Keywords: nerve regeneration, resveratrol, oxidative stress, endoplasmic reticulum stress, neuroinflammation, subarachnoid hemorrhage, nuclear factor-erythroid 2-related factor 2, heme oxygenase-1, glucose-regulated protein 78, neural regeneration
Abstract
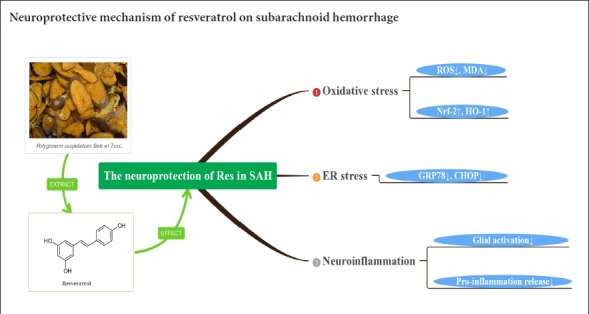
Previous studies have shown that resveratrol, a bioactive substance found in many plants, can reduce early brain injury after subarachnoid hemorrhage, but how it acts is still unclear. This study explored the mechanism using the experimental subarachnoid hemorrhage rat model established by injecting autologous blood into the cerebellomedullary cistern. Rat models were treated with an intraperitoneal injection of 60 mg/kg resveratrol 2, 6, 24 and 46 hours after injury. At 48 hours after injury, their neurological function was assessed using a modified Garcia score. Brain edema was measured by the wet-dry method. Neuronal apoptosis in the prefrontal cortex was detected by terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labeling assay. Levels of reactive oxygen species and malondialdehyde in the prefrontal cortex were determined by colorimetry. CHOP, glucose-regulated protein 78, nuclear factor-erythroid 2-related factor 2 and heme oxygenase-1 mRNA expression levels in the prefrontal cortex were measured by reverse transcription polymerase chain reaction. Tumor necrosis factor-alpha content in the prefrontal cortex was detected by enzyme linked immunosorbent assay. Immunohistochemical staining was used to detect the number of positive cells of nuclear factor-erythroid 2-related factor 2, heme oxygenase 1, glucose-regulated protein 78, CHOP and glial fibrillary acidic protein. Western blot assay was utilized to analyze the expression levels of nuclear factor-erythroid 2-related factor 2, heme oxygenase 1, glucose-regulated protein 78 and CHOP protein expression levels in the prefrontal cortex. The results showed that resveratrol treatment markedly alleviated neurological deficits and brain edema in experimental subarachnoid hemorrhage rats, and reduced neuronal apoptosis in the prefrontal cortex. Resveratrol reduced the levels of reactive oxygen species and malondialdehyde, and increased the expression of nuclear factor-erythroid 2-related factor 2, heme oxygenase-1 mRNA and protein in the prefrontal cortex. Resveratrol decreased glucose-regulated protein 78, CHOP mRNA and protein expression and tumor necrosis factor-alpha level. It also activated astrocytes. The results suggest that resveratrol exerted neuroprotective effect on subarachnoid hemorrhage by reducing oxidative damage, endoplasmic reticulum stress and neuroinflammation. The study was approved by the Animals Ethics Committee of Shandong University, China on February 22, 2016 (approval No. LL-201602022).
Chinese Library Classification No. R453; R364; R741
Introduction
Early brain injury following subarachnoid hemorrhage (SAH) has been considered the main contributor to neurological outcomes (Bederson et al., 2009; Suzuki, 2015). Recent studies reported that oxidative damage and endoplasmic reticulum stress contribute to the pathogenesis of early brain injury (Ayer and Zhang, 2008; Nakka et al., 2016). Therefore, anti-oxidative stress or anti-endoplasmic reticulum stress intervention may be an effective therapeutic strategy for early brain injury.
Resveratrol (3, 5, 4′-trihydroxystilbene), is a polyphenol produced in several plants (Juan et al., 2012). The studies indicated that resveratrol showed various bioactivities, including as an antioxidant, anti-inflammatory, cardioprotective, antiapoptotic and anticancer (Zhang et al., 2010; Orsu et al., 2013; Tomé-Carneiro et al., 2013). Resveratrol is not harmful to normal cells or tissues, and can traverse the blood-brain barrier via simple diffusion. Resveratrol exerts a neuroprotective effect against central nervous system damage, such as Alzheimer’s disease (Wang et al., 2006; Albani et al., 2009), traumatic brain injury (Ates et al., 2007) or cerebral ischemia (Della-Morte et al., 2009). Importantly, resveratrol has been shown to attenuate cerebral vasospasm and early brain injury after experimental SAH (Shao et al., 2014; Zhou et al., 2014). However, the molecular mechanism of resveratrol has not been clarified.
Endoplasmic reticulum stress can activate apoptotic signals, resulting in cell apoptosis and deterioration of neurological functions (Tan et al., 2018). Under endoplasmic reticulum stress conditions, misfolded proteins accumulate in the endoplasmic reticulum, and glucose-regulated protein 78 (GRP78) is released by the endoplasmic reticulum stress sensor. The stress sensor is activated and up-regulates GRP78 and anti-C/EBP homologous protein (CHOP) expression (Son et al., 2010). Increasing evidence has shown that up-regulation of anti-nuclear factor-erythroid 2-related factor 2 (Nrf2) is involved in the antioxidant protection in various central nervous system diseases. Following oxidative insults, Nrf2 rapidly migrates to and accumulates in the nucleus, where it activates a battery of anti-oxidant genes, including those for anti-heme oxygenase-1 (HO-1), nicotinamide adenine dinucleotide phosphate (NADPH), quinone oxidoreductase 1, glutamate-cysteine ligase, and glutathione peroxidase (Shu et al., 2016). Several studies have indicated that inflammation is invariably associated with brain damage after SAH (Zhang et al., 2010; Orsu et al., 2013; Tomé-Carneiro et al., 2013). The immune response to SAH is characterized by a rapid activation of microglial cells, mast cells and the production of pro-inflammatory factors, including pro-apoptotic proteins, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, reactive oxygen species (ROS) and nitric oxide.
