Key Points
Question
Is there evidence to guide the treatment decision between transcatheter or surgical aortic valve replacement for patients with aortic stenosis at intermediate operative risk and a history of coronary artery bypass graft (CABG) surgery?
Findings
In this post hoc analysis of 273 intermediate-risk patients with prior CABG surgery enrolled in a randomized clinical trial, both transcatheter and surgical aortic valve replacement were safe and provided symptom relief. The transcatheter approach provided more rapid improvement in quality of life and better exercise tolerance at 1-year follow-up.
Meaning
Transcatheter and surgical aortic valve replacement are appropriate treatments in patients with prior CABG surgery, and the less-invasive transcatheter approach may facilitate faster recovery.
This post hoc analysis of a randomized clinical trial compares outcomes after transcatheter vs surgical aortic valve replacement in patients at intermediate operative risk with a history of coronary artery bypass graft surgery.
Abstract
Importance
Surgical aortic valve replacement (SAVR) has increased risk for patients with aortic stenosis (AS) and a history of coronary artery bypass graft (CABG) surgery. Transcatheter aortic valve replacement (TAVR) may be an alternative.
Objective
To compare TAVR with SAVR outcomes in patients at intermediate operative risk with prior CABG surgery.
Design, Setting, and Participants
In this post hoc analysis of the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) noninferiority randomized clinical trial, patients with severe, symptomatic AS at intermediate operative risk were enrolled from 87 centers across the United States, Europe, and Canada from June 2012 to June 2016 and followed-up with up to July 2017. Those with a history of CABG surgery were considered for analysis. Data were analyzed from September to December 2017.
Interventions
A total of 1746 patients were enrolled and randomized 1:1 to self-expanding TAVR or SAVR. An implant was attempted in 1660 patients, of whom 273 had prior CABG surgery, including 136 who underwent attempted TAVR and 137 who underwent attempted SAVR.
Main Outcomes and Measures
The primary outcome was all-cause mortality or disabling stroke at 1-year follow-up. Efficacy outcomes included quality of life, measured using the Kansas City Cardiomyopathy Questionnaire at 30 days, 6 months, and 1 year, and distance walked in 6 minutes, measured using the 6-minute walk test at 30 days and 1 year.
Results
Of the 136 patients in the TAVR cohort, 111 (81.6%) were male, and the mean (SD) age was 76.9 (6.5) years; of the 137 in the SAVR cohort, 117 (85.4%) were male, and the mean (SD) age was 76.6 (6.5) years. The mean (SD) Society of Thoracic Surgeons Predicted Risk of Mortality score was 5.0% (1.6%) in the TAVR cohort and 5.2% (1.7%) in the SAVR cohort. All-cause mortality or disabling stroke at 1-year follow-up was 8.9% (95% CI, 5.2-15.2) in the TAVR cohort and 6.7% (95% CI, 3.5-12.8) in the SAVR cohort (log-rank P = .53). Compared with patients receiving SAVR, the mean (SD) Kansas City Cardiomyopathy Questionnaire summary score was significantly better among patients receiving TAVR at 30 days (81.4 [19.2] vs 69.7 [22.6]; P < .001); treatments were similar at 1 year (85.7 [14.6] vs 82.8 [18.4]; P = .19). Compared with patients in the SAVR cohort, those in the TAVR cohort showed greater mean (SD) improvement in distance walked at 1 year (48.3 [120.6] m vs 16.8 [88.7] m; P = .04).
Conclusions and Relevance
Both TAVR and SAVR were safe for intermediate-risk patients with AS and prior CABG surgery. The transcatheter approach facilitated faster improvement in quality of life and better exercise capacity at 1-year follow-up.
Trial Registration
ClinicalTrials.gov identifier: NCT01586910
Introduction
Coronary artery disease previously treated with coronary artery bypass graft (CABG) surgery is common in patients with severe, symptomatic aortic stenosis (AS) and increases the complexity of surgical aortic valve replacement (SAVR) owing to the risks of sternal re-entry with cardiac or graft injury.1,2,3,4,5 Transcatheter aortic valve replacement (TAVR) is a less invasive approach that may be particularly well-suited for these patients. The purpose of this study was to compare outcomes after TAVR using a self-expanding device with outcomes after SAVR in patients with AS at intermediate operative risk and a history of CABG surgery.
