Abstract
Objectives/Hypothesis
Surgical tracheostomy (ST) with creation of an inferiorly based U‐shaped tracheal flap, known as the Björk flap, is the most commonly performed. The purpose of this study was to evaluate whether outcome was different in patients who underwent low ST with retraction and preservation of the thyroid isthmus compared to those who underwent high ST with ligation of the thyroid isthmus.
Study Design
Retrospective cohort study.
Methods
We included 1,143 patients who underwent ST with creation of a Björk flap between 2008 and 2015. Different outcome parameters, including complications, decannulation, inpatient mortality, and surgical characteristics, such as length of surgery and height of tracheal incision, were assessed comparing low and high ST.
Results
Complications occurred in 7.7% of patients, of which persistent stoma (4.1%) and hemorrhages (2.7%) were the most common. Low tracheostomy with retraction and preservation of thyroid isthmus was done in 31.4% of cases. Complications did not significantly differ between low and high tracheostomies (8.0% vs. 7.0%, P = .468). Moreover, decannulation rate and inpatient mortality were also not significantly different in low compared to high tracheostomies (P = .816 and P = .152, respectively). However, low tracheostomies were associated with significantly shorter operation times (33.0 ± 0.8 min vs. 38.7 ± 0.5 min, P < .001) and lower tracheal incisions for creation of a Björk flap (P < .001) compared to high tracheostomies.
Conclusions
Low tracheostomies are as safe as high tracheostomies regarding complications. Due to the fact that low tracheostomies are associated with shorter operation times and lower tracheal incisions, we recommend performong low tracheostomies whenever feasible.
Level of Evidence
4 Laryngoscope, 128:2783–2789, 2018
Keywords: Surgical tracheostomy, Björk flap, low tracheostomy, thyroid isthmus, complications, outcome
INTRODUCTION
Tracheostomy is defined as a surgical procedure with creation of an opening into the trachea through the neck, with tracheal mucosa being brought into continuity with skin, whereas tracheotomy is defined as an incision in the trachea.1 Despite this divergent nomenclature, tracheostomy and tracheotomy are often used synonymously, and little differentiation is made in these definitions in daily practice.1
Traditionally, tracheostomies were performed as open surgical tracheostomies (STs) by a surgical team at the intensive care unit (ICU) or within the operating room (OR). Nowadays, tracheostomies are among the most commonly conducted procedures in critically ill patients, and an increasing number of tracheostomies are performed as percutaneous dilatational tracheotomies (PDTs) by nonsurgical physicians.1, 2, 3 Numerous reviews and meta‐analyses already compared outcome of these techniques, showing a trend toward fewer complications in PDTs compared to STs.4, 5, 6
However, an analysis of PDTs compared to STs was not the purpose of this study. Herein we focused on STs with creation of an inferiorly based U‐shaped tracheal flap, which is known as the Björk flap. A ST typically starts with a horizontal incision through skin and platysma, followed by identification of midline raphe, bilateral retraction of strap muscles laterally continued by exact midline dissection until the thyroid isthmus is encountered.7, 8 The thyroid isthmus can be either retracted superiorly and can be preserved, or it has to be divided to get better exposure of the anterior tracheal wall. Once the anterior tracheal wall is exposed, there exist different techniques to perform the tracheostomy. On the one hand, vertical, horizontal, or combined vertical and horizontal tracheal incisions can be done. On the other hand, tracheal window resection, which is a vertical tracheostomy with stay sutures to the anterior chest wall, or horizontal tracheostomy with creation of a Björk flap, can be performed. The latter one is the most common.7
Significant complications are reported in 3.2% to 40% of STs, among which hemorrhage is the most common.9, 10, 11 Hemorrhages are mainly attributed to injuries of the anterior jugular veins or to thyroid isthmus bleedings. Although some authors recommend Heffner's subthyroidal tracheostomy with retraction and preservation of the thyroid isthmus, the majority of surgeons suture ligate or divide the thyroid isthmus with cauterization accompanied by meticulous hemostasis to prevent hemorrhage.1, 7, 12, 13, 14
The main purpose of this retrospective, descriptive, single‐center study was to evaluate whether Heffner's subthyroidal tracheostomy with retraction and preservation of thyroid isthmus, herein defined as low tracheostomy, is associated with a higher complication rate and worse clinical outcome compared to patients who underwent conventional ST with suture ligation of the thyroid isthmus, which is classified as high tracheostomy.
