Abstract
Cynomolgus monkeys (Macaca fascicularis) are a valuable model organism for human disease modeling because human physiology and pathology are closer to those of cynomolgus monkeys than rodents. It has been widely reported that mature oocytes can be recovered from cynomolgus monkeys through ovarian stimulation by human follicle-stimulating hormone (hFSH). However, it is unknown whether mature oocytes can be effectively obtained through a second ovarian stimulation by hFSH. Here, we report that some ovaries (eight ovaries from 14 female monkeys) were stimulated effectively by hFSH even after the first ovum pick up, whereas the others were stimulated poorly by hFSH. Furthermore, we found antibodies against hFSH only in the serum of female monkeys with poorly stimulated ovaries. Collectively, these data suggest that anti-hFSH antibodies in serum may cause a poor ovarian response to hFSH stimulation. Finally, detection of such antibodies as well as observation of the ovary over the course of hFSH administration might be useful to predict favorable second ovarian stimulation by hFSH.
Keywords: Cynomolgus monkey, Follicular-stimulating hormone (FSH), MII oocyte, Ovarian stimulation
There are several nonhuman primates that are widely used as laboratory animals, including New World monkeys such as the common marmoset (Callithrix jacchus) [1, 2] and Old World monkeys such as the rhesus monkey (Macaca mulatta) [3, 4], Japanese monkey (Macaca fuscata) [5, 6], and cynomolgus monkey (Macaca fascicularis) [7, 8]. Old World monkeys are closer to humans in terms of organ size and anatomical structure than New World monkeys. Therefore, they have been used for modeling diseases, such as Parkinson’s [9, 10] and Huntington’s [11, 12] diseases, and transplantation studies [13, 14]. In particular, the cynomolgus monkey is superior to other macaque monkeys because they breed throughout the year rather than seasonally like the rhesus macaque.
Follicle-stimulating hormone (FSH) is a heterodimeric glycoprotein that is secreted by the pituitary gland and is important for follicle development in females and spermatogenesis in males [15,16,17]. FSH consists of a specific subunit (FSHβ) and common alpha subunit (FSHα) shared with luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and chorionic gonadotropin (CG) [15, 18]. FSH facilitates the development of immature pre-antral follicles to pre-ovulatory follicles, as characterized by the formation of a fluid-filled antrum within a follicle [16]. In the female, FSH binds to G protein-coupled FSH receptors (FSHRs) belonging to the glycoprotein hormone receptor family expressed on ovarian granulosa cells and promotes estrogen production [19, 20]. Both physiological and genetic studies of rodent models and human patients carrying mutations in the hormone-specific β-subunit and FSHR-encoding genes indicate that FSH actions are essential for antral-stage follicle development during ovarian folliculogenesis and, consequently, female fertility [21,22,23,24].
Although many studies have reported that mature oocytes can be recovered from cynomolgus monkeys through ovarian stimulation by human FSH (hFSH) [25,26,27], it is unknown whether mature oocytes can be effectively obtained repeatedly from cynomolgus monkeys through ovarian stimulation by hFSH. Here, we report that ovaries were stimulated effectively in a certain group of cynomolgus monkeys by hFSH even after the first ovum pick up (OPU), but ovaries in the other monkeys were stimulated poorly by hFSH. Furthermore, we found antibodies against hFSH only in the serum of female monkeys with poorly stimulated ovaries.
Materials and Methods
Animals
All experimental procedures were approved by the Animal Care and Use Committee of Shiga University of Medical Science, and the procedures were carried out in accordance with the approved guidelines (Approval number: 2018-9-8). Oocytes were collected from 14 sexually mature female cynomolgus monkeys aged 4–10 years and weighing 2.1–3.9 kg. Semen was collected from three sexually mature male monkeys aged 8–17 years and weighing 5.2–6.6 kg. Temperature and humidity in the animal rooms were maintained at 25 ± 2°C and 50 ± 5%, respectively. The light cycle was 12 h of artificial light from 0800 to 2000 h. In the morning, each animal was fed 20 g/kg of body weight of commercial pellet monkey chow (CMK-1; CLEA Japan, Tokyo, Japan) supplemented with 20–50 g sweet potato in the afternoon. Water was available ad libitum.
