Abstract
Alcohol expresses its reinforcing properties by activating areas of the mesolimbic dopamine system, which consists of dopaminergic neurons projecting from the ventral tegmental area to the nucleus accumbens. The findings that reward induced by food and addictive drugs involve common mechanisms raise the possibility that gut–brain hormones, which control appetite, such as amylin, could be involved in reward regulation. Amylin decreases food intake, and despite its implication in the regulation of natural rewards, tenuous evidence support amylinergic mediation of artificial rewards, such as alcohol. Therefore, the present experiments were designed to investigate the effect of salmon calcitonin (sCT), an amylin receptor agonist and analogue of endogenous amylin, on various alcohol‐related behaviours in rodents. We showed that acute sCT administration attenuated the established effects of alcohol on the mesolimbic dopamine system, particularly alcohol‐induced locomotor stimulation and accumbal dopamine release. Using the conditioned place preference model, we demonstrated that repeated sCT administration prevented the expression of alcohol's rewarding properties and that acute sCT administration blocked the reward‐dependent memory consolidation. In addition, sCT pre‐treatment attenuated alcohol intake in low alcohol‐consuming rats, with a more evident decrease in high alcohol consumers in the intermittent alcohol access model. Lastly, sCT did not alter peanut butter intake, blood alcohol concentration and plasma corticosterone levels in mice. Taken together, the present data support that amylin signalling is involved in the expression of alcohol reinforcement and that amylin receptor agonists could be considered for the treatment of alcohol use disorder in humans.
Keywords: addiction, food intake, gut–brain axis, mesolimbic dopamine system, reward, salmon calcitonin
Introduction
Alcohol use disorder is a co‐morbid and highly prevalent disorder, causing severe health problems for both the society and the individual (Grant et al. 2015). However, existing pharmacotherapy has shown limited efficacy (Anton et al. 2006); therefore, further investigation of possible alcohol neurochemical targets could lead to new pharmacological interventions (Heilig & Egli 2006; Litten et al. 2012). Alcohol targets components of the mesolimbic dopamine system (Larsson & Engel 2004; Vengeliene et al. 2008; Soderpalm, Lof, & Ericson 2009), which is involved in the expression of its reinforcing properties (Adinoff 2004). Beyond the mesolimbic dopamine system, which includes dopamine projections from the ventral tegmental area (VTA) to nucleus accumbens (NAc) (Koob & Bloom 1988; Kelley & Berridge 2002), recent studies show that alcohol activates the cholinergic inputs from the laterodorsal tegmental nucleus (LDTg) (Larsson et al. 2005).
Emerged evidence suggests that feeding and reward behaviours share common complex neurochemical mechanisms and that these are mediated by the mesolimbic dopamine system (Rada, Mark, & Hoebel 1998; Hoebel et al. 1999; Addolorato et al. 2006; Jerlhag et al. 2006; Abizaid et al. 2011; Dickson et al. 2011; Leggio et al. 2011; Jerlhag et al. 2011a; Edwards & Abizaid 2016). More specifically, gut–brain peptides, which have been traditionally known to regulate food intake and energy balance (Ahima & Antwi 2008), seem to play a pivotal role in mediating the reinforcing properties of alcohol and other drugs of abuse (Jerlhag et al. 2010; Abizaid et al. 2011; Jerlhag & Engel 2011; Clifford et al. 2012; Egecioglu, Engel, & Jerlhag 2013a; Suchankova et al. 2013a; Engel & Jerlhag 2014; Vadnie et al. 2014; Vallof et al. 2016c). Notably, ghrelin, glucagon‐like peptide‐1 and neuromedin U have been shown to alter alcohol‐induced reward phenotypes by acting on the mesolimbic dopamine system (Kraus et al. 2005; Leggio et al. 2011; Jerlhag et al. 2011b; Landgren et al. 2012; Suchankova et al. 2013b; Leggio et al. 2014; Vallof et al. 2016a; Vallof et al. 2016b). Other hormones, for example, amylin, have been recently studied for their role to control energy balance through gut–brain axis regulation (Reda, Geliebter, & Pi‐Sunyer 2002; Lutz 2009). Amylin seems to regulate food intake through central mechanisms, by acting in areas of the mesolimbic dopamine system such as the VTA and LDTg (Mietlicki‐Baase et al. 2015b; Reiner et al. 2017), identifying it an interesting candidate for exploring its potential reward‐regulating properties.
Amylin is a 37‐amino acid hormone, co‐secreted with insulin in the pancreatic β‐cells (Westermark, Andersson, & Westermark 2011), and its physiological role includes insulin secretion inhibition, inhibition of gastric emptying and glucagon secretion (Hay et al. 2015). Endogenous amylin and its analogue and receptor agonist, salmon calcitonin (sCT), exert anorexigenic properties by signalling satiation (Lutz et al. 1995; Lutz et al. 2000; Reidelberger et al. 2004; Mack et al. 2007; Lutz 2012). Amylin receptors have been identified in various brain areas including area postrema, nucleus of the solitary tract and dorsal raphe among others (Sexton et al. 1994). In fact, amylin and sCT express their anorexigenic effects through central mechanisms involving the area postrema and the nucleus of the solitary tract (Lutz et al. 2001a; Potes & Lutz 2010; Braegger et al. 2014). Recent studies show that amylin receptors in the NAc, VTA and LDTg (Baisley & Baldo 2014; Mietlicki‐Baase et al. 2015a; Reiner et al. 2017) and more specifically amylin receptors on ventral tegmental dopaminergic neurons mediate the effect of sCT on the control of energy balance (Mietlicki‐Baase et al. 2013; Mietlicki‐Baase et al. 2015b). Thus, amylin's action on the mesolimbic dopamine system suggests a potential role that extends beyond regulation of natural rewards, and tenuous evidence supports a possible amylinergic mediation of artificial rewards, such as alcohol. Therefore, in the present study, we investigated the effects of sCT on alcohol‐mediated behaviours in mice, namely, locomotor stimulation and accumbal dopamine release as well as the expression of alcohol's rewarding properties and reward‐dependent memory consolidation in the alcohol‐induced conditioned place preference (CPP) paradigm. Moreover, the ability of sCT to influence alcohol intake in low and high alcohol‐consuming rats was explored. Finally, we evaluated the involvement of stress, alcohol metabolism and caloric content as possible mechanisms through which sCT influences alcohol‐induced reward. We hence investigated the effects of sCT on plasma corticosterone levels, blood alcohol concentration and peanut butter consumption, respectively.