In this study, our goals were to test whether resveratrol administration suppressed oxidative stress and endoplasmic reticulum stress; whether it improved outcomes following SAH; and to examine the potential mechanisms of resveratrol following SAH.
Materials and Methods
Experimental animals
One hundred clean male Wistar rats aged 6 weeks and weighing 280 to 350 g were purchased from the Laboratory Animal Center, Shandong University, China (license number: SCXK (Lu) 20130009). All experiments were approved by the Ethics Committee of Shandong University on February 22, 2016 (approval No. LL-201602022).
Experimental SAH was induced in animals using the double blood injection model (Xia et al., 2006). Briefly, rats were anesthetized using isoflurane (RWD Life Science Co., Shenzhen, China) during the surgical procedure. A total of 200 μL blood from the femoral artery was injected into the cerebellomedullary cistern over a 3-minute period.
Drug administration and experimental groups
The rats were randomly allocated into four groups. In the sham group (n = 24), rats were administered vehicle only (1% ethanol; Xinteng Pharmaceutical Co., Xintai, China). The sham + resveratrol group (n = 24) received 60 mg/kg resveratrol. Rats in the SAH group (n = 26) were administered vehicle only (1% ethanol). SAH + resveratrol group (n = 26) received 60 mg/kg resveratrol. Resveratrol (Sigma-Aldrich, St. Louis, MO, USA) was first dissolved in ethanol, and then diluted with 0.9% NaCl. Ethanol did not exceed 1% of the total volume. The drug solution or vehicle was intraperitoneally injected at 2, 6, 24 and 46 hours after SAH.
Modified Garcia scores
Blind scoring was used for a modified Garcia system to observe neurological function at 48 hours after induction of SAH (Hasegawa et al., 2011). The test consisted of seven subtests: spontaneous activity, the rat’s reaction to side stroking, vibrissae touch, limb symmetry, forelimb outstretching, its climbing and beam walking ability. Higher scores demonstrated better neurological function.
Brain edema analysis
Rats were sacrificed with excess isoflurane. The brain tissue was prepared for further analysis at 48 hours after SAH. The wet/dry method (Yoon et al., 2010) was used to measure brain edema. Brain water content was calculated by (wet weight – dry weight)/ wet weight × 100%.
Terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labeling (TUNEL) assay
To observe the effect of resveratrol on neuronal apoptosis following SAH, TUNEL-positive neurons in the prefrontal cortex were evaluated using a TUNEL staining kit (KeyGEN BioTECH, Jiangsu, China). 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich) was used for counterstaining to visualize all cells. Ten random fields were captured for one section per animal (n = 4 each group) with a fluorescence microscope (IX71; Olympus, Tokyo, Japan). The proportion of TUNEL-positive cells (with a green fluorescent nucleus) was expressed as the percentage of the total cells counted. The TUNEL-positive cell results for rats in each subgroup were averaged.
Oxidative stress analysis
ROS and malondialdehyde (MDA) contents in prefrontal cortex homogenates were determined with the commercial DHE assay kit (Sigma-Aldrich) in accordance with the manufacturer’s instructions. The ROS level was indicated as a percent of ROS obtained relative to that of sham group. The DHE-staining results were pixilated and quantified using the Image-Pro Plus image analysis system. Values were expressed relative to the fluorescence signal of respective controls. The MDA level was expressed as per mg protein in the homogenate.
Reverse transcription-polymerase chain reaction
Prefrontal cortex tissues were homogenized in TRIzol reagent (CWBIO, Nanjing, China). The concentrations of nucleic acids were determined using a spectrophotometer (OD260/280) (Thermo, Waltham, MA, USA). Equal amounts of mRNA were reverse-transcribed to cDNA with the reverse transcription-polymerase chain reaction (RT-PCR) kit (Fermentas, Vilnius, Lithuania). cDNAs for CHOP, GRP78, Nrf2, HO-1 and β-actin were amplified by PCR with the specific primers. The reaction products were separated by electrophoresis on 1.3% agarose/TAE gel including 0.1% GoldView (vol/vol). The GelDocXR System was used to capture the image. The Image-Pro Plus 6.0 software was used to determine band intensity and each value was normalized to the β-actin. The primers were synthesized by HuaDa Gene (Shenzhen, China) (Table 1).
Table 1.
PCR primers in the text
| Gene | Forward (5′–3′) | Reverse (5′–3′) | Product size (bp) |
|---|---|---|---|
| CHOP | AGC TGG AAG CCT GGT ATG AG | GAC CAC TCT GTT TCC GTT TC | 276 |
| GRP-78 | GAA TCC CTC CTG CTC CCC GT | TTG GTC ATT GGT GAT GGT GAT TTT G | 134 |
| Nrf2 | ATT TGT AGA TGA CCA TGA GTC GC | ACT GTA ACT CGG GAA TGG AAA | 188 |
| HO-1 | GAC AGA AGA GGC TAA GAC CG | TGA ACT AGT GCT GAT CTG GG | 193 |
| β-Actin | CTA TTG GCA ACG AGC GGT TCC | CAG CAC TGT GTT GGC ATA GAG G | 152 |
Enzyme linked immunosorbent assay
The TNF-α level in cerebral cortex was detected using the enzyme linked immunosorbent assay (ELISA) kit (Dakewe, Shenzhen, China) according to the manufacturer’s protocols. TNF-α level was expressed as per mg protein in the homogenate.