Oocyte collection
Ovarian stimulation and oocyte collection were carried out as previously described by Yamasaki et al. [28] with some modifications. Briefly, beginning at menses, the level of sex steroid hormones was reduced by subcutaneous injection of 0.9 mg gonadotropin-releasing hormone antagonist (Leuplin; Takeda Chemical Industries, Osaka, Japan). Two weeks later, a micro-infusion pump (iPRECIO SMP-200, Primetech Corp, Tokyo, Japan) with 15 IU/kg hFSH (Asuka Pharmaceutical, Tokyo, Japan) was embedded subcutaneously in the back under anesthesia (ketamine and xylazine) and operated at 7 μl/h for 10 days. On the day after the last hFSH injection, 400 IU/kg human chorionic gonadotropin (hCG; Asuka Pharmaceutical) was injected intramuscularly. Oocytes were collected by follicular aspiration at 40 h after hCG treatment using a laparoscope (LA-6500, Machida Endoscope, Chiba, Japan). Cumulus-oocyte complexes were recovered in α-modification of Eagle’s medium (MP Biomedicals LLC, Solon, OH, USA) containing 10% Serum Substitute Supplement (Irvine Scientific, Santa Ana, CA, USA) at 38°C in a humidified atmosphere with5% CO2 for 1–2 h. Cumulus cells were removed from oocytes by mechanical pipetting after brief exposure (< 1 min) to 0.5 mg/ml hyaluronidase (Sigma Chemical, St. Louis, MO, USA) adjusted with m-TALP (pH 7.4), a modified Tyrode solution containing lactate, pyruvate, 0.3% bovine serum albumin (Sigma Chemical), and HEPES. Then, oocytes were transferred to m-TALP without hyaluronidase at 38°C with 5% CO2 until further use. Oocytes were classified into the four stages: germinal vesicle (GV), metaphase I (MI), metaphase II (MII), or degenerate (DG).
Detection of antibodies specific for hFSH or hCG by ELISA
The antibody titers of plasma samples against hFSH or hCG were determined using an ELISA [29]. A 0.5 ml blood sample was collected from the femoral vein using a 27 G needle and centrifuged at 1,730 × g for 15 min to separate the plasma. Then, 96-well plates were coated with 50 μl human FSH (20 IU/ml; Asuka Pharmaceutical) or hCG (20 IU/ml; Asuka Pharmaceutical) diluted with saline at 4°C overnight. After washing five times with PBS containing 0.05% Tween-20 (PBS-T), 50 μl of 1/10 diluted samples were incubated overnight in the coated plates. After washing five times with PBS-T, 50 μl horseradish peroxidase (HRP)-conjugated anti monkey IgG (1:1,000; MP Biomedicals, Santa Ana, CA, USA) was added, followed by incubation for 1 h at room temperature. HRP activity was assessed using 3, 3′, 5, 5′-tetramethyl benzidine substrate. The reaction was stopped by addition of 1 M hydrogen chloride. Optical density was measured using an iMark microplate reader (Bio-Rad, Hercules, CA, USA) at 450 nm.