MATERIALS AND METHODS
Study Population
This study was conducted at the Department of Otorhinolaryngology, Head and Neck Surgery of the Medical University of Vienna. We identified 1,447 consecutive patients who underwent ST between January 2008 and December 2015. Those patients who received PDTs or patients in whom specialists other than ear, nose, and throat surgeons performed ST were excluded from this analysis. We further excluded 304 patients who did not receive ST with creation of the Björk flap. Finally, medical reports of 1,143 patients were available for analysis.
We analyzed several clinical characteristics (sex, age, indication for tracheostomy), time to tracheostomy (early vs. late tracheostomy), height of tracheal incision, length of surgery, site of surgery (ICU vs. OR), urgency of the ST (elective vs. urgent), and different outcome parameters (complications, decannulation, intrahospital mortality), with special emphasis on low compared to high tracheostomies.
Indications for Tracheostomy
With regard to indication for ST, patients were classified into four groups: 1) cardiorespiratory problems, comprising acute respiratory distress syndrome, chronic obstructive pulmonary disease, or prolonged weaning after cardiac surgeries; 2) tumor diseases, especially head and neck carcinomas; 3) neurologic impairments, mainly insult, hypoxic brain damage after resuscitation, or cerebral hemorrhage; and 4) severe burn injuries.
Complications
We differentiated the following complications: persistent stoma, hemorrhage, wound infection with surgical revision of stoma and tracheal stenosis. Persistent stoma was defined as stoma that persists longer than 3 months after decannulation without complete closure. Hemorrhages and wound infections were defined as events, which have made surgical revision or management at the ICU or the OR necessary. Patients only receiving local treatment due to minor hemorrhages or stoma infections were not assessed as hemorrhages or wound infections. Additionally, only patients with symptomatic narrowing of tracheal lumen were counted as tracheal stenosis.
Decannulation and Intrahospital Mortality
Patients who were discharged with cannula and who were not decannulated during the first 6 months after discharge (according to medical reports) were assumed to have not been decannulated. Those patients with cannula, who were lost in follow‐up or who died within inpatient stay were also assessed as missing decannulation.15 Those patients who died during inpatient stay were assessed as intrahospital mortalities.
Statistical Methods
Statistical analyses were performed using SPSS software (version 21; IBM, Armonk, NY). All data within the Results section are reported as mean ± standard error of the mean. A χ2 test was used for comparison of nominal variables, and we used an unpaired Student t test to compare mean of normally distributed variables. Altogether, mainly descriptive statistics were used. All tests were two sided, and P values below .05 were considered as statistically significant.
RESULTS
Patient Cohort
We recruited a total of 1,143 patients, including 748 males (65.4%) and 395 females (34.6%), with a mean age of 60.6 ± 0.44 years (range, 3–90 years), who underwent ST with creation of a Björk flap. Within our cohort, there were 15 young patients (<18 years) and among them, only two were younger than 10 years. The vast majority of STs were performed as elective procedures (n = 1,097, 96.0%), whereas urgent ST was only necessary in 46 patients (4.0%). In 80.9% of cases, STs were conducted as open, bedside tracheostomies in the ICU, and the remaining 19.1% of STs were performed in the OR. The most common indications for ST were cardiorespiratory problems (61.1%), followed by tumor diseases (19.2%), neurologic impairments (12.2%), and burn injuries (7.5%). Early tracheostomies, defined as STs that were performed within day 0 to 7 of intubation, and late tracheostomies (LTs), defined as STs that were performed thereafter, were performed in 47.7% (n = 528) and 52.3% (n = 579) of cases, respectively. Data regarding time to tracheostomy was missing in 36 patients (Table 1).
