Key Points
Question
Is the use of opioids to treat chronic noncancer pain associated with greater benefits or harms compared with placebo and alternative analgesics?
Findings
In this meta-analysis that included 96 randomized clinical trials and 26 169 patients with chronic noncancer pain, the use of opioids compared with placebo was associated with significantly less pain (−0.69 cm on a 10-cm scale) and significantly improved physical functioning (2.04 of 100 points), but the magnitude of the association was small. Opioid use was significantly associated with increased risk of vomiting.
Meaning
Opioids may provide benefit for chronic noncancer pain, but the magnitude is likely to be small.
Abstract
Importance
Harms and benefits of opioids for chronic noncancer pain remain unclear.
Objective
To systematically review randomized clinical trials (RCTs) of opioids for chronic noncancer pain.
Data Sources and Study Selection
The databases of CENTRAL, CINAHL, EMBASE, MEDLINE, AMED, and PsycINFO were searched from inception to April 2018 for RCTs of opioids for chronic noncancer pain vs any nonopioid control.
Data Extraction and Synthesis
Paired reviewers independently extracted data. The analyses used random-effects models and the Grading of Recommendations Assessment, Development and Evaluation to rate the quality of the evidence.
Main Outcomes and Measures
The primary outcomes were pain intensity (score range, 0-10 cm on a visual analog scale for pain; lower is better and the minimally important difference [MID] is 1 cm), physical functioning (score range, 0-100 points on the 36-item Short Form physical component score [SF-36 PCS]; higher is better and the MID is 5 points), and incidence of vomiting.
Results
Ninety-six RCTs including 26 169 participants (61% female; median age, 58 years [interquartile range, 51-61 years]) were included. Of the included studies, there were 25 trials of neuropathic pain, 32 trials of nociceptive pain, 33 trials of central sensitization (pain present in the absence of tissue damage), and 6 trials of mixed types of pain. Compared with placebo, opioid use was associated with reduced pain (weighted mean difference [WMD], −0.69 cm [95% CI, −0.82 to −0.56 cm] on a 10-cm visual analog scale for pain; modeled risk difference for achieving the MID, 11.9% [95% CI, 9.7% to 14.1%]), improved physical functioning (WMD, 2.04 points [95% CI, 1.41 to 2.68 points] on the 100-point SF-36 PCS; modeled risk difference for achieving the MID, 8.5% [95% CI, 5.9% to 11.2%]), and increased vomiting (5.9% with opioids vs 2.3% with placebo for trials that excluded patients with adverse events during a run-in period). Low- to moderate-quality evidence suggested similar associations of opioids with improvements in pain and physical functioning compared with nonsteroidal anti-inflammatory drugs (pain: WMD, −0.60 cm [95% CI, −1.54 to 0.34 cm]; physical functioning: WMD, −0.90 points [95% CI, −2.69 to 0.89 points]), tricyclic antidepressants (pain: WMD, −0.13 cm [95% CI, −0.99 to 0.74 cm]; physical functioning: WMD, −5.31 points [95% CI, −13.77 to 3.14 points]), and anticonvulsants (pain: WMD, −0.90 cm [95% CI, −1.65 to −0.14 cm]; physical functioning: WMD, 0.45 points [95% CI, −5.77 to 6.66 points]).
Conclusions and Relevance
In this meta-analysis of RCTs of patients with chronic noncancer pain, evidence from high-quality studies showed that opioid use was associated with statistically significant but small improvements in pain and physical functioning, and increased risk of vomiting compared with placebo. Comparisons of opioids with nonopioid alternatives suggested that the benefit for pain and functioning may be similar, although the evidence was from studies of only low to moderate quality.
This systematic review and meta-analysis of randomized clinical trials comparing opioid vs nonopioid treatment of chronic noncancer pain estimates the association of opioids with pain intensity, physical function, and incidence of vomiting.
Introduction
In 2016, an estimated 50 million adults in the United States were living with chronic noncancer pain,1 many of whom were prescribed opioid medications.2,3,4 From 2013 to 2016, the United States was the largest per-capita consumer of opioids in the world.5,6 The effects of opioids on chronic pain are uncertain,7 whereas the harms found to be associated with prescription opioids include diversion,8 addiction,9 overdose, and death.10
The most recent systematic review11 of opioids for chronic noncancer pain included only effectiveness trials with 1 year of follow-up or longer and found no eligible randomized clinical trials (RCTs). The most recent review with studies that had less than 1 year of follow-up12 included only studies published up to July 2009 and reported meta-analyses results as the standardized mean difference, which has limitations.13 The review concluded that compared with placebo, opioids provided better pain relief for neuropathic and nociceptive pain than for fibromyalgia; however, no test for interaction was reported.14
This systematic review and meta-analysis of RCTs of opioids for chronic noncancer pain includes more recent data and addresses the limitations of the previous reviews.
Methods
We followed the statement on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for RCTs,15 registered our review (PROSPERO Identifier: CRD42012003023), and published our protocol16 (the protocol also appears in Supplement 1). Prior to the analyses, we made the following changes to the protocol: we excluded RCTs reporting less than 4 weeks follow-up; and we included subgroup analyses of (1) enrichment trials (studies that excluded patients who reported no improvement, had problematic adverse events, or both during an open-label run-in period) vs trials that did not use the enrichment method, (2) parallel study design vs crossover trials, and (3) trials that reported change scores for treatment effects vs trials that only reported end-of-study treatment effects. In addition to these protocol changes made before conducting the analyses, we conducted a post hoc subgroup analysis of trials that administered combination products (opioid and acetaminophen) compared with opioids alone.
Data Sources and Searches
An academic librarian developed the search strategies (eAppendix 1 in Supplement 2) without language restrictions and searched the databases of CENTRAL, CINAHL, EMBASE, MEDLINE, AMED, and PsycINFO from inception to April 1, 2018. We reviewed reference lists of eligible reports and contacted authors to obtain unpublished data.
Eligibility Criteria
The included trials (1) enrolled patients with chronic noncancer pain, (2) randomized them to an oral or transdermal opioid (pure opioid or a combination product) vs any nonopioid control, and (3) conducted follow-up for at least 4 weeks. Conference abstracts and rarely used interventions (such as oral ketamine, mexiletine, propoxyphene, dextropropoxyphene, fedotozine, and asimadoline) for chronic noncancer pain in North America were excluded.
Study Selection
Using a standardized form, 27 reviewers working in 13 teams screened titles, abstracts, and full-text articles reporting potentially eligible studies. Disagreements were resolved by discussion or by consultation with an adjudicator when necessary. We used online systematic review software (DistillerSR, Evidence Partners) to facilitate literature screening.
Data Extraction
A pair of reviewers independently abstracted each article. The included data were study and patient characteristics, dose and duration of treatment, and patient-important outcomes as guided by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.17,18
The primary outcomes were pain, physical functioning, and vomiting incidence (a systematic review of patient values and preferences19 identified nausea and vomiting as the opioid-induced adverse events that were most important to patients). We calculated the morphine-equivalent dose for each opioid by multiplying the quantity × the milligrams per unit dispensed × drug-specific conversion factors (eTable 1 in Supplement 2).20 There are no established conversion factors for buprenorphine or cebranopadol. We used the longest follow-up reported.21
Risk of Bias Assessment
Using a modified Cochrane risk of bias instrument, pairs of reviewers independently assessed the articles for risk of bias.22,23 Response options for each item were “definitely or probably yes” (assigned a low risk of bias) and “definitely or probably no” (assigned a high risk of bias).
Data Synthesis and Analysis
The adjusted κ statistic addressed interrater agreement regarding eligibility.24 Continuous measures were converted to common scales on a domain-by-domain basis as follows: (1) pain intensity was converted to the 10-cm visual analog scale (VAS) for pain; (2) physical functioning to the 100-point 36-item Short Form Survey (SF-36) physical component score; (3) emotional functioning to the 100-point SF-36 mental component score; (4) role functioning to the 100-point SF-36 subscale for role limitations due to physical problems; (5) social functioning to the 100-point SF-36 subscale for social functioning; and (6) sleep to the SF-36 sleep quality 100-mm VAS.25
All continuous outcome measures reported by more than 1 study were pooled and the weighted mean difference and the risk difference of achieving the minimally important difference were calculated. To estimate the probability of achieving greater than or equal to the minimally important difference in the control group, we used (1) the median or mean and standard deviation of the control group and (2) the established minimally important difference for each outcome in the treatment group. We used the pooled mean difference to estimate the mean in the treatment group and calculated the probability of achieving greater than or equal to the minimally important difference in the treatment group. We used probabilities in both groups to acquire the risk difference for achieving greater than or equal to the minimally important difference.13
The minimally important difference is the smallest amount of improvement in a treatment outcome that patients would recognize as important.26 For example, the minimally important difference is about 1.0 cm for the 10-cm VAS for pain.27 For the SF-36 items, the minimally important difference of 10 points was used for the individual domains (ie, role functioning and social functioning), 5 points for the summary scores (ie, physical functioning and emotional functioning), and 10 mm for sleep quality (measured using the 100-mm VAS).28
Adverse events are reported as binary outcomes. Due to the large number of adverse event types reported (n = 114), we explored the frequency distribution of adverse events and decided prior to analysis to pool the adverse events reported by 20 or more RCTs. Associations between treatment and adverse events are reported as relative risks (RRs) and risk differences and were determined using random-effects modeling and the DerSimonian-Laird method.29 We conducted a post hoc sensitivity analysis using the Hartung-Knapp-Sidik-Jonkman method.30 Change scores from baseline to the end of follow-up were used to account for interpatient variability. If the change scores were not reported, we calculated them using the baseline and end-of-study scores and the associated SDs using a correlation coefficient derived from the largest trial at the lowest risk of bias that reported a change score. We conducted subgroup analyses for reported vs converted change scores and only used the reported change score if a significant subgroup effect was found.
