Key Points
Question
Does premedication with propofol reduce the frequency of prolonged desaturation during nasotracheal intubation in neonates compared with the combination of a rapid-onset short-acting opioid and a muscle relaxant?
Findings
In this randomized clinical trial involving 173 neonates undergoing nonemergency nasotracheal intubation, the frequency of a pulse oximetry value less than 80% for at least 60 consecutive seconds for atropine with propofol was 60% vs 66% for atropine with atracurium and sufentanil. Although this difference was not statistically significant, the confidence interval included a clinically important difference.
Meaning
Although this study did not find a statistically significant difference between interventions, it may have been underpowered, and further research may be warranted.
Abstract
Importance
Propofol or a combination of a synthetic opioid and muscle relaxant are both recommended for premedication before neonatal intubation but have yet to be compared.
Objective
To compare prolonged desaturation during neonatal nasotracheal intubation after premedication with atropine-propofol vs atropine-atracurium-sufentanil treatment.
Design, Setting, and Participants
Multicenter, double-blind, randomized clinical trial (2012-2016) in 6 NICUs in France that included 173 neonates requiring nonemergency intubation. The study was interrupted due to expired study kits and lack of funding.
Interventions
Eighty-nine participants were randomly assigned to the atropine-propofol group and 82 to the atropine-atracurium-sufentanil group before nasotracheal intubation.
Main Outcomes and Measures
The primary outcome was prolonged desaturation (Spo2 <80% lasting > 60 seconds), using intention-to-treat analysis using mixed models. Secondary outcomes assessed the characteristics of the procedure and its tolerance.
Results
Of 173 neonates randomized (mean gestational age, 30.6 weeks; mean birth weight, 1502 g; 71 girls), 171 (99%) completed the trial. Of 89 infants, 53 (59.6%) in the atropine-propofol group vs 54 of 82 (65.9%) in the atropine-atracurium-sufentanil group achieved the primary outcome (adjusted RD, −6.4; 95% CI, −21.0 to 8.1; P = .38). The atropine-propofol group had a longer mean procedure duration than did the atropine-atracurium-sufentanil group (adjusted RD, 1.7 minutes; 95% CI, 0.1-3.3 minutes; P = .04); a less frequent excellent quality of sedation rate, 51.7% (45 of 87) vs 92.6% (75 of 81; P < .001); a shorter median time to respiratory recovery, 14 minutes (IQR, 8-34 minutes) vs 33 minutes (IQR, 15-56 minutes; P = .002), and shorter median time to limb movement recovery, 18 minutes (IQR, 10-43 minutes) vs 36 minutes (IQR, 19-65 minutes; P = .003). In the 60 minutes after inclusion, Spo2 was preserved significantly better in the atropine-propofol group (time × treatment interaction P = .02). Of the atropine-propofol group 20.6% had head ultrasound scans that showed worsening intracranial hemorrhaging (any or increased intraventricular hemorrhage) in the 7 days after randomization vs 17.6% in the atropine-atracurium-sufentanil group (adjusted RD, 1.2; 95% CI, −13.1 to 15.5, P = .87). Severe adverse events occurred in 11% of the atropine-propofol group and in 20% of the atropine-atracurium-sufentanil group.
Conclusions and Relevance
Among neonates undergoing nonemergency nasotracheal intubation, the frequency of prolonged desaturation did not differ significantly between atropine used with propofol or atropine used with atracurium and sufentanil. However, the study may have been underpowered to detect a clinically important difference, and further research may be warranted.
Trial Registration
ClinicalTrials.gov Identifier: NCT01490580, EudraCT number: 2009-014885-25
This randomized trial compares the effect of premedication with atropine and propofol vs atropine, atracurium, and sufentanil on prolonged desaturation of neonates in the NICU requiring nonemergency nasotracheal intubation.
Introduction
Academic societies consider that all neonates requiring endotracheal intubation should receive premedication, except in life-threatening situations.1,2 In practice, awake intubation was common in years 2005 and 2006 in some units,3 and inadequate data about candidate drugs is a barrier to premedication use.4 The American Academy of Pediatrics has proposed several drugs, but no consensus exists.1 In 2011, the Canadian Paediatric Society considered the most suitable option was a combination of a rapid-onset, short-acting analgesic and a short-duration muscle relaxant, preceded by a vagolytic.2 Consistent with this approach, a pilot study was conducted to evaluate a protocol combining atropine, sufentanil, and atracurium in premature infants born before 32 weeks of gestation5; their intubation conditions and hemodynamics were good, but 51% experienced prolonged desaturation as measured by pulse oximetry (Spo2 <80% for more than 60 seconds).
Propofol is considered an acceptable alternative option1 by the American Academy of Pediatrics and is used by many neonatologists.6,7,8 A previous randomized clinical trial comparing propofol alone to atropine, suxamethonium, and morphine together as premedication for nonemergency neonatal intubation found significantly higher intraprocedure oxygen saturation in the propofol group.9 The slow onset of morphine’s maximal effect makes it unsuitable for tracheal intubation, however.1
Thus a double-blind randomized clinical trial was designed to compare the effectiveness against prolonged desaturation of a combination of a vagolytic and propofol vs a combination of a vagolytic, a rapid-onset, short-acting opioid, and a short-acting muscle relaxant.
Methods
Study Design and Setting
This multicenter, double-blind, randomized clinical trial with parallel groups took place in 6 French academic perinatal centers (see the Protocol in Supplement 1). Enrollment occurred between 2012 and 2016, with 2 interruptions for logistic issues related to the investigational drug production process (between February and April 2013, and between August 2014 and March 2016, see eMethods in Supplement 2). An appropriate ethics committee (Paris Ile de France 3) and the French Medicinal Products Agency (ANSM) both approved this study. Parents of all infants provided written informed consent.
Participants
Infants were eligible if they were hospitalized in the neonatal intensive care unit (NICU), had a corrected postmenstrual age younger than 45 weeks and intravenous access, and required nonemergency (defined by an acceptable delay of 30 minutes from decision to intubation) or planned intubation. Exclusion criteria were sedative or anesthetic administration in the previous 24 hours, hemodynamic failure, defined as mean arterial blood pressure less than corrected gestational age or capillary refill time exceeding 3 seconds, upper airway malformation, life-threatening situation requiring immediate intubation, inclusion in another trial, any contraindication to any study drug, and previous inclusion in this trial.