Table 1.
Clinical Characteristics Comparing High and Low Tracheostomy
| Characteristics | Total, 1,143 (100) | High Tracheostomy, 715 (68.6) | Low Tracheostomy, 327 (31.4) | P Value |
|---|---|---|---|---|
| Age, yr, mean ± SD (range, 3–90 yr) | 60.6 ± 14.9 | 61.3 ± 14.8 | 59.5 ± 15.2 | .081a |
| Sex, no. (%) | ||||
| Male | 748 (65.4) | 484 (70.7) | 201 (29.3) | |
| Female | 395 (34.6) | 231 (64.7) | 126 (35.3) | .057b |
| Operation time, min, mean ± SD | 36.8 ± 13.1 | 38.7 ± 12.3 | 33.0 ± 13.4 | <.001a |
| Indication for tracheostomy, no. (%) | ||||
| Cardiorespiratory problem | 698 (61.1) | 463 (72.5) | 176 (27.5) | |
| Malignant disease | 219 (19.2) | 105 (51.7) | 98 (48.3) | |
| Neurologic impairment | 140 (12.2) | 90 (72.0) | 35 (28.0) | |
| Burn injury | 86 (7.5) | 57 (76.0) | 18 (24.0) | <.001b |
| Time to tracheostomy, no. (%)c | ||||
| Early tracheostomy | 528 (47.7) | 302 (63.4) | 174 (36.6) | |
| Late tracheostomy | 579 (52.3) | 385 (72.1) | 149 (27.9) | .003b |
| Urgency of tracheostomy, no. (%) | ||||
| Elective | 1097 (96.0) | 691 (69.0) | 311 (31.0) | |
| Urgent | 46 (4.0) | 24 (60.0) | 16 (40.0) | .231b |
| Site of tracheostomy, no. (%) | ||||
| ICU | 925 (80.9) | 608 (72.3) | 233 (27.7) | |
| OR | 218 (19.1) | 107 (53.2) | 94 (46.8) | <.001b |
| Experience of surgeon, no. (%) | ||||
| Resident | 577 (50.5) | 393 (72.5) | 149 (27.5) | |
| Consultant | 566 (49.5) | 320 (64.4) | 178 (35.6) | .005b |
| Height of tracheostomy, no. (%)c | ||||
| First and second tracheal ring | 128 (15.6) | 105 (17.9) | 19 (8.9) | |
| Second and third tracheal ring | 550 (67.1) | 419 (71.5) | 118 (55.1) | |
| Below third tracheal ring | 142 (17.3) | 62 (10.6) | 77 (36.0) | <.001b |
Unpaired Student t test.
χ2 test.
Data regarding type of surgery, time to tracheostomy, and height of tracheostomy were missing in 101, 36, and 323 patients, respectively.
ICU = intensive care unit; OR = operating room; SD = standard deviation.
Type of Surgery
Length of surgery, height of tracheostomy, and site of surgery
High and low tracheostomies were performed in 68.6% and 31.4% of cases, respectively. Low tracheostomies were significantly more often performed by consultants (35.6% vs. 27.5%), whereas high tracheostomies were consequently more often done by residents (72.5% vs. 64.4%, P = .005). Low tracheostomies were associated with shorter operation times (33.0 ± 0.8 min vs. 38.7 ± 0.5 min, P < .001) (Fig. 1A). Moreover, compared to high tracheostomies, low tracheostomies were associated with significantly lower tracheal incisions for creation of a Björk flap (P < .001). In particular, high tracheostomies were performed between the first and second, second and third, and below the third tracheal ring in 17.9%, 71.5%, and 10.6% of cases compared to 8.9%, 55.1%, and 36% in low tracheostomies, respectively (Fig. 1B, Table 1). According to the site of surgery, 46.8% of STs were performed as low tracheostomies in the OR, whereas 72.3% of STs at ICUs were done as high tracheostomies, respectively (P < .001). Conversely, urgency of ST did not significantly influence type of surgery (P = .231). Although not significant, low tracheostomies were more often performed in females compared to males (38.5% vs. 32.3%, P = .057), whereas mean age at time of ST did not significantly differ between low compared to high tracheostomies (59.5 ± 0.8 years vs. 61.3 ± 0.6 years, P = .081) (Table 1).