We contacted authors to obtain unpublished data for nonsignificant findings. If these data were unavailable, we addressed the risk of overestimating the magnitude of the association by imputing a weighted mean difference of 0 or an RR of 1 for the effect estimates and an associated variance using the hot-deck approach.31 The sensitivity analyses excluded imputation for nonsignificant effects. Stata statistical software version 13.1 (StataCorp) was used. Comparisons were 2-tailed using a P ≤ .05 threshold.
Subgroup Analyses and Meta-Regression
The Cochran Q test and the I2 statistic were used to examine statistical heterogeneity and explore treatment associations according to the following subgroups: (1) crossover vs parallel trials; (2) trials at risk of bias (on an item-by-item basis); (3) reported vs converted change scores for effect estimates; (4) studies of participants receiving disability benefits or involved in litigation vs those who were not receiving disability benefits or involved in litigation; (5) enriched enrollment trials vs not enriched; (6) trials with longer vs shorter (<3 months vs ≥3 months) follow-up; (7) higher vs lower doses of opioid, and (8) trials of combination products (opioid and acetaminophen) vs trials of opioids alone.
A clinical expert committee32 blinded to the study results provided clinical categories for subgroup analysis and adjudicated trial populations as follows: (1) conditions associated with objective findings or not; (2) neuropathic vs nociceptive vs central sensitization; and (3) neuropathic vs nonneuropathic. Neuropathic pain results from injury to the nervous system (eg, diabetic neuropathy). Injury to other tissues producing noxious stimulus is defined as nociceptive pain (eg, osteoarthritis). Pain present without tissue damage is considered central sensitization (eg, fibromyalgia).
We conducted subgroup analyses if there were 2 or more studies in a given subgroup and conducted tests of interaction to establish whether the subgroups differed significantly from one another. We assessed the credibility of significant subgroup effects (P < .05) using previously suggested criteria (eTable 2 in Supplement 2).14 Meta-regression was performed for length of follow-up, opioid dose, and loss to follow-up.
Quality of Evidence
The Grading of Recommendations Assessment, Development and Evaluation was used to summarize the quality of evidence on an outcome-by-outcome basis as high, moderate, low, or very low.33 We did not rate down the quality of evidence for risk of bias if the subgroup analysis showed no association of treatment effects with risk of bias. When there were at least 10 studies for meta-analysis,34 we assessed publication bias by visual assessment of funnel plot asymmetry and by using the results from the Begg test.35
Results
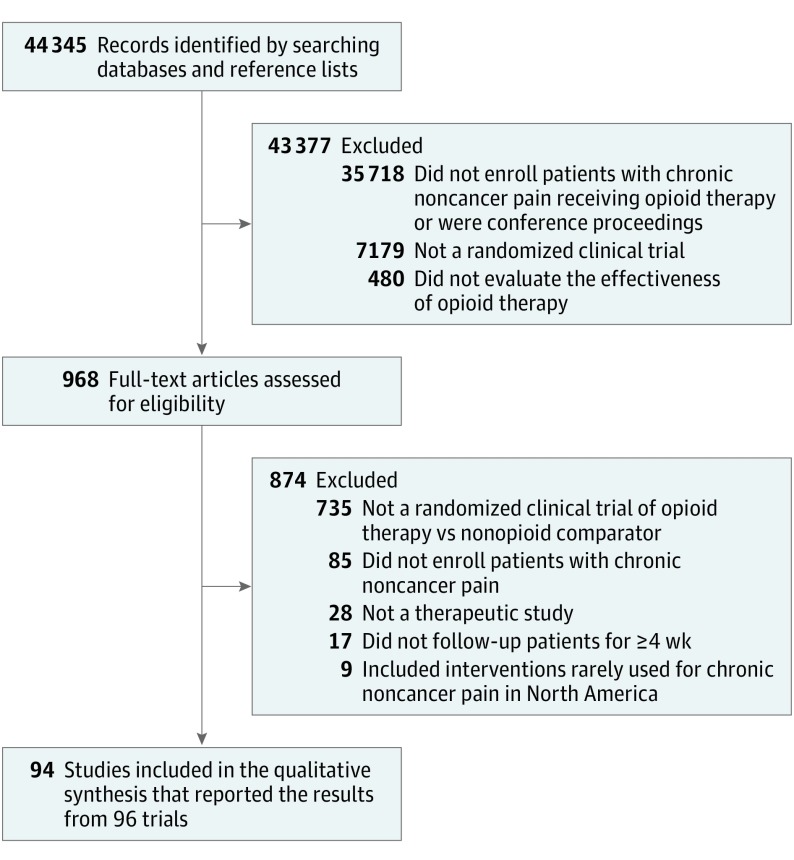
Of 44 345 citations, 88 English and 5 non-English reports met eligibility criteria. Three articles reported 2 RCTs each, resulting in 96 trials with 26 169 patients (Figure 1 and eAppendix 2 in Supplement 2). There was agreement between reviewers at the full-text review stage (κ = 0.78). Of the 3 authors contacted for clarification of eligibility criteria, only 1 responded. Of the 11 authors contacted for additional data, only 2 responded.
Figure 1. Diagram of the Study Selection Process for the Systematic Review and Meta-analysis.
Study Characteristics
Among patients in the eligible trials, the median of the mean age was 58 years (interquartile range [IQR], 51-61 years). Among the 91 trials reporting sex distribution, 61% (15 397 of 25 462) of enrolled patients were female (median of individual trials, 58%; IQR, 47%-64%). Six trials included patients with different types of chronic pain, 25 included patients with neuropathic pain, 32 included patients with nociceptive pain, and 33 included patients with central sensitization (pain present in the absence of tissue damage; eTable 3 in Supplement 2).
Among 51 nonenrichment trials, the mean pain score at baseline was 6.54 cm on a 10-cm VAS (median of individual trials, 6.38 cm [IQR, 5.72-6.96 cm]; eTable 4 in Supplement 2). Of 83 trials comparing opioids with placebo, 14 added opioids or placebo to the pretrial analgesic therapy, 44 allowed additional analgesics on a limited basis, 5 were unclear regarding additional analgesic therapy, and 20 did not permit participants to receive additional analgesic therapy.
Among patients in the opioid groups in 87 trials, the median of the average morphine-equivalent dose per day was 45.0 mg (IQR, 28.2-78.3 mg; range, 7.5-242.7 mg). The median follow-up was 60 days (IQR, 30-84 days). There were 9 RCTs (9%) that reported no industry funding, 76 (79%) that reported receiving industry funding, and 11 (12%) that did not specify funding type.
Six trials (6%) reported whether patients were involved in litigation or receiving disability benefits36,37,38,39,40,41; one of these trials39 enrolled 20 patients receiving compensation benefits and 1 patient with ongoing litigation. Sixty-nine trials (72%) excluded patients with current or prior substance use disorder and 45 trials (47%) excluded patients who had a diagnosed mental illness or were taking a psychotropic medication. No trials reported rates of addiction or enrollment of patients with a substance use disorder or other mental illness. Two trials reported rates of accidental opioid overdose. One trial of buprenorphine reported no accidental overdoses among 254 patients.42 Another trial reported 1 accidental overdose with respiratory arrest among 191 patients in a trial of extended-release hydrocodone.38
Risk of Bias
All trials were at risk of bias for at least 1 of the following domains; however, 51 (53%) adequately generated their randomization sequence, 48 (50%) adequately concealed allocation, 84 (88%) blinded patients, 84 (88%) blinded caregivers, 83 (87%) blinded data collectors, 82 (85%) blinded outcome assessors, and 6 (6%) included a blinded data analyst. There were 73 trials (76%) with frequent (≥20%) missing outcome data (eTable 5 in Supplement 2).
Outcomes for Opioids vs Placebo
Pain Relief
Although the difference did not reach the minimally important difference of 1 cm, opioids were associated with pain relief compared with placebo (weighted mean difference, −0.79 cm [95% CI, −0.90 to −0.68 cm] on a 10-cm VAS for pain, P < .001; modeled risk difference for achieving the minimally important difference, 13.6% [95% CI, 11.8% to 15.4%]). Studies with longer follow-up reported less pain relief (eFigures 1 and 2 in Supplement 2; P = .04 for interaction).
