Abstract
Patient reporting suggests that the physical and psychological effects of autoimmune hepatitis (AIH) can be substantial. However, health‐related quality of life (HRQOL) in patients with AIH remains incompletely characterized, and health utility remains to be explored. Treatment for AIH often includes the use of corticosteroids, which are agents that can be associated with significant adverse effects. Here we explore the impact of AIH and its treatments on patient‐reported HRQOL and health utility in a large cohort of prevalent cases from the United Kingdom Autoimmune Hepatitis (UK‐AIH) national study. Data were collected from 990 adult participants with a clinical diagnosis of AIH using validated HRQOL tools including the European Quality‐of‐Life 5‐Dimension 5‐Level (EQ‐5D‐5L) and clinical data forms. The EQ‐5D‐5L dimension scores were compared with UK population norms and with a disease control cohort with primary biliary cholangitis (PBC). Within the AIH cohort, regression analysis was used to explore associations between HRQOL and demographic and clinical variables with a particular focus on the impact of AIH therapies including corticosteroid use. HRQOL, measured by the EQ‐5D‐5L utility index, is shown to be significantly impaired in our cohort of AIH patients compared with population norms. Within the AIH cohort, corticosteroid use was found to be significantly associated with impaired HRQOL, even when controlling for biochemical disease activity status. Conclusion: Our data show evidence of HRQOL impairment in a large cohort of AIH patients compared with the general population. Furthermore, corticosteroid use is strongly associated with decreased HRQOL, independent of remission status. This highlights the need for better corticosteroid‐free therapy approaches and it emphasizes the need for future novel therapeutic trials in AIH. (Hepatology 2018; 00:000‐000).
Autoimmune hepatitis (AIH) is a rare immune‐mediated chronic liver disease that, if under‐treated, results in progressive liver injury leading to cirrhosis, hepatic failure or death. AIH remains a diagnostic and therapeutic challenge with at least a third of patients presenting with cirrhosis, a fifth having relapsing disease, and 30%‐50% developing cirrhosis despite treatment.( 1 ) AIH therefore has the potential to cause significant medical and economic burdens on affected patients and health care delivery systems, respectively. Current management of AIH largely comprises corticosteroids alone (mainly prednisolone) or in combination with azathioprine, with 38%‐93% of patients achieving remission,( 2, 3 ) but up to 90% having a disease relapse after withdrawal of therapy.( 4 ) Other second‐line immunosuppressants include mycophenolate mofetil (MMF), cyclosporine, and tacrolimus, but these lack evidence based on randomized controlled trials. All these agents, particularly corticosteroids, can be associated with important adverse effects such as the metabolic syndrome with its sequelae, osteoporosis, weight gain, and disturbance in sleep and mood.( 5, 6, 7, 8, 9 )
In addition to its clinical impact as a result of disease progression to cirrhosis, up to 50% of patients with AIH are symptomatic with fatigue, general ill health, abdominal pain, and joint pain, despite treatment.( 10, 11 ) Patient reports suggest that the physical and psychological effects of AIH on patients can be substantial. These remain, however, incompletely characterized,( 4 ) and it can be challenging to separate these from adverse effects attributable to the treatment used for AIH. The nature and extent of the impact of AIH and its symptoms and treatments on health‐related quality of life (HRQOL) has not been widely reported. A survey among members of the Dutch liver patient association evaluated HRQOL in liver disease of different etiologies using the Dutch Short Form Survey‐36 (SF‐36), the Liver Disease Symptom Index 2.0 and the Multidimensional Fatigue Index‐20. The subset of patients with AIH (n = 142) had significantly lower scores (and thus worse HRQOL) in all SF‐36 scales (particularly, physical problems or general health scales), as well as significantly worse fatigue scores (reported via the Multidimensional Fatigue Index‐20 questionnaire) compared with Dutch healthy controls.( 12 ) A study of 24 children with AIH or with Primary Sclerosing Cholangitis/AIH overlap using the Pediatric Quality of Life (PedsQL) 4.0 questionnaire showed significant impairment of HRQOL. This was associated with the presence of frequent liver disease‐related symptoms (particularly, abdominal pain, fatigue, and mood symptoms).( 13 ) More recently, a German single‐center study (n = 103) using patient‐reported HRQOL data found higher rates of depression and anxiety in AIH patients compared with the general population. A major associated factor was concern regarding the risk and implications of progressive liver disease. There was also a correlation found between prednisolone use and depression.( 14 ) A Polish single‐center study (reported in abstract form) found that a population of patients (52 with AIH had reduced HRQOL according to the SF‐36, Modified Fatigue Impact Scale, and Patient Health Questionnaire‐9) compared with a matched healthy‐control group.( 15 ) Finally, a Canadian study (n = 52) described an association between psychosocial distress, nonadherence to treatment, and incomplete response to therapy in AIH.( 16 ) Studies on health‐related quality of life (HRQOL) in AIH have been mainly single‐center with small numbers, but these suggest that there is a significant issue with quality of life (QOL) in AIH and that further formal exploration of its impact is warranted.( 12, 13, 14, 15, 16 )
QOL is a critically‐important issue for patients, and one that is increasingly prioritized by regulatory bodies when evaluating the benefits of new drugs. A deeper understanding of HRQOL is therefore essential if we are to make progress with therapy in AIH. In this study, we utilized a unique cohort of prevalent cases in the United Kingdom‐AIH (UK‐AIH) study cohort to explore the impact of AIH and its treatments on patient life quality using the European Quality‐of‐Life 5‐Dimension 5‐Level (EQ‐5D‐5L) tool to evaluate HRQOL.( 17 ) We also used the data to calculate health state utilities, values which represent an individual’s preferred value for specific health states relative to full health, which are fundamental in assessing cost‐effectiveness and cost utility of the management of disease.( 18 ) As fatigue, cognitive impairment, anxiety and depression are important symptoms that have been reported in chronic liver diseases and have significant impact on QOL,( 12, 14, 19, 20, 21 ) we used three other qualitative tools (the Fatigue Impact Scale [FIS],( 22 ) the Cognitive Failure Questionnaire [CFQ],( 23 ) and the Hospital Anxiety Depression Scale [HADS])( 24 ) to explore the impact of specific symptom sets on HRQOL and health utilities.
Patients and Methods
STUDY POPULATION
UK‐AIH is a cross‐sectional cohort study open to adult patients (≥16 years) with a current clinical diagnosis of AIH (as evaluated by their treating clinician) who were recruited from a secondary hospital care setting between March 2014 and January 2017. Patients had to be living in the United Kingdom and all patients provided written informed consent for use of data. The protocol was approved by the National Health Service (NHS) Health Research Authority (IRAS ID: 144806, REC reference: 14/LO/0303) and was conducted in accordance with the International Council for Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki.
