Shigella causes diarrheal disease with serious public health implications. However, conventional Shigella identification methods are laborious and time-consuming and can be erroneous due to the high similarity between Shigella and enteroinvasive Escherichia coli (EIEC) and cross-reactivity between serotyping antisera. Further, serotype interpretation is complicated for inexperienced users. To develop an easier method with higher accuracy based on whole-genome sequencing (WGS) for Shigella serotyping, we systematically examined genomic information of Shigella isolates from 53 serotypes to define rules for differentiation and serotyping. We created ShigaTyper, an automated pipeline that accurately and rapidly excludes non-Shigella isolates and identifies 59 Shigella serotypes using Illumina paired-end WGS reads. A serotype can be unambiguously predicted at a data processing speed of 538 MB/min with 98.2% accuracy from a regular laptop. Once it is installed, training in bioinformatics analysis and Shigella genetics is not required. This pipeline is particularly useful to general microbiologists in field laboratories.
KEYWORDS: Shigella, in silico, serotying, whole-genome sequencing
ABSTRACT
Bacteria of the genus Shigella, consisting of 4 species and >50 serotypes, cause shigellosis, a foodborne disease of significant morbidity, mortality, and economic loss worldwide. Classical Shigella identification based on selective media and serology is tedious, time-consuming, expensive, and not always accurate. A molecular diagnostic assay does not distinguish Shigella at the species level or from enteroinvasive Escherichia coli (EIEC). We inspected genomic sequences from 221 Shigella isolates and observed low concordance rates between conventional designation and molecular serotyping: 86.4% and 80.5% at the species and serotype levels, respectively. Serotype determinants for 6 additional serotypes were identified. Examination of differentiation gene markers commonly perceived as characteristic hallmarks in Shigella showed high variability among different serotypes. Using this information, we developed ShigaTyper, an automated workflow that utilizes limited computational resources to accurately and rapidly determine 59 Shigella serotypes using Illumina paired-end whole-genome sequencing (WGS) reads. Shigella serotype determinants and species-specific diagnostic markers were first identified through read alignment to an in-house curated reference sequence database. Relying on sequence hits that passed a threshold level of coverage and accuracy, serotype could be unambiguously predicted within 1 min for an average-size WGS sample of ∼500 MB. Validation with WGS data from 380 isolates showed an accuracy rate of 98.2%. This pipeline is the first step toward building a comprehensive WGS-based analysis pipeline of Shigella spp. in a field laboratory setting, where speed is essential and resources need to be more cost-effectively dedicated.
IMPORTANCE Shigella causes diarrheal disease with serious public health implications. However, conventional Shigella identification methods are laborious and time-consuming and can be erroneous due to the high similarity between Shigella and enteroinvasive Escherichia coli (EIEC) and cross-reactivity between serotyping antisera. Further, serotype interpretation is complicated for inexperienced users. To develop an easier method with higher accuracy based on whole-genome sequencing (WGS) for Shigella serotyping, we systematically examined genomic information of Shigella isolates from 53 serotypes to define rules for differentiation and serotyping. We created ShigaTyper, an automated pipeline that accurately and rapidly excludes non-Shigella isolates and identifies 59 Shigella serotypes using Illumina paired-end WGS reads. A serotype can be unambiguously predicted at a data processing speed of 538 MB/min with 98.2% accuracy from a regular laptop. Once it is installed, training in bioinformatics analysis and Shigella genetics is not required. This pipeline is particularly useful to general microbiologists in field laboratories.
INTRODUCTION
Bacteria of the genus Shigella cause bacillary dysentery (shigellosis), one of the leading diarrheal diseases worldwide, disproportionately affecting children under 5 years of age from low- and middle-income countries (1–3). Shigella is transmitted through the fecal-oral route at an extremely low infectious dose (4) and manifests clinical symptoms, including fever, abdominal pain, watery or bloody diarrhea, vomiting, and potentially death (5). Although primarily a disease of the poor, shigellosis is still a public health concern in developed countries. An estimated 450,000 cases occur annually in the United States (6), bringing an economic loss of $257 million (7). The actual impact may be higher, as culture-based diagnosis underestimates shigellosis ∼2-fold (2), and a substantial increase in culture-confirmed shigellosis cases was reported in recent years (8). There is no licensed shigellosis vaccine, and >90% of Shigella isolates are antimicrobial resistant (9), leaving those exposed at risk.
Shigella consists of 4 species (serogroups) and >54 serotypes: Shigella dysenteriae (15 serotypes), S. flexneri (18 serotypes), S. boydii (20 serotypes), and S. sonnei (1 serotype). These serotypes are distinguished solely through the somatic (O) antigen, or lipopolysaccharide, expressed on the cell surface. Shigella is believed to acquire the O antigen from commensal Escherichia coli strains (10). Shigella serodiversity is further expanded through acquiring genes from other enteric bacteria and mobile genetic elements to either lose or replace the O-antigen biosynthetic genes or modify the O antigen (10–15).
It is important to determine the distribution of Shigella species and serotypes in time and space for disease burden tracking and outbreak investigation and to inform and evaluate policies aimed for disease reduction and vaccine development (16). Conventional Shigella identification relies on a combination of biochemical and serological assessment. Biochemical assays are conducted to distinguish Shigella from E. coli, the results of which roughly identify Shigella to the species level. Serological testing (slide agglutination) follows to determine the serotype. Serological differentiation is essential but is laborious, time-consuming, and expensive and can be erroneous. Intra- and interspecies cross-reactivity is common, and commercial antisera are at best 91% accurate (17). Rough strains that do not express O antigen and newly emerged Shigella serotypes without antisera that recognize them are nontypeable, accounting for 6 to 10% of annual Shigella isolates in the United States (8).
Molecular typing has been in development to replace conventional Shigella identification. The multilocus virulence gene ipaH, which has been employed by many institutions as a molecular target for Shigella (18, 20), does not differentiate Shigella and enteroinvasive E. coli (EIEC), a virulence clade of E. coli that shares many biochemical properties and virulence genes with Shigella (21, 22). Serotypes of the same Shigella species are not necessarily genetically closer than those from another species. Consequently, methods relying on genetic relatedness often cannot successfully place Shigella into clearly segregated clades by species (23–27). Although multilocus sequence typing (MLST) showed promise in Shigella classification (28), some MLST sequence types (STs) consist of multiple serotypes, which can lead to loss of critical information for vaccine development, as immunity against Shigella O-antigen is associated with protection from shigellosis (29). Molecular assays directly targeting the O-antigen-specific biosynthetic genes, such as PCR-restriction fragment length polymorphism (RFLP) (30), multiplex PCR (31, 32), and microarray (33), have been developed. However, these methods require additional biochemical assays to differentiate Shigella from E. coli, as many Shigella serotypes share identical surface O antigens with commensal E. coli (10).
Whole-genome sequencing (WGS) is a promising technology to replace conventional assays for microbial typing. With the cost of WGS decreasing precipitously (34), it is increasingly used in clinical diagnosis and disease surveillance. The bottleneck for adopting WGS, however, resides in WGS analysis, a skill not often possessed by analysts trained as general microbiologists. Furthermore, interpretation of Shigella serotypes is complicated, as it is determined by the combination of O-antigen synthesis and modification enzymes. An easy, simple serotyping pipeline with a user-friendly interface is needed for a WGS-based Shigella surveillance program.
Genoserotyping of Shigella requires information on both genetic determinants for serotype and those that differentiate from E. coli, particularly EIEC. Nevertheless, Shigella-specific genetic markers were often only studied in common serotypes but not rare serotypes. As Shigella underwent convergent evolution to arrive at similar phenotypes (23, 27, 35), conclusions drawn from type strains cannot reflect all serotypes. Here we report a comprehensive examination of Shigella genomic data covering 53 different serotypes, from which we derived results for the development of an in silico serotyping pipeline, ShigaTyper, that can make a direct prediction for 59 Shigella serotypes. ShigaTyper was specifically designed to meet the need of general microbiologists in field laboratories, where resources for Shigella identification are often limited. Such a pipeline is especially useful when species and serotype information is essential in quickly identifying organisms in outbreak situations.
RESULTS
We examined genetic determinants from a development set of 48 genome assemblies and the raw reads of 221 Shigella isolates, 56 of which were generated in-house and 165 were downloaded from the National Center for Biotechnology Institute (NCBI), collectively representing 53 different serotypes. There were 97 isolates designated S. boydii (including 6, 4, 4, 2, 2, 5, 2, 4, 7, 7, 5, 3, 3, 3, 3, 2, 3, 6, 4, 7, and 2 isolates typed to serotypes 1 to 20 and E1621-54, respectively, and 10 untyped isolates), 55 isolates designated S. dysenteriae (including 4, 5, 2, 3, 3, 1, 1, 2, 2, 2, 2, 3, 3, 2, and 2 isolates typed to serotypes 1 to 15, respectively, and 18 untyped isolates), 49 isolates designated S. flexneri (including 2, 1, 3, 2, 2, 7, 3, 3, 2, 2, 3, 1, 1, 3, 1, 2, and 3 isolates typed to serotypes Y, X, 1a, 1b, 1c [7a], 2a, 2b, 3a, 3b, 3, 4a, 4bv, 4, 5b, 5, 6, and provisional, respectively, and 7 untyped isolates), and 19 isolates designated S. sonnei. Additionally, 38 isolates of 14 Gram-positive and -negative foodborne bacteria were used as an exclusion group, including 13 EIEC isolates, 8 non-EIEC E. coli isolates (including 1 Shiga toxin-producing E. coli [STEC] isolate), 2 enterobacterial species that share O antigen with Shigella (Escherichia albertii and Plesiomonas shigelloides), 8 other enterobacteria (Salmonella enterica, Klebsiella pneumoniae, Enterobacter cloacae, and Yersinia enterocolitica), 3 nonenterobacterial Gram-negative diarrheagenic bacteria (Vibrio parahaemolyticus and Campylobacter jejuni), and 4 Gram-positive pathogens (Staphylococcus aureus, Listeria monocytogenes, and Enterococcus faecium). The distribution of species and serotypes of these strains (the development set) is described in Table 1 and in Tables S1 and S2.
TABLE 1.
Summary of Shigella WGS development set used in this studya
| Strain designation | No. of strains | No. (%) with concordant species designation | No. of serotypes included | No. of strains with serotype designation | No. (%) with concordant serotype designation |
|---|---|---|---|---|---|
| S. boydii | 97 | 79 (81.4) | 21 | 87 | 68 (78.2) |
| S. dysenteriae | 55 | 48 (87.3) | 15 | 37 | 31 (83.8) |
| S. flexneri | 49 | 47 (95.9) | 13 | 42 | 33 (78.6)b |
| S. sonnei | 19 | 17 (89.5) | 1 | 19 | 17 (89.5) |
| Shigella sp. | 1 | ||||
| EIEC | 13 | 13 (100) | |||
| Non-Shigella/EIEC | 25 | 25 (100) | |||
| Shigella only | 221 | 191 (86.4) | 50 | 185 | 149 (80.5) |
| Total | 259 | 229 (88.4) |
Strains were sequenced from an in-house collection (n = 58) or their WGSs were downloaded from the NCBI (n = 201).
Partial agreement between designation and in silico serotyping for S. flexneri was considered concordance (e.g., serotype 5 versus 5a).
Genetic determinants for Shigella serotypes.
Genetic determinants for most Shigella O antigens have been characterized. Within the O-antigen biosynthetic gene cluster (rfb), genes encoding O-antigen flippase, wzx, and polymerase, wzy, are serotype specific; their sequences were obtained from published reports (10, 12–15, 36–53). For serotypes without O-antigen information, rfb sequence located between the conserved galF and gnd genes was first extracted from assembled genomes (54). Sequences of wzx and wzy on rfb were determined by gene annotation, BLAST search, and protein secondary structure analysis. S. dysenteriae 14 and S. boydii 19 each possessed a unique rfb sequence with no close homolog in another bacterial species. The rfb sequences from S. dysenteriae 15 and provisional serotypes 96-265, E670-74, and S. boydii E1621-54 were nearly identical to those of enterotoxigenic E. coli (ETEC) OgN15 strain E819, E. albertii strain SP140152, E. coli O170, and E. coli O7. S. boydii 20 shares identical rfb sequences with S. boydii 1. A chromosomally carried rfb gene was not found for S. dysenteriae 93-119 and 204-96.