Methods
Patients and Design
The Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial (NCT01586910) enrolled patients across 87 sites in the United States, Europe, and Canada between June 2012 and June 2016.6 Aortic valve replacement was attempted in 1660 patients, of whom 864 had an attempted TAVR and 796 had an attempted SAVR. The trial complied with the Declaration of Helsinki, all local ethics committees approved the research protocol, and written informed consent was obtained from all patients. The trial protocol can be found in Supplement 1. A total of 136 patients who received TAVR and 137 who received SAVR underwent CABG surgery prior to enrollment in the trial and were included in this post hoc analysis (eFigure 1 in Supplement 2).
End Points
The primary end point was all-cause mortality or disabling stroke at 1-year follow-up, with disabling stroke defined according to the Valve Academic Research Consortium–2 (VARC-2).7 Other safety and efficacy end points were explored through 1-year follow-up, including symptom status defined by New York Heart Association (NYHA) class, quality of life assessed by the Kansas City Cardiomyopathy Questionnaire,8 and exercise capacity determined by the 6-minute walk test.9
Statistical Analysis
Categorical variables were compared using χ2 or Fisher exact tests. Continuous variables were presented as means with SDs and compared using the t test. Kaplan-Meier estimates were used to construct all-cause mortality or disabling stroke for the time-to-event analysis. The log-rank test was used to compare the time to events. All testing used a 2-sided α level less than .05. Cox proportional hazard modeling was used to test for an interaction between prior CABG surgery and no prior CABG surgery for any safety outcome at 30 days and 1 year. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Patients
The baseline characteristics are presented in Table 1. Of the 136 patients in the TAVR cohort, 111 (81.6%) were male, and the mean (SD) age was 76.9 (6.5) years; of the 137 in the SAVR cohort, 117 (85.4%) were male, and the mean (SD) age was 76.6 (6.5) years. The mean (SD) Society of Thoracic Surgeons Predicted Risk of Mortality score was 5.0% (1.6%) in the TAVR cohort and 5.2% (1.7%) in the SAVR cohort.
Table 1. Baseline Demographic Characteristics and Medical History.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| TAVR (n = 136) | SAVR (n = 137) | ||
| Demographic Characteristics | |||
| Age, mean (SD), y | 76.9 (6.5) | 76.6 (6.5) | .70 |
| Male | 111 (81.6) | 117 (85.4) | .40 |
| STS-PROM score, mean (SD), % | 5.0 (1.6) | 5.2 (1.7) | .60 |
| NYHA class | .65 | ||
| II | 61 (44.9) | 58 (42.3) | |
| III | 70 (51.5) | 73 (53.3) | |
| IV | 5 (3.7) | 6 (4.4) | |
| 6-Minute walk test distance, mean (SD), m | 281.0 (116.8) | 277.1 (113.1) | .79 |
| Diabetes | 53 (39.0) | 57 (41.6) | .66 |
| Creatinine level >2 mg/dL | 1 (0.7) | 3 (2.2) | .62 |
| Hypertension | 130 (95.6) | 130 (94.9) | .79 |
| Peripheral vascular disease | 61 (44.9) | 60 (43.8) | .86 |
| Cerebrovascular disease | 29 (21.3) | 27 (19.7) | .74 |
| Prior stroke | 14 (10.3) | 13 (9.5) | .82 |
| Chronic lung disease | 45 (33.3)a | 49 (35.8) | .67 |
| Medical History | |||
| Time since CABG surgery, mean (SD), y | 13.0 (6.3) | 13.0 (6.4) | .93 |
| Operative incidence | .99 | ||
| First reoperation cardiovascular surgery | 134 (98.5) | 135 (98.5) | |