Immunohistochemistry
Rats (n = 4–6/group) from each group were anesthetized and perfused with 4% paraformaldehyde (pH 7.4). Brains were collected and placed in the same fixative at 4°C for 24 hours. Brain samples were sliced into 4 µm thick coronal sections. The sections between –1.60 to –2.00 mm from the bregma were selected for immunohistochemistry. The sections were deparaffinized using a standard procedure and washed with phosphate-buffered saline as described previously. Briefly, after blocking for 30 minutes at room temperature, the sections were incubated with the following primary antibodies: Nrf2 (rabbit monoclonal antibody, 1:100; Cell Signaling, Beverly, MA, USA), HO-1 (rabbit monoclonal antibody, 1:100; Cell Signaling), GRP78 (rabbit polyclonal antibody, 1:100; Cell Signaling), and CHOP (rabbit polyclonal antibody, 1:100; Proteintech Group, Rosemont, IL, USA), ionized calcium binding adaptor molecule 1 (Iba-1) (rabbit monoclonal antibody, 1:100; Abcam, Cambridge, MA, USA) and glial fibrillary acidic protein (GFAP) (rabbit polyclonal antibody, 1:200; Abcam) at 4°C overnight. The slices were then treated with a commercial assay kit as secondary antibodies (Gene Tech, Shanghai, China) according to the manufacturer’s protocols. The number of positive cells was measured in three randomly selected microscopic fields at 200× magnification. Numbers of positive cells within each section were expressed as the average of three images per section. The value calculated was then expressed as the percent of positive cells obtained relative to that of the sham group.
Western blot assay
The prefrontal cortex was extracted and frozen at –120°C. For immunoblots, the tissue was weighed and cracked on ice. Following homogenization in a radioimmune precipitation assay buffer containing protease/phosphatase inhibitors and phenylmethyl sulfonylfluoride, the tissue was centrifuged at 13,800 × g for 10 minutes at 4°C. The resulting supernatant was removed and 5× loading buffer was added to the supernatant. Bicinchoninic acid Protein Assay Kits were used to quantify total protein concentrations. After dilution with 5× loading buffer, equal amounts of protein were run on sodium dodecyl sulfate polyacrylamide gel electrophoresis gels. Proteins were electrophoresed initially at 80 V for 30 minutes, and then altered to 120 V for 1 hour or longer. They were wet transferred to polyvinylidene fluoride membranes at 300 mA for 1 hour or longer. The membranes were incubated for 1 hour in 5% non-fat milk. Membranes were then incubated overnight at 4°C with primary antibodies for Nrf2 (rabbit monoclonal antibody, 1:1000; Proteintech Group, Rosemont, IL, USA), HO-1 (rabbit monoclonal antibody, 1:2000; Proteintech Group), GRP78 (rabbit polyclonal antibody, 1:1000; Proteintech Group) and CHOP (rabbit polyclonal antibody, 1:1000; Proteintech Group). These membranes were incubated with secondary antibodies at room temperature for 1 hour. Enhanced chemiluminescence kit reagents (Millipore Sigma, Burlington, MA, USA) were then used to develop chemiluminescent signals, which were detected using the Tanon Imaging System (Tanon-4600).
Statistical analysis
All data are presented as the mean ± SD. Statistical differences between groups was performed with one-way analysis of variance using the post hoc Tukey’s test. Data were analyzed using SPSS v.19.0 for Windows (IBM, Armonk, NY, USA). P value less than 0.05 was considered statistically significant.
Results
Resveratrol treatment attenuates mortality, neurological deficits, brain edema in SAH rats
In total, 52 surgeries were performed on the operated groups of rats (not including the sham group). Four rats died within 1 hour after SAH (4/52 operated animals). The mortality was 7.7 % (2/26) in the SAH group, and 7.7 % (2/26) in the SAH + resveratrol group, within 48 hours following surgeries. No rat died in the sham or sham + resveratrol groups. Neurological scores significantly decreased (P < 0.001), and brain edema (P < 0.05) significantly increased in the SAH groups compared with the sham group at 48 hours after SAH. Neurological scores were higher, while brain water content was lower in the SAH + resveratrol group than in the SAH group (P < 0.01 or P < 0.05; Figure 1A).
Figure 1.
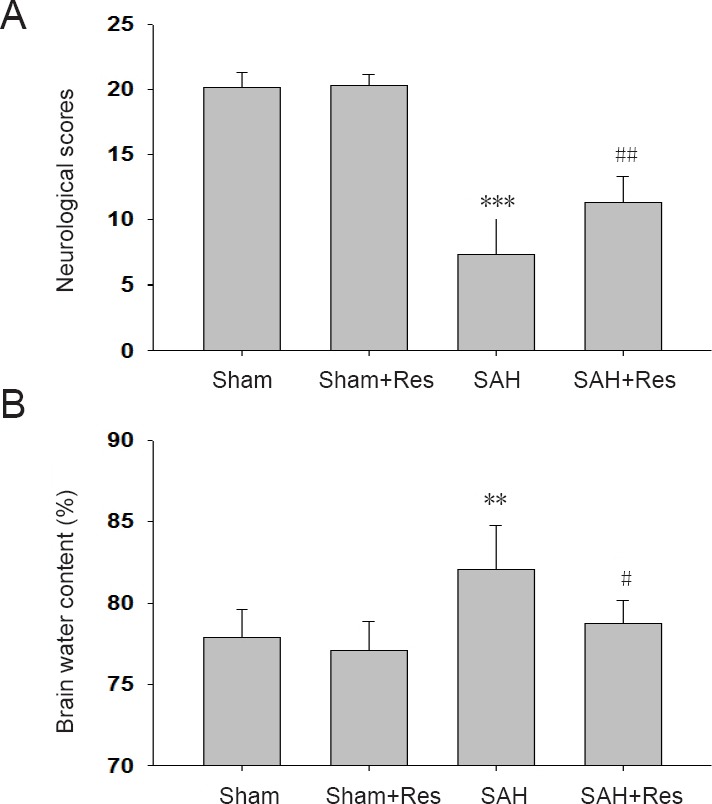
Effect of Res on neurological deficits and brain edema 48 hours following SAH.