To further differentiate between serotypes, we collected the sequences of O-antigen modification enzymes of S. flexneri (12, 15, 52, 53), a chromosomally encoded S. sonnei-specific putative methylase (this sequence is hereafter referred to as Ss_methylase) (55), wbaM of S. boydii 10 (48), and the plasmid-borne rfp of S. dysenteriae 1 (14, 38). Genome comparison of S. boydii 1 and 20 revealed a unique, nonchromosomal heparinase gene in all S. boydii 20 isolates (n = 7) but not S. boydii 1 (n = 6), which we tentatively included as the S. boydii 20 marker.
Source, coordinates, and references for sequences included in the reference sequence database are listed in Table 2.
TABLE 2.
Sources of sequences included in the reference sequence database for Shigella serotypinga
| Sequence | Source | Accession no. | Gene identifier(s) | Beginning position | Ending position | Complement | Length (bp) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| ipaH_C | S. flexneri 2a 301 chromosome | AE005674.2 | ipaH_7 | 2686919 | 2687698 | 780 | 91 | |
| ipaB | S. sonnei pSS_046 | CP000039.1 | ipaB | 84468 | 86210 | x | 1,743 | 92 |
| Ss_methylase | S. sonnei SS046 chromosome | CP000038.1 | SSON_1583 | 1663456 | 1665288 | 1,833 | 55 | |
| Ss_wzx | S. sonnei pSS_046 | CP000039.1 | wzx | 197086 | 198354 | 1,269 | 13, 92 | |
| Ss_wzy | S. sonnei pSS_046 | CP000039.1 | wzy | 198423 | 199595 | 1,173 | 13, 92 | |
| Sf_wzx | S. flexneri 2a 301 chromosome | AE005674.2 | rfbE | 2113945 | 2115201 | x | 1,257 | 36, 91 |
| Sf_wzy | S. flexneri 2a 301 chromosome | AE005674.2 | rfc | 2110959 | 2112107 | x | 1,149 | 36, 91 |
| Sf6_wzx | S. flexneri 6 NCTC9779 O-Ag cluster | EU118169 | wzx | 4509 | 5741 | 1,233 | 10 | |
| Sf6_wzy | S. flexneri 6 NCTC9779 O-Ag cluster | EU118169 | wzy | 5797 | 6984 | 1,188 | 10 | |
| GtrI | S. flexneri 1a 0439 chromosome | CP020342.1 | BS647_00210 | 24836 | 25787 | x | 952 | 39, 42 |
| GtrII | S. flexneri 2a 301 chromosome | AE005674.2 | gtrII | 319152 | 320612 | 1,461 | 91 | |
| GtrIV | Shigella phage SfIV | NC_022749 | V416_gp25 | 20408 | 21721 | x | 1,314 | 93 |
| GtrV | S. flexneri 5 8401 chromosome | CP000266 | gtrV | 274667 | 275920 | x | 1,254 | 40, 94 |
| GtrX | S. flexneri 4c 1205 chromosome | CP012140 | AD871_01870 | 346525 | 347790 | x | 1,266 | 95 |
| GtrIC | S. flexneri 7b 3007 | CP024473 | Unannotated | 4289665 | 4291260 | 1,596 | 96 | |
| Oac | Enterobacterial phage Sf6 | AF547987 | 15 | 15624 | 16625 | x | 1,002 | 37, 97 |
| Oac1b | Partial sequence from S. flexneri 1b | JF450728.1 | oac | 2673 | 3674 | 1,002 | 53 | |
| Xv | pSFxv_2 from strain 2002017 | NC_017320.1 | SFXV_RS26800 | 1523 | 3043 | x | 1,521 | 15, 98 |
| Sd1_wzx | S. dysenteriae 1 Sd197 chromosome | CP000034 | rfbX | 2010259 | 2011449 | 1,191 | 14, 99 | |
| Sd1_wzy | S. dysenteriae 1 Sd197 chromosome | CP000034 | rfc | 2011446 | 2012588 | 1,143 | 14, 99 | |
| Sd1_rfp | S. dysenteriae 1 pSD197_spA | CP000640 | rfpB | 2979 | 4112 | x | 1,134 | 14, 38, 99 |
| Sd2_wzx | S. dysenteriae 2 O-Ag cluster | EU296404 | wzx | 5775 | 7202 | 1,428 | 10 | |
| Sd2_wzy | S. dysenteriae 2 O-Ag cluster | EU296404 | wzy | 1867 | 2952 | 1,086 | 10 | |
| Sd3_wzx | S. dysenteriae 3 O-Ag cluster | EU296415 | wzx | 1111 | 2625 | 1,515 | 10 | |
| Sd3_wzy | S. dysenteriae 3 O-Ag cluster | EU296415 | wzy | 7014 | 8117 | 1,104 | 10 | |
| Sd4_wzx | S. dysenteriae 4 O-Ag cluster | EU296402 | wzx | 1081 | 2352 | 1,272 | 10 | |
| Sd4_wzy | S. dysenteriae 4 O-Ag cluster | EU296402 | wzy | 4769 | 6001 | 1,233 | 10 | |
| Sd5_wzx | S. dysenteriae 5 O-Ag cluster | EU294174 | wzx | 4728 | 5915 | 1,188 | 10 | |
| Sd5_wzy | S. dysenteriae 5 O-Ag cluster | EU294174 | wzy | 9803 | 11134 | 1,332 | 10 | |
| Sd6_wzx | S. dysenteriae 6 O-Ag gene cluster | EU296414 | wzx | 340 | 1614 | 1,275 | 10 | |
| Sd6_wzy | S. dysenteriae 6 O-Ag gene cluster | EU296414 | wffH_5 (including the fusion junction between wzy and wffH) | 3592 | 4722 | 1,131 | 10 | |
| Sd7_wzx | S. dysenteriae 7 O-Ag gene cluster, strain M1354 | AY380835 | wzx | 7707 | 9194 | 1,488 | 45 | |
| Sd7_wzy | S. dysenteriae 7 O-Ag gene cluster, strain M1354 | AY380835 | wzy | 10646 | 11836 | 1,191 | 45 | |
| Sd8_wzx | S. dysenteriae 8 O-Ag gene cluster | EU294166 | wzx | 3180 | 4349 | 1,170 | 10 | |
| Sd8_wzy | S. dysenteriae 8 O-Ag gene cluster | EU294166 | wzy | 1899 | 3125 | 1,227 | 10 | |
| Sd9_wzx | S. dysenteriae 9 O-Ag gene cluster | EU296416 | wzx | 7775 | 9055 | 1,281 | 10 | |
| Sd9_wzy | S. dysenteriae 9 O-Ag gene cluster | EU296416 | wzy | 5373 | 6578 | 1,206 | 10 | |
| Sd10_wzx | S. dysenteriae 10 O-Ag gene cluster | EU294178 | wzx | 4706 | 5866 | 1,161 | 10 | |
| Sd10_wzy | S. dysenteriae 10 O-Ag gene cluster | EU294178 | wzy | 6933 | 8354 | 1,422 | 10 | |
| Sd11_wzx | S. dysenteriae 11 O-Ag gene cluster | EU294172 | wzx | 3741 | 5141 | 1,401 | 10 | |
| Sd11_wzy | S. dysenteriae 11 O-Ag gene cluster | EU294172 | wzy | 5138 | 6301 | 1,164 | 10 | |
| Sd12_wzx | S. dysenteriae 12 O-Ag gene cluster | EU294169 | wzx | 5663 | 6874 | 1,212 | 10 | |
| Sd12_wzy | S. dysenteriae 12 O-Ag gene cluster | EU294169 | wzy | 7882 | 8955 | 1,074 | 10 | |
| Sd13_wzx | S. dysenteriae 13 O-Ag cluster | EU294167 | wzx | 5631 | 7133 | 1,503 | 10 | |
| Sd13_wzy | S. dysenteriae 13 O-Ag cluster | EU294167 | wzy | 11011 | 12108 | 1,098 | 10 | |
| Sd14_wzx | S. dysenteriae 14 ATCC 49346 chromosome | CP026832 | Unannotated | 2829534 | 2830799 | x | 1,266 | 54, this study |
| Sd14_wzy | S. dysenteriae 14 ATCC 49346 chromosome | CP026832 | Unannotated | 2831775 | 2832977 | x | 1,203 | 54, this study |
| Sd15_wzx | S. dysenteriae 15 ATCC 49347 chromosome | CP026834.1 | Unannotated | 965436 | 966623 | x | 1,188 | 54, this study |
| Sd15_wzy | S. dysenteriae 15 ATCC 49347 chromosome | CP026834.1 | Unannotated | 966613 | 967854 | x | 1,242 | 54, this study |
| SdProv_wzx | S. dysenteriae provisional 96-265 chromosome | CP026819.1 | Unannotated | 2893541 | 2894860 | 1,320 | 54, this study | |
| SdProv_wzy | S. dysenteriae provisional 96-265 chromosome | CP026819.1 | Unannotated | 2899151 | 2900362 | 1,212 | 54, this study | |
| SdProvE_wzx | S. dysenteriae provisional E670-74 chromosome | CP027027.1 | Unannotated | 3878013 | 3879260 | x | 1,248 | 54, this study |
| SdProvE_wzy | S. dysenteriae provisional E670-74 chromosome | CP027027.2 | Unannotated | 3874004 | 3875176 | x | 1,173 | 54, this study |
| Sb1_wzx | S. boydii 1 O-Ag cluster | AY630255 | wzx | 4570 | 5754 | 1,185 | 49 | |
| Sb1_wzy | S. boydii 1 O-Ag cluster | AY630255 | wzy | 6605 | 7669 | 1,065 | 49 | |
| Sb2_wzx | S. boydii 2 O-Ag cluster | EU296418 | wzx | 4106 | 5353 | 1,248 | 10 | |
| Sb2_wzy | S. boydii 2 O-Ag cluster | EU296418 | wzy | 6127 | 7314 | 1,188 | 10 | |
| Sb3_wzx | S. boydii 3 O-Ag cluster | EU296407 | wzx | 1774 | 3039 | 1,266 | 10 | |
| Sb3_wzy | S. boydii 3 O-Ag cluster | EU296407 | wzy | 7165 | 8415 | 1,251 | 10 | |
| Sb4_wzx | S. boydii Sb227 chromosome | CP000036 | wzx | 877026 | 878255 | x | 1,230 | 43, 99 |
| Sb4_wzy | S. boydii Sb227 chromosome | CP000036 | wzy | 874635 | 875927 | x | 1,293 | 43, 99 |
| Sb5_wzx | S. boydii 5 O-Ag cluster | AF402313 | wzx | 7263 | 8411 | 1,149 | 43 | |
| Sb5_wzy | S. boydii 5 O-Ag cluster | AF402313 | wzy | 5379 | 6470 | 1,092 | 43 | |
| Sb6_wzx | S. boydii 6 O-Ag cluster | AF402314 | wzx | 11771 | 13183 | x | 1,413 | 43 |
| Sb6_wzy | S. boydii 6 O-Ag cluster | AF402314 | wzy | 3195 | 4247 | 1,053 | 43 | |
| WbaM | S. boydii 10 O-Ag cluster | AY693427 | wbaM | 9468 | 10424 | 957 | 48 | |
| Sb7_wzx | S. boydii 7 O-Ag cluster | EU296411 | wzx | 6714 | 7928 | 1,215 | 10 | |
| Sb7_wzy | S. boydii 7 O-Ag cluster | EU296411 | wzy | 8889 | 10184 | 1,296 | 10 | |
| Sb8_wzx | S. boydii 8 O-Ag cluster | EU294163 | wzx | 4664 | 5869 | 1,206 | 10 | |
| Sb8_wzy | S. boydii 8 O-Ag cluster | EU294163 | wzy | 7853 | 8935 | 1,083 | 10 | |
| Sb9_wzx | S. boydii 9 O-Ag cluster | AF402315 | wzx | 6944 | 8191 | 1,248 | 43 | |
| Sb9_wzy | S. boydii 9 O-Ag cluster | AF402315 | wzy | 4922 | 6139 | 1,218 | 43 | |
| Sb11_wzx | S. boydii 11 O-Ag cluster | AY529126 | wzx | 10367 | 11794 | x | 1,428 | 46 |
| Sb11_wzy | S. boydii 11 O-Ag cluster | AY529126 | wzy | 5432 | 6460 | 1,029 | 46 | |
| Sb12_wzx | S. boydii 12 O-Ag cluster | EU296406 | wzx | 6264 | 7580 | 1,317 | 10 | |
| Sb12_wzy | S. boydii 12 O-Ag cluster | EU296406 | wzy | 7561 | 8700 | 1,140 | 10 | |
| Sb13_wzx | S. boydii 13 O-Ag cluster | AY369140 | wzx | 1713 | 3032 | 1,320 | 44 | |
| Sb13_wzy | S. boydii 13 O-Ag cluster | AY369140 | wzy | 3019 | 4197 | 1,179 | 44 | |
| Sb14_wzx | S. boydii 14 O-Ag cluster | EU296409 | wzx | 1054 | 2511 | 1,458 | 10 | |
| Sb14_wzy | S. boydii 14 O-Ag cluster | EU296409 | wzy | 2515 | 3726 | 1,212 | 10 | |
| Sb15_wzx | S. boydii 15 O-Ag cluster | EU296412 | wzx | 1042 | 2433 | 1,392 | 10 | |
| Sb15_wzy | S. boydii 15 O-Ag cluster | EU296412 | wzy | 2453 | 3631 | 1,179 | 10 | |
| Sb16_wzx | S. boydii 16 O-Ag cluster | DQ371800 | wzx | 2385 | 3785 | 1,401 | 50 | |
| Sb16_wzy | S. boydii 16 O-Ag cluster | DQ371800 | wzy | 3796 | 4953 | 1,158 | 50 | |
| Sb17_wzx | S. boydii 17 O-Ag cluster | DQ875941 | wzx | 926 | 2191 | 1,266 | 51 | |
| Sb17_wzy | S. boydii 17 O-Ag cluster | DQ875941 | wzy | 4681 | 5892 | 1,212 | 51 | |
| Sb18_wzx | S. boydii CDC 3083-94 chromosome | CP001063 | wzx | 1103246 | 1104463 | 1,218 | 47, 54 | |
| Sb18_wzy | S. boydii CDC 3083-94 chromosome | CP001063 | wzy | 1105612 | 1106757 | 1,146 | 47, 54 | |
