Abstract
Background
Patients with symptomatic severe aortic stenosis and a history of chest radiation therapy represent a complex and challenging cohort. It is unknown how transcatheter aortic valve replacement (TAVR) compares with surgical aortic valve replacement in this group of patients, which was the objective of this study.
Methods and Results
We retrospectively reviewed all patients with severe aortic stenosis who underwent either TAVR or surgical aortic valve replacement at our institution with a history of mediastinal radiation (n=55 per group). End points were echocardiographic and clinical outcomes in‐hospital, at 30 days, and at 1 year. Inverse propensity weighting analysis was used to account for intergroup baseline differences. TAVR patients had a higher STS score than surgical aortic valve replacement patients (5.1% [3.2, 7.7] versus 1.6% [0.8, 2.6], P<0.001) and more often (P<0.01 for all) a history of atrial fibrillation (45.5% versus 12.7%), chronic lung disease (47.3% versus 7.3%), peripheral arterial disease (38.2% versus 7.3%), heart failure (58.2% versus 18.2%), and pacemaker therapy (23.6% versus 1.8%). Postoperative atrial fibrillation was less frequent (1.8% versus 27.3%; P<0.001) and hospital stay was shorter in TAVR patients (4.0 [2.0, 5.0] versus 6.0 [5.0, 8.0] days; P<0.001). The ratio of observed‐to‐expected 30‐day mortality was lower after TAVR as was 30‐day mortality in inverse propensity weighting–adjusted Kaplan–Meier analyses.
Conclusions
In patients with severe aortic stenosis and a history of chest radiation therapy, TAVR performs better than predicted along with less adjusted 30‐day all‐cause mortality, postoperative atrial fibrillation, and shorter hospitalization compared with surgical aortic valve replacement. These data support further studies on the preferred role of TAVR in this unique patient population.
Keywords: aortic valve implantation, aortic valve stenosis, radiation, transcatheter aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Cardiovascular Surgery, Catheter-Based Coronary and Valvular Interventions
Clinical Perspective
What Is New?
In patients with symptomatic severe aortic stenosis after chest radiation therapy, surgical aortic valve replacement performs worse and transcatheter aortic valve replacement performs better than predicted by Society of Thoracic Surgery score.
In this patient population, transcatheter aortic valve replacement is associated with lower adjusted 30‐day and 1‐year mortality, less postoperative atrial fibrillation, and shorter duration of hospital stay but higher readmission rates.
What Are the Clinical Implications?
Transcatheter aortic valve replacement might be the preferred choice for patients with symptomatic severe aortic stenosis and history of chest radiation.
Further studies should be conducted on the preferred role of transcatheter aortic valve replacement in this unique patient population, preferably in a randomized clinical trial design.
Introduction
Radiation therapy to the chest is an important element in the treatment of various malignancies such Hodgkin's lymphoma and breast cancer. Efforts have been made to reduce radiation injury to chest structures, but most of these came into effect only in the new millennium. In addition, clinical consequences of radiation‐induced heart disease are not seen until ≈10 to 20 years after radiation therapy.1, 2 Thus, at present, radiation‐induced heart disease remains a clinical reality, an element of which is valvular heart disease in nearly half of the patients.3 Management of valve disease in these patients is complicated by the presence of both, regurgitation and stenosis, of multiple cardiac valves, as well as concomitant coronary artery, myocardial, and pericardial disease. Furthermore, mediastinal, pulmonary, and/or pericardial fibrosis pose particular challenges for surgical interventions in this patient population. Percutaneous approaches may thus present a more favorable option for these patients (eg, transcatheter aortic valve replacement [TAVR]) in case of severe aortic stenosis (AS).
Since its introduction in 2002 by Cribier, TAVR has become an important treatment strategy for patients with severe AS.4 According to the updated American Heart Association/American College of Cardiology valve guidelines,5, 6 the recommendation for either surgical aortic valve replacement (SAVR) or TAVR among high‐risk patients with severe symptomatic AS (stage D) was changed from Class IIa (LOE B) to Class I (LOE A).5, 6 For patients with severe symptomatic AS (stage D) and intermediate surgical risk, TAVR has been shown to be a reasonable alternative to SAVR (Class IIa, LOE B‐R). In patients with radiation‐induced heart disease, TAVR may have higher feasibility and higher clinical effectiveness than SAVR.7 Supporting data, however, are not available because retrospective analyses have not been performed and clinical trials such as the PARTNER (Placement of Aortic Transcatheter Valve) trial were not stratified to a level that would allow for randomized comparisons of TAVR versus SAVR in this patient population. The current retrospective cohort study was performed to address this gap in knowledge.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure unless approved by all authors after review and conclusion of a reasonable request.
Patient Population
The Mayo Clinic STS (Society of Thoracic Surgery) and TVT (Transcatheter Valve Therapy) registries were screened for patients who underwent TAVR or SAVR for severe AS and had a history of chest radiation for malignancy (total number of patients screened from February 2011 to April 2018, n=1210 for TAVR and n=1707 for SAVR).8 The Mayo Clinic Institutional Review Board approved this study as well as a waiver of the requirement to obtain informed consent in accordance with 45 CFR 46.116 and a waiver of HIPAA authorization in accordance with applicable HIPAA regulations.
Clinical and Laboratory Measurements
The STS‐TVT database review included age, sex, current smoking status, hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, heart failure, New York Heart Association class 2 weeks before procedure, chronic lung disease, prior myocardial infarction, prior percutaneous coronary angioplasty, prior coronary artery bypass grafting (CABG), prior stroke, peripheral arterial disease (PAD), radiation history, and immunosuppression. Porcelain aorta was defined according to published guidelines.9 STS risk score was calculated for all patients as per standard practice.9 The STS/American College of Cardiology TVR registry‐based risk score was used to calculate expected in‐hospital mortality among cancer patients.10 Clinical records were reviewed to determine the timing and type of malignancy for which the patients received chest radiation.