Statistical analysis
Statistical analyses of all data comparisons were carried out by t-test using GraphPad Prism 8 software (https://www.graphpad.com/scientific-software/prism/). P < 0.05 was considered statistically significant.
Results
Poor second ovarian stimulations by hFSH in a group of cynomolgus monkeys
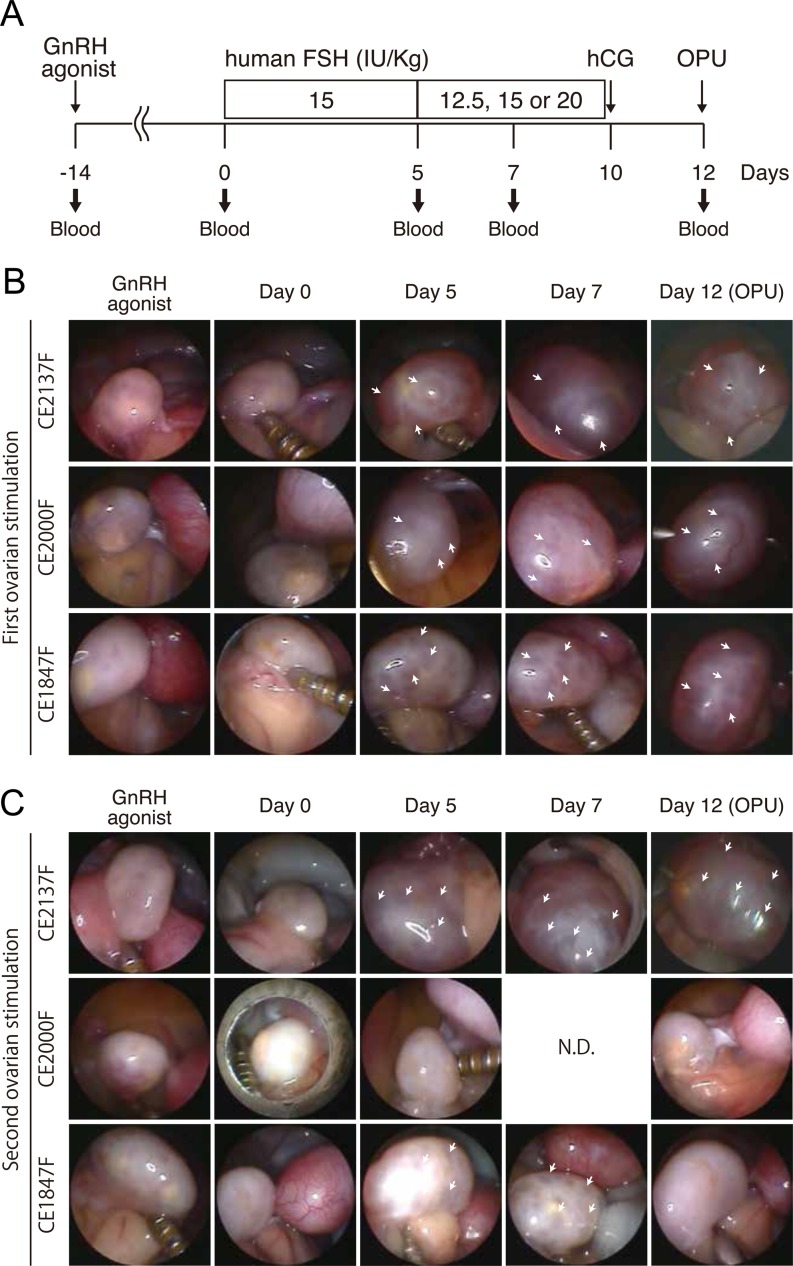
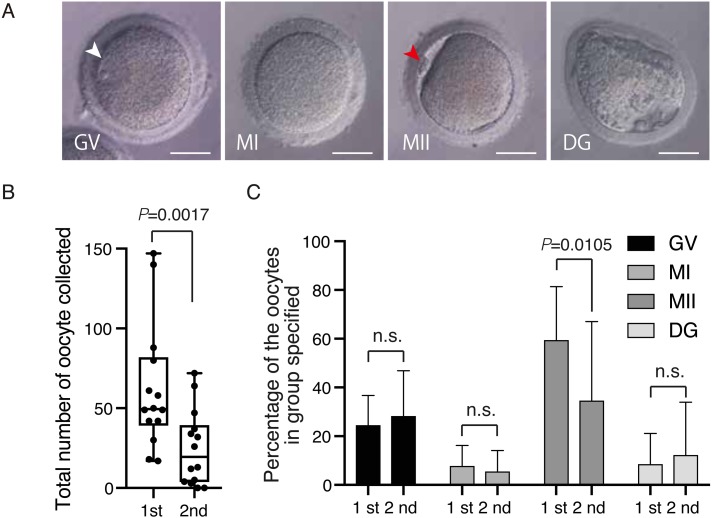
It has been widely reported, including by the current authors, that mature oocytes can be recovered effectively from cynomolgus monkeys through ovarian stimulation by hFSH [25, 27, 30]. However, it is unknown whether mature oocytes can be effectively obtained repeatedly by hFSH stimulation. Therefore, in this study, we investigated whether ovaries are stimulated by hFSH administration after the first ovum pick up (OPU). Fourteen female cynomolgus monkeys were subjected to first ovarian stimulation by successive hFSH administration for 10 days, followed by OPU at day 12 as described in Fig. 1A. Laparoscopic observation indicated that their ovaries responded well to hFSH and follicles developed normally (Fig. 1B). At least 6 months later, these female monkeys were subjected to second ovarian stimulations according to the same protocol used for the first stimulation (Fig. 1A). Blood was collected at various time points of the first and second hFSH administrations (Fig. 1A). While some monkeys showed normal follicle development during the hFSH administration (i.e. translucent follicles enlarged and maturated over the course of hFSH administration) (Fig. 1C, upper panel), we found that some monkeys showed severely impaired follicle development (Fig. 1C, middle panel), and other monkeys showed normal follicle development until day 7, but failed to develop normally (Fig. 1C, lower panel). Four types of oocytes (GV, MI, MII and DG) were obtained by OPU after the second ovarian stimulation (Fig. 2A). As a result, a reduced number of total oocytes was obtained at the second ovarian stimulation compared with the first (Fig. 2B, Table 1), and the percentage of MII oocytes was selectively reduced at the second ovarian stimulation (Fig. 2C, Table 1). However, the quality of the MII oocytes obtained from the second OPU appeared to be maintained normally, because fertilization and developmental rates of oocytes obtained from the first and second oocyte collections were similar [1st OPU vs. 2nd OPU fertilization rate: 76.3% (90/118: 2-cell embryo /MII oocytes) vs. 88.9% (8/9: 2-cell embryo /MII oocytes); blastocyst rate: 64.4% (58/90: blastocyst/2-cell embryo) vs. 50% (4/8: blastocyst/2-cell embryo)], although the number was small and needs to be increased for significance.