| Second reoperation cardiovascular surgery | 2 (1.5) | 2 (1.5) | |
| LIMA grafts | 118 (86.8) | 114 (83.2) | .41 |
| Prior PCI | 48 (35.3) | 51 (37.2) | .74 |
| Pre-existing IPG/ICD | 14 (10.3) | 18 (13.1) | .47 |
| Angina | 26 (19.1) | 27 (19.7) | .90 |
| Prior myocardial infarction | 43 (31.6) | 47 (34.3) | .64 |
| Atrial fibrillation/flutter | 41 (30.1) | 37 (27.0) | .57 |
| Echocardiography, mean (SD) | |||
| Left ventricular ejection fraction, % | 58.7 (11.1) | 57.5 (11.8) | .38 |
| Aortic valve area, cm2 | 0.79 (0.21) | 0.82 (0.21) | .40 |
| Mean gradient, mm Hg | 43.0 (11.7) | 44.5 (12.7) | .31 |
| Anatomical factors | |||
| Severe aortic calcification | 20 (14.7) | 20 (14.6) | .98 |
| Prohibitive chest deformity | 1 (0.7) | 0 | .50 |
Abbreviations: CABG, coronary artery bypass grafting; ICD, implantable cardioverter-defibrillator; IPG, implantable pulse generator; LIMA, left internal mammary artery; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SAVR, surgical aortic valve replacement; STS-PROM, Society of Thoracic Surgeons’ Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement.
SI conversion factor: To convert creatinine level to micromoles per liter, multiply by 88.4.
Data were available for 135 patients.
Clinical Outcomes
Clinical outcomes are presented in Table 2. All-cause mortality or disabling stroke at 1-year follow-up did not differ between treatments (TAVR: 8.9%; 95% CI, 5.2-15.2; SAVR: 6.7%; 95% CI, 3.5-12.8; P = .53) (eFigure 2 in Supplement 2). There was no significant difference in all-cause mortality or all stroke at 30 days.
Table 2. 30-Day and 1-Year Adjudicated Outcomesa.
| Outcome | No. (%) | P Value | |
|---|---|---|---|
| TAVR (n = 136) | SAVR (n = 137) | ||
| 30-d Outcomes | |||
| All-cause mortality or disabling stroke | 1 (0.7) | 4 (2.9) | .18 |
| All-cause mortality | 0 | 1 (0.7) | .32 |
| Cardiovascular mortality | 0 | 1 (0.7) | .32 |
| All stroke | 5 (3.7) | 7 (5.1) | .55 |
| Disabling stroke | 1 (0.7) | 3 (2.2) | .31 |
| Life-threatening or disabling bleeding | 7 (5.1) | 8 (5.9) | .80 |
| Major bleeding | 10 (7.4) | 11 (8.1) | .83 |
| Transfusion of PRBCs, unitsb | 13 (9.6) | 53 (38.7) | <.001 |
| 2-3 | 6 (4.4) | 26 (19.0) | <.001 |
| ≥4 | 4 (2.9) | 17 (12.4) | <.001 |
| Major vascular complications | 7 (5.1) | 3 (2.2) | .20 |
| Acute kidney injury stage 2 or 3 | 0 | 4 (2.9) | .04 |
| Myocardial infarction | 1 (0.7) | 1 (0.7) | >.99 |
| Permanent pacemaker | 33 (24.3) | 7 (5.2) | <.001 |
| Atrial fibrillation | 13 (9.6) | 55 (37.5) | <.001 |
| 1-y Outcomes | |||
| All-cause mortality or disabling stroke | 12 (8.9) | 10 (6.7) | .53 |
| All-cause mortality | 8 (5.9) | 6 (4.5) | .60 |
| Cardiovascular mortality | 6 (4.5) | 6 (4.5) | .99 |
| All stroke | 9 (6.7) | 10 (7.5) | .79 |
| Disabling stroke | 4 (3.0) | 4 (3.0) | .99 |
| Life-threatening or disabling bleeding | 9 (6.7) | 11 (8.2) | .65 |
| Major bleeding | 14 (10.4) | 17 (12.0) | .70 |
| Myocardial infarction | 2 (1.5) | 4 (3.0) | .40 |
| Permanent pacemaker | 41 (29.0) | 12 (9.0) | <.001 |
| Atrial fibrillation | 22 (13.3) | 63 (39.0) | <.001 |
| Aortic valve hospitalization | 18 (6.7) | 8 (6.0) | .82 |
| Reintervention | 4 (2.2) | 1 (0.7) | .32 |
Abbreviations: PRBC, packed red blood cell; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Data are presented as events (Kaplan-Meier estimates), and P values are calculated based on the log-rank test.
