Abstract
Background
Tube‐assisted feeding in infancy is common in patients with single‐ventricle physiology (SVP). Postnatal brain development is delayed, and injury is common, in patients with SVP. The role of brain findings in feeding outcomes remains unclear. We sought to determine the association between neonatal perioperative brain injury and postnatal brain maturation with feeding‐tube dependency in patients with SVP at neonatal discharge and just before the stage‐2 palliation.
Methods and Results
We evaluated a cohort of 48 term neonates with SVP who underwent pre‐ and postoperative brain magnetic resonance imaging. Perioperative brain injury and microstructural brain development were measured with diffusion tensor imaging including fractional anisotropy in white matter and apparent diffusion coefficient in gray matter. The primary outcome was defined as being 100% orally fed (binary). Of the patients 79% (38/48) were tube fed at hospital discharge, and 27% (12/45) were tube fed before stage‐2 palliation. Perioperative brain injury did not differ by group. Orally fed patients had a faster rate of decrease in apparent diffusion coefficient (3%, 95% CI 1.7% to 4.6%, P<0.001) at discharge and a faster rate of increase in fractional anisotropy (1.4%, 95% CI 0.6% to 2.2%, P=0.001) at the time of stage‐2 palliation compared with tube‐fed patients, denoting more robust brain development.
Conclusions
Slower rate of postnatal brain maturation but not perioperative brain injury is associated with feeding modality in infancy. These results support the importance of brain health in optimizing feeding outcomes in patients with SVP.
Keywords: brain development, brain injury, feeding, single‐ventricle physiology
Subject Categories: Congenital Heart Disease, Magnetic Resonance Imaging (MRI)
Clinical Perspective
What Is New?
This is the first study to investigate the association between brain injury or brain maturation and feeding modality in patients with single‐ventricle physiology.
Single‐ventricle patients who were dependent on tube feeding in the interstage period had slower neonatal brain development than those who were completely orally fed.
Perioperative brain injury was not associated with feeding modality in single‐ventricle patients in the interstage period.
What Are the Clinical Implications?
Early measures of brain development by diffusion tensor imaging are associated with feeding abilities in single‐ventricle patients.
Brain health in the neonatal period is important in optimizing feeding outcomes in single‐ventricle patients.
Introduction
Complex congenital heart disease (CHD) including single‐ventricle lesions affects 6 per 1000 live births each year.1 Advances in surgical technique and perioperative care have led to a drastic increase in survival in this patient population.2 Increased survival is paralleled with increased morbidity including impaired neurodevelopmental outcomes3 and challenges with feeding and growing during the infancy period.4, 5, 6, 7
Growth failure and feeding dysfunction are common in infants with complex CHD, with up to 50% of neonates requiring tube‐assisted feeding at hospital discharge from their neonatal operation.8 Similar to reports in premature infants and those with neonatal brain injury,9, 10, 11 early feeding dysfunction and growth failure have been linked to poor neurodevelopmental outcomes in patients with complex CHD.12, 13 In particular, both motor and cognitive outcomes appear to be affected by poor somatic growth and the need for tube‐assisted feeding.
Several studies of the neonatal brain utilizing magnetic resonance imaging (MRI) in the context of CHD have revealed an increased prevalence of brain injury in the form of white matter injury and/or focal stroke as well as relative brain immaturity despite full‐term birth.14, 15, 16 Although feeding dysfunction in these patients is multifactorial, 1 proposed mechanism is delay in oromotor development.6, 8 Coordination of feeding and sucking is thought to be 1 of the earlier manifestations of oromotor control in the newborn.17 Thus, a natural question is to what extent neonatal brain injury or dysmaturation in the patient with complex CHD contributes to the high prevalence of feeding dysfunction in infancy.
Our primary aim for this study was to assess the association between perioperative brain injury and/or brain development as measured by MRI with feeding modality at discharge from the hospitalization following neonatal congenital heart surgery and before the stage‐2 surgical palliation (S2P) in patients with single‐ventricle physiology. We hypothesized that an increasing severity of neonatal brain injury and slower rate of neonatal brain development would be associated with an increased need for tube‐assisted feeding.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Between 2003 and 2016, newborns with critical CHD at the University of California San Francisco were consecutively invited to participate in a prospective protocol studying brain development and brain injury in CHD using MRI. Brain‐imaging findings from this cohort have previously been reported.15, 16, 18 Patients who were born before 36 weeks’ gestation, had a suspected congenital infection, or showed clinical evidence of a congenital malformation or syndrome and/or a suspected or confirmed genetic or chromosomal anomaly were excluded. Once written informed consent was received, patients underwent a brain MRI before and after their neonatal cardiac surgery. The institutional committee on human research at the University of California San Francisco approved the study protocol (IRB #10‐03479).
For the current study all enrolled patients from the MRI study were screened for eligibility. Patients diagnosed as having single‐ventricle physiology (SVP) were included in the current study (n=59). SVP was defined as the presence of 1 functioning ventricle requiring a palliative surgical intervention for survival in the newborn period with either aortic or pulmonary obstruction. Only subjects alive at S2P and with available feeding data at the time of neonatal hospital discharge and/or immediately before the S2P were included in the current analysis (n=48). A minority of subjects remained in the hospital between the neonatal operation and S2P (n=2). In these subjects, feeding status in the 24 hours before the S2P was recorded. SVP was defined as the presence of 1 functioning ventricle requiring a palliative surgical intervention for survival in the newborn period with either aortic or pulmonary obstruction.