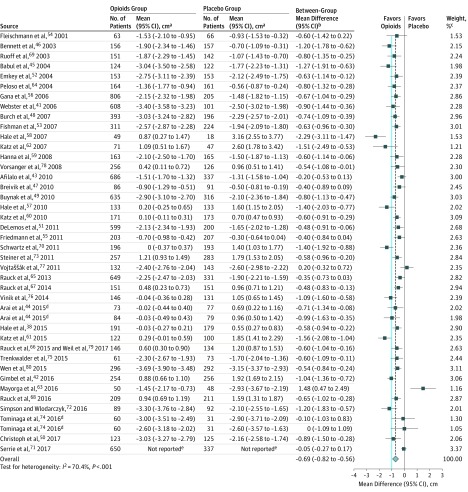
High-quality evidence from 42 RCTs that followed up patients for 3 months or longer (16 617 patients)38,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80 found that opioids were associated with reduced pain vs placebo (weighted mean difference, −0.69 cm [95% CI, −0.82 to −0.56 cm] on a 10-cm VAS for pain, P < .001; modeled risk difference for achieving the minimally important difference, 11.9% [95% CI, 9.7% to 14.1%]; Figure 2, the Table, and eFigure 3 in Supplement 2). The original data for pain relief appear in eTable 6 in Supplement 2. There were no differences in the magnitude of association based on category of chronic noncancer pain (eFigures 4-6 and eTable 7 in Supplement 2; range, P = .13 to P = .45 for interaction).
Figure 2. Pain Relief on a 10-cm Visual Analog Scale Among Patients With Chronic Noncancer Pain Who Received Opioids vs Placebo in 42 High-Quality Randomized Clinical Trials.
The blue line represents the minimally important difference of 1 cm on the 10-cm visual analog scale for pain. The dashed vertical line represents the overall pooled measure of association.
aWithin-group change from baseline data.
bBetween-group differences in change from baseline data.
cWeights are from random-effects analyses.
dArticle reported results from 2 randomized clinical trials.
eArticle only reported between-group mean difference and 95% CI.
Table. GRADE Evidence Profile of Opioids vs Placebo for Patients With Chronic Noncancer Pain Included in Randomized Clinical Trials.
| Outcome Measure | No. of Trials (N = 96) |
No. of Patients (N = 26 169) |
Follow-up, mo | Serious Risk of Bias?a |
I2 (95% CI), %b | Serious Indirectness or Imprecision?c |
P Value for Publication Biasd |
No. (%) of Patients Who Achieved the MID | Risk Difference for Achieving the MID (95% CI), % |
WMD (95% CI) | Quality of Evidence |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Opioids | |||||||||||
| Paine | 42 | 16 617 | 3-6f | No | 70.4 (59.5 to 78.4) |
No | .07 | 3232 (48.7) | 6048 (60.6) | 11.9 (9.7 to 14.1) |
−0.69 cm (−0.82 to −0.56) |
High |
| Physical functioningg | 51 | 15 754 | 1-6 | No | 65.7 (53.9 to 74.4) |
No | .06 | 2992 (46.1) | 5058 (54.6) | 8.5 (5.9 to 11.2) |
2.04 points (1.41 to 2.68) |
High |
| Emotional functioningh | 23i | 8962 | 1-4 | No | 47.2 (14.0 to 67.6) |
Noj | .86 | 1141 (35.0) | 1899 (33.3) | −1.7 (−4.2 to 0.8) |
−0.44 points (−1.09 to 0.20) |
High |
| Role functioningk | 16i | 5329 | 1-4 | No | 18.3 (0 to 54.6) |
Noj | .89 | 1113 (48.0) | 1475 (49.0) | 1.0 (−0.7 to 2.6) |
0.87 points (−0.54 to 2.28) |
High |
| Social functioningl | 29 | 7623 | 1-4 | No | 19.7 (0 to 49.4) |
No | .84 | 1527 (43.8) | 1920 (46.4) | 2.6 (0.7 to 4.5) |
1.58 points (0.45 to 2.70) |
High |
| Sleep qualitym | 15 | 6585 | 3-6f | No | 60.3 (30.0 to 77.5) |
No | .06 | 1232 (47.2) | 2111 (53.1) | 5.9 (2.8 to 9.1) |
3.42 mm (1.58 to 5.26) |
High |
| Vomitingn | ||||||||||||
| Enrichment trials | 18 | 5961 | 1.5-4 | No | 0 (0 to 41.3) |
No | .65 | 68 (2.3)o | 179 (5.9)o | 3.6 (2.1 to 5.4) |
RR, 2.50 (1.89 to 3.30) |
High |
| Nonenrichment trials | 33 | 11 268 | 1-6 | No | 0 (0 to 36.4) |
No | .23 | 96 (2.3)o | 667 (9.4)o | 7.1 (5.4 to 9.3) |
RR, 4.12 (3.34 to 5.07) |
High |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development and Evaluation; MID, minimally important difference; RR, relative risk; SF-36, 36-item Short Form; WMD, weighted mean difference.
An I2 value between 75% and 100% indicates that heterogeneity may be considerable.
Indirectness results if the intervention, patients, or outcomes are different from the research question under investigation. Imprecision refers to situations in which the 95% CI includes both benefit and harm.
Tested using a symmetric funnel plot and the Begg test. Publication bias was not detected in any of the studies.
Measured using a visual analog scale (VAS). Score range, 0 to 10 cm; lower is better (the MID is 1 cm).27
Compared with placebo, opioids were associated with less pain relief (P = .04 for interaction) and less improvement in sleep quality (P = .03 for interaction) in studies with longer follow-up.
Measured using the SF-36 physical component score. Score range, 0 to 100 points; higher is better (the MID is 5 points).28
Measured using the SF-36 mental component score. Score range, 0 to 100 points; higher is better (the MID is 5 points).28
Compared with placebo, opioids were associated with less improvement in emotional functioning (P = .01 for interaction) and role functioning (P = .007 for interaction) in studies that reported change scores directly vs studies for which change scores were converted from baseline and end-of-study scores.
Rating down for imprecision was not performed because the 95% CI did not include patient-important effects for either benefit or harm.
Measured using the SF-36 subscale for role limitations due to physical problems. Score range, 0 to 100 points; higher is better (the MID is 10 points).28
Measured using the SF-36 subscale for social functioning. Score range, 0 to 100 points; higher is better (the MID is 10 points).28
Measured using the SF-36 sleep quality 100-mm VAS. Score range, 0 to 100 mm; higher is better (the MID is 10 mm).28
Enrichment trials precede randomization with an open-label treatment phase during which patients with problematic adverse events, poor response to treatment, or both are excluded. Compared with placebo, opioids were associated with vomiting, but less so for enrichment vs nonenrichment trials (P = .007 for interaction).
These patients experienced vomiting.
Physical Functioning
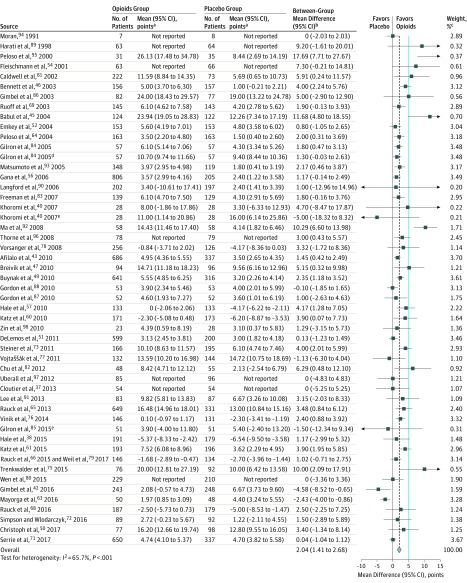
High-quality evidence from 51 RCTs (15 754 patients)37,38,40,42,43,45,46,47,49,50,51,52,54,56,57,60,61,63,64,65,66,68,69,71,72,73,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98 showed opioids were associated with a small improvement in physical functioning compared with placebo, but did not meet the criterion for the minimally important difference (weighted mean difference, 2.04 points [95% CI, 1.41-2.68 points] on the 100-point SF-36 physical component score, P < .001; minimally important difference, 5 points; modeled risk difference for achieving the minimally important difference, 8.5% [95% CI, 5.9%-11.2%]; the Table, Figure 3, and eFigure 7 in Supplement 2). Two trials reporting only P values with significant improvement in physical functioning were excluded from the pooled analysis.41,55
Figure 3. Physical Functioning Assessed by the 100-Point 36-Item Short Form Physical Component Score Among Patients With Chronic Noncancer Pain Who Received Opioids vs Placebo in 51 High-Quality Randomized Clinical Trials.
The blue line represents the minimally important difference of 5 points on the 100-point 36-item Short Form physical component score. The dashed vertical line represents the overall pooled measure of association.
aWithin-group change from baseline data.
bBetween-group differences in change from baseline data.
cWeights are from random-effects analyses.
dComparison was morphine plus gabapentin vs gabapentin alone.
eComparison was morphine plus nortriptyline vs nortriptyline alone.
