Abstract
Background
Coronary artery bypass grafting for acute coronary syndrome complicated by cardiogenic shock (CS) is associated with a high mortality. This registry study aimed to distinguish between early surgical outcomes of CS patients with non–ST‐segment–elevation myocardial infarction (NSTEMI) and ST‐segment–elevation myocardial infarction (STEMI).
Methods and Results
Patients with NSTEMI (n=1218) or STEMI (n=618) referred for coronary artery bypass grafting were enrolled in a prospective multicenter registry between 2010 and 2017. CS was present in 227 NSTEMI (18.6%) and 243 STEMI patients (39.3%). Key clinical end points were in‐hospital mortality (IHM) and major adverse cardiocerebral events (MACCEs). Predictors for IHM and MACCEs were identified using multivariable logistic regression analysis. STEMI patients with CS were younger, had a lower prevalence of diabetes mellitus and multivessel disease, and exhibited higher myocardial injury (troponin 9±17 versus 3±6 ng/mL) before surgery compared with patients with NSTEMI (P<0.05). Emergency coronary artery bypass grafting was performed more often in STEMI (58%) versus NSTEMI (40%; P=0.002). On‐pump surgery with cardioplegia was the preferred surgical technique in CS. IHM and MACCE rates were 24% and 49% in STEMI patients with CS and were higher compared with NSTEMI (IHM 15% versus MACCE 34%; P<0.001). Predictors for IHM and MACCE in CS were a reduced ejection fraction and a higher European System for Cardiac Operative Risk Evaluation score.
Conclusions
Surgical revascularization in NSTEMI and STEMI patients with CS is associated with a substantial but not prohibitive IHM and MACCE rate. Worse early outcomes were found for patients with STEMI complicated by CS compared with NSTEMI patients.
Keywords: acute coronary syndrome, acute myocardial infarction, cardiogenic shock, coronary artery bypass grafting, surgical myocardial revascularization
Subject Categories: Cardiovascular Surgery, Revascularization
Clinical Perspective
What Is New?
This prospective multicenter registry study demonstrates that surgical revascularization of patients with non–ST‐segment–elevation myocardial infarction and ST‐segment–elevation myocardial infarction complicated by cardiogenic shock (CS) is still associated with a substantial but not prohibitive rate of in‐hospital mortality and major adverse cardiocerebral events.
Importantly, our registry data revealed distinct differences with respect to surgical outcomes for patients with non–ST‐segment–elevation myocardial infarction and ST‐segment–elevation myocardial infarction complicated by CS.
Here, rates of in‐hospital mortality and major adverse cardiocerebral events were both significantly higher in patients with ST‐segment–elevation myocardial infarction complicated by CS when compared with non–ST‐segment–elevation myocardial infarction patients.
What Are the Clinical Implications?
Surgical revascularization in patients with non–ST‐segment–elevation myocardial infarction and ST‐segment–elevation myocardial infarction complicated by CS remains a viable option in patients with CS who are not amenable to primary reperfusion with percutaneous coronary intervention.
On‐pump surgery with cardioplegic cardiac arrest, single internal thoracic artery use, and multiple venous grafting is currently considered the be the safest strategy in this high‐risk patient cohort.
Neither the surgical revascularization technique (on‐ versus off‐pump surgery) nor the use of multiple arterial grafts were predictive for in‐hospital mortality and major adverse cardiocerebral events, and the optimal timing interval for coronary artery bypass grafting in patients with acute coronary syndrome complicated by CS still needs to be elucidated by future trials.
Introduction
Cardiogenic shock (CS) occurs in 6% to 10% of patients with acute coronary syndrome (ACS) and remains the most common cause of in‐hospital mortality (IHM), despite the implementation of guideline‐directed therapies and early myocardial reperfusion by primary percutaneous coronary intervention (PCI).1, 2, 3 Randomized controlled trials investigating the results of primary PCI in ACS patients complicated by CS report early mortality rates between 39% and 56%.4, 5, 6 Coronary artery bypass grafting (CABG) in ACS patients complicated by CS is still considered to be a valiant treatment option in the current European Society of Cardiology/European Association for Cardio‐Thoracic Surgery guidelines on myocardial revascularization, especially in the presence of complex multivessel coronary artery disease (CAD) or coronary lesions not amenable for PCI.7 In a subanalysis of the SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) trial and a recent review of the literature, CABG had comparable mortality rates to PCI, despite the fact that more complex CAD was treated in the CABG subgroups.8, 9 Nonetheless, the proportion of ACS patients with CS referred to CABG has remained unaltered during the past decades at ≈5%.10
In contrast to the wealth of evidence supporting PCI therapy, where optimal treatment strategies for patients with ST‐segment–elevation myocardial infarction (STEMI) and non–ST‐segment–elevation myocardial infarction (NSTEMI) complicated by CS are well established,3, 11, 12 current surgical strategies are driven by less robust data from observational trials that are prone to unacceptably high variability with regards to both techniques and outcomes. Early mortality rates between 16% and 54% have been reported after CABG in ACS‐related CS,13, 14, 15 with various studies suggesting better outcomes after off‐pump or on‐pump beating‐heart surgery.15, 16, 17 Unlike for PCI, there are a complete lack of surgical prospective data that discriminate between NSTEMI and STEMI in this patient population and a paucity of publications in the literature with regard to the contemporary characteristics of CABG in CS. Therefore, our study analyzed current trends and surgical practice patterns in NSTEMI and STEMI complicated by CS based on the prospective data of a multicenter surgical myocardial infarction registry and aimed to identify predictors for CS, early mortality, and major adverse events.
Patients and Methods
Study Population
The surgical myocardial infarction registry was instigated by 4 university‐affiliated cardiac surgery centers within North‐Rhine‐Westphalia, the most populous state of Germany. It is a multicenter all‐comers registry for all consecutive adult patients (age >18 years) requiring CABG for ACS. After obtaining ethical approval and waiver of informed patient consent for the registry study protocol (protocol no. 15‐6553‐BO; 2010), a total of 2616 adult patients with ACS were entered prospectively and anonymously into the registry database between January 2010 and December 2017, including patients with unstable angina, NSTEMI, and STEMI. Over 120 perioperative clinical variables were collected for each patient in prespecified spreadsheets. The type of ACS was assigned by the local investigators for each patient according to current guidelines.1, 2
This subgroup analysis included a total of 1836 patients with NSTEMI (n=1218) and STEMI (n=618) that had complete data sets with respect to the primary and key secondary outcome measures. Data of patients with ACS successfully treated by primary PCI or optimal medical therapy without referral for surgical revascularization were not collected by our registry.
Outcome Measures and Definitions
Patients with NSTEMI and STEMI were stratified by the presence of CS before CABG. Preoperative CS was defined by current recommendations of the American Heart Association3: systemic hypotension with the need for continuous intravenous inotropic support (dobutamine or epinephrine) to maintain systolic arterial pressure above 90 mm Hg or cardiac index above 2.0 L/min per m2, the need for mechanical circulatory support (intra‐aortic balloon pump (IABP) or venoarterial extracorporeal membrane oxygenation (ECMO), cardiac arrest with the need for cardiopulmonary rescuscitation (CPR) or end‐organ hypoperfusion with lactate levels >3 mmol/L.