Randomization, Concealment, and Masking
The data manager used 4D statistical software (4D SAS, Clichy, France) to create the randomization sequence, with a 1:1 allocation ratio, a fixed block size of 4, and stratification by center and weight (≤1000 g or >1000 g). To ensure allocation concealment, randomization was centralized through a dedicated website and only the pharmacists from the manufacturing organizations had access to the randomization list. Trialists were not informed of the block size throughout the trial. A double-dummy approach was used with intralipids (white emulsion) as a placebo for propofol and normal saline as a placebo for atracurium and sufentanil so that treatments contained in the masked vials in both groups appeared identical (eFigure 1 in Supplement 2). Parents, physicians, nurses, and external statisticians were unaware of treatment allocation.
Interventions
The interventions and the double-dummy approach are depicted in eFigure 1 (Supplement 2). Briefly, 6 syringes were prepared for all participants. The first 4 syringes contained a series of active drugs and placebo according to the treatment group: atropine, then placebo, then placebo, and then propofol in the atropine-propofol group or atropine, then atracurium, then sufentanil, and then placebo in the atropine-atracurium-sufentanil group. These 4 syringes had to be injected successively. If anesthesia was not adequate 2 minutes after the last injection, the 2 additional syringes were injected: placebo then propofol in the atropine-propofol group or atracurium then placebo in the atropine-atracurium-sufentanil group. If adequate anesthesia was still not obtained 2 minutes after the sixth syringe injection, open-label drugs could be used at the operator’s request, and the participant remained in the study. Adequate anesthesia was defined as no facial expression, spontaneous movement, or reaction to light tactile stimulation before attempting laryngoscopy.
Atropine was administered at 15 μg/kg in both groups. In the atropine-propofol group, the first propofol dose was 2.5 mg/kg in infants more than 1000 g, as previously reported.9 Because of concerns about this dose in the smallest infants,7,10,11 we used 1 mg/kg as a first dose in infants 1000 g or less. The additional propofol dose was 1 mg/kg for all infants. In the atropine-atracurium-sufentanil group, the first atracurium dose was 0.3 mg/kg and the additional dose 0.1 mg/kg.5,12 Results from the pilot study5 led us to use a lower sufentanil dose of 0.1 μg/kg in infants 1000 g or less to prevent thoracic rigidity. We used 0.2 μg/kg of sufentanil for those more than 1000 g, as previously reported.5
Intubation was performed according to the center’s local standard practice, usually through the nasotracheal route. No level of experience for the first operator was required, but a senior physician familiar with airway management in neonates had to attend all intubations and be ready to take over if required. Preoxygenation was recommended to obtain an Spo2 of at least 95% before the first attempt and at least 90% for subsequent attempts (see Protocol in Supplement 1).
Primary Outcome
The primary outcome was prolonged desaturation, defined as Spo2 of less than 80% for 60 consecutive seconds between the first drug injection and completion of the intubation. This threshold of 80% has been considered clinically significant in other studies.13,14 The duration of 60 seconds was arbitrarily chosen as clinically relevant and suggestive of a decrease in functional residual capacity.15 This outcome was assessed by a blinded independent observer equipped with a stopwatch to record the exact duration of each desaturation. The protocol required that all infants have an oximetry sensor (LNOP Neo-Pt L or Newborn, Masimo Inc) placed on their right hand for the Spo2 signal; the same monitors were used with a standardized acquisition algorithm (Viridia, Philips MedicalSystems) in all participating units.
Secondary Outcomes Included
Intubation Conditions
The independent observer collected the number of intubation attempts, the duration of the procedure, and times to recovery of spontaneous respiratory and limb movements. The operator scored the quality of sedation with a standardized scale (Supplement 2).
Vital Signs
The independent observer collected heart rate, Spo2, mean arterial blood pressure, transcutaneous partial carbon dioxide pressure (when available) at predefined time points (1 minute before the first injection, at the first injection, and at 3, 6, 9, 12, 15, 30, 45, and 60 minutes after it), and the lowest heart rate and Spo2 values during intubation. All these values were then compared with the monitor recordings for confirmation.
Worsening of Head Ultrasound Scans
In the 7 days after intubation from the preinclusion evaluation, ultrasounds of the neonates’ heads were defined as a normal scan before inclusion and any grade intraventricular hemorrhage afterward or as a preinclusion grade 1 or 2 scan deteriorating to grade 3 or 4 according to the Papile classification.16 This analysis was not centralized but was performed in each center according to its usual protocols.
Long-term follow-up is planned with evaluation of neurodevelopmental outcomes at a corrected age of 2 years.17
Adverse Events
Predefined stopping rules were set and approved by the French authorities (see Supplement 2); an independent data and safety monitoring board examined adverse events and severe adverse events every 6 months to determine application of these rules. Adverse events were collected within 60 minutes of the first drug injection and severe adverse events were collected up to 7 days after inclusion. A certified company (For Drug Consulting) collected, analyzed, and graded all severe adverse events and directed the sponsor to report suspected unexpected serious adverse reactions to the French and European authorities. The development safety update report was sent annually to the French authorities.
Statistical Analysis
Because a previous study5 suggested that the primary outcome (prolonged desaturation) would decrease from 50% in the atropine-atracurium-sufentanil group to 30% in the atropine-propofol group, the required sample size was estimated to be 186 patients, with a 2-sided α error of .05 and a power of 0.8. To compensate for dropouts (randomized patients not undergoing intubation), the number of planned inclusions was rounded up to 200 to enroll around 100 infants per group.
Baseline characteristics were described by percentages for qualitative variables and mean (SDs) or median (interquartile ranges [IQRs]), as appropriate, for quantitative variables.
The primary outcome was analyzed according to the intent-to-treat principle with a generalized mixed model adjusted for weight at inclusion (≤1000 g, >1000 g) and treating center as a random effect (exchangeable within-center correlation structure). We used a log-binomial distribution to obtain absolute risk differences.
Worsening of head ultrasound scans was analyzed with a model similar to that used for the primary outcome, duration of intubation with a generalized mixed-linear model, and the number of intubation attempts with a generalized mixed-model and log-Poisson distribution that accounted for the possibility of several attempts per child. Median number of intubation attempts, median duration of intubation, quality of sedation, and the times to recovery of respiratory and limb movements were compared with the Kruskal-Wallis test. Differences between groups for the median number of intubation attempts, the median duration of intubation, and the median time to recovery of respiratory and limb movements were calculated using the Hodges-Lehmann estimation of location shift with associated 95% CIs. Variations of the physiological parameters recorded at the predefined time points were analyzed with a generalized linear model for repeated data, including treatment group, time, time × treatment interaction, baseline parameter value, weight at inclusion (≤1000 g, >1000 g), and treating center as a random effect (exchangeable within-center correlation structure). Several predefined time points of interest, chosen for their clinical relevance, were considered for the physiological parameters to limit the multiplicity of tests: for heart rate and Spo2, early changes (6 and 9 minutes after the first injection) were considered, and for mean arterial blood pressure and transcutaneous partial carbon dioxide pressure, later time points (15 and 30 minutes after the first injection).