| Sb19_wzx | S. boydii 83-578 chromosome | CP026814.1 | Unannotated | 3586585 | 3587835 | 1,251 | 54, this study | |
| Sb19_wzx | S. boydii 83-578 chromosome | CP026814.1 | Unannotated | 3588633 | 3589772 | 1,140 | 54, this study | |
| SbProv_wzx | S. boydii provisional 54-1621 chromosome | CP026810 | Unannotated | 1495194 | 1496618 | 1,425 | 54, this This study | |
| SbProv_wzy | S. boydii provisional 54-1621 chromosome | CP026810 | Unannotated | 1499881 | 1501056 | 1,176 | 54, this This study | |
| Heparinase | E. coli isolate Co6114 plasmid pCo6114_2 | CP016036 | Heparinase II/III-like protein | 2803 | 4428 | x | 1,626 | This study |
| EclacY | E. coli DH1 (ME8569) chromosome | AP012030 | lacY | 362350 | 363603 | 1,254 | 100 | |
| cadA | S. dysenteriae 1 Sd197 chromosome | CP000034 | cadA | 4179987 | 4182129 | 2,143 | 99 | |
| Stx1 | S. dysenteriae 1 3818T Shiga toxin sequence | M19437 | stxA, stxB | 161 | 1387 | 1,227 | 62 | |
| Stx2 | E. coli O157:H7 EDL933 Stx2 sequence | X07865 | sltIIA, sltIIB | 239 | 1479 | 1,241 | 62 | |
| ShET1 | S. flexneri 2a 301 chromosome | AE005674.2 | set1B, set1A | 3069555 | 3070277 | 723 | 91, 101 | |
| ShET2 | S. sonnei pSS_046 | CP000039.1 | sen/ospD2 | 5491 | 7200 | x | 1,710 | 92, 102 |
| Sat_N | S. dysenteriae 10 ATCC 12039 chromosome | CP026831.1 | espC | 4214643 | 4217642 | 3,000 | 54, 103 |
Annotated reference genome sequences or O-antigen gene clusters based on which original characterization was published were chosen unless there was a mistake in the sequence (for example, EU118169 was chosen over EU294165, because the EU294165 differs from the rest of S. flexneri 6 O-antigen gene cluster sequences by 5 nucleotides). When annotation was not available, sequences were annotated by RAST (87–89) and serotype-specific determinants were identified. Publications from which the sequence was generated and the O-antigen gene cluster was characterized are included as references. Accession numbers refer to GenBank or SRA.
Genetic determinants to differentiate Shigella and EIEC.
Differentiation of Shigella from E. coli is an indispensable part of Shigella identification. The highly conserved 3′ end of ipaH genes (ipaH_C) was employed as an indicator for Shigella/EIEC (56–62). Most Shigella organisms are impaired for lactose fermentation and lysine decarboxylation. Therefore, sequences of E. coli lacY (EclacY) and lysine decarboxylase (cadA) were used as differentiation markers for Shigella from EIEC. These sequences were also included in the reference sequence database (Table 2).
We hypothesized that genetic markers ipaH_C, EclacY, cadA, and Ss_methylase can be used for EIEC differentiation and Shigella identification. As the same defective phenotype in Shigella can be caused by different types of mutations and in different genes, we sought to identify exceptions in the Shigella and EIEC genomes, summarized in Table 3.
TABLE 3.
Exceptions to the inclusion/exclusion criteria used for Shigella serotypinga
| Gene marker | Function and use | Serotype | Description of exception | No. of strains with exception (accession number of strains) | No. of strains examined |
|---|---|---|---|---|---|
| ipaH_C | Conserved virulence gene Shigella/EIEC inclusion marker | S. boydii 13 | Absent | 3 (SRR4178425, SRR4181329, SRR4181518) | 3 |
| EclacY | Lactose permease Shigella exclusion marker | S. boydii 9 | Full-length lacY | 4 (CP026836.1, SRR4180506, SRR6760302, SRR8186698) | 7 |
| 373-bp 5′ end of lacY | 3 (SRR4176997, SRR4180898, SRR4181342) | ||||
| S. boydii 15 | lacY with 72-bp deletion at 5′ end | 3 (ASM296813v1 [GCF_002968135.1], SRR4179879, SRR8186662) | 3 | ||
| S. dysenteriae 1 | 366- to 475-bp 5′ end of lacY | 8 (CP000034, CP006736, DRR015930, SRR1811629, SRR5330538, SRR6373753, SRR8186696, SRR8186588) | 8 | ||
| S. sonnei | 270-bp 5′ end of lacY | 21 (CP000038.1, CP023645.1, ERR1762061, ERR1762062, SRR4180904, SRR6927290, SRR6954223, SRR6982834, SRR7013788, SRR7013790, SRR7013792, SRR7013793, SRR7013794, SRR7013797, SRR7013799, SRR8186598, SRR8186617, SRR8186670, SRR8186671, SRR8186733, SRR8186738) | 22 | ||
| No lacY | 1 (SRR6927273) | ||||
| cadA | Lysine decarboxylase, Shigella exclusion marker | S. boydii 11 | Full-length cadA | 4 (CP026846.1, SRR4176974, SRR4180810, SRR4180822) | 5 |
| 258-bp 3′ end of cadA | 1 (DRR015925) | ||||
| S. dysenteriae 1 | Full-length cadAb | 8 (see above for accession numbers) | 8 | ||
| S. dysenteriae 8 | Full-length cadA | 6 (CP026827.1, DRR015992, SRR2994193, SRR8186616, SRR8186618, SRR8186667) | 6 | ||
| S. dysenteriae 10 | 127- to 171-bp 3′ end of cadA | 3 (CP026831.1, DRR015994, SRR8186726) | 3 | ||
| S. sonnei | Full-length cadAc | 22 (see above for accession numbers) | 22 | ||
| Ss_methylase | Putative (DNA) methylase, S. sonnei-specific marker | S. dysenteriae 10 | Full-length Ss_methylase | 3 (see above for accession numbers) | 3 |
| EIEC | Full-length Ss_methylase | 2 (DRR015801, SRR5330536) | 14 |
Gene markers ipaH, EclacY, cadA, and Ss_methylase were examined from the genomes of 53 Shigella serotypes and EIEC. Serotypes displaying a genotype(s) that is an exception to the rule for Shigella/EIEC inclusion/exclusion, description of the exception, number of strains with the exception, accession numbers of the isolates, and total number of strains examined are listed.
The full-length cadA in the S. dysenteriae 1 genome has a 4-bp deletion and the gene product is prematurely truncated.
The full-length cadA in the S. sonnei genome is disrupted by two insertional elements and the gene product is prematurely truncated.
(i) ipaH. We detected ipaH_C in all Shigella and EIEC strains as expected, except S. boydii 13 (n = 3). This is consistent with previous findings that S. boydii 13 is noninvasive and more closely related to E. albertii than to Shigella (35, 63).
(ii) lacY. EclacY was reported to be absent from Shigella organisms except S. sonnei and S. dysenteriae 1 while present in most EIEC isolates (64). A remnant from the 5′ end of EclacY was detected in 21 of 22 S. sonnei (107 to 270 bp) and 8 of 8 S. dysenteriae 1 (361 to 475 bp) genomes, respectively. We observed full-length EclacY in 4 S. boydii serotype 9 isolates (n = 7) and 373 bp of 5′-EclacY in the other 3. S. boydii serotype 15 (n = 3) carried nearly full-length EclacY except a 72-bp deletion at the 5′ end. Eleven EIEC isolates carried full-length EclacY (n = 14).
(iii) cadA. It was reported that cadA was deleted in most Shigella isolates but present in the genomes of 70% of EIEC isolates (65). We observed that all S. sonnei (n = 22) and S. dysenteriae (n = 8) isolates carry full-length cadA as previously reported (66, 67). S. dysenteriae 8 (n = 5) also harbored full-length cadA. S. dysenteriae 10 (n = 3) carried a 3′ remnant of cadA. Among S. boydii 11 isolates (n = 5), 4 harbored full-length cadA and 1 carried a 258-bp remnant at the 3′ end. Ten EIEC isolates harbored full-length cadA (n = 14).
(iv) Ss_methylase. We detected Ss_methylase in all 22 S. sonnei genomes. However, Ss_methylase was also detected in all S. dysenteriae 10 (n = 3) and 2 EIEC (n = 14) isolates that we examined.
Virulence factors.
Virulence of Shigella/EIEC is attributed to pINV (56), an invasion plasmid that carries genes allowing enteroinvasion. Sequence of ipaB, an essential gene for invasion, was included as a marker for pINV. Shiga toxin expressed from S. dysenteriae 1 is associated with hemolytic-uremic syndrome. Both type 1 (stx1) (57–60) and type 2 (stx2) (61) Shiga toxins have been reported for other Shigella serotypes. Therefore, we included the reference sequences of stx1 (stx/stx1a) and stx2 (stx2a) (62). In addition, sequences encoding the Shigella enterotoxins 1 and 2 (ShET1 and ShET2) and the N terminus of autotransporter toxin Sat (sat_N) were included (Table 2).
Comparison of results from conventional and molecular Shigella serotyping.
We manually inspected WGS reads of 259 isolates in the development set to molecularly determine their species and serotype based on their O-antigen synthesis and modification genes, Shigella/EIEC differentiation markers, and MLST profile. Overall, a serotype can be assigned to 253 isolates (97.7%) based on their molecular profiles. Sequences of wzx gene for multiple serotypes were observed in 30 (11.6%) isolates. Nevertheless, reads mapped to minor wzx genes were usually <2% of that mapped to a dominant wzx gene, indicating low levels of contamination, and a serotype could be assigned in 25 cases. Five genomes had multiple wzx genes present at comparable levels and 1 had no recognizable serotype determinant genes. By molecular profiling, there were 83 S. boydii isolates, with 8, 5, 4, 6, 2, 1, 2, 4, 6, 7, 4, 1, 3, 3, 2, 2, 2, 5, 4, 9, and 3 isolates belonging to serotypes 1 to 20 and E1621-54, respectively (21 serotypes), 55 S. dysenteriae isolates, with 6, 10, 6, 2, 3, 2, 1, 5, 4, 2, 2, 3, 3, 3, 2, and 1 isolates belonging to serotypes 1 to 15 and 96-265, respectively (16 serotypes), 50 S. flexneri isolates, with 3, 1, 2, 3, 4, 11, 3, 7, 3, 1, 3, 1, 3, 6, 1 isolates belonging to serotypes Y, Xv (4c), 1a, 1b, 1c (7a), 2a, 2b, 3a, 3b, 4a, 4av, 4bv, 5a, 6, and 7b, respectively (15 serotypes), 20 S. sonnei isolates, and 14 EIEC isolates. Six isolates designated Shigella were ipaH negative and therefore considered non-Shigella/EIEC. No isolates carried wzx and wzy belonging to different O antigens as was reported for E. coli (68).
