Abstract
Circadian rhythms control many biochemical and physiological functions within the body of an organism. These circadian rhythms are generated by a molecular clock that is located in almost every cell of the body. Accumulating data indicate that dysfunction of the circadian clock negatively affects the health status of the tissue in which the circadian clock has been disabled. The eye also contains a complex circadian system that regulates many important functions such as the processing of light information, the release of neurotransmitters, and phagocytic activity by the retinal pigment epithelium, to name just a few. Emerging experimental evidence indicates that dysfunction of the circadian clock within the retina has severe consequence for retinal function and photoreceptor viability. The aim of this review is to provide the reader with a summary of current knowledge about the eye circadian system and what effects emerge with a disruption of this system.
Keywords: circadian clock, Bmal1, retina, RPE, cornea
Introduction
Circadian rhythms are present in nearly all organisms on the planet. These rhythms are generated by series of transcriptional-translational feedback loops [1]. The circadian clock plays a key role in the regulation of the metabolic processes within a cell, hence it is not surprising that dysfunction of circadian rhythms – by genetic or environmental factors – are believed to be a cofactor in the development of several pathologies such as: metabolic disorders [2], cancer [3,4], inflammation [5], and premature aging [6-8] to name just a few. Indeed, the importance of the circadian clock in regulating numerous functions within organisms has been recently underscored by awarding of the 2017 Nobel Prize in Physiology or Medicine to Drs. Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for their discoveries of molecular mechanisms controlling the circadian rhythm. The aims of this review are to describe the role that circadian clocks play in the regulation of ocular functions and the consequences that circadian disruption may have on the eye of mammals.
Molecular Clockwork in Mammals
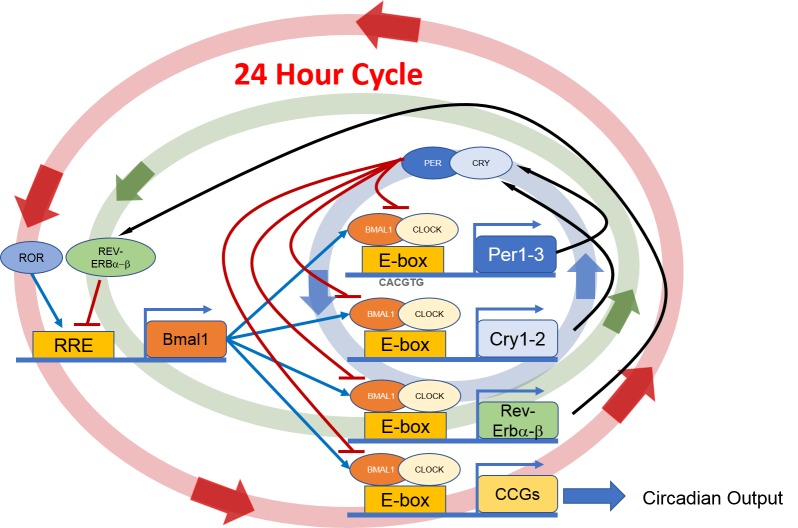
The accepted model for the molecular clockwork in mammals has two interlocking transcription-translation feedback loops involving several “clock genes” and their protein products, which ultimately regulate the transcription of other genes called clock-controlled genes (CCGs). These feedback loops consist of positive and negative components. The positive components include the basic helix-loop-helix-PAS domain transcription factors, CLOCK and BMAL1. These transcription factors heterodimerize and bind to E-box promoter elements that enhance the transcription of genes encoding the negative components PERIOD (PER) 1, 2 and CRYPTOCHROME (CRY) 1, 2. The PER and CRY proteins feedback inhibit the transcription of the Per and Cry genes by blocking CLOCK/BMAL1-mediated trans-activation (Figure 1). The second feedback loop involves the transactivation of the Rev-Erbα, Rev-Erbβ, and Rora genes by CLOCK/BMAL1. The protein products of these genes compete for binding to RRE elements in the Bmal1 promoter, driving a daily rhythm of Bmal1 transcription and closing the second feedback loop [1]. The circadian clock directly controls the rhythmic transcription of CCGs via the BMAL1/CLOCK complex that binds to DNA sequences (E-boxes) that are present on the promoter region of these genes that ultimately regulate physiological functions.
Figure 1.