A prespecified subgroup analysis was performed for infants weighing 1000 g or less or more than 1000 g at inclusion, and interaction between weight and treatment effect was tested within the generalized mixed model used for the primary outcome.
Analysis of adverse events and severe adverse events was descriptive only and included all infants who actually received the allocated drug.
Post hoc analyses of oxygen desaturation time were performed after imputation of 3 different values (0, 30, or 59 seconds) for the duration of Spo2 less than 80% in infants who did not experience a prolonged desaturation for more than 60 seconds. Exact duration was collected for infants who achieved the primary outcome (prolonged desaturation).
To examine the possibility of bias due to the 19-month interruption in the study, we described baseline characteristics of participants and analyzed the primary outcome before and after the interruption post hoc.
Imputation was not used for missing data for secondary outcomes, except for the desaturation time analysis (no data were missing for the primary outcome). Statistical analyses were performed with SAS version 9.4 (SAS Institute Inc). P < .05 (2-sided) was considered statistically significant; because there was no adjustment for multiple comparisons, analyses of secondary end points should be interpreted as exploratory.
Results
Study Population
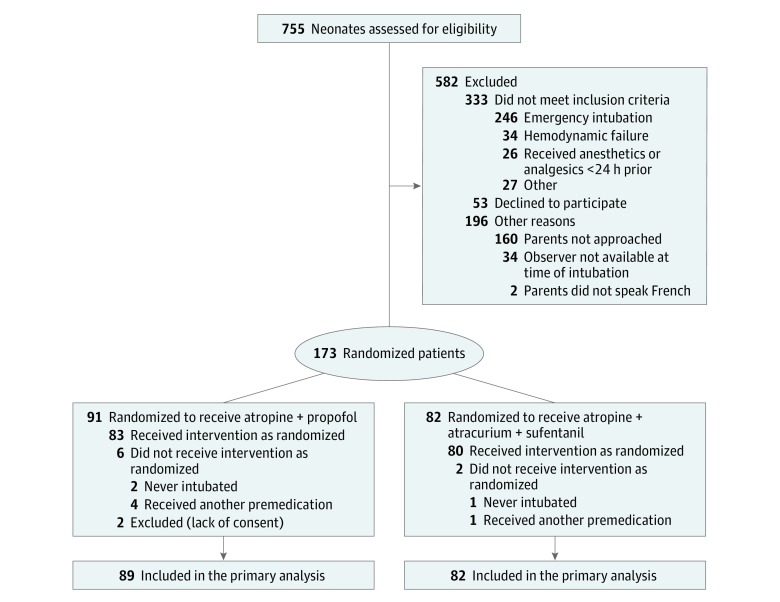
The trial was prematurely interrupted for logistic reasons in July 2016: a fourth drug manufacturing run was necessary because the intralipids had passed their expiration date and funding had run out. Intralipids have a relatively short validity (12 months), and serial production was required because the placebo for propofol expires rapidly. During the study period, 755 patients were screened for eligibility, and 173 were randomized (Figure 1). Subsequent exclusion of 2 randomized patients for missing or withdrawn parental consent left 89 patients in the atropine-propofol group and 82 in the atropine-atracurium-sufentanil group. Table 1 summarizes patients’ characteristics by group. The groups were well balanced, except that the atropine-atracurium-sufentanil group had a higher proportion of boys and the atropine-propofol group a higher median weight at intubation.
Figure 1. Population Flowchart of Premedication Prior to Neonatal Intubation.
Table 1. Baseline Characteristics.
| Characteristics | No. (%)a | |
|---|---|---|
| Atropine-Propofol Group (n = 89) |
Atropine-Atracurium-Sufentanil Group (n = 82) |
|
| Gestational age at birth, median (IQR), wk | 30 (28-34) | 29 (26-32) |
| Birth weight, median (IQR), g | 1310 (815-2285) | 1130 (850-1685) |
| Boys | 44 (49.4) | 57 (69.5) |
| Postnatal age at inclusion, median (IQR), d | 1 (0-9) | 1 (0-10) |
| Weight at inclusion, median (IQR), g | 1400 (950-2315) | 1273 (960-1805) |
| Weight categories at inclusion, g | ||
| ≤1000 | 27 (30.3) | 25 (30.5) |
| 1000-1500 | 21 (23.6) | 30 (36.6) |
| >1500 | 41 (46.1) | 27 (32.9) |
| Previous intubation | 32 (36.0) | 33 (40.2) |
| Reason for intubation | ||
| Respiratory distress syndrome | 60 (67.4) | 50 (61.0) |
| Apnea | 3 (3.4) | 9 (11.0) |
| Surgery | 20 (22.5) | 16 (19.5) |
| Other | 6 (6.7) | 7 (8.5) |
| Ventilatory mode before intubation | ||
| Invasive ventilationb | 10 (11.2) | 11 (13.4) |
| Noninvasive ventilation | 59 (66.3) | 61 (74.4) |
| Nasal o2, low flow | 1 (1.1) | 0 |
| Room air spontaneous ventilation | 15 (16.9) | 8 (9.8) |
| Unknown | 4 (4.5) | 2 (2.4) |
| Initial Fio2, median (IQR), % | 48 (25-99)c | 50 (30-100)d |
| Initial Spo2, median (IQR), % | 98 (94-100)e | 97 (94-99)f |
| First operator’s previous experience | ||
| <10 intubations | 35 (39.3) | 35 (42.7) |
| 10-50 intubations | 42 (47.2) | 32 (39.0) |
| >50 intubations | 10 (11.2) | 13 (15.9) |
| Unknown | 2 (2.2) | 2 (2.4) |
Abbreviation: Fio2, fraction of inspired oxygen; IQR, interquartile range; Spo2, oxygen saturation measured by pulse oximetry.
Percentages may not sum to 100 due to rounding.
Intubated patients who received no analgesic or anesthetic in the 24 h preceding randomization. These patients had their endotracheal tube changed.
Fio2 before intubation was missing for 7 patients.
Fio2 before intubation was missing for 5 patients.
Spo2 before intubation was missing for 3 patients.
Spo2 before intubation was missing for 2 patients in the atropine-atracurium-sufentanil group.
Interventions Received
The proportion of patients who required additional doses differed significantly between the 2 groups with 48 of 89 patients (53.9%) in the atropine-propofol group and 8 of 82 (9.8%) in the atropine-atracurium-sufentanil group receiving 6 syringes or more (P < .001) (Table 2). All intubations were performed through the nasotracheal route.