Materials and Methods
For detailed protocol description, see Supporting Information.
Animals
For the locomotor activity, CPP, in vivo microdialysis, peanut butter intake, blood alcohol concentration and corticosterone analysis experiments, adult postpubertal age‐matched male NMRI mice (8–12 weeks old and 25–30 g body weight; Charles River, Susfeldt, Germany) were used. The mice were group housed, fed ad libitum and maintained at a 12/12 hour light/dark cycle and at 20°C with 50 percent humidity. Mice were used for the present experiments, because we have extensive experience working with mice and have previously obtained robust locomotor stimulation, CPP and accumbal dopamine release in response to alcohol and other addictive drugs (Vallof et al. 2016c).
For the intermittent access, 20 percent alcohol two‐bottle‐choice drinking paradigm, adult postpubertal age‐matched male outbred RccHan Wistar rats (Envigo, Huntingdon, Cambridgeshire, UK) were used. The rats, for the whole duration of this experiment, were maintained on a 12‐hour reversed light/dark cycle (lights off at 8 am) and were housed individually in high Macrolon III cages. They were maintained in rooms with 20°C and 50 percent humidity and fed ad libitum. Outbred Rcc Han Wistar rats were selected for the intermittent access paradigm because they display a voluntary high and stable alcohol intake, causing pharmacologically relevant blood alcohol concentrations in the intermittent access model (Vallof et al. 2016a).
All experiments were approved by the Swedish Ethical Committee on Animal Research in Gothenburg. Each experiment used an independent set of animals.
Drugs
Salmon calcitonin (Tocris Bioscience, Bristol, UK) was diluted in vehicle (0.9 percent sodium chloride solution) and administered intraperitoneally (IP) at the doses of 1 or 5 μg/kg always 30 minutes prior to alcohol injection. Alcohol (96 percent, VWR International AB, Stockholm, Sweden) was diluted in vehicle (0.9 percent sodium chloride solution) to 15 percent v/v and was administered at a dose of 1.75 g/kg, IP.
Locomotor activity
Locomotor activity tests were conducted to investigate the effects of two different doses of sCT (1 or 5 μg/kg, IP) on per se locomotor activity in mice and the effects of a high (5 μg/kg, IP) or a low (1 μg/kg, IP) sCT dose on alcohol‐induced locomotor stimulation in mice. For protocol description, see Supporting Information.
Briefly, mice were allowed to habituate to the activity boxes for 60 minutes, and sCT or an equal volume of vehicle (saline solution, IP) was administered 30 minutes prior to alcohol (1.75 g/kg, IP) or vehicle injection. The subsequent 60‐minute cumulative locomotor activity was registered.
In vivo microdialysis and dopamine release measurements
For the measurements of accumbal dopamine release, the mice were implanted with a microdialysis custom‐made probe (Blomqvist et al. 1993) positioned in NAc shell (coordinates relative to bregma of 1.5 mm AP, ±0.6 ML and 4.7 mm DV were used (Paxinos & Watson 1998)), after surgical procedures that have been previously described in detail (Jerlhag et al. 2009). For protocol description, see Supporting Information.
After 1 hour of habituation to the microdialysis set‐up, perfusion samples were collected in 20‐minute intervals during the entire experimental protocol (from −40 to 260 minutes). The baseline dopamine level was defined as the average of three consecutive samples (−40 to 0 minutes) before the first alcohol (1.75 g/kg, IP) or vehicle (saline solution, IP) challenge (time 0). The dopamine release was determined as the percent increase from baseline.
In the first experiment, an initial alcohol challenge was given to establish that the mice respond with an accumbal dopamine release to alcohol compared with vehicle treatment. Seven consecutive 20‐minute samples were collected after this initial challenge. At 150 minutes, sCT (5 μg/kg, IP) or an equal volume of vehicle (saline solution, IP) was administered. Thirty minutes later, vehicle (saline solution, IP) or alcohol (1.75 g/kg, IP) was administered (180 minutes). Thereafter, four additional samples were collected (experiment terminated at 260 minutes). Collectively, the following three treatment groups were created: alcohol–vehicle–alcohol (Alc‐Veh‐Alc), alcohol–sCT–alcohol (Alc‐sCT‐Alc) and vehicle–sCT–vehicle (Veh‐sCT‐Veh).
In the second microdialysis experiment, the effects of sCT on the initial alcohol‐induced accumbal dopamine release were investigated in animals that received only a single alcohol injection, in order to identify the ability of sCT to affect the initial alcohol‐induced accumbal dopamine release. Firstly, sCT (5 μg/kg, IP) or an equal volume of vehicle (saline solution, IP) was administered after the collection of the three baseline samples at 10 minutes, and 30 minutes later, an alcohol injection (1.75 g/kg, IP) was administered (40 minutes). Thereafter, nine additional samples were collected (experiment terminated at 220 minutes). Collectively, the following two treatment groups were created: vehicle–alcohol (Veh‐Alc) and sCT–alcohol (sCT‐Alc).
Verification of probe placement
Following each microdialysis experiment, the probes' location in the brain (NAc shell) was verified. For protocol description, see Supporting Information. Figure 1 shows a schematic representation of placements within NAc shell as well as one representative brain slice verification. Mice with misplaced probe placements were excluded from the statistical analysis.
Figure 1.
