30 seconds at 96 °C, annealing for 1 min at 60 °C, and extension for 2 min at 72 °C, followed by a single cycle at 72 °C for 15 min. The PCR products were electrophoresed on 2% agarose gel and stained with ethidium bromide. All real‐time PCR reactions were performed in triplicate. The gene expression levels were normalized by dividing the resulting mRNA values by the value for β‐actin mRNA isolated at the same time‐point. Finally, the electrophoresis strips were analyzed with Smart‐view 2001 software.
Western Blotting
Next, 2 × 106–5 × 106 P2 generation osteoblasts were lysed in 200 μL ice cold lysis buffer (1 M Tris‐HCl, pH = 7.4, 150 mM NaCl, 0.1% sodium dodecylsulfate, 1% triton X‐100, protease inhibitor, 1% sodium deoxycholate, 0.1 mM ethylenediaminetetraacetic acid and 1 mM phenylmethylsulfonyl fluoride (Gibco, Gaithersburg, MD, USA). After 30 min incubation at 4 °C, the lysates were sonicated for 30 min and centrifuged at 12 000 g for 10 min at 4 °C. The supernatant was removed and denatured at 100 °C for 5 min before being subjected to a gel. Proteins (100 μg/well) were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis and then converted to a nitrocellulose membrane (Sigma) by electroblotting. The membranes were blocked for 1 hour at room temperature with 5% defatted milk in tris‐buffered saline with 0.1% Tween‐20 (Sigma). Subsequently, the membranes were immersed overnight at 4 °C with 1:1000 rabbit polyclonal anti‐Runx2 (Bioworld, USA), and 1:5000 mouse anti‐GAPDH antibody (Bioworld, USA). Following incubation with the primary antibody, membranes were washed carefully and re‐incubated for 2 h at room temperature with 1:5000 goat anti‐rabbit or anti‐mouse secondary antibodies (Bioworld, Visalia, CA, USA). After careful washing, reactive bands were detected using a western blotting chemiluminescence kit (Bioworld) according to the manufacturer's specifications and analyzed with an Amersham Imaging System and software (Amersham Biosciences, Piscataway, NJ, USA).
Statistical Analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences (version 12.0; SPSS, Chicago, IL, USA). Data were expressed as mean ± standard deviation. Different groups were compared by using Student's t‐test. Correlations between the expression level of Runx2 and the anthropometric data and BMD data were assessed by Pearson correlation analysis. In this study, the level of statistical significance was set at P < 0.05.
Results
Anthropometric Data
Twelve patients with normal BMD and ten with reduced BMD were recruited into the study. Their physical characteristics, including age, Risser sign, body weight, and BMI in AIS patients are shown according to bone mass in Table 1. Student's t‐test showed these variables did not differ significantly between the two study groups (P > 0.05). However, the mean Cobb angle of the main curve in patients with normal BMD was significantly lower than that in patients with reduced BMD (46.9° vs 55.2°, P < 0.05).
Table 1.
Profiles of AIS patients with normal or reduced BMD (mean ± s.d.)
| Index | Normal BMD (12 cases) | Reduced BMD (10 cases) |
|---|---|---|
| Age (yrs) | 14.3 ± 1.7 | 14.7 ± 1.1 |
| Height (cm) | 158.8 ± 5.7 | 156.3 ± 9.7 |
| Corrected height (cm) | 161.9 ± 5.3 | 158.9 ± 9.3 |
| Body weight (kg) | 44.5 ± 4.6 | 41.3 ± 2.9 |
| BMI (kg/m2) | 16.9 ± 1.3 | 16.3 ± 1.0 |
| Risser sign | 2.0 ± 0.9 | 2.5 ± 0.9 |
| Cobb angle (°) | 46.9 ± 4.4 * | 55.2 ± 5.8 * |
*P < 0.05.
BMD Measurement
The mean LS‐BMD and FN‐BMD were 0.993 g/m2 and 0.911 g/m2, respectively, in patients with normal BMD, and 0.757 g/m2 and 0.733 g/m2, respectively, in patients with reduced BMD. Both the mean LS‐BMD and FBMD were significantly different between two groups (P < 0.05).
Verification of Primary Cultured Osteoblasts
After 7–10 days culture, a few irregular, multiform or spindle shaped cells that had migrated from bone fragments were observed by inverted microscope (Fig. 1A). Once the cells had reached full confluence after 4 weeks culture, their morphology was uniformly short spindle shapes.