QUALITY OF LIFE AND SYMPTOM IMPACT MEASURES
Health‐related quality of life and symptom impact information was collected using patient‐reported EQ‐5D‐5L, FIS, CFQ and HADS tools.
The European Quality‐of‐life 5‐Dimension 5‐Level (EQ‐5D‐5L) tool is a simple, generic HRQOL instrument comprising five health dimensions which generates a health utility index (UI) and a visual analogue scale (VAS). The European Quality‐of‐life 5‐Dimension (EQ‐5D) tool was first introduced as a three‐level version (EQ‐5D‐3L) in 1990 with three levels of severity (no problems, some problems, and extreme problems), and was subsequently revised in 2009 to include five levels of severity (EQ‐5D‐5L) in order to improve the instrument’s sensitivity and reduce ceiling effects compared with EQ‐5D‐3L.( 17, 25 ) The EQ‐5D tool has been used to evaluate HRQOL in a wide spectrum of diseases including liver disease, notably hepatitis C,( 26 ) hepatitis B,( 27 ) liver transplantation,( 28 ) and other chronic liver diseases. The EQ‐5D‐5L is widely used internationally as a patient‐reported outcome measure and is the preferred tool of England’s health authority, the National Institute for Health and Care Excellence (NICE), for use in cost‐effectiveness analysis.( 29 ) The EQ‐5D‐5L tool comprises the EQ descriptive system of five health dimensions (mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression) and the EQ‐visual analogue scale (EQ‐VAS). Each health dimension has five levels (scored from 1 to 5): no problems, slight problems, moderate problems, severe problems and extreme problems. The respondent indicates his/her health state by ticking the box against the most appropriate statement in each of the five health dimensions. Utility Index (UI) is then calculated from these five health dimensions using the EQ‐5D‐5L Value Set for England.( 30 ) The UI ranges from ‐0.28 (‘worst possible health’) to 1.00 (‘best possible health’). The EQ‐VAS records the participants’ self‐rated health on a vertical VAS with end‐points labelled “the worst health you can imagine” (0) at the bottom of the scale and “the best health you can imagine” (100) at the top of the scale, respectively. A higher UI and VAS denote a higher HRQOL. For the purpose of this manuscript, the term UI is used to represent HRQOL.
The FIS tool is an assessment tool developed to evaluate the impact of fatigue on the activities and quality of daily life. It has been validated in chronic fatigue syndrome, multiple sclerosis,( 22 ) and primary biliary cholangitis (PBC).( 31 ) It consists of 40 items addressing the impact of fatigue on aspects of daily life (maximum score 160), containing three intermixed domains addressing physical, cognitive (maximum score 40 each) and psychosocial (maximum score 80) elements of fatigue. The FIS is scored on a 5‐point Likert scale (0 = no problem to 4 = extreme problem). The higher the total score, the higher the impact of fatigue.
The CFQ is a tool that assesses the prevalence of cognitive symptoms by measuring the frequency of cognitive slips or failures occurring in everyday life. These include memory, attention, concentration, forgetfulness, word‐finding abilities, and confusion. The questionnaire consists of 25 questions encompassing failures in perception, memory and motor function. The patient rates how often these failures occur on a five‐point Likert scale of 0‐4 (0 = never, 4 = very often). The responses are summed to obtain a total CFQ score. The higher the score, the greater the cognitive impairment (overall range 0‐100).( 23 )
The HADS tool is a validated 14‐item measure of current anxiety (HADS‐A) and depression (HADS‐D) optimized for use in patients with chronic disease.( 24 ) It was developed as a screening instrument for use in a hospital outpatient setting and is aimed at detecting the presence and severity of depression and anxiety in nonpsychiatric settings.( 32 ) Anxiety and depression subscales (comprising 7 items) are scored separately (ranging 0‐21 for each subscale). For each subscale, a score of 0‐7 indicates no anxiety or depression, 8‐10 borderline “caseness,” and a score of ≥11 is clinically significant, indicating “caseness” for depression or anxiety.( 19, 24 )
CLINICAL DATA COLLECTION, GROUP COMPARISON AND GROUP SUBTYPES
Demographic and clinical data were collected from patient records by study team members using clinical data forms. Patients with previous liver transplantations were excluded from this analysis. Clinical data was collected on sex,age at inclusion of study, year of diagnosis, biochemical results on recruitment date (serum alanine transferase [ALT] and Immunoglobulin G [IgG]) or within 12 months of recruitment date, number of flares in the past 12 months, immunosuppressive treatment, presence of cirrhosis (defined histologically or clinically by radiological evidence or transient elastography) and diagnosis of osteoporosis (on diagnosis of AIH and since diagnosis). Biochemical remission was defined as normal ALT and IgG at the time of recruitment (and within the preceding 12 months) with no documented flares in the last 12 months. A flare was defined as an abnormal ALT above the upper limit of normal (ULN) requiring an increase or addition of corticosteroid treatment. The upper limit of normal (ULN) used for ALT and IgG levels were based on each site’s pathology laboratory ULN.
Ethnicity and data on other medical conditions including PBC, primary sclerosing cholangitis (PSC), ulcerative colitis or Crohn’s disease, and rheumatoid arthritis (RA) were collected using patient‐reported “tick‐box” questionnaires.
A cohort of PBC patients obtained from the UK‐PBC cohort (n = 1665) with known age, sex, European quality‐of‐life 5‐dimension 5‐level utility index (EQ‐5D‐5L UI) and EQ‐VAS scores were used for comparison.( 33 )
STATISTICAL ANALYSIS
The cohort was characterized descriptively, with categorical variables presented as frequencies and percentages and continuous variables presented as medians and ranges due to underlying distributional assumptions. To compare HRQOL with UK population norms, the EQ‐5D‐5L dimension scores in our cohort were converted to EQ‐5D‐3L index values using the crosswalk calculator, as there are currently no published population norms available using the EQ‐5D‐5L value set. The crosswalk calculator determines a UI value for each EQ‐5D‐5L state by first predicting the likelihood of being in each EQ‐5D‐3L state and, secondly, calculating the weighted sum of the UI values across the EQ‐5D‐3L states. This is based on an analysis of data from coadministering both tools to 3,691 respondents.( 34 ) These values were then compared with EQ‐5D‐3L values of UK population norms( 35 ) using the two sample t test after standardization for age and sex and reported as mean (standard deviation).