Table 2. Primary Outcome, Intervention Received, and Secondary Exploratory Outcomes.
| Outcomes | No. (%)a | Adjusted Absolute Differences (95% CI)b | P Valuec | |
|---|---|---|---|---|
| Atropine- Propofol Group (n = 89) |
Atropine- Atracurium- Sufentanil Group (n = 82) |
|||
| Primary outcome | ||||
| Spo2 <80% for >60 s | 53 (59.6) | 54 (65.9) | −6.4 (−21.0 to 8.1) | .38 |
| No. of allocated syringes injected | ||||
| None | 6 (6.7) | 2 (2.4) | <.001 | |
| 4 | 35 (39.3) | 72 (87.8) | ||
| 6 | 32 (36.0) | 6 (7.3) | ||
| 6 plus open-label drug(s) | 16 (18.0) | 2 (2.4) | ||
| Secondary Exploratory Outcomes | ||||
| No. of intubation attempts | ||||
| No. with data available | 87 (97.8) | 81 (98.8) | ||
| 1 | 41 (47.1) | 47 (58.0) | 1.7 (1.4 to 2.0) | .37 |
| 2 | 25 (28.7) | 23 (28.4) | ||
| >2 | 21 (24.1) | 11 (13.6) | ||
| Median (IQR) | 2 (1-2) | 1 (1-2) | 0 | .10d |
| Duration of intubation | ||||
| No. with data available | 84 (94.4) | 80 (97.6) | ||
| Median (IQR), min | 6.0 (2.8 to 9.1) | 3.5 (1.3 to 6.0) | 1.7 (0.6 to 3.0) | .003d |
| Mean (SD), min | 6.6 (5.3) | 4.9 (5.7) | 1.7 (0.1 to 3.3) | .04 |
| Duration of intubation according to the number of intubation attempts, mean (SD), min | ||||
| 1 Attempt | 3.1 (3.6) | 2.3 (1.9) | 0.7 (−0.3 to 1.7) | .17 |
| 2 Attempts | 8.5 (4.7) | 5.4 (2.2) | 3.2 (0.9 to 5.4) | .006 |
| >2 Attempts | 10.8 (4.4) | 14.4 (10.1) | −3.8 (−8.4 to 0.8) | .10 |
| Quality of sedatione | ||||
| No. with data available | 87 (97.8) | 81 (98.8) | ||
| Excellent | 45 (51.7) | 75 (92.6) | <.001 | |
| Good | 29 (33.3) | 5 (6.2) | ||
| Acceptable | 9 (10.3) | 1 (1.2) | ||
| Poor | 4 (4.6) | 0 | ||
| Time to respiratory movement recovery | ||||
| No. with data available | 68 (76.4) | 66 (80.5) | ||
| Median (IQR), min | 14 (8-34) | 33 (15-56) | −10.8 (−19.4 to −3.8) | .002c |
| Time to spontaneous limb movement recovery | ||||
| No. with data available | 67 (75.3) | 66 (80.5) | ||
| Median (IQR), min | 18 (10-43) | 36 (19-65) | −12.8 (−21.7 to −3.6) | .003c |
| Change From Baseline in Physiologic Parameters Before and After Injection, Adjusted Mean (SD) | ||||
| Heart rate | ||||
| 1 Min before to 6 min after | ||||
| No. with data available | 86 (96.6) | 80 (97.6) | ||
| Difference, beats/min | 3.3 (19.5) | 11.5 (19.6) | −8.2 (−13.5 to −2.9) | .003 |
| 1 Min before to 9 min after | ||||
| No. with data available | 86 (96.6) | 80 (97.6) | ||
| Difference, beats/min | 1.6 (25.2) | 11.7 (25.3) | −10.1 (−17.3 to −2.9) | .007 |
| Mean arterial blood pressure | ||||
| 1 Min before to 15 min after | ||||
| No. with data available | 80 (89.9) | 77 (93.9) | ||
| Difference, mm Hg | −6.8 (12.7) | 0.2 (12.7) | −7.0 (−11.1 to −3.0) | <.001 |
| 1 Min before to 30 min after | ||||
| No. with data available | 76 (85.4) | 74 (90.2) | ||
| Difference, mm Hg | −9.1 (9.3) | −3.3 (9.4) | −5.8 (−8.8 to −2.8) | <.001 |
| Spo2 | ||||
| 1 Min before to 6 min after | ||||
| No. with data available | 85 (95.5) | 80 (97.6) | ||
| Difference, % | −6.0 (20.1) | −12.0 (20.1) | 6.0 (−0.1 to 12.1) | .05 |
| 1 Min before to 9 min after | ||||
| No. with data available | 84 (94.4) | 80 (97.6) | ||
| Difference, % | −8.7 (22.3) | −15.9 (22.2) | 7.2 (0.5 to 14.0) | .04 |
| Transcutaneous partial carbon dioxide pressure | ||||
| 1 Min before to 15 min after | ||||
| No. with data available | 32 (36.0) | 29 (35.4) | ||
| Difference, mm Hg | 8.0 (14.4) | 14.1 (14.4) | −6.1 (−13.5 to 1.3) | .10 |
| 1 Min before to 30 min after | ||||
| No. with data available | 30 (33.7) | 29 (35.4) | ||
| Difference, mm Hg | 5.1 (19.1) | 16.2 (19.3) | −11.1 (−21.1 to −1.0) | .03 |
| Worsening of Head Ultrasoundf | ||||
| No. with data available | 68 (76.4) | 68 (82.9) | ||
| No. of cases | 14 (20.6) | 12 (17.6) | 1.2 (−13.1 to 15.5) | .87 |
| Preintubation Scan | ||||
| Change from normal scan to any intraventricular hemorrhage grade | ||||
| No. with data available | 49 | 49 | ||
| No. of cases | 11 (22.4) | 5 (10.2) | 11.0 (−5.5 to 27.6) | .19 |
| Change from grade 1 or 2 intraventricular hemorrhage to grade 3 or 4 | ||||
| No. with data available | 19 | 19 | ||
| No. of cases | 3 (15.8) | 7 (36.8) | −24.0 (−50.9 to 3.0) | .08 |
Abbreviation: IQR, interquartile range; Spo2, oxygen saturation measured by pulse oximetry.
Percentages may not sum to 100 due to rounding.
Absolute risk differences and mean differences were calculated with a generalized mixed model adjusted for weight at inclusion (≤1000 g, >1000 g) taking into account within-center correlation; median difference, Hodges-Lehmann estimation and represents atropine-propofol group minus atropine-atracurium-sufentanil group.