All participants underwent echocardiography before the aortic valve procedure. Left ventricular ejection fraction was measured by the biplane Simpson method (biplane method of disk summation).11 Stroke volume was determined by simplified continuity equation using the Doppler method. Aortic valve area (AVA) and gradient were measured by simplified continuity and Bernoulli equations, respectively.12, 13 Valvular regurgitation was graded by experienced echocardiographers at our institution according to established guidelines.14 Low left ventricular stroke volume index (LV‐SVI) was defined as <35 mL/m2 and low‐flow low‐gradient (LF‐LG) AS by the combination of AVA ≤1 mm2, mean gradient <40 mm Hg, and low LV‐SVI.15, 16
Outcome Parameters
In‐hospital outcomes included all‐cause mortality, myocardial infarction, stroke, major adverse cardiac events, postoperative atrial fibrillation, pacemaker placement, respiratory arrest, renal failure requiring dialysis, cardiac tamponade, bleeding, and length of hospital stay. In general, these were analyzed in accordance with the standards set forth by the Valve Academic Research Consortium (VARC‐2).9 Major adverse cardiac events were defined as the composite of death, myocardial infarction, or stroke. Postoperative atrial fibrillation was defined as new onset (ie, normal sinus rhythm before and atrial fibrillation after valve replacement). Postdischarge mortality and readmission events were calculated based on registry and chart review data. Echocardiographic estimations of aortic valve gradient and presence of at least moderate prosthetic or periprosthetic regurgitation at discharge as well as at 6 months and 1 year postprocedure were collected as supplementary outcome data.
Statistical Analyses
Normally distributed continuous variables were presented as mean±SD. Variables with a skewed distribution as determined by a Shapiro–Wilk test were shown as median accompanied by the interquartile range. Categorical variables were expressed as frequency and percentages. T test or Kruskal–Wallis rank sum tests were used for continuous variables depending on variable distribution. Chi‐square tests with Yate's correction for continuity were used to compare categorical variables. The Kaplan–Meier method was used to illustrate the timing of events during follow‐up, and statistical assessment was performed with the log‐rank test. Inverse probability of treatment weighting was utilized to minimize the effects of confounding in Kaplan–Meier analysis by accounting for differences in STS score. Differences in 30‐day observed crude and STS defined expected all‐cause mortality in the SAVR and TAVR groups were analyzed using a Poisson test. Predictors of long‐term mortality were assessed by univariate Cox proportional‐hazards analyses. The proportional‐hazards assumptions of Cox proportional‐hazards analysis were assessed with Schoenfeld Residuals tests and no relevant violations were observed. Hazard ratios and 95% CI were reported. A multivariable Cox regression analysis of long‐term mortality was fit to STS score, TAVR, and LF‐LG. P values of <0.05 were considered to be statistically significant. SPSS (v.22), JMP (v.13), and R (3.4.2) were used for data analysis.
Results
Patient Population
A total of 110 patients with symptomatic severe AS and a history of chest radiation were identified, 55 patients in each group (ie, 4.6% and 3.2% of all patients undergoing TAVR and SAVR, respectively) (Figure S1). Lymphoma (56.4%) and breast cancer (34.6%) were the 2 most common indications for radiation therapy. On average, these patients were 26.4±13.2 years out from chest radiation therapy, 26.7±12.4 years in the SAVR and 26.2±13.6 years in the TAVR group (P=0.91). Of the 55 patients who underwent TAVR, 8 (14.5%) received the self‐expandable Medtronic CoreValve (Medtronic, Minneapolis, MN) and the remainder the balloon‐expandable Edwards Lifesciences Sapien Valve (Edwards Lifesciences, Irvine, CA). Twelve patients (21.8%) in the TAVR group underwent a transapical TAVR, and a transfemoral approach was utilized in the remainder. No patient in the TAVR group had percutaneous coronary intervention within a month or at the time of the valve procedure, compared with 14 patients (25.5%) in the SAVR group who underwent concomitant CABG.
The characteristics of the study population by procedural group are summarized in Table 1. Patients in the TAVR group were older and had a more extensive cardiovascular history including atrial fibrillation, PAD, heart failure, or prior pacemaker implantation. These patients also more commonly had chronic lung disease. The average mean aortic valve gradient was lower in the TAVR group (Table 1), whereas no difference was found for AVA, LV‐SVI, right ventricular systolic pressure, or other echocardiographic parameters. There was a numeric but not statistically significant difference in the prevalence of LF‐LG AS between the TAVR and SAVR groups.
Table 1.
Baseline Characteristics of the Study Cohort
| Variable | TAVR Group (n=55) | SAVR Group (n=55) | P Value |
|---|---|---|---|
| Clinical, demographic, and symptom variables | |||
| Age, y | 72.0 (62.0, 81.5) | 60.0 (55.5, 73.0) | <0.001 |
| Female sex, n (%) | 34 (61.8) | 34 (61.8) | 1.00 |
| BMI, kg/cm2 | 25.3 (22.1, 30.0) | 28.7 (25.5, 31.8) | 0.001 |
| STS score, % | 5.1 (3.2, 7.7) | 1.6 (0.8, 2.6) | <0.001 |
| Smoking history, n (%) | 20 (36.4) | 24 (43.6) | 0.56 |
| Hypertension, n (%) | 34 (61.8) | 29 (52.7) | 0.44 |
| Diabetes mellitus, n (%) | 9 (16.4) | 12 (21.8) | 0.63 |
| Hyperlipidemia, n (%) | 25 (45.5) | 36 (65.5) | 0.06 |