Regression analysis was used to explore the association of various demographic and clinical covariates with HRQOL in our cohort. The primary outcome measure of interest was UI, a continuous measure of HRQOL. Due to the highly‐skewed nature of this variable, we used quantile regression with the median as the chosen quantile. Regression models were developed for each covariate of interest and models were adjusted for age, sex, and biochemical remission status, as appropriate.
Further regression analysis was used to explore the relationship between various other HRQOL outcome measures and corticosteroid use. These outcome measures included the five individual health dimensions of the EQ‐5D‐5L (analyzed using ordinal regression), continuous measures FIS and CFQ (analyzed using quantile regression), and the three‐level classification of HADS‐A and HADS‐D (analyzed using ordinal regression). Data were analyzed using STATA version 14.1, R version 3.3.0.
Results
DEMOGRAPHIC, CLINICAL AND HRQOL CHARACTERISTICS
Data from 990 patients were analyzed from 39 hospitals (32 nontransplant centers and 7 transplant centers). Table 1 shows the clinical characteristics of the study population and self‐reported comorbidities. For further details on therapy regimens, see Supporting Table S1. Table 2 summarizes the HRQOL and symptom severity characteristics.
Table 1.
Clinical Characteristics of the Study Population
| Biochemical Tests | |
| ALT (IU/L) (n = 965) | 25.0 (4.0‐1315.0) |
| ALT:ULN ratio | 0.6 (0.1‐35.0) |
| IgG (g/L) (n = 742) | 12.5 (2.1‐51.1) |
| IgG:ULN ratio | 0.8 (0.2‐3.2) |
| Biochemical Remission | |
| Normal ALT only (according to each site’s ULN [n = 965]) | 758 (78.5) |
| Normal ALT and IgG (n = 736) | 449 (61.0) |
| Normal ALT and IgG with no documented flares last 12 months (used in regression analysis [n = 990]) | 558 (56.4) |
| Therapy and Dose per day (n = 990) | |
| Prednisolone | 499 (50.4) |
| Dose, mg | 5 (0.2‐60) |
| Budesonide | 47 (4.7) |
| Dose, mg | 3 (0.4‐9) |
| Azathioprine | 581 (58.7) |
| Dose, mg | 100 (25‐250) |
| Dose/weight, mg/kg | 1.1 (0.2‐2.8) |
| 6‐Mercaptopurine | 48 (4.8) |
| Dose, mg | 50 (12‐150) |
| Dose/weight, mg/kg | 0.7 (0.1‐2.0) |
| Mycophenolate (MMF/MA) | 166 (16.8%) |
| Dose, mg | 1,000 (200‐3,000) |
| Tacrolimus | 41 (4.1) |
| Dose, mg | 2 (0.5‐9) |
| Cyclosporine | 6 (0.6) |
| Dose, mg | 125 (50‐300) |
| Self‐reported comorbidities (n = 956) | |
| Primary biliary cholangitis | 64 (6.7) |
| Primary sclerosing cholangitis | 22 (2.3) |
| Ulcerative colitis/Crohn’s | 48 (5.0) |
| Rheumatoid arthritis | 60 (6.3) |
| Osteoporosis | 147 (15.4) |
Note: Data are presented as n (%) or median (range), unless noted otherwise.
Table 2.
HRQOL and Symptom Impact Characteristics
| HRQOL Measures | |
| EQ‐5D‐5L UI (n = 986) | 0.89 (‐0.19 ‐ 1.00) |
| EQ‐VAS (n = 985) | 80 (10‐100) |
| FIS (n = 950) | 27 (0‐160) |
| CFQ (n = 950) | 32 (0‐100) |
| HADS‐A score (n = 966) | 5 (0‐21) |
| HADS‐D score (n = 965) | 3 (0‐18) |
| HADS‐A score classifications (n = 966) | |
| No anxiety (0‐7) | 637 (65.9) |
| Borderline (8‐10) | 181 (18.7) |
| Clinically significant (≥11) | 148 (15.3) |
| HADS‐D score classifications (n = 965) | |
| No depression (0‐7) | 816 (84.6) |
| Borderline (8‐10) | 96 (9.9) |
| Clinically significant (≥11) | 53 (5.5) |
Note: Data are presented as n (%) or median (range), unless noted otherwise.
Of the study participants, 795 (80%) were female and 92% of Caucasian ethnicity. The median age at inclusion in the study was 58 years (17‐95) and median duration of disease was 7 years (0‐57). A total of 79% of those with available data had positive antinuclear antibody (ANA), or smooth muscle antibody (SMA), or soluble liver antigen (SLA) antibodies, and 1% had anti‐Liver‐Kidney Microsomal (LKM) positivity. Altogether, 558 (56%) were in biochemical remission, 545 (55%) of patients were on corticosteroids, and 330 (33%) had cirrhosis. There were 25 (3%) patients on triple immunosuppression (i.e., taking two immunosuppressants and corticosteroids).
HRQOL AND UTILITY ABNORMALITY IN AIH
The median EQ‐5D‐5L UI value was 0.89 (‐0.19‐1.00) and the median EQ‐VAS was 80 (10‐100). The distribution of EQ‐5D‐5L responses can be seen in Fig. 1, split by health domain. Of the five domains, the pain/discomfort domain had the highest proportion of patients reporting problems (57%), while the self‐care domain had the lowest (11%). Following conversion to EQ‐5D‐3L index values using the crosswalk calculator,( 34 ) the mean UI (0.77, SD = 0.23, standardized for sex and age) was significantly lower in our cohort (t = 11.4, P <0.001) compared with UK population norms (0.86, SD = 0.23).
Figure 1.
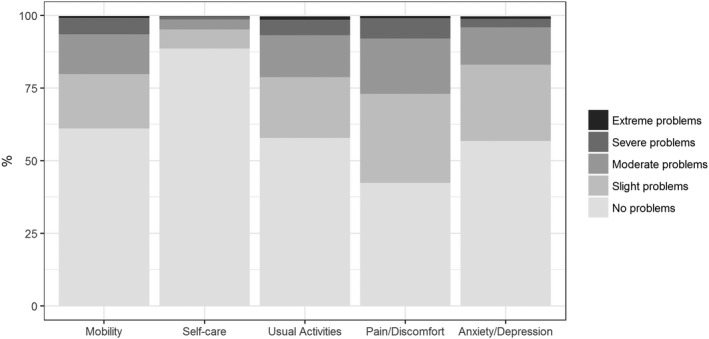
Distribution of EQ‐5D‐5L responses levels, split by health domain (whole cohort).
When comparing our cohort of AIH patients to a large cohort of PBC patients (n = 1665, median age: 67 years [29‐95], 91% female), we found no statistically significant difference in UI between the two groups, after controlling for age and sex (β = 0. 008, P = 0.41). On graphical comparison of the EQ‐5D‐5L responses according to health domains between AIH and PBC, the proportion of patients experiencing problems in each of the subdomains appear similar between the two groups. (See Supporting Fig. S1.)
ASSOCIATION BETWEEN DEMOGRAPHIC AND CLINICAL FACTORS AND HRQOL/UTILITY
The results of univariable quantile regression are presented in Table 3. In the unadjusted regression analysis, we observed that increasing age and body mass index (BMI) are both associated with lower UI (β = ‐0.001, P <0.001 and β = ‐0.006, P <0.001 respectively) and female sex is associated with higher UI (β = 0.034, P = 0.027). We did not find any evidence that the place of care (transplant centers versus nontransplant centers) is associated with UI (β = 0.000, P = 1.000).
Table 3.
Regression Analysis Investigating Factors Associated With EQ‐5D‐5L Utility Index Value.
| Outcome Variable | Predictor | Coefficient (β) | 95% CI | P Value |
|---|---|---|---|---|
| EQ‐5D‐5L UI value | Demographic | |||
| Age at inclusion | ‐0.001 | (‐0.002, ‐0.001) | <0.001 | |
| BMI | ‐0.006 | (‐0.008, ‐0.005) | <0.001 | |
| Sex, female | 0.034 | (0.004, 0.064) | 0.027 | |
| Transplant center | 0.000 | (‐0.021, 0.021) | 1.000 | |
| Clinical covariatesa | ||||
| In biochemical remissionb | 0.024 | (0.004, 0.043) | 0.020 | |
| Has cirrhosis | 0.013 | (‐0.009, 0.035) | 0.245 | |
| Duration of disease | 0.001 | (‐0.000, 0.002) | 0.202 | |
| “Normal ALT” using lower cut‐offs ALT (ALT <30 for males, ALT <19 for females) | 0.006 | (‐0.016, 0.029) | 0.587 | |
| Treatmenta | ||||
| Prednisolone/budesonide | ‐0.030 | (‐0.051, ‐0.009) | 0.006 | |
| Low dose corticosteroid (defined as either <10 mg prednisolone/day or <6 mg budesonide/day) | ‐0.027 | (‐0.050, ‐0.004) | 0.021 | |
| High dose corticosteroid (≥10 mg prednisolone/day or ≥6 mg budesonide/day) | ‐0.035 | (‐0.067, ‐0.004) | 0.028 | |
|
Corticosteroid dose (log‐transformed) (corticosteroid users only, n = 545) |
‐0.006 | (‐0.028, 0.016) | 0.582 | |
| Azathioprine / 6‐mercaptopurine | 0.009 | (‐0.011, 0.030) | 0.356 | |
| MMF/MA | ‐0.007 | (‐0.035, 0.020) | 0.594 | |
| Tacrolimus/cyclosporine (CNIs) | ‐0.077 | (‐0.124, ‐0.030) | 0.002 | |
| Patient reported comorbiditiesa | ||||
| PBC | ‐0.046 | (‐0.088, ‐0.004) | 0.033 | |
| PSC | ‐0.090 | (‐0.154, ‐0.027) | 0.005 | |
| Rheumatoid arthritis | ‐0.053 | (‐0.092, ‐0.014) | 0.008 | |
| Osteoporosis | ‐0.023 | (‐0.052, 0.007) | 0.132 | |
| Ulcerative colitis/Crohn’s | ‐0.031 | (‐0.076, 0.014) | 0.177 |
Controlling for age, sex & remission status.
Controlling for age and sex.
Patients who are in biochemical remission appear to have significantly higher UI (β = 0.024, P = 0.020) than those not in biochemical remission, after controlling for age and sex. In all subsequent models discussed, we controlled for age, sex and remission status. We did not observe any association between UI and having cirrhosis or duration of disease. We also explored lower cut‐offs for normality for ALT, adopting the hepatitis B virus cut‐offs (<30 IU/L for males and <19 for females)( 36 ) and did not find any association between UI and these lower cut‐off levels.
When considering the association between patient‐reported comorbidities and HRQOL, we found that patients who reported having either PBC (β = ‐0.046, P = 0.033), PSC (β = ‐0.090, P = 0.005) or RA (β = ‐0.053, P = 0.008) have impaired UI compared with AIH patients not reporting these comorbidities. We did not find any evidence of an association between self‐reported osteoporosis or inflammatory bowel disease in our cohort.
ASSOCIATION BETWEEN AIH THERAPIES AND HRQOL/UTILITY
We found that corticosteroid use was significantly associated with lower UI (β = ‐0.030, P = 0.006) and this was irrespective of being on low‐dose corticosteroids (<10 mg prednisolone or <6 mg budesonide per day) or high‐dose corticosteroids (≥prednisolone 10 mg or ≥6 mg budesonide per day). (See Table 3) Furthermore, within the group on corticosteroids, we did not find any evidence that dose was associated with material difference in HRQOL impairment, with impact still being seen in patients on low dose corticosteroid (Table 3). We did not find any evidence that the reduction in UI seen in corticosteroid users was different for patients in biochemical remission compared with those not in biochemical remission (this was explored via the addition of an interaction term to the model resulting in a nonsignificant coefficient, (β = ‐0.013, P = 0.486). Additionally, we found that calcineurin inhibitor (CNI) use was also significantly associated with a lower UI (β = ‐0.077, P = 0.002). Even after controlling for corticosteroid use, CNI use remained significantly associated with lower UI (β = ‐0.067, P = 0.008). We did not find any evidence of association between azathioprine/6‐mercaptopurine (6MP) or Mycophenolate mofetil (MMF)/Mycophenolic acid (MA) use and impaired QOL. Furthermore, we found no evidence of a difference between corticosteroid monotherapy and combination therapy with azathioprine (explored via the addition of an interaction term to the model resulting in a nonsignificant coefficient, β = ‐0.024, P = 0.177).
Further investigation of individual health states within the EQ‐5D‐5L (Table 4) showed that corticosteroid use was associated with increased problems in the mobility (β = 0.307, P = 0.026) and usual activities (β = 0.426, P = 0.002) domains. There was evidence of a borderline association between corticosteroid use and increased problems in the anxiety/depression domain (β = 0.267, P = 0.043). We did not find evidence of an association between corticosteroid use and the self‐care or pain/discomfort domains.
Table 4.