Schematic illustration of the molecular circadian clock. BMAL1: CLOCK heterodimer binds to E-box present on the promoter region of Per and Cry. Then PER together with CRY inhibit their own transcript. The second feedback loop involves the transactivation of the Rev-Erbα, Rev-Erbβ, and Rora genes by CLOCK/BMAL1. Then REV-ERBα, REV-ERBβ and RORA compete for binding to RRE elements in the Bmal1 promoter, driving a daily rhythm of Bmal1 transcription and the second feedback loop. These feedback loops generate a 24-hour rhythmic oscillation.
Circadian Clocks in the Mammalian Eye
Several studies have shown that many aspects of mammalian eye physiology are under the control of retinal circadian clocks and these clocks control neurotransmitter release, processing of visual information, and cellular processes [9-11]. A few excellent reviews have recently been published on this topic, hence this review focuses on the effects of dysregulation in circadian rhythms within the eye and impact on the function and health of this organ.
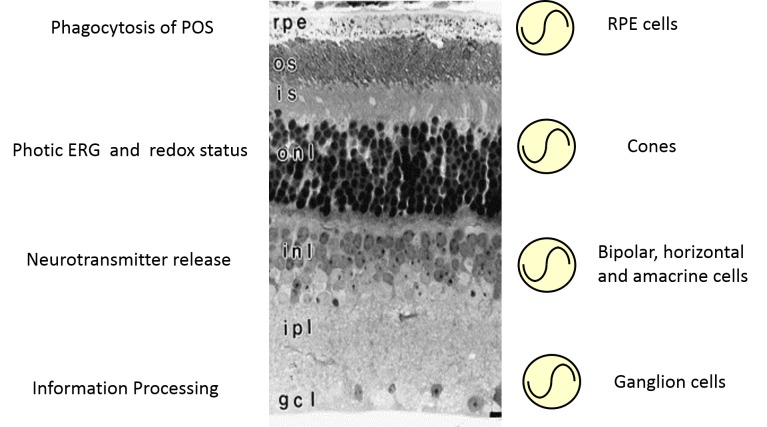
Within the eye, the retina – and more specifically the photoreceptors – is the tissue that perceives light and then via neuronal pathways (i.e., the optic nerve and the retinal hypothalamic tract) communicate this light information to image forming and non-image forming brain nuclei where these signals are further processed to produce vision or to entrain the body’s circadian rhythms [12-14]. The retina is a heterogenous collection of different neuronal cell types that are organized into discreet layers. These layers can be classified based on their physical characteristics with the nuclei of a cell type forming the nuclear layer and the synapses between cell types that form the plexiform layers [15]. Using genetically-modified animal models in which a circadian clock could be monitored in real-time [16], previous studies have shown that circadian rhythms in this tissue can be generated in the photoreceptor layers [17,18], the inner retinal layer and by the ganglion cell layer [17] (Figure 2). However, these studies did not reveal which cells within each retinal layer contains a circadian clock. This question was addressed by a seminal study using single cell RT-PCR, where Ruan et al. [19] reported that within the retina only horizontal, bipolar, amacrine, and ganglion neurons simultaneously expressed all the canonical clock genes, whereas no cell within the photoreceptor layer was found to contain the necessary molecular machinery to produce a circadian oscillation. This somewhat contradictory result (see [17,18]) was later explained by a study in which the distribution of clock proteins was investigated in the mouse retina [20]. The results of this study mostly confirm the previous study by Ruan et al. [19], but it also revealed that cones (which comprise only 2 to 3 percent of the total photoreceptors in the mouse and thus may be easily missed in the sampling by Ruan et al. [19]) also harbor all the molecular machinery necessary to generate a circadian oscillation [20]. The notion that cones contain a circadian clock is further supported by electroretinogram (ERGs) studies which have demonstrated that – at least in the mouse – only the photic (i.e., the cone mediated response) ERG is under circadian control whereas the scotopic ERG (i.e., the rod mediated response) does not show any circadian regulation [21-24]. It is also worthwhile mentioning that in addition to retinal neuronal cells, Mueller cells in the mouse retina contain functional circadian clocks [25]. Hence, it appears that within the retina – as seen in the rest of the body – the majority of cells contain a circadian clock.
Figure 2.
Schematic representation of the circadian organization in the mammalian retina. Circadian clocks are present in many retinal cell types where they control specific cellular functions. Dysfunction of the circadian clock in retinal cells affected the functioning and viability of retinal cells.