| ≥50% left main, n (%) | 7 (13.0) | 6 (12.2) | 1.00 |
| ≥70% 3 vessels CAD, n (%) | 12 (22.2) | 10 (20.4) | 1.00 |
| Atrial fibrillation, n (%) | 25 (45.5) | 7 (12.7) | <0.001 |
| Carotid disease >79%, n (%) | 8 (14.5) | 4 (7.3) | 0.36 |
| PAD, n (%) | 21 (38.2) | 4 (7.3) | <0.001 |
| Prior stroke, n (%) | 6 (10.9) | 2 (3.6) | 0.27 |
| Prior MI, n (%) | 7 (12.7) | 6 (10.9) | 1.00 |
| CHF, n (%) | 32 (58.2) | 10 (18.2) | <0.001 |
| Prior CABG, n (%) | 13 (23.6) | 6 (10.9) | 0.13 |
| Prior PCI, n (%) | 20 (36.4) | 10 (18.2) | 0.05 |
| Prior ICD, n (%) | 4 (7.3) | 0 (0.0) | 0.13 |
| Prior implanted pacemaker, n (%) | 13 (23.6) | 1 (1.8) | 0.002 |
| NYHA class | 3 (2, 3) | 2 (2, 3) | 0.005 |
| Type of malignancy | 0.25 | ||
| Lymphoma | 27 (49.1) | 33 (60.0) | |
| Breast cancer | 19 (34.5) | 19 (34.5) | |
| Other | 9 (16.4) | 3 (5.5) | |
| Immunosuppression, n (%) | 2 (3.6) | 3 (5.5) | 1.00 |
| Chronic lung disease, n (%) | 26 (47.3) | 4 (7.3) | <0.001 |
| Preprocedure medications | |||
| β‐Blockers, n (%) | 30 (54.5) | 30 (54.5) | 1.00 |
| ACEI/ARB, n (%) | 16 (29.1) | 19 (34.5) | 0.85 |
| Statin, n (%) | 32 (58.2) | 30 (54.5) | 0.70 |
| Aspirin, n (%) | 40 (72.7) | 35 (63.6) | 0.41 |
| Anticoagulation, n (%) | 21 (38.2) | 2 (3.6) | <0.001 |
| Laboratory data | |||
| Hematocrit, % | 37.3±5.9 | 38.9±4.3 | 0.12 |
| Creatinine, mg/dL | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.2) | 0.94 |
| Echocardiographic data | |||
| Left ventricular ejection fraction, % | 60.0 (50.0, 65.0) | 62.0 (58.5, 65.0) | 0.10 |
| AVA, mm2 | 0.9 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.36 |
| LV‐SVI, mL/m2 | 44.5 (36.0, 49.8) | 44.0 (40.0, 48.0) | 0.97 |
| LV‐SVI <35 mL/m2, n (%) | 9 (16.4) | 5 (9.1) | 0.39 |
| Low‐flow, low‐gradient AS, n (%) | 8 (14.5) | 3 (5.5) | 0.20 |
| Mean aortic valve gradient, mm Hg | 31.0 (20.5, 37.5) | 43.5 (38.3, 51.8) | <0.001 |
| ≥ Moderate aortic valve regurgitation, n (%) | 20 (36.4) | 15 (27.3) | 0.41 |
| Calcific mitral valve stenosis, n (%) | 8 (14.5) | 11 (20.0) | 0.45 |
| ≥ Moderate mitral valve regurgitation, n (%) | 9 (16.4) | 4 (7.2) | 0.14 |
| Mean mitral valve gradient, mm Hg | 5.0 (3.3, 5.8) | 4.0 (3.0, 5.0) | 0.41 |
| ≥ Moderate tricuspid regurgitation, n (%) | 16 (29.1) | 8 (14.5) | 0.11 |
| RVSP, mm Hg | 39.0 (32.0, 46.0) | 36.0 (28.0, 41.8) | 0.06 |
Data are presented as mean±SD, no. (%), or median (Q1, Q3). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AS, aortic stenosis; AVA, aortic valve area; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; ICD, implantable cardioverter defibrillator; LV‐SVI, left ventricular stroke volume index; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; RVSP, right ventricular systolic pressure; SAVR, surgical transcatheter aortic valve replacement; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement.
Outcome Analyses
As shown in Table 2, the aortic valve area was larger and the mean aortic valve gradient was lower in the TAVR group than in the SAVR group at discharge and at 1 year. In relation to baseline values, the gain in aortic valve area was higher in the TAVR group but the reduction in the mean gradient was lower (Table 2). The change in mean aortic valve (AV) gradient per change in AV area was lower in the TAVR than in the SAVR group, driven by the LF‐LG patients in the TAVR group (mean change in AV gradient per change in AV area was −11.4±5.5 in TAVR patients with LF‐LG versus −22.1±15.8 in TAVR patients without LF‐LG, P=0.003). No difference was found between TAVR and SAVR in regard to the percentage of at least moderate prosthetic or periprosthetic aortic valve regurgitation. A similar decrease in the number of patients with ≥ moderate mitral regurgitation was seen in both groups after aortic valve replacement, with 4 in the TAVR and none in the SAVR group remaining (Table 2).
Table 2.
Echocardiographic Outcomes
| Variable | TAVR Group | SAVR Group | P Value |
|---|---|---|---|
| Discharge echocardiographic data | n=54 | n=54 | |
| AVA, mm2 | 2.1 (1.7, 2.4) | 1.9 (1.6, 2.2) | 0.12 |
| AVA change from baseline, mm2 | 1.2 (0.9, 1.6) | 1.1 (0.9, 1.4) | 0.14 |
| Mean AV gradient, mm Hg | 10.2±3.8 | 15.1±8.5 | <0.001 |
| Mean AV gradient change from baseline, mm Hg | −21.4±13.8 | −29.9±13.0 | 0.001 |
| Mean AV gradient change/mean AVA change | −21.1±15.9 | −29.5±21.8 | 0.03 |
| ≥ Moderate AV prosthetic regurgitation, n (%) | 1 (1.9) | 0 (0.0) | 0.33 |
| ≥ Moderate AV periprosthetic regurgitation, n (%) | 2 (3.7) | 0 (0.0) | 0.17 |
| ≥ Moderate mitral regurgitation, n (%) | 4 (7.4) | 0 (0.0) | 0.04 |
| 6‐mo echocardiographic data | n=47 | n=28 | |
| Mean AV gradient, mm Hg | 10.4±5.7 | 13.1±6.2 | 0.06 |
| ≥ Moderate AV prosthetic regurgitation, n (%) | 0 (0.0) | 0 (0.0) | 1.00 |
| ≥ Moderate AV periprosthetic regurgitation, n (%) | 4 (8.5) | 0 (0.0) | 0.16 |
| ≥ Moderate mitral regurgitation, n (%) | 4 (8.5) | 0 (0.0) | 0.11 |
| 1‐y echocardiographic data | n=24 | n=22 | |
| Mean AV gradient, mm Hg | 9.7±4.1 | 15.1±6.5 | 0.001 |
| ≥ Moderate AV prosthetic regurgitation, n (%) | 0 (0.0) | 1 (4.4) | 0.30 |
| ≥ Moderate AV periprosthetic regurgitation, n (%) | 2 (8.3) | 1 (4.4) | 0.56 |
| ≥ Moderate mitral regurgitation, n (%) | 3 (12.5) | 0 (0.0) | 0.42 |
Data are presented as mean±SD, no. (%), or median (Q1, Q3). The number of patients with available echocardiographic studies at 6 and 12 months is mentioned in the table. AV indicates aortic valve; AVA, aortic valve area; LV‐SVI, left ventricular stroke volume index; RVSP, right ventricular systolic pressure; SAVR, surgical transcatheter aortic valve replacement; TAVR, transcatheter aortic valve replacement.
