Abstract
Allopregnanolone and pregnanolone (together termed allo + pregnan) are neurosteroid metabolites of proges-terone that equipotently facilitate the action of gamma-amino-butyric acid (GABA) at GABAA receptors. The adrenal steroid dehydroepiandrosterone (DHEA) allosterically antagonizes GABAA receptors and facilitates N-methyl-D-aspartate (NMDA) receptor function. In prior research, premenopausal women with posttraumatic stress disorder (PTSD) displayed low cerebrospinal fluid (CSF) levels of allo + pregnan [undifferentiated by the gas chromatography-mass spectrometry (GC-MS) method used] that correlated strongly and negatively with PTSD reexperiencing and negative mood symptoms. A PTSD-related decrease in the ratio of allo + pregnan to 5α-dihydroprogesterone (5α-DHP: immediate precursor for Allopregnanolone) suggested a block in synthesis of these neurosteroids at 3α-hydroxysteroid dehydrogenase (3α-HSD). In the current study, CSF was collected from unmedicated, tobacco-free men with PTSD (n = 13) and trauma-exposed healthy controls (n = 17) after an overnight fast. Individual CSF steroids were quantified separately by GC-MS. In the men with PTSD, allo + pregnan correlated negatively with Clinician-Administered PTSD Scale (CAPS-IV) total (ρ = −0.74, p = 0.006)and CAPS-IV derived Simms dysphoria cluster (ρ = −0.71, p = 0.01) scores. The allo + pregnan to DHEA ratio also was negatively correlated with total CAPS (ρ = −0.74, p = 0.006) and dysphoria cluster (ρ = −0.79, p = 0.002) scores. A PTSD-related decrease in the 5α-DHP to progesterone ratio indicated a block in Allopregnanolone synthesis at 5α-reductase. This study suggests that CSF allo + pregnan levels correlate negatively with PTSD and negative mood symptoms in both men and women, but that the enzyme blocks in synthesis of these neurosteroids may be sex-specific. Consideration of sex, PTSD severity, and function of 5α-reductase and 3α-HSD thus may enable better targeting of neurosteroid-based PTSD treatments.
Keywords: PTSD, Depression, Allopregnanolone, Dehydroepiandrosterone, Cerebrospinal fluid, GABA, Neurosteroids, Gas chromatography-mass spectrometry
1. Introduction
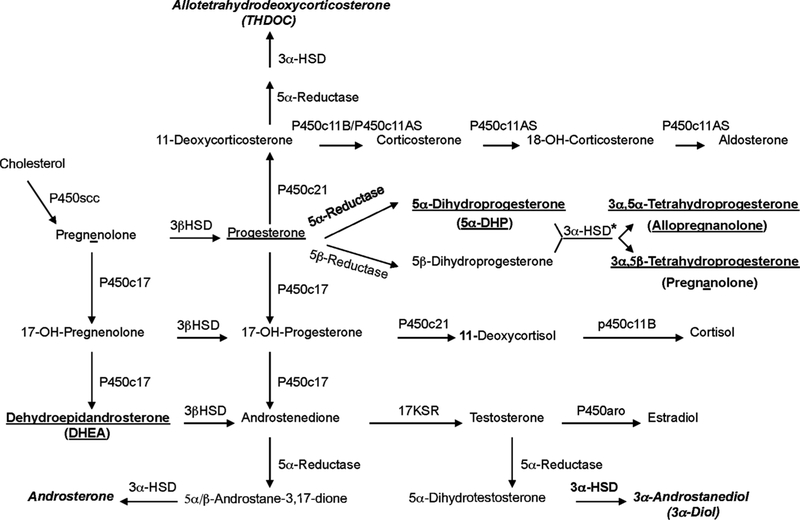
Many interacting neurobiological factors are involved in the pathophysiology of posttraumatic stress disorder (PTSD) (Pitman et al., 2012; Rasmusson et al., 2018, 2017b), including Allopregnanolone and pregnanolone, neurosteroid metabolites of progesterone that equipotently facilitate GABAergic neurotransmission at nanomolar concentrations (Fig. 1) (Pineles et al., 2018; Rasmusson et al., 2006b). In premenopausal women with PTSD, cerebrospinal fluid (CSF) levels of these stereoisomers [undifferentiated by the gas chromatography-mass spectrometry (GC-MS) methodology employed; herein termed allo + pregnan when levels of each are summed] were 40% of levels in healthy non-traumatized women, and correlated strongly and negatively with PTSD re-experiencing and negative mood symptoms (Rasmusson et al., 2006b). The ratio of Allopregnanolone to 5α-dihydroprogesterone (5α- DHP: the immediate steroid precursor for Allopregnanolone) also was low in the women with PTSD, whereas the ratio of 5α-DHP to proges-terone was not low, suggesting a block in the synthesis of these GA-BAergic neurosteroids at 3α-hydroxysteroid dehydrogenase (3αHSD), not 5α-reductase (Fig. 1).
Fig. 1.
Neuroactive Steroid Synthetic Pathways. Bold underlined: Steroids produced in the central nervous system (CNS) and periphery and measured for thecurrent study in cerebrospinal fluid (CSF) by gas chromatography/mass spectrometry (GC-MS). Allopregnanolone and pregnanolone (together designated allo + pregnan) are equipotent stereoisomers that markedly facilitate the action of gamma-amino-butyric acid (GABA) at GABAA receptors. Dehydroepiandrosterone (DHEA) is produced by the adrenal gland and crosses into the CNS, where it allosterically antagonizes GABAA receptor function and facilitates N-methyl-D-aspartate (NMDA) receptor function. Bold italicized: Allotetrahydrodeoxycorticosterone (THDOC), 3α-androstanediol (3α — diol), and androsterone were not measured in the current study, but like Allopregnanolone, are GABAergic neuroactive steroids produced by the serial action of 5α-reductase and 3α-hydroxysteroid dehydrogenase (3α-HSD). Other acronyms from top to bottom, left to right: P450c11AS = P450c11 aldosterone synthase; P450scc = P450 side-chain cleavage enzyme; 3α-HSD = 3α-hydroxysteroid dehydrogenase; 17KSR = 17-ketosteroid reductase; P450aro = P450 aromatase. Note that all pathways are not active in all tissues. *In addition, 3α-HSD is noted to operate solely in the reductive direction. The earlier work on this topic by Penning et al. (2000) was performed in vitro and suggested that 3α-HSD functions in a bidirectional manner based on the oxidative/reductive status of the milieu in which the enzyme is functioning. Follow-up work by Penning et al. (2004) indicated that human Type 3 3α-HSD (AKR1C2: primarily responsible for production of Allopregnanolone and pregnanolone in humans) functioned in vitro and in COS-1 cell lysates in a bi-directional manner. However, in intact COS-1 and prostate cells AKR1C2 functioned solely as a ketosteroid reductase. According to these investigators, the failure to observe AKR1C2-mediated oxidation “…in a cellular context was due to the potent inhibition of this reaction by low concentrations of NADPH that may prevail in the whole cell.”.
Recent work by Izumi et al. (2013) suggests that Allopregnanolone and pregnanolone synthesis in brain neurons (e.g., Agís-Balboa et al., 2006) can be initiated de novo by N-methyl-D-aspartate (NMDA) receptor activation. De novo synthesis of Allopregnanolone and pregnanolone in adrenocortical cells is initiated by diurnal or stress-induced increases in adrenocorticotropic hormone (ACTH) (Purdy et al., 1991), which acts at a G-protein coupled receptor to stimulate intracellular second messenger pathways that lead to neurosteroidogenesis; allo- pregnanolone and pregnanolone are also produced by the ovary and testis in response to luteinizing hormone (LH) (Genazzani et al., 2002). Allopregnanolone and pregnanolone produced peripherally then cross the blood-brain barrier.
Brain Allopregnanolone and pregnanolone levels peak about an hour after initiation of stress (Purdy et al., 1991). In the central nervous system (CNS) Allopregnanolone and pregnanolone have been shown to exert anticonvulsant (Kaminski et al., 2004) and antinociceptive (Charlet et al., 2008) effects, as well as modulate neurophysiological and behavioral reactions to acute stress by increasing GABA-activated GABAA receptor-mediated chloride ion influx (up to 7–10 times) (Majewska et al., 1986; Puia et al., 1990). Allopregnanolone and/or pregnanolone are also thought to promote phase 2 sleep, reduce inflammation, reduce apoptosis, promote neurogenesis and myelination, protect against the negative effects of neurotrauma, and promote processes relevant to memory such as long-term depression (LTD) and long-term potentiation (LTP) interference (reviewed by Rasmusson and Pineles, 2018, and Rasmusson et al., 2017b).