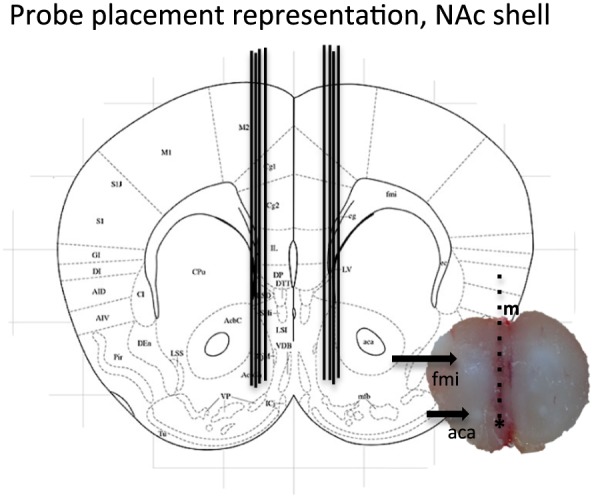
Representative brain slice and schematic representation of probe placement in nucleus accumbens (NAc) shell. A coronal mouse brain section showing eight representative probe placements (illustrated by vertical lines) in the NAc shell. One brain slice shows a representative probe placement in the NAc shell. The asterisk shows the targeted area; vertical lines represent the probe; m, midline; fmi, anterior forceps of corpus callosum; aca, anterior commissure
Conditioned place preference
Two distinct CPP tests were performed in mice as previously described (Jerlhag et al. 2009; Vallof et al. 2016a).
The first CPP test was conducted to evaluate the effect of sCT on the rewarding properties of alcohol. In these experiments, sCT or vehicle was administered on the preconditioning days prior to alcohol injections, in order to define the ability of sCT to affect the rewarding properties of alcohol. This experiment allows us to investigate the ability of sCT to prevent the reward induced by acute alcohol, reflecting the expression of CPP. sCT (5 μg/kg, IP) or vehicle (saline solution, IP) was administered 30 minutes prior to alcohol or vehicle (saline solution, IP) administration on each of the four conditioning days, creating two treatment groups of Veh‐Alc and sCT‐Alc.
The second experiment was conducted in a separate mice group and was designed to investigate the effect of sCT on memory consolidation of alcohol reward. In these experiments, sCT or vehicle was administered on the last post‐conditioning day only, whereas alcohol had been administered daily throughout the conditioning period, allowing the mice to establish a place preference for the least preferred compartment. This design tests the ability of sCT to influence the reward‐dependent memory consolidation. At post‐conditioning, mice received sCT (5 μg/kg, IP) or an equal volume of vehicle solution (saline solution, IP) and 30 minutes later placed on the midline between the two compartments with free access to both compartments for 20 minutes (creating the following treatment groups; alcohol–vehicle and alcohol–sCT). For detailed protocol description, see Supporting Information.
Intermittent access 20 percent alcohol two‐bottle‐choice drinking paradigm
This experiment was conducted to evaluate the effect of sCT (5 μg/kg, IP) on alcohol intake in rats exposed to 12 weeks of alcohol in the intermittent access model.
The rats were given free access to one bottle of 20 percent alcohol and one bottle of water during three 24‐hour sessions per week (Mondays, Wednesdays and Fridays). The rats had unlimited access to two bottles of water between the alcohol‐access periods. All bottles were weighed at 24 hours after the fluids were placed to the rat cages. The body weight of each rat was measured daily prior to bottle presentation, to allow for calculating the grams of alcohol intake per kilogram of body weight (g/kg). The preference for alcohol over water (the ratio of alcohol to total fluid intake) was calculated at all timepoints. In addition, water and food intake was measured. The alcohol intake experiment was conducted after a period of 12 weeks of intermittent access to alcohol.
In this experiment, two separate groups of rats, low and high alcohol consuming, were administered a single injection of sCT (5 μg/kg, IP) or vehicle solution (saline solution, IP) on an alcohol‐drinking day (Monday or Wednesday) in a way that all rats alternately received both sCT and vehicle injections in a balanced design. There was 1 day between each injection (water‐drinking days, Tuesday). After treatment and when returned to their cages, the rats were presented with one bottle of 20 percent alcohol and one bottle of water. Alcohol, water and food intake, as well as alcohol preference measurements, were registered 1 and 24 hours following alcohol presentation. Rat body weight was measured only at the 24‐hour timepoint.
Peanut butter intake experiments
All mice were allowed to familiarize the taste of peanut butter for 1 week before the test. Mice were transferred to novel, empty cages, and two doses of sCT (1 or 5 μg/kg, IP) or vehicle (saline solution, IP) were administered 30 minutes prior to peanut butter exposure. Peanut butter consumption was measured at the timepoint of 1 hour.
Blood alcohol concentration
Mice were injected with sCT (5 μg/kg, IP) or an equal volume of vehicle solution (saline solution, IP), and 30 minutes later, all animals were injected with alcohol (1.75 g/kg, IP). The animals were decapitated 20 minutes later, and trunk blood was collected in microtubes (Vacuette, Greiner Bio‐one, Florence, Italy). The analysis of the blood alcohol concentration was outsourced to Sahlghrenska University Hospital (Gothenburg, Sweden; study agreement BML‐NEURO).
Serum levels of corticosterone
Mice were injected with sCT (5 μg/kg, IP) or an equal volume of vehicle solution (saline solution, IP), and 30 minutes later, capillary blood from the tail was collected in microvettes (Sarstedt, Helsingborg, Sweden). The blood was centrifuged (5 minutes, 10 000 g), and corticosterone was thereafter measured in serum with an Enzo Corticosterone enzyme‐linked immunosorbent assay (ELISA) kit (AH Diagnostic, Stockholm, Sweden).
