ABSTRACT
In addition to SecA of the general Sec system, many Gram-positive bacteria, including mycobacteria, express SecA2, a second, transport-associated ATPase. SecA2s can be subdivided into two mechanistically distinct types: (i) SecA2s that are part of the accessory Sec (aSec) system, a specialized transporter mediating the export of a family of serine-rich repeat (SRR) glycoproteins that function as adhesins, and (ii) SecA2s that are part of multisubstrate systems, in which SecA2 interacts with components of the general Sec system, specifically the SecYEG channel, to export multiple types of substrates. Found mainly in streptococci and staphylococci, the aSec system also contains SecY2 and novel accessory Sec proteins (Asps) that are required for optimal export. Asp2 also acetylates glucosamine residues on the SRR domains of the substrate during transport. Targeting of the SRR substrate to SecA2 and the aSec translocon is mediated by a specialized signal peptide. Multisubstrate SecA2 systems are present in mycobacteria, corynebacteria, listeriae, clostridia, and some bacillus species. Although most substrates for this SecA2 have canonical signal peptides that are required for export, targeting to SecA2 appears to depend on structural features of the mature protein. The feature of the mature domains of these proteins that renders them dependent on SecA2 for export may be their potential to fold in the cytoplasm. The discovery of aSec and multisubstrate SecA2 systems expands our appreciation of the diversity of bacterial export pathways. Here we present our current understanding of the mechanisms of each of these SecA2 systems.
INTRODUCTION
The protein export systems of bacteria deliver proteins from the cytoplasm to the cell envelope or extracellular environment, and in doing so, they play critical roles in bacterial physiology and pathogenesis. In bacteria, the majority of protein export is carried out by the general Sec system (1, 2). The core components of the Sec system are the integral membrane proteins SecY, SecE, and SecG, which form the SecYEG channel through which unfolded proteins traverse the membrane, and the SecA ATPase, which provides energy for export (Fig. 1A). SecA shuttles between the cytoplasm and SecYEG in its role in export. SecDFYajC are auxiliary components that enhance export efficiency. Proteins exported by the Sec pathway are synthesized as preproteins with N-terminal signal peptides that are recognized by the Sec machinery and removed during export to produce the mature protein. Some Gram-positive bacteria, including high-GC Gram-positive actinobacteria such as mycobacteria, possess two SecA proteins. In these cases, SecA (sometimes called SecA1) is the canonical SecA of the Sec pathway, while SecA2 functions in a specialized pathway that exports one or a few proteins. There are at least two evolutionarily and mechanistically distinct types of SecA2 systems: the accessory Sec (aSec) system, which has also been referred to as the SecA2/SecY2 system, and the multisubstrate SecA2 system, which was initially called the SecA2-only system.
FIGURE 1.
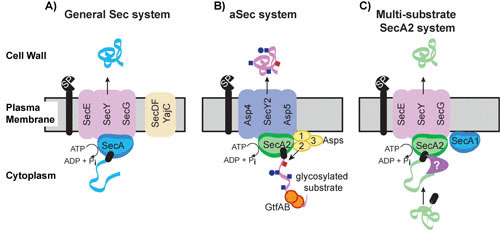
Models for the general Sec system, the aSec system, and the multisubstrate SecA2 system. (A) General Sec system. SecA uses ATP hydrolysis to export cytoplasmic preproteins through the SecYEG channel in an unfolded state. SecDFYajC are auxiliary components that enhance export efficiency. Sec signal peptides (black rectangle) target preproteins (blue ribbon) for export through SecYEG. Following export across the membrane, the signal peptide is cleaved by a signal peptidase (SP) and the resulting mature protein folds into its proper conformation. (B) aSec system. The model depicted is largely based on studies of the S. gordonii SecA2 system. Glycosylation of the preprotein (pink ribbon) with GlcNAc (blue squares) and Glc (blue circles) likely occurs cotranslationally. The positively charged N region of the signal peptide (black rectangle) targets the preprotein to anionic phospholipids, which aids the localization with SecA2. Transport through the SecY2/Asp4/5 channel requires a specific sequence in the mature region of the preprotein, as well as Asp1 to Asp3. Asp2 is a bifunctional protein that also mediates O-acetylation of GlcNAc moieties (red square). Cleavage of the signal peptide is thought to be carried out by the general SP. (C) Multisubstrate SecA2 system. The model depicted is largely based on studies of the mycobacterial SecA2 system. SecA2 works with the canonical SecYEG channel and possibly SecA1 to export its specific subset of preproteins (green ribbon). The majority of SecA2 substrates are synthesized as preproteins with a signal peptide (black rectangle) that is cleaved in association with export. The mature domain, not the signal peptide, of a preprotein determines if a protein is exported by this SecA2 system. It is proposed that the mature domain of a SecA2 substrate has the propensity to fold in the cytoplasm and that the role of SecA2 is to facilitate the export of such proteins, in an unfolded state, through the SecYEG channel. Additional factors are likely to work with SecA2 in the pathway (purple symbol). The role of SecA2 in exporting moonlighting proteins that lack signal peptides is unclear and not depicted in the model.
ACCESSORY SEC SYSTEM
Many species of Gram-positive bacteria express an aSec system. Along with SecA2, the aSec system invariably includes SecY2 (a paralogue of SecY) and three to five accessory Sec proteins (Asps) (Fig. 1B) (3). The latter proteins are essential for substrate transport and are exclusively associated with aSec systems (4, 5). aSec systems transport large, heavily glycosylated cell wall-anchored proteins, known as serine-rich repeat (SRR) glycoproteins (6–8). These substrates undergo extensive O-linked glycosylation intracellularly prior to their transport to the bacterial cell surface, where they function as adhesins important for commensal and pathogenic behavior (9–19).
The gene organization of aSec loci is highly conserved across species and genera (Fig. 2A). Along with the transport components, each aSec locus typically encodes one transported substrate (although up to three have been described) and two or more glycosyltransferase (Gtf) proteins that modify the preprotein in the cytoplasm prior to export (3, 20–22). It is not entirely clear why a dedicated system is necessary for the export of the SRR glycoproteins. One longstanding explanation is that the aSec system transports these unusual substrates because the canonical SecA or SecYEG cannot accommodate glycosylated proteins. Indeed, many aSec substrates cannot undergo canonical Sec transport if glycosylated (23, 24). As discussed below, however, recent studies indicate a more complex role for the aSec system in coordinating transport and posttranslational modification of the SRR glycoprotein, thereby ensuring proper adhesin function.