In contrast to these GABAergic neurosteroids, dehydroepian-drosterone (DHEA) allosterically antagonizes GABAA receptors and facilitates N-methyl-D-aspartate (NMDA) receptor function with micromolar potency. In humans, DHEA is synthesized by the adrenal gland in response to ACTH, peaks in plasma about 10–20 minutes after synthesis initiation and crosses into brain where it may be sulfated to DHEAS, which is 4 times more potent than DHEA in modulating GABAA and NMDA receptor function (Rasmusson et al., 2004). In premenopausal women with PTSD, the ratio of allo + pregnan to DHEA in CSF appeared to be more strongly negatively associated with PTSD reexperiencing and mood symptoms than the sum of allo + pregnan alone, suggesting that a balance between inhibitory and excitatory neuro-transmission in the CNS impacts PTSD symptom expression (Rasmusson et al., 2006b).
CSF Allopregnanolone and pregnanolone levels have not yet been examined in men with PTSD. However, research in male rodents suggests that deficits in Allopregnanolone synthesis may play a role in PTSD pathogenesis in men. A reduction in brain Allopregnanolone levels induced by prolonged social isolation in male (Pibiri et al., 2008) but not female (Pinna et al., 2008) mice was associated with anxiety-like behavior in the plus maze, aggression toward conspecific same-sex intruders, increased expression of conditioned contextual (but not cued) fear, decreased rates of contextual fear extinction, and deficits in extinction retention (Pibiri et al., 2008; Pinna and Rasmusson, 2014). A reduction in brain and serum Allopregnanolone levels induced in male rats by exposure to a series of intense stressors over a few hours also was associated with increased conditioned contextual fear (Zhang et al., 2014).
Given the relationship between CSF allo + pregnan deficits and PTSD symptoms in women, as well as preclinical research linking low brain Allopregnanolone levels to behavioral analogues of PTSD in male rodents, we investigated levels of CSF Allopregnanolone and pregnanolone, as well as the steroid precursors for these neurosteroids in trauma-exposed men with and without PTSD. We hypothesized that the sum of these equipotent GABAergic steroids in CSF (i.e., allo + pregnan) would be low in men with PTSD and correlate negatively with Simms et al. (2002) confirmatory factor analysis-defined PTSD re- experiencing and dysphoria clusters derived from Clinician-Administered PTSD Scale (CAPS-IV) scores (Blake et al., 1995). Given previous findings in women (Rasmusson et al., 2006b), we also explored whether (allo + pregnan)/DHEA and (allo + pregnan)/DHEAS ratios in CSF would correlate negatively with PTSD symptoms.
Plasma Allopregnanolone and pregnanolone levels would be expected to rise in response to diurnal morning spikes in ACTH release, as well as in response to the stress of a lumbar puncture (LP). As morning ACTH spikes or responses to stress were expected to vary among study participants and could correlate with PTSD symptom patterns (Rasmusson et al., 2018), we also examined the relationships between CSF allo + pregnan levels and PTSD symptoms while controlling statistically for plasma ACTH levels. We were concerned that low CNS production of allo + pregnan among the PTSD participants might potentiate hypothalamic-pituitary-adrenal (HPA) axis responses to the stress of an LP session (Barbaccia et al., 2001), resulting in increased adrenal production of Allopregnanolone and pregnanolone, which would cross the blood-brain barrier and obscure possible central deficits in the production of these GABAergic neurosteroids as an artifact of the experimental design.
Finally, based on the reported block in Allopregnanolone synthesis at 5α-reductase in socially isolated male but not female mice (Agís-Balboa et al., 2007) and the observed link between a polymorphism in the 5α-reductase II gene and PTSD risk in men, but not women (Gillespie et al., 2013), we hypothesized that men with PTSD would demonstrate a block in Allopregnanolone synthesis at 5α-reductase, manifest as a low ratio of CSF 5α-DHP to progesterone (Fig. 1).
Most studies of biomarkers in PTSD have focused on mean diagnostic group differences. However, the field of psychiatry is moving toward development of precision medicine treatments for PTSD based on individual variability in the pathophysiological mechanisms that contribute to PTSD clinical phenotypes (e.g., Dalvie et al., 2016). Therefore, in addition to examining average differences in neuroactive steroid levels and ratios between trauma-exposed individuals with and without PTSD, we defined the proportion of PTSD participants with apparent deficiencies in allo + pregnan synthesis in relation to trauma control group indices.
2. Materials and methods
The study took place at the VA National Center for PTSD Women’s Health Science Division, which is located at VA Boston Healthcare System and affiliated with Boston University School of Medicine. The Institutional Review Boards of both institutions approved the study procedures.
2.1. Recruitment
Potential study participants responded to community advertisements on bulletin boards and the internet by contacting the study investigators. After telephone screening, potentially eligible individuals were scheduled for a paid, in-person screening evaluation.
2.2. In-person screening
After completing the informed consent process, participants were assessed for exposure to DSM-IV PTSD Criterion A1/A2 trauma (APA, 1994) using the Traumatic Life Events Questionnaire (TLEQ) (Kubany et al., 2000) and Childhood Trauma Questionnaire (CTQ) (Fink et al., 1995). The Clinician-Administered PTSD Scale (CAPS-IV one-month version) was used to diagnose PTSD (Blake et al., 1995) and the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995) was used to diagnose other Axis I disorders; diagnoses were confirmed at consensus diagnosis meetings by three doctoral-level clinicians trained to criterion on the CAPS and SCID using published manuals, videotapes, and demonstrations. Participants were screened for current or significant past medical illness that could impact psychiatric symptoms or neuroactive steroid levels via medical history, review of systems and physical examination. An electrocardiogram, urinalysis, clinical blood tests including a gamma-glutamyl transferase test sensitive to recent alcohol use, and a urine toxicology test for benzodiazepines, opiates, barbiturates, cocaine, amphetamines, marijuana, and cotinine (a metabolite of nicotine with a 10–27 hour half-life (Jarvis et al., 1988) also were completed.
2.3. Eligibility criteria
PTSD group participants were required to meet DSM-IV symptom criteria for chronic PTSD as determined by the CAPS. Participants with past but not current PTSD, current partial PTSD (Stein et al., 1997), bipolar I disorder, psychotic disorders (except psychosis NOS due to trauma-related sensory hallucinations), and current alcohol, nicotine, or illicit drug dependence were excluded. Participants with homicidal or suicidal ideation requiring intervention were excluded and referred for clinical evaluation. Healthy trauma-exposed participants (TCs) were required to meet the DSM-IV A1 criterion for exposure to a potentially traumatic stressor, but not the criteria for current, partial or lifetime PTSD. Potential TCs with a current Axis I diagnosis or more than one past episode of major depression were excluded.
Participants were required to have normal screening labs, as well as negative urine toxicology and cotinine screens at screening and the LP. Participants were asked to be free from alcohol, illicit drugs, nicotine, and all medication before the LP for ≥ 4 weeks, or ≥ 6 weeks for selective serotonin reuptake inhibitors (SSRIs) with long half-lives. Over-the-counter medications such as acetaminophen, ibuprofen, or loratidine were acceptable if the last dose was > 7 days before the LP.
2.4. Lumbar puncture
Participants fasted except for water intake after midnight until after the LP; they were scheduled to arrive for the LP session at 7:30 am. Urine drug and cotinine screening, an ophthalmologic examination to rule out increased intracranial pressure, and vital signs were completed. Participants then sat quietly upright for ~60mín. (TC: 66.6 ± 25.8 min.; PTSD: 72.4 + 21.6 min.; p = 0.77) before blood was drawn from an antecubital vein, processed and stored at −80 °C. Approximately 30 min. after the blood draw (TC: 34.2 ± 21.9 min.; PTSD: 31.8 ± 12.0 min.; p = 0.67), the LP was performed by an experienced anesthesiologist with the participant in a sitting position. A Sprotte needle was used to minimize risk of post-LP headaches (Strupp et al., 2001). Twenty 1cc CSF aliquots were collected over about 20 min. and kept on dry ice until storage at −80° C immediately after the LP. Participants then lay prone for 30 min. while the CAPS-IV (one-week version) was administered.
2.5. CSF neuroactive steroid and plasma ACTH analyses
CSF neuroactive steroids were measured by GC-MS. Samples were extracted in ethyl acetate and lyophilized. The steroids of interest were purified and separated using high-pressure liquid chromatography (HPLC). Tritiated steroids (American Radiolabeled Chemicals, St. Louis, MO) and deuterated internal standards (CDN Isotopes, Pointe-Claire, QC, and Steraloids, Newport, RI) were added to monitor retention time on the HPLC column and allow quantification of each steroid. Each steroid then was derivatized for GCMS, which was conducted in the standard electron impact mode (EI). To calculate the quantity of the steroid in each fraction, the area under the steroid peak was divided by the area under the deuterated internal standard peak. The detection limit for Allopregnanolone, pregnanolone and the other steroids quantified was ~5 fmol/mL or 1.6 pg/ml. Samples for each analyte were run in one batch. The intra- and inter-assay coefficients of variation (CVs) for each analyte are < 5% and < 10%, respectively (Cheney et al.,1995). As Allopregnanolone and pregnanolone have equipotent effects at GABAA receptors, we added their levels together (termed allo + pregnan) to assess their relationship to the PTSD phenotype, as in previous studies (Pineles et al., 2018; Rasmusson et al., 2006b). Plasma ACTH was measured using a two-site enzyme-linked immunosorbent assay (ELISA) (MD Bioproducts, St. Paul, MN). Low (43.7 pg/mL) and high (191 pg/mL) control samples included in each assay had intra-assay CVs of 8.2% and 7.2% (n = 6), respectively, and inter-assay CVs of 6.7% and 12.9% (n = 5), respectively.