In‐hospital crude all‐cause mortality and major adverse cardiac events rates were numerically but not statistically lower in the TAVR group than in the SAVR group, as detailed in Table 3. The rate of postoperative atrial fibrillation was significantly lower in the TAVR group than in the SAVR group (3.6% versus 32.7%, respectively, P<0.001). Patients who underwent TAVR had a shorter length of hospital stay than patients in the SAVR group (4.0 [2.0, 5.0]) versus 6.0 [5.0, 8.0] days, P<0.001).
Table 3.
Crude Perioperative and Short‐Term Postoperative Outcomes
| Variable | TAVR Group (n=55) | SAVR Group (n=55) | P Value |
|---|---|---|---|
| In‐hospital outcomes | |||
| Mortality, n (%) | 1 (1.8) | 2 (3.6) | 1.00 |
| MI, n (%) | 0 (0.0) | 1 (1.8) | 1.00 |
| Stroke, n (%) | 0 (0.0) | 1 (1.8) | 1.00 |
| MACE, n (%) | 1 (1.8) | 2 (3.6) | 1.00 |
| Postoperative atrial fibrillation, n (%) | 2 (3.6) | 18 (32.7) a | <0.001 |
| Permanent pacemaker placement, n (%) | 8 (14.5) | 4 (7.3) | 0.36 |
| Respiratory arrest, n (%) | 0 (0.0) | 3 (5.5) | 0.24 |
| Renal failure requiring dialysis, n (%) | 0 (0.0) | 2 (3.6) | 0.48 |
| Cardiac tamponade, n (%) | 0 (0.0) | 2 (3.6)b | 0.48 |
| VARC‐2 defined life‐threatening or major bleeding, n (%) | 0 (0.0) | 2 (3.6)b | 0.48 |
| Length of hospital stay, d | 4.0 (2.0, 5.0) | 6.0 (5.0, 8.0) | <0.001 |
| 30‐d outcomes | |||
| All‐cause mortality, n (%) | 1 (1.8) | 5 (9.1)c | 0.21 |
| 30‐d rehospitalization, n (%) | 6 (10.9) | 3 (5.5) | 0.49 |
| 1‐y outcomes | |||
| All‐cause mortality, n (%) | 9 (16.4) | 6 (10.9) | 0.40 |
| 90‐d rehospitalization, n (%) | 17 (30.9) | 3 (5.5) | 0.001 |
Data are presented as mean±SD, no. (%), or median (Q1, Q3). CABG indicates coronary artery bypass graft; MACE, major adverse cardiac events; MI, myocardial infarction; SAVR, surgical transcatheter aortic valve replacement; TAVR, transcatheter aortic valve replacement; VARC‐2, Valve Associated Research Consortium‐2.
Four patients had concomitant SAVR and CABG and developed postoperative atrial fibrillation. The other 14 patients had isolated SAVR.
One patient had concomitant SAVR and CABG and had tamponade and life‐threatening bleeding.
Two patients had concomitant SAVR and CABG; the other 3 had isolated SAVR.
In the crude analysis, there was no difference in 30‐day or 1‐year all‐cause mortality (Figure 1A and B). After inverse probability of treatment weighting adjusting for STS score, 30‐day and 1 year all‐cause mortality were significantly lower in the TAVR group (Figure 1C and D). Compared with the expected mortality based on STS risk score, the observed mortality was lower in the TAVR than in the SAVR group, calculating into a lower observed‐versus‐expected 30‐day all‐cause mortality ratio (TAVR 0.33 [95% CI 0.01–1.86] versus SAVR 5.00 [95% CI 1.62–11.67], P=0.005). In a separate analysis using the STS/American College of Cardiology‐TVT registry TAVR score, observed mortality (1.8%) was lower than expected (3.6%) in the TAVR group (ratio of observed‐versus‐expected 0.5 [95% CI 0.01–2.79]).
Figure 1.
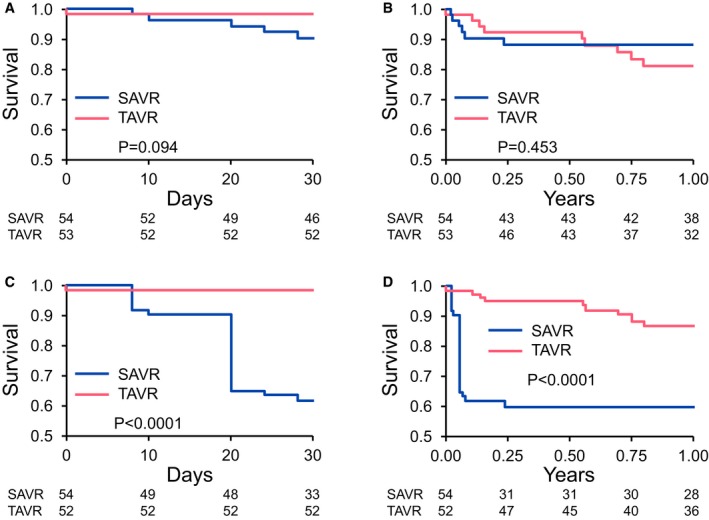
A, Unadjusted Kaplan–Meier curves for survival at 30‐day follow‐up. B, Unadjusted Kaplan–Meier curves for survival at 1‐year follow‐up. C, Kaplan–Meier curves for survival at 30‐day follow‐up after applying inverse propensity treatment weighting. D, Kaplan–Meier curves for survival at 1‐year follow‐up after applying inverse propensity treatment weighting. SAVR indicates surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Cox proportional hazards analysis in the entire cohort identified prior myocardial infarction, PAD, LF‐LG AS, and STS risk score as predictors of mortality. In the multivariate model, LF‐LG AS and STS risk score remained significant predictors of 1‐year mortality adjusting for TAVR (Table 4). LF‐LG AS was predictive of 1‐year mortality in the TAVR group alone in both the unadjusted and the inverse probability of treatment weighting–adjusted analyses (Figure S2). Less than half of the LF‐LG AS patients survived up to 1 year after TAVR (Figure S2).
Table 4.