FIGURE 2.
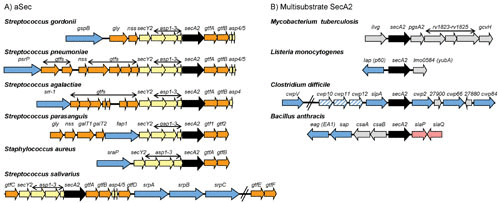
Genomic regions encoding aSec and multisubstrate SecA2 proteins. (A) aSec loci. Shown are representative aSec loci in Gram-positive bacteria. The secA2 gene is shown in black and the other genes encoding core components of the aSec translocase (SecY2 and Asps) are colored yellow. Genes encoding glycosyltransferases (Gtf) and proteins involved in carbohydrate modifications are shown in orange. Genes encoding exported SecA2 substrates are shown in blue. In Streptococcus parasanguis, the Asp orthologues are called Gap1 to Gap3. In Streptococcus salivarius, the gtfEF genes are located distal to the secA2 locus but are required for the first step of O-GlcNAcylation of the substrate (89) and thus may be functionally analogous to the gtfAB pairs found in other aSec loci. (B) Multisubstrate SecA2 loci. Shown are representative multisubstrate secA2 genes and neighboring genomic regions in Gram-positive bacteria. The secA2 gene is shown in black, and genes encoding SecA2 substrates are shown in blue. Candidate genes for additional SecA2 substrates are shown with blue stripes. Substrates encoded elsewhere in the genome are not shown. Additional proteins with roles in SecA2-dependent export are encoded by genes shown in pink. Genes encoding proteins with no known connection to export are shown in gray.
Substrates of the aSec System
The SRR glycoproteins comprise a unique family of adhesins that bind a wide range of ligands and impact biofilm formation and virulence (10, 12, 18, 19, 25–29). The adhesins have a conserved domain organization, with a 90-amino-acid signal peptide at the N terminus followed by a short SRR domain, a ligand binding region (BR), a long SRR domain, and a C-terminal LPXTG cell wall anchoring motif (Fig. 3). The BRs can vary significantly, reflecting their considerable repertoire of ligands. For example, several species of oral streptococci express SRR adhesins with “Siglec (sialic acid-binding immunoglobulin-type lectins)-like” binding regions that mediate binding to sialoglycans (30, 31), while Streptococcus agalactiae expresses SRR glycoproteins that interact with proteins (e.g., human keratin 4 and fibrinogen) (16, 32, 33). This diversity of ligands most likely reflects specific targets for microbial adhesion in different biological niches.
FIGURE 3.
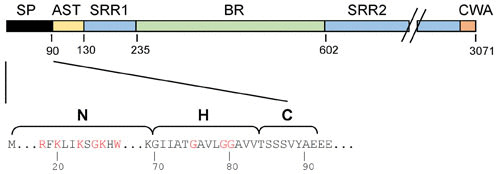
GspB domains and features of the N-terminal signal peptide (SP). (Top) Domains of the SRR glycoprotein GspB. AST, aSec transport domain; SRR1 and SRR2, serine-rich repeat regions 1 and 2, respectively; BR, ligand binding region; CWA, cell wall-anchoring domain. The CWA includes a transmembrane segment, an LPxTG motif, and a charged C-terminal tail (90). (Bottom) The GspB signal peptide has the tripartite structure of canonical signal peptides: the N-terminal (N), hydrophobic core (H), and cleavage (C) regions. However, the N region is substantially longer than typical signal peptides and includes a KxYKxGKxW motif (red). Glycine residues in the H region are also indicated in red.
Preprotein Recognition and Trafficking to the aSec System
The preprotein signal peptide of aSec substrates has a tripartite structure similar to that of general Sec system substrates, but the N region is approximately three times longer and includes a KxYKxGKxW motif (Fig. 3). This polybasic motif, along with additional basic residues in the extended N region, aids in targeting of the preprotein to anionic phospholipid patches in the membrane and is important for the Asp-independent colocalization of the preprotein with SecA2 (34). The hydrophobic core (the H region) of the signal peptide is also important for trafficking to the aSec system and contains three glycine residues essential for substrate delivery to the aSec pathway and away from the canonical Sec system.
In addition to the signal peptide, the SRR adhesin GspB of Streptococcus gordonii has a specific segment (the accessory Sec transport [AST] domain) at the amino terminus of the mature region that is required for transport. Deletion of the AST domain abolishes aSec export (24, 35), and even single amino acid substitutions within the domain can impair this process. The AST domain interacts directly with SecA2 during transport (36), which affects substrate targeting to the translocon and perhaps opening of the Y2 channel. The requirement of a specific segment in the mature region of the preprotein, along with the involvement of the Asps (see below), is a unique feature of aSec transport that may ensure the selectivity of this pathway for SRR glycoproteins.
The aSec Translocase
SecA2 proteins belonging to the aSec system have a 45-amino-acid truncation of the C-terminal domain (CTD), compared with canonical SecAs, and typically have a proline residue at the C terminus (3) (Fig. 4). These SecA2 proteins have 70% similarity (35 to 40% identity) to SecA of Escherichia coli, which includes a high similarity in the preprotein cross-linking domain (PPXD) and the nucleotide binding motifs NBD1 and NBD2 (3). In S. gordonii, SecA2 has a lower basal rate of ATP hydrolysis than its SecA paralogue, and SecA2 requires higher magnesium concentrations for activity (37). These and other findings indicate that streptococcal SecA2 may be more tightly regulated than SecA, which supports the possibility that one or more of the Asps may be required to stimulate ATP binding or hydrolysis, as discussed below.
FIGURE 4.
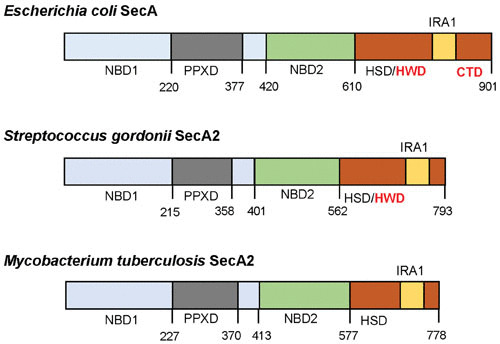
Domain organization in the canonical SecA of Escherichia coli and SecA2 proteins of S. gordonii and M. tuberculosis. Domains were identified in SecA2 proteins by alignment with E. coli SecA using published domain boundaries (91). NBD, nucleotide binding domain; PPXD, preprotein cross-linking domain; HSD, helical scaffold domain; HWD, helical wing domain; IRA, intramolecular regulator of ATPase activity; CTD, C-terminal domain. Compared to the canonical SecA, SecA2 proteins have deletions in the HWD and CTD regions. Amino acid number in the protein sequence is shown below each schematic.