Statistical analysis
The first experiment of locomotor activity was analysed with a one‐way ANOVA. The further alcohol‐induced locomotor activity experiments were analysed with a two‐way ANOVA test followed by Tukey's post hoc test for multiple comparison between treatments. Accumbal dopamine release analyses were performed using a two‐way repeated measures ANOVA followed by Bonferroni post hoc test for the comparison between different treatments at given timepoints. CPP, blood alcohol concentration and plasma corticosterone levels data were assessed with an unpaired t‐test. The data from the intermittent alcohol access experiment were analysed using a paired t‐test. Peanut butter intake was analysed with a one‐way ANOVA followed by a Bonferroni post hoc test. Data are presented as mean ± SEM. A probability value of P < 0.05 was considered as statistically significant.
Results
sCT attenuates alcohol‐induced locomotor stimulation and accumbal dopamine release following a single or repeated alcohol injections in mice
The dose response study showed that there was no effect of sCT (1 or 5 μg/kg, IP) on locomotor activity per se in mice when compared with vehicle treatment (F(1, 21) = 0.25, P = 0.7829); (vehicle: 394 ± 172 cm/60 minutes, sCT 1 μg/kg: 281 ± 64 cm/60 minutes and sCT 5 μg/kg: 317 ± 79 cm/60 minutes).
A high dose of sCT attenuated alcohol‐induced locomotor stimulation. Indeed, an effect of alcohol treatment (F(1, 49) = 23.35, P < 0.0001) and of sCT treatment (F(1, 49) = 8.75, P = 0.0048) was found on locomotor activity after sCT (5 μg/kg, IP) and alcohol (1.75 g/kg, IP) administration. There was an effect of alcohol × sCT treatment interaction (F(1, 49) = 6.97, P = 0.0111; n = 14 for vehicle–vehicle and sCT–vehicle, n = 13 for Veh‐Alc and n = 12 for sCT‐Alc group) on locomotor activity. Further post hoc analysis showed that alcohol significantly increased locomotor activity in comparison with vehicle treatment (P < 0.0001; Fig. 2a. This effect was significantly attenuated by a single injection of sCT prior to alcohol administration (P < 0.01) at a dose with no effect per se on locomotor stimulation as shown by comparison with the vehicle group (P > 0.05). There was no significant difference between the vehicle group and the sCT–alcohol group (P > 0.05).
Figure 2.
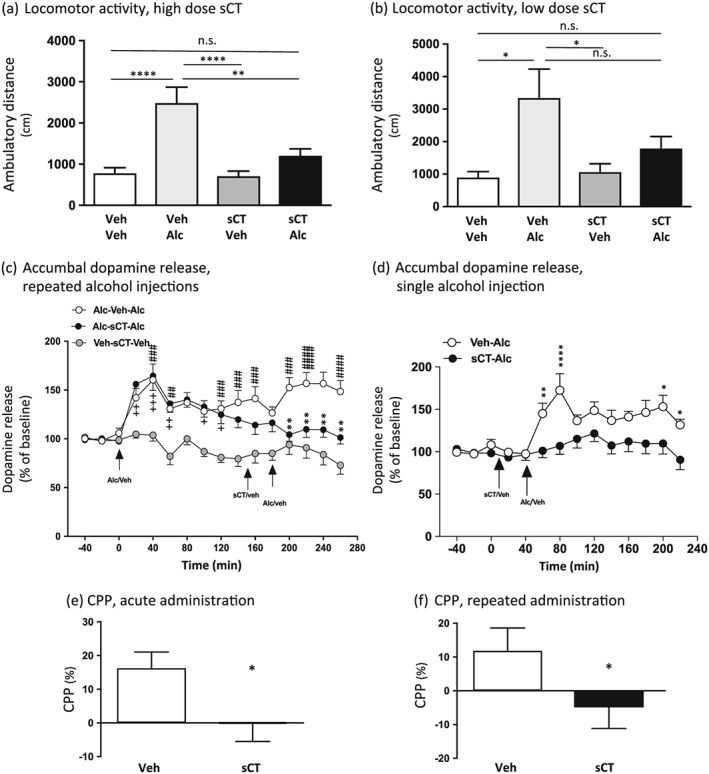
Peripheral injection of salmon calcitonin (sCT) attenuates alcohol‐induced locomotor stimulation, accumbal dopamine release after a single and repeated alcohol injection and the expression of the rewarding properties and reward‐dependent memory consolidation of alcohol‐induced conditioned place preference in mice. (a) Alcohol [Alc, 1.75 g/kg, intraperitoneally (IP)]‐induced locomotor stimulation was blocked by a single peripheral injection of sCT (5 μg/kg, IP), at a dose that does not affect locomotor activity per se compared with vehicle (Veh). (Data are presented as mean ± SEM; **P < 0.01, ****P < 0.0001; n.s., non‐significant.) (b) Acute administration of a low dose of sCT (1 μg/kg, IP), a dose without an effect per se, had a tendency in reducing alcohol‐induced locomotor stimulation in mice. Indeed, alcohol (1.75 g/kg, IP) did not cause a locomotor stimulation in sCT pretreated mice, but there was not a significant difference between Veh‐Alc‐treated and sCT‐Alc‐treated mice. (Data are presented as mean ± SEM; *P < 0.05; n.s., non‐significant.) (c) An initial alcohol (1.75 g/kg, IP) injection increased accumbal dopamine release for both alcohol‐receiving groups Alc‐Veh‐Alc and Alc‐sCT‐Alc at the timepoints of 20, 40, 60 and 100–160 minutes when compared with Veh‐sCT‐Veh. A second alcohol (1.75 g/kg, IP) injection enhanced accumbal dopamine release in the Alc‐Veh‐Alc group at the timepoints of 200–260 minutes compared with Veh‐sCT‐Veh group. Pre‐treatment with a single peripheral injection of sCT (5 μg/kg, IP), decreased alcohol (1.75 g/kg, IP)‐induced accumbal dopamine release at timepoints 200–260 minutes in the Alc‐sCT‐Alc group compared with Alc‐Veh‐Alc group. (Data are presented as mean ± SEM; ##P < 0.01, ###P < 0.001, ####P < 0.0001 for comparisons between the Alc‐Veh‐Alc and Veh‐sCT‐Veh; +P < 0.05, ++P < 0.01, +++P < 0.001 for comparisons between Alc‐sCT‐Alc and Veh‐sCT‐Veh; **P < 0.01 for comparisons between Alc‐Veh‐Alc and Alc‐sCT‐Alc.) (d) Pre‐treatment with an acute sCT injection (5 μg/kg, IP) blocked the initial accumbal dopamine release caused by a single alcohol (1.75 g/kg, IP) injection in the sCT‐Alc group when compared with the Veh‐Alc, at the timepoints of 60, 80, 200 and 220 minutes. (Data are presented as mean ± SEM; **P < 0.01, ****P < 0.0001, *P < 0.05.) (e) Repeated administration of sCT (5 μg/kg, IP) blocks the rewarding effect of alcohol (1.75 g/kg, IP)‐induced conditioned place preference (CPP). (Data are presented as mean ± SEM, *P < 0.05; data calculated as percent of total time spent in the drug‐paired (i.e. less preferred) compartment during post‐conditioning and preconditioning sessions.) (f) A single injection of sCT on the post‐conditioning day blocked the reinforcing memory consolidation of alcohol (1.75 g/kg, IP)‐induced CPP. (Data are presented as mean ± SEM, *P < 0.05.)