Fig. 1.
Poor second ovarian stimulation by hFSH in some female monkeys. A: Schematic overview of ovarian stimulation in a cynomolgus monkey. Day 0 is the start date of hFSH administration. Shown below is the timing of blood sampling. The second ovarian stimulation was performed after at least 6 months in consideration of animal welfare. OPU; ovum pick up. B and C: Laparoscopic observation of ovaries during the first (B) and second (C) treatment by hFSH. Arrows indicate translucent growing follicles. CE2137F, and CE2000F are identification codes for each cynomolgus monkey. N.D., Not determined.
Fig. 2.
Reduced number of total oocytes in some female monkeys in the course of the second hFSH administration. A: Phase contrast images of GV, MI, MII, and DG oocytes recovered from female monkeys receiving the second hFSH administration. Arrowheads in white and red indicate the germinal vesicle in a GV oocyte and the first polar body in a MII oocyte, respectively. Scale bars = 50 µm. B: Number of total oocytes collected. C: Percentages of collected GV, MI, MII and DG oocytes. n.s; not significant.
Table 1. Results of 1st and 2nd superovulation in the individual cynomolgus monkey.
| hFSH Ab (–) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Individual number | Origin* (Female/Male) | OPU | Total | GV | MI | MII | DG | hCG Ab |
| CE862F | V/I | 1st | 42 | 1 | 22 | 12 (28.6) | 7 | N.D. |
| 2nd | 37 | 3 | 22 | 6 (16) | 6 | |||
| CE960F | C/C | 1st | 30 | 0 | 6 | 24 (80) | 0 | N.D. |
| 2nd | 26 | 6 | 9 | 11 (42.3) | 0 | |||
| CE1108F | P/V | 1st | 49 | 3 | 6 | 40 (81.6) | 0 | N.D. |
| 2nd | 47 | 0 | 11 | 36 (76.6) | 0 | |||
| CE1951F | C/C | 1st | 17 | 1 | 4 | 12 (70.6) | 0 | + |
| 2nd | 12 | 0 | 6 | 6 (50.0) | 0 | |||
| CE1956F | C/C | 1st | 18 | 1 | 2 | 15 (83.3) | 0 | – |
| 2nd | 13 | 0 | 1 | 12 (92.3) | 0 | |||
| CE2024F | C/C | 1st | 49 | 9 | 7 | 29 (59.2) | 4 | – |
| 2nd | 34 | 7 | 10 | 8 (23.5) | 0 | |||
| CE2109F | C/C | 1st | 50 | 11 | 9 | 13 (26.0) | 17 | – |
| 2nd | 32 | 5 | 8 | 13 (40.6) | 6 | |||
| CE2137F | C/C | 1st | 80 | 0 | 24 | 56 (70) | 0 | – |
| 2nd | 72 | 1 | 15 | 56 (78) | 0 | |||
| hFSH Ab (+) | ||||||||
| Individual number | Origin* (Female/Male) | OPU | Total | GV | MI | MII | DG | hCG Ab |
| CE866F | V/V | 1st | 42 | 8 | 11 | 23 (54.8) | 0 | + |
| 2nd | 3 | 0 | 1 | 0 (0) | 2 | |||
| CE1723F | P/P | 1st | 61 | 4 | 20 | 15 (24.6) | 21 | N.D. |
| 2nd | 0 | 0 | 0 | 0 (0) | 0 | |||
| CE1810F | P/P | 1st | 140 | 11 | 30 | 85 (60.7) | 14 | N.D. |
| 2nd | 0 | 0 | 0 | 0 (0) | 0 | |||
| CE1847F | C/C | 1st | 147 | 0 | 56 | 89 (60.5) | 2 | + |
| 2nd | 5 | 0 | 1 | 2 (40) | 2 | |||
| CE1831F | V/V | 1st | 58 | 0 | 9 | 49 (84.5) | 0 | + |
| 2nd | 64 | 17 | 22 | 20 (31) | 5 | |||
| CE2000F | C/C | 1st | 88 | 1 | 37 | 43 (48.9) | 7 | + |
| 2nd | 4 | 0 | 2 | 0 (0) | 2 | |||
* V: Vietnam, I: Indonesia, C: China, P: Philippines, N.D.: Not determined.