SecY2 likely forms the transmembrane channel for aSec transport and likely functions similarly to SecY (38, 39). The predicted topology of SecY2 is nearly identical to that of SecY (3), even though SecY2 homologues have low primary sequence similarity to the SecY paralogues (20% identity and 60% similarity). Like its paralogue, SecY2 is likely to interact directly with SecA2 to mediate transport (35). It remains unclear how SecY2 can transport an extensively glycosylated protein, in contrast to SecY.
In most species of streptococci with aSec systems, one or two additional small proteins (Asp4 and Asp5) are likely to form a complex with SecY2 in vivo (5, 39). Although the roles of Asp4 and Asp5 in transport are uncertain, these proteins are predicted to have structural features resembling those of SecE and SecG of other organisms, respectively, suggesting analogous functions. In complex with SecY2, these proteins enhanced the ATPase activity of streptococcal SecA2 in proteoliposomes, paralleling the effects of SecYEG on SecA (39). Asp4 is partially dispensable for the export of truncated or nonglycosylated GspB variants via the aSec route (40), consistent with a role for Asp4 in stabilizing the open state of the transmembrane channel, rather than a role in the initiation of translocation. Some species lack Asp5 (e.g., S. agalactiae) or both Asp4 and Asp5 (e.g., Staphylococcus aureus). It is possible that members of the canonical system substitute for these Asps, and indeed, in S. aureus, there is evidence for interaction between SecY2 and SecG (41).
Asp1 to Asp3
Asp1 to Asp3 are invariable components of the aSec system and are essential for substrate transport by this pathway. These Asps are located in the cytosol but have an affinity for anionic lipids and can localize as a complex with SecA2 at the inner membrane (38, 42, 43). Although Asp1 to Asp3 lack homology to other transport-associated proteins and their roles in aSec transport are not well defined, their interactions provide some insights as to function. Asp2 and Asp3 directly bind the SRR regions of the GspB preprotein (44). This interaction does not require glycosylation of the SRR domain or specific amino acid motifs. Instead, Asp2 and Asp3 appear to recognize the unstructured or nonfolded sections of the preprotein. Although these Asps bind GspB directly, they do not seem to function as conventional chaperones, since they are not required for GspB stability or targeting to the membrane or translocon (42, 43). However, these Asps augment the physical engagement of the AST domain of substrates with SecA2, as indicated by more extensive AST domain-SecA2 cross-linking in vivo, when Asp1 to Asp3 are present (35). Since these interactions are essential for aSec transport, one key role of Asp1 to Asp3 appears to be the enhancement of substrate interactions with the motor protein.
aSec Transport and Posttranslational Modification Are Coordinated Processes
Glycosylation and transport of aSec substrates were initially viewed as independent and sequential processes. Recent studies indicate, however, that these events are coordinated to ensure the proper posttranslational modification and function of the SRR glycoproteins. In addition to its role in transport, Asp2 has been shown to be an acetyltransferase that modifies N-acetylglucosamine moieties on the SRR domains of GspB (45). Targeted mutations of the predicted Asp2 catalytic domain had no effect on transport but abolished acetylation of GspB. Moreover, acetylated GspB was detected only when the glycoprotein had undergone aSec transport, not among cytosolic forms (when aSec transport was blocked) or when GspB was engineered to undergo canonical Sec transport. Thus, Asp2 is a bifunctional protein involved in both the posttranslational modification and transport of SRR glycoproteins. Moreover, these processes appear to be coordinated during the biogenesis of SRR glycoproteins, such that the adhesin is optimally modified for binding. This requirement to couple substrate modification and export may explain the coevolution of the SRR glycoproteins with their specialized glycan modification and export systems.
MULTISUBSTRATE SecA2 SYSTEMS
Multisubstrate SecA2 systems export more than one substrate, although the number of exported substrates is still small compared to that of the general Sec system. The multisubstrate SecA2 systems of Mycobacterium tuberculosis (46, 47), Listeria monocytogenes (48, 49), and likely Bacillus anthracis (50, 51) are required for pathogenesis. In Corynebacterium glutamicum (52) and Clostridium difficile (53) the multisubstrate systems are essential for bacterial viability. There is no SecY2 in multisubstrate SecA2 systems. Instead, the canonical SecYEG channel is used (Fig. 1C) (54, 55). A common finding across multisubstrate systems is that secA2 mutations diminish but do not completely abolish export of SecA2 substrates (56–59). Given that SecA2 works with SecYEG, the residual export observed in the absence of SecA2 may be attributable to the general Sec pathway, although this is unproven.
Unlike for aSec systems, phylogenetic analysis of multisubstrate SecA2 proteins and the genomic regions flanking the secA2 gene do not indicate evolutionary relatedness (Fig. 2B) (3). Thus, there is risk in assuming that there is a single type of multisubstrate SecA2 system with a common mechanism. Nonetheless, there are some intriguing similarities between systems, such that multisubstrate SecA2 systems might be examples of convergent evolution.
Substrates of Multisubstrate SecA2 Systems
Proteomics has been the primary method for identifying substrates of multisubstrate SecA2 systems (48, 56, 58, 60, 61). Proteins exported by multisubstrate systems exhibit a relatively wide variety of functions, with some common themes. Recently, the multisubstrate SecA2 systems of M. tuberculosis and L. monocytogenes were identified as functioning in RNA secretion as well as protein export (62, 63). While the role for SecA2 in secreting RNA is a complete mystery, this discovery emphasizes the substrate diversity of multisubstrate systems.
Actinobacteria (mycobacteria and corynebacteria)
At least 15 mycobacterial proteins clearly depend on SecA2 for their export to the cell wall or extracellular environment (56, 58, 60). While no corynebacterial SecA2 substrates have been identified, the essentiality of secA2 in C. glutamicum predicts SecA2 substrates with vital functions in this species.