Clinical Variables
Clinical data were prospectively collected from the medical records by a team of trained neonatal research nurses and reviewed by a pediatric intensivist and/or cardiologist (P.M., S.P.) blinded to all neuroimaging findings. Clinical variables were extracted from the patient record for each 24‐hour period. Feeding modality was determined by retrospective chart review. The primary outcome was dichotomous and defined as being 100% orally fed versus requiring any amount of tube‐assisted feeding (nasogastric or gastrostomy tube) at 2 time points: (1) at neonatal hospital discharge from the first‐stage palliation and (2) immediately before the S2P. An assessment of heart failure severity was determined by adapting the New York University Pediatric Heart Failure Index, which was previously published by Connolly et al.19 Based on available clinical data, this index was modified to administer points based on number and dose of cardiac medications. These included digoxin, diuretics (low to moderate dose, high dose, or more than 1), ACE inhibitors, non‐ACE inhibitor vasodilators or angiotensin receptor blockers, β‐blockers, and antiarrhythmic agents. A total score based on these medications was derived at each time point (at time of neonatal hospital discharge and immediately before S2P). A higher number of points denoted increasing heart failure.
Study
Preoperative MRI studies were performed as soon as the baby could be safely transported to the MRI scanner as determined by the clinical team. Postoperative studies were performed after completion of perioperative care including removal of temporary pacing leads and before discharge from the hospital. Study methods, including brain imaging, were consistent across the duration of the study (see Data S1 for detailed MRI methods). Studies were performed with pharmacologic sedation, as needed. No adverse events occurred during this protocol. A neuroradiologist (A.J.B.) reviewed each MRI for focal, multifocal, or global changes. Brain injury was characterized as stroke, white matter injury (WMI), intraventricular hemorrhage, and/or global hypoxic‐ischemic injury as previously described.16 Postoperative brain injuries described were limited to newly acquired lesions not evident on the preoperative scan. White matter injury was classified as mild (1‐3 foci each <2 mm), moderate (>3 foci or any focus >2 mm), or severe (estimated >5% of white matter volume).16 Brain injury severity (BIS) was determined for each patient as previously described.20 The BIS was assigned as follows: 0 indicates normal (no injury); 1, minimal injury (minimal WMI and intraventricular hemorrhage grade I or II); 2, stroke (all stroke); and 3, moderate to severe injury (moderate and severe WMI, intraventricular hemorrhage grade III, or global hypoxic‐ischemic injury). The BIS score was assigned based on the worst injury observed on that MRI. To account for multiple injuries in a single subject at both time points, a maximal BIS and WMI severity score was determined, which was the highest score between the preoperative and postoperative scores.
Diffusion Tensor Imaging
Diffusion tensor imaging was performed using a sequence optimized for neonatal brain imaging to measure microstructural brain development. The diffusion tensor is an ellipsoid, the size and form of which manifest the direction and amount of free water diffusion. With increasing microstructural brain development, the magnitude of water diffusion motion (apparent diffusion coefficient [ADC]) decreases, and the regional directionality of water motion (fractional anisotropy [FA]) increases.21 The ADC and FA were calculated for voxels in 5 anatomical regions in the white matter (1‐5) and for voxels in 4 anatomical regions in the gray matter (6‐9) bilaterally using prespecified anatomical references15: (1) anterior white matter; (2) central white matter; (3) posterior white matter; (4) posterior limb of the internal capsule; (5) optic radiations; (6) caudate nucleus; (7) thalamus; (8) calcarine region; and (9) hippocampus. Correct regions of interest placements were confirmed by a neuroradiologist (A.J.B.). The values from the left and right hemispheres were averaged, and a mean value was used for analysis.
Statistical Analyses
Demographic characteristics, descriptors of brain injury, and clinical variables were compared among patients based on their feeding modality at each time point using standard descriptive statistics. Logistic regression analyses were performed to assess the relationship between diffusion parameters at a single time point (postoperative MRI) and feeding modality at each time point. Using generalized estimating equations for repeated measures, change in FA in white matter and change in average diffusivity in gray matter from both scans were compared between the 2 outcome groups. An interaction term for each outcome group by postmenstrual age at MRI was included to determine whether parameters of microstructural brain development in white and gray matter evolved differently across outcome groups. All analyses were performed with Stata version 14.0 statistical software (StataCorp LP, College Station, TX).
Results
A total of 48 infants with SVP were included in the study and were alive at the time of S2P, with a slight male predominance (63%). During the study period, 3 subjects were lost to follow‐up due to relocation or missing clinical data; thus, at the time of S2P, 45 subjects were available for analysis. At the time of hospital discharge, 79% (n=38/48) required tube‐assisted feeding while only 27% (n=12/45) were tube‐fed immediately before the S2P. Demographic and patient characteristics at time of neonatal hospital discharge and before the S2P are summarized in Tables 1 and 2. Length of neonatal hospital stay was significantly longer in the group of infants who were being tube fed at the time of discharge (33 versus 23 days, P=0.02) and tube fed at time of S2P (45.5 versus 28 days, P=0.03). The mean age at the time of the S2P was 24.3 weeks in tube‐fed versus 21.9 weeks in the orally fed group (P=0.57). Otherwise, no clinically significant differences were noted between the groups. In particular, birth weight, weight at discharge, weight before S2P, and other anthropometric measures were similar between the groups. In addition, we assessed the change in weight between the different time points (birth to discharge, birth to S2P, and discharge to S2P) and found no difference based on feeding modality. The frequency of postoperative cardiac arrest and/or the need for extracorporeal life support was similar regardless of feeding status.
Table 1.