All‐cause IHM was the primary outcomes measure. Major adverse cardiocerebral events (MACCEs) were recorded as a composite secondary end point consisting of death from cardiac origin, nonfatal perioperative myocardial infarction, low cardiac output syndrome (LCOS), need for postoperative CPR, and stroke during index hospitalization. Postoperative LCOS was defined by the need for moderate or high inotropic support (intravenous dobutamine >6 mg/kg per minute, epinephrine >0.1 mg/kg per minute) or mechanical support (IABP and ECMO) to maintain systolic arterial pressure >90 mm Hg or cardiac index >2.0 L/min per m2.15 Perioperative markers of myocardial injury, including troponin and creatinine kinase–myocardial band (CK‐MB) were assessed routinely by the local laboratories. Perioperative myocardial infarction was defined as type 5 myocardial infarction following the criteria of the third universal definition of myocardial infarction18, 19: symptoms of myocardial ischemia with new pathological Q‐waves or left bundle branch block, a 5‐fold increase of CK‐MB above the upper limit of normal (ULN) and/or a 10‐fold elevation of cardiac troponin T or I above the ULN or clinical or angiographic evidence for early graft failure requiring repeat revascularization (PCI or CABG). Stroke during index hospitalization was defined by the presence of new‐onset postoperative neurological deficits lasting longer than 24 hours with imaging evidence of new brain injury or ischemia. Renal dysfunction was graded according to the recommendation of the Chronic Kidney Disease Epidemiology Collaboration using creatinine clearance (stage I–II >59 mL/min per 1.73 m2; stage III–V ≤59 mL/min per 1.73 m2). Timing of surgery from onset of ACS symptoms were classified into time intervals that closely reflected the recommendations of the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II: emergency or salvage surgery, ≤24 hours; urgent surgery, >24 to ≤72 hours; or late surgery, >72 hours.
Indication and timing for surgery and the specific surgical technique of revascularization (choice of conduits, off‐pump or on‐pump or beating‐heart CABG), cardioplegic strategy (blood or crystalloid cardioplegia), anesthesia, and postoperative management was left at the discretion of the attending physicians at the participating centers. In general, indication and timing of surgery at all participating centers followed commonly applied ischemia‐guided criteria.1, 2 These included persistent angina or electrocardiographic abnormalities indicating ischemia, hemodynamic instability, persistent rise of cardiac enzymes, or failed PCI.
Statistical Analysis
Statistical analyses of prospectively collected data were performed using the SPSS statistical software package (IBM SPSS Statistics Version 23, IBM Corp, Armonk, NY). Overall completeness of registry data items was 91.5% (ratio of missing to total data items: 8.824/104.241). Missing values were not imputed, and all supporting data are available within the article and its online supplementary files. Continuous variables are expressed as mean values with SD and comparison was performed with 1‐way ANOVA. Categorical variables are given as counts and percentages and are compared using the chi‐squared test. A multivariable logistic regression model was used to identify preoperative predictors for cardiogenic shock and pre‐ and intraoperative predictors of IHM and MACCE in NSTEMI and STEMI. Relevant variables (Tables S1 and S2) were entered into a logistic regression model with backward selection and a significance level of 0.05 for entry into the model. After variable selection, a final multivariable logistic regression was performed to also include patients in the model with missing data. Results are presented as odds ratios (ORs) with corresponding 95% CIs. Logistic regression model discrimination was assessed by the area under the receiver operator characteristic curve (AUC) and calibration by the Lemeshow‐Hosmer goodness‐of‐fit statistic. All reported P values are 2‐sided, and P<0.05 was considered statistically significant.
Results
Preoperative Characteristics
Patients with STEMI were more often in CS (39.3%; n=243) compared with NSTEMI (18.6%; n=227; P<0.001; Table 1). Mean age of NSTEMI patients was 68±10 years, 78% were males, and 34% had diabetes mellitus. Multivessel CAD was present in 83% of patients with NSTEMI, and 46% had a left mainstem disease (LMD). Compared with patients with stable NSTEMI, patients with NSTEMI complicated by CS had a higher proportion of renal dysfunction (Chronic Kidney Disease Epidemiology Collaboration stage 3/4) and prior cardiac surgery. In addition, left ventricular ejection fraction (LVEF) was reduced and logistic EuroSCORE, troponin, and CK‐MB levels higher in NSTEMI patients complicated by CS. Mean age of patients with STEMI was 67±10 years, 77% were males, and 25% had diabetes mellitus. Seventy‐nine percent of patients with STEMI had a multivessel CAD and 40% LMD. STEMI patients with CS had a higher rate of LMD, renal dysfunction (Chronic Kidney Disease Epidemiology Collaboration stage 3/4), acute or failed PCI, and thrombolysis, and a lower proportion had multivessel CAD compared with STEMI patients without CS. Patients with STEMI complicated by CS had a lower LVEF, a higher logistic EuroSCORE, troponin T, and CK‐MB elevation before CABG.
Table 1.
Baseline characteristics of NSTEMI and STEMI patients stratified by cardiogenic shock
| NSTEMI (−) CS (n = 991) | NSTEMI (+) CS (n = 227) | P value | STEMI (−) CS (n = 375) | STEMI (+) CS (n = 243) | P value | |
|---|---|---|---|---|---|---|
| Age, y | 68.3 ± 10.4 | 68.3 ± 10.2 | 0.974 | 67.2 ± 11.5 | 65.6 ± 11.0a | 0.064 |
| Male sex, % (n) | 77.7 (770/991) | 79.3 (180/227) | 0.657 | 76.8 (288/375) | 77.4 (188/243) | 0.922 |
| BMI, kg/m² | 28.2 ± 4.9 | 28.3 ± 5.5 | 0.889 | 27.6 ± 4.9 | 27.4 ± 4.1a | 0.639 |
| Hypertension, % (n) | 85.2 (666/782) | 80.9 (127/157) | 0.185 | 82.8 (289/349) | 73.0 (162/222) | 0.006 |
| Smoking history, % (n) | 38.0 (375/988) | 32.0 (71/222) | 0.106 | 41.4 (154/372) | 32.9 (76/231) | 0.039 |
| Hyperlipidemia, % (n) | 50.9 (397/780) | 51.9 (82/158) | 0.862 | 54.1 (190/351) | 37.4 (82/219)a | <0.001 |
| Diabetes mellitus, % (n) | 32.8 (322/983) | 37.8 (85/225) | 0.160 | 27.3 (102/373) | 20.7 (50/241)a | 0.069 |
| COLD, % (n) | 12.9 (127/988) | 17.8 (40/225) | 0.068 | 9.1 (34/373) | 9.6 (23/240)a | 0.887 |
| Peripheral vascular disease, % (n) | 16.3 (161/989) | 17.9 (40/224) | 0.552 | 10.8 (40/372) | 10.5 (25/238)a | 1.000 |
| Stroke, % (n) | 10.0 (99/986) | 12.8 (29/226) | 0.230 | 8.3 (31/375) | 9.5 (23/241) | 0.662 |
| CKD‐EPI grade III–V, % (n) | 31.1 (301/967) | 41.9 (91/217) | 0.003 | 27.1 (99/365) | 41.6 (94/226) | <0.001 |
| Coronary artery disease, % (n) | ||||||
| Triple‐vessel disease | 82.0 (813/991) | 85.5 (194/227) | 0.468 | 82.4 (309/375) | 72.8 (177/243)a | 0.018 |
| Left mainstem disease | 45.5 (449/987) | 48.9 (110/225) | 0.374 | 36.3 (136/375) | 45.0 (180/240) | 0.035 |
| Prior MI | 26.3 (259/985) | 30.8 (77/227) | 0.185 | 25.9 (97/374) | 28.0 (68/243) | 0.578 |