Emotional Functioning
Opioids were not significantly associated with emotional functioning compared with placebo (weighted mean difference, 0.14 points [95% CI, −0.58 to 0.86 points] on the 100-point SF-36 mental component score, P = .70). We found a subgroup effect among studies with reported vs converted change scores (eFigure 8 in Supplement 2; P = .01 for interaction). High-quality evidence from 23 RCTs (8962 patients) reporting actual change scores indicated that opioids were not associated with emotional functioning (weighted mean difference, −0.44 points [95% CI, −1.09 to 0.20 points] on the 100-point SF-36 mental component score, P = .53; Table).
Role Functioning
Opioids were associated with improved role functioning compared with placebo (weighted mean difference, 2.80 points [95% CI, 0.99 to 4.61 points] on the 100-point SF-36 subscale for role limitations due to physical problems, P < .001); however, the association was smaller in studies with reported vs converted change scores (eFigure 9 in Supplement 2; P = .007 for interaction). When restricted to trials reporting actual change, high-quality evidence from 16 RCTs (5329 patients) demonstrated no association of opioids on role functioning compared with placebo (weighted mean difference, 0.87 points [95% CI, −0.54 to 2.28 points] on the 100-point SF-36 subscale for role limitations due to physical problems, P = .23; Table).
Social Functioning
High-quality evidence from 29 RCTs (7623 patients) showed an association of opioids with improved social functioning compared with placebo but did not meet the minimally important difference criterion (weighted mean difference, 1.58 points [95% CI, 0.45-2.70 points] on the 100-point SF-36 subscale for social functioning, P = .006; minimally important difference, 10 points; modeled risk difference for achieving the minimally important difference, 2.6% [95% CI, 0.7%-4.5%]; Table and eFigure 10 in Supplement 2).
Sleep Quality
Opioids were associated with improved sleep quality compared with placebo (weighted mean difference, 4.56 mm [95% CI, 2.88-6.24 mm] on the SF-36 sleep quality 100-mm VAS, P < .001; minimally important difference, 10 mm; modeled risk difference for achieving the minimally important difference, 5.9% [95% CI, 3.7%-8.1%]; eFigure 11 in Supplement 2); however, the association was smaller in studies with longer follow-up (eFigures 11 and 12 in Supplement 2; P = .03 for interaction). High-quality evidence from 15 RCTs (6585 patients) with follow-up lasting 3 months or longer found that opioids were associated with a small improvement in sleep quality compared with placebo but did not meet the criterion for the minimally important difference (weighted mean difference, 3.42 mm [95% CI, 1.58-5.26 mm] on the SF-36 sleep quality 100-mm VAS, P < .001; modeled risk difference for the minimally important difference, 5.9% [95% CI, 2.8%-9.1%]; Table).
Vomiting
Opioids were associated with an increased incidence of vomiting; however, this association was less in the 18 enrichment RCTs (5961 patients) compared with placebo (RR, 2.50 [95% CI, 1.89-3.30], P < .001; risk difference, 3.6% [95% CI, 2.1%-5.4%]) than in 33 nonenrichment RCTs (11 268 patients) compared with placebo (RR, 4.12 [95% CI, 3.34-5.07], P < .001; risk difference, 7.1% [95% CI, 5.4%-9.3%]; Table and eFigure 13 in Supplement 2; P = .007 for interaction). At least 20 RCTs reported each of the following adverse events: nausea, constipation, dizziness, drowsiness, headache, pruritus, and dry mouth. Except for headache, opioid use was associated with a higher incidence of these adverse events compared with placebo (eTable 8 in Supplement 2).
Outcomes for Opioids vs Active Comparators
Opioids vs Nonsteroidal Anti-Inflammatory Drugs
Moderate-quality evidence from 9 RCTs (1431 patients) showed no difference in the association of opioids vs nonsteroidal anti-inflammatory drugs for pain relief (weighted mean difference, −0.60 cm [95% CI, −1.54 to 0.34 cm] on the 10-cm VAS for pain, P = .21). Moderate-quality evidence from 7 RCTs (1311 patients) suggested no difference in physical functioning between opioids and nonsteroidal anti-inflammatory drugs (weighted mean difference, −0.90 points [95% CI, −2.69 to 0.89 points] on the 100-point SF-36 physical component score, P = .33). High-quality evidence from 5 RCTs (2632 patients) showed an association of opioids with vomiting compared with nonsteroidal anti-inflammatory drugs (RR, 4.71 [95% CI, 2.92 to 7.60], P < .001; risk difference, 6.3% [95% CI, 3.2% to 11.1%]; eTable 9 in Supplement 2).
Opioids vs Tricyclic Antidepressants
Low-quality evidence from 3 RCTs (246 patients) suggested no difference in pain relief between opioids and nortriptyline (weighted mean difference, −0.13 cm [95% CI, −0.99 to 0.74 cm] on the 10-cm VAS for pain, P = .78). Low-quality evidence from 2 trials (158 patients) suggested no difference in physical functioning (weighted mean difference, −5.31 points [95% CI, −13.77 to 3.14 points] on the 100-point SF-36 physical component score, P = .22; eTable 10 in Supplement 2).
Opioids vs Anticonvulsants
Moderate-quality evidence from 3 RCTs (303 patients) suggested opioids were associated with greater pain relief than anticonvulsants (weighted mean difference, −0.90 cm [95% CI, −1.65 to −0.14 cm] on the 10-cm VAS for pain, P = .02; minimally important difference, 1 cm; modeled risk difference for achieving the minimally important difference, 16.2% [95% CI, 2.8% to 26.1%]). Low-quality evidence suggested no difference in physical functioning (weighted mean difference, 0.45 points [95% CI, −5.77 to 6.66 points] on the 100-point SF-36 physical component score, P = .89; eTable 11 in Supplement 2).
Opioids vs Synthetic Cannabinoids
Low-quality evidence from 1 crossover trial suggested no difference between opioids and nabilone for pain relief (73 patients; mean difference, −0.13 cm [95% CI, −1.04 to 0.77 cm] on the 10-cm VAS for pain, P = .77) or physical functioning (71 patients; mean difference, −1.2 points [95% CI, −4.50 to 2.10 points] on the 100-point SF-36 physical component score, P = .48; eTable 12 in Supplement 2).
Opioids vs Usual Care
Compared with usual care, low-quality evidence from 1 trial (111 patients) showed an association of opioids with greater pain relief (mean difference, −2.06 cm [95% CI, −2.65 to −1.48 cm] on the 10-cm VAS for pain, P < .001; minimally important difference, 1 cm; modeled risk difference for achieving the minimally important difference, 21.1% [95% CI, 18.7% to 22.1%]; eTable 13 in Supplement 2). The associations with additional outcomes for opioids vs active comparators appear in eTables 9 through 13 in Supplement 2.
Additional Analyses
Most eligible trials allowed for postrandomization titration of opioid dose, which precluded between-trial subgroup analyses of higher vs lower doses of opioids. In 6 RCTs that compared different doses of opioids, meta-regression of moderate-quality evidence showed no dose response for pain relief (P = .39), functional recovery (P = .22), or gastrointestinal adverse events (P = .12) (eTable 14 and eFigures 14-16 in Supplement 2).
No additional subgroup analyses or meta-regressions proved credible (eTables 15-17 in Supplement 2). Associations were independent of whether trials administered pure opioids or opioids combined with acetaminophen; subgroup analysis found 1 significant test of interaction (P = .002 for interaction), suggesting an association with improved role functioning with combination products, but with low credibility (eTable 15b in Supplement 2).
Sensitivity analyses excluding data imputation for nonsignificant effects showed larger but unimportant differences in measures of association (eTable 18 in Supplement 2). Sensitivity analyses using the Hartung-Knapp-Sidik-Jonkman method for pooling showed consistent results with the DerSimonian-Laird method (eTable 19 in Supplement 2).
Discussion
Compared with placebo, opioids were associated with (1) small improvements in pain, physical functioning, and sleep quality; (2) unimportant improvements in social functioning; and (3) no improvements in emotional functioning or role functioning. Compared with placebo, opioids were associated with increased vomiting, drowsiness, constipation, dizziness, nausea, dry mouth, and pruritus.
Moderate- to low-quality evidence suggested that opioids were associated with similar improvements in pain and physical functioning compared with nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, and synthetic cannabinoids and were associated with small improvements in pain but not physical functioning compared with anticonvulsants. These results were restricted to treatment lasting 1 to 6 months and may not apply to individuals with substance use disorder or other mental illness, to those involved in litigation, or to those receiving disability benefits.
Opioids were associated with less pain relief during longer trials perhaps as a result of opioid tolerance or opioid-induced hyperalgesia (a condition in which opioid use results in hypersensitivity to painful stimuli).99 A reduced association with benefit over time might lead to prescription of higher opioid doses and consequent harms.32 Moreover, long-term opioid therapy causes physical dependence,100,101 and symptoms of opioid withdrawal (including pain) resolve when opioids are resumed. Therefore, patients may continue to use opioids after analgesic benefits have waned to avoid withdrawal.102
Although clinical practice guidelines discourage long-term opioid therapy for headache, fibromyalgia, or axial low back pain,103,104 we found no evidence for differential condition-specific associations with neuropathic, nociceptive, or central sensitization conditions. Prior inferences may have been driven by systematic reviews focusing on average effects alone.105,106 The limitations of calculating the average benefit associated with opioids are (1) the assumption that all patients experience comparable analgesia and (2) lack of consideration for the distribution around the mean and the proportion of patients who achieve the minimally important difference.107 Therefore, we converted the average effects to the proportion of responders. Based on a prior study,19 some patients may find the modeled proportion of 12% for achieving the minimally important difference for pain relief warrants a trial of treatment with opioids.