Demographics by Feeding Modality: Neonatal Hospital Discharge
| Characteristic | Tube Fed (n=38) | 100% Orally Fed (n=10) | P Valuea |
|---|---|---|---|
| Male sex, N (%) | 24 (63.1%) | 6 (60%) | 1.0 |
| Single‐ventricle lesion, left‐sided, N (%) | 36 (94.7%) | 7 (70%) | 0.055 |
| Prenatally diagnosed, N (%) | 25 (65.8%) | 7 (70%) | 1.0 |
| EGA at birth, wk (median, IQR) | 39.0 (38.1‐39.7) | 38.0 (37.6‐38.7) | 0.01 |
| Birth weight, kg (mean, 95% CI) | 3.2 (3.1‐3.4) | 3.1 (2.8‐3.3) | 0.39 |
| Birth head circumference, cm (mean, 95% CI) | 34.2 (33.6‐34.8) | 34 (33.1‐34.9) | 0.72 |
| Discharge weight, kg (mean, 95% CI) | 3.6 (3.3‐3.9) | 3.5 (2.5‐4.4) | 0.73 |
| Weight change birth to discharge, kg (mean, 95% CI) | 0.42 (0.11‐0.73) | 0.31 (−0.7 to 1.3) | 0.76 |
| Post‐op ECLS, N (%) | 4 (10.5%) | 1 (10%) | 1.0 |
| Post‐op arrest, N (%) | 4 (10.5%) | 1 (10%) | 1.0 |
| Day intubated post‐op (median, IQR) | 7 (6, 9.5) | 5 (5, 7) | 0.10 |
| Vocal cord paresis, N (%)b | 12/21 (57.1%) | 1/6 (16.7%) | 0.16 |
| Hospital length of stay, d (median, IQR) | 33 (25, 49) | 23 (19, 28) | 0.02 |
| Heart failure score at discharge (median, IQR)c | 2 (1, 3) | 2 (1, 2) | 0.55 |
| DOL preoperative MRI (median, IQR) | 5.5 (3, 7) | 6 (5, 7) | 0.24 |
| DOL postoperative MRI (median, IQR) | 24 (21, 29) | 21 (20, 28) | 0.76 |
| Corrected GA preoperative MRI (median, IQR) | 39.4 (38.7, 40.1) | 39.3 (38.4, 39.4) | 0.23 |
| Corrected GA post‐operative MRI (median, IQR) | 42.2 (41.4, 43.8) | 41.7 (40.4, 42.3) | 0.31 |
DOL indicates day of life; ECLS, extracorporeal life support; EGA, estimated gestational age; GA, gestational age; IQR, interquartile range; MRI, magnetic resonance imaging; post‐op, postoperative.
P‐value based on Mann‐Whitney test or Student t test for continuous variables and Fisher exact test for categorical variables.
Based on objective testing of vocal cords; data missing for 21 subjects (17 in the tube‐fed group and 4 in the orally fed group).
Heart failure score assigned based on modification of the New York University Pediatric Heart Failure Index. The P‐value represents a test for trends analysis.
Table 2.
Demographics by Feeding Modality—Stage II Operation (S2P)
| Characteristic | Tube Fed (n=12) | Orally Fed (n=33) | P Valuea |
|---|---|---|---|
| Male sex, N (%) | 9 (75%) | 19 (57.6%) | 0.48 |
| Single ventricle lesion, left‐sided, N (%) | 12 (100%) | 29 (87.9%) | 0.56 |
| Prenatally diagnosed, N (%) | 9 (75%) | 22 (66.7%) | 0.73 |
| EGA at birth, wk (median, IQR) | 39.1 (38.4, 39.4) | 38.4 (38, 39.3) | 0.32 |
| Birth weight, kg (mean, 95% CI) | 3.2 (3.0‐3.4) | 3.2 (3.0‐3.4) | 0.96 |
| Birth head circumference, cm (mean, 95% CI) | 33.9 (32.7‐35.1) | 34.3 (33.6‐34.8) | 0.58 |
| Age before stage II, wk (median, IQR) | 24.3 | 21.9 | 0.57 |
| Weight before S2P, kg (mean, 95% CI) | 5.7 (4.9‐6.5) | 5.8 (5.4‐6.1) | 0.88 |
| Head circumference before S2P, cm (mean, 95% CI) | 39.2 (36.5‐41.9) | 40.1 (38.3‐41.5) | 0.48 |
| Length before S2P, cm (mean, 95% CI) | 59.8 (54.8‐64.8) | 61.5 (59.9‐63.1) | 0.37 |
| Weight change birth to S2P, kg (mean, 95% CI) | 2.5 (1.7‐3.3) | 2.5 (2.1‐2.9) | 0.93 |
| Weight change neonatal discharge to S2P, kg (mean, 95% CI) | 2.1 (0.97‐3.2) | 2.5 (2.1‐2.9) | 0.37 |
| Postoperative arrest (after stage I) N (%) | 0 | 5 (15%) | 0.3 |
| Postoperative ECLS (after stage I) N (%) | 0 | 5 (15%) | 0.3 |
| Vocal cord paresis, N (%)b | 5/9 (55.6%) | 8/18 (44.4%) | 0.69 |
| Neonatal hospital length of stay, d (median, IQR) | 45.5 (31.5, 71.5) | 28 (24, 36) | 0.03 |
| Heart failure score at S2P (median, IQR)c | 2 (1.5, 3) | 2 (1, 2) | 0.2 |
| DOL preoperative MRI (median, IQR) | 5 (3.5, 6) | 6 (4, 7.5) | 0.24 |
| DOL postoperative MRI (median, IQR) | 27.5 (21, 39.5) | 23.5 (19.5, 28.5) | 0.19 |
| Corrected GA preoperative MRI (median, IQR) | 39.5 (38.8, 40.1) | 39.3 (38.5, 40.1) | 0.64 |
| Corrected GA postoperative MRI (median, IQR) | 42.3 (41.9, 44.1) | 41.9 (40.7, 43.0) | 0.08 |
DOL indicates day of life; ECLS, extracorporeal life support; EGA, estimated gestational age; GA, gestational age; IQR, interquartile range; MRI, magnetic resonance imaging; S2P, stage‐2 surgical palliation.