We compared in silico and conventional designations for these 259 strains (Table 1). All 24 non-Shigella/EIEC isolates were identified by the absence of ipaH_C. Twelve of the 13 EIEC isolates (92.3%) were identified as EIEC except 1 isolate that carried S. dysenteriae 3 wzx and wzy but lacked EclacY and cadA. As this strain was typed to ST270 by MLST, it is likely to be an EIEC isolate. For the 221 Shigella genomes, 191 (86.4%) were congruent at species level and 6, 1, and 17 were molecularly determined as not Shigella/EIEC, EIEC, and another Shigella species. Of the 185 isolates with a serotype designation, 149 (80.5%) had concordant serotype determinants.
Development of an automated in silico Shigella serotyping pipeline.
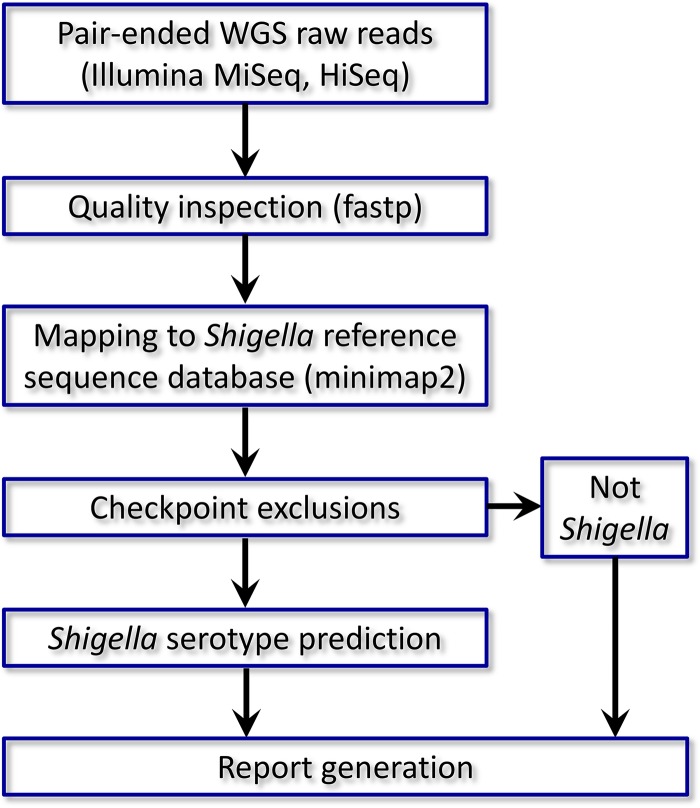
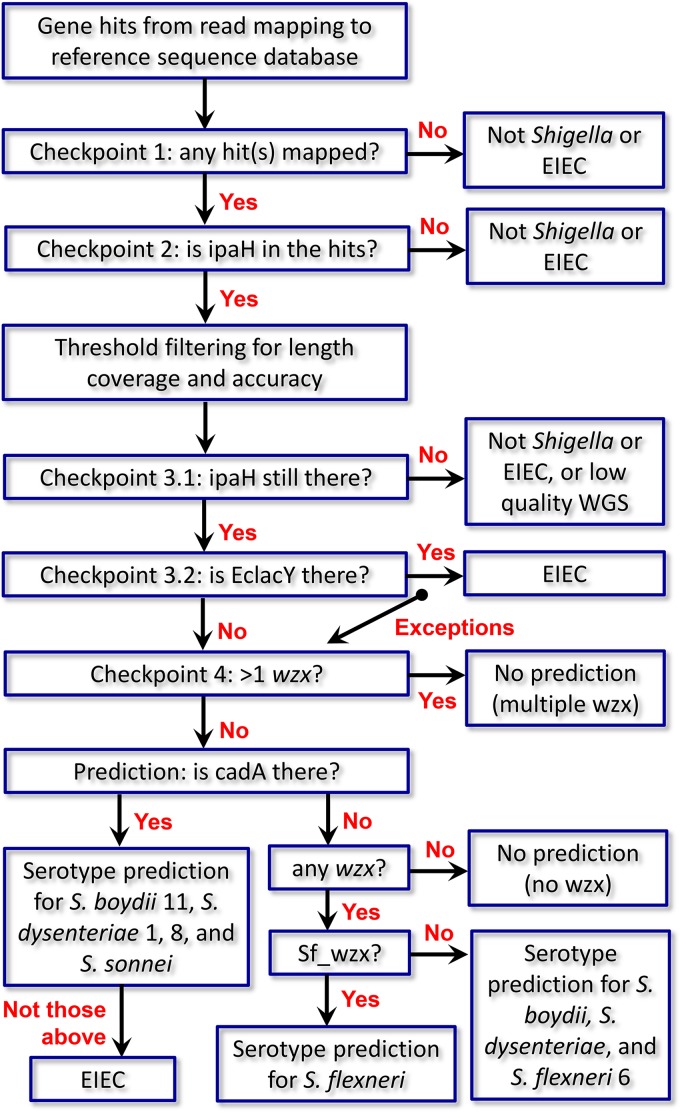
Molecular serotyping of Shigella requires careful consideration of multiple gene determinants, which can be daunting for inexperienced analysts. To automate such a process in a rapid and efficient way, we developed ShigaTyper, an integrative workflow for in silico Shigella serotyping using Illumina paired-end WGS reads (Fig. 1). Jupyter Notebook was used as the user interface so that all command line tools could be prerecorded and executed in one place with the click of a button and output directly printed below each step. A recently reported WGS reads preprocessing package, fastp, was used for quality inspection (69). Comparing results from 95 isolates with and without quality filtering and trimming showed 100% consistency in prediction outcomes. Therefore, quality filtering and trimming were omitted. WGS raw reads were directly aligned to the reference sequence database using minimap2 (70). Shigella differentiation was conducted through exclusion steps before serotype prediction (Fig. 2). Strains that did not carry ipaH_C were considered “not Shigella/EIEC” and eliminated. For ipaH+ strains, length coverage and number of variants for each of the gene hits were determined using samtools (71) and bcftools. Threshold values were set to eliminate gene hits that did not achieve sufficient coverage and accuracy. Gene coverage and accuracy were defined as the fraction of gene length covered by WGS reads (breadth of coverage) and fraction of nucleotide identity to the reference sequence, respectively. We tested 80% and 50% for gene coverage and 80% for gene accuracy. The list of gene hits passing threshold filtering was screened for Shigella/EIEC differentiation markers. Strains deemed to belong to Shigella were then subjected to serotype prediction. In this method, a report is automatically generated for each sample, including name, quality attributes of the WGS reads, a serotype prediction, and a summary table for each of the identified gene hits for final review. In addition, when the virulence plasmid pINV or a toxin is detected, a warning message is also included. An example of report is shown in Fig. 3A and B. An additional summary table listing serotype prediction for each of the strains is listed in the batch processing notebook (Fig. 3C).
FIG 1.
Summary of workflow for ShigaTyper. A detailed description can be found in Results (“Development of an automated in silico Shigella serotyping pipeline”).
FIG 2.
Schematic illustration of a decision tree for Shigella differentiation before serotype prediction employed in ShigaTyper. ShigaTyper was designed to differentiate and exclude non-Shigella or contaminated isolates before predicting serotype for Shigella isolates. Distantly related non-Shigella/EIEC species (such as Listeria) usually have no read mapped to any of the genes in the reference sequence database and fail at checkpoint 1. Enterobacterial species (such as Salmonella) may have one or more hits but not ipaH_C and fail at checkpoint 2. Checkpoint 3 excludes EIEC based on the presence of full-length EclacY gene, with the exception of S. boydii 9 and 15. Last, if there are more than one wzx genes present in the WGS reads, it indicates multiple serotypes and fails checkpoint 4. Details on serotype prediction are provided in Results.
FIG 3.
A representative output for ShigaTyper. (A) QC inspection of WGS reads. Quality inspection results were parsed from reports generated by fastp and are summarized in a table showing number of reads, number of bases, number of bases with >Q20 and >Q30 scores, and average read length. A visual representation of average quality score of each of the 4 bases over sequencing cycle and an estimated average depth for genome coverage are given below the table. (B) Serotype prediction for the sample. A direct serotype prediction is made by ShigaTyper based on threshold filter values passed by gene determinants as described in Results. A warning signal is given if sequence of the pINV-encoded virulence factor IpaB, a Shiga toxin, or an enterotoxin is detected in the WGS reads. The table summarizes characteristics of each of the genetic determinants identified from the WGS data. Those that passed the threshold filter values are shown in blue. All the codes are hidden from view for clarity of reporting but can be toggled to show for examination if needed. (C) Report of ShigaTyper batch processing. The summary table lists outcomes for serotype prediction, invasion plasmid, Shiga toxin, and enterotoxin.
Serotyping prediction by ShigaTyper.
Serotype prediction for ShigaTyper was made primarily through the serotype-specific wzx gene, as O-antigen expression is absolutely dependent on wzx but not wzy (10, 46, 72). Additionally, we observed better gene coverage for wzx than wzy (93.4% ± 8.9% versus 81.7% ± 19.1% for the 46 in-house samples under 1 GB), presumably because of the higher GC content of wzx than wzy (30.2% ± 1.95% versus 28.6% ± 1.52%), as the transposase-based library generation in the current MiSeq workflow disfavors AT-rich sequences (73, 74). For serotypes that cannot be predicted solely by wzx, additional criteria were applied as follows.
S. boydii 1 (Sb1) and 20 contain identical chromosomal rfb genes. For strains carrying Sb1_wzx, those that also carried a heparinase were assigned to S. boydii 20, while those that did not were designated S. boydii 1.
S. boydii 6 and 10 contain identical rfb genes; however, wbaM in S. boydii 6 is disrupted with an insertional element between positions 252 and 253 (48). Therefore, read alignment to wild-type wbaM is expected to be poor at the insertional junction for S. boydii 6, and quality filtering should remove a significant fraction of these bases. Indeed, for the S. boydii 6 strain we examined, only 18.6% of bases passed quality filtering at the junction, while 57.4% of bases did for the overall wbaM gene. In contrast, in S. boydii 10 (n = 3), the percentage of bases passing quality filtering at the junction was comparable to that for the overall wbaM gene (50.0% ± 0.0% versus 51.7% ± 4.3%). A threshold ratio of percent passing filtering at the junction over the entire wbaM was therefore set at 0.5. An isolate with a ratio below the threshold was considered S. boydii 6, and an isolate with a ratio above 0.5 was considered S. boydii 10. We identified another 4 S. boydii isolates that were wbaM+. Sequence alignment showed a contiguous, undisrupted wbaM gene consistent with S. boydii 10 for all 4 strains. All of them had a junction-to-overall ratio above 0.5 (0.955 ± 0.087), validating the use of wbaM junctional quality to distinguish S. boydii 6 and 10. There was only 1 S. boydii 6 isolate in our development set (even though 5 were designated S. boydii 6). However, this strategy later successfully distinguished S. boydii 6 from S. boydii 10 in our validation study.
All S. flexneri serotypes except S. flexneri 6 share the same rfb gene cluster but have different O-antigen modifications by enzymes encoded on bacteriophages or plasmids (12, 75). For strains containing the S. flexneri 1 to 5 wzx gene (Sf_wzx), the list of O-antigen modification genes identified was searched in a Python dictionary containing gene formulas of 19 S. flexneri serotypes. When a serotype had two or more possible gene formulas, all were included for interpretation. (For example, S. flexneri 5a is defined as S. flexneri modified by the glucosyltransferase, GtrV, regardless of the presence of the O-antigen acetylase, Oac. Both gene formulas “gtrV” and “gtrV, oac” were considered S. flexneri 5a.)
S. sonnei carries its rfb on pINV, which is lost at high frequency (76). Therefore, we used the chromosomal Ss_methylase as a diagnostic marker. To distinguish S. sonnei from S. dysenteriae 10 and EIEC, only strains positive for both cadA and Ss_methylase but negative for any wzx except S. sonnei wzx (Ss_wzx) were considered S. sonnei. A strain carrying Ss_wzx or pINV marker ipaB was assigned virulent S. sonnei form I. Otherwise, such a strain was considered form II. Only 5 (25%) S. sonnei strains were form I, consistent with the reported high plasmid instability.