2.6. Analyses
Per protocol, data from two participants diagnosed with PTSD and eligible for the LP at screening, but who no longer met PTSD criteria several weeks later at the LP, were excluded. CSF could not be collected from one trauma control. Thus, CSF neuroactive steroid data from 17 TCs and 13 participants with PTSD were analyzed by GC/MS. The number of participants included in analyses varied across biomarker indices (see Table 2) due to occasional technical problems measuring the analytes of interest; subjects with missing data were excluded on an analysis-wise basis both to make best use of the available data and avoid assumptions about the underlying variable distributions, as generally required for imputation approaches. Demographic characteristics and screening psychometric assessment scores were tabulated for the entire sample and compared between the PTSD and TC groups. In addition, because we were not able to directly measure enzyme activity in the Allopregnanolone and pregnanolone synthesis pathways, we computed the ratio between the neurosteroid product of each enzyme and its neurosteroid precursor (Fig. 1) to reflect enzyme activity indirectly—with and without controlling for plasma ACTH by fitting a linear model of the neurosteroid level or ratio with diagnostic group and ACTH as predictors. To provide a fuller picture of the degree to which distributions of neurosteroid to precursor ratios in the PTSD subjects were shifted relative to the TCs, we calculated five-number summary percentiles for the TCs (minimum, maximum, and middle quartiles—see Table 3), and then calculated the proportion of PTSD subjects with a ratio falling beneath each TC percentile. A relative decrease in the neurosteroid to precursor ratio among PTSD subjects would give rise to a left-shifted distribution (e.g., greater than 25% of the PTSD subjects’ ratios would fall under the 25th percentile of TC values). A relative increase in the neurosteroid to precursor ratio among the PTSD subjects would give rise to a right-shifted distribution (e.g., less than 25% of PTSD subjects’ values would fall under the 25th percentile of TC values).
Table 2.
Results of t-tests comparing PTSD and non-PTSD group means for plasma adrenocorticotropin (ACTH) and cerebrospinal fluid neuroactive steroids and ratios.
| Cerebrospinal Fluid Neuroactive Steroid Indices (pg/ml) | TC Mean ± SD (n) |
PTSD Mean ± SD (n) |
ta | dfa | Pa | Cohen’s d [95% CI]a |
|---|---|---|---|---|---|---|
| Progesterone | 13.0 ± 12.2 | 17.1 ± 11.6 | 0.87 | 23.7 | .39 | 0.34 [−0.47; 1.16] |
| (PROG) | (14) | (12) | 0.61 | 20.0 | .55 | 0.26 [−0.62; 1.13] |
| 5α-Dihydroprogesterone: | 573.7 ± 669.7 | 309.4 ± 293.7 | −1.37 | 20.1 | .19 | −0.53 [−1.34; 0.28] |
| (5α-DHP) | (15) | (12) | −1.68 | 21.0 | .11 | −0.69 [−1.58; 0.19] |
| Allopregnanolone: | 18.2 ± 10.6 | 15.1 ± 9.7 | −0.78 | 24.5 | .44 | −0.30 [−1.10; 0.50] |
| (Allo) | (15) | (12) | −0.98 | 21.0 | .34 | −0.41 [−1.27; 0.46] |
| Pregnanolone: | 5.1 ± 4.1 | 17.0 ± 23.6 | 1.72 | 11.5 | .11 | 0.67 [−0.15; 1.49] |
| (Pregnan) | (15) | (12) | 1.71 | 21.0 | .10 | 0.71 [−0.18; 1.59] |
| Allo + Pregnan | 23.3 ± 10.6 | 32.1 ± 23.7 | 1.20 | 14.5 | .25 | 0.46 [−0.34; 1.27] |
| (15) | (12) | 1.00 | 21.0 | .33 | 0.41 [−0.46; 1.28] | |
| Dehydroepiandrosterone: | 839.4 ± 889.0 | 618.9 ± 479.3 | −0.87 | 25.6 | .39 | −0.32 [−1.08; 0.44] |
| (DHEA) | (17) | (13) | −1.28 | 24.0 | .21 | −0.50 [−1.32; 0.32] |
| DHEA-Sulfate: | 2121 ± 1572 | 2059 ± 2087 | −0.09 | 19.7 | .93 | −0.03 [−0.82; 0.75] |
| (DHEAS) | (16) | (12) | −0.16 | 22.0 | .87 | −0.07 [−0.91; 0.78] |
| Plasma | ||||||
| Adrenocorticotropic Hormone (ACTH) | 26.6 ± 17.8 | 21.5 ± 10.7 | −0.92 | 24.7 | .36 | −0.36 [−1.17; 0.45] |
| (16) | (11) | |||||
| Ratios Indicating Possible Enzyme Blocks in the Allo and Pregnan Synthesis Pathways | ||||||
| 5α-DHP/PROG | 64.1 ± 60.4 | 25.8 ± 24.2 | −2.09 | 16.3 | .05t | −0.86 [−1.75; 0.03] |
| (13) | (11) | −2.14 | 18.0 | <.05* | −0.94 [−1.91; 0.03] | |
| Allo/5α-DHP | 0.11 ± 0.21 | 0.12 ± 0.17 | 0.18 | 23.9 | 0.86 | 0.07 [−0.74; 0.18] |
| (14) | (12) | 0.48 | 20.0 | 0.64 | 0.20 [−0.68; 1.08] | |
| Ratios Integrating Possible Synthesis Blocks Across Neurosteroidogenesis | ||||||
| (Allo + Pregnan)/5α-DHP | 0.13 ± 0.25 | 0.36 ± 0.58 | 1.30 | 14.4 | .21 | 0.51 [−0.31; 1.34] |
| (14) | (12) | 1.55 | 20.0 | .14 | 0.65 [−0.25; 1.55] | |
| PROG/ACTH | 0.75 ± 0.83 | 0.93 ± 0.49 | 0.65 | 19.9 | .52 | 0.27 [−0.61; 1.15] |
| (13) | (10) | |||||
| (Allo + Pregnan)/ACTH | 1.27 ± 1.11 | 1.76 ± 1.84 | 0.75 | 13.6 | .47 | 0.31 [−0.55; 1.17] |
| (14) | (10) | |||||
| Ratios Reflecting | ||||||
| Inhibitory to Excitatory Neurotransmission Balance | ||||||
| (Allo + Pregnan)/DHEA | 0.047 ± 0.03 | 0.116 ± 0.15 | 1.56 | 11.9 | .14 | 0.61 [−0.21; 1.42] |
| (15) | (12) | 1.85 | 21.0 | .08 | 0.76 [−0.12; 1.65] | |
| (Allop + Pregnan)/DHEAS | 0.016 ± 0.01 | 0.035 ± 0.05 | 1.28 | 12.5 | .22 | 0.50 [−0.31; 1.31] |
| (15) | (12) | 1.38 | 21.0 | .18 | 0.57 [−0.31; 1.44] |
Note:
The first line of results for each biomarker is for the unadjusted t-test (using Welch’s correction for unequal variances); the second line reports the adjusted statistics controlling for plasma ACTH levels. t = 0.05 ≤ p < 0.10;
= p < 0.05.
Table 3.