P‐value based on t test or Mann‐Whitney test for continuous variables and Fisher exact test for categorical variables.
Based on objective testing of vocal cords; data missing for 18 subjects (15 in the orally fed group and 3 in the tube‐fed group).
Heart failure score assigned based on modification of the New York University Pediatric Heart Failure Index. The P‐value represents a test for trends analysis.
Vocal cord function was assessed objectively in 27 subjects. Although vocal cord paresis was more common among tube‐fed subjects at the time of neonatal hospital discharge (57.1% in the tube‐fed group versus 16.7% in the orally fed group), this difference did not reach statistical significance (P=0.16). Similarly, the prevalence of vocal cord paresis did not appear to vary based on feeding modality at the time of S2P (55.6% in tube‐fed versus 44.4% in orally fed children, P=0.69).
A surrogate measure of heart failure was assessed based on the number of cardiac medications the subject was taking at the time of discharge and immediately before the S2P as described in Methods. No association was demonstrated between this measure of heart failure and feeding status at each time point (Tables 1 and 2).
The prevalence of any perioperative brain injury was similar between groups at both time points (discharge: 63.2% in tube‐fed and 70% in orally fed patients, P=1.0; S2P: 58.3% in tube‐fed and 69.7% in orally fed patients, P=0.49). Brain injury severity as measured by BIS did not differ by outcome group at either time point. Similarly, white matter injury severity did not differ by outcome group at either time point, and no infants in this cohort had lesions in the brainstem (Tables 3 and 4).
Table 3.
Prevalence and Severity of Neonatal Brain Injury by Feeding Modality, Neonatal Hospital Discharge
| Tube Fed (n=38) | Orally Fed (n=10) | P Value | |
|---|---|---|---|
| Perioperative brain injury, N (%) | 24 (63.2%) | 7 (70%) | 1.0a |
| Max BIS score | |||
| None (0) | 15 (39.5%) | 3 (30%) | 0.5b |
| Mild WMI (1) | 2 (5.3%) | 0 (0%) | |
| Stroke (2) | 9 (23.7%) | 3 (30%) | |
| Moderate to severe WMI (3) | 12 (31.6%) | 4 (40%) | |
| Max WMI score | |||
| None | 24 (63.2%) | 6 (60%) | 0.58b |
| Mild | 3 (7.9%) | 0 (0%) | |
| Moderate | 9 (23.7%) | 2 (20%) | |
| Severe | 2 (5.3%) | 2 (20%) | |
Brain injury severity (BIS) was categorized for each subject in an ordinal scale: 0, no injury; 1, minimal injury (mild white matter injury [WMI] or intraventricular hemorrhage grade I‐II); 2, stroke; 3, moderate to severe WMI. WMI severity was categorized as mild, moderate, or severe. To account for multiple injuries in a single subject at both time points, a maximal BIS score or WMI severity score was determined, which was the higher score between the preoperative and postoperative scans.
Fisher exact test.
Test for trends.
Table 4.
Prevalence and Severity of Neonatal Brain Injury by Feeding Modality, Stage II Operation
| Tube Fed (n=12) | Orally Fed (n=33) | P Value | |
|---|---|---|---|
| Perioperative brain injury, N (%) | 7 (58.3%) | 23 (69.7%) | 0.49a |
| Maximal BIS score | |||
| None | 5 (41.7%) | 11 (33.3%) | 0.83b |
| Mild | 0 (0%) | 2 (6.1%) | |
| Moderate | 3 (25%) | 9 (27.3%) | |
| Severe | 4 (33.3%) | 11 (33.3%) | |
| Max WMI score | |||
| None | 7 (58.3%) | 21 (63.6%) | 0.8b |
| Mild | 1 (8.3%) | 2 (6.1%) | |
| Moderate | 3 (25%) | 7 (21.2%) | |
| Severe | 1 (8.3%) | 3 (9.1%) | |
BIS indicates brain injury severity; WMI, white matter injury.
Fisher exact test.
Test for trends.
To determine the relationship between brain development and feeding‐tube dependency, we first assessed diffusion‐imaging parameters at a single time point (postoperative MRI). No relationship was noted between average FA in white matter or average ADC in gray matter and the odds of requiring tube feeding at discharge or at the time of S2P. To assess the association between change in postnatal brain maturation (eg, rate of brain development) and feeding‐tube dependency at discharge and before the S2P, change in FA in white matter and ADC in gray matter over time from preoperative to postoperative MRI was analyzed in the 2 groups after adjustment for postmenstrual age at MRI. Tube‐fed patients at the time of hospital discharge had a similar rate of change in perioperative white matter FA as the orally fed patients. However, orally fed patients had a faster decline in ADC in gray matter, consistent with more rapid maturation, from the pre‐ to postoperative MRI as compared with the tube‐fed patients despite the fact that tube‐fed patients had their postoperative MRIs performed later (3% increase in the difference with each week of increase in age, 95% CI 1.7% to 4.6%, P<0.001) (Figure 1). Patients who were orally fed at the time of their S2P had a faster rate of increase in perioperative white matter FA (more mature) as compared with those with tube‐assisted feeding (1.4% increase in the difference with each week of increase in age, 95% CI 0.6% to 2.2%, P=0.001), whereas no difference was noted in gray matter ADC between the 2 groups (Figure 2). In addition, WMI severity was not associated with change in postnatal brain maturation. When subjects with no or mild WMI were compared with those with moderate or severe WMI, there was no difference in rate of increase in FA (P=0.6) or rate of decrease in ADC (P=0.89).