In mycobacteria, one category of SecA2 substrates is cell wall proteins involved in importing solutes, such as solute binding proteins (SBPs) and Mce proteins (56, 60). SBPs deliver solutes to ABC transporters in the membrane (64), and Mce proteins are thought to deliver lipids to Mce transporters (65). A second category of SecA2 substrates is proteins with roles in growth and survival of mycobacteria in macrophages, such as SapM, PknG, and LipO, which prevent delivery of mycobacteria to phagolysosomes (56, 58, 66), and SodA and KatG, which protect against oxygen radicals (47). As discussed below, peptidoglycan hydrolases are SecA2 substrates in other multisubstrate systems. While this is not clearly the case in mycobacteria, in M. marinum a peptidoglycan hydrolase (IipA) was identified as SecA2 dependent (58).
Listeria
In listeriae, the secA2 gene is adjacent to the gene encoding the p60 protein (Fig. 2B) (57, 67). p60 is one of a group of listeriae SecA2 substrates that are peptidoglycan hydrolases, including NamA (MurA), SspB, and MltD (57, 61, 67, 68). Another functional category of listeriae SecA2 substrates is adhesins, such as Cbp and LAP (61, 69). Finally, similar to the mycobacterial SecA2 system, export of SBPs and superoxide dismutase (SodA) is associated with SecA2 of L. monocytogenes (48, 61, 70).
Clostridium
In C. difficile, the secA2 gene is in a locus with genes encoding the major S-layer protein (SlpA) and S-layer-related proteins, called cell wall proteins (CwpV, Cwp2, Cwp66, and Cwp84) (Fig. 2B) (53, 71). SlpA and Cwps are exported as SecA2 substrates (53). The finding that slpA is required for C. difficile viability explains, at least in part, why the SecA2 system is essential in this bacterium (71). Peptidoglycan hydrolase and adhesin activities have been assigned or predicted for SlpA and/or Cwp proteins (71–74), which is reminiscent of functional categories of listeriae SecA2 substrates.
Bacillus
Similar to the case with C. difficile, in B. anthracis the SecA2 system exports S-layer proteins (EA1 and Sap) (59), and the secA2 gene is adjacent to genes encoding these proteins (Fig. 2B). While B. anthracis has a secY2 gene, it is not clustered with secA2 in the genome and a secY2 mutant does not exhibit a Sap or EA1 export defect. Thus, there appears to be no SecY2 involvement in SecA2 transport (59). Both Sap and EA1 possess peptidoglycan hydrolase activity (75). Only members of the Bacillus cereus sensu lato group have secA2 or an S-layer (51).
Substrate Recognition by Multisubstrate SecA2 Systems
Most substrates of multisubstrate SecA2 systems have signal peptides that are indistinguishable from canonical Sec signal peptides. Experiments with mycobacteria demonstrate that the signal peptide of a SecA2 substrate is required for export (60, 76). However, the signal peptide does not determine whether a protein is exported by the SecA2 pathway versus another pathway. When signal peptides of SecA2-dependent and SecA1-dependent substrates are swapped, the proteins are still exported by their respective pathways (76). Thus, it is the mature domain of a SecA2-exported protein that determines its transport pathway. These details have been studied only with mycobacterial SecA2 substrates; similar studies are needed for other multisubstrate systems.
In mycobacteria and listeriae there are also examples of proteins lacking signal peptides that depend on SecA2 for export (i.e., PknG, SodA, and KatG in mycobacteria and SodA, LAP, and phosphomannose isomerase in listeriae) (47, 48, 56, 69, 70). For these cases, the proteins are exported as well as localized to the cytoplasm, and they are likely to be “moonlighting” proteins that function in both locations. Nothing is known about the recognition of these proteins by the SecA2 system. Further, it remains possible that the effect of SecA2 on these proteins is indirect. One possibility is that moonlighting proteins might be released from the cytoplasm as a secondary consequence of SecA2-dependent export of peptidoglycan hydrolases that affect cell wall integrity (77).
The Multisubstrate SecA2 Translocase
The mycobacterial SecA2 pathway is the most-studied multisubstrate system, in terms of mechanism. The mycobacterial SecA2 has a role that is distinct from that of SecA1. Even when overexpressed, SecA1 and SecA2 are unable to fulfill the function of one another (78). In addition, M. tuberculosis SecA1 and SecA2 share only 38% identity (54% sequence similarity). Thus, it was a surprise to discover broad similarity between the crystal structures of M. tuberculosis SecA1 and SecA2 (79). However, compared to SecA1, the CTD of SecA2 is truncated, similar to what was found for SecA2 of the aSec system (Fig. 4) (3). In addition, the helical wing domain (HWD) is missing in the mycobacterial SecA2. The lack of an HWD is a conserved feature of actinobacterial SecA2 proteins, but small HWD truncations may also exist in other SecA2 proteins (79). The significance of CTD and HWD truncations to SecA2 function remains to be investigated.
Like canonical SecAs, SecA2 is an ATPase, and amino acid substitutions in the nucleotide binding domain (NBD1) of SecA2 abolish export (53, 80). However, mycobacterial SecA1 and SecA2 differ in ATPase activity. SecA2 has a lower ATPase rate than SecA1 and also binds ADP and ATP with a higher affinity and releases ADP more slowly (80, 81). Moreover, ADP binding to SecA2 induces a structural rearrangement involving the precursor-binding domain (PPXD) that is not observed in ADP-bound SecA1 or conventional SecA proteins. These differences in nucleotide interactions might reflect the existence of additional proteins that stimulate ATP hydrolysis or ADP release or distinct mechanisms of substrate recognition by SecA2.
Data indicate that SecA2 works with the canonical SecYEG. In mycobacteria and listeriae, suppressors of secA2 mutants map to the sole secY gene in these bacteria, which argues for the canonical SecY being used by SecA2 for export (54, 55). In C. difficile, there are also data for SecA2 working with the same SecYEG channel as used by SecA1 (53). Because proteins must be in an unfolded state to transit SecYEG (82, 83), the discovery that SecYEG is used by multisubstrate SecA2 systems implies that the substrates of these systems need to be unfolded for translocation. In mycobacteria, it is demonstrated that the mature domain of a protein dictates the need for SecA2 for export (i.e., not the signal peptide) (76). Further, the mature domain of a mycobacterial SecA2 substrate can be engineered to be exported by the Tat system, a pathway requiring proteins be folded in order to be exported (76). Thus, one possibility is that the mature domain of SecA2 substrates has a propensity to fold or aggregate in the cytoplasm and that the SecA2 system, through currently unknown mechanisms, enables export of such proteins. For example, SecA2 or other players in the multisubstrate system might keep substrates from folding prior to or during export.