(A-D). Proportion of PTSD group values below TC group value at each quartile.
| Percentile | PTSD Group Proportion > 100th Percentile for TC Group | |||||
|---|---|---|---|---|---|---|
| 0th | 25th | 50th | 75th | 100th | ||
| A. PROG/ACTH | ||||||
| Value of TC Group Ratio at Percentile | 0.12 | 0.25 | 0.30 | 0.74 | 2.89 | |
| PTSD Group Proportion Below | 31% | 31% | 31% | 38% | 100% | 0% |
| TC Value | ← | ← | → | → | ||
| B. 5α-DHP/PROG | ||||||
| Value of TC Group Ratio at Percentile | 0.56 | 21.2 | 36.6 | 91.4 | 197.1 | |
| PTSD Group Proportion Below | 15% | 54% | 77% | 100% | 100% | 0% |
| TC Value | ← | ← | ← | ← | ||
| C. (Allo + Pregnan)/5α-DHP | ||||||
| Value of TC Group Ratio at Percentile | 0.01 | 0.03 | 0.06 | 0.08 | 0.97 | |
| PTSD Group Proportion Below | 8% | 15% | 38% | 46% | 85% | 15% |
| TC Value | ← | → | → | → | → | → |
| D. (Allo + Pregnan)/ACTH | ||||||
| Value of TC Group Ratio at Percentile | 0.21 | 0.53 | 0.95 | 1.63 | 4.44 | |
| PTSD Group Proportion Below | 23% | 31% | 62% | 77% | 92% | 8% |
| TC Value | ← | ← | ← | ← | → | → |
Note: PTSD = posttraumatic stress disorder; TC = trauma-exposed but healthy; PROG = progesterone; ACTH = adrenocorticotropic hormone; 5α-DHP = 5α- dihydroprogesterone; Allo + Pregnan = sum of Allopregnanolone and pregna-nolone. Bold left-pointing arrows: PTSD group values shifted to the left relative to TC group values. Bold right-pointing arrows: PTSD group values shifted to the right relative to TC group values.
Finally, for participants with PTSD and the TCs, we calculated Spearman’s rank-order correlations between the one-week total CAPS scores assessed at the LP and: a) CSF allo + pregnan and b) the ratio of CSF allo + pregnan to DHEA(S). We also report correlations between these biomarker indices and the four Simms et al. (2002) PTSD symptom clusters defined by confirmatory factor analysis (instead of the DSM-IV PTSD symptom clusters defined by committee consensus; see the legend for Fig. 2 for the list of DSM-IV PTSD symptoms comprising each of the four Simms PTSD symptom clusters). In addition, we examined the relationships between these biomarker indices and PTSD symptom severity as partial correlations while controlling for plasma ACTH, as discussed in the introduction. An α < .05 was used to judge statistical significance for the primary hypotheses based on previous findings. For exploratory hypotheses, our primary interest was to report the preliminary effect sizes, but we also report p-values for completeness.
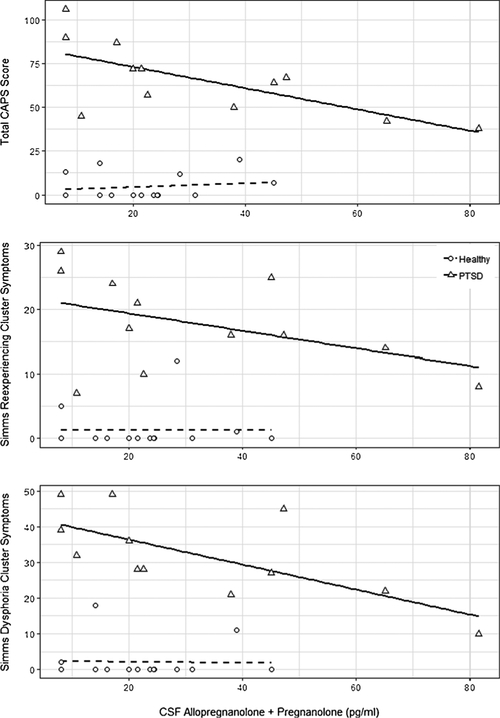
Fig. 2.
Scatter-plots of cerebrospinal fluid (CSF) Allopregnanolone + pregnanolone levels (pg/ml) and PTSD symptoms measured using the Clinician-Administered PTSD Scale (CAPS) one-week version immediately after the lumbar puncture. For the participants with PTSD, correlations were large for CSF Allopregnanolone + pregnanolone levels (pg/ml) and (top) total CAPS scores (Spearman rho = 0.74, p = 0.006), (middle) Simms re-experiencing cluster symptoms (rho = −0.51, p = 0.09), and (bottom) Simms dysphoria cluster symptoms (rho= −0.71, p = 0.01). Correlations for the trauma-ex-posed healthy participants (i.e., trauma controls; TCs) were small and non-significant. Simms et al. (2002) confirmatory factor analysis-defined PTSD symptom clusters comprise the following Diagnostic and Statistical Manual (DSM)-IV PTSD symptoms rated using the CAPS.
Simms reexperiencing cluster: B1-intrusive thoughts, B2-nightmares, B3- flashbacks, B4- emotional distress at trauma cue exposure and B5-physiological reactivity to trauma cues.
Simms active avoidance cluster: C1-avoidance of trauma-related thoughts, feelings, conversations; C2-avoidance of people, places, things.
Simms dysphoria cluster: C3-amnesia for details of the trauma; C4-decreased interest in everyday activities; C5-detachment or estrangement from others; C6- restricted range of affect; C7-sense of foreshortened future; D1-disturbed sleep; D2-irritability; D3-poor concentration.
Simms arousal cluster: D4-hypervigilance; D5-increased startle.
3. Results
3.1. Participant characteristics
As indicated in Table 1, the TC and PTSD groups did not differ significantly in age, weight, body mass index (BMI), ethnicity or education; the PTSD participants were less likely to be employed and differed in marital status. As expected, the PTSD group was exposed to a greater number of trauma types, and had higher TLEQ scores, CAPS scores at screening and the LP, negative mood symptoms at screening, and rates of past alcohol abuse. About half of the PTSD group was diagnosed with current major depression.
Table 1.
Demographic Characteristics for Total Sample and PTSD vs. Trauma Control Groups.
| Total sample (n = 30) |
PTSD (n = 13) |
Trauma Control (n = 17) |
Welch’s t-test / Chi square t | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 37.2 | 10.6 | 40.1 | 9.3 | 35.0 | 11.2 | −1.35 |
| Weight (lbs.) | 188.1 | 37.8 | 198.7 | 33.9 | 180.6 | 39.5 | −1.32 |
| BMI (kg/m2) | 27.0 | 4.7 | 28.5 | 4.0 | 26.0 | 5.0 | −1.49 |
| TLEQ (total trauma exposure) | 13.9 | 14.8 | 21.9 | 15.7 | 7.8 | 11.0 | −2.77* |
| CTQ | 48.2 | 23.9 | 56.2 | 22.8 | 42.1 | 23.6 | −1.67 |
| CAPS | 29.9 | 33.3 | 63.4 | 21.9 | 4.4 | 7.0 | −9.35* |
| BDI | 8.7 | 13.1 | 18.5 | 15.1 | 1.1 | 1.7 | −4.15* |
| n | % | n | % | n | % | χ2 | |
| Veteran Status | 1.63 | ||||||
| Veteran | 8 | 26.67 | 5 | 38.46 | 3 | 17.65 | |
| Not a Veteran | 22 | 73.33 | 8 | 61.54 | 14 | 82.35 | |
| Race/Ethnicity | 0.15 | ||||||
| Caucasian | 13 | 43.33 | 6 | 46.15 | 7 | 41.18 | |
| African American | 15 | 50.00 | 6 | 46.15 | 9 | 52.94 | |
| Hispanic/Latino | 2 | 6.66 | 1 | 7.69 | 1 | 5.88 | |
| Years of Education | 1.83 | ||||||
| High school/GED | 12 | 40.00 | 7 | 53.84 | 5 | 29.41 | |
| Completed part of college | 9 | 30.00 | 3 | 23.08 | 6 | 35.29 | |
| 2 or 4year degree | 6 | 20.00 | 2 | 15.38 | 4 | 23.53 | |
| Graduate school | 3 | 10.00 | 1 | 7.69 | 2 | 11.76 | |
| Employment | 10.92* | ||||||
| Working full-time | 9 | 30.00 | 4 | 30.78 | 5 | 29.41 | |
| Working part-time | 5 | 16.67 | 0 | 0 | 5 | 29.41 | |
| In school part- or full-time | 3 | 10.00 | 0 | 0 | 3 | 17.65 | |
| Unemployed | 12 | 40.00 | 9 | 69.23 | 3 | 17.65 | |
| Marital Status | 8.89* | ||||||
| Married or cohabitating | 2 | 6.67 | 2 | 15.38 | 0 | 0 | |
| Widowed | 1 | 3.33 | 0 | 0 | 1 | 5.88 | |
| Divorced/Separated | 8 | 26.67 | 6 | 46.15 | 2 | 11.76 | |
| Never married | 19 | 63.33 | 5 | 38.46 | 14 | 82.35 | |
| Current Axis I diagnosis | |||||||
| Anxiety disorders | 3 | 10.00 | 3 | 23.08 | 0 | 0 | 4.36 |
| Depression | 7 | 23.33 | 7 | 53.85 | 0 | 0 | 11.94* |
| Alcohol Abuse | 1 | 3.33 | 1 | 7.69 | 0 | 0 | 1.35 |
| Lifetime Axis I diagnoses | |||||||
| Anxiety disorders | 1 | 3.33 | 1 | 7.69 | 0 | 0 | 1.35 |
| Depression | 1 | 3.33 | 1 | 7.69 | 0 | 0 | 1.35 |
| Alcohol Abuse | 9 | 30.00 | 7 | 53.84 | 2 | 11.76 | 6.21* |
| Substance Abuse | 6 | 20.00 | 4 | 30.78 | 2 | 11.76 | 1.66 |
Note:
p < .05 BMI = body mass index; TLEQ = Traumatic Life Events Questionnaire; CTQ = Childhood Trauma Questionnaire; BDI = Beck Depression Inventory; CAPS = Clinician Administered PTSD Scale. Anxiety disorders = panic disorder, specific phobia, obsessive compulsive disorder, social phobia. Although the entire SCID-I was administered, no other diagnoses are reported because no one in the sample met criteria for those diagnoses.