Figure 1.
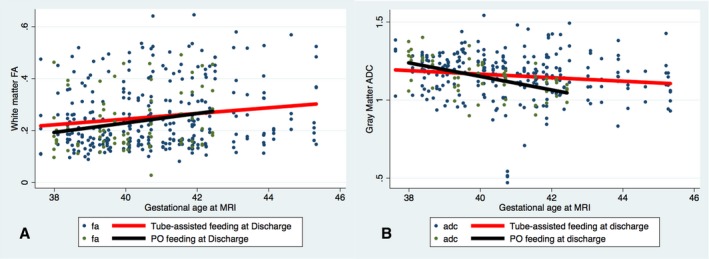
Association of change in fractional anisotropy (FA) and apparent diffusion coefficient (ADC) by feeding modality—neonatal hospital discharge. Scatterplots and linear regression lines of change in FA in white matter voxels (A) and ADC in gray matter voxels (B) demonstrate no difference in the rate of FA increase in white matter voxels between patients who were 100% orally fed (black line) vs those who were tube fed (red line) at the time of neonatal hospital discharge (P=0.3). In contrast, there was a faster rate of decrease in ADC in orally fed patients compared with tube‐fed patients (P<0.001). MRI indicates magnetic resonance imaging; PO, by mouth.
Figure 2.
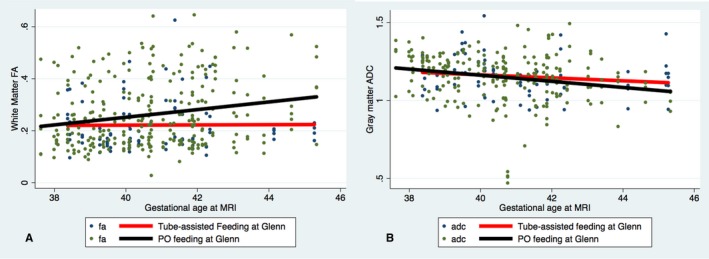
Association of change in fractional anisotropy (FA) and apparent diffusion coefficient (ADC) by feeding modality—stage II operation (Glenn). Scatterplots and linear regression lines of change in FA in white matter voxels (A) and ADC in gray matter voxels (B) demonstrate a faster rate of increase in FA (P=0.001) in patients who were 100% orally fed (black line) at the time of the stage II operation (Glenn) as compared with those who required tube feeding (red line). There was no significant difference in change in ADC in gray matter voxels, although there was a trend toward a faster rate of decrease in ADC in gray matter voxels in 100% orally fed patients (P=0.3). MRI indicates magnetic resonance imaging; PO, by mouth.
Given the potential interaction between vocal cord paresis and our outcome of interest, we performed a sensitivity analysis restricted to those with objective data on vocal cord function. Among these 27 subjects, the analysis was stratified between those with and those without vocal cord paresis. The relationship between postnatal brain maturation and feeding modality at the time of S2P remained significant. In particular, orally fed patients at the time of S2P had a faster rate of increase in perioperative white matter FA than those with tube‐assisted feeding, regardless of vocal cord function (vocal cord paresis [n=13]: 2.8% increase in the difference with each week of increase in age, 95% CI 1.2% to 4.5%, P=0.001; no vocal cord paresis [n=14]: 1.6% increase in the difference with each week of increase in age, 95% CI 0.45% to 2.8%, P=0.004). However, the relationship between perioperative brain maturation and feeding modality at the time of discharge changed based on vocal cord function. In the group with vocal cord paresis there was no difference in the rate of decrease in perioperative ADC in gray matter between orally fed and tube‐fed patients at the time of discharge (1.9% increase in the difference with each week of increase in gestational age, 95% CI −0.2% to 4.2%, P=0.08). In those without vocal cord paresis orally fed patients continued to have a faster rate of decrease in perioperative ADC in gray matter than did tube‐fed patients at discharge (2.0% increase in the difference with each week of increase in gestational age, 95% CI 0.15% to 3.8%, P=0.03).
Discussion
In this prospective cohort of term newborns with SVP studied serially with neonatal brain MRI, our data demonstrate that slower brain maturation detected early in life is associated with the need for tube‐assisted feeding at the time of neonatal hospital discharge and before the S2P. Our study suggests that the risk of feeding‐tube dependency in the interstage period may be increased by delays in neonatal brain development. Perioperative brain injury and pharmacologic surrogate measures of heart failure did not appear to be associated with feeding modality. To our knowledge, this is the first study demonstrating the association between slower neonatal brain growth and feeding‐tube dependency in SVP patients.
Feeding dysfunction is common among patients with SVP. Approximately 50% of patients receive tube‐assisted feeding on discharge from their neonatal hospitalization.6 Beyond the neonatal period, feeding dysfunction persists in ~20% of patients.22 The etiology of feeding dysfunction in this population is multifactorial and typically due to decreased intake or increased energy expenditure as can be seen in the setting of congestive heart failure.23, 24 Decreased nutrition intake can be multifactorial with common causes including vocal cord dysfunction,25, 26 gastroesophageal reflux, genetic factors,27 immature feeding skills, and overall medical complexity.8 Studies have shown downstream effects of feeding dysfunction and poor growth on neurodevelopmental outcomes.12, 28, 29 Our goals were to assess how early neurologic status as measured by neonatal brain MRI is associated with feeding abilities among SVP patients. After adjustment for age at MRI, patients who were able to achieve 100% oral feeding at the time of neonatal hospital discharge and before S2P had more robust neonatal perioperative microstructural brain development (change in FA and ADC from the preoperative to postoperative scan) in both white and gray matter as compared with patients who required tube feeding. In particular, infants who remain dependent on tube feeding at the time of S2P had minimal change in white matter FA in the perioperative period, whereas those who achieved oral feeding showed an increase over time.