There may also be a role for SecA1 in SecA2-dependent export. M. tuberculosis SecA1 and SecA2 form heterodimers in vitro (84). Additionally, in mycobacteria and listeriae, if SecA1 is depleted or inhibited, SecA2-dependent export is compromised (85, 86). However, further studies are required because the effect of SecA1 on SecA2 export could instead be due to a function of SecA1 in transporting SecYEG proteins to the membrane. In C. difficile, SecA1 depletion does not impact SecA2 export, indicating that in this species there is no role for SecA1 in SecA2 transport (53).
Additional Factors Involved in Multisubstrate SecA2 Systems
In mycobacteria, SatS is a cytoplasmic chaperone that works with SecA2 to export a subset of SecA2 substrates (87). SatS stabilizes and prevents aggregation of substrates in the cytoplasm and potentially delivers them to the export machinery. In B. anthracis SlaP and SlaQ, which are encoded by genes adjacent to secA2 (Fig. 2B), are cytoplasmic proteins required for export of SecA2 substrates. The functions of SlaP and SlaQ are unknown (59, 88). In L. monocytogenes, the DivIVA protein that recruits proteins to the poles and septum of Gram-positive bacteria is necessary for septal localization and secretion of the p60 and MurA SecA2 substrates (68). The connection between DivIVA and SecA2 export requires further studies to understand.
CONCLUSION
Many Gram-positive bacteria have SecA2 systems that export a small set of proteins and contribute to pathogenesis. However, it is important to recognize that at least two types of SecA2 systems exist (aSec and multisubstrate systems), each with a distinctive mechanism. In the future, it will be important to clarify the defining features of the respective SecA2 substrates as well as the recognition and translocation events of each type of pathway. For multisubstrate systems, in particular, more studies are needed to determine the degree of mechanistic similarity in the absence of evolutionary relatedness.
ACKNOWLEDGMENTS
We gratefully acknowledge Brittany Miller for review of the manuscript.
We acknowledge support from NIH (grant R01AI41513 to B.A.B. and P.M.S. and grant R01AI054540 to M.B.).
REFERENCES
- 1.Tsirigotaki A, De Geyter J, Šoštaric N, Economou A, Karamanou S. 2017. Protein export through the bacterial Sec pathway. Nat Rev Microbiol 15:21–36. 10.1038/nrmicro.2016.161. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Crane JM, Randall LL. 2017. The Sec system: protein export in Escherichia coli. EcoSal Plus 7:ESP-0002-2017. 10.1128/ecosalplus.ESP-0002-2017. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensing BA, Seepersaud R, Yen YT, Sullam PM. 2014. Selective transport by SecA2: an expanding family of customized motor proteins. Biochim Biophys Acta 1843:1674–1686. 10.1016/j.bbamcr.2013.10.019. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takamatsu D, Bensing BA, Sullam PM. 2004. Genes in the accessory sec locus of Streptococcus gordonii have three functionally distinct effects on the expression of the platelet-binding protein GspB. Mol Microbiol 52:189–203. 10.1111/j.1365-2958.2004.03978.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Takamatsu D, Bensing BA, Sullam PM. 2005. Two additional components of the accessory sec system mediating export of the Streptococcus gordonii platelet-binding protein GspB. J Bacteriol 187:3878–3883. 10.1128/JB.187.11.3878-3883.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bensing BA, Sullam PM. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol Microbiol 44:1081–1094. 10.1046/j.1365-2958.2002.02949.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Wu H, Fives-Taylor PM. 2004. Investigating the role of secA2 in secretion and glycosylation of a fimbrial adhesin in Streptococcus parasanguis FW213. Mol Microbiol 53:843–856. 10.1111/j.1365-2958.2004.04116.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Siboo IR, Chaffin DO, Rubens CE, Sullam PM. 2008. Characterization of the accessory Sec system of Staphylococcus aureus. J Bacteriol 190:6188–6196. 10.1128/JB.00300-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mistou MY, Dramsi S, Brega S, Poyart C, Trieu-Cuot P. 2009. Molecular dissection of the secA2 locus of group B Streptococcus reveals that glycosylation of the Srr1 LPXTG protein is required for full virulence. J Bacteriol 191:4195–4206. 10.1128/JB.01673-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siboo IR, Chambers HF, Sullam PM. 2005. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect Immun 73:2273–2280. 10.1128/IAI.73.4.2273-2280.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seifert KN, Adderson EE, Whiting AA, Bohnsack JF, Crowley PJ, Brady LJ. 2006. A unique serine-rich repeat protein (Srr-2) and novel surface antigen (epsilon) associated with a virulent lineage of serotype III Streptococcus agalactiae. Microbiology 152:1029–1040. 10.1099/mic.0.28516-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Xiong YQ, Bensing BA, Bayer AS, Chambers HF, Sullam PM. 2008. Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb Pathog 45:297–301. 10.1016/j.micpath.2008.06.004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Zeng M, Fives-Taylor P. 2007. The glycan moieties and the N-terminal polypeptide backbone of a fimbria-associated adhesin, Fap1, play distinct roles in the biofilm development of Streptococcus parasanguinis. Infect Immun 75:2181–2188. 10.1128/IAI.01544-06. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Froeliger EH, Fives-Taylor P. 2001. Streptococcus parasanguis fimbria-associated adhesin fap1 is required for biofilm formation. Infect Immun 69:2512–2519. 10.1128/IAI.69.4.2512-2519.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obert C, Sublett J, Kaushal D, Hinojosa E, Barton T, Tuomanen EI, Orihuela CJ. 2006. Identification of a candidate Streptococcus pneumoniae core genome and regions of diversity correlated with invasive pneumococcal disease. Infect Immun 74:4766–4777. 10.1128/IAI.00316-06. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo HS, Mu R, Kim BJ, Doran KS, Sullam PM. 2012. Binding of glycoprotein Srr1 of Streptococcus agalactiae to fibrinogen promotes attachment to brain endothelium and the development of meningitis. PLoS Pathog 8:e1002947. 10.1371/journal.ppat.1002947. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo HS, Xiong YQ, Sullam PM. 2013. Role of the serine-rich surface glycoprotein Srr1 of Streptococcus agalactiae in the pathogenesis of infective endocarditis. PLoS One 8:e64204. 