| Acute PCI < 24 h | 8.0 (55/684) | 13.5 (17/126) | 0.060 | 12.7 (40/316) | 28.4 (60/211)a | <0.001 |
| Failed PCI | 6.6 (45/685) | 7.9 (10/126) | 0.564 | 13.0 (41/315) | 23.2 (49/211)a | 0.003 |
| Thrombolysis | 0.1 (1/683) | 1.6 (2/128) | 0.067 | 1.0 (3/315) | 5.3 (11/207) | 0.004 |
| Atrial fibrillation, % (n) | 6.2 (61/988) | 6.2 (14/225) | 1.000 | 4.3 (16/373) | 10.5 (25/239) | 0.004 |
| Prior cardiac surgery, % (n) | 1.4 (10/702) | 6.6 (9/137) | 0.001 | 0.9 (3/319) | 0.0 (0/217)a | 0.276 |
| LVEF, % | 51.6 ± 14.3 | 41.4 ± 15.3 | <0.001 | 48.7 ± 13.5 | 41.2 ± 15.8 | <0.001 |
| CPR, % (n) | – | 26.1 (59/226) | – | – | 52.9 (128/242)a | – |
| High‐dose inotropes, % (n) | – | 23.8 (29/122) | – | – | 34.0 (69/203) | – |
| IABP support, % (n) | – | 28.4 (64/225) | – | – | 35.0 (85/243) | – |
| ECMO support, % (n) | – | 0 (0/225) | – | – | 0.8 (2/243) | – |
| Logistic EuroSCORE, % | 12.2 ± 12.2 | 28.6 ± 19.5 | <0.001 | 18.7 ± 18.3 | 31.2 ± 20.1 | <0.001 |
| Antiplatelets, % (n) | ||||||
| Acetylsalicylic acid | 95.4 (642/673) | 95.3 (121/128) | 0.651 | 97.4 (299/307) | 91.0 (191/210) | 0.002 |
| Dual antiplatelet therapy | 51.8 (350/676) | 51.6 (66/128) | 1.000 | 58.1 (179/308) | 52.8 (112/212) | 0.244 |
| High‐sensitive Troponin I, ng/mL | 2.4 ± 5.3 | 3.3 ± 5.8 | 0.024 | 8.0 ± 14.7 | 8.6 ± 16.9a | 0.769 |
| High‐sensitive Troponin T, ng/mL | 0.6 ± 1.0 | 1.3 ± 1.3 | <0.001 | 1.3 ± 1.7 | 2.0 ± 3.6 | 0.035 |
| CK‐MB, U/L | 27 ± 40 | 54 ± 65 | <0.001 | 46 ± 73 | 86 ± 105a | <0.001 |
Values are expressed as mean value and standard deviation or percentages with counts as indicated. BMI indicates body mass index; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CK‐MB, creatinine kinase–myocardial band; COLD, chronic obstructive lung disease; CPR, cardiopulmonary resuscitation; CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; EuroSCORE, European System for Cardiac Operative Risk Evaluation; IABP, intra‐aortic balloon pump; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction.
Significant differences (P < 0.05) between NSTEMI and STEMI groups with CS that are provided in detail in the results section.
Comparison of NSTEMI and STEMI subgroups with CS revealed that STEMI patients were younger (P=0.005), had a lower body mass index (P=0.045), a lower rate of multivessel CAD (P=0.001), diabetes mellitus (P<0.001), hyperlipidemia (P=0.006), chronic obstructive lung disease (P=0.010), peripheral vascular disease (P=0.032), and prior cardiac surgery (P<0.001). In contrast, the rate of acute PCI (P=0.002), failed PCI (P<0.001), and CPR before surgery (P<0.001) was higher in STEMI patients with CS, with increased preoperative levels of troponin I (P=0.014) and CK‐MB (P=0.005). Logistic EuroSCORE, LVEF, and the need for inotropic or mechanical circulatory support (IABP, ECMO) did not differ in patients with NSTEMI and STEMI complicated by CS. Similarly, the rate of dual antiplatelet therapy was >50% in the total cohort without any difference among ACS subgroups, regardless of the presence or absence of CS.
Intraoperative Data
Twenty percent of patients with NSTEMI and 41% of patients with STEMI were operated as emergent CABG procedures within 24 hours from onset of symptoms (Table 2). On‐pump CABG using cardioplegic cardiac arrest with the use of left internal thoracic artery and vein grafts was the predominant technique of revascularization in all groups, with an average of 3 bypass grafts per patient. Among patients with NSTEMI, emergency CABG, on‐pump beating‐heart surgery, and concomitant cardiac procedures were higher in patients complicated by CS compared with stable patients, resulting in longer cardiopulmonary bypass (CPB) and aortic cross‐clamp times. Conversely, fewer patients in NSTEMI with CS received off‐pump revascularization or a left internal thoracic artery graft. Similarly, patients with STEMI complicated by CS were more frequently operated on‐pump using the beating‐heart technique, received fewer arterial grafts and more frequently required combined cardiac procedures with longer CPB times compared with stable patients with STEMI. Among both CS subgroups, patients with STEMI were more likely to receive emergency CABG (P=0.002), warm blood cardioplegia (P=0.001), and vein grafts (P<0.001). In contrast, left internal thoracic artery use (P=0.009) and the total number of grafts (P=0.012) was lower in patients with STEMI with CS, resulting in shorter aortic cross‐clamp times (P=0.003) when compared with patients with NSTEMI complicated by CS.
Table 2.
Operative characteristics of NSTEMI and STEMI patients stratified by CS
| NSTEMI (−) CS (n = 991) | NSTEMI (+) CS (n = 227) | P value | STEMI (−) CS (n = 375) | STEMI (+) CS (n = 243) | P value | |
|---|---|---|---|---|---|---|
| Symptoms to CABG | ||||||
| ≤24 h, % (n) | 16.9 (112/664) | 40.1 (49/122) | <0.001 | 30.4 (94/309) | 58.2 (114/196)a | <0.001 |
| 24–72 h, % (n) | 40.7 (262/664) | 32.0 (39/122) | 0.085 | 28.8 (89/309) | 22.4 (44/196) | 0.121 |
| >72 h, % (n) | 41.9 (270/664) | 27.9 (34/122) | 0.003 | 40.8 (126/309) | 19.4 (38/196) | <0.001 |
| Door to CABG ≤ 24 h, % (n) | 58.3 (443/760) | 69.7 (108/155) | 0.009 | 60.7 (202/333) | 73.4 (163/222) | 0.002 |
| On‐pump, cardioplegia, % (n) | 93.6 (928/991) | 93.4 (212/227) | 0.881 | 90.4 (288/375) | 88.9 (216/243) | 0.587 |
| Warm blood cardioplegia, % (n) | 39.4 (366/928) | 25.6 (55/215) | <0.001 | 50.1 (169/337) | 49.5 (107/216)a | 0.931 |
| On‐pump, beating‐heart, % (n) | 1.2 (12/991) | 4.4 (10/227) | 0.003 | 1.6 (6/375) | 6.6 (16/243) | 0.002 |
| Off‐pump, % (n) | 5.1 (50/991) | 1.8 (4/227) | 0.031 | 8.0 (30/375) | 4.5 (11/243) | 0.099 |
| No. of grafts | 3.2 ± 1.0 | 3.2 ± 1.00 | 0.804 | 3.1 ± 1.0 | 3.0 ± 1.0a | 0.109 |
| Vein grafts, % (n) | 85.7 (848/989) | 82.4 (187/227) | 0.215 | 90.1 (336/373) | 92.9 (224/241)a | 0.245 |
| LITA use, % (n) | 97.0 (959/991) | 88.1 (199/227) | <0.001 | 92.2 (344/375) | 78.9 (191/242)a | <0.001 |
| RITA or Radial artery, % (n) | 8.2 (81/989) | 4.4 (10/226) | 0.051 | 9.3 (35/373) | 3.7 (9/242) | 0.010 |
| Total arterial CABG, % (n) | 4.4 (44/989) | 2.7 (6/226) | 0.268 | 7.0 (26/373) | 4.1 (10/242) | 0.162 |
| Concomitant cardiac surgery, % (n) | 3.1 (31/987) | 11.1 (25/226) | <0.001 | 4.8 (18/373) | 9.1 (22/243) | 0.045 |
| Valve surgery, % (n) | 3.1 (31/987) | 9.3 (21/226) | 0.002 | 3.8 (14/373) | 6.6 (16/243) | 0.127 |
| Ventricular septal defect, % (n) | 0.0 (31/987) | 1.8 (4/226) | 0.001 | 1.0 (4/373) | 2.5 (6/243) | 0.204 |
| CPB time, min | 96 ± 39 | 115 ± 42 | <0.001 | 99 ± 41 | 116 ± 53 | <0.001 |
| Aortic cross‐clamp time, min | 57 ± 25 | 64 ± 27 | <0.001 | 56 ± 22 | 56 ± 29a | 0.945 |
Values are expressed as mean and standard deviation or percentages with counts as indicated. CABG indicates coronary artery bypass grafting; CPB, cardiopulmonary bypass; CS, cardiogenic shock; LITA, left internal thoracic artery; NSTEMI, non–ST‐segment–elevation myocardial infarction; RITA, right internal thoracic artery; STEMI, ST‐segment–elevation myocardial infarction.