Transfusion of PRBCs is presented as events (percentage rate).
Several adverse events were more common at 30 days following SAVR compared with TAVR, including stage 2 or 3 acute kidney injury, requirement for blood transfusion, and new or worsening atrial fibrillation. A new permanent pacemaker was required more often following TAVR than SAVR. There was no interaction between prior CABG surgery and no prior CABG surgery status for any outcome at 30 days or 1 year (eTable 1 in Supplement 2).
Aftercare and Symptom Status
Compared with patients undergoing SAVR, patients undergoing TAVR had a shorter index hospital stay (mean [SD] duration, 5.1 [3.8] days vs 9.0 [5.7] days; P < .001). More patients in the TAVR cohort were discharged directly home rather than to another care facility compared with those in the SAVR cohort (125 of 136 [91.9%] vs 99 of 137 [72.3%]; P < .001) (eTable 2 in Supplement 2). At 30 days, 98 of 131 patients receiving TAVR (74.8%) and 70 of 125 patients receiving SAVR (56.0%) were in NYHA class I (P = .002). At 1-year follow-up, there was no difference between groups, with 91 of 125 patients in the TAVR cohort (72.8%) and 80 of 111 in the SAVR cohort (72.1%) in NYHA class I (P = .94) (eFigure 3 in Supplement 2).
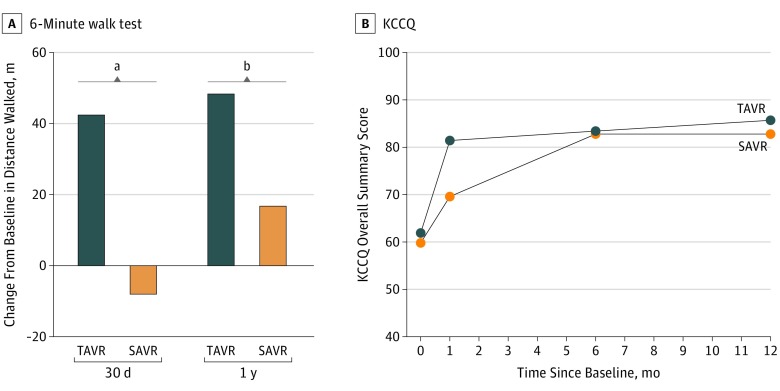
Exercise Capacity and Quality of Life
Patients receiving TAVR walked significantly farther in 6 minutes than patients receiving SAVR at 30 days and 1 year (Figure, A). The Kansas City Cardiomyopathy Questionnaire overall summary score demonstrated clinically meaningful improvement in quality of life over time for patients in both treatment arms (Figure, B). Patients in the TAVR cohort showed a larger improvement at 30 days compared with those in the SAVR cohort, but by 1 year, the degree of improvement was similar between groups.
Figure. Exercise Capacity and Quality-of-Life Outcomes at 1 Year.
A, The mean difference in meters walked at 30 days and 1 year relative to baseline in patients receiving transcatheter aortic valve replacement (TAVR; 112 patients at 30 days and 103 at 1 year) and surgical aortic valve replacement (SAVR; 103 patients at 30 days and 90 at 1 year). B, Mean Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score for patients receiving TAVR and SAVR. Data were available at baseline, 30 days, 6 months, and 1 year for 135, 133, 121, and 116 patients receiving TAVR, respectively, and for 133, 123, 120, and 106 patients receiving SAVR. The difference between groups was significant at 30 days.
aP < .001.
bP = .04.