On the contrary, a low dose of sCT reduced but did not completely attenuate alcohol‐induced locomotor stimulation. There was an effect of alcohol treatment (F(1, 23) = 9.39, P = 0.0055) on locomotor activity following a low dose of sCT (1 μg/kg, IP) and alcohol (1.75 g/kg, IP) administration, but no effect of sCT treatment (F(1, 23) = 2.02, P = 0.1680) or alcohol × sCT treatment interaction (F(1, 23) = 2.36, P = 0.1384; n = 8 per group) was found on locomotor activity (Fig. 2b). Further post hoc analysis showed that alcohol treatment increased locomotor activity in vehicle pretreated mice compared with vehicle treatment (P < 0.05). Albeit alcohol did not increase locomotor activity in mice pretreated with sCT (P > 0.05), there was no significant difference between vehicle–alcohol and sCT–alcohol treatment (P > 0.05). The selected dose of 1 μg/kg had no effect per se on locomotor activity compared with vehicle treatment (P > 0.05).
As shown in Figure 2c, the accumbal dopamine release data showed an overall effect of treatment (F(2, 384) = 103, P < 0.0001), time (F(15, 192) = 5.953, P < 0.0001) and treatment × time interaction (F(30, 384) = 3.065, P < 0.0001; n = 13 per group).
In the first part of the experiment, the responsiveness to alcohol (1.75 g/kg) per se was investigated (alcohol injection at timepoint 0 minutes). This initial injection of alcohol caused a significant increase in accumbal dopamine release compared with vehicle treatment (Veh‐sCT‐Veh) in both groups that received alcohol (Alc‐Veh‐Alc and Alc‐sCT‐Alc). Specifically, in the Alc‐Veh‐Alc group, alcohol significantly increased accumbal dopamine at timepoints 40 (P < 0.001), 60 (P < 0.01), 120 (P < 0.01), 140–160 (P < 0.001) and 180 minutes (P < 0.05). Moreover, alcohol increased dopamine in NAc at timepoints 20 (P < 0.01), 40 (P < 0.001), 60 (P < 0.01) and 100–120 minutes (P < 0.05) in the Alc‐sCT‐Alc group. The subsequent part of the experiment aimed at investigating the ability of sCT to affect alcohol‐induced dopamine release. Administration of sCT (5 μg/kg, IP at 150 minutes) 30 minutes prior to the second alcohol injection (1.75 g/kg, IP at 180 minutes) significantly decreased the alcohol‐induced accumbal dopamine release (Alc‐sCT‐Alc) compared with vehicle pre‐treatment (Alc‐Veh‐Alc) at the timepoints 200–260 (P < 0.01).
The second accumbal dopamine release experiment (Fig. 2d) revealed an overall effect of treatment (F(1, 126) = 54.56, P < 0.0001), time (F(13, 126) = 4.722, P < 0.0001) and time × treatment interaction (F(13, 126) = 2.634, P = 0.0028; n = 10 per group). Post hoc analysis revealed that administration of sCT (5 μg/kg, IP at 10 minutes) 30 minutes prior to a single alcohol injection (1.75 g/kg, IP at 40 minutes) significantly decreased alcohol‐induced accumbal dopamine release (sCT‐Alc) compared with vehicle pre‐treatment (Veh‐Alc) at the timepoints 60 (P < 0.01), 80 (P < 0.0001), 200 (P < 0.05) and 220 (P < 0.05) minutes.
sCT attenuates the expression of alcohol‐induced CPP in mice
The first CPP experiment revealed that sCT significantly attenuated the rewarding properties of alcohol, i.e. expression of CPP (P = 0.0420; n = 8 per group; Fig. 2e). sCT had no effect on CPP in this experimental set‐up as resulted by a separate control experiment [−2 ± 1 percent for vehicle–vehicle (n = 8) and −2 ± 2 percent for sCT–vehicle (n = 7); P = 0.8471]. As shown in Figure 2f, a single injection of sCT (5 μg/kg, IP) on the post‐conditioning day significantly decreased memory consolidation of alcohol reward in the CPP test (P = 0.0453, n = 8 per group). Finally, acute administration of sCT on the post‐conditioning day did not affect CPP per se in mice as resulted from a separate experiment [1 ± 5 percent for vehicle–vehicle (n = 8) and 5 ± 4 percent for vehicle–sCT (n = 7); P = 0.5996].
sCT decreases alcohol and food intake in low alcohol‐consuming rats
A single injection of sCT (5 μg/kg, IP) significantly reduced alcohol intake in low alcohol‐consuming rats (mean baseline alcohol consumption: 2.31 g/kg) compared with vehicle injection, as shown in Figure 3a, at the timepoint of 1 hour (P = 0.0177, n = 17). Alcohol intake scores for the 24‐hour timepoint were not significantly different between the two groups (P = 0.4477; Fig. 3b). Overall, the sCT‐treated group showed a decrease of 15 percent in alcohol consumption (mean between the treatment groups: 0.6741–0.5712 g/kg) for the timepoint of 1 hour and a decrease of 5 percent (mean baseline consumption between the treatment groups: 2.866–2.726 g/kg) for the timepoint of 24 hours.