Significant differences (P < 0.05) between NSTEMI and STEMI groups with CS that are provided in detail in the results section.
Clinical Outcomes
In the overall cohort, all‐cause IHM (no CS, 5.7% versus CS, 19.8%; P<0.001) and MACCEs (no CS, 13.4% versus CS, 41.8%; P<0.001) was 3‐ to 4‐fold higher for patients with CS when compared with stable patients (Table 3; Figures 1 and 2). Among patients with NSTEMI, CS was associated with significantly higher IHM and MACCEs, which was mainly driven by an increase in the rate of cardiac death, LCOS, and CPR. More patients with NSTEMI complicated by CS required inotropic or IABP support, red blood cell transfusion, and dialysis after surgery that resulted in a prolonged mechanical ventilation and length of stay at the intensive care unit and hospital compared with patients with NSTEMI without CS. Similarly, IHM and MACCEs were significantly higher in patients with STEMI complicated by CS compared with stable patients with STEMI. Cardiac death, CPR, LCOS, and stroke rates were higher in patients with STEMI with CS. Moreover, patients with STEMI complicated by CS required more inotropic, IABP, or ECMO support after CABG when compared with patients with STEMI without CS. In addition, the need for blood transfusions, rethoracotomy, and dialysis was increased in patients with STEMI complicated by CS, resulting in prolonged need for mechanical ventilation and length of stay at the intensive care unit.
Table 3.
Clinical outcomes of NSTEMI and STEMI patients stratified by CS
| NSTEMI (−) CS (n = 991) | NSTEMI (+) CS (n = 227) | P value | STEMI (−) CS (n = 375) | STEMI (+) CS (n = 243) | P value | |
|---|---|---|---|---|---|---|
| In‐hospital mortality, n (%) | 5.9 (58/991) | 15.4 (35/227) | <0.001 | 5.3 (20/375) | 23.9 (58/243)a | <0.001 |
| MACCE, n (%) | 12.8 (127/991) | 33.5 (76/227) | <0.001 | 14.9 (56/375) | 49.4 (120/243)a | <0.001 |
| Cardiac death | 3.8 (38/991) | 8.8 (20/227) | 0.003 | 3.2 (12/375) | 22.6 (55/243)a | <0.001 |
| LCOS | 7.6 (75/991) | 26.9 (61/227) | <0.001 | 12.0 (45/375) | 44.0 (107/243)a | <0.001 |
| PMI | 2.5 (25/991) | 2.6 (6/227) | 0.819 | 1.6 (6/375) | 2.5 (6/243) | 0.553 |
| CPR | 3.9 (39/991) | 7.5 (17/227) | 0.033 | 4.0 (15/375) | 9.1 (22/243) | 0.014 |
| Stroke | 2.8 (28/991) | 4.4 (10/227) | 0.209 | 1.3 (5/375) | 4.1 (10/243) | 0.034 |
| Inotropes >48 h, % (n) | 15.9 (174/672) | 59.1 (68/115) | <0.001 | 27.8 (86/309) | 66.5 (127/191) | <0.001 |
| IABP support, n (%) | 7.2 (91/988) | 38.5 (87/227) | <0.001 | 13.7 (51/373) | 55.1 (134/243)a | <0.001 |
| ECMO support, n (%) | 0.8 (8/987) | 2.2 (5/225) | 0.075 | 2.1 (8/373) | 7.8 (19/243)a | 0.001 |
| Rethoracotomy for bleeding, n (%) | 5.6 (55/988) | 6.6 (15/226) | 0.528 | 5.3 (20/374) | 10.5 (25/237) | 0.025 |
| >5 RBC units in 48 h, n (%) | 17.9 (122/681) | 28.8 (36/125) | 0.007 | 21.0 (66/315) | 38.7 (79/204) | <0.001 |
| Mechanical ventilation, h | 47 ± 100 | 123 ± 207 | <0.001 | 50 ± 118 | 112 ± 144 | <0.001 |
| Tracheostomy, n (%) | 5.3 (36/681) | 18.0 (22/122) | <0.001 | 9.8 (31/316) | 20.7 (42/203) | <0.001 |
| Dialysis, n (%) | 8.1 (80/986) | 19.1 (43/225) | <0.001 | 8.0 (30/374) | 28.3 (66/233) | <0.001 |
| ICU stay, d | 5 ± 6 | 8 ± 10 | <0.001 | 5 ± 7 | 8 ± 8 | <0.001 |
| Hospital stay, d | 13 ± 9 | 15 ± 13 | <0.001 | 13 ± 20 | 14 ± 14 | 0.834 |
Values are expressed as mean and standard deviation or percentages with counts as indicated. CPR indicates cardiopulmonary resuscitation; CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; ICU, intensive care unit; LCOS, low cardiac output syndrome; MACCE, major adverse cardiocerebral events; NSTEMI, non–STsegment–elevation myocardial infarction; PMI, perioperative myocardial infarction; RBC, red blood cell unit transfusion; STEMI, ST‐segment–elevation myocardial infarction.
Significant differences (P < 0.05) between NSTEMI and STEMI groups with CS that are provided in detail in the results section.
Figure 1.
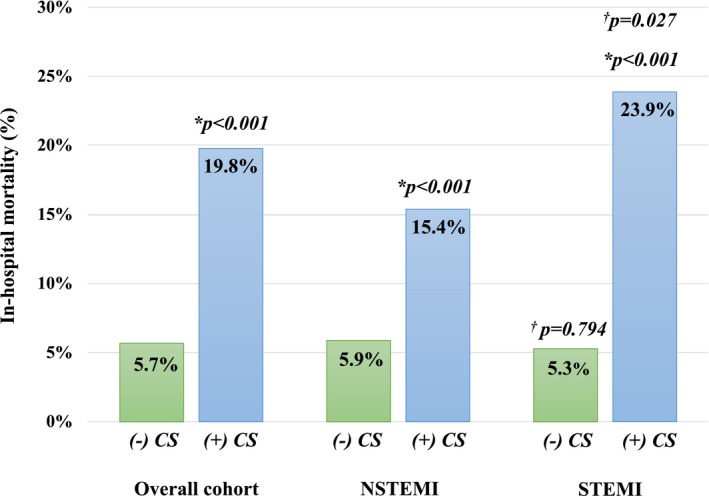
In‐hospital mortality stratified by the presence of cardiogenic shock. * indicates P value compared with the corresponding ACS group without CS; † indicates P value compared with the corresponding NSTEMI group; ACS indicates acute coronary syndrome; + or − CS, with or without cardiogenic shock; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Figure 2.