P values were obtained from adjusted generalized mixed models unless otherwise stated.
Kruskal-Wallis test.
See Supplement 2 for standardized scale.
Defined as either a normal scan before inclusion and any grade intraventricular hemorrhage afterward or a preintubation grade 1 or 2 changing to grade 3 or 4 afterward.
Outcome Assessment
Primary Outcome
Prolonged desaturation did not differ significantly between the groups: 53 of 89 infants (59.6%) in the atropine-propofol group and 54 of 82 infants (65.9%) in the atropine-atracurium-sufentanil group (adjusted risk difference, −6.4; 95% CI, −21.0 to 8.1; P = .38) (Table 2).
Secondary Outcomes
In the atropine-propofol group, the mean (SD) duration of the intubation procedure was significantly longer (6.6 [5.3] minutes vs 4.9 [5.7] minutes), for an adjusted difference of 1.7 minutes (95% CI, 0.1 to 3.3 minutes; P = .04) and the quality of sedation poorer (Table 2), but the median times to spontaneous respiration recovery were 14 minutes (IQR, 8-34 minutes) in the atropine-propofol group vs 33 minutes (IQR, 15-56 minutes) in the atropine-atracurium-sufentanil group (P = .002) and the median times for limb movement recovery were 18 minutes (IQR, 10-43 minutes) for the atropine-propofol group vs 36 minutes (IQR, 19-65 minutes) for the atropine-propofol-atracurium group (P = .003) (Table 2).
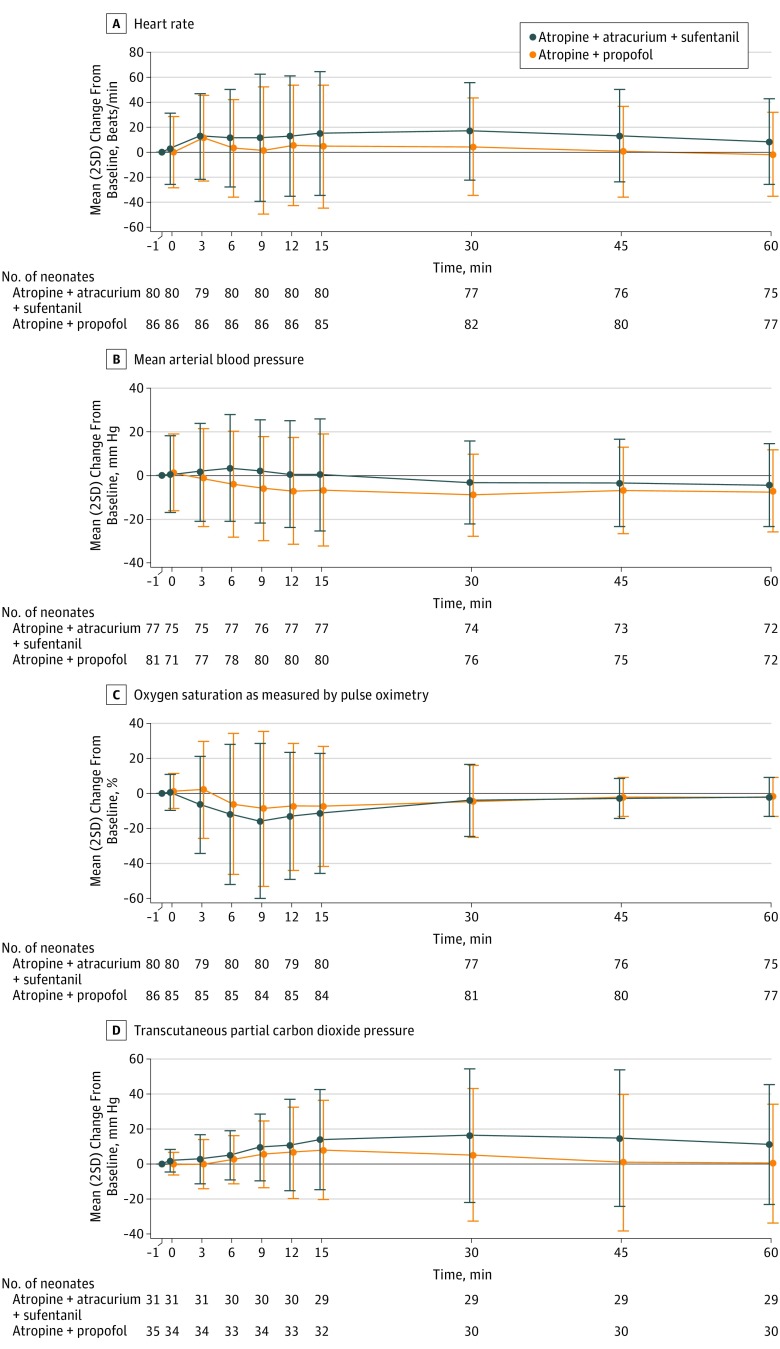
Changes over time in heart rate, mean arterial blood pressure, Spo2, and transcutaneous partial carbon dioxide pressure within each group are illustrated in Figure 2. Significant time × treatment interactions suggested different patterns for heart rate (P < .001), mean arterial blood pressure (P = .009), and Spo2 (P = .02) between the 2 groups but not for transcutaneous partial carbon dioxide pressure (P = .48). In the atropine-atracurium-sufentanil group, heart rate showed a persistent increase from baseline (Figure 2A), and Spo2 decreased more profoundly over time (Figure 2C), whereas in the atropine-propofol group, the mean arterial blood pressure decrease was deeper over time (Figure 2B). These differences were confirmed by the significant adjusted differences observed at all predefined time points for heart rate, mean arterial blood pressure, and Spo2, except for change in Spo2 from 1 minute before to 6 minutes after injection (P = .053) (Table 2). The lowest median heart rates were 136 beats/min (IQR, 115-152 beats/min) in the atropine-propofol group and 148 beats/min (IQR 104-160 beats/min) in the atropine-atracurium-sufentanil group and the lowest median Spo2 values were 61% (IQR, 35%-78%) in the atropine-propofol group and 56% (IQR, 24%-69%) in the atropine-atracurium-sufentanil group.
Figure 2. Changes From Baseline Over Time.
The x-axis includes the time before and after first drug injection (denoted as “0”) in minutes. The y-axis shows difference from the baseline value. Each point represents the mean value and the error bars, 2 standard deviations.