Figure 1.
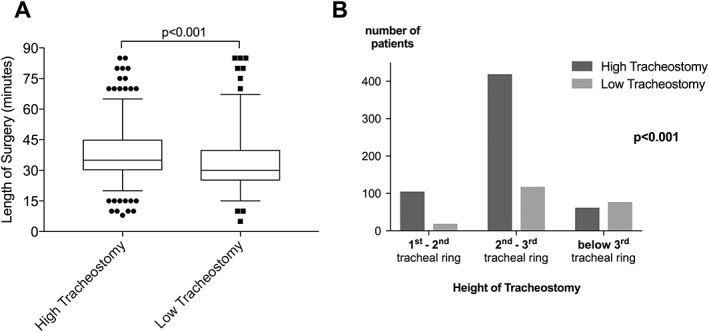
Length of surgery and height of tracheostomy. Low tracheostomies were associated with significantly shorter operation times (A) and significantly lower tracheal incisions for creation of a Björk flap (B) compared to high tracheostomies.
Regarding indication for STs, 48.3% of STs were performed as low tracheostomies in patients with malignant diseases, which was significantly higher compared to 24.0%, 27.5%, and 28.0% in patients with burn injuries, cardiorespiratory problems, and neurologic impairments, respectively (P < .001) (Table 1).
Outcome Analysis
Complications
Complications occurred in 88 out of 1,143 patients (7.7%). Persistent stoma and hemorrhage represented the main complications in 4.1% and 2.7% of cases, followed by tracheal stenosis (0.5%) and wound infection with revision of stoma (0.3%) (Table 2). Overall complication rate did not significantly differ in patients with low compared to those with high tracheostomy (8.0% vs. 7.0%, P = .468). Interestingly, complications occurred significantly more often in females compared to males (10.4% vs. 5.7%, P = .013). In particular, 6.1% of females developed persistent stoma, which was twice as much compared to 3.1% in males (P = .013). Incidences of hemorrhages (3.3% vs. 2.4%, P = .381), wound infections with surgical revision (0.8% vs. 0.1%, P = .088), and tracheal stenosis (0.5% vs. 0.5%, P = .950) did not significantly differ in females compared to males (Table 3). Site of surgery (P = .079), urgency of tracheostomy (P = .760), experience of the surgeon (P = .154), and indication for ST (P = .246) had no significant impact on complication rate (Table 3).
Table 2.
Outcome Parameters
| Outcome | No. | % |
|---|---|---|
| Complications | ||
| No | 1,055 | 92.3 |
| Yes | 88 | 7.7 |
| Persistent stoma | 47 | 4.1 |
| Hemorrhage | 31 | 2.7 |
| Tracheal stenosis | 6 | 0.5/1.4a |
| Wound infection with surgical revision | 4 | 0.3 |
| Decannulation | ||
| No | 644 | 56.3 |
| Yes | 499 | 43.7 |
| Intrahospital mortality | ||
| No | 728 | 63.7 |
| Yes | 415 | 36.3 |
Overall incidence rate of tracheal stenosis is indicated for the whole patient cohort (n = 1,143) and for those 435 patients at risk, who were successfully decannulated and free of intubation for 8 weeks.
Table 3.
Complications According to Different Clinical Variables
| Clinical Variables | Complications | ||||
|---|---|---|---|---|---|
| Persistent Stoma | Hemorrhage | Tracheal Stenosis | Wound Infection | P Value | |
| Type of surgery | |||||
| Low | 13 (4.0) | 19 (2.7) | 5 (0.7) | 4 (0.6) | |
| High | 26 (3.6) | 12 (3.7) | 1 (0.3) | 0 (0.0) | .468 |
| Sex | |||||
| Female | 24 (6.1) | 13 (3.3) | 2 (0.5) | 3 (0.8) | |
| Male | 23 (3.1) | 18 (2.4) | 4 (0.5) | 1 (0.1) | .013 |
| Site of surgery | |||||
| OR | 8 (3.7) | 8 (3.7) | 1 (0.5) | 1 (0.5) | |
| ICU | 39 (4.2) | 23 (2.5) | 5 (0.5) | 3 (0.3) | .079 |
| Urgency of surgery | |||||
| Elective | 46 (4.2) | 30 (2.7) | 6 (0.6) | 4 (0.4) | |
| Urgent | 1 (2.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) | .760 |
| Experience of surgeon | |||||
| Resident | 25 (4.3) | 13 (2.3) | 2 (0.3) | 1 (0.2) | |
| Consultant | 22 (3.9) | 18 (3.2) | 4 (0.7) | 3 (0.5) | .154 |
| Indication for tracheostomy | |||||
| Cardiorespiratory problems | 29 (4.2) | 18 (2.6) | 3 (0.4) | 1 (0.2) | |
| Malignant disease | 8 (3.7) | 8 (3.7) | 1 (0.5) | 1 (0.5) | |
| Neurologic impairment | 8 (5.7) | 4 (2.9) | 2 (1.4) | 1 (0.7) | |
| Burn injury | 2 (2.3) | 1 (1.1) | 0 (0.0) | 1 (1.1) | .246 |
| Total, 88 (7.7) | 47 (4.1) | 31 (2.7) | 6 (0.5) | 4 (0.3) | |
Data are indicated as absolute numbers and percentage within parentheses. χ2 test has been used for statistical analysis.
ICU = intensive care unit; OR = operating room.
Tracheal stenosis
Symptomatic tracheal stenosis requiring surgical intervention was diagnosed in six (two females, four males) of 1,143 patients (0.5%), of which one had undergone low and five had undergone high tracheostomies, respectively. Tracheal stenosis occurred in 0.7% of high tracheostomies and 0.3% of low tracheostomies (P = .436). Moreover, if considering only those patients with successful decannulation who were free of intubation for at least 8 weeks (n = 435), incidence of tracheal stenosis was 1.4%, which did not significantly differ between low and high tracheostomies (0.8% vs. 1.9%, P = .372). Age was not significantly different in patients with tracheal stenosis compared to those without (51.8 ± 8.9 years vs. 60.6 ± 0.4 years, P = .147). Among these patients with tracheal stenosis, one patient underwent cricotracheal resection, whereas the five remaining patients underwent tracheal resection.
Decannulation
Successful decannulation was achieved in 499 patients (43.7%) with a mean time to decannulation of 56.1 ± 6.6 days. Among those, 90.2% (n = 453) and 87.2% (n = 435) of successfully decannulated patients had uneventful courses with intubation‐free survival 6 and 8 weeks after decannulation. Success of decannulation was not significantly affected by type of ST (P = .816) or height of tracheal incision (P = .215). Among those 56.3% of patients (n = 644) in whom decannulation failed, 401 patients died within inpatient stay and 243 patients were not decannulated due to vital necessity. This was either necessary for life‐long ventilation support after hypoxic brain damage, locked‐in syndrome, or other neurologic impairments, as well as in patients with head and neck malignancies.
Intrahospital Mortality
Of the 1,143 patients, 415 died during inpatient stay (36.3%), with a mean time between ST and intrahospital death of 36.3 ± 3.9 days. Inpatient mortality was lower but not significantly different in low compared to high tracheostomies (34.3% vs. 38.9%; P = .152).