Echocardiographic Findings
At each time point after the procedure, the mean aortic valve gradient was lower (mean [SD] gradient at 1-year follow-up: TAVR, 8.2 [3.5] mm Hg; SAVR, 11.6 [5.1] mm Hg; P < .001). Additionally, the aortic valve area was larger for patients receiving TAVR compared with those receiving SAVR (mean [SD] aortic valve area at 1-year follow-up: TAVR, 2.2 [0.6] cm2; SAVR, 1.8 [0.6] cm2; P < .001) (eFigure 4 in Supplement 2).
Discussion
This post hoc analysis from the SURTAVI trial6 suggests that for intermediate-risk patients with symptomatic, severe AS and prior CABG surgery, TAVR using the self-expanding valve and SAVR are both safe. The rate of all-cause mortality and disabling stroke as well as the individual components of this primary end point were similar at 30 days and 1 year. Complications of the procedures differed, with those receiving SAVR having more transfusions, acute kidney injury, and atrial fibrillation. Transcatheter aortic valve replacement led to a higher pacemaker rate but also to significantly better aortic valve area and mean gradient and a more rapid improvement in symptoms, exercise capacity, and quality of life.
Reoperation in patients with prior CABG surgery carries increased risk both because these patients tend to be older and have more comorbidities and because of the risk of cardiac or graft injury during sternal re-entry.1,2,3,4,5 Real-world clinical practice is steering away from repeated surgery in favor of TAVR. A 2018 analysis from the National Inpatient Sample database between 2012 and 201410 showed that for patients with prior CABG surgery, the number of TAVRs increased from 1615 in 2012 to 4400 in 2014, while the number of SAVRs decreased from 2285 to 1895 during this period. In Germany, TAVR was the preferred therapy in nearly 90% of patients with prior CABG in 2013.11 The available clinical evidence generally supports this trend. Outcomes of the Placement of Aortic Transcatheter Valves IA randomized clinical trial favored SAVR,12 while those in the CoreValve Pivotal Trial favored TAVR.13 A 2016 review and meta-analysis14 found that patients with previous CABG surgery treated with TAVR or SAVR had similar short-term and long-term survival, but TAVR resulted in a shorter hospital stay and a greater need for postprocedure pacemakers.
It is interesting that although quality-of-life improvement for SAVR caught up to that for TAVR by 1 year, the exercise capacity was better for TAVR throughout follow-up. There are at least 2 potential reasons for this. Some patients may believe themselves to be disabled even if they are objectively doing well. Also, TAVR’s superior hemodynamics may have facilitated increased transvalvular flow needed for exercise.
Limitations
This study has limitations. The cohort in this post hoc analysis represents 16.4% of the total SURTAVI trial population with an attempted implant. Firm conclusions cannot be drawn because of the limited statistical power.
Conclusions
Our study suggests that both SAVR and TAVR are safe for patients with prior CABG surgery at intermediate operative risk. Treatment modality influenced the postoperative course, with TAVR facilitating faster improvement in quality of life and more robust exercise tolerance, which persisted at 1-year follow-up.
Trial protocol.
eTable 1. Outcomes with P values of interaction of prior CABG (yes/no) and treatment (TAVR/SAVR).
eTable 2. Resource utilization.
eFigure 1. Randomization and analysis populations.
eFigure 2. Kaplan-Meier all-cause mortality or disabling stroke estimates.
eFigure 3. New York Heart Association symptom status.
eFigure 4. Aortic valve hemodynamics.