There were no differences in the associations of opioid dose with outcomes. This result was consistent with a prior RCT.108 Although prescription of high-dose opioids (≥200 mg of a morphine-equivalent dose per day) is common,109,110 only 21 of 96 trials addressed mean or median morphine-equivalent doses per day of 90 mg or greater.
Strengths of this review included (1) a comprehensive search for eligible RCTs in any language; (2) data imputation for missing nonsignificant outcomes; (3) use of minimally important differences; and (4) sensitivity analyses that addressed methodological differences, length of follow-up, and reported vs converted change scores.
Limitations
This review has several limitations. First, it was not possible to assess the long-term associations of opioids with chronic noncancer pain because no trial followed up patients for longer than 6 months. Second, none of the included studies provided rates of developing opioid use disorder and only 2 reported rates of overdose. Third, numerous outcomes and comparisons were evaluated, including subgroup analyses. Some findings might be statistically significant by chance. Fourth, subgroup effects could not be evaluated for opioids vs active comparators when there were less than 2 trials in each subgroup. Fifth, the modeling of risk difference for achieving the minimally important difference was based on assumptions that could not be directly assessed and might not have been met.
Sixth, heterogeneity associated with pooled estimates for pain relief and functional improvement among trials of opioids vs placebo may have reduced evidence quality. Evidence quality was not downgraded because the magnitude and direction of the effects was largely consistent. Seventh, the quality of the evidence ratings are largely subjective and some might disagree with our assessments. Eighth, although litigation and wage replacement benefits likely influence treatment effects, there were insufficient data in the included trials to make conclusions regarding these issues. Ninth, trials of opioid therapy for chronic noncancer pain excluded patients with current or prior substance use disorders or other active mental illness; however, more than half of opioids prescribed in the United States are for patients diagnosed with a mental health disorder.111,112
Conclusions
In this meta-analysis of RCTs of patients with chronic noncancer pain, evidence from high-quality studies showed that opioid use was associated with statistically significant but small improvements in pain and physical functioning, and increased risk of vomiting compared with placebo. Comparisons of opioids with nonopioid alternatives suggested that the benefit for pain and functioning may be similar, although the evidence was from studies of only low to moderate quality.
Trial protocol
eAppendix 1: Literature search strategies
eAppendix 2: Reference list of eligible trials
eTable 1. Conversion factors for opioids
eTable 2. Criteria for assessing the credibility of significant subgroup effects
eTable 3. Characteristics of 96 eligible randomized clinical trials
eTable 4. Summary of baseline pain scores in 23 enrichment trials and 51 non-enrichment trials
eTable 5. Risk of bias assessment of 96 eligible randomized clinical trials
eTable 6. Original data for pain relief reported by 80 randomized clinical trials of opioids vs. placebo
eTable 7. Multiple meta-regression of length of follow-up, clinical condition (neuropathic vs. non-neuropathic conditions) and pain relief
eTable 8. GRADE evidence profile of adverse events for opioids vs. placebo and patients with chronic noncancer pain from 83 randomized clinical trials
eTable 9. GRADE evidence profile of opioids vs. NSAIDs for patients with chronic noncancer pain from 12 randomized clinical trials
eTable 10. GRADE evidence profile of opioids vs. tricyclic antidepressants for patients with chronic noncancer pain from 3 randomized clinical trials
eTable 11. GRADE evidence profile of opioids vs. anticonvulsants for patients with chronic noncancer pain from 3 randomized clinical trials
eTable 12. GRADE evidence profile of opioids vs. nabilone for patients with chronic noncancer pain from 1 randomized clinical trial
eTable 13. GRADE evidence profile of opioids vs. usual care for patients with chronic noncancer pain from 1 randomized clinical trial
eTable 14. Results of within-trial comparisons of different opioid doses
eTable 15a. Subgroup analyses for pain, physical and emotional functioning for randomized clinical trials of opioids vs. placebo
eTable 15b. Subgroup analyses for role and social functioning, and sleep quality for randomized clinical trials of opioids vs. placebo
eTable 15c. Subgroup analyses for vomiting, nausea and constipation for randomized clinical trials of opioids vs. placebo
eTable 15d. Subgroup analyses for dizziness, drowsiness, and headache for randomized clinical trials of opioids vs. placebo
eTable 15e. Subgroup analyses for pruritus and dry mouth for randomized clinical trials of opioids vs. placebo
eTable 16a. Subgroup analyses for pain, physical functioning and vomiting for randomized clinical trials of opioids vs. NSAIDs
eTable 16b. Subgroup analyses for nausea, constipation and dizziness for randomized clinical trials of opioids vs. NSAIDs
eTable 16c. Subgroup analyses for drowsiness, headache and pruritus for randomized clinical trials of opioids vs. NSAIDs
eTable 17. Meta-regressions of length of follow-up and proportion of loss to follow-up for randomized clinical trials of opioids vs. placebo and opioids vs. NSAIDs
eTable 18. Sensitivity analysis by excluding studies with data imputed for non-significant results
eTable 19. Sensitivity analysis of DerSimonian-Laird method vs. Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis
eFigure 1. Meta-regression for pain relief and length of follow-up for 80 randomized clinical trials of opioids vs. placebo
eFigure 2. Subgroup analysis for pain relief and length of follow-up (<3 months vs. ≥3 months) from 80 randomized clinical trials of opioids vs. placebo
eFigure 3. Funnel plot for pain relief at ≥ 3 months follow-up for 42 randomized clinical trials of opioids vs. placebo
eFigure 4. Subgroup analysis for pain relief and objective vs. subjective conditions from 76 randomized clinical trials of opioids vs. placebo
eFigure 5. Subgroup analysis for pain relief and neuropathic vs. nociceptive vs. central sensitization from 76 randomized clinical trials of opioids vs. placebo
eFigure 6. Subgroup analysis for pain relief and neuropathic vs. non-neuropathic conditions from 76 randomized clinical trials of opioids vs. placebo
eFigure 7. Funnel plot for physical function for 51 randomized clinical trials of opioids vs. placebo
eFigure 8. Emotional functioning on the SF-36 mental component summary scale among chronic noncancer pain patients receiving opioids vs. placebo from 37 randomized clinical trials
eFigure 9. Role functioning on the SF-36 role limitations due to physical problems sub-scale among chronic noncancer pain patients receiving opioids vs. placebo from 32 randomized clinical trials
eFigure 10. Social functioning on the SF-36 social functioning sub-scale among chronic noncancer pain patients receiving opioids vs. placebo from 29 randomized clinical trials
eFigure 11. Sleep quality on a 100 mm VAS among chronic noncancer pain patients receiving opioids vs. placebo from 31 randomized clinical trials
eFigure 12. Meta-regression for sleep quality and length of follow-up for 31 randomized clinical trials of opioids vs. placebo
eFigure 13. Vomiting among chronic noncancer pain patients receiving opioids vs. placebo from 51 randomized clinical trials
eFigure 14. Meta-regression for pain relief and opioid dose among 5 trials with 14 within-study comparisons
eFigure 15. Meta-regression for physical function and opioid dose among 4 trials with 11 within-study comparisons
eFigure 16. Meta-regression for gastrointestinal adverse events and opioid dose among 5 trials with 14 within-study comparisons
References
- 1.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. doi: 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227. doi: 10.2105/AJPH.2007.131714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, McLellan AT. Opioid abuse in chronic pain. N Engl J Med. 2016;374(13):1253-1263. doi: 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- 4.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. doi: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 5.International Narcotics Control Board Narcotic drugs: estimated world requirements for 2017: statistics for 2015. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2016/Narcotic_Drugs_Publication_2016.pdf. Accessed October 25, 2018.
- 6.International Narcotics Control Board Narcotic drugs: estimated world requirements for 2018: statistics for 2016. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2017/Narcotic_drugs_technical_publication_2017.pdf. Accessed October 25, 2018.
- 7.Centers for Disease Control and Prevention Key messages: prescription opioid data. https://www.cdc.gov/drugoverdose/data/prescribing.html. Accessed October 25, 2018.