10.1371/journal.pone.0064204. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi Y, Takashima E, Shimazu K, Yagishita H, Aoba T, Konishi K. 2006. Contribution of sialic acid-binding adhesin to pathogenesis of experimental endocarditis caused by Streptococcus gordonii DL1. Infect Immun 74:740–743. 10.1128/IAI.74.1.740-743.2006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Sorge NM, Quach D, Gurney MA, Sullam PM, Nizet V, Doran KS. 2009. The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J Infect Dis 199:1479–1487. 10.1086/598217. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bensing BA, Gibson BW, Sullam PM. 2004. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J Bacteriol 186:638–645. 10.1128/JB.186.3.638-645.2004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takamatsu D, Bensing BA, Sullam PM. 2004. Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J Bacteriol 186:7100–7111. 10.1128/JB.186.21.7100-7111.2004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou M, Wu H. 2009. Glycosylation and biogenesis of a family of serine-rich bacterial adhesins. Microbiology 155:317–327. 10.1099/mic.0.025221-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 23.Bensing BA, Takamatsu D, Sullam PM. 2005. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol Microbiol 58:1468–1481. 10.1111/j.1365-2958.2005.04919.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, Sun B, Wu H, Peng Z, Fives-Taylor PM. 2007. Differential roles of individual domains in selection of secretion route of a Streptococcus parasanguinis serine-rich adhesin, Fap1. J Bacteriol 189:7610–7617. 10.1128/JB.00748-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez CJ, Shivshankar P, Stol K, Trakhtenbroit S, Sullam PM, Sauer K, Hermans PW, Orihuela CJ. 2010. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog 6:e1001044. 10.1371/journal.ppat.1001044. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyburn TM, Bensing BA, Xiong YQ, Melancon BJ, Tomasiak TM, Ward NJ, Yankovskaya V, Oliver KM, Cecchini G, Sulikowski GA, Tyska MJ, Sullam PM, Iverson TM. 2011. A structural model for binding of the serine-rich repeat adhesin GspB to host carbohydrate receptors. PLoS Pathog 7:e1002112. 10.1371/journal.ppat.1002112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lizcano A, Sanchez CJ, Orihuela CJ. 2012. A role for glycosylated serine-rich repeat proteins in gram-positive bacterial pathogenesis. Mol Oral Microbiol 27:257–269. 10.1111/j.2041-1014.2012.00653.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo HS, Minasov G, Seepersaud R, Doran KS, Dubrovska I, Shuvalova L, Anderson WF, Iverson TM, Sullam PM. 2013. Characterization of fibrinogen binding by glycoproteins Srr1 and Srr2 of Streptococcus agalactiae. J Biol Chem 288:35982–35996. 10.1074/jbc.M113.513358. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Six A, Bellais S, Bouaboud A, Fouet A, Gabriel C, Tazi A, Dramsi S, Trieu-Cuot P, Poyart C. 2015. Srr2, a multifaceted adhesin expressed by ST-17 hypervirulent group B Streptococcus involved in binding to both fibrinogen and plasminogen. Mol Microbiol 97:1209–1222. 10.1111/mmi.13097. [PubMed] [DOI] [PubMed] [Google Scholar]
- 30.Bensing BA, Khedri Z, Deng L, Yu H, Prakobphol A, Fisher SJ, Chen X, Iverson TM, Varki A, Sullam PM. 2016. Novel aspects of sialoglycan recognition by the Siglec-like domains of streptococcal SRR glycoproteins. Glycobiology 26:1222–1234. 10.1093/glycob/cww042. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng L, Bensing BA, Thamadilok S, Yu H, Lau K, Chen X, Ruhl S, Sullam PM, Varki A. 2014. Oral streptococci utilize a Siglec-like domain of serine-rich repeat adhesins to preferentially target platelet sialoglycans in human blood. PLoS Pathog 10:e1004540. 10.1371/journal.ppat.1004540. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shivshankar P, Sanchez C, Rose LF, Orihuela CJ. 2009. The Streptococcus pneumoniae adhesin PsrP binds to keratin 10 on lung cells. Mol Microbiol 73:663–679. 10.1111/j.1365-2958.2009.06796.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samen U, Eikmanns BJ, Reinscheid DJ, Borges F. 2007. The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect Immun 75:5405–5414. 10.1128/IAI.00717-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bensing BA, Siboo IR, Sullam PM. 2007. Glycine residues in the hydrophobic core of the GspB signal sequence route export toward the accessory Sec pathway. J Bacteriol 189:3846–3854. 10.1128/JB.00027-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bensing BA, Sullam PM. 2010. Transport of preproteins by the accessory Sec system requires a specific domain adjacent to the signal peptide. J Bacteriol 192:4223–4232. 10.1128/JB.00373-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bensing BA, Yen YT, Seepersaud R, Sullam PM. 2012. A Specific interaction between SecA2 and a region of the preprotein adjacent to the signal peptide occurs during transport via the accessory Sec system. J Biol Chem 287:24438–24447. 10.1074/jbc.M112.378059. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bensing BA, Sullam PM. 2009. Characterization of Streptococcus gordonii SecA2 as a paralogue of SecA. J Bacteriol 191:3482–3491. 10.1128/JB.00365-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen YT, Cameron TA, Bensing BA, Seepersaud R, Zambryski PC, Sullam PM. 2013. Differential localization of the streptococcal accessory sec components and implications for substrate export. J Bacteriol 195:682–695. 10.1128/JB.01742-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandara M, Corey RA, Martin R, Skehel JM, Blocker AJ, Jenkinson HF, Collinson I. 2016. Composition and activity of the non-canonical Gram-positive SecY2 complex. J Biol Chem 291:21474–21484. 10.1074/jbc.M116.729806. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seepersaud R, Bensing BA, Yen YT, Sullam PM. 2010. Asp3 mediates multiple protein-protein interactions within the accessory Sec system of Streptococcus gordonii. Mol Microbiol 78:490–505. 10.1111/j.1365-2958.2010.07346.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sibbald MJ, Winter T, van der Kooi-Pol MM, Buist G, Tsompanidou E, Bosma T, Schäfer T, Ohlsen K, Hecker M, Antelmann H, Engelmann S, van Dijl JM. 2010. Synthetic effects of secG and secY2 mutations on exoproteome biogenesis in Staphylococcus aureus. J Bacteriol 192:3788–3800. 