The next question that many scientists around the world started to focus on was the effect of removing clock genes (i.e., disruption of circadian clockwork, Figure 1) on retinal cell function and health. The first study that tried to address this question was performed in Dr. Weitz’s laboratory [22]. In this very elegant study, the authors reported that in the mouse retina many genes (more than a thousand) show a circadian rhythm and a large fraction of these genes were no longer rhythmic in the retina of mice lacking Bmal1. They also reported that removal of Bmal1 abolished the circadian rhythm in the amplitude of the b-wave of the photic ERG [22]. Interestingly, they did not find any significant alteration in retinal cell morphology, at least in young mice. Since these experiments were performed on a global Bmal1 Knock-Out (KO) mouse, the authors could not conclude whether the results obtained were due to a dysfunctional retinal clock or whether the master circadian clock (i.e., superchiasmatic nucleus) located in the brain could have also contributed to the observed results. To address this important question, the authors decided to produce a mouse in which Bmal1 was only removed from the retina (Chx10Cre; Bmal1Fl/Fl mice [22]). The data obtained with this retinal specific Bmal1 KO mouse confirmed previous findings in the global Bmal1 KO mouse, thus demonstrating that the retinal circadian clock is actually controlling the circadian rhythms in the photic ERGs [22]. The next study that tackled this important question investigated the effects of Per1 and Per2 removal on the health of retina [26]. This investigation indicated that deletion of both Per1 and Per2 somewhat affected the organization of the retinal tissue and down-regulated the expression and the distribution of cone opsin (specifically, blue cone opsin) mRNA and protein. In an additional study, Dr. Hicks’ laboratory investigated the effect of Rev-Erbα removal on the retina, and similarly to Per1 and Per2 removal, their results show that a dysfunctional clock affects the processing of the visual information (where an increase in light sensitivity was observed), but there were no significant changes on the morphology of the retina [27]. Finally, the effects of Cryptochrome 1 (Cry1) or 2 (Cry2) removal from the retina was investigated by Stuart Peirson’s laboratory using global Cryptochrome 1 and 2 knock-out mice [28]. The authors reported that contrary to previously published data [29], CRY1 immunoreactivity was observed in all retinal layers and within the photoreceptor layer only in the cone photoreceptors. When Cry 1 or Cry2 KOs were crossed with Period 2::luciferase transgenic mice, the circadian rhythm in bioluminescence was less robust and stable in Cry1 KOs retinas, whereas in Cry2 retinas the circadian rhythm in bioluminescence had a significantly longer period. Cry1 KO mice did not show a circadian rhythm in the amplitude of b-wave under photopic conditions, whereas in Cry2 KO mice they only observed a small reduction in the amplitude of the b-wave of the scotopic ERGs. The rhythms in contrast sensitivity and pupillary light response were also attenuated or even abolished in Cry1 KO mice, but significant effects were observed in Cry2 KO mice. Hence it appears that at least within the retinal clockwork, Cry1 plays a key role in the circadian regulation of retinal functions, whereas the role of Cry2 is marginal.
The observation that CRY1 is only present in the cones agrees well with those of Storch et al. [22] and Liu et al. [20] in supporting the presence of a circadian clock in cone photoreceptors. Hence, from these studies it was concluded that removal of clock genes from the retina has a modest effect and these effects seem to be concentrated on the circadian regulation of visual information processing rather than on retinal cell morphology and/or viability.
This view has been recently challenged by a series of new and exciting studies in which the role of Bmal1 has been further investigated. In the first of these new studies, the authors used a transgenic mice line in which Bmal1 was selectively removed only from the neural retina (Chx10-Cre; Bmal1Fl/Fl mice) or cone photoreceptors (HRGP-Cre; Bmal1Fl/Fl mice) and reported that such a removal has dramatic consequences on the spatial distribution of the cone opsin [30]. In addition, they reported that Bmal1 regulates the expression of thyroid hormone-activating enzyme type 2 iodothyronine deiodinase (Dio2) and treatment with thyroid hormone partially rescued the phenotype produced by Bmal1 removal. This result supports the notion that Bmal1 and Dio2 are involved in determining cone photoreceptor identity [30]. However, apart from a small reduction in the length of the cones, the morphological analysis of photoreceptors revealed that the retina of young (2 to 3 months old) Bmal1 KO mice was mostly unaffected compared to same age controls [30]. An additional study using 8 to 9 month old Bmal1 KO (i.e., the maximum life span for these KO mice [8]) reported a significant reduction (about 30 percent) in the thickness of the outer nuclear layer, but no significant alterations were observed in the thickness of the inner retinal layer and ganglion cells layer [31]. Furthermore, the authors also reported that removal of Clock and Npas2 (i.e., disabling the formation of the Bmal1/Clock or Npas2 dimer, Figure 1) produced an almost identical effect of Bmal1 removal, thus indicating that this effect is due to action of the Bmal1/Clock (Npas2) dimer and not to possible pleiotropic effects of Bmal1 that are independent from the circadian clock.