Generation of antibodies against hFSH in a group of female monkeys during the second hFSH administration
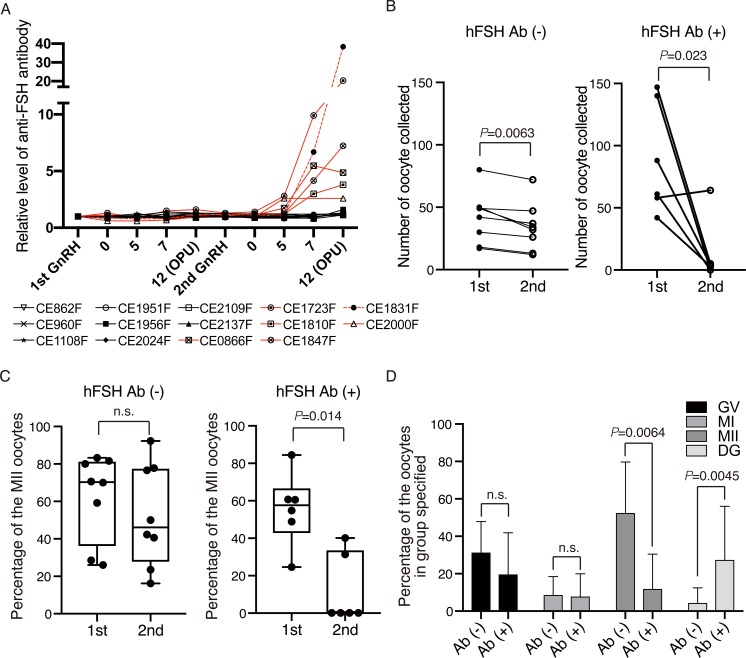
Because some monkeys showed poor second ovarian stimulation by hFSH (Fig. 1B), we considered that the antibodies against hFSH had been generated in the serum and hampered the actions of hFSH in these monkeys. To test this hypothesis, we measured antibodies against hFSH by an ELISA and found such antibodies in six out of 14 female monkeys, which increased over the course of the second hFSH administration (Fig. 3A). When the female monkeys were divided into two groups, hFSH antibody-minus [Ab (–)] and -plus [Ab (+)], monkeys without the antibodies showed a similar number of total oocytes with slight decrease, whereas monkeys with the antibodies showed a markedly reduced number of total oocytes (Fig. 3B). The percentage of MII oocytes was severely reduced in monkeys with the antibodies (Fig. 3C), although MI and GV were not changed significantly (Fig. 3D).
Fig. 3.
Production of antibodies against hFSH is associated with poor second ovarian response. A: Detection of antibodies against hFSH in serum. Because the level of antibodies against hFSH from first to second GnRH administrations was 1 ± 0.46, we considered that the antibody level was elevated significantly when the level of antibodies against hFSH exceeded 1.46. Red and black lines indicate Ab (+) and Ab (–), respectively. B: Numbers of total oocytes collected from cynomolgus monkeys without or with the antibody production. C: Percentages of MII oocytes collected from cynomolgus monkeys without or with the antibody production. D: Percentages of GV, MI, MII, and DG oocytes collected from cynomolgus monkeys without or with the antibody production at the second superovulation. n.s; not significant.
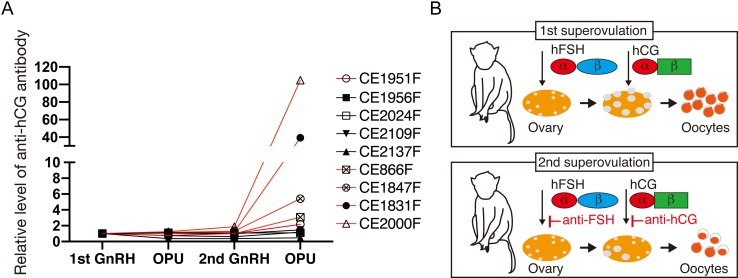
Since the percentage of MII oocytes was reduced in monkeys with the antibodies against hFSH (Fig. 3D, Table 1), we considered that hCG, a hormone required for the maturation from MI into MII oocytes, is also neutralized by antibodies. In fact, when we evaluated the generation of the antibodies against hCG in the serum from nine monkeys (five hFSH Ab (–) and four hFSH Ab (+)) (Table 1), we found that antibodies against hCG were detected in all of four hFSH Ab (+) samples and one out of five hFSH Ab (–) samples (Fig. 4A, Table 1), suggesting that antibodies against hFSH and hCG are generated simultaneously in the most of the samples. We could not measure 4 samples due to the loss of the serum. Collectively, these data indicate that the antibodies hamper hCG action.