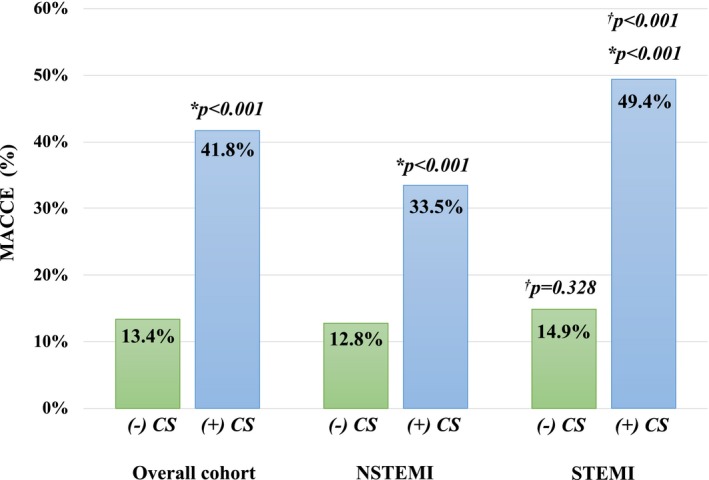
Major adverse cardiocerebral events (MACCEs) stratified by the presence of cardiogenic shock. * indicates P value compared with the corresponding ACS group without CS; † indicates P value compared with the corresponding NSTEMI group; + or − CS indicates with or without cardiogenic shock; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Direct comparison of NSTEMI and STEMI subgroups with CS showed a higher IHM and MACCE rate in patients with STEMI (Figures 1 and 2). Cardiac death (P<0.001), LCOS (P<0.001), and mechanical circulatory support (IABP, P<0.001; ECMO, P<0.005) were all significantly higher in patients with STEMI complicated by CS, compared with patients with NSTEMI complicated by CS. The impact of timing of surgery on IHM and MACCEs is presented in Figures S1 and S2. IHM and MACCEs were comparable between NSTEMI and STEMI subgroups with CS for the respective timing intervals. Similarly, timing of surgery had no impact on IHM or MACCE rates for patients with NSTEMI or STEMI without CS.
Predictors of Cardiogenic Shock
Key preoperative predictors for CS before CABG in the total cohort (Table 4) were younger age, peripheral vascular disease, LMD, STEMI, acute PCI, troponin levels >2‐fold of the ULN, a reduced LVEF, and a higher logistic EuroSCORE. For NSTEMI and STEMI subgroups, younger age, peripheral vascular disease, acute PCI, reduced LVEF, and a higher logistic EuroSCORE were predictive for CS. LMD and troponin levels >2‐fold of the ULN were identified as predictors for CS only in patients with STEMI. The area under the receiver operator characteristic curve was 0.86, indicating a good discrimination of the model with satisfactory calibration in the Lemeshow‐Hosmer goodness‐of‐fit test (χ2=12.3; P=0.137).
Table 4.
Preoperative predictors of CS
| Overall ACS cohort | NSTEMI | STEMI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value |
| Age (y) | 0.93 | 0.91–0.95 | <0.001 | 0.92 | 0.89–0.94 | <0.001 | 0.94 | 0.91–0.96 | <0.001 |
| Hyperlipidemia | 1.79 | 1.23–2.61 | 0.002 | 2.55 | 1.49–4.35 | 0.001 | |||
| Peripheral vascular disease | 3.92 | 2.15–7.14 | <0.001 | 4.78 | 2.14–10.8 | <0.001 | 3.92 | 2.15–7.14 | <0.001 |
| Atrial fibrillation | 3.60 | 1.31–9.88 | 0.013 | ||||||
| PCI within 24 h | 2.53 | 1.53–4.18 | <0.001 | 2.99 | 1.34–6.67 | 0.008 | 2.57 | 1.34–4.91 | 0.004 |
| Left mainstem disease | 1.68 | 1.16–2.44 | 0.006 | 1.65 | 0.99–2.74 | 0.054 | |||
| Troponin > 2x ULN | 2.20 | 1.26–3.85 | 0.006 | 3.25 | 1.45–7.30 | 0.004 | |||
| LVEF | 0.98 | 0.97–0.99 | <0.001 | 0.97 | 0.96–0.99 | 0.002 | 0.98 | 0.96–1.00 | 0.039 |
| STEMI | 1.94 | 1.35–2.80 | <0.001 | n.a. | n.a. | ||||
| Logistic EuroSCORE | 1.08 | 1.07–1.09 | <0.001 | 1.10 | 1.08–1.11 | <0.001 | 1.05 | 1.04–1.07 | <0.001 |
ACS indicates acute coronary syndrome; CS, cardiogenic shock; EuroSCORE, European System for Cardiac Operative Risk Evaluation; LVEF, left ventricular ejection fraction; NSTEMI, non–ST‐segment–elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; ULN, upper limit of normal.
Predictors of IHM and MACCE
Predictors of IHM (Table 5) in the overall cohort and in STEMI with CS were a reduced LVEF, a higher logistic EuroSCORE, CPB time, and lower aortic cross‐clamp time. History of smoking, prior myocardial infarction, CPB time, and aortic cross‐clamp time were independently predictive for IHM in patients with NSTEMI complicated by CS.
Table 5.
Predictors of IHM and MACCE in NSTEMI and STEMI patients with cardiogenic shock
| Overall cohort (+) CS | NSTEMI (+) CS | STEMI (+) CS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| In‐hospital Mortality | |||||||||
| Smoker | 3.06 | 1.12–8.33 | 0.029 | ||||||
| Prior MI | 2.90 | 1.37–6.15 | 0.005 | ||||||
| LVEF | 0.98 | 0.96–1.00 | 0.038 | 0.97 | 0.93–1.00 | 0.033 | |||
| Logistic EuroSCORE | 1.04 | 1.03–1.06 | <0.001 | 1.06 | 1.03–1.08 | <0.001 | |||
| CPB time (min) | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.02–1.05 | <0.001 | 1.03 | 1.02–1.04 | <0.001 |
| Aortic clamp time (min) | 0.96 | 0.95–0.98 | <0.001 | 0.97 | 0.94–0.99 | 0.003 | 0.96 | 0.94–0.98 | <0.001 |
| MACCE | |||||||||
| Diabetes mellitus | 3.13 | 1.29–7.61 | 0.012 | ||||||
| Peripheral vascular disease | 4.11 | 1.56–10.84 | 0.004 | 3.54 | 1.11–11.25 | 0.032 | 7.02 | 1.75–28.19 | 0.006 |
| CKD‐EPI Stage 3–4 | 2.06 | 1.14–3.71 | 0.016 | 2.93 | 1.37–6.28 | 0.006 | |||
| Prior MI | 4.21 | 1.51–11.73 | 0.006 | ||||||
| Prior stroke | 2.93 | 1.08–7.93 | 0.035 | ||||||
| LVEF | 0.97 | 0.95–0.98 | <0.001 | 0.96 | 0.93–0.98 | 0.001 | |||
| Troponin >2x ULN | 4.66 | 1.27–17.17 | 0.021 | ||||||
| CPB time (min) | 1.02 | 1.01–1.03 | <0.001 | 1.03 | 1.01–1.04 | <0.001 | 1.03 | 1.01–1.04 | <0.001 |
| Aortic clamp time (min) | 0.97 | 0.96–0.99 | 0.001 | 0.97 | 0.95–0.99 | 0.001 | 0.97 | 0.95–0.99 | 0.001 |
| Warm vs cold blood CP | 2.29 | 1.27–4.15 | 0.006 | 1.17 | 0.55–2.50 | 0.008 | |||
CKD‐EPI indicates Chronic Kidney Disease Epidemiology Collaboration; CP, cardioplegia; CPB, cardiopulmonary bypass; EuroSCORE, European System for Cardiac Operative Risk Evaluation; IHM, in‐hospital mortality; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiocerebral events; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; OR, odds ratio; STEMI, ST‐segment–elevation myocardial infarction; ULN, upper limit of normal.