Performance of ShigaTyper.
We determined the prediction accuracy for ShigaTyper, excluding the 6 uninterpretable samples from the development set. When we used 80% as the threshold level for gene length coverage and accuracy, as previously reported for S. flexneri (77), we achieved 95.7% (242/253) and 94.5% (239/253) accuracies at the species and serotype levels. Sequence accuracy was >97% for all serotype determinants identified. In 9 out of the 11 isolates with inaccurate species designation, it could be attributed to low length coverage of one or more gene hits, leading to no prediction (n = 7) or misdesignation (n = 2). Prediction accuracies increased to 98.8% (250/253) and 98.0% (248/253) at the species and serotype levels, respectively, when the threshold gene coverage was reduced to 50%. Isolates that could not be serotyped at 50% gene coverage by ShigaTyper were manually examined. Two isolates had low-level contamination of the genetic determinant(s) from another serotype or EIEC that were >50% covered. One isolate did not have a wzx gene and therefore could not be typed. One isolate was predicted to be S. flexneri 5b because it carried O-antigen modification genes gtrV, gtrX, and oac. However, this strain was phenotypically S. flexneri 3a due to a 1-bp insertion in gtrV. Another isolate was a S. flexneri carrying unconventional gene formula not included in the prediction script and was designated “S. flexneri novel serotype.”
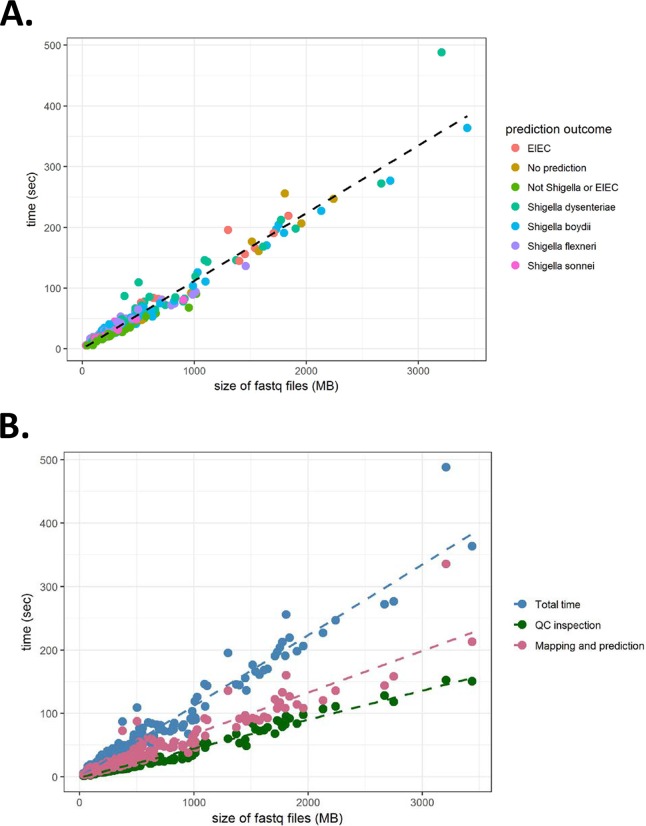
The turnaround time for ShigaTyper was directly proportional to the size of the paired end fastq files irrespective of the prediction outcome (Fig. 4A). On average, the pipeline processed WGS raw reads at 538.1 MB/min, translating to a time to prediction of ∼1 min for an average-size sample (509.9 ± 538.1 MB). Most of the time was spent on executing the three command line tools, fastp, minimap2 and samtools, accounting for 35.8% ± 4.9% and 45.7% ± 4.8% of the total time. As a result, 36.6% ± 4.9% and 63.4% ± 4.9% of the total time were spent on quality inspection and mapping and prediction, respectively (Fig. 4B).
FIG 4.
Speed for serotype prediction is directly proportional to the size of WGS files. (A) Total time spent for Shigella serotyping was plotted against the sum of size of the paired-end WGS reads in fastq.gz format. Outcomes of serotype prediction are indicated on the right. A linear regression line is shown in black. (B) Total time, time spent on quality (QC) inspection, and time spent on mapping and prediction are plotted against the sum of size of the paired-end WGS reads in fastq.gz format. Linear regression lines of the same color are also shown. The average size for the sum of the paired-end WGS reads was 509.9 ± 538.1 MB and ranged from 30.7 to 3,436.7 MB.
Validation of ShigaTyper.
ShigaTyper was validated using a separate collection of Shigella strains, including 62 well-designated clinical isolates (16), 33 reference strains, and WGS reads of 287 isolates downloaded from the NCBI, comprising 49 designated serotypes and 32 non-Shigella isolates. Specifically, the validation set included 94 isolates designated S. sonnei and 87 isolates designated S. boydii, of which 10, 8, 2, 9, 2, 1, 1, 5, 0, 3, 2, 4, 3, 6, 3, 0, 0, 6, 4, 11, 3, 2, and 2 isolates typed to serotypes 1 to 20, E1621-54, E140634-99, and E25411-82 (20 serotypes), respectively. Eighty-four isolates designated S. dysenteriae included 3, 9, 8, 7, 2, 1, 1, 3, 2, 5, 1, 1, and 1 isolate typed to serotypes 1 to 4, 6 to 9, 11, 12, 14, E11207-96 (96-265), and E670-74 (13 serotypes), respectively, and 40 untyped isolates. Eighty-five isolates designated S. flexneri included 1, 1, 2, 9, 9, 17, 3, 7, 3, 2, 1, 1, 2, 7, and 1 isolate typed to serotypes Y, Xv (4c), 1a, 1b, 1c (7a), 2a, 2b, 3a, 3b, 4a, 4b, 4, 5a, 5b, 6, and 7 (15 serotypes), respectively, and 19 untyped isolates. Thirty-two isolates that were common diarrheagenic or foodborne bacteria were selected as the exclusion group, including 26 E. coli isolates (of which 10, 3, and 1 were designated EIEC, ETEC, and STEC), 1 P. shigelloides isolate, 1 Salmonella enterica isolate, 1 K. pneumoniae isolate, 2 S. aureus isolates, and 1 L. monocytogenes isolate. After correction by manual inspection, there were 90 S. boydii isolates, with 9, 9, 3, 11, 2, 1, 1, 4, 0, 4, 2, 2, 2, 7, 3, 1, 0, 5, 6, 13, and 5 isolates belonging to serotypes 1 to 20 and E1621-54, respectively, 74 S. dysenteriae isolates, with 5, 19, 12, 11, 0, 2, 1, 1, 7, 0, 2, 8, 1, 1, 0, 3, and 1 isolate belonging to serotypes 1 to 15, 96-265, and E670/74, respectively, 87 S. flexneri isolates, with 1, 2, 1, 17, 12, 27, 1, 3, 8, 4, 2, 1, 1, 6, and 1 isolate belonging to serotypes Y, Xv (4c), 1a, 1b, 1c (7a), 2a, 2av, 2b, 3a, 3b, 4av, 5a, 5b, 6, and 7b, respectively, 93 S. sonnei isolates, and 14 EIEC isolates. Two isolates designated Shigella were ipaH negative and therefore considered non-Shigella/EIEC. One isolate had gene determinants from multiple serotypes (wzx or wzy) and another had no gene determinants, and the two were deemed uninterpretable. This corresponded to 95.7% concordance rate at the species level and 90% at the serotype level. After correction and removal of the 2 uninterpretable isolates, the validation set contained 344 Shigella isolates of 49 serotypes, 14 EIEC isolates, and 22 non-Shigella/EIEC isolates (Table 4).
TABLE 4.
Summary of Shigella WGS validation sets used in this studya
| Strain designation (molecular) | No. of strains | No. of serotypes included | Prediction accuracy, no. of strains (%) |
|||
|---|---|---|---|---|---|---|
| 80% length coverage |
50% length coverage |
|||||
| Species | Serotype | Species | Serotype | |||
| S. boydii | 90 | 19 | 79 (87.7) | 76 (84.4) | 87 (96.7) | 87 (96.7) |
| S. dysenteriae | 74 | 13 | 72 (97.3) | 72 (97.3) | 74 (100) | 74 (100) |
| S. flexneri | 87 | 15 | 87 (100) | 82 (88.9) | 87 (100) | 84 (96.6) |
| S. sonnei | 93 | 1 | 92 (98.9) | 92 (98.9) | 92 (98.9) | 92 (98.9) |
| Shigella subtotal | 344 | 49 | 330 (95.9) | 322 (93.6) | 340 (98.8) | 337 (98.0) |
| EIEC | 14 | 14 (100) | 14 (100) | |||
| Non-Shigella/EIEC | 22 | 22 (100) | 22 (100) | |||
| Overall | 380 | 366 (96.3) | 373 (98.2) | |||
Strains were sequenced from an in-house collection of 62 clinical isolates and 33 reference strains (n = 95), or their WGSs were downloaded from the NCBI (n = 255).
The validation set was subjected to automated serotype prediction by ShigaTyper. At the 80% gene coverage threshold, the accuracy rates for the 344 Shigella strains were 95.9% and 93.6% at species and serotype levels, respectively. At the 50% gene coverage level, the accuracy rates increased to 98.8% and 98.0% at the species and serotype levels, respectively. At both threshold levels ShigaTyper differentiated 14 out of 14 EIEC isolates and 22 out of 22 non-Shigella/EIEC isolates (100%). The overall accuracies for the 380 isolates were 96.3% and 98.2% at 80% and 50% gene coverage thresholds, respectively. Of the 7 Shigella isolates that could not be identified at the 50% gene coverage level, 4 had low-level contamination of another serotype, 1 was an S. sonnei isolate without a full-length cadA, 1 was an S. flexneri 3b isolate carrying an unconventional O-antigen acetylase gene (oac1b instead of oac), and 1 was an S. flexneri 4av carrying Ss_methylase, rendering the algorithm unable to make a correct prediction. The presence of Ss_methylase was not due to contamination with S. sonnei, S. dysenteriae 10, or EIEC, because no read was mapped to EclacY, cadA, Sd10_wzx, or Sd10_wzy.
Four of the 10 clinical S. sonnei isolates (40%) were completely devoid of EclacY sequence, while only 6 (5.8%) out of the remaining 103 S. sonnei isolates from the developed world did not carry an EclacY remnant. This allelic polymorphism did not affect the serotype prediction outcome.
The ability of Shigella to absorb Congo red to form red colonies is linked to its virulence plasmid pINV (56). We examined the predictive value of ipaB and ShET2 as a pINV-indicative marker in 83 in-house Shigella isolates. Sixty-four of the 83 isolates formed red or dark pink colonies (pINV+) in the presence of Congo red, and 59 and 61 of them were positive for ipaB and ShET2, respectively. Sixty-one of them were positive for either ipaB or ShET2. Of the 19 isolates that formed light pink or white colonies (pINV−), 8 and 10 were positive for ipaB and ShET2, respectively. Twelve of them were positive for either ipaB or ShET2. Both ipaB and ShET2 had an overall accuracy of 84.3%. However, ipaB has a slightly higher positive predictive value (88.1%) than ShET2 (85.9%) or ipaB or ShET2 (83.6%) in predicting pINV.
A subset of 68 S. sonnei isolates in the validation set were characterized for the presence of Shiga toxin-producing bacteriophage (78). ShigaTyper detected stx1 in 42 out of the 42 Shiga toxin-positive isolates and did not detect stx1 in 26 out of the 26 Shiga toxin-negative isolates, 100% consistent with the previous report.
Genetic variation due to bacteriophages.
Ss_methylase was observed in the genomes of all S. sonnei (n = 115) and S. dysenteriae 10 (n = 3) isolates, 4 EIEC isolates (n = 28), and 1 S. flexneri 4av isolate (n = 5). Therefore, we investigated if this gene is associated with mobile genetic elements. Ss_methylase in S. sonnei was present within an ∼9.7-kbp contig flanked by insertion sequence 1 (IS1) sequence between the ynfF and ydf loci, suggesting a transposon-mediated insertion event in the founding ancestor. In contrast, Ss_methylase in S. dysenteriae 10, 2 EIEC, and the S. flexneri 4av resided in an ∼40-kbp lambdoid prophage integrated between the potB and potC loci. The prophage from another EIEC isolate (SRA accession number SRR6049563) was integrated between the mtfA and zinT loci. Prophages from the EIEC and S. flexneri 4av isolates shared gene organizations similar to the one from the S. dysenteriae 10 genome but were nonconserved in genes encoding structural phage proteins.