Multiple studies have demonstrated that newborns with complex CHD have delayed brain maturation using both quantitative15 and semiquantitative techniques.14 Normally, FA increases in white matter with maturation of the oligodendrocyte lineage and early myelination.30 Term newborns with complex CHD have been shown to have lower levels of FA in their white matter compared with normal newborns, suggesting delays in myelination.15 Maturation of the gray matter is related to the development of complex dendritic arbors and horizontal connections between neurons, leading to a decrease in ADC. Volumetric studies have noted a progressive falloff in cortical and subcortical gray matter in fetuses with hypoplastic left heart syndrome.31 In addition, growth of gray matter appears to be impaired postnatally in neonates with hypoplastic left heart syndrome.32 In our study impaired maturation in both the white matter (as noted by the slower rate of increase in FA) and gray matter (as noted by the slower rate of decrease in ADC) appears to be related to failure in achieving full oral feeding in infancy among SVP patients. Our results are not entirely surprising, given the relative brain immaturity observed in this population. In preterm infants impaired suck‐and‐swallow coordination has been associated with younger postmenstrual age,33 suggesting that successful coordination involved in feeding may be an indicator of neurologic maturation.34 In fact, oromotor development and coordination of feeding and sucking are thought to be among the earliest manifestations of motor control in the newborn.17 Studies utilizing semiquantitative measures of brain development in the neonate with CHD have observed an increased prevalence of an “open operculum,” a region thought to be associated with oral motor coordination and language skills.14 In other populations functional brain‐imaging studies have suggested that a complex network of signals involving both the brainstem and cortex are involved in oromotor coordination.35
Somatic growth in the context of a complex CHD such as SVP is known to be impaired, with most patients exhibiting early deficits in weight, length, and head circumference.4 Somatic growth, in turn, has been linked to neurodevelopmental outcomes. Both poor linear growth and smaller head circumference have been associated with motor impairments in infancy.28, 36 In addition to somatic growth, device‐assisted feeding in infants with a complex CHD is thought to be independently associated with motor and cognitive delays at 6 and 12 months of age.12, 29 Interestingly, device‐assisted feeding, thought to provide adequate nutrition and calories to patients with SVP, has been associated with poor weight gain in the interstage period when compared with those who achieve full oral feeds.36 These studies, in combination with our findings, complicate the causal pathways among brain development, feeding modality, growth, and ultimate neurodevelopmental outcomes. Given our findings, it is possible that early delays in brain maturation set the groundwork for poor oral feeding skills, leading to delays in somatic growth and ultimately to poor neurodevelopmental outcomes. However, given our relatively small sample size, we are unable to establish causality in this study.
Brain injury incidence and severity were not associated with our outcome of interest. Although brain injury is common, the vast majority of neonates with complex CHD exhibit mild to moderate forms of white matter injury or small focal strokes,16, 20 which may not affect feeding outcomes given the widely distributed brain networks involved. However, increasing severity of brain injury may affect measures of microstructural brain development (both FA and ADC) as noted in a earlier study by our group.20 Thus, although brain injury may not directly affect feeding modality, it may influence perioperative and ongoing brain development and lead to difficulties with oromotor skills. Interestingly, we did not find an association between WMI severity and change in FA or ADC in the perioperative period; however, our sample size is small, particularly for those with moderate to severe WMI, limiting our conclusions regarding the complex relationships between brain injury and brain development. Our modified assessment of heart failure did not appear to be associated with feeding modality. Prior studies have suggested that congestive heart failure is an independent risk factor for feeding dysfunction.36, 37 Our findings may be limited due to the lack of granular data to define heart failure among this cohort, which forced us to use medications as a surrogate measure of heart failure. Thus, we are unable to exclude the possibility that heart failure remains associated with feeding skills. Consistent with earlier studies, a longer neonatal hospital length of stay was significantly associated with tube‐assisted feeding at each time point.36, 37, 38 This finding is intuitive in that patients requiring tube‐assisted feeding require additional resources, ancillary therapies, and social support before being discharged home.
The strengths of our study are in the well‐characterized group of subjects with SVP and quantitative MRI measures of brain development. There are, however, some notable limitations. We lacked granular data on other clinical risk factors that may influence feeding modality such as the prevalence of gastroesophageal reflux. It is worth noting that of the 5 neonates who arrested postoperatively and required extracorporeal membrane oxygenation, all were able to achieve full oral feeding at the time of S2P, suggesting that significant postoperative instability may not be a risk factor for feeding outcomes. However, our sample size is too small to draw any firm conclusions about this point. Second, only a subgroup of subjects had objective data on vocal cord function. In the subgroup of patients with data on vocal cord function, we were, however, able to demonstrate that vocal cord paresis did not affect our findings at the time of S2P. This suggests that the effects of neonatal brain maturity may be stronger in feeding outcomes in infancy than mechanical factors such as vocal cord paresis. Third, the findings at the time of neonatal hospital discharge are limited given the lack of data on when full oral feeding was achieved, and the timing of the postnatal MRI was later in the tube‐fed group compared with the orally fed group. Finally, because our study spanned a long period, we were unable to assess the impact of era on feeding outcomes given our sample size.