10.1128/JB.01452-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spencer C, Bensing BA, Mishra NN, Sullam PM. 2019. Membrane trafficking of the bacterial adhesin GspB and the accessory Sec transport machinery. J Biol Chem 294:1502–1515. 10.1074/jbc.RA118.005657. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Bensing BA, Seepersaud R, Mi W, Liao M, Jeffrey PD, Shajahan A, Sonon RN, Azadi P, Sullam PM, Rapoport TA. 2018. Unraveling the sequence of cytosolic reactions in the export of GspB adhesin from Streptococcus gordonii. J Biol Chem 293:5360–5373. 10.1074/jbc.RA117.000963. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yen YT, Seepersaud R, Bensing BA, Sullam PM. 2011. Asp2 and Asp3 interact directly with GspB, the export substrate of the Streptococcus gordonii accessory Sec system. J Bacteriol 193:3165–3174. 10.1128/JB.00057-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seepersaud R, Sychantha D, Bensing BA, Clarke AJ, Sullam PM. 2017. O-acetylation of the serine-rich repeat glycoprotein GspB is coordinated with accessory Sec transport. PLoS Pathog 13:e1006558. 10.1371/journal.ppat.1006558. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurtz S, McKinnon KP, Runge MS, Ting JP, Braunstein M. 2006. The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response. Infect Immun 74:6855–6864. 10.1128/IAI.01022-06. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braunstein M, Espinosa BJ, Chan J, Belisle JT, Jacobs WR, Jr. 2003. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol Microbiol 48:453–464. 10.1046/j.1365-2958.2003.03438.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 48.Lenz LL, Mohammadi S, Geissler A, Portnoy DA. 2003. SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc Natl Acad Sci U S A 100:12432–12437. 10.1073/pnas.2133653100. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandrabos C, M’Homa Soudja S, Weinrick B, Gros M, Frangaj A, Rahmoun M, Jacobs WR Jr, Lauvau G. 2015. The p60 and NamA autolysins from Listeria monocytogenes contribute to host colonization and induction of protective memory. Cell Microbiol 17:147–163. 10.1111/cmi.12362. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang YT, Oh SY, Hendrickx AP, Lunderberg JM, Schneewind O. 2013. Bacillus cereus G9241 S-layer assembly contributes to the pathogenesis of anthrax-like disease in mice. J Bacteriol 195:596–605. 10.1128/JB.02005-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Missiakas D, Schneewind O. 2017. Assembly and function of the Bacillus anthracis S-layer. Annu Rev Microbiol 71:79–98. 10.1146/annurev-micro-090816-093512. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caspers M, Freudl R. 2008. Corynebacterium glutamicum possesses two secA homologous genes that are essential for viability. Arch Microbiol 189:605–610. 10.1007/s00203-008-0351-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 53.Fagan RP, Fairweather NF. 2011. Clostridium difficile has two parallel and essential Sec secretion systems. J Biol Chem 286:27483–27493. 10.1074/jbc.M111.263889. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ligon LS, Rigel NW, Romanchuk A, Jones CD, Braunstein M. 2013. Suppressor analysis reveals a role for SecY in the SecA2-dependent protein export pathway of mycobacteria. J Bacteriol 195:4456–4465. 10.1128/JB.00630-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durack J, Burke TP, Portnoy DA. 2015. A prl mutation in SecY suppresses secretion and virulence defects of Listeria monocytogenes secA2 mutants. J Bacteriol 197:932–942. 10.1128/JB.02284-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feltcher ME, Gunawardena HP, Zulauf KE, Malik S, Griffin JE, Sassetti CM, Chen X, Braunstein M. 2015. Label-free quantitative proteomics reveals a role for the Mycobacterium tuberculosis SecA2 pathway in exporting solute binding proteins and Mce transporters to the cell wall. Mol Cell Proteomics 14:1501–1516. 10.1074/mcp.M114.044685. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lenz LL, Portnoy DA. 2002. Identification of a second Listeria secA gene associated with protein secretion and the rough phenotype. Mol Microbiol 45:1043–1056. 10.1046/j.1365-2958.2002.03072.x. [DOI] [PubMed] [Google Scholar]
- 58.van der Woude AD, Stoop EJ, Stiess M, Wang S, Ummels R, van Stempvoort G, Piersma SR, Cascioferro A, Jiménez CR, Houben EN, Luirink J, Pieters J, van der Sar AM, Bitter W. 2014. Analysis of SecA2-dependent substrates in Mycobacterium marinum identifies protein kinase G (PknG) as a virulence effector. Cell Microbiol 16:280–295. 10.1111/cmi.12221. [PubMed] [DOI] [PubMed] [Google Scholar]
- 59.Nguyen-Mau SM, Oh SY, Kern VJ, Missiakas DM, Schneewind O. 2012. Secretion genes as determinants of Bacillus anthracis chain length. J Bacteriol 194:3841–3850. 10.1128/JB.00384-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibbons HS, Wolschendorf F, Abshire M, Niederweis M, Braunstein M. 2007. Identification of two Mycobacterium smegmatis lipoproteins exported by a SecA2-dependent pathway. J Bacteriol 189:5090–5100. 10.1128/JB.00163-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Renier S, Chambon C, Viala D, Chagnot C, Hébraud M, Desvaux M. 2013. Exoproteomic analysis of the SecA2-dependent secretion in Listeria monocytogenes EGD-e. J Proteomics 80:183–195. 10.1016/j.jprot.2012.11.027. [PubMed] [DOI] [PubMed] [Google Scholar]
- 62.Cheng Y, Schorey JS. 2018. Mycobacterium tuberculosis-induced IFN-β production requires cytosolic DNA and RNA sensing pathways. J Exp Med 215:2919–2935. 10.1084/jem.20180508. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdullah Z, Schlee M, Roth S, Mraheil MA, Barchet W, Böttcher J, Hain T, Geiger S, Hayakawa Y, Fritz JH, Civril F, Hopfner KP, Kurts C, Ruland J, Hartmann G, Chakraborty T, Knolle PA. 2012. RIG-I detects infection with live Listeria by sensing secreted bacterial nucleic acids. EMBO J 31:4153–4164. 10.1038/emboj.2012.274. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui J, Davidson AL. 2011. ABC solute importers in bacteria. Essays Biochem 50:85–99. 10.1042/bse0500085. [PubMed] [DOI] [PubMed] [Google Scholar]