Fig. 4.
Production of antibodies against hCG. A: Detection of antibodies against hCG in serum. Because the level of antibodies against hCG from first to second GnRH administrations was 1 ± 0.31, we considered that the antibody level was elevated significantly when the level of antibodies against hCG exceeded 1.31. Red and black lines indicate Ab (+) and Ab (–), respectively. B: Summary of this study. Data are shown as the mean ± SD.
Discussion
In this study, we found that mature oocytes can be recovered effectively from a group of female monkeys even after the first OPU, but at a markedly decreased level in the other group of female monkeys. We also found that generation of antibodies against hFSH was strongly associated with poor second ovarian stimulation by hFSH. Furthermore, we found generation of antibodies against hCG. We presumed that antibody generation may hamper hFSH and hCG actions (Fig. 4B). Currently, it is unknown which factor triggers antibody generation in a specific group of female monkeys.
FSH consists of a specific subunit, FSHβ, and common alpha subunit, FSHα, shared with LH, TSH, and CG [15, 18]. Although we found generation of antibodies against hFSH, it is unclear whether these antibodies recognize either FSHα or FSHβ, or both subunits. Comparison of amino acid sequences among human and monkey orthologs of FSHα and FSHβ revealed high conservation of FSHβ (96%) and moderate conservation of FSHα (84%) (Supplementary Fig. 1: online only). Considering the lower conservation in FSHα, antibodies against FSHα may be generated more easily. Consistent with this idea, our data indicate that antibodies against hCG consisting of FSHα are also generated in the serum of female monkeys with poor second ovarian stimulation. This may account for the fact that the percentage of MII oocytes was selectively reduced at the second ovarian stimulation.
hFSH is used worldwide for treating human infertility [31,32,33]. hFSH used in the clinic is purified from human urea. It is reported that anti-FSH antibodies are elevated in infertile women and antibodies are associated with dysregulation of immune reactions and repeatedly performed IVF procedures [34,35,36]. In marmoset subjects, Marshall and coworkers reported the generation of antibodies against hFSH, although mature oocytes were recovered effectively from marmosets at multiple times [37]. Currently, it is unclear why antibody generation does not halt hFSH actions in marmosets. However, because the common marmoset and cynomolgus monkey weigh 0.3 and 3 kg, respectively, the dose for marmosets (50 IU/day) corresponds to 500 IU/kg for cynomolgus monkeys, which is 10-fold higher. Thus, it would be very interesting to investigate whether a very high dose of hFSH administration may overcome antibodies against hFSH. In rhesus and cynomolgus monkey subjects, repeatedly treatment of hFSH or hCG cause anti-FSH antibodies [38, 39] but it is not shown the correlation between anti-hFSH production, follicular development and oocyte maturation.
Although the repeated hFSH and hCG administrations are likely to be the cause of the reduced number of oocytes in second OPU, there are several approaches to address this issue. One is the production and purification of FSH and CG from cynomolgus monkey, avoiding antibody generation. The other is the use of anti-inhibin serum that is widely used in many kinds of animals to increase the number of oocytes [40,41,42].
Taken together, our results clearly indicate that the production of anti-hFSH antibodies in a specific group of cynomolgus monkeys could cause a poor ovarian response to hFSH stimulation. Further, detection of such antibodies as well as observation of the ovary over the course of hFSH administration could be useful for predicting favorable second ovarian stimulation by hFSH.
Conflict of Interests
The authors declare no competing financial interests.
Supplementary
Acknowledgments
We thank the Research Center for Animal Life Science research support team for animal care at Shiga University of Medical Science. We also thank Dr Nakaya for critical comments on this manuscript.
This study was supported in part by JSPS KAKENHI Grant Number JP17K14977 to YS, and by an in-house grant from Shiga University of Medical Science to ME and YS.