In conclusion, early measures of brain development appear to be associated with feeding abilities among SVP patients. Our findings contribute further to the literature in refining the relationships among brain health, feeding abilities, and neurodevelopmental outcomes. Further studies are planned with this cohort to assess neurodevelopmental outcome as it pertains to neonatal brain maturity, feeding abilities, and somatic growth. The incorporation of developmental care in the cardiac intensive care unit during the neonatal period for patients with complex CHD is emerging as a critical component of care for this vulnerable population.39 Interestingly, comprehensive programs that are focused on developmental and family integration have been successful in improving neurodevelopmental and feeding outcomes among premature infants.40, 41 Our findings support the importance of brain health, such as these comprehensive developmental programs, early in life in patients with complex CHD to optimize outcomes including nutrition, growth, and neurodevelopment.
Sources of Funding
This work was supported by grants K23 NS099422, R01 NS40117, R01NS063876, R01EB009756, R01HD07274, and P01 NS082330 from the National Institutes of Health.
Disclosures
None.
Supporting information
Data S1. Supplemental Methods
Acknowledgments
We thank the neonatal nurses of the Neonatal Clinical Research Center, including Cassandra Williams at the University of California at San Francisco, whose skills made this study possible; Trevor Flynn for acquiring and processing MRI data; and Yensy Zetino for obtaining clinical data for the study.
(J Am Heart Assoc. 2019;8:e012291. DOI: 10.1161/JAHA.119.012291.)
References
- 1. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 2. Karamlou T, Diggs BS, Ungerleider RM, Welke KF. Evolution of treatment options and outcomes for hypoplastic left heart syndrome over an 18‐year period. J Thorac Cardiovasc Surg. 2010;139:119–126; discussion 126–127. [DOI] [PubMed] [Google Scholar]
- 3. Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE, Mital S, Pike N, Sood E, Mahle WT, Cooper DS, Dunbar‐Masterson C, Krawczeski CD, Lewis A, Menon SC, Pemberton VL, Ravishankar C, Atz TW, Ohye RG, Gaynor JW; Pediatric Heart Network Investigators . Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daymont C, Neal A, Prosnitz A, Cohen MS. Growth in children with congenital heart disease. Pediatrics. 2013;131:e236–e242. [DOI] [PubMed] [Google Scholar]
- 5. Davis D, Davis S, Cotman K, Worley S, Londrico D, Kenny D, Harrison AM. Feeding difficulties and growth delay in children with hypoplastic left heart syndrome versus d‐transposition of the great arteries. Pediatr Cardiol. 2008;29:328–333. [DOI] [PubMed] [Google Scholar]
- 6. Medoff‐Cooper B, Naim M, Torowicz D, Mott A. Feeding, growth, and nutrition in children with congenitally malformed hearts. Cardiol Young. 2010;20(suppl 3):149–153. [DOI] [PubMed] [Google Scholar]
- 7. Kelleher DK, Laussen P, Teixeira‐Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006;22:237–244. [DOI] [PubMed] [Google Scholar]
- 8. Medoff‐Cooper B, Ravishankar C. Nutrition and growth in congenital heart disease: a challenge in children. Curr Opin Cardiol. 2013;28:122–129. [DOI] [PubMed] [Google Scholar]
- 9. Slattery J, Morgan A, Douglas J. Early sucking and swallowing problems as predictors of neurodevelopmental outcome in children with neonatal brain injury: a systematic review. Dev Med Child Neurol. 2012;54:796–806. [DOI] [PubMed] [Google Scholar]
- 10. Adams‐Chapman I, Bann CM, Vaucher YE, Stoll BJ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Association between feeding difficulties and language delay in preterm infants using Bayley Scales of Infant Development‐Third Edition. J Pediatr. 2013;163:680–685.e1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taine M, Charles M‐A, Beltrand J, Rozé JC, Léger J, Botton J, Heude B. Early postnatal growth and neurodevelopment in children born moderately preterm or small for gestational age at term: a systematic review. Paediatr Perinat Epidemiol. 2018;32:268–280. [DOI] [PubMed] [Google Scholar]
- 12. Medoff‐Cooper B, Irving SY, Hanlon AL, Golfenshtein N, Radcliffe J, Stallings VA, Marino BS, Ravishankar C. The association among feeding mode, growth, and developmental outcomes in infants with complex congenital heart disease at 6 and 12 months of age. J Pediatr. 2015;169:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mussatto KA, Hoffmann R, Hoffman G, Tweddell JS, Bear L, Cao Y, Tanem J, Brosig C. Risk factors for abnormal developmental trajectories in young children with congenital heart disease. Circulation. 2015;132:755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536; discussion 536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. [DOI] [PubMed] [Google Scholar]
- 16. McQuillen PS, Barkovich AJ, Hamrick SEG, Perez M, Ward P, Glidden DV, Azakie A, Karl T, Miller SP. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. [DOI] [PubMed] [Google Scholar]
- 17. McFarland DH, Tremblay P. Clinical implications of cross‐system interactions. Semin Speech Lang. 2006;27:300–309. [DOI] [PubMed] [Google Scholar]
- 18. Peyvandi S, Chau C, Guo T, Xu D, Glass H, Synnes A, Poskitt K, Barkovich A, Miller SP, McQuillen PSM. Neonatal brain injury and timing of neurodevelopmental assessment in patients with congenital heart disease. J Am Coll Cardiol. 2018;71:1986–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Connolly D, Rutkowski M, Auslender M, Artman M. The New York University Pediatric Heart Failure Index: a new method of quantifying chronic heart failure severity in children. J Pediatr. 2001;138:644–648. [DOI] [PubMed] [Google Scholar]