Predictors of MACCE in the overall CS cohort were peripheral vascular disease, renal dysfunction (Chronic Kidney Disease Epidemiology Collaboration stage 3/4), prior stroke, a reduced LVEF, an elevated logistic EuroSCORE, and a troponin I increase of >2‐fold of the ULN before CABG. Intraoperative predictors of MACCEs were CPB time, aortic cross‐clamp time, and the use of warm blood cardioplegia. Peripheral vascular disease, CPB time, and aortic cross‐clamp time were common predictors of MACCEs in patients with NSTEMI and STEMI complicated by CS. Of note, the use of warm blood cardioplegia and a reduced LVEF were independent predictors of MACCEs only in patients with STEMI. Discrimination and calibration of the logistic regression model for IHM and MACCEs was good (IHM: area under the receiver operator characteristic curve area under the receiver operator characteristic curve=0.87; MACCE: area under the receiver operator characteristic curve=0.84; Lemeshow‐Hosmer goodness‐of‐fit test: IHM: χ2=9.5; P=0.31; MACCE: χ2=10.7; P=0.22).
Multivariable analysis of IHM and MACCE stratified by ACS subtypes are summarized in Table S3. Briefly, preoperative CS was one of the strongest predictors of IHM and MACCEs in the entire cohort (IHM: OR, 2.88; 95% CI, 1.63–5.09; P<0.001; MACCE: OR, 2.32; 95% CI, 1.57–3.43; P<0.001) and in patients with STEMI (IHM: OR, 2.90; 95% CI, 1.26–6.65; P=0.012; MACCE: OR, 3.88; 95% CI, 2.25–6.68; P<0.001), but not in patients with NSTEMI.
Discussion
Our all‐comers registry study demonstrates that cardiogenic shock is present in over 20% of patients referred to CABG surgery for NSTEMI or STEMI, and that surgical myocardial revascularization in CS continues to be linked to high rates of IHM and MACCE when compared with stable patients with ACS. Importantly, our analysis revealed distinct differences with respect to surgical outcomes between patients with NSTEMI and STEMI complicated by CS. Here, rates of IHM and MACCEs were both significantly higher in patients with STEMI complicated by CS when compared with patients with NSTEMI.
Cardiogenic Shock in Patients With NSTEMI and STEMI
Current guidelines still consider CABG to be a viable option for patients presenting with ACS complicated by CS. However, in contemporary practice, surgery is often considered as the last resort for myocardial revascularization. In the IABP‐SHOCK II (Intraaortic Balloon Pump in Cardiogenic Shock II) trial, only 3.3% of patients with CS were directly referred to surgery.5, 10 Registry data using the Society of Thoracic Surgeons Adult Cardiac Surgery Database demonstrated that 1% to 2% of all CABG patients present with CS before surgery and that this subgroup accounts for 14% of all CABG deaths.13 We found an overall CS rate of 26% in our exclusively surgical NSTEMI and STEMI cohort that was comparable to a previous single‐center study reporting 16%15 but higher than the usually observed CS rates of 6% to 10% in not‐exclusively‐surgical ACS populations.3, 10 In addition, patients with STEMI showed a significantly higher propensity to develop CS (39.3%) when compared with patients with NSTEMI (18.6%). Among other variables, the presence of a STEMI was independently predictive for the development of CS. The observed ratio of 2:1 for CS between STEMI and NSTEMI patients in our study was roughly comparable to the CULPRIT‐SHOCK (Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock) and IABP‐SHOCK II trial.4, 5 In contrast, the proportion of CS in STEMI or NSTEMI referred to CABG was up to 15‐fold higher in our registry and when compared with other STEMI (3%–8%)10, 20, 21 or NSTEMI cohorts (1–3%).22, 23 This observation suggests that patients with ACS‐related CS undergoing CABG often represent a negative selection with more complex CAD that is not amenable to PCI, as indicated by the higher rate of LMD or failed PCI, especially, in our STEMI group.
Operative Mortality and Major Adverse Events in ACS Subtypes
The overall IHM of 19.8% in our surgical registry was congruent with a recently reported 17.7% IHM from the nationwide Society of Thoracic Surgeons surgical database.14 Other studies that did not discriminate between ACS subtypes show rates of IHM between 22% and 41% for patients with CS.13, 24 In a retrospective analysis by the Leipzig group, operative mortality was almost halved within the past decade from 42% to 25% in patients with CS.15 Thus, optimal adherence to evidence‐based guidelines, including the use of early PCI and dual antiplatelet therapy,3 may account for improvements in operative survival as shown by the relatively low IHM reported from a recent Society of Thoracic Surgeons database analysis and by our registry data.14
Our study is unique in that it not only provides detailed information with regard to the current characteristics of surgical patients with CS, but it also demonstrates modern CABG strategies. We show that CABG is associated with higher IHM and MACCE rates in patients with STEMI complicated by CS and that preoperative CS was one of the strongest predictors of IHM and MACCE in this group. This confirms previous reports that also identified STEMI, albeit not NSTEMI, as predictive for IHM.15 While our registry data cannot provide a definite explanation for this observation, the lower extent of nontransmural myocardial injury and clinical acuity in NSTEMI patients with CS, as suggested by the preoperative markers for myocardial injury and the lower rate of emergency CABG compared with STEMI, may have translated to better outcomes.25, 26 Only a few surgical trials have separately reported outcomes of patients with NSTEMI and STEMI complicated by CS. In a review of the California Discharge Data from 1999 to 2005, Weiss et al27 demonstrated a comparable 24% IHM in CABG with cardiogenic shock and “transmural MI,” while a 43% operative mortality rate was found for STEMI with CS in a subanalysis of the SHOCK trial.8 For NSTEMI, a lower IHM of 3.8% to 5.1% after CABG surgery was demonstrated in 2 observational studies. However, the studies did not differentiate between patients with and without CS.28, 29 To the best of our knowledge, our registry data are unique in their ability to provide separate outcome data of patients with NSTEMI complicated by CS, indicating an ≈3‐fold increase in IHM compared with stable patients with NSTEMI. In our NSTEMI cohort without CS, IHM was 5.9% and comparable to a previously reported IHM rate of 4.6%.28
Timing of Surgery and Surgical Strategy
Another focus of our multicenter registry was the preoperative therapy, timing, and specific surgical technique applied in each ACS subgroup. Dual antiplatelet therapy was present in over 50% of patients and comparable among all groups. Despite this higher dual antiplatelet therapy rate compared with other surgical cohorts,15, 30 our rethoracotomy rate was similar to previous reports. Furthermore, preoperative IABP therapy was implemented in 32% of patients and increased in patients with CS after surgery. Although our data confirm a decline of preoperative IABP use in CS, as also shown by Davierwala et al,15 IABP therapy remains an accepted treatment option for numerous cardiac surgeons in the post IABP‐SHOCK II trial era.5, 31 Of note, preoperative IABP use was not found to be predictive for IHM in our overall ACS cohort (OR, 0.686; 95% CI, 0.312–1.506; P=0.347).
In contrast to PCI, the evidence derived from surgical trials aiming to elucidate optimal timing to operate on patients with NSTEMI or STEMI complicated by CS is scarce. While the substantial benefits of early surgical revascularization are well documented for patients with STEMI in the SHOCK trial,6 the optimal surgical timing, especially for patients with NSTEMI complicated by CS remains elusive.1, 2, 7 Emergency (<24 hours) or early (<72 hours) CABG was associated with lower survival and was a predictor of early mortality in studies that did not discriminate between ACS subtypes.27, 30, 32, 33 For NSTEMI, no differences in IHM were found between emergency and late surgery (<24 hours versus >72 hours).28, 29 Comparable to previous reports,14, 27 51% of our patients with CS underwent emergent CABG and 77% were operated within 72 hours of onset of symptoms. Indicative for the delay in the decision pathway until referral of patients to surgery is the fact that the rate of emergency CABG increased to 72% when considering only the time interval between admission to the cardiac surgery unit and skin incision (door‐to‐CABG time). Thus, the main reason for postponed surgery can be found in the delayed referral of patients from external hospitals, where patients were initially admitted. Importantly, we also show that emergency CABG was more frequently required in patients with STEMI complicated by CS. While this observation implies a higher degree of clinical acuity, when compared with NSTEMI patients,27, 32, 33 emergency surgery had no impact on IHM or MACCE rates between CS subgroups.