DISCUSSION
We investigated the clinical outcome of low tracheostomies, with retraction and preservation of thyroid isthmus, compared to high tracheostomies, with suture ligation of thyroid isthmus, in 1143 patients. To the best of our knowledge, this is the first study that assessed outcome of low compared to high tracheostomies.
Former studies reported overall incidences of complications between 3.2% and 40%.9, 10 We found complications in 88 out of 1,143 patients (7.7%), of which persistent stoma (4.1%) and hemorrhage (2.7%) were the most common. We assessed persistent stoma as tracheostomy‐related complication, which was in contrast to other large series.9, 11 After exclusion of persistent stoma as a tracheostomy‐related complication, our overall complication rate was 3.2%. This was similar to reported complication rates of 3.2% and 4.3% by Shah et al. and Goldenberg et al.9, 11
Low tracheostomies were performed in 31.4% of cases. Importantly, low tracheostomies with retraction and preservation of thyroid isthmus were not associated with higher numbers of hemorrhages, persistent stomas, wound infections with surgical revisions, tracheal stenosis, or overall complications compared to high tracheostomies. Additionally, chosen surgical procedure had no significant impact on decannulation or inpatient mortality.
In our unmatched series, we could further show that preservation of thyroid isthmus was associated with a significantly shorter mean operation time of 33.0 ± 0.8 minutes compared to 38.7 ± 0.5 minutes in high tracheostomies, regardless of whether tracheostomies were performed by experienced consultants or residents. Although significant, this time saving will be rarely crucial for daily practice and reflects only the results of our series. Nonetheless, this time savings might be of particular interest for health economics due to limited healthcare resources and steadily increasing costs with increasing demand for evidence of cost‐effectiveness and optimization of resources.16 Therefore, we strongly recommend performing prospective matched series to further evaluate the possible economic benefit of low tracheostomies over high tracheostomies.
Low tracheostomies were significantly more often performed by experienced consultants than by residents. This might be most likely caused by the fact that STs are still commonly taught as high tracheostomies to young residents. Consequently, residents typically perform high STs until they are experienced enough to augment their surgical armamentarium. However, after exclusion of STs that were done by consultants, operation time was still significantly shorter in low tracheostomies compared to high tracheostomies (32.9 ± 1.08 minutes vs. 40.6 ± 0.61 minutes, P < .001). This clearly indicates that low tracheostomies are associated with shorter operation time regardless of the surgeon's experience. Importantly, low tracheostomies were performed preferentially in patients without neck deformities or thyromegaly and normal neck anatomy. Moreover, low tracheostomies were significantly more often performed in patients with malignant diseases, in whom tracheostomy is typically performed in ORs as elective procedures, prior to scheduled tumor resections to achieve a safe airway situation.
Tracheal stenosis is reported as a major late complication of tracheostomy that is defined as abnormal narrowing of the tracheal lumen.7, 17, 18 Incidences of symptomatic postoperative airway stenosis, which ultimately require intervention, range from 0.8% to 12%.9, 11, 19 We detected tracheal stenosis in only six patients (0.5%), which was lower compared to what has been previously reported. However, if considering only those patients at risk, who were successfully decannulated and who lived intubation free for 8 weeks, incidence of tracheal stenosis was 1.4%, which was still low but in line with the literature. Among these patients, three experienced stenosis at the stoma site and three developed subglottic tracheal stenosis. Incidences of tracheal stenosis were lower but not significantly different in low compared to high tracheostomies, whereas sex, age, and height of tracheostomy did not significantly differ in patients with tracheal stenosis compared to those without.
Amongst others, tracheotomies placed too high with potential damage of the first tracheal ring or cricoid cartilage can cause subglottic or tracheal stenosis.20, 21, 22 Therefore, high tracheostomies between the first and second tracheal ring should be avoided. Within our cohort, 17.9% of high tracheostomies but only 8.9% of low tracheostomies were performed between the first and second tracheal ring. Consequently, low tracheostomies with lower tracheal incisions for creation of a Björk flap could help to further minimize the already low risk of tracheal stenosis.