Regression Analysis Investigating the Relationship Between Corticosteroid Use and Further HRQOL Outcome Measures
| Outcome variable | Predictor | Coefficient (β) | 95% CI | P Value |
|---|---|---|---|---|
| EQ‐5D‐5L health states: | Corticosteroid usea | |||
| Mobility | 0.307 | (0.037, 0.578) | 0.026 | |
| Self‐care | 0.373 | (‐0.054, 0.800) | 0.087 | |
| Usual activities | 0.426 | (0.162, 0.690) | 0.002 | |
| Pain/discomfort | 0.203 | (‐0.040, 0.447) | 0.102 | |
| Anxiety/depression | 0.267 | (0.009, 0.525) | 0.043 | |
| Total FIS score | 9.50 | (4.12, 14.9) | <0.001 | |
| CFQ score | 2.46 | (‐0.562, 5.48) | 0.111 | |
| HADS‐Anxiety score | 0.162 | (‐0.118, 0.443) | 0.256 | |
| HADS‐Depression score | 0.327 | (‐0.059, 0.713) | 0.096 |
Controlling for age, sex, and remission status.
Additional HRQOL measures (FIS, CFQ, HADS‐A and HADS‐D) are considered in Table 4, where their associations with corticosteroid use are explored. We observe impaired HRQOL for corticosteroid users with respect to all HRQOL measures, with the association between corticosteroid use and FIS (β = 9.50, P <0.001) reaching statistical significance.
Discussion
This large and comprehensive study of quality of life in AIH explores health utility in this disease. Health utility is a critical parameter that plays an integral role in the assessment of the value of current and emerging therapies in disease. The multi‐center nature of the patient population (a combination of liver transplant centers, tertiary hospitals and district general hospitals) ensures a comprehensive spectrum of patients and provides important real‐world data. Our key findings are that quality of life and health utility impairment in AIH are significant (and similar in degree to those seen in PBC)( 19 ) and that the nature of the treatment used in AIH, in particular the use of corticosteroids, is potentially a key driver for impaired quality of life and health utility. Health utility in AIH should be included in the assessment of treatment approaches in AIH and improvement of health states should be a goal for future therapy approaches, alongside the conventional target of prevention of disease progression.
Achieving a state of biochemical remission, in which surrogate serum markers of disease activity are normalized, was associated with significantly better health utility outcomes after controlling for age and sex. In the German study by Schramm and colleagues, which found reduced mental wellbeing in their patients with AIH, the majority (77%) were in biochemical remission.( 14 ) This compares with the 56% remission rate in our “real world” nationwide patient cohort. Our relatively lower proportion of patients in remission may be due to the stricter definition for biochemical remission applied in our study, which takes into account the reported trend of the biochemical parameters in the previous 12 months. Incomplete biochemical remission (failure of transaminases and IgG levels to normalize) is predictive of relapse after treatment withdrawal, histological activity, progression to cirrhosis, and poor outcome.( 4 ) Ongoing inflammation in a patient not in remission would be an obvious potential explanation for reduced utility. Patients who have not achieved complete biochemical remission are more likely to be on dual or triple immunosuppression (potentially at higher doses) and have had more courses of corticosteroids, and they are at increased risk of complications of progressive disease. All these factors could also impact quality of life and utility. In our analysis, cirrhosis did not emerge as a cofactor associated with poorer utility, even after controlling for age, sex and remission status. Schramm et al. also found that the presence of cirrhosis in AIH patients was not significantly associated with depressive symptoms and the physical component score of the 12‐Item Short‐Form Health Survey (SF‐12) in the AIH patients did not differ between those with and without cirrhosis.( 14 )
The most striking finding in our study was that corticosteroids were associated with significantly lower levels of utility, even after controlling for age, sex and, crucially, biochemical remission status. This effect was seen for both high‐dose and low‐dose corticosteroid therapy. On further analysis of the five EQ‐5D‐5L health domains, corticosteroid use was associated with increased problems with mobility and usual activities. One of the well‐known adverse effects of corticosteroids is weight gain. Almost a third (31%) of our patients on corticosteroids were obese with BMI ≥30, which may contribute to problems in mobility and usual activities. Corticosteroids were the first therapy shown to improve the outcome of patients with AIH by significantly reducing mortality compared with placebo in the early controlled trials. However, these trials reported a high proportion of corticosteroid‐related adverse effects including Cushingoid features in 20%‐50% of patients, diabetes in 15%‐20%, hypertension, cataracts, psychosis, and osteoporotic vertebral collapses in 5%‐10%.( 1, 5, 6, 7, 8 ) Subsequent studies reported a 30%‐53% prevalence of corticosteroid‐related side‐effects.( 9, 37, 38 ) Despite the adverse effects associated with corticosteroids, a significant proportion (38%‐85%) of patients remain on corticosteroids in the long term.( 39, 40 ) The impact of corticosteroids on QOL has been studied in other diseases dependent on long‐term corticosteroids, such as systemic lupus erythematosus,( 41 ) sarcoidosis,( 42 ) liver transplantation( 43 ) and, historically, rheumatoid arthritis.( 44 ) The recognition of the impact of oral corticosteroids in systemic lupus erythematosus has even catalyzed the development of a Systemic Lupus Erythematosus‐Specific Steroid Questionnaire.( 45 ) There have been no previous studies in AIH, to our knowledge, that have explored the specific impact of corticosteroids on QOL. The study by Schramm and colleagues found a correlation between rates of depression with prednisolone use in their AIH cohort.( 14 ) However, no correlation was found between corticosteroid use and HADS‐A or HADS‐D in our study. A key conclusion of our study is that clinicians should be more aware of the potential for impaired life quality and health utility in patients with AIH who are on corticosteroids. This should be factored into decisions regarding the appropriateness of long‐term corticosteroid therapy with clinicians being aware that steroid minimization, as opposed to discontinuation, may not confer benefit.
The use of CNIs (tacrolimus or cyclosporine) in AIH appears to be growing, although the evidence base is limited (case‐series in refractory patients). CNIs are well‐known to have associated adverse effects such as renal impairment, neurotoxicity, hypertension, and gum hypertrophy.( 4, 46, 47 ) CNIs are often second‐ or third‐line therapies for azathioprine or mycophenolate mofetil intolerant patients or those who have failed to respond to conventional therapies. Although only 4% of our population were on CNIs, it is striking to note that this still emerged as a covariate significantly associated with lower UI even after controlling for corticosteroid use. This highlights the need for therapies that are better tolerated with better efficacy.