Figure 3.
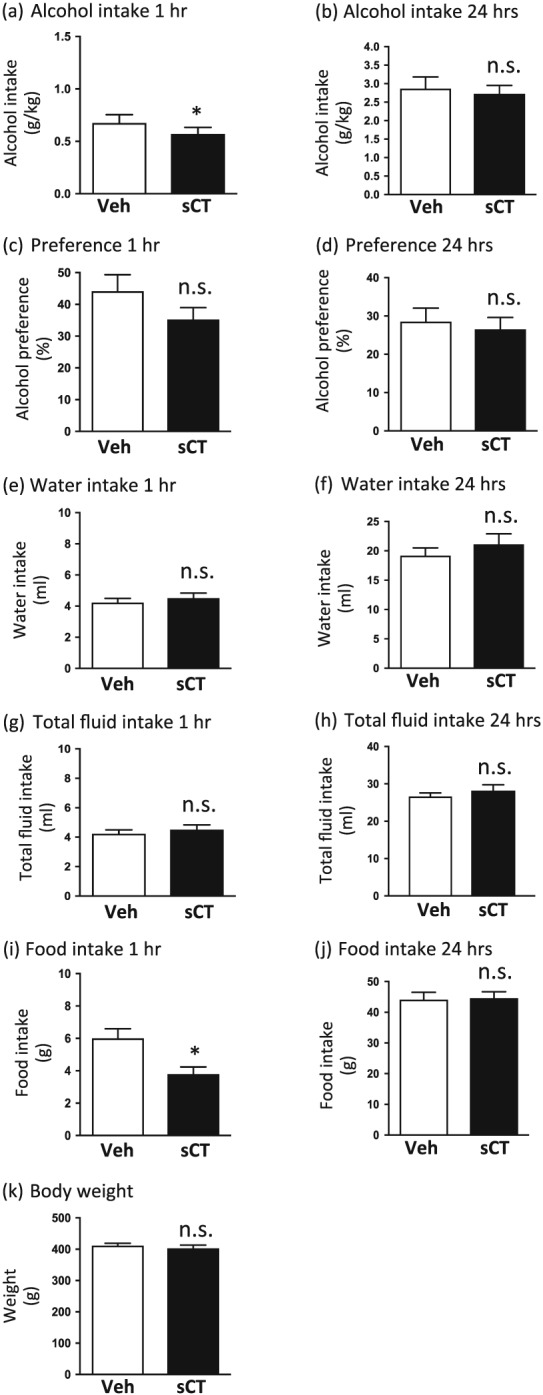
Salmon calcitonin (sCT) administration decreases alcohol and food intake in low alcohol‐consuming rats. (a) A single sCT injection [5 μg/kg, intraperitoneally (IP)] decreased alcohol intake in low alcohol‐consuming rats in the intermittent access 20 percent alcohol two‐bottle‐choice drinking paradigm at the timepoint of 1 hour compared with vehicle (Veh). (b) sCT administration did not have an effect on alcohol intake at the timepoint of 24 hours. There was no effect of sCT on alcohol preference in either timepoint of (c) 1 hour or (d) 24 hours. sCT did not alter water intake at either measured timepoint of (e) 1 hour or (f) 24 hours. sCT did not alter the total fluid intake at either timepoint of (g) 1 hour or (h) 24 hours. A single sCT injection decreased food intake at the timepoint of (i) 1 hour but had no effect at the timepoint of (j) 24 hours. sCT did not alter 24‐hour rat body weight in the low alcohol‐consuming group (k). (Data are presented as mean ± SEM; *P < 0.05; n.s., non‐significant.)
sCT administration did not alter alcohol preference in either measured timepoint of 1 hour (P = 0.0917; Fig. 3c) or 24 hours (P = 0.2401; Fig. 3d). sCT did not alter water intake at any measured timepoint of 1 hour (P = 0.1255; Fig. 3e) or 24 hours (P = 0.0783; Fig. 3f). There was no effect of sCT on the total fluid consumption in the rats at any measured timepoint of 1 or 24 hours (P = 0.3976 and P = 0.182, respectively; Fig. 3g & h).
Acute administration of sCT significantly decreased food intake at the timepoint of 1 hour after administration compared with vehicle administration (P = 0.0075) as presented in Figure 3i. Food intake scores at the timepoint of 24 hours after administration did not significantly differ between the two conditions (P = 0.8946; Fig. 3j). sCT administration did not have an effect on rat weight (P = 0.1980) in low alcohol‐consuming rats (Fig. 3k).
sCT robustly decreases alcohol, food intake and rat body weight in high alcohol‐consuming rats
A single injection of sCT (5 μg/kg, IP) significantly reduced alcohol intake in high alcohol‐consuming rats (mean baseline alcohol consumption: 6.14 g/kg) compared with vehicle injection, as shown in Figure 4a, at the timepoint of 1 hour (P = 0.001, n = 25). Alcohol intake scores for the 24‐hour timepoint showed that sCT significantly reduced alcohol intake when compared with the vehicle group (P = 0.0001; Fig. 4b) in these high alcohol‐consuming rats. Collectively, the sCT‐treated group showed a 43 percent decrease in alcohol intake (mean consumption between the treatment groups: 1.317–0.7484 g/kg) for the timepoint of 1 hour and 39 percent decrease at the 24‐hour timepoint (mean consumption between the treatment groups: 6.343–3.862 g/kg).
Figure 4.