One possible explanation for the lower occurrence of tracheal stenosis in our cohort might be the 1.5 times higher mortality rate of 36.3% compared to 19.2% to 24.2% in other studies11, 15 with omission of patients and potential sequelae of tracheostomy. Due to the fact that we had no tracheostomy‐related deaths, we assume that patients most likely died from underlying disease rather than by tracheostomy‐related complications. Moreover, although we checked the common and comprehensive medical database used in the majority of Vienna hospitals, we could not exclude missing or incomplete documentation causing false and/or lower incidences of tracheal stenosis.
Conversely, formation of trachea‐innominate artery fistula (TIF) represents a rare but fatal complication of tracheostomy that occurs in less than 0.5% of patients, with reported mortality rates of 80% to 100%.9, 18, 23 Long‐term tracheal intubation, malposition of tube, overinflated cuff, high‐riding innominate artery, and abnormally low placement of the tracheostomy (below the third tracheal ring) can lead to formation of TIF. Therefore, tracheotomies between the second and third tracheal ring are recommended.1, 7, 8 Regardless of whether high or low tracheostomy was performed, we detected no TIFs. In particular, 10.6% of high and 36.0% of low tracheostomies were performed below the third tracheal ring without occurrence of TIF.
Interestingly, we found significantly higher complication rates in females compared to males (10.4% vs. 5.7%). Especially, incidence of persistent stoma was almost two times higher in females compared to males (6.1% vs. 3.1%), whereas other complication subtypes did not significantly differ. We did not find significant differences regarding type of surgery, age, decannulation, and inpatient mortality between females and males, which might be an explanation for this phenomenon. Hence, the underlying mechanism causing this significant difference remains unclear. Whether gender‐specific factors are responsible for significantly higher incidences of persistent stomas needs to be further evaluated.
Nonetheless, there are several limitations in our study that warrant discussion. The main limitation of the retrospective study design and the use of medical reports for analysis of different outcome parameters is the inherent risk of selection and information bias. Due to wrong or missing documentation, we may have overlooked some patients or may have misinterpreted data leading us to wrong conclusions. However, the large patient number may mitigate such biases. The strength of our study is the large number of patients who underwent surgical tracheostomy with creation of a Björk flap, and that all tracheostomies were performed by our Department.
CONCLUSION
We evaluated low compared to high tracheostomies with the creation of Björk flap in 1,143 patients to analyze whether complication and outcome differed. Complications occurred in 7.7% of patients but did not significantly differ in low compared to high tracheostomies (8.0% vs. 7.0%). Moreover, low tracheostomies were associated with significantly shorter operation times (33.0 ± 0.8 min vs. 38.7 ± 0.5 min) and significantly lower tracheostomies for creation of a Björk flap. Importantly, regardless of whether thyroid isthmus could be preserved or not, tracheostomies below the third tracheal ring are principally safe and reliable procedures.
Presented at the 61th Annual Meeting of the Austrian Society of Otorhinolaryngology–Head and Neck Surgery, Vienna, Austria, September 13–17, 2017.
The study was supported and funded by the Department of Otorhinolaryngology–Head and Neck Surgery, Medical University of Vienna, Vienna, Austria.