Figure 1.

Verification of osteoblasts cultured from a 14‐year‐old AIS with normal BMD. (A) Light microscopy of specimen cultured for 10 days shows many cells migrating from the bone fragment (×100). (B) Alkaline phosphatase staining shows many brownish‐black grains in the cytoplasm (×200). (C) After culturing for 4 weeks, mineralized nodules stain red with alizarin red (×100).
With alkaline phosphatase staining, brownish‐black grains were observed in the cytoplasm with a positivity rate of 85% (Fig. 1B). No brownish‐black grains were found in the controls.
After 4 weeks culture, formed mineralized nodules were observed and these stained red with alizarin red (Fig. 1C).
Runx2 Expression
The mean amount of Runx2 mRNA was 0.49 ± 0.12 in patients with normal BMD and 0.35 ± 0.12 in patients with reduced BMD, whereas the mean amounts of protein were 0.062 ± 0.020 and 0.042 ± 0.006, respectively (Fig. 2). Expression of both mRNA and protein of Runx2 in osteoblasts of patients with reduced BMD was significantly lower than in osteoblasts of patients with normal BMD (P < 0.05).
Figure 2.
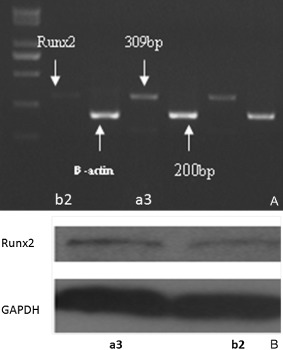
The mRNA and protein expression of Runx2 in girls with AIS. The mRNA expression (A) was determined by reverse transcription PCR while the protein expression (B) was assessed by western blotting. The expression of Runx2 of osteoblasts from an AIS girl (14.5 years, Cobb angle of major curve is 56º) with reduced BMD (b2) was lower than another AIS girl (14.4 years, Cobb angle of major curve is 48º) with normal BMD (a3).
Correlation Analysis
Correlations between Runx2 expression and anthropometric variables (age, height, weight and BMI) and BMD data are shown in Table 2. There were no correlations between expression of Runx2 and chronologic age, height and BMI at either mRNA or protein level. Correlations between Runx2 expression and body height were marginally significant at the mRNA level and significant at the protein level. In addition, Runx2 expression was positively correlated with LS‐BMD and FN‐BMD at both mRNA and protein levels. There was no significant correlation between Runx2 expression and curve magnitude.
Table 2.
Correlations between Runx2 and anthropometric variables in girls with AIS
| Index | Age (years) | Weight (kg) | Height (cm) | Cobb angle | BMI | LS‐BMD | FN‐BMD | |
|---|---|---|---|---|---|---|---|---|
| mRNA | r value | 0.319 | 0.518 | 0.296 | −0.170 | 0.368 | 0.619 | 0.780 |
| P value | 0.159 | 0.016 | 0.193 | 0.462 | 0.070 | 0.007 | 0.000 | |
| Protein | r value | 0.276 | 0.351 | 0.136 | −0.401 | 0.244 | 0.737 | 0.800 |
| P value | 0.238 | 0.129 | 0.567 | 0.080 | 0.261 | 0.000 | 0.000 |
Correlations according to Pearson correlation analysis.
Bold font indicates significant correlations.
Correlations between BMD and anthropometric variables ae listed in Table 2. The BMDs of girls with AIS correlated positively with BMI and body weight. After controlling for age, weight and BMI, the FN‐BMD and LS‐BMD still correlated positively with Runx2 (Tables 3 and 4).
Table 3.
Correlations between anthropometric variables and BMD in girls with AIS
| Index | Age (years) | Risser | Weight (kg) | Height (cm) | BMI | |
|---|---|---|---|---|---|---|
| LS‐BMD | r value | 0.158 | 0.120 | 0.365 | −0.420 | 0.213 |
| P value | 0.505 | 0.615 | 0.113 | 0.860 | 0.329 | |
| FN‐BMD | r value | 0.050 | 0.087 | 0.479 | 0.129 | 0.510 |
| P value | 0.834 | 0.716 | 0.033 | 0.589 | 0.013 |
Correlations according to Pearson correlation analysis.
Bold font indicates significant correlations.
Table 4.