- 20. Dimitropoulos A, McQuillen PS, Sethi V, Moosa A, Chau V, Xu D, Brant R, Azakie A, Campbell A, Barkovich AJ, Poskitt KJ, Miller SP. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hüppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–497. [DOI] [PubMed] [Google Scholar]
- 22. Maurer I, Latal B, Geissmann H, Knirsch W, Bauersfeld U, Balmer C. Prevalence and predictors of later feeding disorders in children who underwent neonatal cardiac surgery for congenital heart disease. Cardiol Young. 2011;21:303–309. [DOI] [PubMed] [Google Scholar]
- 23. Li J, Zhang G, Herridge J, Holtby H, Humpl T, Redington AN, Van Arsdell GS. Energy expenditure and caloric and protein intake in infants following the Norwood procedure. Pediatr Crit Care Med. 2008;9:55–61. [DOI] [PubMed] [Google Scholar]
- 24. Schwalbe‐Terilli CR, Hartman DH, Nagle ML, Gallagher PR, Ittenbach RF, Burnham NB, Gaynor JW, Ravishankar C. Enteral feeding and caloric intake in neonates after cardiac surgery. Am J Crit Care. 2009;18:52–57. [DOI] [PubMed] [Google Scholar]
- 25. Skinner ML, Halstead LA, Rubinstein CS, Atz AM, Andrews D, Bradley SM. Laryngopharyngeal dysfunction after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;130:1293–1301. [DOI] [PubMed] [Google Scholar]
- 26. Hehir DA, Rudd N, Slicker J, Mussatto KA, Simpson P, Li S‐H, Frommelt MA, Tweddell JS, Ghanayem NS. Normal interstage growth after the Norwood operation associated with interstage home monitoring. Pediatr Cardiol. 2012;33:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burnham N, Ittenbach RF, Stallings VA, Gerdes M, Zackai E, Bernbaum J, Clancy RR, Gaynor JW. Genetic factors are important determinants of impaired growth after infant cardiac surgery. J Thorac Cardiovasc Surg. 2010;140:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ravishankar C, Zak V, Williams IA, Bellinger DC, Gaynor JW, Ghanayem NS, Krawczeski CD, Licht DJ, Mahony L, Newburger JW, Pemberton VL, Williams RV, Sananes R, Cook AL, Atz T, Khaikin S, Hsu DT; Pediatric Heart Network Investigators . Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. 2013;162:250–256.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y, Brosig C. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133:e570–e577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drobyshevsky A, Song S‐K, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clouchoux C, du Plessis AJ, Bouyssi‐Kobar M, Tworetzky W, McElhinney DB, Brown DW, Gholipour A, Kudelski D, Warfield SK, McCarter RJ, Robertson RL, Evans AC, Newburger JW, Limperopoulos C. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 2012;23:2932–2943. [DOI] [PubMed] [Google Scholar]
- 32. Peyvandi S, Kim H, Lau J, Barkovich AJ, Campbell A, Miller S, Xu D, McQuillen P. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J Thorac Cardiovasc Surg. 2017;155:291–300.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gewolb IH, Vice FL, Schwietzer‐Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Dev Med Child Neurol. 2001;43:22–27. [DOI] [PubMed] [Google Scholar]
- 34. Gewolb IH, Bosma JF, Reynolds EW, Vice FL. Integration of suck and swallow rhythms during feeding in preterm infants with and without bronchopulmonary dysplasia. Dev Med Child Neurol. 2003;45:344–348. [DOI] [PubMed] [Google Scholar]
- 35. Ludlow CL. Central nervous system control of voice and swallowing. J Clin Neurophysiol. 2015;32:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Maria MV, Glatz AC, Ravishankar C, Quartermain MD, Rush CH, Nance M, William Gaynor J, Goldberg DJ. Supplemental tube feeding does not mitigate weight loss in infants with shunt‐dependent single‐ventricle physiology. Pediatr Cardiol. 2013;34:1350–1356. [DOI] [PubMed] [Google Scholar]
- 37. Lambert LM, Pike NA, Medoff‐Cooper B, Zak V, Pemberton VL, Young‐Borkowski L, Clabby ML, Nelson KN, Ohye RG, Trainor B, Uzark K, Rudd N, Bannister L, Korsin R, Cooper DS, Pizarro C, Zyblewski SC, Bartle BH, Williams RV; Pediatric Heart Network Investigators . Variation in feeding practices following the Norwood procedure. J Pediatr. 2014;164:237–242.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slicker J, Sables‐Baus S, Lambert LM, Peterson LE, Woodard FK, Ocampo EC; National Pediatric Cardiology‐Quality Improvement Collaborative Feeding Work Group . Perioperative feeding approaches in single ventricle infants: a survey of 46 centers. Congenit Heart Dis. 2016;11:707–715. [DOI] [PubMed] [Google Scholar]
- 39. Butler SC, Huyler K, Kaza A, Rachwal C. Filling a significant gap in the cardiac ICU: implementation of individualised developmental care. Cardiol Young. 2017;27:1797–1806. [DOI] [PubMed] [Google Scholar]
- 40. Als H, Gilkerson L, Duffy FH, McAnulty GB, Buehler DM, Vandenberg K, Sweet N, Sell E, Parad RB, Ringer SA, Butler SC, Blickman JG, Jones KJ. A three‐center, randomized, controlled trial of individualized developmental care for very low birth weight preterm infants: medical, neurodevelopmental, parenting, and caregiving effects. J Dev Behav Pediatr. 2003;24:399–408. [DOI] [PubMed] [Google Scholar]
- 41. Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, Warfield SK, Hüppi PS, Butler SC, Conneman N, Fischer C, Eichenwald EC. Early experience alters brain function and structure. Pediatrics. 2004;113:846–857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods