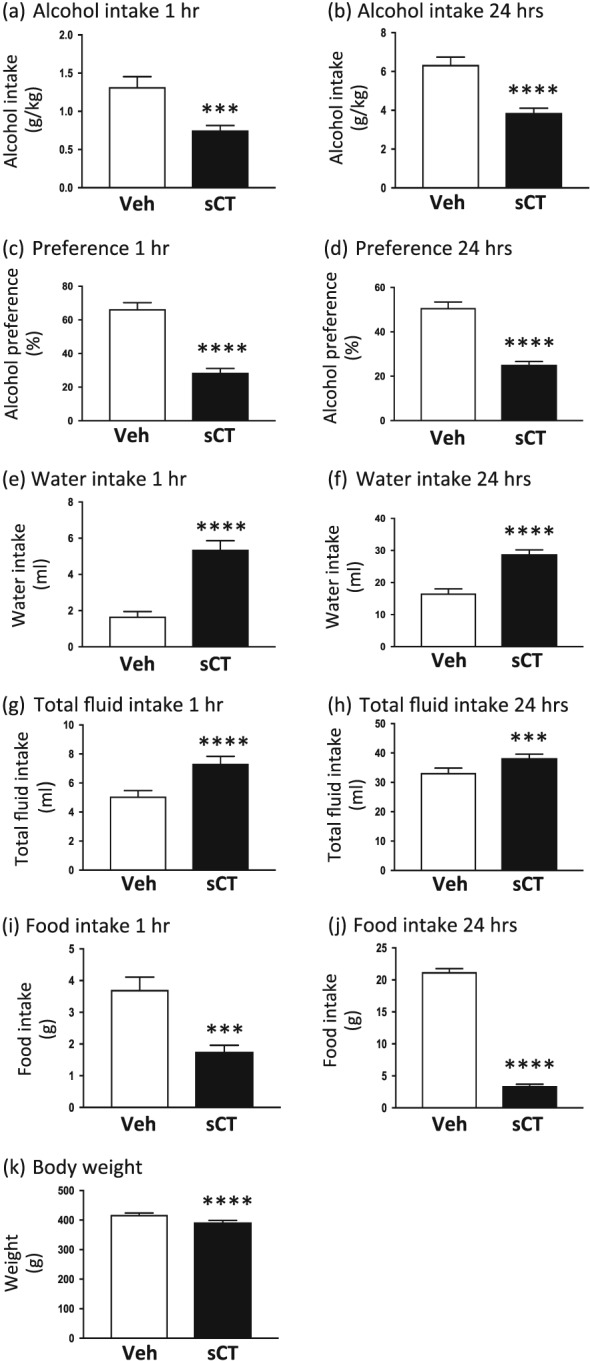
Salmon calcitonin (sCT) administration decreases alcohol and food intake, as well as body weight in high alcohol‐consuming rats. Acute sCT administration [5 μg/kg, intraperitoneally (IP)] reduced alcohol intake in high alcohol‐consuming rats in the intermittent access 20 percent alcohol two‐bottle‐choice drinking paradigm at the timepoint of (a) 1 hour and (b) 24 hours compared with vehicle (Veh). sCT pre‐treatment decreased preference for alcohol at the timepoints of both (c) 1 hour and (d) 24 hours. sCT increased (e) 1‐hour and (f) 24‐hour water intake and similarly increased total fluid intake at the timepoints of (g) 1 hour and (h) 24 hours. A single sCT injection decreased food intake at the timepoint of (i) 1 hour and (j) 24 hours. sCT administration decreased body weight at 24 hours in the high alcohol‐consuming group (k). (Data are presented as mean ± SEM; ****P < 0.0001, ***P < 0.001.)
Acute sCT administration decreased alcohol preference at the timepoint of 1 hour (P < 0.0001; Fig. 4c) and 24 hours (P < 0.0001; Fig. 4d). sCT significantly increased water intake at timepoints of both 1 hour (P < 0.0001; Fig. 4e) and 24 hours (P < 0.0001; Fig. 4f) compared with the vehicle‐treated group. Moreover, total fluid intake was significantly increased after sCT administration at the timepoint of 1 hour (P < 0.0001; Fig. 4g) and 24 hours (P = 0.001; Fig. 4h) when compared with the vehicle group.
Acute administration of sCT significantly reduced food intake at the 1‐hour (P = 0.0001; Fig. 4i) and 24‐hour (P < 0.0001; Fig. 4j) timepoints compared with vehicle in the high alcohol‐consuming rats. Moreover, a single sCT injection significantly decreased the 24‐hour values of rat body weight (P < 0.0001; Fig. 4k).
sCT does not alter peanut butter intake in mice
sCT administration in two doses (low: 1 μg/kg, IP and high: 5 μg/kg, IP) did not alter peanut butter intake compared with vehicle as shown in Figure 5, at the timepoint of 1 hour (F(2, 29) = 0.9771, P = 0.3884; n = 10 for the vehicle and n = 11 for both sCT groups).
Figure 5.
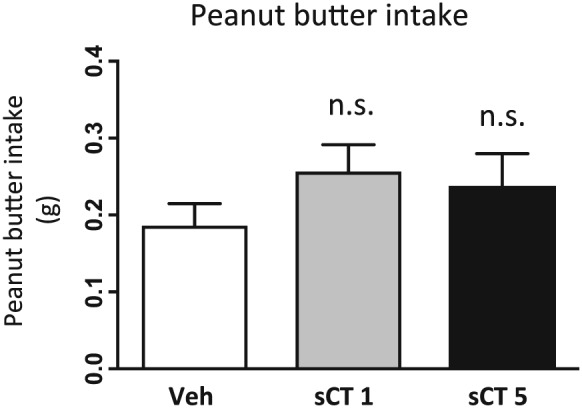
Salmon calcitonin (sCT) administration does not affect intake of palatable food in satiated mice. Acute sCT administration in two different doses [1 and 5 μg/kg, intraperitoneally (IP)] did not change peanut butter consumption compared with vehicle (Veh) administration. (Data are presented as mean ± SEM; n.s., non‐significant.)
sCT does not alter blood alcohol concentration and corticosterone levels
Figure 6a shows the blood alcohol concentration levels between vehicle (saline solution, IP) and sCT (5 μg/kg, IP)‐treated groups. sCT administration did not affect blood alcohol concentration (P = 0.2733, n = 8 per group).