Preintubation and postintubation head ultrasounds were obtained for 78% of the infants: 68 in each group. Findings worsened in 14 of 68 infants (20.6%) of the atropine-propofol group and 12 of 68 (17.6%) of the atropine-atracurium-sufentanil group (adjusted risk difference, 1.2; 95% CI, −13.1 to 15.5; P = .87) (Table 2).
Tolerance and Adverse Events
Adverse events were reported for 25 of 83 infants (30.1%) and 28 of 80 infants (35%) and severe adverse events for 9 of 83 infants (10.8%) and 16 of 80 infant (20.0%) in the atropine-propofol group and atropine-atracurium-sufentanil group, respectively (Table 3). In the atropine-propofol group, the most frequently reported adverse event was hypotension, in 11 of 83 infants (13.3%). Nine infants recovered without intervention, 1 received two 10 mL/kg normal saline fluid boluses, and 1, dopamine (10 μg/kg/min), started 19 hours after the first syringe injection. In the atropine-atracurium-sufentanil group, chest rigidity was the most frequently reported adverse event, in 11 of 80 infants (13.8%); all were managed by increasing peak inspiratory pressure. Four of these events were accompanied by bradycardia less than 100 beats/min and lasting for more than 60 seconds. Five infants died in the 7 days after inclusion, 2 in the atropine-propofol group and 3 in the atropine-atracurium-sufentanil group; no deaths were attributed to the study products (Table 3).
Table 3. Adverse Events and Severe Adverse Events.
| Events | No. (%) | |
|---|---|---|
| Atropine-Propofol Group (n = 83) | Atropine-Atracurium- Sufentanil Group (n = 80) |
|
| No. of infants with adverse events | 25 (30.1) | 28 (35) |
| Type of adverse events | ||
| Hypotension (untreated) | 9 (10.8) | 1 (1.3) |
| Hypertension | 7 (8.4) | 7 (8.8) |
| Bradycardia <100/min >60 s | 1 (1.2) | 6 (7.5) |
| Prolonged hypoxiaa | 2 (2.4) | 5 (6.3) |
| Upper airway trauma | 4 (4.8) | 3 (3.8) |
| Hypercapnia | 3 (3.6) | 5 (6.3) |
| Myoclonia | 3 (3.6) | 0 |
| Hypocapnia | 0 | 1 (1.3) |
| Hypothermia | 1 (1.2) | 0 |
| Laryngospasm | 1 (1.2) | 0 |
| No. of infants with severe adverse events | 9 (10.8) | 16 (20.0) |
| Type of severe adverse events | ||
| Death | 2 (2.4)b | 3 (3.8)c |
| Thoracic rigidity | 3 (3.6) | 11 (13.8) |
| Pneumothorax | 2 (2.4) | 4 (5.0) |
| Sepsis | 4 (4.8) | 1 (1.3) |
| Digestive tract perforationd | 3 (3.6) | 1 (1.3) |
| Hypotension (treated) | 2 (2.4) | 0 |
| Pulmonary hemorrhage | 1 (1.2) | 2 (2.5) |
| Cardiac arrest | 1 (1.2) | 1 (1.3) |
| Supraventricular tachycardia | 1 (1.2) | 0 |
| Pulmonary hypertension | 0 | 1 (1.3) |
| Aspiration syndrome | 0 | 1 (1.3) |
| Hyponatremia | 1 (1.2) | 0 |
No standardized definition for prolonged hypoxia. These events did not fulfill any of the criteria for being considered severe, namely, causing death, threatening life, causing temporary or permanent disability or incapacity, requiring or extending the hospitalization of a patient.
One patient was included on day 7 of life and died on day 9 due to multiple organ failure with suspected sepsis; 1 patient was included on day 1 of life and died on day 3 due to pulmonary hemorrhage. None of these deaths was attributed to the study drugs.
One patient was included on day 0 of life and died on day 2 due to multiple organ failure without sepsis; 1 patient was included on day 6 of life before surgery and died on day 7 due to postoperative multiple organ failure; 1 patient was included on day 7 of life and died on day 9 due to pulmonary hemorrhage. None of these deaths was attributed to the study drugs.
All perforations occurred in the small bowel; none were in the esophagus.
Subgroup Analysis
The primary outcome did not differ by weight at inclusion (≤1000 g or >1000 g) (interaction test, P = .90) (eFigure 2 in Supplement 2).
Post Hoc Analysis
The median desaturation time did not differ significantly between the atropine-propofol and the atropine-atracurium-sufentanil groups after imputing 0, 30, or 59 seconds for infants without the primary outcome (P = .16 for 0 seconds, P = .15 for 30 seconds, and P = .15 for 59 seconds) (eTable 1 in Supplement 2).
As in the main analysis, some imbalance was observed between the treatment groups both before and after the 19-month interruption (eTable 2 in Supplement 2). The primary outcome did not differ before and after this interruption and was consistent with the main analysis (eTable 3 in Supplement 2).
Discussion
This trial found no significant difference in the prolonged desaturation rate during nonemergency intubation in the NICU between atropine-propofol and atropine-atracurium-sufentanil. This study also provided information about the tolerance of these 2 regimens. In the atropine-propofol group, Spo2 appeared to be better preserved and both spontaneous respiration and limb movements recovered faster than in the atropine-atracurium-sufentanil group, but reinjections were required much more often. In the atropine-atracurium-sufentanil group, however, mean arterial blood pressure was better, the duration of intubation shorter, and the quality of sedation higher, although thoracic rigidity and severe adverse events were more frequent than in the atropine-propofol group.
To our knowledge, this was the first double-blind, multicenter, randomized clinical trial to compare 2 premedication regimens for neonatal intubation. This design reflects daily practice in many NICUs, with intubation usually performed by operators with limited experience5,18 and a number of attempts consistent with observational studies.3,18,19
Although the results showed no statistical difference in terms of desaturation, they confirm the risk of adverse events associated with neonatal intubation, even when premedication is used.19 They also show that these adverse events can be safely managed. The goal of premedication is to prevent pain, because multiple painful experiences in the neonatal period are associated with poor neurodevelopmental outcome.20,21 The use of a muscle relaxant (atracurium) in one of the treatment groups precluded the clinical assessment of pain in this study. Nevertheless, multiple injections were required much more frequently in the atropine-propofol group, in more than half the cases compared with less than 10% in the atropine-atracurium-sufentanil group. This finding is consistent with the wide interindividual variability in the neonatal pharmacodynamics of propofol.22 It also suggests that a higher dose might have provided better sedation, but a design that mimicked titration23 while maintaining double-blinding was preferred, to limit potential adverse events. The prolonged increase in heart rate observed in the atropine-atracurium-sufentanil suggests that infants in this group might not have received adequate pain relief during paralysis. This study thus raises questions about the use of a muscle relaxant in a premedication regimen, because, unlike propofol, it prevents opioid titration.23 The better quality of sedation in the atropine-atracurium-sufentanil group again underlines that the operator’s likelihood of successfully completing the procedure does not necessarily correlate with the patient’s comfort (pain relief and stress) when paralysis is used.24
In the largest previously published randomized clinical trial comparing propofol with atropine-morphine-suxamethonium, the authors found that propofol was significantly associated with faster intubation and higher Spo2 during intubation.9 In this present study, the time × treatment interaction was significant for Spo2 with values consistent with those from a previous study.9 Absolute difference between groups for Spo2 at 6 minutes after induction was clinically significant, and both statistically and clinically significant at 9 minutes after induction. The possibility that the present study was underpowered to detect a difference in the primary outcome is thus plausible.