Shiga toxin has never been reported for S. boydii. We observed stx1 in 3 S. boydii 19 (n = 10). Sequence comparison of the assembled genomes revealed that the stx1 coding sequence resided within a prophage integrated between the ynfG and ynfF loci that is 99.9% identical to the POCJ13 phage, a lysogenic bacteriophage that infects and converts S. dysenteriae 4 and S. flexneri into Shiga toxin producers (58).
Shigella identification through MLST and biochemical analyses.
All isolates from the development and validation sets were screened for their MLST profiles (n = 637). Most Shigella serotypes belonged to STs previously reported (28), except that ST1753 was previously assigned to S. flexneri, while we observed that only S. boydii E1621-54 typed to this ST (n = 7). ST groups for some Shigella serotypes were not known. Isolates from some serotypes returned no or previously unreported STs. Overall, 78 of the 552 Shigella isolates (14.1%) could not be properly categorized by MLST (Table S4).
Fifty-one Shigella isolates from 42 different serotypes were selected for automated microbial identification through biochemical properties. Except S. sonnei isolates that could be identified to species level, most Shigella isolates were identified as “Shigella group” (non-S. sonnei Shigella). Five (9.8%) isolates from 3 serotypes were identified as E. coli and 2 isolates from 2 serotypes showed low confidence in discrimination between Shigella and E. coli. A control isolated identified as EIEC by molecular profiling was subjected to the same analysis and was identified as E. coli (Table S5).
DISCUSSION
Shigella is a serious threat to public health, despite the low number of cases in developed countries. However, it can be expensive to maintain a pathogen-specific surveillance program, factoring in the time required for analyst training, reagent preparation, and maintenance, especially for rare Shigella serotypes. Conventional Shigella identification method is labor-intensive, potentially subjective, and not sufficiently accurate. Using molecular profiling, we showed that conventional Shigella serotyping was at best 90% accurate, consistent with a previous report of an upper limit at 91% (17). Similarly, biochemical identification could erroneously assign some Shigella isolates as E. coli. A WGS-based identification method has a universal workflow for all pathogens and provides high-resolution data with better accuracy. Therefore, it is ideal to replace the conventional methods. The same sequencing data can be used in screenings for virulence genes and antimicrobial resistance (AMR), MLST, and single nucleotide polymorphism (SNP) analysis, further reducing the cost in pathogen characterization and outbreak investigation.
In this study, we conducted a comprehensive examination of genome information from 56 Shigella serotypes. By identifying gene determinants for novel Shigella serotypes, as well as setting criteria for E. coli and EIEC differentiation, we provided information enabling in silico Shigella serotyping. We further demonstrated the feasibility of this approach with a proof-of-concept WGS serotyping pipeline, ShigaTyper, using bioinformatic and programming tools freely available online. ShigaTyper provides a prototype for simple and rapid identification of clinical Shigella isolates with high accuracy.
The lack of lactose fermentation and the lack of lysine decarboxylation represent two hallmark traits of Shigella. Nevertheless, we observed considerable variation in the gene structure of the EclacY and cadA, confirming the previous observation that Shigella is not a homogeneous group and the seemingly identical phenotype was often caused by different inactivation mechanisms (67, 79). Variability was even present within serotype (Table 3), suggesting further genome rearrangement postspeciation. This high genome variability, together with the observation that the putative S. sonnei-specific marker, Ss_methylase (2, 55), was present in multiple Shigella and EIEC serotypes associated with bacteriophages, suggests that no single genetic marker alone should be used for Shigella identification at the species or serotype level. Rather, a combination of genes should be taken into consideration for proper EIEC differentiation and Shigella designation. WGS is an especially valuable tool for this purpose, as it provides abundant information and the data can always be reevaluated with additional gene makers. For example, serotype determinants of EIEC can be included for better differentiation.
Shigella is a highly dynamic group of bacteria. Annually, 6 to 10% of Shigella isolates are untypeable (8), suggesting that novel serotypes are constantly emerging and Shigella evolution is an ongoing process. We identified serotype determinants from six previously uncharacterized Shigella serotypes. Four of them had nearly identical O-antigen genes of another enterobacterium and likely arose from horizontal transfer. The absence of rfb in some serotypes (S. dysenteriae 93-119 and 204-96) and the presence of additional serotype determinants on mobile elements (S. boydii 20) indicate that there are multiple mechanisms at work for Shigella serotype diversification.
Direct target mapping using WGS reads has been successfully employed for predicting bacterial serotypes for E. coli, S. flexneri, and Salmonella (68, 77, 80) and for inferring AMR (81, 82). We developed a similar pipeline for Shigella using a short-read mapping approach that has been used for microbial MLST (83) and Salmonella serotyping (80). The assembly-free approach reduced analysis time and is less computation intensive, enabling resource-limited field labs to perform in silico serotyping on a regular office laptop. The average fastq file for validation was 446.7 ± 296.7 MB, and took 49.8 ± 33.1 s to prediction, or 31.6 ± 21.0 s had quality inspection been omitted. We attribute the higher speed than SeqSero for Salmonella (80) to the fact that Shigella serotype determinants are unique enough and did not require subsequent rounds of alignment/BLAST to discriminate multiple probable alleles. Because the sequence aligner in ShigaTyper, minimap2, is capable of aligning long DNA sequences efficiently (70), assembled genomes in fasta format can be used for serotype prediction in a similar fashion. We included codes processing genome assemblies in ShigaTyper. However, it might not be as time-effective, as genome assembly usually takes more than 1 min to complete.
ShigaTyper is particularly suitable for general microbiologists. The use of Jupyter Notebook consolidated all codes in one place. Once installed, there will be no need for bioinformatic and programming skills. A direct serotype prediction is made without operator interpretation, reducing user subjectivity and ensuring reproducibility. This pipeline is highly flexible. For example, by including sequences of Shiga toxins in the reference sequence database, we identified S. boydii 19 as another Shiga toxin-producing serotype. Detection of novel serotypes or additional virulence genes can be easily achieved by updating the reference sequence database. We determined the current threshold setting optimal at 50% gene length coverage, as this setting captured most of the serotype gene determinants but allowed tolerance for low-level contamination. Additional filter settings can be adopted to meet regulatory requirement as necessary.
The scope of our work was limited by the availability of well-designated Shigella WGS raw reads. Of the 59 serotypes that ShigaTyper was designed to identify, we were unable to obtain WGS reads of S. flexneri Yv, X, and 4b for examination. As our cohort was small and some serotypes were represented by only a few strains, larger-scale confirmation and validation are needed for the implementation of in silico Shigella serotyping. Nevertheless, our work contributed to the transition of public health surveillance into molecular technologies and can be integrated with other WGS-based tools for detection and investigation of enteric pathogens.
MATERIALS AND METHODS
Strains.
In-house strains used in this study are described in Table S1. Most strains used for ShigaTyper development were provided by the FDA Pacific Regional Laboratory Southwest. Shigella strains used for validation were generously provided by the Global Enteric Multicenter Study consortium (16) and California Department of Public Health. Strains were propagated in brain heart infusion or on tryptic soy agar (TSA) with 0.1 mg/ml of Congo red. All strains were screened by PCR for the presence of ipaH and Ss_methylase. Selected isolates were examined using serotype-specific PCR primers. Strains corresponding to the sequences downloaded from the National Center for Biotechnology Institute (NCBI) are described in Tables S2 (genomes or sequence assemblies) and 3 (WGS reads).
Whole-genome sequencing.
Genomic DNA was extracted from 1 to 2 ml of overnight culture using QIAamp DNA minikit on a QiaCube (Qiagen, Hilden, Germany) and fragmented and indexed using Nextera XT DNA sample preparation and DNA index kits (Illumina, San Diego, CA). DNA concentration was determined using a Qubit dsDNA BR Assay system (Thermo Fisher, Waltham, MA). Libraries were normalized and pooled for sequencing on an Illumina MiSeq system using 500 V.2 reagent cartridges.
Bioinformatic analyses.
Local computational analyses were conducted on a Dell laptop (Intel core i7-6600U CPU, 16 GB of memory) with a Windows 7 host and an Ubuntu 18.04 guest addition (4 processor cores, 4.3 GB of memory) on a VMware Player 14.1.1. Bioinformatic packages were installed and managed by Anaconda 4.4.11 with Python 3.6.5 through Bioconda, including fastp 0.12.2 (69), minimap2 2.13 (70), and htslib/samtools/bcftools 1.7 (71, 84). All command line and python codes were maintained in Jupyter Notebooks and run on Jupyter 1.0.0 and nbconvert 5.3.1. Papermill 0.14.2 was used for batch processing of samples. MLST of scheme “ecoli1” was determined using stringMLST 0.5.1 (85) with 12 GB memory allocation. When needed, de novo genome assembly, gene annotation, and E. coli serotyping were performed using Spades 3.11.1 (86) on GalaxyTrakr, RAST (87–89), and SerotypeFinder (68). Mauve (90) 2015-02-26 was used for genome comparison.
Biochemical identification.
A Vitek 2 Compact automated system with GN ID card (bioMérieux, Marcy-l'Étoile, France) was used for microbial identification per manufacturer’s instruction.
Data availability.
Sequences generated in this study have been deposited in the NCBI Sequence Read Archive under the BioProject number PRJNA490540; accession numbers for each of the strains are listed in Table S1. The stand-alone Shigella serotyping pipeline, together with instructions for system setup and running, is available by request or at https://github.com/CFSAN-Biostatistics/shigatyper. An online version will be made available soon on GalaxyTrakr (https://galaxytrakr.org).
Supplementary Material
ACKNOWLEDGMENTS
We thank Sharon Tennant of University of Maryland School of Medicine, Baltimore, MD, Edward Desmond and Gregory B. Inami of California Department of Public Health, Kai-Shun Chen of FDA Winchester Engineering and Analytical Center, Wen Lin of FDA Center for Tobacco Products, and Chong-Ming Cheng, a former member of FDA Pacific Southwest Laboratory, for providing Shigella strains. We are grateful to Rachel Binet of FDA CFSAN, Selen Stromgren of FDA Office of Regulatory Affairs, and Christopher J. Webb of Dupont for critical reading of the manuscript. We thank Okumu Kaluoch, Elaine Yeh, and Jonathan Yenovkian of FDA San Francisco Laboratory for technical assistance and discussion.
This work was supported by FDA Commissioner’s Fellowship to Y.W. and FDA CARTS project number IR01650 to H.K.L.
The contents of this article do not reflect any position of the U.S. government or the FDA. Reference to any commercial materials, equipment, or process does not in any way constitute approval, endorsement, or recommendation by the FDA.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00165-19.
REFERENCES
- 1.Pires SM, Fischer-Walker CL, Lanata CF, Devleesschauwer B, Hall AJ, Kirk MD, Duarte AS, Black RE, Angulo FJ. 2015. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS One 10:e0142927. doi: 10.1371/journal.pone.0142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque AS, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD Diarrhoeal Diseases Collaborators. 2017. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 17:909–948. doi: 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuPont HL, Levine MM, Hornick RB, Formal SB. 1989. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis 159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 5.Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. 2017. Shigellosis. Lancet 391:801–812. doi: 10.1016/S0140-6736(17)33296-8. [DOI] [PubMed] [Google Scholar]
- 6.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.091101p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharff RL. 2012. Economic burden from health losses due to foodborne illness in the United States. J Food Prot 75:123–131. doi: 10.4315/0362-028X.JFP-11-058. [DOI] [PubMed] [Google Scholar]
- 8.CDC. 2018. National enteric disease surveillance: Shigella annual report, 2016. https://www.cdc.gov/nationalsurveillance/pdfs/LEDS-Shig-2016-REPORT-508.pdf.
- 9.CDC. 2014. 2014 annual human isolates report. https://wwwcdcgov/narms/pdf/2014-Annual-Report-narms-508cpdf.