Another aspect of our registry was the analysis of surgical techniques in patients who developed CS. This was driven by the results from a few studies showing better outcomes of off‐pump or on‐pump beating‐heart surgery when compared with conventional on‐pump surgery with cardioplegic cardiac arrest. Rastan et al16 demonstrated a decrease of IHM from 33% to 19% with on‐pump beating‐heart surgery compared with on‐pump CABG using cardioplegia in patients with CS, while beating‐heart surgery was not predictive for improved survival in this study. In a report of the Japan Adult Cardiovascular Surgery Database, off‐pump surgery was predominantly performed in low‐risk ACS patients, whereas the rate of on‐pump beating‐heart surgery increased in the higher‐risk cohort that included patients with CS.17 This trend was also echoed in our subgroup comparison, along with lower rates in the use of arterial grafts in CS.15, 32, 34 The rapid availability of vein grafts facilitates a faster coronary reperfusion in salvage or emergent CABG situations when compared with the more time‐consuming harvesting of arterial conduits. Consequently, our data demonstrate that on‐pump surgery with cardioplegic cardiac arrest, single internal thoracic artery use, and multiple venous grafting is still considered the safest strategy in patients with CS. The latter is also supported by the fact that neither the surgical revascularization technique nor the use of multiple arterial grafts were predictive for IHM and MACCE.
Limitations
When compared with well‐controlled clinical trials, the quality of our all‐comers, multicenter registry data set is not as good and not specifically powered for the investigated outcome measures. Long‐term clinical outcomes were not captured in our registry. Completeness of data items varied as indicated in the method section and within the counts of our tables. Thus, missing data may have influenced our results, despite our efforts to acquire all prespecified data items. In addition, early treatment decisions for ACS (PCI, dual antiplatelet therapy, etc) and possible time delays until referral to the cardiac surgery unit were beyond the control of the participating centers. Furthermore, completeness of myocardial revascularization was not acquired in our registry. It is therefore not possible to exclude differences among subgroups that may have influenced our outcome measures. Finally, we cannot rule out an institutional bias with regard to the timing of surgery and the applied surgical revascularization technique among the participating centers. In contrast to the predominantly retrospective, single‐center design of existing surgical trials in the literature, our large sample size and multicenter approach may have minimized this bias as much as possible.
Conclusion
Our registry study demonstrates that >20% of patients referred to CABG surgery with NSTEMI or STEMI are in CS. We confirm that surgical revascularization in this high‐risk cohort is associated with a substantial but not prohibitive rate of IHM and MACCE. In light of these outcomes, we advocate that CABG should remain a viable option in patients with CS who are not amenable to primary reperfusion with PCI. Distinct differences with respect to surgical outcomes were observed for patients with NSTEMI and STEMI complicated by CS, including a higher rate of IHM and MACCE in patients with STEMI. Timing of surgery and surgical revascularization technique had no impact on postoperative adverse outcomes.
Disclosures
None.
Supporting information
Table S1. Preoperative Variables Entered Into the Logistic Regression Model for Cardiogenic Shock
Table S2. Pre‐ and Intraoperative Variables Entered Into the Logistic Regression Model for In‐Hospital Mortality and MACCE
Table S3. Predictors of IHM and MACCE in ACS Subgroups
Figure S1. In‐hospital mortality stratified by the timing of surgery from onset of symptoms.
Figure S2. Major adverse cardio‐cerebral events (MACCE) stratified by the timing of surgery from onset of symptoms.
Acknowledgments
The authors thank all investigators of the participating centers cordially for their dedicated efforts in this investigator‐initiated registry.
(J Am Heart Assoc. 2019;8:e012049. DOI: 10.1161/JAHA.119.012049.)
References
- 1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P; ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 2. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 3. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research . Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 4. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer‐Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Savonitto S, Torremante P, Vrints C, Schneider S, Desch S, Zeymer U; CULPRIT‐SHOCK Investigators . PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. [DOI] [PubMed] [Google Scholar]
- 5. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Bohm M, Ebelt H, Schneider S, Schuler G, Werdan K; IABP‐SHOCK II Trial Investigators . Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. [DOI] [PubMed] [Google Scholar]
- 6. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625–634. [DOI] [PubMed] [Google Scholar]
- 7. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 8. White HD, Assmann SF, Sanborn TA, Jacobs AK, Webb JG, Sleeper LA, Wong CK, Stewart JT, Aylward PE, Wong SC, Hochman JS. Comparison of percutaneous coronary intervention and coronary artery bypass grafting after acute myocardial infarction complicated by cardiogenic shock: results from the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial. Circulation. 2005;112:1992–2001. [DOI] [PubMed] [Google Scholar]
- 9. Mehta RH, Lopes RD, Ballotta A, Frigiola A, Sketch MH, Bossone E, Bates ER. Percutaneous coronary intervention or coronary artery bypass surgery for cardiogenic shock and multivessel coronary artery disease? Am Heart J. 2010;159:141–147. [DOI] [PubMed] [Google Scholar]
- 10. Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, Fonarow GC. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST‐elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590 DOI: 10.1161/JAHA.113.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Auffret V, Cottin Y, Leurent G, Gilard M, Beer JC, Zabalawi A, Chague F, Filippi E, Brunet D, Hacot JP, Brunel P, Mejri M, Lorgis L, Rouault G, Druelles P, Cornily JC, Didier R, Bot E, Boulanger B, Coudert I, Loirat A, Bedossa M, Boulmier D, Maza M, Le Guellec M, Puri R, Zeller M, Le Breton H; ORBI and RICO Working Groups . Predicting the development of in‐hospital cardiogenic shock in patients with ST‐segment elevation myocardial infarction treated by primary percutaneous coronary intervention: the ORBI risk score. Eur Heart J. 2018;39:2090–2102. [DOI] [PubMed] [Google Scholar]
- 12. Dangas G, Guedeney P. Prediction, staging, and outcomes of ischaemic cardiogenic shock after STEMI: a complex clinical interplay. Eur Heart J. 2018;39:2103–2105. [DOI] [PubMed] [Google Scholar]
- 13. Mehta RH, Grab JD, O'Brien SM, Glower DD, Haan CK, Gammie JS, Peterson ED; Society of Thoracic Surgeons National Cardiac Database Investigators . Clinical characteristics and in‐hospital outcomes of patients with cardiogenic shock undergoing coronary artery bypass surgery: insights from the Society of Thoracic Surgeons National Cardiac Database. Circulation. 2008;117:876–885. [DOI] [PubMed] [Google Scholar]