Finally, our laboratory has further expanded our knowledge on the roles that Bmal1 plays in the regulation of retinal functions [32] by showing that removal of Bmal1 from the retina by using Chx10-Cre; Bmal1Fl/Fl mice has multiple effects on both rod pathway and cone viability. In the rod pathway, we observed that Bmal1 removal caused stunting of rod bipolar cell dendrites and thinning of the outer plexiform layer in both young and old mice. Such an effect is likely to be due to the action of Bmal1 during the development and/or differentiation of these cells since the dendritic abnormalities of the rod bipolar cells are already present in 28-day old mice. In addition, we also reported that the viability of cone photoreceptors during aging is greatly affected by removal of this gene since retinal specific-Bmal1 KO mice at the age of 24 to 26 months have a dramatic reduction in the number of cones (40 to 50 percent) with respect to control mice of the same age [32]. Since loss of cones during aging is a hallmark of age-related macular degeneration, we hope that this new data will stimulate research in humans to investigate whether circadian dysfunctions (such as genetic mutations or shift-work) could be a risk factor for age-related macular degeneration.
Circadian Clock Energy Metabolism and Cone Viability
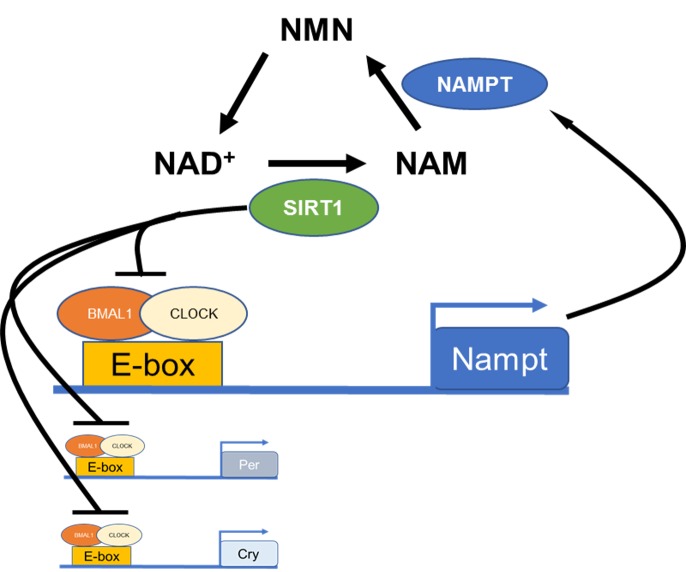
Accumulating evidence indicates that cellular metabolism is under direct control of the circadian clock [2]. Indeed, redox status, adenosine triphosphate (ATP) production, and gene expression in mitochondria are under control of the circadian clock [33]. In mice, clock gene deletion alters metabolism and mitochondrial energy expenditure, and increases oxidative damage in the heart and liver [34-39]. Moreover, several investigations have also shown that levels of the oxidoreductase factor nicotinamide adenine dinucleotide (NAD+) are under direct control of the circadian clock via the transcriptional regulation of nicotinamide phosphoribosyltransferase (NAMPT; [40,41]), the rate-limiting enzyme in NAD+ biosynthesis (Figure 3). Experimental data also indicate that NAMPT modulates cellular metabolism, senescence and photoreceptor survival via the regulation of NAD+-dependent deacetylase SIRTUIN 1 (SIRT1; [42-44]). SIRT1 is expressed in the retina [45] and may be involved in the modulation of photoreceptor health and aging [46-49].
Figure 3.