- 10.Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Wang Q, Reeves PR, Wang L. 2008. Structure and genetics of Shigella O antigens. FEMS Microbiol Rev 32:627–653. doi: 10.1111/j.1574-6976.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- 11.Lai V, Wang L, Reeves PR. 1998. Escherichia coli clone Sonnei (Shigella sonnei) had a chromosomal O-antigen gene cluster prior to gaining its current plasmid-borne O-antigen genes. J Bacteriol 180:2983–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allison GE, Verma NK. 2000. Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri. Trends Microbiol 8:17–23. doi: 10.1016/S0966-842X(99)01646-7. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd JG, Wang L, Reeves PR. 2000. Comparison of O-antigen gene clusters of Escherichia coli (Shigella) sonnei and Plesiomonas shigelloides O17: sonnei gained its current plasmid-borne O-antigen genes from P. shigelloides in a recent event. Infect Immun 68:6056–6061. doi: 10.1128/IAI.68.10.6056-6061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng L, Perepelov AV, Zhao G, Shevelev SD, Wang Q, Senchenkova SN, Shashkov AS, Geng Y, Reeves PR, Knirel YA, Wang L. 2007. Structural and genetic evidence that the Escherichia coli O148 O antigen is the precursor of the Shigella dysenteriae type 1 O antigen and identification of a glucosyltransferase gene. Microbiology 153:139–147. doi: 10.1099/mic.0.2006/001107-0. [DOI] [PubMed] [Google Scholar]
- 15.Sun Q, Knirel YA, Lan R, Wang J, Senchenkova SN, Jin D, Shashkov AS, Xia S, Perepelov AV, Chen Q, Wang Y, Wang H, Xu J. 2012. A novel plasmid-encoded serotype conversion mechanism through addition of phosphoethanolamine to the O-antigen of Shigella flexneri. PLoS One 7:e46095. doi: 10.1371/journal.pone.0046095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, Antonio M, Hossain A, Mandomando I, Ochieng JB, Oundo JO, Qureshi S, Ramamurthy T, Tamboura B, Adegbola RA, Hossain MJ, Saha D, Sen S, Faruque AS, Alonso PL, Breiman RF, Zaidi AK, Sur D, Sow SO, Berkeley LY, O'Reilly CE, Mintz ED, Biswas K, Cohen D, Farag TH, Nasrin D, Wu Y, Blackwelder WC, Kotloff KL, Nataro JP, Levine MM. 2014. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis 59:933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefebvre J, Gosselin F, Ismail J, Lorange M, Lior H, Woodward D. 1995. Evaluation of commercial antisera for Shigella serogrouping. J Clin Microbiol 33:1997–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsay B, Ochieng JB, Ikumapayi UN, Toure A, Ahmed D, Li S, Panchalingam S, Levine MM, Kotloff K, Rasko DA, Morris CR, Juma J, Fields BS, Dione M, Malle D, Becker SM, Houpt ER, Nataro JP, Sommerfelt H, Pop M, Oundo J, Antonio M, Hossain A, Tamboura B, Stine OC. 2013. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J Clin Microbiol 51:1740–1746. doi: 10.1128/JCM.02713-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.Venkatesan MM, Buysse JM, Kopecko DJ. 1989. Use of Shigella flexneri ipaC and ipaH gene sequences for the general identification of Shigella spp. and enteroinvasive Escherichia coli. J Clin Microbiol 27:2687–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasqua M, Michelacci V, Di Martino ML, Tozzoli R, Grossi M, Colonna B, Morabito S, Prosseda G. 2017. The intriguing evolutionary journey of enteroinvasive E. coli (EIEC) toward pathogenicity. Front Microbiol 8:2390. doi: 10.3389/fmicb.2017.02390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Beld MJ, Reubsaet FA. 2012. Differentiation between Shigella, enteroinvasive Escherichia coli (EIEC) and noninvasive Escherichia coli. Eur J Clin Microbiol Infect Dis 31:899–904. doi: 10.1007/s10096-011-1395-7. [DOI] [PubMed] [Google Scholar]
- 23.Pupo GM, Lan R, Reeves PR. 2000. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc Natl Acad Sci U S A 97:10567–10572. doi: 10.1073/pnas.180094797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahl JW, Morris CR, Emberger J, Fraser CM, Ochieng JB, Juma J, Fields B, Breiman RF, Gilmour M, Nataro JP, Rasko DA. 2015. Defining the phylogenomics of Shigella species: a pathway to diagnostics. J Clin Microbiol 53:951–960. doi: 10.1128/JCM.03527-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prosseda G, Di Martino ML, Campilongo R, Fioravanti R, Micheli G, Casalino M, Colonna B. 2012. Shedding of genes that interfere with the pathogenic lifestyle: the Shigella model. Res Microbiol 163:399–406. doi: 10.1016/j.resmic.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Lan R, Alles MC, Donohoe K, Martinez MB, Reeves PR. 2004. Molecular evolutionary relationships of enteroinvasive Escherichia coli and Shigella spp. Infect Immun 72:5080–5088. doi: 10.1128/IAI.72.9.5080-5088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pupo GM, Karaolis DK, Lan R, Reeves PR. 1997. Evolutionary relationships among pathogenic and nonpathogenic Escherichia coli strains inferred from multilocus enzyme electrophoresis and mdh sequence studies. Infect Immun 65:2685–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chattaway MA, Schaefer U, Tewolde R, Dallman TJ, Jenkins C. 2017. Identification of Escherichia coli and Shigella species from whole-genome sequences. J Clin Microbiol 55:616–623. doi: 10.1128/JCM.01790-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McArthur MA, Maciel M Jr, Pasetti MF. 2017. Human immune responses against Shigella and enterotoxigenic E. coli: current advances and the path forward. Vaccine 35:6803–6806. doi: 10.1016/j.vaccine.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coimbra RS, Grimont F, Grimont PA. 1999. Identification of Shigella serotypes by restriction of amplified O-antigen gene cluster. Res Microbiol 150:543–553. doi: 10.1016/S0923-2508(99)00103-5. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q, Lan R, Wang Y, Zhao A, Zhang S, Wang J, Wang Y, Xia S, Jin D, Cui Z, Zhao H, Li Z, Ye C, Zhang S, Jing H, Xu J. 2011. Development of a multiplex PCR assay targeting O-antigen modification genes for molecular serotyping of Shigella flexneri. J Clin Microbiol 49:3766–3770. doi: 10.1128/JCM.01259-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Ploeg CA, Roge AD, Bordagorria XL, de Urquiza MT, Celi Castillo AB, Bruno SB. 2017. Design of two multiplex PCR assays for serotyping Shigella flexneri. Foodborne Pathog Dis 15:33–38. doi: 10.1089/fpd.2017.2328. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Cao B, Liu B, Liu D, Gao Q, Peng X, Wu J, Bastin DA, Feng L, Wang L. 2009. Molecular detection of all 34 distinct O-antigen forms of Shigella. J Med Microbiol 58:69–81. doi: 10.1099/jmm.0.000794-0. [DOI] [PubMed] [Google Scholar]
- 34.Wetterstrand K. 2017. DNA sequencing costs: data from the NHGRI Genome Sequencing Program (GSP). https://www.genome.gov/27541954/dna-sequencing-costs-data/.
- 35.Hyma KE, Lacher DW, Nelson AM, Bumbaugh AC, Janda JM, Strockbine NA, Young VB, Whittam TS. 2005. Evolutionary genetics of a new pathogenic Escherichia species: Escherichia albertii and related Shigella boydii strains. J Bacteriol 187:619–628. doi: 10.1128/JB.187.2.619-628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons DA, Romanowska E. 1987. Structure and biology of Shigella flexneri O antigens. J Med Microbiol 23:289–302. doi: 10.1099/00222615-23-4-289. [DOI] [PubMed] [Google Scholar]
- 37.Clark CA, Beltrame J, Manning PA. 1991. The oac gene encoding a lipopolysaccharide O-antigen acetylase maps adjacent to the integrase-encoding gene on the genome of Shigella flexneri bacteriophage Sf6. Gene 107:43–52. doi: 10.1016/0378-1119(91)90295-M. [DOI] [PubMed] [Google Scholar]
- 38.Gohmann S, Manning PA, Alpert CA, Walker MJ, Timmis KN. 1994. Lipopolysaccharide O-antigen biosynthesis in Shigella dysenteriae serotype 1: analysis of the plasmid-carried rfp determinant. Microb Pathog 16:53–64. doi: 10.1006/mpat.1994.1005. [DOI] [PubMed] [Google Scholar]
- 39.Bastin DA, Lord A, Verma NK. 1997. Cloning and analysis of the glucosyl transferase gene encoding type I antigen in Shigella flexneri. FEMS Microbiol Lett 156:133–139. doi: 10.1111/j.1574-6968.1997.tb12718.x. [DOI] [PubMed] [Google Scholar]
- 40.Huan PT, Bastin DA, Whittle BL, Lindberg AA, Verma NK. 1997. Molecular characterization of the genes involved in O-antigen modification, attachment, integration and excision in Shigella flexneri bacteriophage SfV. Gene 195:217–227. doi: 10.1016/S0378-1119(97)00143-1. [DOI] [PubMed] [Google Scholar]
- 41.Guan S, Verma NK. 1998. Serotype conversion of a Shigella flexneri candidate vaccine strain via a novel site-specific chromosome-integration system. FEMS Microbiol Lett 166:79–87. doi: 10.1111/j.1574-6968.1998.tb13186.x. [DOI] [PubMed] [Google Scholar]
- 42.Adhikari P, Allison G, Whittle B, Verma NK. 1999. Serotype 1a O-antigen modification: molecular characterization of the genes involved and their novel organization in the Shigella flexneri chromosome. J Bacteriol 181:4711–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Qu W, Reeves PR. 2001. Sequence analysis of four Shigella boydii O-antigen loci: implication for Escherichia coli and Shigella relationships. Infect Immun 69:6923–6930. doi: 10.1128/IAI.69.11.6923-6930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng L, Senchenkova SN, Yang J, Shashkov AS, Tao J, Guo H, Zhao G, Knirel YA, Reeves P, Wang L. 2004. Structural and genetic characterization of the Shigella boydii type 13 O antigen. J Bacteriol 186:383–392. doi: 10.1128/JB.186.2.383-392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng L, Tao J, Guo H, Xu J, Li Y, Rezwan F, Reeves P, Wang L. 2004. Structure of the Shigella dysenteriae 7 O antigen gene cluster and identification of its antigen specific genes. Microb Pathog 36:109–115. doi: 10.1016/j.micpath.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Tao J, Feng L, Guo H, Li Y, Wang L. 2004. The O-antigen gene cluster of Shigella boydii O11 and functional identification of its wzy gene. FEMS Microbiol Lett 234:125–132. doi: 10.1016/j.femsle.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 47.Feng L, Senchenkova SN, Wang W, Shashkov AS, Liu B, Shevelev SD, Liu D, Knirel YA, Wang L. 2005. Structural and genetic characterization of the Shigella boydii type 18 O antigen. Gene 355:79–86. doi: 10.1016/j.gene.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Senchenkova SN, Feng L, Yang J, Shashkov AS, Cheng J, Liu D, Knirel YA, Reeves PR, Jin Q, Ye Q, Wang L. 2005. Structural and genetic characterization of the Shigella boydii type 10 and type 6 O antigens. J Bacteriol 187:2551–2554. doi: 10.1128/JB.187.7.2551-2554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao J, Wang L, Liu D, Li Y, Bastin DA, Geng Y, Feng L. 2005. Molecular analysis of Shigella boydii O1 O-antigen gene cluster and its PCR typing. Can J Microbiol 51:387–392. doi: 10.1139/w05-015. [DOI] [PubMed] [Google Scholar]
- 50.Liu B, Senchenkova SN, Feng L, Perepelov AV, Xu T, Shevelev SD, Zhu Y, Shashkov AS, Zou M, Knirel YA, Wang L. 2006. Structural and molecular characterization of Shigella boydii type 16 O antigen. Gene 380:46–53. doi: 10.1016/j.gene.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 51.Senchenkova SN, Feng L, Wang Q, Perepelov AV, Qin D, Shevelev SD, Ren Y, Shashkov AS, Knirel YA, Wang L. 2006. Structural and genetic characterization of Shigella boydii type 17 O antigen and confirmation of two new genes involved in the synthesis of glucolactilic acid. Biochem Biophys Res Commun 349:289–295. doi: 10.1016/j.bbrc.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 52.Stagg RM, Tang SS, Carlin NI, Talukder KA, Cam PD, Verma NK. 2009. A novel glucosyltransferase involved in O-antigen modification of Shigella flexneri serotype 1c. J Bacteriol 191:6612–6617. doi: 10.1128/JB.00628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Q, Lan R, Wang Y, Wang J, Xia S, Wang Y, Zhang J, Yu D, Li Z, Jing H, Xu J. 2012. Identification of a divergent O-acetyltransferase gene oac 1b from Shigella flexneri serotype 1b strains. Emerg Microbes Infect 1:e21. doi: 10.1038/emi.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J, Lindsey RL, Garcia-Toledo L, Loparev VN, Rowe LA, Batra D, Juieng P, Stoneburg D, Martin H, Knipe K, Smith P, Strockbine N. 2018. High-quality whole-genome sequences for 59 historical Shigella strains generated with PacBio sequencing. Genome Announc 6:e00282-18. doi: 10.1128/genomeA.00282-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho MS, Ahn TY, Joh K, Kwon OS, Jheong WH, Park DS. 2012. A novel marker for the species-specific detection and quantitation of Shigella sonnei by targeting a methylase gene. J Microbiol Biotechnol 22:1113–1117. doi: 10.4014/jmb.1111.11006. [DOI] [PubMed] [Google Scholar]