References
- 1.Mansfield K. Marmoset models commonly used in biomedical research. Comp Med 2003; 53: 383–392. [PubMed] [Google Scholar]
- 2.Miller CT, Freiwald WA, Leopold DA, Mitchell JF, Silva AC, Wang X. Marmosets: a neuroscientific model of human social behavior. Neuron 2016; 90: 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itell HL, Kaur A, Deere JD, Barry PA, Permar SR. Rhesus monkeys for a nonhuman primate model of cytomegalovirus infections. Curr Opin Virol 2017; 25: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colman RJ. Non-human primates as a model for aging. Biochim Biophys Acta Mol Basis Dis 2018; 1864 (9 Pt A): 2733–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miura M, Yasunaga J, Tanabe J, Sugata K, Zhao T, Ma G, Miyazato P, Ohshima K, Kaneko A, Watanabe A, Saito A, Akari H, Matsuoka M. Characterization of simian T-cell leukemia virus type 1 in naturally infected Japanese macaques as a model of HTLV-1 infection. Retrovirology 2013; 10: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pennesi ME, Garg AK, Feng S, Michaels KV, Smith TB, Fay JD, Weiss AR, Renner LM, Hurst S, McGill TJ, Cornea A, Rittenhouse KD, Sperling M, Fruebis J, Neuringer M. Measuring cone density in a Japanese macaque (Macaca fuscata) model of age-related macular degeneration with commercially available adaptive optics. Adv Exp Med Biol 2014; 801: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Takahashi K, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 2009; 460: 1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentes GA, Guimarães JR, Volotão EM, Fialho AM, Hooper C, Ganime AC, Gardinali NR, Lanzarini NM, da Silva ADS, Pitcovski J, Leite JP, Pinto MA. Cynomolgus monkeys (Macaca fascicularis) as an experimental infection model for human group a rotavirus. Viruses 2018; 10: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, Balzamo E, Bezard E, Tison F, Ghorayeb I. Sleep disorders in Parkinson’s disease: the contribution of the MPTP non-human primate model. Exp Neurol 2009; 219: 574–582. [DOI] [PubMed] [Google Scholar]
- 10.Soderstrom K, O’Malley J, Steece-Collier K, Kordower JH. Neural repair strategies for Parkinson’s disease: insights from primate models. Cell Transplant 2006; 15: 251–265. [DOI] [PubMed] [Google Scholar]
- 11.Isacson O, Riche D, Hantraye P, Sofroniew MV, Maziere M. A primate model of Huntington’s disease: cross-species implantation of striatal precursor cells to the excitotoxically lesioned baboon caudate-putamen. Exp Brain Res 1989; 75: 213–220. [DOI] [PubMed] [Google Scholar]
- 12.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990; 13: 281–285. [DOI] [PubMed] [Google Scholar]
- 13.Kisu I, Mihara M, Banno K, Hara H, Masugi Y, Araki J, Iida T, Yamada Y, Kato Y, Shiina T, Suganuma N, Aoki D. Uterus allotransplantation in cynomolgus macaque: a preliminary experience with non-human primate models. J Obstet Gynaecol Res 2014; 40: 907–918. [DOI] [PubMed] [Google Scholar]
- 14.Enskog A, Johannesson L, Chai DC, Dahm-Kähler P, Marcickiewicz J, Nyachieo A, Mwenda JM, Brännström M. Uterus transplantation in the baboon: methodology and long-term function after auto-transplantation. Hum Reprod 2010; 25: 1980–1987. [DOI] [PubMed] [Google Scholar]
- 15.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem 1981; 50: 465–495. [DOI] [PubMed] [Google Scholar]
- 16.McGee EA, Perlas E, LaPolt PS, Tsafriri A, Hsueh AJ. Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol Reprod 1997; 57: 990–998. [DOI] [PubMed] [Google Scholar]
- 17.Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev 2000; 21: 551–583. [DOI] [PubMed] [Google Scholar]
- 18.Dias JA, Cohen BD, Lindau-Shepard B, Nechamen CA, Peterson AJ, Schmidt A. Molecular, structural, and cellular biology of follitropin and follitropin receptor. Vitam Horm 2002; 64: 249–322. [DOI] [PubMed] [Google Scholar]
- 19.Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 2006; 18: 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest 2010; 120: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA 1998; 95: 13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huhtaniemi IT, Themmen APN. Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine 2005; 26: 207–217. [DOI] [PubMed] [Google Scholar]
- 23.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 1997; 15: 201–204. [DOI] [PubMed] [Google Scholar]