3.2. CSF neuroactive steroid levels and ratios
There was a trend for the unadjusted CSF 5α-DHP to progesterone ratio to be lower in the PTSD group compared to the TC group (t=−2.09, p = 0.05); the difference was statistically significant after con- trolling for plasma ACTH levels (t=−2.14, p = 0.047; Table 2). There were no other statistical group differences in steroid levels or ratios, including possible indices of 3α-HSD deficiency such as the Allopregnanolone to 5α-DHP or (allo + pregnan)/5α-DHP ratios.
Table 3 shows the proportion of PTSD participants with neurosteroid to precursor ratios falling below each TC percentile. About a third of the PTSD group had progesterone to ACTH ratios that were lower than the lowest ratio in the TC group. About 15% of the PTSD group had a lower 5α-DHP to progesterone ratio than the lowest ratio in the TC group. The efficiency of 3α-HSD (indicated by the allo + pregnan/5α-DHP ratio) appeared to be right-shifted (i.e., greater) in the PTSD group. The CSF allo + pregnan to plasma ACTH ratio, perhaps indicating net allo + pregnan synthesis efficiency, was lower than the lowest TC ratio in 23% of the PTSD participants.
3.3. Correlations between CSF neuroactive steroid indices and PTSD symptoms
As can be seen in Fig. 2, CSF allo + pregnan levels in the PTSD group correlated strongly and negatively with one-week total CAPS (ρ = −0.74, p = 0.006) and Simms dysphoria cluster ρ = −0.71, p = 0.01) scores assessed at the LP. There were large and moderate (but not statistically significant) effect sizes, respectively, for correlations between CSF allo + pregnan levels and the Simms reexperiencing (ρ = −0.51, p = 0.09) and arousal (ρ = −0.49, p = 0.11) cluster scores. There was a small non-significant negative correlation between CSF allo + pregnan and the Simms active avoidance cluster (ρ = −0.27, p = 0.39). The results were similar when controlling for plasma ACTH, with large effect sizes for the negative partial correlations between CSF allo + pregnan and one-week total CAPS (ρ = −0.82, p = 0.007) and Simms dysphoria cluster (ρ = −0.88, p < 0.002). There were large, moderate and small effect sizes, respectively, for the negative partial correlations between CSF allo + pregnan levels and the Simms reexperiencing (ρ = −0.55, p = 0.08), arousal (ρ = −0.48, p = 0.14) and active avoidance (ρ = −0.25, p = 0.49) cluster scores. Within the TC group, Spearman corre- lations between CSF allo + pregnan levels and total CAPS or Simms PTSD symptom cluster scores were small and non-significant (all ps < 0.2, ps > 0.5).
The PTSD group also showed strong correlations between the CSF (allo + pregnan)/DHEA ratio and total CAPS (ρ = −0.74, p = 0.006) and Simms dysphoria cluster (ρ = −0.79, p = 0.002) scores. There were similar negative correlations between the CSF (allo + pregnan)/DHEAS ratio and total CAPS (ρ = −0.70, p = 0.01) and Simms dysphoria cluster (ρ = −0.80, p = 0.002) scores. There were medium to small (non-significant) effect sizes for correlations between the CSF allo + pregnan/ DHEA(S) ratios and Simms reexperiencing, arousal, and avoidance cluster scores.
4. Discussion
4.1. Evidence for the impact of allo + pregnan on PTSD severity in men
This study found strong negative associations between CSF allo + pregnan levels and total PTSD symptoms in men currently meeting criteria for PTSD. Correlation magnitudes were similar across Simms et al (2002) confirmatory factor analysis-defined PTSD reexperiencing, dysphoria and arousal cluster symptoms. Controlling for plasma ACTH levels (to account for possible confounding effects of morning ACTH surges and variable stress-stimulated ACTH effects on allo + pregnan synthesis in reaction to the LP) increased these associations somewhat. This LP study in men free from confounding psychotropics thus replicates and extends previous studies in premenopausal women (Rasmusson et al., 2006b; Pineles et al., 2018) suggesting a role for deficient synthesis of these GABAergic neurosteroids in the pathophysiology of PTSD.
Also consistent with the previous findings in women, there was a more robust correlation between PTSD-related depressive symptoms (captured by the Simms dysphoria cluster) and the CSF (allo + pregnan)/DHEA ratio, as well as the (allo + pregnan)/DHEAS ratio than CSF allo + pregnan levels alone. At first this seems paradoxical. Several previous studies have demonstrated a negative relationship between plasma DHEA(S) resting levels or reactivity and PTSD or PTSD-related depressive symptoms, as well as a positive relationship between plasma DHEA(S) levels and stress resilience (reviewed in Pitman et al., 2012; Rasmusson and Pineles, 2018; Rasmusson et al., 2017b). However, as previously suggested (e.g., Rasmusson et al., 2006b), a balance between the inhibitory effects of allo + pregnan and the excitatory effects of DHEA(S) in the central nervous system may influence PTSD symptoms severity; i.e., DHEA(S) may confer stress resilience only when balanced by adequate synthesis of allo + pregnan. However, it is also possible that a lower ratio of allo + pregnan to DHEA(S) in CSF reflects reduced conversion of DHEA to other stress protective compounds: a) androsterone (Dor et al., 2015), another potent GABAergic neurosteroid produced by the serial activity of 5α-reductase and 3α-HSD (Fig. 1), and b) 5-androsten-3β,17β-diol (ADIOL) (Saijo et al.,2011). ADIOL promotes estrogen receptor (ER)β-mediated recruitment of C-terminal binding protein (CtBP) co-repressor complexes to AP-1 dependent promoters present in genes involved in GABAergic neurosteroid synthesis as well as suppression of microglia-mediated inflammation. Notably, recent research demonstrates clear causal links between inflammation and depressive symptoms (Miller and Raison, 2016).
4.2. Possible sex differences in the enzyme sites at which GABAergic neurosteroid synthesis may be blocked in PTSD
In contrast to the current findings in men with PTSD, premenopausal women with PTSD have not demonstrated 5α-reductase deficiency in either CSF or plasma (Rasmusson et al., 2006b; Pineles et al., 2018). Given the inability to separate Allopregnanolone from pregnanolone, or measure 5α-DHP in CSF, Rasmusson et al. (2006b) suggested, but could not confirm, that the low ratio of allopreg + pregnan to 5α-DHP observed in premenopausal women with PTSD was consistent with 3α-HSD deficiency. Pineles et al. (2018) investigated the relationship between PTSD symptoms and both the (allo + pregnan)/5α-DHP and Allopregnanolone/5α-DHP ratios in plasma, and found that only the (allo + pregnan)/5α-DHP ratio was associated with PTSD. We therefore concluded that the (allo + pregnan)/5α-DHP ratio might be a more robust biomarker for PTSD (even though it may not be the most specific metric for 3α-HSD deficiency) because it reflects the composite effects of two opposing PTSD-related factors: 1) upstream excitatory pressures that push production of 5α-DHP (e.g., amygdala hyperreactivity that drives HPA axis activation and peripheral production of 5α-DHP during stress), and 2) deficient 3α-HSD function that blocks conversion of 5α-DHP and (unmeasured) 5β-HDP to Allopregnanolone and pregnanolone, respectively. Indeed, a particularly striking finding was the failure of the women with PTSD to increase the plasma ratio of allo + pregnan to 5α-DHP in reaction to a moderately stressful psychophysiology task, whereas this ratio increased robustly in the healthy controls.
In contrast, in the current study, neither the Allopregnanolone/5α-DHP ratio nor the (allo + pregnan)/5α-DHP ratio differed the PTSD and TC groups, suggesting that 3α-HSD activity was normal in the men with PTSD. Instead, the men with PTSD exhibited deficient 5α-reductase activity (Fig. 1, Table 2). As indicated in Table 3, 77% of the men with PTSD had 5α-DHP/progesterone ratios below the median 5α-DHP/progesterone ratio in TCs. These results are thus consistent with: a) the social isolation model of PTSD vulnerability in male mice wherein brain 5α-reductase I expression and Allopregnanolone levels are diminished (Agís-Balboa et al., 2007; Pinna and Rasmusson, 2014), and b) the association between PTSD risk and a 5α-reductase II gene polymorphism in men but not women (Gillespie et al., 2011).