- 14. Cox ML, Gulack BC, Thibault DP, He X, Williams ML, Thourani VH, Jacobs JP, Brennan JM, Daneshmand MA, Acharya D. Outcomes after coronary artery bypass grafting in patients with myocardial infarction, cardiogenic shock and unresponsive neurological state: analysis of the Society of Thoracic Surgeons Database. Eur J Cardiothorac Surg. 2018;54:710–716. [DOI] [PubMed] [Google Scholar]
- 15. Davierwala PM, Leontyev S, Verevkin A, Rastan AJ, Mohr M, Bakhtiary F, Misfeld M, Mohr FW. Temporal trends in predictors of early and late mortality after emergency coronary artery bypass grafting for cardiogenic shock complicating acute myocardial infarction. Circulation. 2016;134:1224–1237. [DOI] [PubMed] [Google Scholar]
- 16. Rastan AJ, Eckenstein JI, Hentschel B, Funkat AK, Gummert JF, Doll N, Walther T, Falk V, Mohr FW. Emergency coronary artery bypass graft surgery for acute coronary syndrome: beating heart versus conventional cardioplegic cardiac arrest strategies. Circulation. 2006;114:I477–I485. [DOI] [PubMed] [Google Scholar]
- 17. Kawamoto S, Miyata H, Motomura N, Tanemoto K, Takamoto S, Saiki Y. Surgical outcomes of isolated coronary artery bypass grafting for acute coronary syndrome—based on the Japan Adult Cardiovascular Surgery Database. Circ J. 2017;82:123–130. [DOI] [PubMed] [Google Scholar]
- 18. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force of Universal Definition of Myocardial Infraction . Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 19. Thielmann M, Sharma V, Al‐Attar N, Bulluck H, Bisleri G, Bunge JJH, Czerny M, Ferdinandy P, Frey UH, Heusch G, Holfeld J, Kleinbongard P, Kunst G, Lang I, Lentini S, Madonna R, Meybohm P, Muneretto C, Obadia JF, Perrino C, Prunier F, Sluijter JPG, Van Laake LW, Sousa‐Uva M, Hausenloy DJ. ESC Joint Working Groups on Cardiovascular Surgery and the Cellular Biology of the Heart Position Paper: perioperative myocardial injury and infarction in patients undergoing coronary artery bypass graft surgery. Eur Heart J. 2017;38:2392–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, James S, Kellerth T, Lindahl B, Ravn‐Fischer A, Rydberg E, Yndigegn T, Jernberg T. Improved outcomes in patients with ST‐elevation myocardial infarction during the last 20 years are related to implementation of evidence‐based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur Heart J. 2017;38:3056–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scholz KH, Maier SKG, Maier LS, Lengenfelder B, Jacobshagen C, Jung J, Fleischmann C, Werner GS, Olbrich HG, Ott R, Mudra H, Seidl K, Schulze PC, Weiss C, Haimerl J, Friede T, Meyer T. Impact of treatment delay on mortality in ST‐segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT‐STEMI trial. Eur Heart J. 2018;39:1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, James S, Kellerth T, Lindahl B, Ravn‐Fischer A, Rydberg E, Yndigegn T, Jernberg T. Relations between implementation of new treatments and improved outcomes in patients with non‐ST‐elevation myocardial infarction during the last 20 years: experiences from SWEDEHEART registry 1995 to 2014. Eur Heart J. 2018;39:3766–3776. [DOI] [PubMed] [Google Scholar]
- 23. Kolte D, Khera S, Dabhadkar KC, Agarwal S, Aronow WS, Timmermans R, Jain D, Cooper HA, Frishman WH, Menon V, Bhatt DL, Abbott JD, Fonarow GC, Panza JA. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating non‐ST‐elevation myocardial infarction. Am J Cardiol. 2016;117:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Hussain F, Philipp RK, Ducas RA, Elliott J, Džavík V, Jassal DS, Tam JW, Roberts D, Garber PJ, Ducas J. The ability to achieve complete revascularization is associated with improved in‐hospital survival in cardiogenic shock due to myocardial infarction: Manitoba cardiogenic SHOCK Registry Investigators. Catheter Cardiovasc Interv. 2011;78:540–548. [DOI] [PubMed] [Google Scholar]
- 25. Thielmann M, Massoudy P, Neuhauser M, Tsagakis K, Marggraf G, Kamler M, Mann K, Erbel R, Jakob H. Prognostic value of preoperative cardiac troponin I in patients undergoing emergency coronary artery bypass surgery with non‐ST‐elevation or ST‐elevation acute coronary syndromes. Circulation. 2006;114:I448–I453. [DOI] [PubMed] [Google Scholar]
- 26. Jacobs AK, French JK, Col J, Sleeper LA, Slater JN, Carnendran L, Boland J, Jiang X, LeJemtel T, Hochman JS. Cardiogenic shock with non‐ST‐segment elevation myocardial infarction: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36:1091–1096. [DOI] [PubMed] [Google Scholar]
- 27. Weiss ES, Chang DD, Joyce DL, Nwakanma LU, Yuh DD. Optimal timing of coronary artery bypass after acute myocardial infarction: a review of California discharge data. J Thorac Cardiovasc Surg. 2008;135:503–511. [DOI] [PubMed] [Google Scholar]
- 28. Davierwala PM, Verevkin A, Leontyev S, Misfeld M, Borger MA, Mohr FW. Does timing of coronary artery bypass surgery affect early and long‐term outcomes in patients with non–ST‐segment–elevation myocardial infarction? Circulation. 2015;132:731–740. [DOI] [PubMed] [Google Scholar]
- 29. Parikh SV, de Lemos JA, Jessen ME, Brilakis ES, Ohman EM, Chen AY, Wang TY, Peterson ED, Roe MT, Holper EM. Timing of in‐hospital coronary artery bypass graft surgery for non‐ST‐segment elevation myocardial infarction patients results from the National Cardiovascular Data Registry ACTION Registry‐GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry‐Get With The Guidelines). JACC Cardiovasc Interv. 2010;3:419–427. [DOI] [PubMed] [Google Scholar]
- 30. Nichols EL, McCullough JN, Ross CS, Kramer RS, Westbrook BM, Klemperer JD, Leavitt BJ, Brown JR, Olmstead E, Hernandez F, Sardella GL, Frumiento C, Malenka D, DiScipio A; Northern New England Cardiovascular Disease Study Group . Optimal timing from myocardial infarction to coronary artery bypass grafting on hospital mortality. Ann Thorac Surg. 2017;103:162–171. [DOI] [PubMed] [Google Scholar]
- 31. Deppe AC, Weber C, Liakopoulos OJ, Zeriouh M, Slottosch I, Scherner M, Kuhn EW, Choi YH, Wahlers T. Preoperative intra‐aortic balloon pump use in high‐risk patients prior to coronary artery bypass graft surgery decreases the risk for morbidity and mortality—a meta‐analysis of 9,212 patients. J Card Surg. 2017;32:177–185. [DOI] [PubMed] [Google Scholar]
- 32. Axelsson TA, Mennander A, Malmberg M, Gunn J, Jeppsson A, Gudbjartsson T. Is emergency and salvage coronary artery bypass grafting justified? The Nordic Emergency/Salvage coronary artery bypass grafting study. Eur J Cardiothorac Surg. 2016;49:1451–1456. [DOI] [PubMed] [Google Scholar]
- 33. Lee DC, Oz MC, Weinberg AD, Lin SX, Ting W. Optimal timing of revascularization: transmural versus nontransmural acute myocardial infarction. Ann Thorac Surg. 2001;71:1198–1204. [DOI] [PubMed] [Google Scholar]
- 34. Ito H, Mizumoto T, Tempaku H, Fujinaga K, Sawada Y, Teranishi S, Shimpo H. Emergency off‐pump coronary artery bypass graft surgery for patients on preoperative intraaortic balloon pump. Ann Thorac Surg. 2016;102:821–828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Preoperative Variables Entered Into the Logistic Regression Model for Cardiogenic Shock
Table S2. Pre‐ and Intraoperative Variables Entered Into the Logistic Regression Model for In‐Hospital Mortality and MACCE
Table S3. Predictors of IHM and MACCE in ACS Subgroups
Figure S1. In‐hospital mortality stratified by the timing of surgery from onset of symptoms.
Figure S2. Major adverse cardio‐cerebral events (MACCE) stratified by the timing of surgery from onset of symptoms.