- 65.Casali N, Riley LW. 2007. A phylogenomic analysis of the Actinomycetales mce operons. BMC Genomics 8:60. 10.1186/1471-2164-8-60. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zulauf KE, Sullivan JT, Braunstein M. 2018. The SecA2 pathway of Mycobacterium tuberculosis exports effectors that work in concert to arrest phagosome and autophagosome maturation. PLoS Pathog 14:e1007011. 10.1371/journal.ppat.1007011. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra KK, Mendonca M, Aroonnual A, Burkholder KM, Bhunia AK. 2011. Genetic organization and molecular characterization of secA2 locus in Listeria species. Gene 489:76–85. 10.1016/j.gene.2011.08.015. [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Halbedel S, Hahn B, Daniel RA, Flieger A. 2012. DivIVA affects secretion of virulence-related autolysins in Listeria monocytogenes. Mol Microbiol 83:821–839. 10.1111/j.1365-2958.2012.07969.x. [DOI] [PubMed] [Google Scholar]
- 69.Burkholder KM, Kim KP, Mishra KK, Medina S, Hahm BK, Kim H, Bhunia AK. 2009. Expression of LAP, a SecA2-dependent secretory protein, is induced under anaerobic environment. Microbes Infect 11:859–867. 10.1016/j.micinf.2009.05.006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 70.Archambaud C, Nahori MA, Pizarro-Cerda J, Cossart P, Dussurget O. 2006. Control of Listeria superoxide dismutase by phosphorylation. J Biol Chem 281:31812–31822. 10.1074/jbc.M606249200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Fagan RP, Fairweather NF. 2014. Biogenesis and functions of bacterial S-layers. Nat Rev Microbiol 12:211–222. 10.1038/nrmicro3213. [PubMed] [DOI] [PubMed] [Google Scholar]
- 72.Calabi E, Ward S, Wren B, Paxton T, Panico M, Morris H, Dell A, Dougan G, Fairweather N. 2001. Molecular characterization of the surface layer proteins from Clostridium difficile. Mol Microbiol 40:1187–1199. 10.1046/j.1365-2958.2001.02461.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 73.Waligora AJ, Hennequin C, Mullany P, Bourlioux P, Collignon A, Karjalainen T. 2001. Characterization of a cell surface protein of Clostridium difficile with adhesive properties. Infect Immun 69:2144–2153. 10.1128/IAI.69.4.2144-2153.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bradshaw WJ, Kirby JM, Roberts AK, Shone CC, Acharya KR. 2017. The molecular structure of the glycoside hydrolase domain of Cwp19 from Clostridium difficile. FEBS J 284:4343–4357. 10.1111/febs.14310. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahn JS, Chandramohan L, Liou LE, Bayles KW. 2006. Characterization of CidR-mediated regulation in Bacillus anthracis reveals a previously undetected role of S-layer proteins as murein hydrolases. Mol Microbiol 62:1158–1169. 10.1111/j.1365-2958.2006.05433.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 76.Feltcher ME, Gibbons HS, Ligon LS, Braunstein M. 2013. Protein export by the mycobacterial SecA2 system is determined by the preprotein mature domain. J Bacteriol 195:672–681. 10.1128/JB.02032-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ebner P, Gotz F. 12 November 2018. Bacterial excretion of cytoplasmic proteins (ECP): occurrence, mechanism, and function. Trends Microbiol 10.1016/j.tim.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 78.Braunstein M, Brown AM, Kurtz S, Jacobs WR Jr. 2001. Two nonredundant SecA homologues function in mycobacteria. J Bacteriol 183:6979–6990. 10.1128/JB.183.24.6979-6990.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swanson S, Ioerger TR, Rigel NW, Miller BK, Braunstein M, Sacchettini JC. 2015. Structural similarities and differences between two functionally distinct SecA proteins: the Mycobacterium tuberculosis SecA1 and SecA2. J Bacteriol 198:720–730. 10.1128/JB.00696-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hou JM, D’Lima NG, Rigel NW, Gibbons HS, McCann JR, Braunstein M, Teschke CM. 2008. ATPase activity of Mycobacterium tuberculosis SecA1 and SecA2 proteins and its importance for SecA2 function in macrophages. J Bacteriol 190:4880–4887. 10.1128/JB.00412-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D’Lima NG, Teschke CM. 2014. ADP-dependent conformational changes distinguish Mycobacterium tuberculosis SecA2 from SecA1. J Biol Chem 289:2307–2317. 10.1074/jbc.M113.533323. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonardi F, Halza E, Walko M, Du Plessis F, Nouwen N, Feringa BL, Driessen AJ. 2011. Probing the SecYEG translocation pore size with preproteins conjugated with sizable rigid spherical molecules. Proc Natl Acad Sci U S A 108:7775–7780. 10.1073/pnas.1101705108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Randall LL, Hardy SJ. 1986. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell 46:921–928. 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- 84.Prabudiansyah I, Kusters I, Driessen AJ. 2015. In vitro interaction of the housekeeping SecA1 with the accessory SecA2 protein of Mycobacterium tuberculosis. PLoS One 10:e0128788. 10.1371/journal.pone.0128788. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rigel NW, Gibbons HS, McCann JR, McDonough JA, Kurtz S, Braunstein M. 2009. The accessory SecA2 system of mycobacteria requires ATP binding and the canonical SecA1. J Biol Chem 284:9927–9936. 10.1074/jbc.M900325200. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halbedel S, Reiss S, Hahn B, Albrecht D, Mannala GK, Chakraborty T, Hain T, Engelmann S, Flieger A. 2014. A systematic proteomic analysis of Listeria monocytogenes house-keeping protein secretion systems. Mol Cell Proteomics 13:3063–3081. 10.1074/mcp.M114.041327. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller BK, Hughes R, Ligon LS, Rigel NW, Malik S, Anjuwon-Foster BR, Sacchettini JC, Braunstein M. 2019. Mycobacterium tuberculosis SatS is a chaperone for the SecA2 protein export pathway. eLife 8:e40063. 10.7554/eLife.40063. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen-Mau SM, Oh SY, Schneewind DI, Missiakas D, Schneewind O. 2015. Bacillus anthracis SlaQ promotes S-layer protein assembly. J Bacteriol 197:3216–3227. 10.1128/JB.00492-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Couvigny B, Lapaque N, Rigottier-Gois L, Guillot A, Chat S, Meylheuc T, Kulakauskas S, Rohde M, Mistou MY, Renault P, Doré J, Briandet R, Serror P, Guédon E. 2017. Three glycosylated serine-rich repeat proteins play a pivotal role in adhesion and colonization of the pioneer commensal bacterium, Streptococcus salivarius. Environ Microbiol 19:3579–3594. 10.1111/1462-2920.13853. [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Navarre WW, Schneewind O. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev 63:174–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Papanikou E, Karamanou S, Economou A. 2007. Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol 5:839–851. 10.1038/nrmicro1771. [PubMed] [DOI] [PubMed] [Google Scholar]