- 56.Maurelli AT, Blackmon B, Curtiss R III.. 1984. Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect Immun 43:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beutin L, Strauch E, Fischer I. 1999. Isolation of Shigella sonnei lysogenic for a bacteriophage encoding gene for production of Shiga toxin. Lancet 353:1498. doi: 10.1016/S0140-6736(99)00961-7. [DOI] [PubMed] [Google Scholar]
- 58.Gray MD, Lampel KA, Strockbine NA, Fernandez RE, Melton-Celsa AR, Maurelli AT. 2014. Clinical isolates of Shiga toxin 1a-producing Shigella flexneri with an epidemiological link to recent travel to Hispaniola. Emerg Infect Dis 20:1669–1677. doi: 10.3201/eid2010.140292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta SK, Strockbine N, Omondi M, Hise K, Fair MA, Mintz E. 2007. Emergence of Shiga toxin 1 genes within Shigella dysenteriae type 4 isolates from travelers returning from the island of Hispanola. Am J Trop Med Hyg 76:1163–1165. doi: 10.4269/ajtmh.2007.76.1163. [DOI] [PubMed] [Google Scholar]
- 60.Lamba K, Nelson JA, Kimura AC, Poe A, Collins J, Kao AS, Cruz L, Inami G, Vaishampayan J, Garza A, Chaturvedi V, Vugia DJ. 2016. Shiga toxin 1-producing Shigella sonnei infections, California, United States, 2014-2015. Emerg Infect Dis 22:679–686. doi: 10.3201/eid2204.151825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nyholm O, Lienemann T, Halkilahti J, Mero S, Rimhanen-Finne R, Lehtinen V, Salmenlinna S, Siitonen A. 2015. Characterization of Shigella sonnei isolate carrying Shiga toxin 2-producing gene. Emerg Infect Dis 21:891–892. doi: 10.3201/eid2105.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, Strockbine NA, Melton-Celsa AR, Sanchez M, Persson S, O’Brien AD. 2012. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol 50:2951–2963. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walters LL, Raterman EL, Grys TE, Welch RA. 2012. Atypical Shigella boydii 13 encodes virulence factors seen in attaching and effacing Escherichia coli. FEMS Microbiol Lett 328:20–25. doi: 10.1111/j.1574-6968.2011.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lobersli I, Wester AL, Kristiansen A, Brandal LT. 2016. Molecular differentiation of Shigella spp. from enteroinvasive E. coli. Eur J Microbiol Immunol 6:197–205. doi: 10.1556/1886.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hazen TH, Leonard SR, Lampel KA, Lacher DW, Maurelli AT, Rasko DA. 2016. Investigating the relatedness of enteroinvasive Escherichia coli to other E. coli and Shigella isolates by using comparative genomics. Infect Immun 84:2362–2371. doi: 10.1128/IAI.00350-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casalino M, Latella MC, Prosseda G, Ceccarini P, Grimont F, Colonna B. 2005. Molecular evolution of the lysine decarboxylase-defective phenotype in Shigella sonnei. Int J Med Microbiol 294:503–512. doi: 10.1016/j.ijmm.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Day WA Jr, Fernandez RE, Maurelli AT. 2001. Pathoadaptive mutations that enhance virulence: genetic organization of the cadA regions of Shigella spp. Infect Immun 69:7471–7480. doi: 10.1128/IAI.69.12.7471-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joensen KG, Tetzschner AM, Iguchi A, Aarestrup FM, Scheutz F. 2015. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J Clin Microbiol 53:2410–2426. doi: 10.1128/JCM.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H. 2018. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grozdanov L, Zahringer U, Blum-Oehler G, Brade L, Henne A, Knirel YA, Schombel U, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, Rietschel ET, Dobrindt U. 2002. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J Bacteriol 184:5912–5925. doi: 10.1128/JB.184.21.5912-5925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kia A, Gloeckner C, Osothprarop T, Gormley N, Bomati E, Stephenson M, Goryshin I, He MM. 2017. Improved genome sequencing using an engineered transposase. BMC Biotechnol 17:6. doi: 10.1186/s12896-016-0326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green B, Bouchier C, Fairhead C, Craig NL, Cormack BP. 2012. Insertion site preference of Mu, Tn5, and Tn7 transposons. Mob DNA 3:3. doi: 10.1186/1759-8753-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knirel YA, Sun Q, Senchenkova SN, Perepelov AV, Shashkov AS, Xu J. 2015. O-antigen modifications providing antigenic diversity of Shigella flexneri and underlying genetic mechanisms. Biochemistry 80:901–914. doi: 10.1134/S0006297915070093. [DOI] [PubMed] [Google Scholar]
- 76.Sansonetti PJ, Kopecko DJ, Formal SB. 1981. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun 34:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chattaway MA, Greig DR, Gentle A, Hartman HB, Dallman TJ, Jenkins C. 2017. Whole-genome sequencing for national surveillance of Shigella flexneri. Front Microbiol 8:1700. doi: 10.3389/fmicb.2017.01700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Svab D, Balint B, Vasarhelyi B, Maroti G, Toth I. 2017. Comparative genomic and phylogenetic analysis of a Shiga toxin producing Shigella sonnei (STSS) strain. Front Cell Infect Microbiol 7:229. doi: 10.3389/fcimb.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Martino ML, Fioravanti R, Barbabella G, Prosseda G, Colonna B, Casalino M. 2013. Molecular evolution of the nicotinic acid requirement within the Shigella/EIEC pathotype. Int J Med Microbiol 303:651–661. doi: 10.1016/j.ijmm.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Zhang S, Yin Y, Jones MB, Zhang Z, Deatherage Kaiser BL, Dinsmore BA, Fitzgerald C, Fields PI, Deng X. 2015. Salmonella serotype determination utilizing high-throughput genome sequencing data. J Clin Microbiol 53:1685–1692. doi: 10.1128/JCM.00323-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hunt M, Mather AE, Sanchez-Buso L, Page AJ, Parkhill J, Keane JA, Harris SR. 2017. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genom 3:e000131. doi: 10.1099/mgen.0.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inouye M, Dashnow H, Raven LA, Schultz MB, Pope BJ, Tomita T, Zobel J, Holt KE. 2014. SRST2: rapid genomic surveillance for public health and hospital microbiology labs. Genome Med 6:90. doi: 10.1186/s13073-014-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li H. 2011. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta A, Jordan IK, Rishishwar L. 2017. stringMLST: a fast k-mer based tool for multilocus sequence typing. Bioinformatics 33:119–121. doi: 10.1093/bioinformatics/btw586. [DOI] [PubMed] [Google Scholar]
- 86.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Thomason JA III, Stevens R, Vonstein V, Wattam AR, Xia F. 2015. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365. doi: 10.1038/srep08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jin Q, Yuan Z, Xu J, Wang Y, Shen Y, Lu W, Wang J, Liu H, Yang J, Yang F, Zhang X, Zhang J, Yang G, Wu H, Qu D, Dong J, Sun L, Xue Y, Zhao A, Gao Y, Zhu J, Kan B, Ding K, Chen S, Cheng H, Yao Z, He B, Chen R, Ma D, Qiang B, Wen Y, Hou Y, Yu J. 2002. Genome sequence of Shigella flexneri 2a: insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Res 30:4432–4441. doi: 10.1093/nar/gkf566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang Y, Yang F, Zhang X, Yang J, Chen L, Yan Y, Nie H, Xiong Z, Wang J, Dong J, Xue Y, Xu X, Zhu Y, Chen S, Jin Q. 2005. The complete sequence and analysis of the large virulence plasmid pSS of Shigella sonnei. Plasmid 54:149–159. doi: 10.1016/j.plasmid.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 93.Jakhetia R, Talukder KA, Verma NK. 2013. Isolation, characterization and comparative genomics of bacteriophage SfIV: a novel serotype converting phage from Shigella flexneri. BMC Genomics 14:677. doi: 10.1186/1471-2164-14-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huan PT, Whittle BL, Bastin DA, Lindberg AA, Verma NK. 1997. Shigella flexneri type-specific antigen V: cloning, sequencing and characterization of the glucosyl transferase gene of temperate bacteriophage SfV. Gene 195:207–216. doi: 10.1016/S0378-1119(97)00144-3. [DOI] [PubMed] [Google Scholar]
- 95.Huan PT, Taylor R, Lindberg AA, Verma NK. 1995. Immunogenicity of the Shigella flexneri serotype Y (SFL 124) vaccine strain expressing cloned glucosyl transferase gene of converting bacteriophage SfX. Microbiol Immunol 39:467–472. doi: 10.1111/j.1348-0421.1995.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 96.Tang SS, Carlin NI, Talukder KA, Cam PD, Verma NK. 2016. Shigella flexneri serotype 1c derived from serotype 1a by acquisition of gtrIC gene cluster via a bacteriophage. BMC Microbiol 16:127. doi: 10.1186/s12866-016-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Casjens S, Winn-Stapley DA, Gilcrease EB, Morona R, Kuhlewein C, Chua JE, Manning PA, Inwood W, Clark AJ. 2004. The chromosome of Shigella flexneri bacteriophage Sf6: complete nucleotide sequence, genetic mosaicism, and DNA packaging. J Mol Biol 339:379–394. doi: 10.1016/j.jmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 98.Ye C, Lan R, Xia S, Zhang J, Sun Q, Zhang S, Jing H, Wang L, Li Z, Zhou Z, Zhao A, Cui Z, Cao J, Jin D, Huang L, Wang Y, Luo X, Bai X, Wang Y, Wang P, Xu Q, Xu J. 2010. Emergence of a new multidrug-resistant serotype X variant in an epidemic clone of Shigella flexneri. J Clin Microbiol 48:419–426. doi: 10.1128/JCM.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang F, Yang J, Zhang X, Chen L, Jiang Y, Yan Y, Tang X, Wang J, Xiong Z, Dong J, Xue Y, Zhu Y, Xu X, Sun L, Chen S, Nie H, Peng J, Xu J, Wang Y, Yuan Z, Wen Y, Yao Z, Shen Y, Qiang B, Hou Y, Yu J, Jin Q. 2005. Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res 33:6445–6458. doi: 10.1093/nar/gki954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suzuki S, Ono N, Furusawa C, Ying BW, Yomo T. 2011. Comparison of sequence reads obtained from three next-generation sequencing platforms. PLoS One 6:e19534. doi: 10.1371/journal.pone.0019534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fasano A, Noriega FR, Maneval DR Jr, Chanasongcram S, Russell R, Guandalini S, Levine MM. 1995. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J Clin Invest 95:2853–2861. doi: 10.1172/JCI117991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nataro JP, Seriwatana J, Fasano A, Maneval DR, Guers LD, Noriega F, Dubovsky F, Levine MM, Morris JG Jr. 1995. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect Immun 63:4721–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guyer DM, Radulovic S, Jones FE, Mobley HL. 2002. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect Immun 70:4539–4546. doi: 10.1128/IAI.70.8.4539-4546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences generated in this study have been deposited in the NCBI Sequence Read Archive under the BioProject number PRJNA490540; accession numbers for each of the strains are listed in Table S1. The stand-alone Shigella serotyping pipeline, together with instructions for system setup and running, is available by request or at https://github.com/CFSAN-Biostatistics/shigatyper. An online version will be made available soon on GalaxyTrakr (https://galaxytrakr.org).