Correlations between age, weight and BMI‐adjusted BMD and Runx2 mRNA and protein
| Index | mRNA | Protein | |
|---|---|---|---|
| LS‐BMD | Partial correlation | 0.827 | 0.705 |
| P value | 0.000 | 0.002 | |
| FN‐BMD | Partial correlation | 0.680 | 0.815 |
| P value | 0.005 | 0.000 |
Discussion
Since the first report of Burner et al.1, generalized low bone mass and osteopenia in both the axial and peripheral skeleton of subjects with AIS have been widely documented in published reports2, 3, 4, 5, 6, 7. In addition, osteopenia reportedly persists in more than 80% of AIS girls with previously documented osteopenia5. Furthermore, a low BMD has been found to be a risk factor for curve progression29. In a very recent study evaluating BMD among girls with AIS and their siblings, it was found that AIS patients had osteopenia and osteoporosis whereas their siblings with normal spines had normal bone mass30. In the current study, 45.4% (10/22) of AIS patients had lower LS‐BMD and FN‐BMD than do healthy Han Chinese adolescents, whose normal values have previously been ascertained by our team26. In our study, the prevalence of osteopenia was similar to that previously reported by Lee et al., who reported that up to 47% of AIS girls with severe scoliosis (Cobb angle > 40°) are osteopenic29.
Many possible explanations for the reduced BMD in patients with AIS have been put forward. Guo et al. suggested that endochondral bone formation is faster than intramembranous ossification in patients with AIS31. Thus, bone mineral deposition cannot keep pace with the speed of endochondral bone formation, resulting in generalized low bone mass. Our previous study showed that the growth potential of AIS patients is one of the key factors influencing bone mineral accrual26. Because BMD accumulation is a process of bone formation by osteoblasts differentiation and bone absorption by osteoclasts, the activities of both osteoblasts and osteoclasts have been studied. Suh et al. found that serum RANKL concentrations are higher in patients with AIS than in control subjects, suggesting an abnormal activity of osteoblasts in AIS patients14. In addition, AIS patients reportedly have significantly less capacity for osteogenic differentiation and less alkaline phosphatase (ALP) in mesenchymal stem cells than normal subjects; this could be one of the key mechanisms leading to low bone mass32. Runx2 is a key transcription modulator of osteoblasts differentiation and maturation15. In the current study, we demonstrated significantly lower expressions of Runx2 mRNA and protein in AIS patients with low bone mass than in those with normal BMD. In addition, we found that Runx2 expression is positively correlated with LS‐BMD and FN‐BMD, indicating that marked decrease of Runx2 in osteoblasts might be involved in the pathogenesis of abnormal differentiation process of osteoblasts, and contribute to the lower bone mass in girls with AIS.
A relationship between BMD and Runx2 has been reported19, 20, 21, 22, 23. Studies of gene polymorphism have shown that many sites (P1 and P2 promoters) of the Runx2 gene are strongly associated with BMD in ethnically distinct groups of subjects19, 20, 21, 22. Additionally, knockout mice reportedly have significantly lower BMD than do heterozygous Runx2‐II (one of the important isoform of Runx2) mice23. In the current study, we also found a positive association between expression of Runx2 in osteoblasts and BMD of both LS and FN in AIS patients. Again, this correlation remains significant after adjustment for known covariates, namely, age, body weight and BMI.
Osteoblasts affect bone mass and are the predominant cells involved in bone formation. Runx2, a transcription factor, is essential for osteoblasts differentiation and skeletal morphogenesis33. Mice with a mutated Runx2 locus have complete lack of ossification, both intramembranous and endochondral ossification being completely blocked as a result of maturational arrest of osteoblasts18. Accordingly, Runx2 knockout mice have no osteoblasts or bone formation34. Additionally, Runx2 reportedly binds to osteoblasts‐specific elements 2, which is found in the promoter region of all major osteoblasts‐related genes, including osteocalcin, alkaline phosphatase, collagenase‐3, bone sialoprotein and collagen type I alpha1 and controls their expression25. Based on the above findings, it can be theoretically inferred that the lower levels of Runx2 in AIS patients may lead to a relative deficiency in osteoblasts differentiation and maturation, which might decrease ALP activity, extracellular matrix synthesis and bone mineralization, and thus decisively result in lower bone mass in these patients. The histomorphometric study of Cheng et al. supports such an inference: they demonstrated that the osteoblasts from iliac bone and vertebra body of AIS patients with low bone mass have impaired differentiation and/or function, their osteoblasts are few in number and contain reduced alkaline phosphatase5.