References
- 1.Biancari F, Onorati F, Mariscalco G, et al. First-time, isolated surgical aortic valve replacement after prior coronary artery bypass surgery: results from the RECORD multicenter registry. J Card Surg. 2014;29(4):450-454. doi: 10.1111/jocs.12365 [DOI] [PubMed] [Google Scholar]
- 2.Vohra HA, Pousios D, Whistance RN, et al. Aortic valve replacement in patients with previous coronary artery bypass grafting: 10-year experience. Eur J Cardiothorac Surg. 2012;41(3):e1-e6. doi: 10.1093/ejcts/ezr212 [DOI] [PubMed] [Google Scholar]
- 3.Khaladj N, Shrestha M, Peterss S, et al. Isolated surgical aortic valve replacement after previous coronary artery bypass grafting with patent grafts: is this old-fashioned technique obsolete? Eur J Cardiothorac Surg. 2009;35(2):260-264. doi: 10.1016/j.ejcts.2008.09.051 [DOI] [PubMed] [Google Scholar]
- 4.Odell JA, Mullany CJ, Schaff HV, Orszulak TA, Daly RC, Morris JJ. Aortic valve replacement after previous coronary artery bypass grafting. Ann Thorac Surg. 1996;62(5):1424-1430. doi: 10.1016/0003-4975(96)00635-2 [DOI] [PubMed] [Google Scholar]
- 5.Byrne JG, Karavas AN, Filsoufi F, et al. Aortic valve surgery after previous coronary artery bypass grafting with functioning internal mammary artery grafts. Ann Thorac Surg. 2002;73(3):779-784. doi: 10.1016/S0003-4975(01)03456-7 [DOI] [PubMed] [Google Scholar]
- 6.Reardon MJ, Van Mieghem NM, Popma JJ, et al. ; SURTAVI Investigators . Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321-1331. doi: 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 7.Kappetein AP, Head SJ, Généreux P, et al. ; Valve Academic Research Consortium-2 . Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145(1):6-23. doi: 10.1016/j.jtcvs.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Arnold SV, Reynolds MR, Wang K, et al. ; CoreValve US Pivotal Trial Investigators . Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US pivotal trial. JACC Cardiovasc Interv. 2015;8(9):1207-1217. doi: 10.1016/j.jcin.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 10.Gupta T, Khera S, Kolte D, et al. Transcatheter versus surgical aortic valve replacement in patients with prior coronary artery bypass grafting: trends in utilization and propensity-matched analysis of in-hospital outcomes. Circ Cardiovasc Interv. 2018;11(4):e006179. doi: 10.1161/CIRCINTERVENTIONS.117.006179 [DOI] [PubMed] [Google Scholar]
- 11.Reinöhl J, Kaier K, Reinecke H, et al. Transcatheter aortic valve replacement: the new standard in patients with previous coronary bypass grafting? JACC Cardiovasc Interv. 2016;9(20):2137-2143. doi: 10.1016/j.jcin.2016.07.035 [DOI] [PubMed] [Google Scholar]
- 12.Greason KL, Mathew V, Suri RM, et al. Transcatheter versus surgical aortic valve replacement in patients with prior coronary artery bypass graft operation: a PARTNER trial subgroup analysis. Ann Thorac Surg. 2014;98(1):1-7. doi: 10.1016/j.athoracsur.2014.02.079 [DOI] [PubMed] [Google Scholar]
- 13.Conte JV, Gleason TG, Resar JR, et al. Transcatheter or surgical aortic valve replacement in patients with prior coronary artery bypass grafting. Ann Thorac Surg. 2016;101(1):72-79. doi: 10.1016/j.athoracsur.2015.06.067 [DOI] [PubMed] [Google Scholar]
- 14.Ando T, Briasoulis A, Holmes AA, Afonso L, Schreiber T, Kondur A. Transcatheter aortic valve replacement versus surgical aortic valve replacement in patients with previous coronary artery bypass surgery: a systematic review and meta-analysis. Int J Cardiol. 2016;215:14-19. doi: 10.1016/j.ijcard.2016.04.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eTable 1. Outcomes with P values of interaction of prior CABG (yes/no) and treatment (TAVR/SAVR).
eTable 2. Resource utilization.
eFigure 1. Randomization and analysis populations.
eFigure 2. Kaplan-Meier all-cause mortality or disabling stroke estimates.
eFigure 3. New York Heart Association symptom status.
eFigure 4. Aortic valve hemodynamics.