Intubation in this study lasted longer than others have reported.13,18 Despite different definitions of intubation time, many factors influence the duration of the procedure, including the operator’s experience13,25 and the type of premedication.26 One factor likely to be important is the intubation route. This study used the nasotracheal route because it was standard practice at all participating centers. Nevertheless, it presents problems in intubating the nostrils of the smallest infants27 and the orotracheal route may be faster. Intubation routes have been insufficiently studied in neonates and deserve further research.28
The most frequent adverse effect in the atropine-propofol group was hypotension, as previously reported in some studies6,7,8 but not others.9,29 Most of these episodes of hypotension recovered spontaneously. No currently available data provide certainty about whether the low blood pressure value following propofol administration reflects a drop in tissue oxygenation,22,30,31 but additional studies are required to assess its safety, especially if higher doses are used. Adverse respiratory events were frequent in the atropine-atracurium-sufentanil group, as the pilot study showed.5 Although atracurium was injected before sufentanil in this trial, thoracic rigidity, a known adverse effect of synthetic opioids, was still observed sometimes.32,33
Limitations
This study has several limitations. First, the trial was stopped for logistic reasons before the full planned inclusion of patients, and the observed rate of desaturation in both groups was higher than expected, which may have affected its power. The 95% CI of the absolute risk difference between groups for the primary outcome included the prespecified clinically important difference of 20 percentage points and thus supports the possibility that the study was underpowered. Second, this study might have been biased by the imbalance between groups at baseline for some variables, including weight and sex, and by its premature discontinuation. Third, there may be disagreement about the definition of the primary outcome, but no sound evidence is currently available to set a precise threshold, duration, or both that define an episode of desaturation as severe. Nevertheless, desaturation less than 80% during intubation in the pediatric intensive care unit has been associated with prolonged mechanical ventilation.34 Fourth, the opioid and muscle relaxant used can be debated. Sufentanil was used because it is the most popular synthetic opioid in France for neonatal intubation,3,35 although premedication with fentanyl has been studied more extensively.13,14,26,33,36 Therefore, it may not be possible to extrapolate these results to fentanyl or other synthetic opioids. Atracurium has a good safety profile in neonates,37 but suxamethonium is also frequently used.9,33,36,38,39 Again extrapolation of these results to other muscle relaxants may not be possible. Fifth, the nasotracheal route was always used in this study. The results may not be extrapolated to orotracheal intubation, which differs in technique and timing from the nasotracheal route. Sixth a subgroup analysis was planned based on weight at inclusion. Testing for a weight × treatment effect interaction was null, but interaction tests generally lack power. Nevertheless, this analysis is difficult to interpret because there was no statistical difference for the primary outcome. Seventh, because analyses of secondary end points were not adjusted for multiple comparisons, there is the potential for type I error. Significant secondary end point findings should be interpreted as exploratory.
Conclusions
Among neonates undergoing nonemergency tracheal intubation, the frequency of prolonged desaturation did not differ significantly when atropine was used with propofol or with atracurium and sufentanil. However, the study may have been underpowered to detect a clinically important difference, and further research may be warranted.
Trial Protocol
eMethods. Reasons for interruptions, secondary outcomes definitions, stopping rules
eFigure 1. Treatment administration and blinding procedure
eFigure 2. Subgroup analysis according to weight at randomization
eTable1. Desaturation time per treatment group after imputing different values for infants without the primary outcome
eTable 2. Baseline characteristics of participants before and after the 19-month interruption
eTable 3. Primary outcome before and after the 19-month interruption
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Kumar P, Denson SE, Mancuso TJ; Committee on Fetus and Newborn, Section on Anesthesiology and Pain Medicine . Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics. 2010;125(3):608-615. [DOI] [PubMed] [Google Scholar]
- 2.Barrington K. Premedication for endotracheal intubation in the newborn infant. Paediatr Child Health. 2011;16(3):159-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durrmeyer X, Daoud P, Decobert F, et al. Premedication for neonatal endotracheal intubation: results from the epidemiology of procedural pain in neonates study. Pediatr Crit Care Med. 2013;14(4):e169-e175. [DOI] [PubMed] [Google Scholar]
- 4.Muniraman HK, Yaari J, Hand I. Premedication use before nonemergent intubation in the newborn infant. Am J Perinatol. 2015;32(9):821-824. [DOI] [PubMed] [Google Scholar]
- 5.Durrmeyer X, Dahan S, Delorme P, et al. Assessment of atropine-sufentanil-atracurium anaesthesia for endotracheal intubation: an observational study in very premature infants. BMC Pediatr. 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papoff P, Mancuso M, Caresta E, Moretti C. Effectiveness and safety of propofol in newborn infants. Pediatrics. 2008;121(2):448. [DOI] [PubMed] [Google Scholar]
- 7.Welzing L, Kribs A, Eifinger F, Huenseler C, Oberthuer A, Roth B. Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm neonates. Paediatr Anaesth. 2010;20(7):605-611. [DOI] [PubMed] [Google Scholar]
- 8.Simons SH, van der Lee R, Reiss IK, van Weissenbruch MM. Clinical evaluation of propofol as sedative for endotracheal intubation in neonates. Acta Paediatr. 2013;102(11):e487-e492. [DOI] [PubMed] [Google Scholar]
- 9.Ghanta S, Abdel-Latif ME, Lui K, Ravindranathan H, Awad J, Oei J. Propofol compared with the morphine, atropine, and suxamethonium regimen as induction agents for neonatal endotracheal intubation: a randomized, controlled trial. Pediatrics. 2007;119(6):e1248-e1255. [DOI] [PubMed] [Google Scholar]