4.3. PTSD-related 5a-reductase deficiency previously reported for the gucocorticoid pathway
A PTSD-related deficiency in 5α-reductase activity that impacts cortisol metabolism has been reported previously in mixed-sex samples (Yehuda et al., 2009a, b). These studies used GC-MS to discriminate cortisol from its metabolites and demonstrated: a) a reduction in the cortisol metabolite 5α —tetrahydrocortisol (produced by 5α-reductase), but not in cortisol itself, in aging Holocaust survivors with lifetime PTSD (Yehuda et al., 2009b); b) negative correlations between 5α-re- ductase activity (indexed as the ratio of 5α-tetrahydrocortisol to cortisol) and PTSD severity in both Holocaust survivors and 2001 World Trade Center Attack victims (Yehuda et al., 2009a, and 2009b) and c) a positive correlation between 5α-reductase activity and resistance to the therapeutic effects of Prolonged Exposure in the 2001 World Trade Center Attack victims (Yehuda et al., 2009a). Future work will therefore have to determine whether 5α-reductase deficiency in the glucocorti-coid system is sex-dependent, and whether 5α-reductase deficiency, if present in any pathway, manifests across all 5α-reductase dependent neuroactive steroid synthesis pathways (Fig. 1). As previously discussed (Rasmusson and Pineles, 2018), 5α-reductase deficiency in the gluco-corticoid pathway raises cortisol levels and may beneficially compensate for primary enzyme deficiencies that reduce cortisol production (e.g., 21-hydroxylase deficiency)—while simultaneously compromising Allopregnanolone and pregnanolone synthesis and increasing risk for PTSD and depression.
4.4. Study limitations
Experimental design characteristics and methodologies limit conclusions that can be drawn from the current study. Although the findings are bolstered by careful experimental controls and convergence with both preclinical and clinical lines of evidence, replication in larger samples will be important.
As noted above (Section 2.6), enzyme activities in the allopregna-nolone and preganolone synthesis pathways were not measured directly. Instead, as common in clinical neuroendocrine testing, the activity of specific enzymes was inferred from steroid product to precursor ratios. Confirmatory studies assessing enzyme activity directly in PTSD are warranted. It will also be important to investigate whether PTSD-related Allopregnanolone and pregnanolone synthesis deficits can be detected in plasma in men (as for women; Pineles et al., 2018), as plasma is more readily accessible than CSF.
The current study does note discriminate between deficient activity of the two 5α-reductase isoenzymes involved in Allopregnanolone and pregnanolone synthesis. Both 5α-reductase I and 5α-reductase II are expressed in distinct as well as overlapping brain regions relevant to PTSD, including PFC, hippocampus, the amygdala and thalamus (Eicheler et al., 1994; Torres et al., 2002; Torres and Ortega, 2003; Castelli et al., 2013). The expression of 5α-reductase I, which has micromolar affinity for its steroid substrates (Normington and Russell, 1992), increases in male rodents when testosterone levels decrease (Torres and Ortega, 2003)—as occurs in men during intense stress (Morgan et al., 2000)—thereby facilitating catabolism of the increased quantities of steroids produced during stress (Normington and Russell, 1992). In contrast, the expression of 5α-reductase II, which has nano-molar affinity for its steroid substrates (Normington and Russell, 1992), has been shown to decrease in rodent PFC in response to decreases in testosterone (Torres and Ortega, 2003). It is therefore conceivable that a loss-of-function 5α-reductase II gene polymorphism might increase PTSD risk in men by a) compromising Allopregnanolone formation even before trauma exposure (thus enabling more extreme physiological and behavioral reactions to traumatic stress), and b) preventing return 5α-reductase II activity downregulated by intense stress to normal after trauma. Future studies of 5α-reductase I and 5α-reductase II gene ex- pression in humans exposed to extreme stress and in rodent models PTSD will help evaluate these possibilities.
Another limitation of the current study was our inability to measure 5β-DHP and thus the potential role of 5β-reductase in the pathophysiology of PTSD. As seen in Table 1, pregnanolone levels were about three times higher in the participants with PTSD than in the TCs; the group mean standard deviations also were high. Inspection of the data revealed that the pregnanolone levels of 1 TC and 4 PTSD participants were 4–10 times higher than the uniformly low levels seen in the remaining participants (~ 4 pg/ml). This suggests that “compensatory” shunting of progesterone into the 5β-reductase pathway to produce pregnanolone may be protective for some individuals exposed to trauma, particularly if 5α-reductase deficiency is present.
The observed additive relationship of Allopregnanolone and pregnanolone to PTSD symptom severity (likely mediated via facilitation GABA effects at the GABAA receptor), while strong, also does not preclude separate effects of pregnanolone and Allopregnanolone on other processes relevant to PTSD, such as memory processing or risk for chronic refractory PTSD and depression or even long-term neurodegeneration. For example, although the sulfated metabolites of both Allopregnanolone and pregnanolone potently antagonize NMDA receptors, pregnanolone sulfate may be somewhat more potent than Allopregnanolone sulfate in this respect (Park-Chung et al., 1997, 1999). The sulfotransferases or sulfatases that regulate levels of these sulfated neurosteroids also may interact more or less effectively with one or the other neurosteroid precursor or sulfated metabolite. As discussed by Rasmusson and Pineles (2018), rising intracellular levels Allopregnanolone and pregnanolone acting at GABAA receptors, as well as antagonism of NMDA receptors by rising intracellular levels of Allopregnanolone sulfate and pregnanolone sulfate may contribute to LTD or LTP interference (Izumi et al., 2011), which is thought to protect newly modulated synapsis from further excitation during memory consolidation. The quantities of Allopregnanolone and pregnanolone, as well as their sulfated metabolites produced during reexposure to stimuli previously associated with trauma thus may influence extinction and extinction retention, memory processes central to normal recovery from traumatic stress and PTSD. In future studies, measurement of 5β-DHP to more directly gauge 5β-reductase function, and measurement of Allopregnanolone sulfate and pregnanolone sulfate will be important in addressing these important questions. For example, higher pregnano-lone levels could be associated with increased production of pregna- nolone sulfate, but also could result from reduced metabolism pregnanolone to pregnanolone sulfate.
Interestingly, this relatively small study did not demonstrate a mean difference in the sum of CSF Allopregnanolone and pregnanolone between the PTSD and TC participants, even though CSF allo + pregnan levels correlated strongly and negatively with PTSD symptom severity in the PTSD group. There may be two reasons for these seemingly discrepant observations. First, the range in total CAPS scores among the small group of PTSD participants was large (Fig. 2). This suggests that group mean differences in CSF allo + pregnan levels might emerge in a study including a greater proportion of PTSD participants with severe symptoms. Secondly, even quantitatively normal allo + pregnan levels in the PTSD group may not be high enough to counter other (unmeasured) PTSD-related neurobiological factors that drive PTSD symptoms, such as noradrenergic hyperreactivity, low GABA levels and/or high DHEA(S) levels (as discussed above). There are also 3β-hydroxy isomers of Allopregnanolone and pregnanolone (not measured) that negatively modulate GABAA receptor function (Egebjerg et al., 2001; Lundgren et al., 2003) and may have diminished effects of Allopregnanolone and pregnanolone among the participants with PTSD. Studies quantifying these many factors within single participants are needed to identify possible interactions among them that impact PTSD symptom burden.
This cross-sectional study cannot discriminate between neuroactive steroid system abnormalities present before trauma exposure and those resulting from trauma exposure.
It is also important to point out that the enzyme site(s) at which allo + pregnan synthesis appears to be blocked in this tobacco free, male sample without current substance abuse or use of other psycho-tropics may not generalize to all male PTSD patients. For example, male mice exposed to high intermittent doses of alcohol followed by abrupt alcohol withdrawal showed reduced brain 5-reductase I and 3α-HSD gene expression, as well as lower brain Allopregnanolone levels (Cagetti et al., 2004). Alcohol, like Allopregnanolone and pregnanolone, activates extrasynaptic GABAA receptors at low doses, but also stimulates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), thereby reducing levels of NADPH (Kono et al., 2000; Qin and Crews, 2012). NADPH is a necessary coenzyme for 5α-reductase 1 and II, as well as 3α-HSD. If PTSD patients compensate for inadequate allo + pregnan synthesis in the aftermath of traumatic stress by abusing alcohol, blocks in allo + pregnan synthesis at both 5α-reductase I and 3α- HSD may emerge. Nicotine use also may confound studies of these GABAergic neurosteroids in PTSD unless the timing of recent use and the overall intensity of use are controlled and matched, respectively, across diagnostic groups. While nicotine acutely activates the adrenal gland, chronic nicotine use blunts adrenal reactivity to exogenous and endogenous stressors (Rasmusson et al., 2006a). Nicotine also enhances pregnane xenobiotic receptor (PXR)-induced expression of p450 enzymes such as CYP3A4 that metabolize steroids, including allopregna-nolone, pregnanolone and their precursors (Lamba et al., 2004). As noted below, prescribed psychotropics also may impact allopregnano-lone and pregnanolone levels.