- 8.Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003-2013. JAMA. 2015;314(14):1468-1478. doi: 10.1001/jama.2015.11859 [DOI] [PubMed] [Google Scholar]
- 9.Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain. Pain. 2015;156(4):569-576. doi: 10.1097/01.j.pain.0000460357.01998.f1 [DOI] [PubMed] [Google Scholar]
- 10.Ray WA, Chung CP, Murray KT, et al. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423. doi: 10.1001/jama.2016.7789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain. Ann Intern Med. 2015;162(4):276-286. doi: 10.7326/M14-2559 [DOI] [PubMed] [Google Scholar]
- 12.Furlan A, Chaparro LE, Irvin E, Mailis-Gagnon A. A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic noncancer pain. Pain Res Manag. 2011;16(5):337-351. doi: 10.1155/2011/465281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busse JW, Bartlett SJ, Dougados M, et al. Optimal strategies for reporting pain in clinical trials and systematic reviews. J Rheumatol. 2015;42(10):1962-1970. doi: 10.3899/jrheum.141440 [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:c117. doi: 10.1136/bmj.c117 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 16.Busse JW, Schandelmaier S, Kamaleldin M, et al. Opioids for chronic non-cancer pain. Syst Rev. 2013;2(66):66. doi: 10.1186/2046-4053-2-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi: 10.1016/j.pain.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 18.Turk DC, Dworkin RH, Revicki D, et al. Identifying important outcome domains for chronic pain clinical trials. Pain. 2008;137(2):276-285. doi: 10.1016/j.pain.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 19.Goshua A, Craigie S, Guyatt GH, et al. Patient values and preferences regarding opioids for chronic noncancer pain: a systematic review [published online November 22, 2017]. Pain Med. doi: 10.1093/pm/pnx274 [DOI] [PubMed] [Google Scholar]
- 20.Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi: 10.1002/pds.3945 [DOI] [PubMed] [Google Scholar]
- 21.Tendal B, Nüesch E, Higgins JP, Jüni P, Gøtzsche PC. Multiplicity of data in trial reports and the reliability of meta-analyses: empirical study. BMJ. 2011;343:d4829. doi: 10.1136/bmj.d4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akl EA, Sun X, Busse JW, et al. Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol. 2012;65(3):262-267. doi: 10.1016/j.jclinepi.2011.04.015 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 25.Thorlund K, Walter SD, Johnston BC, et al. Pooling health-related quality of life outcomes in meta-analysis—a tutorial and review of methods for enhancing interpretability. Res Synth Methods. 2011;2(3):188-203. doi: 10.1002/jrsm.46 [DOI] [PubMed] [Google Scholar]
- 26.Schünemann HJ, Guyatt GH. Commentary—goodbye M(C)ID! hello MID, where do you come from? Health Serv Res. 2005;40(2):593-597. doi: 10.1111/j.1475-6773.2005.0k375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 28.Ward MM, Guthrie LC, Alba MI. Clinically important changes in Short Form 36 health survey scales for use in rheumatoid arthritis clinical trials. Arthritis Care Res (Hoboken). 2014;66(12):1783-1789. doi: 10.1002/acr.22392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murad MH, Montori VM, Ioannidis JPA, Prasad K, Cook DJ, Guyatt GH. Fixed-effects and random-effects models In: Users' Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. 3rd ed New York, NY: McGraw-Hill Education; 2015:507-514. [Google Scholar]
- 30.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelman A, Hill J. Missing-data imputation In: Data Analysis Using Regression and Multilevel/Hierarchical Models. New York, NY: Cambridge University Press; 2007:529-543. [Google Scholar]
- 32.Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189(18):E659-E666. doi: 10.1503/cmaj.170363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions: version 5.1.0; updated March 2011. http://handbook-5-1.cochrane.org/. Accessed October 25, 2018.
- 35.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 36.Caldwell JR, Hale ME, Boyd RE, et al. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs. J Rheumatol. 1999;26(4):862-869. [PubMed] [Google Scholar]
- 37.Cloutier C, Taliano J, O’Mahony W, et al. Controlled-release oxycodone and naloxone in the treatment of chronic low back pain: a placebo-controlled, randomized study [published correction appears in Pain Res Manag. 2013;18(6):328]. Pain Res Manag. 2013;18(2):75-82. doi: 10.1155/2013/164609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hale ME, Zimmerman TR, Eyal E, Malamut R. Efficacy and safety of a hydrocodone extended-release tablet formulated with abuse-deterrence technology in patients with moderate-to-severe chronic low back pain. J Opioid Manag. 2015;11(6):507-518. doi: 10.5055/jom.2015.0304 [DOI] [PubMed] [Google Scholar]
- 39.Jamison RN, Raymond SA, Slawsby EA, Nedeljkovic SS, Katz NP. Opioid therapy for chronic noncancer back pain: a randomized prospective study. Spine (Phila Pa 1976). 1998;23(23):2591-2600. doi: 10.1097/00007632-199812010-00014 [DOI] [PubMed] [Google Scholar]
- 40.Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs placebo in patients with chronic lumbar root pain. Pain. 2007;130(1-2):66-75. doi: 10.1016/j.pain.2006.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webster LR, Butera PG, Moran LV, et al. Oxytrex minimizes physical dependence while providing effective analgesia. J Pain. 2006;7(12):937-946. doi: 10.1016/j.jpain.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 42.Gimbel J, Spierings EL, Katz N, et al. Efficacy and tolerability of buccal buprenorphine in opioid-experienced patients with moderate to severe chronic low back pain [published correction appears in Pain. 2017;158(12):2366]. Pain. 2016;157(11):2517-2526. doi: 10.1097/j.pain.0000000000000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee. Clin Drug Investig. 2010;30(8):489-505. doi: 10.2165/11533440-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 44.Arai T, Kashimoto Y, Ukyo Y, Tominaga Y, Imanaka K. Two placebo-controlled, randomized withdrawal studies to evaluate the fentanyl 1 day patch in opioid-naïve patients with chronic pain. Curr Med Res Opin. 2015;31(12):2207-2218. doi: 10.1185/03007995.2015.1092127 [DOI] [PubMed] [Google Scholar]
- 45.Babul N, Noveck R, Chipman H, et al. Efficacy and safety of extended-release, once-daily tramadol in chronic pain. J Pain Symptom Manage. 2004;28(1):59-71. doi: 10.1016/j.jpainsymman.2003.11.006 [DOI] [PubMed] [Google Scholar]
- 46.Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain. Am J Med. 2003;114(7):537-545. doi: 10.1016/S0002-9343(03)00116-5 [DOI] [PubMed] [Google Scholar]
- 47.Breivik H, Ljosaa TM, Stengaard-Pedersen K, et al. A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of a low-dose 7-day buprenorphine transdermal patch in osteoarthritis patients naïve to potent opioids. Scand J Pain. 2010;1(3):122-141. doi: 10.1016/j.sjpain.2010.05.035 [DOI] [PubMed] [Google Scholar]
- 48.Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of tramadol contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage. 2007;34(3):328-338. doi: 10.1016/j.jpainsymman.2006.11.017 [DOI] [PubMed] [Google Scholar]
- 49.Buynak R, Shapiro DY, Okamoto A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain. Expert Opin Pharmacother. 2010;11(11):1787-1804. doi: 10.1517/14656566.2010.497720 [DOI] [PubMed] [Google Scholar]
- 50.Christoph A, Eerdekens MH, Kok M, et al. Cebranopadol, a novel first-in-class analgesic drug candidate. Pain. 2017;158(9):1813-1824. doi: 10.1097/j.pain.0000000000000986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeLemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip. Am J Ther. 2011;18(3):216-226. doi: 10.1097/MJT.0b013e3181cec307 [DOI] [PubMed] [Google Scholar]
- 52.Emkey R, Rosenthal N, Wu SC, et al. Efficacy and safety of tramadol/acetaminophen tablets (ultracet) as add-on therapy for osteoarthritis pain in subjects receiving a COX-2 nonsteroidal antiinflammatory drug. J Rheumatol. 2004;31(1):150-156. [PubMed] [Google Scholar]
- 53.Fishman RL, Kistler CJ, Ellerbusch MT, et al. Efficacy and safety of 12 weeks of osteoarthritic pain therapy with once-daily tramadol (tramadol contramid OAD). J Opioid Manag. 2007;3(5):273-280. doi: 10.5055/jom.2007.0015 [DOI] [PubMed] [Google Scholar]
- 54.Fleischmann RM, Caldwell JR, Roth SH, et al. Tramadol for the treatment of joint pain associated with osteoarthritis. Curr Ther Res Clin Exp. 2001;62(2):113-128. doi: 10.1016/S0011-393X(01)80021-7 [DOI] [Google Scholar]
- 55.Friedmann N, Klutzaritz V, Webster L. Efficacy and safety of an extended-release oxycodone (remoxy) formulation in patients with moderate to severe osteoarthritic pain. J Opioid Manag. 2011;7(3):193-202. doi: 10.5055/jom.2011.0062 [DOI] [PubMed] [Google Scholar]
- 56.Gana TJ, Pascual ML, Fleming RR, et al. Extended-release tramadol in the treatment of osteoarthritis. Curr Med Res Opin. 2006;22(7):1391-1401. doi: 10.1185/030079906X115595 [DOI] [PubMed] [Google Scholar]