Univariate and Multivariate Predictors of 1‐Year Mortality in All Patients
| Predictor | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age (y) | 1.0 (0.96–1.05) | 0.7 | NA | |
| Men | 0.8 (0.27–2.31) | 0.7 | NA | |
| ≥ Moderate chronic lung disease | 2.5 (0.90–6.84) | 0.08 | NA | |
| Prior MI | 3.7 (1.16–11.57) | 0.03 | NA | |
| Baseline atrial fibrillation | 1.1 (0.38–3.28) | 0.8 | NA | |
| NYHA class III–IV | 1.7 (0.54–5.49) | 0.4 | NA | |
| Baseline LVEF | 1.0 (0.93–1.00) | 0.07 | NA | |
| LV‐SVI | 1.0 (0.91–1.03) | 0.3 | NA | |
| LF‐LG aortic stenosis | 4.8 (1.64–14.07) | 0.004 | 4.6 (1.53–14.02) | 0.006 |
| ≥ Moderate mitral valve regurgitation | 0.9 (0.27–3.34) | 0.9 | NA | |
| ≥ Moderate tricuspid valve regurgitation | 0.9 (0.26–3.26) | 0.9 | NA | |
| PAD | 4.0 (1.45–11.00) | 0.008 | NA | |
| Prior stroke | 2.7 (0.60–11.88) | 0.2 | NA | |
| STS score | 1.2 (1.07–1.27) | <0.001 | 1.2 (1.08–1.35) | 0.001 |
| TAVR | 1.5 (0.53–4.17) | 0.5 | Included in all | |
| Concomitant CABG | 0.6 (0.17–2.15) | 0.5 | NA | |
| Postoperative atrial fibrillation | 0.7 (0.17–3.27) | 0.7 | NA | |
Data are presented as mean±SD, no. (%), or median (Q1, Q3). CABG indicates coronary artery bypass grafting; LF‐LG, low‐flow low‐gradient; LVEF, left ventricular ejection fraction; LV‐SVI, left ventricular stroke volume index; MI, myocardial infarction; NA, not applicable; NYHA, New York Heart Association; PAD, peripheral arterial disease; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement.
Regarding readmission rates, these were higher in the TAVR than in the SAVR group, about 2‐fold at 30 days and more than 5‐fold at 90 days (Table 3). The reasons for readmissions are listed in Table S1. Heart failure was the leading readmission diagnosis in TAVR patients.
Discussion
The present study demonstrates that TAVR compared with SAVR for native severe AS in patients with prior chest radiation is associated with (1) older age, higher STS scores, and more baseline comorbidities; (2) lower incidence of postprocedural atrial fibrillation and shorter hospital stay; and (3) lower adjusted 30‐day and 1‐year all‐cause mortality.
A recent study from the Cleveland Clinic on a matched cohort of more than 300 SAVR patients showed that those with prior chest radiation therapy more commonly have more severe coronary artery and pulmonary disease.17 In a smaller study of 26 TAVR patients with a history of prior chest radiation therapy, matched 1:1 to patients without such history, radiation therapy patients presented more frequently with PAD, pacemaker therapy, and moderate/severe MR (mitral regurgitation).18 On the contrary, the very first analysis on TAVR in this patient cohort noted a lower cardiovascular risk factor burden, less PAD, and less atrial fibrillation.7 In our study, we found that patients in the TAVR group were older, had a higher prevalence of atrial fibrillation and prior pacemaker implantation, PAD, CABG‐treated coronary artery disease, heart failure, and chronic lung disease than SAVR patients. Also, their New York Heart Association functional class status was more advanced. The STS score was higher in the TAVR and lower in the SAVR group than previously reported. Collectively, these data indicate that patients with prior chest radiation therapy are more complex, and that those with the highest degree of comorbidity are more likely to be directed to TAVR.
It is interesting that TAVR patients had a lower mean aortic gradient change per mean aortic valve area change than SAVR patients. Their mean gradient, however, was already lower before the procedure despite similar aortic valve areas. This constellation was previously recognized as a unique feature of patients who underwent SAVR with a history of chest radiation and was attributed to lower LV‐SVIs.17 Similarly, in TAVR patients with a history of chest radiation, Donnellan et al noted that 63% of patients had a low LV‐SVI.19 A LV‐SVI <35 mL/m2 is the defining characteristic for LF‐LG AS (ie, AVA ≤1 mm2 but mean gradient <40 mm Hg.15, 16 As reported before, patients with radiation exposure to the chest generally have a preserved ejection fraction and the reduction in stroke volume is the consequence of restrictive changes.20 In combination with severely elevated afterload over time, as in case of severe AS, these changes could be more profound. Indeed, the prevalence of LF‐LG AS was 2 to 4 times higher in our population (20%) than the reported 5% to 10% in the general AS population.17 It was this subgroup of TAVR patients with LF‐LG that experienced the least reduction in mean AV gradient per AVA gained after valve replacement. Future studies will have to explore these hemodynamic aspects further.
Donnellan et al pointed out that patients undergoing SAVR with a history of chest radiation require more inotropic support and blood transfusions, have longer intensive care unit and overall length of hospital stay, more frequently undergo pacemaker therapy, and experience a higher rate of atrial fibrillation, stroke, and mortality.17 Furthermore, Ghoneim et al noted a 10% in‐hospital mortality in nearly 50 patients with a history of chest radiation therapy after SAVR.21 Postprocedural deaths were seen exclusively with combined SAVR and CABG.21 Bouleti et al found that the procedural success of TAVR was lower in the radiation than in the nonradiation group.18 In our study, TAVR and SAVR patients had generally similar in‐hospital outcomes with the following exceptions. The rate of postoperative atrial fibrillation was significantly lower and the length of hospital stay was significantly shorter in the TAVR group. The latter finding is remarkable in view of the more comorbid nature of this cohort of patients. A lower rate of atrial fibrillation is very meaningful, given its association with an increased risk of in‐hospital mortality, higher stroke incidence, and prolonged hospital stay.22, 23, 24, 25, 26, 27 Importantly, the preprocedural prevalence of active atrial fibrillation was not higher in the TAVR group, and the rate of atrial fibrillation in the SAVR group is in keeping with previous reports.22, 28, 29
Whereas patients without prior chest radiation have a survival after SAVR that matches the general population, those with prior chest radiation have a median life expectancy of only 7 years (48% versus 7% mortality at 6±3 years of follow‐up, P<0.001).17 Ghoneim et al recorded similar outcomes over similar follow‐up times: postdischarge mortality was 47% and the average time to death was 2±1 and 1.4±1.6 years (average 5‐year survival rates 65% versus 37%) after isolated SAVR and SAVR+CABG, respectively.21 Despite these numbers, it is important to note the life‐extending benefit of SAVR in this patient population.17 For TAVR, Bouleti et al showed that patients with a history of chest radiation more frequently had severe bleeding and developed heart failure with hemodynamic complications; 30‐day mortality was 8% versus 5% based on the STS score.18 Over a median follow‐up of 3 to 4 years, 58% in the radiation group and 42% in the control group died; average 5‐year survival rates were 33% and 42%, respectively. This compares to 3‐ and 5‐year survival rates of 61% and 46%, respectively, in the UK TAVR registry.30 Dijos et al found no differences in major outcome parameters in TAVR patients with and without chest radiation at 30 days and 6 months.7 In the most recent analysis on AS patients with a history of chest radiation, Donnellan et al observed in‐hospital, 1‐year, and 2‐year survival rates of 96%, 91%, and 86%, respectively, after TAVR compared with 96%, 86%, and 80% after SAVR.19 In our study, we likewise found no significant difference in in‐hospital mortality, but 30‐day and 1‐year mortality was lower with TAVR than with SAVR once adjusted for intergroup baseline STS score differences. Importantly, chest radiation patients undergoing SAVR had a higher than predicted mortality, which confirms the clinical intuition that this represents a high‐risk population. On the contrary, those undergoing TAVR had a lower than predicted mortality. The better than expected performance of the TAVR group was also seen using the STS‐American College of Cardiology‐TVR registry risk score; however, this score was designed to stratify for in‐hospital mortality only.10 Finally, because percutaneous coronary intervention can be safely performed in patients with severe AS and those undergoing TAVR,19, 31 a transcatheter approach might be particularly attractive in patients in need of interventions on both aortic valve and coronary arteries, given the above‐outlined outcome data for SAVR and CABG.