(A) Modified Garcia scores: The higher the score, the better the neurological function. Scores after SAH are comparable with a previous study (Fujii et al., 2014); (B) brain water content. Data are expressed as the mean ± SD (n = 6). **P < 0.01, ***P < 0.001, vs. sham group; #P < 0.05, ##P < 0.01, vs. SAH group (one-way analysis of variance followed by post hoc Tukey’s test). Res: Resveratrol; SAH: subarachnoid hemorrhage.
Resveratrol alleviates neuronal cell apoptosis in the prefrontal cortex of SAH rats
At 48 hours following SAH, the number of apoptotic cells significantly increased compared with the sham group (P < 0.001; Figure 2A). The number of apoptotic cells was significantly attenuated in the SAH + resveratrol group compared with the SAH group at 48 hours following injury (P < 0.05; Figure 2A).
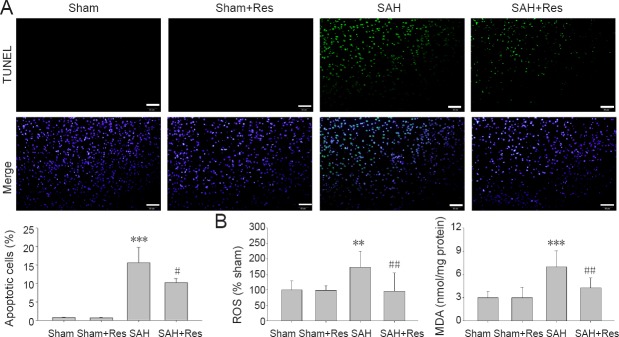
Figure 2.
Res attenuates apoptosis and oxidative stress in prefrontal cortex 48 hours after SAH.
(A) TUNEL-positive cells: SAH increased TUNEL-positive cells (green). Res treatment markedly decreased apoptotic cells. Scale bars: 50 μm. (B) ROS and MDA content: Res treatment noticeably decreased ROS and MDA content. Data are expressed as the mean ± SD (n = 8). **P < 0.01, ***P < 0.001, vs. sham group; #P < 0.05, ##P < 0.01, vs. SAH group (one-way analysis of variance followed by post hoc Tukey’s test). Res: Resveratrol; SAH: subarachnoid hemorrhage; TUNEL: terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labeling; ROS: reactive oxygen species; MDA: malondialdehyde.
Resveratrol decreases ROS and MDA levels in the prefrontal cortex of SAH rats
As shown in Figure 2B, the contents of ROS and MDA in prefrontal cortex homogenates were significantly elevated at 48 hours following SAH (P < 0.01, P < 0.001). After SAH, resveratrol treatment significantly reversed these changes compared with the SAH group (P < 0.01, P < 0.01).
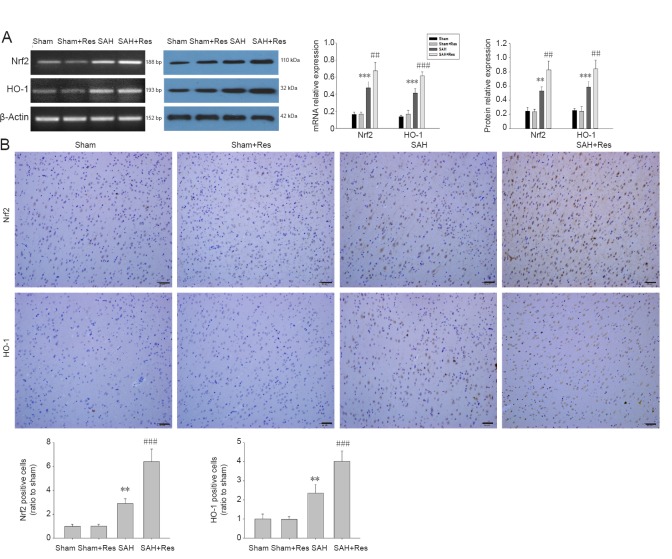
Resveratrol promotes Nrf2 and HO-1 expression in the prefrontal cortex of SAH rats
Nrf2 and HO-1 expression was up-regulated in the SAH group. After SAH, resveratrol treatment further up-regulated Nrf2 and HO-1 expression (Figure 3A). Similarly, immunohistochemical analysis showed that resveratrol administration significantly increased the number of Nrf2/HO-1-positive cells in the prefrontal cortex compared with the SAH group (P < 0.001, P < 0.001; Figure 3B).
Figure 3.
Nrf2 and HO-1 expression in prefrontal cortex further up-regulates following Res treatment 48 hours after SAH.
(A) Expression of Nrf2 and HO-1 in the prefrontal cortex at 48 hours after SAH measured with reverse transcription-polymerase chain reaction and western blot assay. (B) Nrf2- and HO-1-positive cells (immunohistochemical staining). Positive cells are brown. Scale bars: 50 μm. Data are expressed as the mean ± SD (n = 4). **P < 0.01, ***P < 0.001, vs. sham group; ##P < 0.01, ###P < 0.001, vs. SAH group (one-way analysis of variance followed by post hoc Tukey’s test). Nrf2: Nuclear factor-erythroid 2-related factor 2; HO-1: heme oxygenase-1; Res: resveratrol; SAH: subarachnoid hemorrhage.