- 24.Tapanainen JS, Vaskivuo T, Aittomäki K, Huhtaniemi IT. Inactivating FSH receptor mutations and gonadal dysfunction. Mol Cell Endocrinol 1998; 145: 129–135. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, Yoon SB, Jeong KJ, Sim BW, Choi SA, Lee SI, Jin YB, Song BS, Lee SR, Kim SU, Chang KT. Superovulatory responses in cynomolgus monkeys (Macaca fascicularis) depend on the interaction between donor status and superovulation method used. J Reprod Dev 2017; 63: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimozawa N, Okada H, Hatori M, Yoshida T, Sankai T. Comparison of methods to stimulate ovarian follicular growth in cynomolgus and African green monkeys for collection of mature oocytes. Theriogenology 2007; 67: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 27.Seita Y, Tsukiyama T, Iwatani C, Tsuchiya H, Matsushita J, Azami T, Okahara J, Nakamura S, Hayashi Y, Hitoshi S, Itoh Y, Imamura T, Nishimura M, Tooyama I, Miyoshi H, Saitou M, Ogasawara K, Sasaki E, Ema M. Generation of transgenic cynomolgus monkeys that express green fluorescent protein throughout the whole body. Sci Rep 2016; 6: 24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamasaki J, Iwatani C, Tsuchiya H, Okahara J, Sankai T, Torii R. Vitrification and transfer of cynomolgus monkey (Macaca fascicularis) embryos fertilized by intracytoplasmic sperm injection. Theriogenology 2011; 76: 33–38. [DOI] [PubMed] [Google Scholar]
- 29.Kida H, Webster RG, Yanagawa R. Inhibition of virus-induced hemolysis with monoclonal antibodies to different antigenic areas on the hemagglutinin molecule of A/seal/Massachusetts/1/80 (H7N7) influenza virus. Arch Virol 1983; 76: 91–99. [DOI] [PubMed] [Google Scholar]
- 30.Hayes ES, Curnow EC, Trounson AO, Danielson LA, Unemori EN. Implantation and pregnancy following in vitro fertilization and the effect of recombinant human relaxin administration in Macaca fascicularis. Biol Reprod 2004; 71: 1591–1597. [DOI] [PubMed] [Google Scholar]
- 31.Practice Committee of the American Society for Reproductive Medicine.Progesterone supplementation during the luteal phase and in early pregnancy in the treatment of infertility: an educational bulletin. Fertil Steril 2008; 89: 789–792. [DOI] [PubMed] [Google Scholar]
- 32.Amer S. Gonadotropin induction of ovulation. Obstetrics, Gynaecol Reprod Med 2007; 17: 205–210. [Google Scholar]
- 33.Leão RB, Esteves SC. Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech. Clinics (Sao Paulo) 2014; 69: 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gobert B, Jolivet-Reynaud C, Dalbon P, Barbarino-Monnier P, Faure GC, Jolivet M, Béné MC. An immunoreactive peptide of the FSH involved in autoimmune infertility. Biochem Biophys Res Commun 2001; 289: 819–824. [DOI] [PubMed] [Google Scholar]
- 35.Shatavi SV, Llanes B, Luborsky JL. Association of unexplained infertility with gonadotropin and ovarian antibodies. Am J Reprod Immunol 2006; 56: 286–291. [DOI] [PubMed] [Google Scholar]
- 36.Haller K, Salumets A, Uibo R. Anti-FSH antibodies associate with poor outcome of ovarian stimulation in IVF. Reprod Biomed Online 2008; 16: 350–355. [DOI] [PubMed] [Google Scholar]
- 37.Marshall VS, Browne MA, Knowles L, Golos TG, Thomson JA. Ovarian stimulation of marmoset monkeys (Callithrix jacchus) using recombinant human follicle stimulating hormone. J Med Primatol 2003; 32: 57–66. [DOI] [PubMed] [Google Scholar]
- 38.Platia MP, Bloomquist G, Williams RF, Hodgen GD. Refractoriness to gonadotropin therapy: how to distinguish ovarian failure versus pseudoovarian resistance caused by neutralizing antibodies. Fertil Steril 1984; 42: 779–784. [PubMed] [Google Scholar]
- 39.Ottobre JS, Stouffer RL. Antibody production in rhesus monkeys following prolonged administration of human chorionic gonadotropin. Fertil Steril 1985; 43: 122–128. [DOI] [PubMed] [Google Scholar]
- 40.Takedomi T, Kishi H, Medan MS, Aoyagi Y, Konishi M, Itoh T, Yazawa S, Watanabe G, Taya K. Active immunization against inhibin improves superovulatory response to exogenous FSH in cattle. J Reprod Dev 2005; 51: 341–346. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa A, Mochida K, Inoue H, Noda Y, Endo T, Watanabe G, Ogura A. High-yield superovulation in adult mice by anti-inhibin serum treatment combined with estrous cycle synchronization1. Biol Reprod 2016; 94: 21. [DOI] [PubMed] [Google Scholar]
- 42.Takeo T, Nakagata N. Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One 2015; 10: e0128330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.