In exploring the potential factors which might underpin poor quality of life and health utility in AIH, we found that the overall median fatigue impact scale (FIS) score for our AIH cohort was 27 (maximum possible score of 160). Although direct comparisons cannot be made, this score is not dissimilar to the median FIS score seen in community controls( 28 ) and AIH controls (21 in a cohort of 38 patients) in a previous study exploring the impact of fatigue in PBC (median FIS 40).( 48 ) Fatigue scores were higher in patients not in remission and in patients taking corticosteroids, although fatigue did not appear to be the major driving force between corticosteroid‐associated impairment of health utility. The median score for the CFQ tool was 32 (0‐100) with a mean of 34 (SD 18). For comparison, a study evaluating QOL in a cohort of 103 patients with liver transplantation (median time since transplantation of 40 months [range 2‐155]) reported a mean CFQ score of 38 (SD 25.2).( 49 ) There was no association between corticosteroid use and worse CFQ scores in our study. These observations suggest that in AIH, unlike in PBC, cognitive impairment symptoms and fatigue do not appear to be major factors in impaired health utility.
Our study has limitations. Firstly, it is cross‐sectional in nature, therefore any associations found between HRQOL and other factors must be interpreted cautiously, especially with respect to causality. Ideally, we would have longitudinal data on the patients in our cohort, which would allow the effect of treatment to be studied over time. In addition, the population norms used for comparison were based on data from the United Kingdom’s most recent survey in 1993. It is possible that average health has improved over time across the age groups, with the published population norms therefore likely to be conservative. In this large cohort, we observed that the association between corticosteroid use and reduced UI is independent of remission status and other known confounders (age, sex, comorbidity, and so on). However, there may be other unknown and unmeasured confounders that we have been unable to adjust for. Ideally, we would provide an interpretation of the effect size of corticosteroid use on UI at a patient level; however, to our knowledge the minimally important clinical difference in UI has not previously been studied in this population. Another limitation of the study is that our data on comorbidities are self‐reported data by patients rather than data based on more accurate diagnostic criteria which would be highly ideal, particularly for those with overlap or concurrent PBC or PSC. We did not collect socioeconomic data, which would be desirable for more detailed health economic analysis.
There is increasing recognition that quality of life management should be a priority in managing patients with AIH, as it can have an impact on compliance to medication, as well as outcomes. Despite established therapies, little information has been published on HRQOL and utilities for health states resulting from AIH. This current study is the start of bridging this gap and it highlights the impaired HRQOL in patients with AIH. In addition, it can be used to inform on cost‐effectiveness of current treatment regimens in the United Kingdom. There may also be a role for the development of AIH‐specific HRQOL measures, similar to the PBC‐40 (a PBC‐specific HRQOL tool).( 50 )
In summary, our data show evidence of HRQOL impairment in a large cohort of AIH patients compared with the general population, as well as impairment similar to that seen in patients with PBC. Furthermore, corticosteroid use shows an association with decreased HRQOL which is independent of remission status. This highlights the need for better, and ideally corticosteroid‐free, future therapy approaches and emphasizes the need for future novel therapeutic trials in AIH.
Author names in bold designate shared co‐first authorship.
Abbreviations
- AIH
autoimmune hepatitis
- ALT
alanine transferase
- BMI
body mass index
- CFQ
cognitive failure questionnaire
- CNI
calcineurin inhibitor
- EQ‐5D
European quality‐of‐life 5‐dimension
- EQ‐5D‐3L
European quality‐of‐life 5‐dimension 3 level
- EQ‐5D‐5L
European quality‐of‐life 5‐dimension 5 level
- EQ‐5D‐5L UI
European quality‐of‐life 5‐dimension 5‐level utility index
- EQ‐VAS
European quality‐of‐life visual analogue scale
- FIS
fatigue impact scale
- HADS
hospital anxiety depression scale
- HADS‐A
hospital anxiety depression scale‐anxiety
- HADS‐D
hospital anxiety depression scale‐depression
- HRQOL
health‐related quality of life
- IgG
immunoglobulin G
- MA
mycophenolic acid
- MMF
mycophenolate mofetil
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
- QOL
quality of life
- SF‐36
Short Form Survey‐36
- UI
utility index
- ULN
upper limit of normal
- VAS
visual analogue scale
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.30031/suppinfo.
Supporting Information
Acknowledgment
Dr Shirley English (Aberdeen Royal Infirmary), Dr Graeme Alexander/Dr George Mells (Addenbrooke’s Hospital), Dr Debabrata Majumdar (St Peters Hospital), Dr Vinay Sathyanarayana (Barnsley Hospital), Professor John Ramage (Basingstoke & North Hampshire Hospital), Dr Christopher Shorrock (Blackpool Victoria Hospital), Dr James Maggs (Buckinghamshire Hospital), Dr David Elphick (Chesterfield Royal Hospital), Dr Chris Macdonald (Cumberland Infirmary), Professor Matthew Cramp (Derriford Hospital), Dr Joanne Sayer (Doncaster Royal Infirmary), Dr James Jupp (Dorset County Hospital), Dr Jessica Dyson (Freeman Hospital), Dr Coral Hollywood (Gloucestershire Royal Hospital), Dr Alexandra Daley (Heartlands Hospital), Dr Lynsey Corless (Hull Royal Infirmary), Dr Darren Craig (James Cook University Hospital), Dr Jane Collier (John Radcliffe Hospital), Professor Michael Heneghan (King’s College Hospital), Dr Sharat Misra (King’s Mill Hospital), Dr Chris Corbett (New Cross Hospital), Professor John Dillon (Ninewells Hospital), Dr Simon Rushbrook (Norfolk and Norwich University Hospital), Dr Thomas Lee (North Tyneside General Hospital), Dr Nicholas M Sharaer (Poole Hospital), Dr Kara Rye (Princess Royal Hospital), Dr Andrew Fowell (Queen Alexandra Hospital, Portsmouth), Dr Andrea Broad/Dr Dina Mansour (Queen Elizabeth Hospital, Gateshead), Dr Andy Douds (Queen Elizabeth Hospital, King’s Lynn), Dr Stephen Ryder (Queen’s Medical Centre), Dr Richard Keld (Royal Albert Edward Infirmary), Dr Earl Williams (Royal Bournemouth Hospital), Dr William Stableforth (Royal Cornwall Hospital), Dr Andrew Austin (Royal Derby Hospital), Professor Dermot Gleeson (Royal Hallamshire Hospital), Dr Kenneth Simpson (Royal Infirmary of Edinburgh), Dr Imran Patanwala (Royal Liverpool University Hospital), Dr Alison Brind (Royal Stoke University Hospital), Dr Shanika de Silva (Russells Hall Hospital), Dr Aqueel Jamil (Salisbury District Hospital), Dr Saket Singhal (Sandwell General Hospital), Dr Chin Lye Ch’ng (Singleton Hospital), Dr Joanne Topping (South Tyneside District Hospital), Dr Mark Wright (Southampton General Hospital), Dr Talal Valliani (Southmead Hospital), Dr Rebecca Jones (St. James’s University Hospital, Leeds), Dr Harriet Mitchison (Sunderland Royal Hospital), Dr Douglas Thorburn (The Royal Free Hospital), Professor Aftab Ala (The Royal Surrey County Hospital), Dr Ye Htun Oo (University Hospital Birmingham NHS Foundation Trust), Dr Sushma Sakena/Dr Francisco Porras‐Perez (University Hospital of North Durham), Prof Jane Metcalf/Dr Stephen Mitchell (University Hospital of North Tees), Dr Esther Unitt/Dr Victoria Gordon (University Hospitals Coventry & Warwick), and Dr Jeremy Shearman (Warwick Hospital, South Warwickshire NHS Foundation Trust).