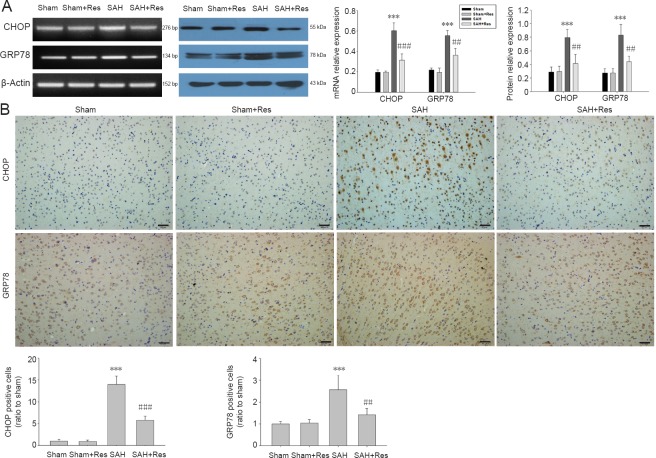
Effects of resveratrol on GRP78 and CHOP expression 48 hours post SAH
The expressions of CHOP and GRP78 increased in the prefrontal cortex following SAH. Levels of CHOP and GRP78 were remarkably reduced in the SAH + resveratrol group compared with the SAH group (Figure 4A). Immunohistochemical analysis in Figure 4B similarly revealed that resveratrol administration significantly decreased the numbers of CHOP-positive cells and GRP78-positive cells in the prefrontal cortex compared with the SAH group (P < 0.001 or P < 0.01).
Figure 4.
Res treatment down-regulates GRP78 and CHOP expression in prefrontal cortex 48 hours after SAH.
(A) Expression of GRP78 and CHOP (reverse transcription-polymerase chain reaction and western blot assay); (B) numbers of GRP78- and CHOP-positive cells (immunohistochemical staining). Positive cells are brown. Scale bars: 50 μm. Data are expressed as the mean ± SD (n = 4). **P < 0.01, ***P < 0.001, vs. sham group; ##P < 0.01, ###P < 0.001, vs. SAH group (one-way analysis of variance followed by post hoc Tukey’s test). GRP78: Glucose-regulated protein 78; CHOP: C/EBP homologous protein; Res: resveratrol; SAH: subarachnoid hemorrhage.
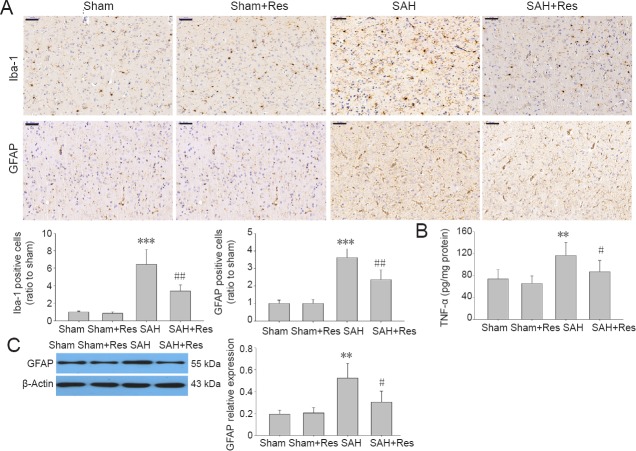
Effects of resveratrol on microglial activation 48 hours post SAH
SAH caused microglial activation, evidenced by changed morphology in the prefrontal cortex and an increase in the microglia marker, Iba-1, positive cells (P < 0.001, vs. sham group) (Figure 5A). The changes were significantly reversed by resveratrol administration (P < 0.01, vs. SAH). TNF-α production significantly increased in the SAH group compared with the sham group (P < 0.01). Resveratrol treatment attenuated TNF-α levels in the prefrontal cortex compared with the SAH group (P < 0.05; Figure 5B).
Figure 5.
Res attenuates glial activation in prefrontal cortex 48 hours following SAH.
(A) Iba-1- and GFAP-positive cells. Upper panels: examples of stained sections. Positive cells are brown. Lower panels: analysis of immunohistochemical staining (n = 4). Scale bars: 50 μm. (B) TNF-α levels (enzyme linked immunosorbent assay; n = 8); (C) GFAP expression (western blot assay; n = 4): Data are expressed as the mean ± SD. **P < 0.01, ***P < 0.001, vs. sham group; #P < 0.05, ##P < 0.01, vs. SAH group (one-way analysis of variance followed by post hoc Tukey’s test). Iba-1: Ionized calcium binding adaptor molecule 1.
Effects of resveratrol on reactive astrogliosis 48 hours post SAH
GFAP is an astrocyte activation marker (Jessen et al., 1984). GFAP staining increased in SAH rats compared with the sham group (P < 0.001). Activated astrocytes were significantly suppressed by resveratrol treatment in the SAH + resveratrol group compared with the SAH group (P < 0.01; Figure 5A). Moreover, GFAP expression was reduced in the SAH + resveratrol group compared with the SAH group (P < 0.05; Figure 5C).
Discussion
We found that resveratrol treatment inhibited neuronal apoptosis, attenuated brain edema and improved neurological deficits following SAH. Importantly, resveratrol decreased ROS and MDA levels, as well as elevating Nrf2 and HO-1 expression in the prefrontal cortex. Resveratrol also significantly down-regulated the expressions of GRP78 and CHOP, and suppressed inflammatory responses after SAH.