Schematic illustration of circadian clock regulation in the NAD+ salvage pathway. BMAL1: CLOCK heterodimer binds to an E-box present on the promoter region of Nampt to regulate the rhythmic transcription of this gene and thus the levels of NAD+ and SIRT1. SIRT1 also regulates clock gene transcript via deacetylation of histones.
In this context is worth noting that cone photoreceptors are among the cells in the body with the highest metabolic demand [50] and they contain more mitochondria, produce more ATP, show more cytochrome c oxidase reactivity and thus more production of reactive oxygen species that any other retinal cell type [51]. Hence, even a relatively small perturbation of cellular metabolism may have significant negative effects on the cell and therefore we believe that the loss of cones observed in aged retinal specific-Bmal1 KO mice may be a consequence of a dysfunctional circadian clock on mitochondrial functions and thus decreases the capability of cone photoreceptors to respond to oxidative stress [32].
Finally, a recent study has reported that genetic removal of Nampt from rod or cone photoreceptors produces degeneration of these cells and exogenous administration of nicotinamide mononucleotide (NMN, a NAD+ precursor) prevents the degeneration of the photoreceptors in Nampt KO mice [52]. Thus, it is plausible to speculate that administration of exogenous NMN can prevent the loss of cones in retinal specific-Bmal1 KO during aging [32] and thus mitigate the effects of circadian clock dysfunction on cone metabolism.
In addition to the retina, the retinal pigment epithelium (RPE) displays a circadian rhythm in the disk shedding and phagocytic activity of rod and cone photoreceptors outer segments [53-55] that is independent from the master circadian clock located in the brain [56]. However, it still unclear whether the circadian clock controlling this rhythm is located in the RPE or in the retina. Our recent work has shown that the RPE also contains a circadian clock that is independent of the master circadian clock located in the brain [57], albeit a signal from the retina is responsible for entrainment of the RPE circadian clock [58]. No study so far has investigated whether disruption of the circadian clock has any effect on the function and health of this tissue. However, indirect evidence suggests that removing the burst of phagocytic activity after the onset of light [59] or by changing the time of the of peak [60] is detrimental to this function of the RPE and its ability to support retinal photoreceptors. In addition, our study with global and retinal specific-Bmal1 KOs suggests that the RPE clock may affect rod viability since global Bmal1 KO show a severe phenotype with respect to photoreceptor loss compared to retinal specific-Bmal1 KO mice [31,32].
Finally, we would like to mention that the circadian rhythm in the retina, RPE, and cornea showed a significant reduction in the amplitude in aged mice and such a reduction seems to be more pronounced in the retina than in the RPE or the cornea [61]. However, it is not known whether the reduction of these rhythms is due to a decrease in the strength of the circadian clock that may occur with aging or whether the loss of synchrony amongst the different cells is responsible for the decrease in the amplitude.
Conclusions
Over the last twenty years, the field of circadian biology has grown tremendously and it is now clear that this system has a tremendous impact on the life of an organism. As stated previously, the importance of this biological system was recognized with awarding of the 2017 Nobel Prize in Medicine or Physiology to three circadian biologists.
The study of circadian rhythms within the eye (Figure 2) is an important topic of investigation and the data so far collected indicates that circadian dysfunctions produce significant alterations in eye function and health. We hope that awarding of the 2017 Nobel prize in Medicine or Physiology to the field of circadian biology will generate even greater emphasis on the role that this biological system plays in health and disease of an organism and how physicians and other health care providers will translate this new knowledge into new medical treatments [62].
Glossary
- ATP
adenosine triphosphate
- BMAL1
brain and muscle arnt-like 1
- CCGs
clock-controlled genes
- CLOCK
circadian locomotor output cycles protein kaput
- Cry
cryptochrome
- Dio2
type 2 iodothyronine deiodinase
- ERG
electroretinogram
- KO
knock-out
- mRNA
messenger ribonucleic acid
- NAD+
nicotinamide adenine dinucleotide
- NAMPT
nicotinamide phosphoribosyltransferase
- Per
period
- RPE
retinal pigment epithelium
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SIRT1
sirtuin 1
Author Contributions
This work was supported by grants from the National Institutes of Health: EY026291 to GT and GM116760 to KB and by NS083932. CD, KB, and GT, wrote the paper. KB and GT designed the Figures.