Potential conflict of interest: M. A. Heneghan discloses a potential conflict with Intercept, Novartis, Falk, Astellis; S. Kendrick is a GSK employee and stock holder; J. K. Dyson is supported by the NIHR Rare Diseases Translational Research Collaboration, D. E. J. Jones has received funding from GSK, Intercept and Pfizer, provided consultancy advice to Novartis, GSK and Intercept and has given sponsored lectures for Falk.
The UK‐AIH study is supported by the National Institute for Health Research (NIHR) Rare Diseases Translational Research Collaboration (BH 140 575/ PD 00401) and the Newcastle NIHR Biomedical Research Centre (BH 137821/ PD 0250).
[The copyright for this article was changed on April 29, 2019, after original online publication.]
REFERENCES
- 1. Gleeson D, Heneghan MA, British Society of G. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut 2011;60:1611‐1629. [DOI] [PubMed] [Google Scholar]
- 2. Czaja AJ. Sustained remission after corticosteroid therapy for type 1 autoimmune hepatitis: A retrospective analysis. Hepatology 2002;35:890‐897. [DOI] [PubMed] [Google Scholar]
- 3. Hoeroldt B, McFarlane E, Dube A, Basumani P, Karajeh M, Campbell MJ, et al. Long‐term outcomes of patients with autoimmune hepatitis managed at a nontransplant center. Gastroenterology 2011;140:1980‐1989. [DOI] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver . European Association for the Study of the Liver clinical practice guidelines: autoimmune hepatitis. JHepatol 2015;63:971‐1004. [DOI] [PubMed] [Google Scholar]
- 5. Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med 1971;40:159‐185. [DOI] [PubMed] [Google Scholar]
- 6. Murray‐Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet 1973;1:735‐737. [DOI] [PubMed] [Google Scholar]
- 7. Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnick GL, Elveback IR, et al. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology 1972;63:820‐833. [PubMed] [Google Scholar]
- 8. Summerskill WH, Korman MG, Ammon HV, Baggenstoss AH. Prednisone for chronic active liver disease: dose titration, standard dose, and combination with azathioprine compared. Gut 1975;16:876‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manns MP, Woynarowski M, Kreisel W, Lurie Y, Rust C, Zuckerman E et al. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology 2010;139:1198‐1206. [DOI] [PubMed] [Google Scholar]
- 10. van Gerven NM, Verwer BJ, Witte BI, van Erpecum KJ, van Buuren HR, Maijers I, et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand J Gastroenterol 2014;49:1245‐1254. [DOI] [PubMed] [Google Scholar]
- 11. Kogan J, Safadi R, Ashur Y, Shouval D, Ilan Y. Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. JClin Gastroenterol 2002;35:75‐81. [DOI] [PubMed] [Google Scholar]
- 12. van der Plas SM, Hansen BE, de Boer JB. Generic and disease‐specific health related quality of life in non‐cirrhotic, cirrhotic and transplanted liver patients: a cross‐sectional study. BMC Gastroenterol 2007;16:375‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gulati R, Radhakrishnan KR, Hupertz V, Wyllie R, Alkhouri N, Worley S, Feldstein AE. Health‐related quality of life in children with autoimmune liver disease. J Pediatr Gastroenterol Nutr 2013;57:444‐450. [DOI] [PubMed] [Google Scholar]
- 14. Schramm C, Wahl I, Weiler‐Normann C, Voigt K, Wiegard C, Glaubke C, Brahler E, et al. Health‐related quality of life, depression, and anxiety in patients with autoimmune hepatititis. J Hepatol 2014;60:618‐624. [DOI] [PubMed] [Google Scholar]
- 15. Kruk B, Janik MK, Raszeja‐Wyszomirska J, Krawczyk M, Milkiewicz P. Health related quality of life (HRQoL) in patients with AIH: a prospective, single centre study [Abstract]. J Hepatol 2017;66[Suppl] SAT 375:S546‐S547. doi: 10.1016/S0168-8278(17)31501-5. [DOI] [Google Scholar]
- 16. Sockalingam S, Blank D, Abdelhamid N, Abbey SE, Hirschfield GM. Identifying opportunities to improve management of autoimmune hepatitis: evaluation of drug adherence and psychosocial factors. J Hepatol 2012;57:1299‐1304. [DOI] [PubMed] [Google Scholar]
- 17. EuroQoL Group . EuroQoll ‐‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199‐208. [DOI] [PubMed] [Google Scholar]
- 18. McLernon DJ, Dillon J, Donnan PT. Health‐state utilities in liver disease: a systematic review. Med Decis Making 2008;28:582‐592. [DOI] [PubMed] [Google Scholar]
- 19. Mells GF, Pells G, Newton JL, Bathgate AJ, Burroughs AK, Heneghan MA, et al. Impact of primary biliary cirrhosis on perceived quality of life: the UK‐PBC national study. Hepatology 2013;58:273‐283. [DOI] [PubMed] [Google Scholar]
- 20. Newton JL, Jones DE, Henderson E, Kane L, Wilton K, Burt AD, et al. Fatigue in non‐alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut 2008;57:807‐813. [DOI] [PubMed] [Google Scholar]
- 21. Newton JL, Hollingsworth KG, Taylor R, El‐Sharkawy AM, Khan ZU, Pearce R, et al. Cognitive impairment in primary biliary cirrhosis: Symptom impact and potential etiology. Hepatology 2008;48:541‐549. [DOI] [PubMed] [Google Scholar]
- 22. Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis 1994;18 Suppl 1:S79‐S83. [DOI] [PubMed] [Google Scholar]
- 23. Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol 1982;21:1‐16. [DOI] [PubMed] [Google Scholar]
- 24. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361‐370. [DOI] [PubMed] [Google Scholar]
- 25. Janssen MF, Birnie E, Haagsma JA, Bonsel GJ. Comparing the standard EQ‐5D three‐level system with a five‐level version. Value in Health 2008;11:275‐284. [DOI] [PubMed] [Google Scholar]
- 26. Samp JC, Perry R, Piercy J, Wood R, Baran RW. Patient health utility, work productivity, and lifestyle impairment in chronic hepatitis C patients in France. Clin Res Hepatol Gastroenterol 2015;39:307‐314. [DOI] [PubMed] [Google Scholar]