- 57.Hale M, Khan A, Kutch M, Li S. Once-daily OROS hydromorphone ER compared with placebo in opioid-tolerant patients with chronic low back pain. Curr Med Res Opin. 2010;26(6):1505-1518. doi: 10.1185/03007995.2010.484723 [DOI] [PubMed] [Google Scholar]
- 58.Hale ME, Ahdieh H, Ma T, et al. Efficacy and safety of OPANA ER (oxymorphone extended release) for relief of moderate to severe chronic low back pain in opioid-experienced patients. J Pain. 2007;8(2):175-184. doi: 10.1016/j.jpain.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 59.Hanna M, O’Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur J Pain. 2008;12(6):804-813. doi: 10.1016/j.ejpain.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 60.Katz N, Hale M, Morris D, Stauffer J. Morphine sulfate and naltrexone hydrochloride extended release capsules in patients with chronic osteoarthritis pain. Postgrad Med. 2010;122(4):112-128. doi: 10.3810/pgm.2010.07.2179 [DOI] [PubMed] [Google Scholar]
- 61.Katz N, Kopecky EA, OʼConnor M, Brown RH, Fleming AB. A phase 3, multicenter, randomized, double-blind, placebo-controlled, safety, tolerability, and efficacy study of Xtampza ER in patients with moderate-to-severe chronic low back pain. Pain. 2015;156(12):2458-2467. doi: 10.1097/j.pain.0000000000000315 [DOI] [PubMed] [Google Scholar]
- 62.Katz N, Rauck R, Ahdieh H, et al. A 12-week, randomized, placebo-controlled trial assessing the safety and efficacy of oxymorphone extended release for opioid-naive patients with chronic low back pain. Curr Med Res Opin. 2007;23(1):117-128. doi: 10.1185/030079906X162692 [DOI] [PubMed] [Google Scholar]
- 63.Mayorga AJ, Wang S, Kelly KM, Thipphawong J. Efficacy and safety of fulranumab as monotherapy in patients with moderate to severe, chronic knee pain of primary osteoarthritis. Int J Clin Pract. 2016;70(6):493-505. doi: 10.1111/ijcp.12807 [DOI] [PubMed] [Google Scholar]
- 64.Peloso PM, Fortin L, Beaulieu A, et al. Analgesic efficacy and safety of tramadol/acetaminophen combination tablets (ultracet) in treatment of chronic low back pain. J Rheumatol. 2004;31(12):2454-2463. [PubMed] [Google Scholar]
- 65.Rauck R, Rapoport R, Thipphawong J. Results of a double-blind, placebo-controlled, fixed-dose assessment of once-daily OROS® hydromorphone ER in patients with moderate to severe pain associated with chronic osteoarthritis. Pain Pract. 2013;13(1):18-29. doi: 10.1111/j.1533-2500.2012.00555.x [DOI] [PubMed] [Google Scholar]
- 66.Rauck RL, Hale ME, Bass A, et al. A randomized double-blind, placebo-controlled efficacy and safety study of ALO-02 (extended-release oxycodone surrounding sequestered naltrexone) for moderate-to-severe chronic low back pain treatment. Pain. 2015;156(9):1660-1669. doi: 10.1097/j.pain.0000000000000230 [DOI] [PubMed] [Google Scholar]
- 67.Rauck RL, Nalamachu S, Wild JE, et al. Single-entity hydrocodone extended-release capsules in opioid-tolerant subjects with moderate-to-severe chronic low back pain. Pain Med. 2014;15(6):975-985. doi: 10.1111/pme.12377 [DOI] [PubMed] [Google Scholar]
- 68.Rauck RL, Potts J, Xiang Q, Tzanis E, Finn A. Efficacy and tolerability of buccal buprenorphine in opioid-naive patients with moderate to severe chronic low back pain. Postgrad Med. 2016;128(1):1-11. doi: 10.1080/00325481.2016.1128307 [DOI] [PubMed] [Google Scholar]
- 69.Ruoff GE, Rosenthal N, Jordan D, et al. Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain. Clin Ther. 2003;25(4):1123-1141. doi: 10.1016/S0149-2918(03)80071-1 [DOI] [PubMed] [Google Scholar]
- 70.Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy. Curr Med Res Opin. 2011;27(1):151-162. doi: 10.1185/03007995.2010.537589 [DOI] [PubMed] [Google Scholar]
- 71.Serrie A, Lange B, Steup A. Tapentadol prolonged-release for moderate-to-severe chronic osteoarthritis knee pain. Curr Med Res Opin. 2017;33(8):1423-1432. doi: 10.1080/03007995.2017.1335189 [DOI] [PubMed] [Google Scholar]
- 72.Simpson RW, Wlodarczyk JH. Transdermal buprenorphine relieves neuropathic pain. Diabetes Care. 2016;39(9):1493-1500. doi: 10.2337/dc16-0123 [DOI] [PubMed] [Google Scholar]
- 73.Steiner DJ, Sitar S, Wen W, et al. Efficacy and safety of the seven-day buprenorphine transdermal system in opioid-naïve patients with moderate to severe chronic low back pain. J Pain Symptom Manage. 2011;42(6):903-917. doi: 10.1016/j.jpainsymman.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 74.Tominaga Y, Koga H, Uchida N, et al. Methodological issues in conducting pilot trials in chronic pain as randomized, double-blind, placebo-controlled studies. Drug Res (Stuttg). 2016;66(7):363-370. doi: 10.1055/s-0042-107669 [DOI] [PubMed] [Google Scholar]
- 75.Trenkwalder C, Chaudhuri KR, Martinez-Martin P, et al. Prolonged-release oxycodone-naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA). Lancet Neurol. 2015;14(12):1161-1170. doi: 10.1016/S1474-4422(15)00243-4 [DOI] [PubMed] [Google Scholar]
- 76.Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37(8):2302-2309. doi: 10.2337/dc13-2291 [DOI] [PubMed] [Google Scholar]
- 77.Vojtaššák J, Vojtaššák J, Jacobs A, et al. A phase IIIb, multicentre, randomised, parallel-group, placebo-controlled, double-blind study to investigate the efficacy and safety of OROS hydromorphone in subjects with moderate-to-severe chronic pain induced by osteoarthritis of the hip or the knee. Pain Res Treat. 2011;2011:239501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vorsanger GJ, Xiang J, Gana TJ, et al. Extended-release tramadol (tramadol ER) in the treatment of chronic low back pain. J Opioid Manag. 2008;4(2):87-97. doi: 10.5055/jom.2008.0013 [DOI] [PubMed] [Google Scholar]
- 79.Weil AJ, Masters ET, Barsdorf AI, et al. Patient-reported health-related quality of life, work productivity, and activity impairment during treatment with ALO-02 (extended-release oxycodone and sequestered naltrexone) for moderate-to-severe chronic low back pain. Health Qual Life Outcomes. 2017;15(1):202. doi: 10.1186/s12955-017-0749-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wen W, Sitar S, Lynch SY, He E, Ripa SR. A multicenter, randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of single-entity, once-daily hydrocodone tablets in patients with uncontrolled moderate to severe chronic low back pain. Expert Opin Pharmacother. 2015;16(11):1593-1606. doi: 10.1517/14656566.2015.1060221 [DOI] [PubMed] [Google Scholar]
- 81.Caldwell JR, Rapoport RJ, Davis JC, et al. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain. J Pain Symptom Manage. 2002;23(4):278-291. doi: 10.1016/S0885-3924(02)00383-4 [DOI] [PubMed] [Google Scholar]
- 82.Chu LF, D’Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia. Pain. 2012;153(8):1583-1592. doi: 10.1016/j.pain.2012.02.028 [DOI] [PubMed] [Google Scholar]
- 83.Freeman R, Raskin P, Hewitt DJ, et al. ; CAPSS-237 Study Group . Randomized study of tramadol/acetaminophen versus placebo in painful diabetic peripheral neuropathy. Curr Med Res Opin. 2007;23(1):147-161. doi: 10.1185/030079906X162674 [DOI] [PubMed] [Google Scholar]
- 84.Gilron I, Bailey JM, Tu D, et al. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352(13):1324-1334. doi: 10.1056/NEJMoa042580 [DOI] [PubMed] [Google Scholar]
- 85.Gilron I, Tu D, Holden RR, et al. Combination of morphine with nortriptyline for neuropathic pain. Pain. 2015;156(8):1440-1448. doi: 10.1097/j.pain.0000000000000149 [DOI] [PubMed] [Google Scholar]
- 86.Gimbel JS, Richards P, Portenoy RK. Controlled-release oxycodone for pain in diabetic neuropathy. Neurology. 2003;60(6):927-934. doi: 10.1212/01.WNL.0000057720.36503.2C [DOI] [PubMed] [Google Scholar]
- 87.Gordon A, Callaghan D, Spink D, et al. Buprenorphine transdermal system in adults with chronic low back pain. Clin Ther. 2010;32(5):844-860. doi: 10.1016/j.clinthera.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 88.Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15(3):169-178. doi: 10.1155/2010/216725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50(6):1842-1846. doi: 10.1212/WNL.50.6.1842 [DOI] [PubMed] [Google Scholar]
- 90.Langford R, McKenna F, Ratcliffe S, et al. Transdermal fentanyl for improvement of pain and functioning in osteoarthritis. Arthritis Rheum. 2006;54(6):1829-1837. doi: 10.1002/art.21884 [DOI] [PubMed] [Google Scholar]
- 91.Lee JH, Lee CS; Ultracet ER Study Group . A randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of the extended-release tramadol hydrochloride/acetaminophen fixed-dose combination tablet for the treatment of chronic low back pain. Clin Ther. 2013;35(11):1830-1840. doi: 10.1016/j.clinthera.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 92.Ma K, Jiang W, Zhou Q, Du DP. The efficacy of oxycodone for management of acute pain episodes in chronic neck pain patients. Int J Clin Pract. 2008;62(2):241-247. doi: 10.1111/j.1742-1241.2007.01567.x [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto AK, Babul N, Ahdieh H. Oxymorphone extended-release tablets relieve moderate to severe pain and improve physical function in osteoarthritis. Pain Med. 2005;6(5):357-366. doi: 10.1111/j.1526-4637.2005.00057.x [DOI] [PubMed] [Google Scholar]