BIBLIOGRAPHY
- 1. McWhorter AJ. Tracheotomy: timing and techniques. Curr Opin Otolaryngol Head Neck Surg 2003;11:473–479. [DOI] [PubMed] [Google Scholar]
- 2. Cox CE, Carson SS, Holmes GM, Howard A, Carey TS. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993‐2002. Crit Care Med 2004;32:2219–2226. [DOI] [PubMed] [Google Scholar]
- 3. Esteban A, Anzueto A, Alía I, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000;161:1450–1458. [DOI] [PubMed] [Google Scholar]
- 4. Higgins KM, Punthakee X. Meta‐analysis comparison of open versus percutaneous tracheostomy. Laryngoscope 2007;117:447–454. [DOI] [PubMed] [Google Scholar]
- 5. Putensen C, Theuerkauf N, Guenther U, Vargas M, Pelosi P. Percutaneous and surgical tracheostomy in critically ill adult patients: a meta‐analysis. Crit Care 2014;18:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta‐analysis. Crit Care 2006;10:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scurry WC Jr, McGinn JD. Operative tracheotomy. Oper Tech Otolaryngol 2007;18:85–89. [Google Scholar]
- 8. Kinley CE. A technique of tracheostomy. Can Med Assoc J 1965;92:79–81. [PMC free article] [PubMed] [Google Scholar]
- 9. Goldenberg D, Ari EG, Golz A, Danino J, Netzer A, Joachims HZ. Tracheotomy complications: a retrospective study of 1130 cases. Otolaryngol Head Neck Surg 2000;123:495–500. [DOI] [PubMed] [Google Scholar]
- 10. Miller JD, Kapp JP. Complications of tracheostomies in neurosurgical patients. Surg Neurol 1984;22:186–188. [DOI] [PubMed] [Google Scholar]
- 11. Shah RK, Lander L, Berry JG, Nussenbaum B, Merati A, Roberson DW. Tracheotomy outcomes and complications: a national perspective. Laryngoscope 2012;122:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. François B, Clavel M, Desachy A, Puyraud S, Roustan J, Vignon P. Complications of tracheostomy performed in the ICU: subthyroid tracheostomy vs surgical cricothyroidotomy. Chest 2003;123:151–158. [DOI] [PubMed] [Google Scholar]
- 13. Heffner JE, Miller KS, Sahn SA. Tracheostomy in the intensive care unit. Part 1: indications, technique, management. Chest 1986;90:269–274. [DOI] [PubMed] [Google Scholar]
- 14. Calhoun KH, Weiss RL, Scott B, Guendert D, Hokanson JA. Management of the thyroid isthmus in tracheostomy: a prospective and retrospective study. Otolaryngol Head Neck Surg 1994;111:450–452. [DOI] [PubMed] [Google Scholar]
- 15. Rayess HM, Revenaugh PC, Benninger MS, Knott PD. Predictive factors for patient outcomes following open bedside tracheotomy. Laryngoscope 2013;123:923–928. [DOI] [PubMed] [Google Scholar]
- 16. Kruper L, Kurichi JE, Sonnad SS. Methodologic quality of cost‐effectiveness analyses of surgical procedures. Ann Surg 2007;245:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med 1981;70:65–76. [DOI] [PubMed] [Google Scholar]
- 18. Epstein SK. Late complications of tracheostomy. Respir Care 2005;50:542–549. [PubMed] [Google Scholar]
- 19. Streitz JM Jr, Shapshay SM. Airway injury after tracheotomy and endotracheal intubation. Surg Clin North Am 1991;71:1211–1230. [DOI] [PubMed] [Google Scholar]
- 20. Anand VK, Alemar G, Warren ET. Surgical considerations in tracheal stenosis. Laryngoscope 1992;102:237–243. [DOI] [PubMed] [Google Scholar]
- 21. Grillo HC, Donahue DM, Mathisen DJ, Wain JC, Wright CD. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486–492. [DOI] [PubMed] [Google Scholar]
- 22. Couraud L, Jougon JB, Velly JF. Surgical treatment of nontumoral stenoses of the upper airway. Ann Thorac Surg 1995;60:250–259. [DOI] [PubMed] [Google Scholar]
- 23. Cooper JD. Trachea‐innominate artery fistula: successful management of 3 consecutive patients. Ann Thorac Surg 1977;24:439–447. [DOI] [PubMed] [Google Scholar]