4.5. Implications for treatment
The study findings may have important treatment implications. Interventions that enhance Allopregnanolone and/or pregnanolone synthesis or otherwise augment levels of these GABAergic neurosteroids may be beneficial to PTSD patients with deficit GABAergic neurosteroid synthesis. Given the potential for epigenetic regulation of 5 α — reductase, short-term treatment with deacetylase inhibitors or other interventions that epigenetically enhance 5 α-reductase expression may be beneficial to men with PTSD (Rasmusson and Pineles, 2018; Rasmusson et al., 2017a). Work by Torres and Ortega (2003) suggests that a short course of testosterone may reverse 5α-reductase II down- regulation. SSRIs at doses < 1/10th the EC50 for serotonin reuptake blockade have been shown to normalize downregulated 5α-reductase I expression (5α-reductase II not assessed) and brain Allopregnanolone levels, as well as PTSD-like behaviors in socially isolated male mice (Pinna et al., 2009). Likewise, in humans with major depression (PTSD not assessed), fluoxetine and fluvoxamine increased CSF allo + pregnan levels in association with decreases in depressive symptoms (Uzunova et al., 1998). Other compounds that induce neurosteroidogenesis [e.g., 18kDa translocator protein (TSPO) ligands (Rupprecht et al., 2010) or agonists at the cannabinoid type 1 (CB1) receptor or peroxisome pro- liferator-activated receptor alpha (PPAR-α (Pinna, 2018)] also may be therapeutic in PTSD, but perhaps only if downstream blocks in Allopregnanolone and/or pregnanolone synthesis don’t interfere. example, in healthy individuals, acutely administered pregnenolone compared to placebo, as well as the serum ratio of Allopregnanolone pregnenolone, were associated with increased dmPFC to amygdala connectivity, which in turn was associated with better emotion regulation (Sripada et al., 2013). In 22 veterans with mild traumatic brain injury (mTBI) ± PTSD, pregnenolone was not more efficacious in reducing PTSD symptoms than placebo, but the direction of effect favored pregnenolone. In addition, increases in pregnenolone, pregnanolone and Allopregnanolone across both conditions were associated with improvements in DSM-IV defined Cluster D symptoms, while increases pregnenolone and Allopregnanolone in the pregnenolone group associated with reductions in total CAPS scores (Marx et al., 2016).
Intravenous Allopregnanolone and a novel oral Allopregnanolone analog were recently reported to show efficacy in post-partum depression (Kanes et al., 2017) and major depression (https://www.businesswire.com/news/home/20180207005417/en/), respectively. Whether direct augmentation of Allopregnanolone levels reduces PTSD symptoms is yet unknown, however.
Consistent with a precision medicine approach to treating PTSD, may be important to target Allopregnanolone-or pregnanolone-based treatments to individuals with demonstrated blocks in Allopregnanolone and/or pregnanolone synthesis, as pre-clinical work shows no advantage of Allopregnanolone-based treatments in male rodents with normal lopregnanolone synthesis (Pibiri et al., 2008; Pinna and Rasmusson, 2014). Allopregnanolone-and/or pregnanolone-based treatments may be more effective in PTSD if dosed to coincide with PTSD-specific recovery processes, such as cognitive reprocessing and extinction may occur in response to trauma reminders in safe therapeutic contexts (Pinna and Rasmusson, 2014)—rather than when dosed to achieve increased steady state levels (e.g., Rasmusson et al., 2017a; Rasmusson and Pineles, 2018).
Finally, this study adds weight to an emerging public health concern (Singh and Avram, 2014) highlighted by the Post-Finasteride Syndrome Foundation (http://www.pfsfoundation.org). A subpopulation of treated with finasteride for premature hair loss or prostate conditions develop disabling and sometimes refractory depression, anxiety, pain, insomnia, sexual dysfunction, suicidal ideation and a variety of metabolic abnormalities—conditions often comorbid with PTSD in men. Finasteride competitively antagonizes 5α-reductase type I at sub-micromolar concentrations (Ki value of 300 nM), and acts as a mechanism-based inactivator of 5α-reductase II at sub-nanomolar concentrations (Drury et al., 2009). Whether risk for off-target effects of finasteride greater among men exposed to trauma, men with PTSD, or men pre-existing 5α-reductase II deficiency or other causes of deficient lopregnanolone or pregnanolone synthesis merits further investigation, as well as clinical caution.
5. Conclusion
This CSF study in men with PTSD replicates a previous study premenopausal women with PTSD demonstrating a strong inverse relationship between CSF allo + pregnan levels and PTSD as well as depressive symptoms. In addition, it demonstrates an apparent PTSD-re- lated block in the Allopregnanolone synthesis pathway at 5α-reductase in men, instead of at 3α-HSD as suggested by previous studies in women with PTSD. This study thus suggests that sex, PTSD severity, and identification of specific enzymatic blocks in the synthesis of Allopregnanolone and/or pregnanolone may help with precision medicine targeting of a variety of pharmacotherapeutics or other interventions aimed at augmenting levels of GABAergic neurosteroids for the prevention and treatment of PTSD.
Acknowledgements
We would like to express our gratitude to the study participants who generously contributed to this project, Marianela Nelson who contributed much time and expertise to the GC-MS measurement of the CSF neuroactive steroids, and Benjamin Johnides, Maggie Bauer, Leah Brogan, Rachel Maskin and Joyce Ambrosino, our dedicated clinical research assistants and nurse who recruited and guided study participants through the study with great care.
Footnotes
Conflict of interest
This research was supported by NIMH R21 MH31113 (Rasmusson, PI) and the National Center for PTSD, Women’s Health Science Division, Department of Veterans Affairs. Over the past three years, Dr. Rasmusson has been compensated as a scientific advisor to Resilience Therapeutics and Cohen Veterans Bioscience. Dr. Pinna has been compensated for measurement of neurosteroid levels for Marinus Pharmaceuticals. Dr. Anderson has consulted to Pepper Hamilton representing the Eli Lilly Co. The remaining authors declare no disclosures or conflicts of interest.