- 94.Moran C. MST continus tablets and pain control in severe rheumatoid arthritis. Br J Clin Res. 1991;2:1-12. [Google Scholar]
- 95.Peloso PM, Bellamy N, Bensen W, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip or knee. J Rheumatol. 2000;27(3):764-771. [PubMed] [Google Scholar]
- 96.Thorne C, Beaulieu AD, Callaghan DJ, et al. A randomized, double-blind, crossover comparison of the efficacy and safety of oral controlled-release tramadol and placebo in patients with painful osteoarthritis. Pain Res Manag. 2008;13(2):93-102. doi: 10.1155/2008/165421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uberall MA, Mueller-Schwefe GH, Terhaag B. Efficacy and safety of flupirtine modified release for the management of moderate to severe chronic low back pain. Curr Med Res Opin. 2012;28(10):1617-1634. doi: 10.1185/03007995.2012.726216 [DOI] [PubMed] [Google Scholar]
- 98.Zin CS, Nissen LM, O’Callaghan JP, et al. A randomized, controlled trial of oxycodone versus placebo in patients with postherpetic neuralgia and painful diabetic neuropathy treated with pregabalin. J Pain. 2010;11(5):462-471. doi: 10.1016/j.jpain.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 99.Chen L, Sein M, Vo T, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag. 2014;10(6):383-393. doi: 10.5055/jom.2014.0235 [DOI] [PubMed] [Google Scholar]
- 100.Salsitz EA. Chronic pain, chronic opioid addiction: a complex nexus. J Med Toxicol. 2016;12(1):54-57. doi: 10.1007/s13181-015-0521-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ballantyne JC. Opioid therapy in chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):201-218. doi: 10.1016/j.pmr.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 102.Juurlink DN. Rethinking “doing well” on chronic opioid therapy. CMAJ. 2017;189(39):E1222-E1223. doi: 10.1503/cmaj.170628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Häuser W, Schug S, Furlan AD. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain. Pain Rep. 2017;2(3):e599. doi: 10.1097/PR9.0000000000000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318-328. doi: 10.1136/annrheumdis-2016-209724 [DOI] [PubMed] [Google Scholar]
- 105.Ballantyne JC. Avoiding opioid analgesics for treatment of chronic low back pain. JAMA. 2016;315(22):2459-2460. doi: 10.1001/jama.2016.6753 [DOI] [PubMed] [Google Scholar]
- 106.Abdel Shaheed C, Maher CG, Williams KA, et al. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain. JAMA Intern Med. 2016;176(7):958-968. doi: 10.1001/jamainternmed.2016.1251 [DOI] [PubMed] [Google Scholar]
- 107.Guyatt GH, Juniper EF, Walter SD, et al. Interpreting treatment effects in randomised trials. BMJ. 1998;316(7132):690-693. doi: 10.1136/bmj.316.7132.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naliboff BD, Wu SM, Schieffer B, et al. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. J Pain. 2011;12(2):288-296. doi: 10.1016/j.jpain.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 109.Gomes T, Mamdani MM, Paterson JM, et al. Trends in high-dose opioid prescribing in Canada. Can Fam Physician. 2014;60(9):826-832. [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandes K, Martins D, Juurlink D, et al. High-dose opioid prescribing and opioid-related hospitalization. PLoS One. 2016;11(12):e0167479. doi: 10.1371/journal.pone.0167479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Edlund MJ, Martin BC, Devries A, et al. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders. Clin J Pain. 2010;26(1):1-8. doi: 10.1097/AJP.0b013e3181b99f35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30(4):407-417. doi: 10.3122/jabfm.2017.04.170112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eAppendix 1: Literature search strategies
eAppendix 2: Reference list of eligible trials
eTable 1. Conversion factors for opioids
eTable 2. Criteria for assessing the credibility of significant subgroup effects
eTable 3. Characteristics of 96 eligible randomized clinical trials
eTable 4. Summary of baseline pain scores in 23 enrichment trials and 51 non-enrichment trials
eTable 5. Risk of bias assessment of 96 eligible randomized clinical trials
eTable 6. Original data for pain relief reported by 80 randomized clinical trials of opioids vs. placebo
eTable 7. Multiple meta-regression of length of follow-up, clinical condition (neuropathic vs. non-neuropathic conditions) and pain relief
eTable 8. GRADE evidence profile of adverse events for opioids vs. placebo and patients with chronic noncancer pain from 83 randomized clinical trials
eTable 9. GRADE evidence profile of opioids vs. NSAIDs for patients with chronic noncancer pain from 12 randomized clinical trials
eTable 10. GRADE evidence profile of opioids vs. tricyclic antidepressants for patients with chronic noncancer pain from 3 randomized clinical trials
eTable 11. GRADE evidence profile of opioids vs. anticonvulsants for patients with chronic noncancer pain from 3 randomized clinical trials
eTable 12. GRADE evidence profile of opioids vs. nabilone for patients with chronic noncancer pain from 1 randomized clinical trial
eTable 13. GRADE evidence profile of opioids vs. usual care for patients with chronic noncancer pain from 1 randomized clinical trial
eTable 14. Results of within-trial comparisons of different opioid doses
eTable 15a. Subgroup analyses for pain, physical and emotional functioning for randomized clinical trials of opioids vs. placebo
eTable 15b. Subgroup analyses for role and social functioning, and sleep quality for randomized clinical trials of opioids vs. placebo
eTable 15c. Subgroup analyses for vomiting, nausea and constipation for randomized clinical trials of opioids vs. placebo
eTable 15d. Subgroup analyses for dizziness, drowsiness, and headache for randomized clinical trials of opioids vs. placebo
eTable 15e. Subgroup analyses for pruritus and dry mouth for randomized clinical trials of opioids vs. placebo
eTable 16a. Subgroup analyses for pain, physical functioning and vomiting for randomized clinical trials of opioids vs. NSAIDs
eTable 16b. Subgroup analyses for nausea, constipation and dizziness for randomized clinical trials of opioids vs. NSAIDs
eTable 16c. Subgroup analyses for drowsiness, headache and pruritus for randomized clinical trials of opioids vs. NSAIDs
eTable 17. Meta-regressions of length of follow-up and proportion of loss to follow-up for randomized clinical trials of opioids vs. placebo and opioids vs. NSAIDs
eTable 18. Sensitivity analysis by excluding studies with data imputed for non-significant results
eTable 19. Sensitivity analysis of DerSimonian-Laird method vs. Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis
eFigure 1. Meta-regression for pain relief and length of follow-up for 80 randomized clinical trials of opioids vs. placebo
eFigure 2. Subgroup analysis for pain relief and length of follow-up (<3 months vs. ≥3 months) from 80 randomized clinical trials of opioids vs. placebo
eFigure 3. Funnel plot for pain relief at ≥ 3 months follow-up for 42 randomized clinical trials of opioids vs. placebo
eFigure 4. Subgroup analysis for pain relief and objective vs. subjective conditions from 76 randomized clinical trials of opioids vs. placebo
eFigure 5. Subgroup analysis for pain relief and neuropathic vs. nociceptive vs. central sensitization from 76 randomized clinical trials of opioids vs. placebo
eFigure 6. Subgroup analysis for pain relief and neuropathic vs. non-neuropathic conditions from 76 randomized clinical trials of opioids vs. placebo
eFigure 7. Funnel plot for physical function for 51 randomized clinical trials of opioids vs. placebo
eFigure 8. Emotional functioning on the SF-36 mental component summary scale among chronic noncancer pain patients receiving opioids vs. placebo from 37 randomized clinical trials
eFigure 9. Role functioning on the SF-36 role limitations due to physical problems sub-scale among chronic noncancer pain patients receiving opioids vs. placebo from 32 randomized clinical trials
eFigure 10. Social functioning on the SF-36 social functioning sub-scale among chronic noncancer pain patients receiving opioids vs. placebo from 29 randomized clinical trials
eFigure 11. Sleep quality on a 100 mm VAS among chronic noncancer pain patients receiving opioids vs. placebo from 31 randomized clinical trials
eFigure 12. Meta-regression for sleep quality and length of follow-up for 31 randomized clinical trials of opioids vs. placebo
eFigure 13. Vomiting among chronic noncancer pain patients receiving opioids vs. placebo from 51 randomized clinical trials
eFigure 14. Meta-regression for pain relief and opioid dose among 5 trials with 14 within-study comparisons
eFigure 15. Meta-regression for physical function and opioid dose among 4 trials with 11 within-study comparisons
eFigure 16. Meta-regression for gastrointestinal adverse events and opioid dose among 5 trials with 14 within-study comparisons