In regard to mortality predictors, Donnellan et al outlined the risk associated with a history of chest radiation for AS patients in general; post SAVR, the STS score remained the only other independent predictor.17 After TAVR, the same group found the STS score and baseline LV‐SVI to be predictive.19 Bouleti et al identified the following to be independent predictors of mortality: PAD, no β‐blocker before TAVR, serum creatinine, and infectious complications.18 Herein, we identified the STS score as an independent predictor of 1‐year mortality for both SAVR and TAVR patients. Though not designed for TAVR, the STS score has been shown to perform equally well, especially after transfemoral TAVR.32 Indeed, the STS score is one of the strongest predictors of survival after TAVR in the general AS population.33, 34, 35 In the current analysis, though, the strongest predictor of 1‐year mortality in the overall and especially in the TAVR cohort was LF‐LG AS. It remains to be shown if and how this information could be utilized to guide patient management. Prior studies have outlined the poor long‐term survival of patients with LF‐LG AS (32% versus 66% 10‐year survival) and the doubling of 5‐year survival rates with SAVR despite higher operative mortality.36 Recent studies outline the favorable performance of TAVR in this patient population and very likely it will play an increasingly important role in LF‐LG AS patients.16, 37, 38
In terms of morbidity, a nearly 3‐fold higher rate of readmissions within 90 days was reported for AS patients with prior chest radiation after SAVR.17 Following TAVR in AS in general, readmission rates as high as 24% at 30 days and 44% at 1 year have been reported, relating to both noncardiac (especially respiratory disease, infections, and bleeding) and cardiac (eg, heart failure and arrhythmias) causes.39 In the current study, readmission rates after TAVR were not quite as high, but still significantly higher than for SAVR. Of interest, all readmissions in the SAVR group and nearly all readmissions in the TAVR group were cardiac‐ or surgery‐related. Proper postprocedural follow‐up is therefore very important, and providers need to be prepared to meet the higher demands of this patient population.
Limitations
The limitations of this study include its nonrandomized and observational nature, which could have significantly affected the results because of confounding factors and significant comorbidities in the TAVR compared with the SAVR group. Inverse probability of treatment weighting was thus used to adjust for confounders, but any such statistical technique remains limited with small cohort sizes. Moreover, 25.5% of patients in the SAVR group underwent concomitant CABG, which could have worsened the clinical outcomes of this group. However, there were no observed numerical differences in outcomes between combined SAVR and CABG and isolated SAVR in our study. Of further note, 1‐year echocardiographic data were not available in significant number of patients in both groups. This being said, this study still provides the first and largest comparison of clinical outcomes post TAVR versus SAVR in patients with a history of chest radiation. A larger multicenter study with larger sample population and longer follow‐up will be necessary to confirm the results of this study. Because of dropout in follow‐up, proportional hazard assumption was violated when analyzing long‐term outcome data up to 3 years, and thus these were not reported. Finally, we could not determine the association of AS and radiation dose because such details of radiation history were not available in the majority of patients.
Conclusions
In patients with severe AS and a history of chest radiation for cancer, TAVR is associated with a favorable short‐ and intermediate‐term prognosis. TAVR is associated with lower mortality than predicted by STS score along with less postoperative atrial fibrillation and shorter duration of hospital stay. In this complex and challenging patient population, TAVR might be the preferred choice of valve replacement. Larger cohort studies and ideally adequately powered randomized clinical trials are needed to further consolidate such a recommendation.
Sources of Funding
This study was supported by research funding from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL116952 to Herrmann) and National Cancer Institute (1 RO1 CA233610‐01 to Herrmann) and the Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN.
Disclosures
None.
Supporting information
Table S1. Reasons for Hospital Readmission in SAVR and TAVR Patients
Figure S1. Patient flow chart for the cohort of patients included in this study.
Figure S2. A, Unadjusted Kaplan–Meier curves for survival at 1‐year follow‐up for patients undergoing transcatheter aortic valve replacement (TAVR) with and without low flow‐low gradient aortic stenosis (LF‐LG AS), (HR 4.81, 95% CI 1.29–17.91, P=0.02); B, Inverse probability of treatment weighting–adjusted Kaplan–Meier curves for survival at 1‐year follow‐up for the 2 specified groups, (HR 4.80, 95% CI 1.96–11.73, P=0.0006).
(J Am Heart Assoc. 2019;8:e012110 DOI: 10.1161/JAHA.119.012110.)