Accumulated evidence has demonstrated that oxidative stress is strongly associated with the progression of early brain injury (Ayer and Zhang, 2008; Duan et al., 2016; Nomoto et al., 2017). The features of oxidative stress, including ROS production, lipid peroxidation, protein inactivation, and DNA damage, have been found in the early stages of brain injury (Wu et al., 2014; Zhang et al., 2016). Our results after SAH showed elevation of ROS and MDA levels, indications of increased oxidative stress. Resveratrol treatment dramatically decreased the excessive production of ROS and MDA, in parallel with improvement in neurological deficits. Resveratrol is reported to inhibit protein carbonylation and nitration, as well as lipid peroxidation, thereby attenuating oxidative stress. Moreover, the anti-oxidant activity of resveratrol is involved in the activation of powerful endogenous mechanisms, such as upregulating Nrf2 and HO-1 expression (Niwa et al., 2016). These possibly contributed to the neuroprotective role of resveratrol in cerebral ischemic damage (Ren et al., 2011). Various studies demonstrated that Nrf2 plays an important role in the antioxidative response to stress and damage of the central nervous system, including that by SAH (Li et al., 2015; Zhang et al., 2018). Under the pathological conditions, the Nrf2 pathway is activated to trigger antioxidant enzyme expression (Lv et al., 2017), including HO-1, and attenuate cellular oxidative stress (Magesh et al., 2012). HO-1 is the stress-induced enzyme that exerts antioxidant properties by catalyzing the degradation of heme (Syapin, 2008). Therefore, induction of the Nrf2/HO-1 system exerts protective effects against a variety of cellular stresses (Chen et al., 2011). In the present study, SAH insult was found to up-regulate Nrf2 and HO-1 expression, which was consistent with a previous study (Chen et al., 2011). Importantly, resveratrol administration dramatically not only attenuated the oxidation damage and early brain injury, but we also found it increased Nrf2 and HO-1 expression further.
Studies have found that endoplasmic reticulum stress-induced apoptosis contributes to various diseases, including diabetes, ischemia, cardiovascular diseases, and SAH (Groenendyk et al., 2013; Roussel et al., 2013; Liu et al., 2016). The endoplasmic reticulum stress is a complex cellular response, triggering the activation and expression of several proteins, including the transcription factor CHOP, which is considered a precipitator of stress-induced apoptosis (Sano and Reed, 2013; Xu et al., 2018b). Therapies that block endoplasmic reticulum stress could be a promising strategy for inhibiting early brain injury-induced apoptosis (Xu et al., 2018a). The current study demonstrated that SAH increased the expression of GRP78 and CHOP in the prefrontal cortex, reinforcing previous studies (Liu et al., 2016). In addition, resveratrol treatment reduced SAH-induced apoptosis and endoplasmic reticulum stress, as evident by attenuating TUNEL-positive cells and CHOP and GRP78 expression in the prefrontal cortex. Liu et al. (2016) demonstrated that resveratrol suppressed endoplasmic reticulum stress-mediated apoptosis in rotenone-induced Parkinson’s disease. This was associated with down-regulating CHOP and GRP78 expression and inhibiting caspase-3 activation in the brain of rotenone exposed rats, which was consistent with a previous study (Gaballah et al., 2016). However, the mechanism of inhibiting endoplasmic reticulum stress by resveratrol still needs further investigation.
The interaction of oxidative stress and endoplasmic reticulum stress is found in various pathological conditions, such as metabolic disease, hyper-homocysteinemia, atherosclerosis, neurodegeneration and acute central nervous system injuries (Malhotra and Kaufman, 2007; Nakka et al., 2016). Studies indicate that oxidative stress and endoplasmic reticulum stress are critical features underlying SAH pathogenesis. SAH causes ROS overproduction, thus leads to the disorder of endoplasmic reticulum redox and cell apoptosis (Zhao et al., 2015). Persistent endoplasmic reticulum stress leads to ROS overproduction, and further aggravates the oxidative damage (Malhotra and Kaufman, 2007). It is possible that oxidative damage and endoplasmic reticulum stress modify the progression and severity of SAH. Combination therapies that inhibit both these stresses might provide a potentially better therapeutic strategy for protecting the brain after SAH. The current report demonstrated that the therapeutic effect of resveratrol treatment post-SAH is strongly linked to the reduction of both oxidative stress and endoplasmic reticulum stress.
Some clinical studies reported that elevated proinflammatory mediators, such as TNF-α and interleukin-6, in serum at 2–3 days post SAH were correlated with poor clinical outcomes (Chou et al., 2012; Savarraj et al., 2018). In addition, increases in interleukin-6 and monocyte chemoattractant protein-1 levels in patient cerebrospinal fluid at 14 days post SAH are linked to delayed ischemic neurological deficits (Niwa et al., 2016). Parallel studies in an animal model showed that SAH insult activates glial cell activation and caused pro-inflammatory cytokine release, which are associated with oxidative stress and neuronal death (Prunell et al., 2005). Our findings confirm previous reports that glial cell activation and increased TNF-α production were presented at 48 hours following SAH, but they were reversed by resveratrol administration. Many studies have highlighted that resveratrol plays a neuroprotective role, due to its anti-inflammatory property, after various central nervous system injuries such as SAH insults (Zhang et al., 2017). Previous studies showed that resveratrol inhibits SAH-induced microglial activation and inflammatory cytokine secretion in the rat (Shao et al., 2014; Zhang et al., 2017). In the present study, resveratrol evidently suppressed SAH-induced glial activation and TNF-α release.
Our study has several limitations. First, the mechanism of activation of the Nrf2/HO-1 system by resveratrol has not been completely elucidated and should be investigated in further studies. Second, the interaction of oxidative stress and endoplasmic reticulum stress in the effect on resveratrol against SAH insult was not investigated. Third, only one dose of resveratrol was used in this study. It will be important in future studies to investigate these aspects. Hypoxic-ischemic insult was not investigated.