References
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A, 2006. Characterization of brain neurons that express enzymes mediating neuro-steroid biosynthesis. Proc. Natl. Acad. Sci 103, 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A, 2007. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social iso-lation-induced behavior in mice. Proc. Natl. Acad. Sci 104, 18736–18741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G, 2001. Stress and neuroactive steroids. Int. Rev. Neurobiol 46, 243–272. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM, 1995. The development of a clinician-administered PTSD scale. J. Traum. Stress 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW, 2004. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippo-campus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacol 46, 570–579. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Casti A, Casu A, Frau R, Bortolato M, Spiga S, Ennas MG, 2013. Regional distribution of 5α-reductase type 2 in the adult rat brain: an im-munohistochemical analysis. Psychoneuroendocrinology 38, 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet A, Lasbennes F, Darbon P, Poisbeau P, 2008. Fast non-genomic effects of progesterone-derived neurosteroids on nociceptive thresholds and pain symptoms. Pain 139, 603–609. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Uzunov D, Costa E, Guidotti A, 1995. Gas chromatographic-mass fragmentographic quantitation of 3 alpha-hydroxy-5 alpha-pregnan-20-one (Allopregnanolone) and its precursors in blood and brain of adrenalectomized and ca-strated rats. J. Neurosci 15, 4641–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvie S, Koen N, McGregor N, O’Connell K, Warnich L, Ramesar R, Nievergelt CM, Stein DJ, 2016. Toward a global roadmap for precision medicine in psy- chiatry: challenges and opportunities. Omics: J. Integr. Biol 20, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor RB, Marx CE, Shampine LJ, Rubinow DR, Schmidt PJ, 2015. DHEA meta-bolism to the neurosteroid androsterone: a possible mechanism of DHEA’s anti-depressant action. Psychopharmacology 232, 3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury JE, Di Costanzo L, Penning TM, Christianson DW, 2009. Inhibition ofhuman steroid 5a-reductase (AKR1D1) by finasteride and structure of the enzyme-inhibitor complex. J. Biol. Chem 284, 19786–19790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egebjerg J, Schousboe A, Krogsgaard-Larsen P, 2001. Glutamate and GABA Receptors and Transporters: Structure, Function and Pharmacology CRC Press, Boca Raton, Florida, pp. 248. [Google Scholar]
- Eicheler W, Tuohimaa P, Vilja P, Adermann K, Forssmann WG, Aumüller G, 1994. Immunocytochemical localization of human 5 alpha-reductase 2 with polyclonal antibodies in androgen target and non-target human tissues. J. Histochem. Cytochem 42, 667–675. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MW, Williams JB, 1995. Structured Clinical Interview for DSM-IV Axis I Disorders New York State Psychiatric Institute, New York. [Google Scholar]
- Genazzani AD, Luisi M, Malavasi B, Strucchi C, Luisi S, Casarosa E, Bernardi F, Genazzani AR, Petraglia F, 2002. Pulsatile secretory characteristics of allo- pregnanolone, a neuroactive steroid, during the menstrual cycle and in amenorrheic patients. Eur. J. Endocr 146, 347–356. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Almli LM, Smith AK, Bradley B, Kerley K, Crain DF, Mercer KB, Weiss T, Phifer J, Tang Y, Cubells JF, 2013. Sex dependent influence of a functional polymorphism in steroid 5-α-reductase type 2 (SRD5A2) on post-traumatic stress symptoms. Am. J. Med. Genet. B Neuropsychiatr. Genet 162, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, O’Dell KA, Zorumski CF, 2013. Metaplastic LTP inhibition after LTD in- duction in CA1 hippocampal slices involves NMDA receptor-mediated neuroster-oidogenesis. Physiol. Rep 1 e00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MJ, Russell MA, Benowitz NL, Feyerabend CO, 1988. Elimination of coti- nine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am. J. Public Health 78, 696–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA, 2004. Allopregnanolone analogs that positively modulate GABAa receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia 45, 864–867. [DOI] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A,Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, Hoffmann E, 2017. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 390, 480–489. [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, 2000. NADPH oxidase-der-ived free radicals are key oxidants in alcohol-induced liver disease. J. Clin. Invest 1 (106), 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schutetz EG, 2004. PXR (NR112) splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol. Appl. Pharmacol 199, 251–265. [DOI] [PubMed] [Google Scholar]
- Lundgren P, Strömberg J, Bäckström T, Wang M, 2003. Allopregnanolone-stimulated GABA mediated chloride ion flux is inhibited by 3β-hydroxy-5α-pregnan-20-one (isoAllopregnanolone). Brain Res 982, 45–53. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrision ND, Schwartz RD, Barker JL, Paul SM, 1986. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 323, 1004–1007. [DOI] [PubMed] [Google Scholar]
- Marx CE, Naylor JC, Kilts JD, Dunn CE, Tupler LA, Szabo ST, Capehart BP, Morey RA, Shampine LJ, Acheson SK, 2016. Neurosteroids and traumatic brain injury; Translating biomarkers to therapeutics; Overview and pilot investigations, in: Iraq and Afghanistan era veterans in In: Laskowitz D, Grant G (Eds.), Translational Research in Traumatic Brain Injury CRC Press/Taylor and Francis Group, Boca Raton, FL: Chapter 7. [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolu- tionary imperative to modern treatment target. Nat. Rev. Immunol 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, Wang S, Mason J, Southwick SM, Fox P, Hazlett G, Charney DS, Greenfield G, 2000. Hormone profiles in humans experiencing military survival training. Biol. Psychiatry 47, 891–901. [DOI] [PubMed] [Google Scholar]
- Normington K, Russell DW, 1992. Tissue distribution and kinetic characteristics of rat steroid 5α-reductase isozyme. J. Biol. Chem 267, 19548–19554. [PubMed] [Google Scholar]
- Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH, 1997. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol. Pharmacol 52, 1113–1123. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH, 1999. Sulfated and unsulfated steroids modulate γ-aminobutyric acid A receptor function through distinct sites. Brain Res 830, 72–87. [DOI] [PubMed] [Google Scholar]
- Penning TM, Burczynski ME, Jez JM, Hung C-F, Lin H-K, Ma H, Moore M, Palackal N, Ratnam K, 2000. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J 351, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Jin Y, Steckelbroeck S, Rizner TL, Lewis M, 2004. Structure-function of human 3α-hydroxysteroid dehydrogenases: genes and proteins. Mol. Cell. Endocrin 215, 63–72. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G, 2008. Decreased corticolimbic Allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc. Natl. Acad. Sci 2105, 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Hall KA, Hauger R, Miller MW, Resick PA, Orr SP, Rasmusson AM, 2018. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allo- pregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology 93, 133–141. [DOI] [PubMed] [Google Scholar]
- Pinna G, 2018. Biomarkers for PTSD at the interface of the endocannabinoid and neurosteroid axis. Front. Neurosci(06 August). 10.3389/fnins.2018.00482. [DOI] [PMC free article] [PubMed]
- Pinna G, Rasmusson AM, 2014. Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Front. Cell. Neurosci 8, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E, 2008Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem. Res 33, 1990–2007. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A, 2009. SSRIs act as selective brain steroidogenic sti- mulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr. Opinion Pharmacol 9, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I, 2012. Biological studies of post-traumatic stress disorder. Nature Rev. Neurosci 13, 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Santi M, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E, 1990. Neurosteroids act on recombinant human GABAA receptors. Neuron 4, 759–765. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Paul SM, 1991. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci 88, 4553–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Crews FT, 2012. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J. Neuroinflammation 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Pineles SL, 2018. Neurotransmitter, peptide and steroid hormone abnormalities in PTSD: biological enodphenotypes relevant to treatment. Curr. Psychiatry Rep 20, 52 10.1007/s11920-018-0908-9. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Vasek J, Lipschitz DS, Vojvoda D, Mustone ME, Shi Q, Gudmundsen G, Morgan CA, Wolfe J, Charney DS, 2004. An increased capacity for adrenal DHEA release is associated negatively with avoidance symptoms and negative mood in women with PTSD. Neuropsychopharmacology 29, 1546–1557. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Picciotto MR, Krishnan-Sarin S, 2006a. Smoking as a complex but critical covariate in neurobiological studies of posttraumatic stress disorders: a re- view. J. Psychopharmacol 20, 693–707. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A, 2006b. Decreased cerebrospinal fluid Allopregnanolone levels in women with posttraumatic stress disorder. Biol. Psychiatry 60, 704–713. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Marx CE, Jain S, Farfel GM, Tsai J, Sun X, Geracioti TD, Hamner MB, Lohr J, Rosse R, Summerall L, Naylor JC, Cusin C, Lang AJ, Raman R, Stein MB, 2017a. A randomized controlled trial of ganaxolone in posttraumatic stress disorder. Psychopharmacol 234, 2245–2257. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Marx CE, Pineles SL, Locci A, Scioli-Salter ER, Nillni YI, Liang JJ, Pinna G, 2017b. Neuroactive steroids and PTSD treatment. Neurosci. Lett 649, 156–163. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M, 2010. Translocator protein (18 kDa) (TSPO) as a ther- apeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov 9, 971–988. [DOI] [PubMed] [Google Scholar]
- Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK, 2011. An ADIOL-ERβ- CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 145, 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms LJ, Watson D, Doebbeling BN, 2002. Confirmatory factor analyses of post-traumatic stress symptoms in deployed and nondeployed veterans of the Gulf War. J. Abn. Psychol 111, 637–647. [DOI] [PubMed] [Google Scholar]
- Singh MK, Avram M, 2014. Persistent sexual dysfunction and depression in finasteride users for male pattern hair loss: a serious concern or red herring? J. Clin. Aesthetic Derm 7, 51–55. [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I, 2013Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biol. Psychiatry 73, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Hazen AL, Forde DR, 1997. Full and partial posttraumatic stress disorder: findings from a community survey. Am. J. Psychiatry 154, 1114–1119. [DOI] [PubMed] [Google Scholar]
- Strupp M, Schueler O, Straube A, Von Stuckrad-Barre S, Brandt T, 2001. “Atraumatic” Sprotte needle reduces the incidence of post-lumbar puncture head- aches. Neurology 57, 2310–2312. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E, 2003. Differential regulation of steroid 5α-reductase isozymes expression by androgens in the adult rat brain. FASEB J 17, 1428–1433. [DOI] [PubMed] [Google Scholar]
- Torres JM, Gomez-Capilla JA, Ortega E, 2002. Quantitative reverse-transcriptase polymerase chain reaction assay for mRNA levels of steroid 5α-reductase isozymes. Biochem. Anal. Biochem 307, 177–180. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A, 1998. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci 95, 3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Andrew R, Schmeidler J, Seckl JR, 2009a. Enduring effects of severe developmental adversity, including nutritional deprivation, on cortisol metabolism in aging Holocaust survivors. J. Psychiatric Res 43, 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Sarapas C, Makotkine I, Andrew R, Seckl JR, 2009bCortisol metabolic predictors of response to psychotherapy for symptoms of PTSD in survivors of the World Trade Center attacks on September 11, 2001. Psychoneuroendocrinology 34, 1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LM, Qiu ZK, Zhao N, Chen HX, Liu YQ, Xu JP, Zhang YZ, Yang RF, Li YF, 2014. Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post-traumatic stress disorder. Int. J. Neuropsychopharm 17, 1659–1669. [DOI] [PubMed] [Google Scholar]