The present study has some potential limitations. First, the patients in the control group were not “real” controls, in that they were not healthy young female adolescents, but also had AIS. For another, the bone biopsies were taken from the iliac crest but not vertebrae of AIS patients. For ethical reasons, it is difficult to obtain bone samples from normal adolescent girls, or even cancellous bone from normal vertebrae of control subjects with other diseases. In patients with AIS, because osteopenia has been found not only in the lumbar spine, but also in other regions including the femoral neck and peripheral bones35, it is thought to be a systemic problem.
Despite these limitations, to our knowledge, this is the first study regarding an association between expression of Runx2 in osteoblasts and BMD in girls with AIS. Our findings suggest that abnormal expression of Runx2 in osteoblasts is a possible cause and pathogenetic factor in reduced BMD in patients with AIS. As previously stated, skeletal growth and bone remodeling are very complicated processes. During the peripubertal stage, many proinflammatory cytokines, molecules, and proteins are involved in their regulation36. Because Runx2 is only one of the key factors responsible for bone formation at this stage, further studies in which other factors are measured might clarify the relationships between such factors and bone mass and Runx2 expression in patients with AIS.
Disclosure: This work was supported by the National Natural Science Foundation of China (81101335), National Post‐doctoral Foundation of China (2012 M52101062), National Key Clinical Specialty Construction Project in Orthopaedics and Jiangsu Province's Key Medical Talents Project (RC2011149).
References
- 1. Burner WL 3rd, Badger VM, Sherman FC. Osteoporosis and acquired back deformities. J Pediatr Orthop, 1982, 2: 383–385. [DOI] [PubMed] [Google Scholar]
- 2. Cook SD, Harding AF, Morgan EL, et al Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop, 1987, 7: 168–174. [DOI] [PubMed] [Google Scholar]
- 3. Lee WT, Cheung CS, Tse YK, et al Generalized low bone mass of girls with adolescent idiopathic scoliosis is related to inadequate calcium intake and weight bearing physical activity in peripubertal period. Osteoporos Int, 2005, 16: 1024–1035. [DOI] [PubMed] [Google Scholar]
- 4. Cheng JC, Guo X. Osteopenia in adolescent idiopathic scoliosis. A primary problem or secondary to the spinal deformity? Spine, 1997, 22: 1716–1721. [DOI] [PubMed] [Google Scholar]
- 5. Cheng JC, Tang SP, Guo X, Chan CW, Qin L. Osteopenia in adolescent idiopathic scoliosis: a histomorphometric study. Spine, 2001, 26: E19–E23. [DOI] [PubMed] [Google Scholar]
- 6. Cheng JC, Qin L, Cheung CS, et al Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res, 2000, 15: 1587–1595. [DOI] [PubMed] [Google Scholar]
- 7. Cheng JC, Guo X, Sher AH. Persistent osteopenia in adolescent idiopathic scoliosis. A longitudinal follow up study. Spine, 1999, 24: 1218–1222. [DOI] [PubMed] [Google Scholar]
- 8. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J, 2008, 17: 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hung VW, Qin L, Cheung CS, et al Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am, 2005, 87: 2709–2716. [DOI] [PubMed] [Google Scholar]
- 10. Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S. Genetic determinants of bone mass in adults. A twin study. J Clin Invest, 1987, 80: 706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horlick M, Wang J, Pierson RN Jr, Thornton JC. Prediction models for evaluation of total‐body bone mass with dual‐energy X‐ray absorptiometry among children and adolescents. Pediatrics, 2004, 114: e337–e345. [DOI] [PubMed] [Google Scholar]
- 12. Eun IS, Park WW, Suh KT, Kim JI, Lee JS. Association between osteoprotegerin gene polymorphism and bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J, 2009, 18: 1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suh KT, Eun IS, Lee JS. Polymorphism in vitamin D receptor is associated with bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J, 2010, 19: 1545–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suh KT, Lee SS, Hwang SH, Kim SJ, Lee JS. Elevated soluble receptor activator of nuclear factor‐kappaB ligand and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J, 2007, 16: 1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ziros PG, Basdra EK, Papavassiliou AG. Runx2: of bone and stretch. Int J Biochem Cell Biol, 2008, 40: 1659–1663. [DOI] [PubMed] [Google Scholar]