In summary, resveratrol treatment has a therapeutic effect against early brain injury. The neuroprotective effect is probably mediated in part by decreased oxidative damage, endoplasmic reticulum stress and inflammatory response and thus ameliorates apoptosis.
Additional file: Open peer review report 1 (98.9KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81671213 (to ZW); the Key Research and Development Foundation of Shandong Province of China, No. 2017GSF218091 (to ZW); the Fundamental Research Funds of Shandong University of China, No. 2015JC008 (to ZW). The funders had no involvement in study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Institutional review board statement: The study was approved by the Animals Ethics Committee of Shandong University, China on February 22, 2016 (approval No. LL-201602022).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Lijie Zhai, University of Illinois at Chicago, USA.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81873768 and 81671213 (both to ZW); the Key Research and Development Foundation of Shandong Province of China, No. 2017GSF218091 (to ZW); the Fundamental Research Funds of Shandong University of China, No. 2015JC008 (to ZW).
P-Reviewer: Zhai L; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Dawes EA, Robens J, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 2.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 3.Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm. Acta Neurochir Suppl. 2008;104:33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr, Harbaugh RE, Patel AB, Rosenwasser RH American Heart Association. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Fang Q, Zhang J, Zhou D, Wang Z. Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J Neurosci Res. 2011;89:515–523. doi: 10.1002/jnr.22577. [DOI] [PubMed] [Google Scholar]
- 6.Chou SH, Feske SK, Atherton J, Konigsberg RG, De Jager PL, Du R, Ogilvy CS, Lo EH, Ning M. Early elevation of serum tumor necrosis factor-alpha is associated with poor outcome in subarachnoid hemorrhage. J Investig Med. 2012;60:1054–1058. doi: 10.231/JIM.0b013e3182686932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan X, Wen Z, Shen H, Shen M, Chen G. Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid Med Cell Longev. 2016;2016:1203285. doi: 10.1155/2016/1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii M, Sherchan P, Krafft PR, Rolland WB, Soejima Y, Zhang JH. Cannabinoid type 2 receptor stimulation attenuates brain edema by reducing cerebral leukocyte infiltration following subarachnoid hemorrhage in rats. J Neurol Sci. 2014;342:101–106. doi: 10.1016/j.jns.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaballah HH, Zakaria SS, Elbatsh MM, Tahoon NM. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem Biol Interact. 2016;251:10–16. doi: 10.1016/j.cbi.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Groenendyk J, Agellon LB, Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol. 2013;75:49–67. doi: 10.1146/annurev-physiol-030212-183707. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa Y, Suzuki H, Altay O, Zhang JH. Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011;42:477–483. doi: 10.1161/STROKEAHA.110.597344. [DOI] [PubMed] [Google Scholar]
- 13.Jessen KR, Thorpe R, Mirsky R. Molecular identity, distribution and heterogeneity of glial fibrillary acidic protein: an immunoblotting and immunohistochemical study of Schwann cells, satellite cells, enteric glia and astrocytes. J Neurocytol. 1984;13:187–200. doi: 10.1007/BF01148114. [DOI] [PubMed] [Google Scholar]
- 14.Juan ME, Alfaras I, Planas JM. Colorectal cancer chemoprevention by trans-resveratrol. Pharmacol Res. 2012;65:584–591. doi: 10.1016/j.phrs.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Li T, Sun KJ, Wang HD, Zhou ML, Ding K, Lu XY, Wei WT, Wang CX, Zhou XM. Tert-butylhydroquinone ameliorates early brain injury after experimental subarachnoid hemorrhage in mice by enhancing Nrf2-independent autophagy. Neurochem Res. 2015;40:1829–1838. doi: 10.1007/s11064-015-1672-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Zhao D, Ji YX, Huang XY, Yang P, Wang YZ, Lei T. Role of glucose-regulated protein 78 in early brain injury after experimental subarachnoid hemorrhage in rats. J Huazhong Univ Sci Technolog Med Sci. 2016;36:168–173. doi: 10.1007/s11596-016-1561-3. [DOI] [PubMed] [Google Scholar]
- 17.Lv H, Liu Q, Zhou J, Tan G, Deng X, Ci X. Daphnetin-mediated Nrf2 antioxidant signaling pathways ameliorate tert-butyl hydroperoxide (t-BHP)-induced mitochondrial dysfunction and cell death. Free Radic Biol Med. 2017;106:38–52. doi: 10.1016/j.freeradbiomed.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012;32:687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 20.Nakka VP, Prakash-Babu P, Vemuganti R. Crosstalk Between endoplasmic reticulum stress, oxidative stress, and autophagy: potential therapeutic targets for acute CNS injuries. Mol Neurobiol. 2016;53:532–544. doi: 10.1007/s12035-014-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwa A, Osuka K, Nakura T, Matsuo N, Watabe T, Takayasu M. Interleukin-6, MCP-1, IP-10, and MIG are sequentially expressed in cerebrospinal fluid after subarachnoid hemorrhage. J Neuroinflammation. 2016;13:217. doi: 10.1186/s12974-016-0675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomoto H, Miyoshi H, Nakamura A, Atsumi T, Manda N, Kurihara Y, Aoki S on behalf of SAIS Study Group. Impact of incretin-related agents on endothelial cell function. Clin Trials Degener Dis. 2017;2:7–11. [Google Scholar]