- 27. Kim JH, Kwon SY, Lee YS, Lee JH, Lee YS, Lee CH. Virologic response to therapy increases health‐related quality of life for patients with chronic hepatitis B. Clin Gastroenterol Hepatol 2012;10:291‐296. [DOI] [PubMed] [Google Scholar]
- 28. Bryan S, Ratcliffe J, Neuberger JM, Burroughs AK, Gunson BK, Buxton MJ. Health‐related quality of life following liver transplantation. Qual Life Res 1998;7:115‐120. [DOI] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence (NICE) . Guide to the methods of technology appraisal 2013. https://www.nice.org.uk/process/pmg9/chapter/foreword 2013:page 41. Published April 2013. Accessed May 2018. [PubMed]
- 30. Devlin N, Shah K, Feng Y, Mulhern B, Van Hout B. Valuing Health‐Related Quality of Life: An EQ‐5D‐5L Value Set for England. OHE Research Paper 16/01 London: Office of Health Economics 2016. https://www.ohe.org/publications/valuing-health-related-quality-life-eq-5d-5l-value-set-england# [DOI] [PMC free article] [PubMed]
- 31. Prince MI, James OFW, Holland NP, Jones DEJ. Validation of a fatigue impact score in primary biliary cirrhosis: towards a standard for clinical and trial use. J Hepatol 2000;32:368‐373. [DOI] [PubMed] [Google Scholar]
- 32. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. JPsychosom Res 2002;52:69‐77. [DOI] [PubMed] [Google Scholar]
- 33. Rice SJ, Albani VJ, Fattakhova GE, Mells GF, Sandford R, Shirley M, et al. Cost and utility estimates for patients at different stages in the natural history of Primary Biliary Cirrhosis (PBC) from the UK‐PBC Cohort [Abstract]. Hepatology 2016;64[Suppl] 1721:850a‐851a. doi: 10.1002/hep.28800 [Google Scholar]
- 34. van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ‐5D‐5L: mapping the EQ‐5D‐5L to EQ‐5D‐3L value sets. Value Health 2012;15:708‐715. [DOI] [PubMed] [Google Scholar]
- 35. Kind P, Hardman G, Macran S. UK population norms for EQ‐5D. University of York, 1999. https://www.researchgate.net/publication/5004008_UK_population_norms_for_EQ-5D
- 36. National Institute for Health and Care Excellence (NICE) . Hepatitis B (chronic): diagnosis and management. Clinical Guidelines 2013;CG 165 https://www.nice.org.uk/guidance/cg165 [PubMed] [Google Scholar]
- 37. Stellon AJ, Keating JJ, Johnson PJ, McFarlane IG, Williams R. Maintenance of remission in autoimmune chronic active hepatitis with azathioprine after corticosteroid withdrawal. Hepatology 1988;8:781‐784. [DOI] [PubMed] [Google Scholar]
- 38. Johnson PJ, McFarlane IG, Williams R. Azathioprine for long‐term maintenance of remission in autoimmune hepatitis. N Engl J Med 1995;333:958‐963. [DOI] [PubMed] [Google Scholar]
- 39. Werner M, Prytz H, Ohlsson B, Almer S, Bjornsson E, Bergquist A, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: A nationwide study. Scand J Gastroenterol 2008;43:1232‐1240. [DOI] [PubMed] [Google Scholar]
- 40. Landeira G, Morise S, Fassio E, Ramonet M, Alvarez E, Caglio P, et al. Effect of cirrhosis at baseline on the outcome of type 1 autoimmune hepatitis. Ann Hepatol 2012;11:100‐106. [PubMed] [Google Scholar]
- 41. Kuriya B, Gladman DD, Ibañez D, Urowitz MB. Quality of life over time in patients with systemic lupus erythematosus. Arthritis Rheum 2008;59:181‐185. [DOI] [PubMed] [Google Scholar]
- 42. Judson MA, Chaudhry H, Louis A, Lee K, Yucel R. The effect of corticosteroids on quality of life in a sarcoidosis clinic: the results of a propensity analysis. Respir Med 2015;109:526‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaydfudim V, Feurer ID, Landman MP, Moore DE, Wright JK, Pinson CW. Reduction in corticosteroids is associated with better health‐related quality of life after liver transplantation. Journal of the American College of Surgeons 2012;214:164‐173. [DOI] [PubMed] [Google Scholar]
- 44. McDougall R, Sibley J, Haga M, Russell A. Outcome in patients with rheumatoid arthritis receiving prednisone compared to matched controls. J Rheumatol 1994;21:1207‐1213. [PubMed] [Google Scholar]
- 45. Mathias SD, Berry P, De Vries J, Askanase A, Pascoe K, Colwell HH, et al. Development of the Systemic Lupus Erythematosus Steroid Questionnaire (SSQ): a novel patient‐reported outcome tool to assess the impact of oral steroid treatment. Health Qual Life Outcomes 2017;15:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sciveres M, Caprai S, Palla G, Ughi C, Maggiore G. Effectiveness and safety of ciclosporin as therapy for autoimmune diseases of the liver in children and adolescents. Aliment Pharmacol Ther 2004;19:209‐217. [DOI] [PubMed] [Google Scholar]
- 47. Tannous MM, Cheng J, Muniyappa K, Farooq I, Bharara A, Kappus M, et al. Use of tacrolimus in the treatment of autoimmune hepatitis: a single centre experience. Aliment Pharmacol Ther 2011;34:405‐407. [DOI] [PubMed] [Google Scholar]
- 48. Goldblatt J, Taylor PJ, Lipman T, Prince MI, Baragiotta A, Bassendine MF, et al. The true impact of fatigue in primary biliary cirrhosis: a population study. Gastroenterology 2002;122:1235‐1241. [DOI] [PubMed] [Google Scholar]
- 49. Elliott C, Frith J, Pairman J, Jones DEJ, Newton JL. Reduction in functional ability is significant postliver transplantation compared with matched liver disease and community dwelling controls. Transpl Int 2011;24:588‐595. [DOI] [PubMed] [Google Scholar]
- 50. Jacoby A, Rannard A, Buck D, Bhala N, Newton JL, James OFW, et al. Development, validation and evaluation of the PBC‐40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut 2005;54:1622‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.30031/suppinfo.
Supporting Information