- 10.Meyer S, Gottschling S, Gortner L. Propofol compared with the morphine, atropine, and suxamethonium regimen as induction agents for neonatal endotracheal intubation: a randomized, controlled trial. Pediatrics. 2007;120(4):932-933. [DOI] [PubMed] [Google Scholar]
- 11.van den Anker JN. Timing of dose-finding studies: before or after completion of a randomized clinical trial? Pediatrics. 2007;120(3):691-692. [DOI] [PubMed] [Google Scholar]
- 12.Meakin G, Shaw EA, Baker RD, Morris P. Comparison of atracurium-induced neuromuscular blockade in neonates, infants and children. Br J Anaesth. 1988;60(2):171-175. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey EM, Al Hazzani F, Faucher D, Barrington KJ. Facilitation of neonatal endotracheal intubation with mivacurium and fentanyl in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):F279-F282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemyre B, Cheng R, Gaboury I. Atropine, fentanyl and succinylcholine for non-urgent intubations in newborns. Arch Dis Child Fetal Neonatal Ed. 2009;94(6):F439-F442. [DOI] [PubMed] [Google Scholar]
- 15.Tourneux P, Léké A, Kongolo G, et al. Relationship between functional residual capacity and oxygen desaturation during short central apneic events during sleep in “late preterm” infants. Pediatr Res. 2008;64(2):171-176. [DOI] [PubMed] [Google Scholar]
- 16.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. [DOI] [PubMed] [Google Scholar]
- 17.Skellern CY, Rogers Y, O’Callaghan MJ. A parent-completed developmental questionnaire: follow up of ex-premature infants. J Paediatr Child Health. 2001;37(2):125-129. [DOI] [PubMed] [Google Scholar]
- 18.Haubner LY, Barry JS, Johnston LC, et al. Neonatal intubation performance: room for improvement in tertiary neonatal intensive care units. Resuscitation. 2013;84(10):1359-1364. [DOI] [PubMed] [Google Scholar]
- 19.Hatch LD, Grubb PH, Lea AS, et al. Endotracheal intubation in neonates: a prospective study of adverse safety events in 162 infants. J Pediatr. 2016;168:62-6e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinall J, Grunau RE. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr Res. 2014;75(5):584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine Prevention and management of procedural pain in the neonate: an Update. Pediatrics. 2016;137(2):e20154271. [DOI] [PubMed] [Google Scholar]
- 22.Smits A, Thewissen L, Caicedo A, Naulaers G, Allegaert K.. Propofol dose-finding to reach optimal effect for (semi-)elective intubation in neonates. J Pediatr. 2016;179:54-60.e59. [DOI] [PubMed] [Google Scholar]
- 23.Shah PS, Shah VS. Propofol for procedural sedation/anaesthesia in neonates. Cochrane Database Syst Rev. 2011;3(3):CD007248. [DOI] [PubMed] [Google Scholar]
- 24.Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet. 1987;1(8524):62-66. [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell CP, Kamlin CO, Davis PG, Morley CJ. Endotracheal intubation attempts during neonatal resuscitation: success rates, duration, and adverse effects. Pediatrics. 2006;117(1):e16-e21. [DOI] [PubMed] [Google Scholar]
- 26.Roberts KD, Leone TA, Edwards WH, Rich WD, Finer NN. Premedication for nonemergent neonatal intubations: a randomized, controlled trial comparing atropine and fentanyl to atropine, fentanyl, and mivacurium. Pediatrics. 2006;118(4):1583-1591. [DOI] [PubMed] [Google Scholar]
- 27.McMillan DD, Rademaker AW, Buchan KA, Reid A, Machin G, Sauve RS. Benefits of orotracheal and nasotracheal intubation in neonates requiring ventilatory assistance. Pediatrics. 1986;77(1):39-44. [PubMed] [Google Scholar]
- 28.Spence K, Barr P. Nasal versus oral intubation for mechanical ventilation of newborn infants. Cochrane Database Syst Rev. 2000;(2):CD000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penido MG, de Oliveira Silva DF, Tavares EC, Silva YP. Propofol versus midazolam for intubating preterm neonates: a randomized controlled trial. J Perinatol. 2011;31(5):356-360. [DOI] [PubMed] [Google Scholar]
- 30.Vanderhaegen J, Naulaers G, Van Huffel S, Vanhole C, Allegaert K. Cerebral and systemic hemodynamic effects of intravenous bolus administration of propofol in neonates. Neonatology. 2010;98(1):57-63. [DOI] [PubMed] [Google Scholar]
- 31.Fleck T, Schubert S, Ewert P, Stiller B, Nagdyman N, Berger F. Propofol effect on cerebral oxygenation in children with congenital heart disease. Pediatr Cardiol. 2015;36(3):543-549. [DOI] [PubMed] [Google Scholar]
- 32.Fahnenstich H, Steffan J, Kau N, Bartmann P. Fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med. 2000;28(3):836-839. [DOI] [PubMed] [Google Scholar]
- 33.Choong K, AlFaleh K, Doucette J, et al. Remifentanil for endotracheal intubation in neonates: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2010;95(2):F80-F84. [DOI] [PubMed] [Google Scholar]
- 34.Parker MM, Nuthall G, Brown C III, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . relationship between adverse tracheal intubation associated events and PICU outcomes. Pediatr Crit Care Med. 2017;18(4):310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones P, Peters MJ, Pinto da Costa N, et al. Atropine for critical care intubation in a cohort of 264 children and reduced mortality unrelated to effects on bradycardia. PLoS One. 2013;8(2):e57478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrington KJ, Byrne PJ. Premedication for neonatal intubation. Am J Perinatol. 1998;15(4):213-216. [DOI] [PubMed] [Google Scholar]
- 37.Nightingale DA. Use of atracurium in neonatal anaesthesia. Br J Anaesth. 1986;58(suppl 1):32S-36S. [DOI] [PubMed] [Google Scholar]
- 38.Norman E, Wikström S, Hellström-Westas L, Turpeinen U, Hämäläinen E, Fellman V. Rapid sequence induction is superior to morphine for intubation of preterm infants: a randomized controlled trial. J Pediatr. 2011;159(6):893-899.e1. [DOI] [PubMed] [Google Scholar]
- 39.Oei J, Hari R, Butha T, Lui K. Facilitation of neonatal nasotracheal intubation with premedication: a randomized controlled trial. J Paediatr Child Health. 2002;38(2):146-150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Reasons for interruptions, secondary outcomes definitions, stopping rules
eFigure 1. Treatment administration and blinding procedure
eFigure 2. Subgroup analysis according to weight at randomization
eTable1. Desaturation time per treatment group after imputing different values for infants without the primary outcome
eTable 2. Baseline characteristics of participants before and after the 19-month interruption
eTable 3. Primary outcome before and after the 19-month interruption