References
- 1. Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. [DOI] [PubMed] [Google Scholar]
- 2. Carlson RG, Mayfield WR, Normann S, Alexander JA. Radiation‐associated valvular disease. Chest. 1991;99:538–545. [DOI] [PubMed] [Google Scholar]
- 3. Gujral DM, Lloyd G, Bhattacharyya S. Radiation‐induced valvular heart disease. Heart. 2016;102:269–276. [DOI] [PubMed] [Google Scholar]
- 4. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. [DOI] [PubMed] [Google Scholar]
- 5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; Members AATF . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 6. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM III, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 7. Dijos M, Reynaud A, Leroux L, Reant P, Cornolle C, Roudaut R, Dos Santos P, Lafitte S. Efficacy and follow‐up of transcatheter aortic valve implantation in patients with radiation‐induced aortic stenosis. Open Heart. 2015;2:e000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carroll JD, Edwards FH, Marinac‐Dabic D, Brindis RG, Grover FL, Peterson ED, Tuzcu EM, Shahian DM, Rumsfeld JS, Shewan CM, Hewitt K, Holmes DR Jr, Mack MJ. The STS‐ACC transcatheter valve therapy national registry: a new partnership and infrastructure for the introduction and surveillance of medical devices and therapies. J Am Coll Cardiol. 2013;62:1026–1034. [DOI] [PubMed] [Google Scholar]
- 9. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodes‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. Eur Heart J. 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 10. Edwards FH, Cohen DJ, O'Brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, Brennan JM, Brindis RG, Rumsfeld J, Holmes DR Jr; Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry . Development and validation of a risk prediction model for in‐hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1:46–52. [DOI] [PubMed] [Google Scholar]
- 11. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M; American Society of Echocardiography and European Association of Echocardiography . Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23; quiz 101‐2. [DOI] [PubMed] [Google Scholar]
- 12. Currie PJ, Seward JB, Reeder GS, Vlietstra RE, Bresnahan DR, Bresnahan JF, Smith HC, Hagler DJ, Tajik AJ. Continuous‐wave Doppler echocardiographic assessment of severity of calcific aortic stenosis: a simultaneous Doppler‐catheter correlative study in 100 adult patients. Circulation. 1985;71:1162–1169. [DOI] [PubMed] [Google Scholar]
- 13. Baumgartner H, Kratzer H, Helmreich G, Kuehn P. Determination of aortic valve area by Doppler echocardiography using the continuity equation: a critical evaluation. Cardiology. 1990;77:101–111. [DOI] [PubMed] [Google Scholar]
- 14. Zoghbi WA, Asch FM, Bruce C, Gillam LD, Grayburn PA, Hahn RT, Inglessis I, Islam AM, Lerakis S, Little SH, Siegel RJ, Skubas N, Slesnick TC, Stewart WJ, Thavendiranathan P, Weissman NJ, Yasukochi S, Zimmerman KG. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2019. Available at: https://www.onlinejase.com/article/S0894-7317(19)30003-3/fulltext. Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 15. Pibarot P, Dumesnil JG. Low‐flow, low‐gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. [DOI] [PubMed] [Google Scholar]
- 16. Saybolt MD, Fiorilli PN, Gertz ZM, Herrmann HC. Low‐flow severe aortic stenosis: evolving role of transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2017;10:e004838. [DOI] [PubMed] [Google Scholar]
- 17. Donnellan E, Masri A, Johnston DR, Pettersson GB, Rodriguez LL, Popovic ZB, Roselli EE, Smedira NG, Svensson LG, Griffin BP, Desai MY. Long‐term outcomes of patients with mediastinal radiation‐associated severe aortic stenosis and subsequent surgical aortic valve replacement: a matched cohort study. J Am Heart Assoc. 2017;6:e005396 DOI: 10.1161/JAHA.116.005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouleti C, Amsallem M, Touati A, Himbert D, Iung B, Alos B, Brochet E, Urena M, Ghodbane W, Ou P, Dilly MP, Nataf P, Vahanian A. Early and late outcomes after trans‐catheter aortic valve implantation in patients with previous chest radiation. Heart. 2016;102:1044–1051. [DOI] [PubMed] [Google Scholar]
- 19. Donnellan E, Krishnaswamy A, Hutt‐Centeno E, Johnston DR, Aguilera J, Kapadia SR, Mick S, Svensson LG, Griffin BP, Desai MY. Outcomes of patients with mediastinal radiation‐associated severe aortic stenosis undergoing transcatheter aortic valve replacement. Circulation. 2018;138:1752–1754. [DOI] [PubMed] [Google Scholar]
- 20. Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, Ruddy KJ, Yan E, Redfield MM. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghoneim A, Bouhout I, Perrault LP, Bouchard D, Pellerin M, Lamarche Y, Demers P, Carrier M, Cartier R, El‐Hamamsy I. Reexamining the role of surgical aortic valve replacement after mediastinal radiation therapy. Ann Thorac Surg. 2017;104:485–492. [DOI] [PubMed] [Google Scholar]
- 22. Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061–1073. [DOI] [PubMed] [Google Scholar]
- 23. Motloch LJ, Reda S, Rottlaender D, Khatib R, Muller‐Ehmsen J, Seck C, Strauch J, Madershahian N, Erdmann E, Wahlers T, Hoppe UC. Postprocedural atrial fibrillation after transcatheter aortic valve implantation versus surgical aortic valve replacement. Ann Thorac Surg. 2012;93:124–131. [DOI] [PubMed] [Google Scholar]
- 24. Sannino A, Stoler RC, Lima B, Szerlip M, Henry AC, Vallabhan R, Kowal RC, Brown DL, Mack MJ, Grayburn PA. Frequency of and prognostic significance of atrial fibrillation in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2016;118:1527–1532. [DOI] [PubMed] [Google Scholar]
- 25. Furuta A, Lellouche N, Mouillet G, Dhanjal T, Gilard M, Laskar M, Eltchaninoff H, Fajadet J, Iung B, Donzeau‐Gouge P, Leprince P, Leuguerrier A, Prat A, Dubois‐Rande JL, Teiger E. Prognostic value of new onset atrial fibrillation after transcatheter aortic valve implantation: a FRANCE 2 registry substudy. Int J Cardiol. 2016;210:72–79. [DOI] [PubMed] [Google Scholar]