References
- Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgs L, Beukelaers P, Vandenbosch R, Belachew S, Nguyen L, Malgrange B. Cell “circadian” cycle: new role for mammalian core clock genes. Cell Cycle. 2009;8(6):832–7. [DOI] [PubMed] [Google Scholar]
- Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY). 2011;3(5):479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong KK, Lam MT, Grandner MA, Sassoon CS, Malhotra A. Timing matters: circadian rhythm in sepsis, obstructive lung disease, obstructive sleep apnea, and cancer. Ann Am Thorac Soc. 2016;13(7):1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY). 2010;2(12):936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khapre RV, Kondratova AA, Susova O, Kondratov RV. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle. 2011. December;10(23):4162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20(14):1868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder-Schmittbuhl M-P, Buhr ED, Dkhissi-Benyahya O, Hicks D, Peirson N, Ribelayga CP, et al. Ocular clocks: adapting mechanisms for eye functions and health. Invest Ophthalmol Vis Sci. 2018; 59(12): 4856-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY. Circadian regulation in the retina: from molecules to network [Epub ahead of print] Eur J Neurosci. 2018: 10.1111/ejn.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39:58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476(7358):92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2(31):31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KN, Saafir TB, Tosini G. The role of retinal photoreceptors in the regulation of circadian rhythms. Rev Endocr Metab Disord. 2009;10(4):271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. Simple anatomy of the retina In: Kolb H, Fernandez E, Nelson R. Webvision: The organization of the retina and visual system. Salt Lake City (UT): University of Utah Health Sciences Center; 1995. [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101(15):5339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger C, Sandu C, Malan A, Mellac K, Hicks D, Felder-Schmittbuhl MP. Circadian organization of the rodent retina involves strongly coupled, layer-specific oscillators. FASEB J. 2015;29(4):1493–504. [DOI] [PubMed] [Google Scholar]
- Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21(14):3866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci USA. 2006;103(25):9703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ribelayga CP. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS One. 2012;7(11):e50602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Baba K, Mazzoni F, Pozdeyev NV, Strettoi E, Iuvone PM, et al. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS One. 2011;6(9):e24483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130(4):730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MA, Barnard AR, Hut RA, Bonnefont X, van der Horst GT, Hankins MW, et al. Electroretinography of wild-type and cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. J Biol Rhythms. 2008;23(6):489–501. [DOI] [PubMed] [Google Scholar]
- Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, et al. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci. 2012;32(27):9359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ruan G, Dai H, Liu AC, Penn J, McMahon DG. Mammalian retinal Müller cells have circadian clock function. Mol Vis. 2016;22:275–83. [PMC free article] [PubMed] [Google Scholar]
- Ait-Hmyed Hakkari O, Felder-Schmittbuhl MP, Garcia-Garrido M, Beck S, Seide C, Sothilingam V, et al. Mice lacking period 1 and period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci. 2013;37(7):1048–60. [DOI] [PubMed] [Google Scholar]
- Ait-Hmyed Hakkari O, Acar N, Savier E, Spinnhirny P, Bennis M, Felder-Schmittbuhl MP, et al. Rev-Erbα modulates retinal visual processing and behavioral responses to light. FASEB J. 2016;30(11):3690–701. [DOI] [PubMed] [Google Scholar]
- Wong JC, Smyllie NJ, Banks GT, Pothecary CA, Barnard AR, Maywood ES, et al. Differential roles for cryptochromes in the mammalian retinal clock. FASEB J. 2018;32(8):4302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci USA. 1998;95(11):6097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant OB, Horton AM, Zucaro OF, Chan R, Bonilha VL, Samuels IS, et al. The circadian clock gene bmal1 controls thyroid hormone-mediated spectral identity and cone photoreceptor function. Cell Rep. 2017;21(3):692–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Ribelayga CP, Michael Iuvone P, Tosini G. The retinal circadian clock and photoreceptor viability. Adv Exp Med Biol. 2018;1074:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Piano I, Lyuboslavsky P, Chrenek MA, Sellers JT, Zhang S, et al. Removal of clock gene Bmal1 from the retina affects retinal development and accelerates cone photoreceptor degeneration during aging. Proc Natl Acad Sci USA. 2018;115(51):13099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella G, Asher G. The circadian nature of mitochondrial biology. Front Endocrinol. 2016;7:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294(2):H1036–47. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, et al. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289(4):H1530–41. [DOI] [PubMed] [Google Scholar]
- Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, et al. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 2015;22(4):709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Das P, Hashimoto I, Nakao T, Deguchi Y, Gouraud SS, et al. The circadian clock maintains cardiac function by regulating mitochondrial metabolism in mice. PLoS One. 2014;9(11):e112811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoogian EN, Panda S. Circadian clock, nutrient quality, and eating pattern tune diurnal rhythms in the mitochondrial proteome. Proc Natl Acad Sci USA. 2016;113(12):3127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342(6158):1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324(5927):654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324(5927):651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N, Li XY, Zhang HM, Yang Z, Su Q. microRNA-199a-5p mediates high glucose-induced reactive oxygen species production and apoptosis in INS-1 pancreatic β-cells by targeting SIRT1. Eur Rev Med Pharmacol Sci. 2017;21(5):1091–8. [PubMed] [Google Scholar]
- Wang J, Zhang Y, Tang L, Zhang N, Fan D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci Lett. 2011;503(3):250–5. [DOI] [PubMed] [Google Scholar]
- Yang W, Nagasawa K, Münch C, Xu Y, Satterstrom K, Jeong S, et al. Mitochondrial sirtuin network reveals dynamic sirt3-dependent deacetylation in response to membrane depolarization. Cell. 2016;167(4):985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban N, Ozawa Y, Inaba T, Miyake S, Watanabe M, Shinmura K, et al. Light–dark condition regulates sirtuin mRNA levels in the retina. Exp Gerontol. 2013;48(11):1212–7. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA. 2003;100(19):10794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaliffa C, Ameqrane I, Dansault A, Leemput J, Vieira V, Lacassagne E, et al. Sirt1 involvement in rd10 mouse retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50(8):3562–72. [DOI] [PubMed] [Google Scholar]
- Lin R, Yan D, Zhang Y, Liao X, Gong G, Hu J, et al. Common variants in SIRT1 and human longevity in a Chinese population. BMC Med Genet. 2016;17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Kaji Y, Noma H, Funatsu H, Okamoto S. The role of SIRT1 in ocular aging. Exp Eye Res. 2013;116:17–26. [DOI] [PubMed] [Google Scholar]
- Cepko C, Punzo C. Cell metabolism: sugar for sight. Nature. 2015;522(7557):428–9. [DOI] [PubMed] [Google Scholar]
- Perkins BD, Fadool JM, Dowling JE. Photoreceptor structure and development: analyses using GFP transgenes. Methods Cell Biol. 2004;76:315–31. [DOI] [PubMed] [Google Scholar]
- Lin JB, Kubota S, Ban N, Yoshida M, Santeford A, Sene A, et al. NAMPT-mediated NAD+ biosynthesis is essential for vision in mice. Cell Rep. 2016;17(1):69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobu C, Hicks D. Regulation of retinal photoreceptor phagocytosis in a diurnal mammal by circadian clocks and ambient lighting. Invest Ophthalmol Vis Sci. 2009;50(7):3495–502. [DOI] [PubMed] [Google Scholar]
- Grace MS, Chiba A, Menaker M. Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis Neurosci. 1999;16(5):909–18. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194(4269):1071–4. [DOI] [PubMed] [Google Scholar]
- Su Terman J, Reme´ CE, Terman M. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993;605(2):256–64. [DOI] [PubMed] [Google Scholar]
- Baba K, Sengupta A, Tosini M, Contreras-Alcantara S, Tosini G. Circadian regulation of the PERIOD 2:LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis. 2010;16:2605–11. [PMC free article] [PubMed] [Google Scholar]
- Baba K, DeBruyne JP, Tosini G. Dopamine 2 receptor activation entrains circadian clocks in mouse retinal pigment epithelium. Sci Rep. 2017;7(1):5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med. 2004;200(12):1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Sengupta A, Sánchez-Bretaño A, Hicks D, Tosini G. Melatonin signaling affects the timing in the daily rhythm of phagocytic activity by the retinal pigment epithelium. Exp Eye Res. 2017;165:90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Tosini G. Aging alters circadian rhythms in the mouse eye. J Biol Rhythms. 2018;33(4):441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW. Circadian clocks: tips from the tip of the iceberg. Nature. 2008;456(7224):881–3. [DOI] [PubMed] [Google Scholar]