- 23.Orsu P, Murthy BV, Akula A. Cerebroprotective potential of resveratrol through anti-oxidant and anti-inflammatory mechanisms in rats. J Neural Transm (Vienna) 2013;120:1217–1223. doi: 10.1007/s00702-013-0982-4. [DOI] [PubMed] [Google Scholar]
- 24.Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Inflammation in the brain after experimental subarachnoid hemorrhage. Neurosurgery. 2005;56:1082–1092. [PubMed] [Google Scholar]
- 25.Ren J, Fan C, Chen N, Huang J, Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem Res. 2011;36:2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 26.Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013;12:105–118. doi: 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- 27.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savarraj J, Parsha K, Hergenroeder G, Ahn S, Chang TR, Kim DH, Choi HA. Early brain injury associated with systemic inflammation after subarachnoid hemorrhage. Neurocrit Care. 2018;28:203–211. doi: 10.1007/s12028-017-0471-y. [DOI] [PubMed] [Google Scholar]
- 29.Shao AW, Wu HJ, Chen S, Ammar AB, Zhang JM, Hong Y. Resveratrol attenuates early brain injury after subarachnoid hemorrhage through inhibition of NF-kappaB-dependent inflammatory/MMP-9 pathway. CNS Neurosci Ther. 2014;20:182–185. doi: 10.1111/cns.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shu L, Wang C, Wang J, Zhang Y, Zhang X, Yang Y, Zhuo J, Liu J. The neuroprotection of hypoxic preconditioning on rat brain against traumatic brain injury by up-regulated transcription factor Nrf2 and HO-1 expression. Neurosci Lett. 2016;611:74–80. doi: 10.1016/j.neulet.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA, Johnson JA, Greig NH, Mattson MP. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H. What is early brain injury? Transl Stroke Res. 2015;6:1–3. doi: 10.1007/s12975-014-0380-8. [DOI] [PubMed] [Google Scholar]
- 33.Syapin PJ. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol. 2008;155:623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan HP, Guo Q, Hua G, Chen JX, Liang JC. Inhibition of endoplasmic reticulum stress alleviates secondary injury after traumatic brain injury. Neural Regen Res. 2018;13:827–836. doi: 10.4103/1673-5374.232477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomé-Carneiro J, Larrosa M, Yáñez-Gascón MJ, Dávalos A, Gil-Zamorano J, Gonzálvez M, García-Almagro FJ, Ruiz Ros JA, Tomás-Barberán FA, Espín JC, García-Conesa MT. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol Res. 2013;72:69–82. doi: 10.1016/j.phrs.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- 37.Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li W, Zhou ML, Wang XL. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar Drugs. 2014;12:6125–6141. doi: 10.3390/md12126125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia JX, Shen B, Yang WD, Chen JM. Establishing models of subarachnoid hemorrhage in rats by double injections of blood into cistern magna. Ningxia Yixue Zazhi. 2006;28:410–412,481. [Google Scholar]
- 39.Xu W, Gao L, Li T, Zheng J, Shao A, Zhang J. Apelin-13 alleviates early brain injury after subarachnoid hemorrhage via suppression of endoplasmic reticulum stress-mediated apoptosis and blood-brain barrier disruption: possible involvement of ATF6/CHOP pathway. Neuroscience. 2018a;388:284–296. doi: 10.1016/j.neuroscience.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 40.Xu W, Lu X, Zheng J, Li T, Gao L, Lenahan C, Shao A, Zhang J, Yu J. Melatonin protects against neuronal apoptosis via suppression of the ATF6/CHOP pathway in a rat model of intracerebral hemorrhage. Front Neurosci. 2018b;12:638. doi: 10.3389/fnins.2018.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon S, Woo SU, Kang JH, Kim K, Kwon MH, Park S, Shin HJ, Gwak HS, Chwae YJ. STAT3 transcriptional factor activated by reactive oxygen species induces IL6 in starvation-induced autophagy of cancer cells. Autophagy. 2010;6:1125–1138. doi: 10.4161/auto.6.8.13547. [DOI] [PubMed] [Google Scholar]
- 42.Zhang F, Liu J, Shi JS. Anti-inflammatory activities of resveratrol in the brain: role of resveratrol in microglial activation. Eur J Pharmacol. 2010;636:1–7. doi: 10.1016/j.ejphar.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Wu Q, Zhang Q, Lu Y, Liu J, Li W, Lv S, Zhou M, Zhang X, Hang C. Resveratrol attenuates early brain injury after experimental subarachnoid hemorrhage via inhibition of NLRP3 inflammasome activation. Front Neurosci. 2017;11:611. doi: 10.3389/fnins.2017.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Wu Q, Lu Y, Wan J, Dai H, Zhou X, Lv S, Chen X, Zhang X, Hang C, Wang J. Cerebroprotection by salvianolic acid B after experimental subarachnoid hemorrhage occurs via Nrf2- and SIRT1-dependent pathways. Free Radic Biol Med. 2018;124:504–516. doi: 10.1016/j.freeradbiomed.2018.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Sun J, Zhu S, Xu T, Lu J, Han H, Zhou C, Yan J. The role of rhynchophylline in alleviating early brain injury following subarachnoid hemorrhage in rats. Brain Res. 2016;1631:92–100. doi: 10.1016/j.brainres.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 46.Zhao D, Liu Q, Ji Y, Wang G, He X, Tian W, Xu H, Lei T, Wang Y. Correlation between nitric oxide and early brain injury after subarachnoid hemorrhage. Int J Neurosci. 2015;125:531–539. doi: 10.3109/00207454.2014.951442. [DOI] [PubMed] [Google Scholar]
- 47.Zhou XM, Zhou ML, Zhang XS, Zhuang Z, Li T, Shi JX, Zhang X. Resveratrol prevents neuronal apoptosis in an early brain injury model. J Surg Res. 2014;189:159–165. doi: 10.1016/j.jss.2014.01.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.