- 26. Biviano AB, Nazif T, Dizon J, Garan H, Fleitman J, Hassan D, Kapadia S, Babaliaros V, Xu K, Parvataneni R, Rodes‐Cabau J, Szeto WY, Fearon WF, Dvir D, Dewey T, Williams M, Mack MJ, Webb JG, Miller DC, Smith CR, Leon MB, Kodali S. Atrial fibrillation is associated with increased mortality in patients undergoing transcatheter aortic valve replacement: insights from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Interv. 2016;9:e002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanawuttiwat T, O'Neill BP, Cohen MG, Chinthakanan O, Heldman AW, Martinez CA, Alfonso CE, Mitrani RD, Macon CJ, Carrillo RG, Williams DB, O'Neill WW, Myerburg RJ. New‐onset atrial fibrillation after aortic valve replacement: comparison of transfemoral, transapical, transaortic, and surgical approaches. J Am Coll Cardiol. 2014;63:1510–1519. [DOI] [PubMed] [Google Scholar]
- 28. Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–549. [DOI] [PubMed] [Google Scholar]
- 29. Filardo G, Hamilton C, Hamman B, Hebeler RF Jr, Adams J, Grayburn P. New‐onset postoperative atrial fibrillation and long‐term survival after aortic valve replacement surgery. Ann Thorac Surg. 2010;90:474–479. [DOI] [PubMed] [Google Scholar]
- 30. Duncan A, Ludman P, Banya W, Cunningham D, Marlee D, Davies S, Mullen M, Kovac J, Spyt T, Moat N. Long‐term outcomes after transcatheter aortic valve replacement in high‐risk patients with severe aortic stenosis: the U.K. Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv. 2015;8:645–653. [DOI] [PubMed] [Google Scholar]
- 31. Goel SS, Agarwal S, Tuzcu EM, Ellis SG, Svensson LG, Zaman T, Bajaj N, Joseph L, Patel NS, Aksoy O, Stewart WJ, Griffin BP, Kapadia SR. Percutaneous coronary intervention in patients with severe aortic stenosis: implications for transcatheter aortic valve replacement. Circulation. 2012;125:1005–1013. [DOI] [PubMed] [Google Scholar]
- 32. Balan P, Zhao Y, Johnson S, Arain S, Dhoble A, Estrera A, Smalling R, Nguyen TC. The society of thoracic surgery risk score as a predictor of 30‐day mortality in transcatheter vs surgical aortic valve replacement: a single‐center experience and its implications for the development of a TAVR risk‐prediction model. J Invasive Cardiol. 2017;29:109–114. [PubMed] [Google Scholar]
- 33. Hemmann K, Sirotina M, De Rosa S, Ehrlich JR, Fox H, Weber J, Moritz A, Zeiher AM, Hofmann I, Schachinger V, Doss M, Sievert H, Fichtlscherer S, Lehmann R. The STS score is the strongest predictor of long‐term survival following transcatheter aortic valve implantation, whereas access route (transapical versus transfemoral) has no predictive value beyond the periprocedural phase. Interact Cardiovasc Thorac Surg. 2013;17:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hermiller JB Jr, Yakubov SJ, Reardon MJ, Deeb GM, Adams DH, Afilalo J, Huang J, Popma JJ; CoreValve United States Clinical Investigators . Predicting early and late mortality after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:343–352. [DOI] [PubMed] [Google Scholar]
- 35. Pilgrim T, Kalesan B, Wenaweser P, Huber C, Stortecky S, Buellesfeld L, Khattab AA, Eberle B, Gloekler S, Gsponer T, Meier B, Juni P, Carrel T, Windecker S. Predictors of clinical outcomes in patients with severe aortic stenosis undergoing TAVI: a multistate analysis. Circ Cardiovasc Interv. 2012;5:856–861. [DOI] [PubMed] [Google Scholar]
- 36. Mohty D, Magne J, Deltreuil M, Aboyans V, Echahidi N, Cassat C, Pibarot P, Laskar M, Virot P. Outcome and impact of surgery in paradoxical low‐flow, low‐gradient severe aortic stenosis and preserved left ventricular ejection fraction: a cardiac catheterization study. Circulation. 2013;128:S235–S242. [DOI] [PubMed] [Google Scholar]
- 37. Maes F, Lerakis S, Barbosa Ribeiro H, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez‐Sarano M, Cheema AN, Nombela‐Franco L, Amat‐Santos I, Munoz‐Garcia AJ, Garcia Del Blanco B, Zajarias A, Lisko JC, Hayek S, Babaliaros V, Le Ven F, Gleason TG, Chakravarty T, Szeto W, Clavel MA, de Agustin A, Serra V, Schindler JT, Dahou A, Salah‐Annabi M, Pelletier‐Beaumont E, Cote M, Puri R, Pibarot P, Rodes‐Cabau J. Outcomes from transcatheter aortic valve replacement in patients with low‐flow, low‐gradient aortic stenosis and left ventricular ejection fraction less than 30%: a substudy from the TOPAS‐TAVI registry. JAMA Cardiol. 2019;4:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez‐Sarano M, Cheema AN, Nombela‐Franco L, Amat‐Santos I, Munoz‐Garcia AJ, Garcia Del Blanco B, Zajarias A, Lisko JC, Hayek S, Babaliaros V, Le Ven F, Gleason TG, Chakravarty T, Szeto WY, Clavel MA, de Agustin A, Serra V, Schindler JT, Dahou A, Puri R, Pelletier‐Beaumont E, Cote M, Pibarot P, Rodes‐Cabau J. Transcatheter aortic valve replacement in patients with low‐flow, low‐gradient aortic stenosis: the TOPAS‐TAVI registry. J Am Coll Cardiol. 2018;71:1297–1308. [DOI] [PubMed] [Google Scholar]
- 39. Nombela‐Franco L, del Trigo M, Morrison‐Polo G, Veiga G, Jimenez‐Quevedo P, Abdul‐Jawad Altisent O, Campelo‐Parada F, Biagioni C, Puri R, DeLarochelliere R, Dumont E, Doyle D, Paradis JM, Quiros A, Almeria C, Gonzalo N, Nunez‐Gil I, Salinas P, Mohammadi S, Escaned J, Fernandez‐Ortiz A, Macaya C, Rodes‐Cabau J. Incidence, causes, and predictors of early (</=30 days) and late unplanned hospital readmissions after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8:1748–1757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Reasons for Hospital Readmission in SAVR and TAVR Patients
Figure S1. Patient flow chart for the cohort of patients included in this study.
Figure S2. A, Unadjusted Kaplan–Meier curves for survival at 1‐year follow‐up for patients undergoing transcatheter aortic valve replacement (TAVR) with and without low flow‐low gradient aortic stenosis (LF‐LG AS), (HR 4.81, 95% CI 1.29–17.91, P=0.02); B, Inverse probability of treatment weighting–adjusted Kaplan–Meier curves for survival at 1‐year follow‐up for the 2 specified groups, (HR 4.80, 95% CI 1.96–11.73, P=0.0006).