- 16. Xu YX, Xu B, Wu CL, Wu Y, Tong PJ, Xiao LW. Dynamic expression of DKK1 protein in the process whereby Epimedium‐derived flavonoids up‐regulate osteogenic and down‐regulate adipogenic differentiation of bone marrow stromal cells in ovariectomized rats. Orthop Surg, 2011, 3: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma XL, Liu ZP, Ma JX, Han C, Zang JC. Dynamic expression of Runx2, Osterix and AJ18 in the femoral head of steroid‐induced osteonecrosis in rats. Orthop Surg, 2010, 2: 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Komori T, Yagi H, Nomura S, et al Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell, 1997, 89: 755–764. [DOI] [PubMed] [Google Scholar]
- 19. Vaughan T, Reid DM, Morrison NA, Ralston SH. RUNX2 alleles associated with BMD in Scottish women; interaction of RUNX2 alleles with menopausal status and body mass index. Bone, 2004, 34: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 20. Doecke JD, Day CJ, Stephens AS, et al Association of functionally different RUNX2 P2 promoter alleles with BMD. J Bone Miner Res, 2006, 21: 265–273. [DOI] [PubMed] [Google Scholar]
- 21. Bustamante M, Nogués X, Agueda L, et al Promoter 2 ‐1025 T/C polymorphism in the RUNX2 gene is associated with femoral neck bmd in Spanish postmenopausal women. Calcif Tissue Int, 2007, 81: 327–332. [DOI] [PubMed] [Google Scholar]
- 22. Lee HJ, Koh JM, Hwang JY, et al Association of a RUNX2 promoter polymorphism with bone mineral density in postmenopausal Korean women. Calcif Tissue Int, 2009, 84: 439–445. [DOI] [PubMed] [Google Scholar]
- 23. Xiao Z, Awad HA, Liu S, et al Selective Runx2‐II deficiency leads to low‐turnover osteopenia in adult mice. Dev Biol, 2005, 283: 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science, 2000, 289: 1501–1504. [DOI] [PubMed] [Google Scholar]
- 25. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell, 1997, 89: 747–754. [DOI] [PubMed] [Google Scholar]
- 26. Qiu Y, Sun X, Cheng JC, et al Bone mineral accrual in osteopenic and non‐osteopenic girls with idiopathic scoliosis during bracing treatment. Spine, 2008, 33: 1682–1689. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt C, Ignatius AA, Claes LE. Proliferation and differentiation parameters of human osteoblasts on titanium and steel surfaces. J Biomed Mater Res, 2001, 54: 209–215. [DOI] [PubMed] [Google Scholar]
- 28. Kaspar D, Seidl W, Neidlinger‐Wilke C, Beck A, Claes L, Ignatius A. Proliferation of human‐derived osteoblast‐like cells depends on the cycle number and frequency of uniaxial strain. J Biomech, 2002, 35: 873–880. [DOI] [PubMed] [Google Scholar]
- 29. Lee WT, Cheung CS, Tse YK, et al Association of osteopenia with curve severity in adolescent idiopathic scoliosis: a study of 919 girls. Osteoporos Int, 2005, 16: 1924–1932. [DOI] [PubMed] [Google Scholar]
- 30. Sadat‐Ali M, Al‐Othman A, Bubshait D, Al‐Dakheel D. Does scoliosis causes low bone mass? A comparative study between siblings. Eur Spine J, 2008, 17: 944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo X, Chau WW, Chan YL, Cheng JC. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis. Results of disproportionate endochondral‐membranous bone growth. J Bone Joint Surg Br, 2003, 85: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 32. Park WW, Suh KT, Kim JI, Kim SJ, Lee JS. Decreased osteogenic differentiation of mesenchymal stem cells and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J, 2009, 18: 1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ducy P, Starbuck M, Priemel M, et al A Cbfa1‐dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev, 1999, 13: 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Otto F, Thornell AP, Crompton T, et al Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell, 1997, 89: 765–771. [DOI] [PubMed] [Google Scholar]
- 35. Lam TP, Hung VW, Yeung HY, et al Abnormal bone quality in adolescent idiopathic scoliosis: a case‐control study on 635 subjects and 269 normal controls with bone densitometry and quantitative ultrasound. Spine, 2011, 36: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 36. Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci, 2006, 1092: 385–396. [DOI] [PubMed] [Google Scholar]