Figure 6.
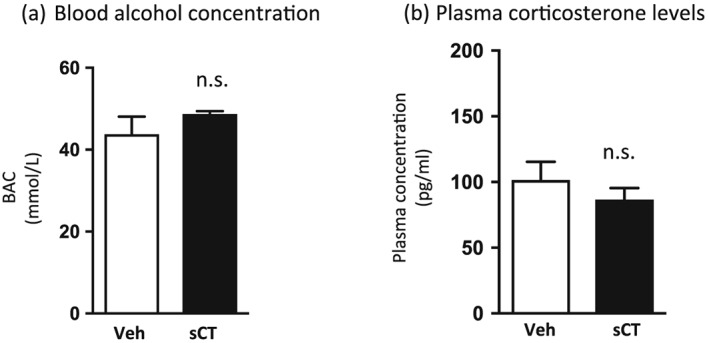
Salmon calcitonin (sCT) does not affect blood alcohol concentration and plasma corticosterone levels in mice. A single sCT injection [5 μg/kg, intraperitoneally (IP)] did not alter (a) the blood alcohol (1.75 g/kg, IP) concentration (BAC) and (b) the plasma levels of corticosterone compared with vehicle (Veh) administration. (Data are presented as mean ± SEM; n.s., non‐significant.)
A single sCT (5 μg/kg, IP) injection did not affect plasma corticosterone levels compared with vehicle injection, as presented in Figure 6b (P = 0.3549, n = 6 in sCT and n = 8 in vehicle group).
Discussion
To the best of our knowledge this is the first study presenting association between amylin receptor signalling and alcohol‐mediated behaviours in rodents. Indeed, we show that sCT, an amylin receptor agonist, attenuates alcohol‐induced locomotor stimulation, accumbal dopamine release, after both a single and a repeated alcohol injection, and the expression of reward‐associated as well as reinforcing memory‐dependent CPP in mice. Moreover, we demonstrate that sCT reduces alcohol and food consumption in low as well as high alcohol‐consuming rats with a more robust effect in the high consumers. Our findings also show that sCT alters neither blood alcohol concentration nor plasma corticosterone levels in mice. Finally, we demonstrate that sCT does not affect peanut butter intake in mice.
The present data show that positive regulation of amylin receptors affects dopaminergic neurotransmission at the level of the mesolimbic dopamine system by preventing alcohol to activate this system. Importantly, the present microdialysis experiments show that sCT decreased alcohol‐induced dopamine release after both a single and two repeated alcohol injections, thus indicating that sCT blocks the initial dopamine release caused by alcohol as well as subsequent dopamine release after a second alcohol injection. In support for a role of amylin in reward regulation are previous data linking dopamine with amylin‐mediated natural rewards, as shown by amylinergic regulation of sexual behaviour in rats through inhibition of dopamine transmission (Clementi et al. 1999), as well as by activation of dopamine D2 receptors in neurons of the AP in regard to amylin's satiety effects (Lutz et al. 2001b). Moreover, activation of the amylin receptors on dopaminergic neurons of the VTA in rats decreases phasic dopamine in NAc core (Mietlicki‐Baase et al. 2013). Another study showing decreased amphetamine‐induced locomotor stimulation in rats after intracerebroventicular sCT administration (Twery et al. 1986) supports that amylin signalling activation regulates drug‐induced reward. However, in this study, sCT decreased locomotion and that is in contrast with our results that revealed no effect of sCT on locomotor activity per se. Our data revealed that a low dose of sCT (1 μg/kg) did not block but presented a dose‐dependent effect in regard to alcohol‐induced locomotor stimulation. Experiments in rhesus monkeys have showed that administration of lower doses of sCT, in the range of 0.032 to 1 μg/kg, decreases food intake in a dose–response manner (Bello, Kemm, & Moran 2008), suggesting a potential enhanced effect of higher doses of the drug. However, the absence of significant sCT effect in our locomotor activity experiments could potentially be explained by the requirement of higher doses of sCT in order to exert its alcohol antagonizing effects. Although this is the first study demonstrating a direct link between peripheral administration of sCT and decreased alcohol intake in rats, indirect data have showed that the calcitonin gene‐related peptide, a member of the calcitonin family, was detected in lower levels in the hippocampus and frontal cortex of alcohol‐preferring rats compared with non‐preferring (Ehlers et al. 1999).
In the present study, we showed that sCT administered on the post‐conditioning day blocked reinforcing memory consolidation in the CPP paradigm in mice. In support for a role of amylin receptor activation in memory processes are the data showing that peripherally administered amylin enhances memory in mice under training conditions in a T‐maze paradigm (Flood & Morley 1992; Flood et al. 1995). More recent studies show that long‐term peripheral amylin treatment enriched learning and memory in mouse models of Alzheimer's disease (Zhu et al. 2015; Qiu 2017; Zhu et al. 2017), suggesting amylin receptors as a drug target for potential treatment of the disease (Qiu 2017). Moreover, a human study showed a positive correlation of plasma amylin and improved cognitive function in elderly population, suggesting a defensive role of amylin receptors against cognitive inclination (Qiu et al. 2014). A possible, although speculative, explanation of our results could suggest that amylinergic activation leads to correction of reward‐dependent memory consolidation and diminishes its expression in the CPP paradigm. However, the brain areas and mechanisms involved in amylinergic memory regulation remain to be investigated in detail in further studies.
It could be hypothesized that the noted effects of alcohol‐mediated behaviours could fall beyond the scope of reward. A possible explanation could be that sCT changes alcohol metabolism or causes stress‐related symptoms that could alter alcohol consumption. However, our data show that sCT administration did not affect the levels of alcohol concentration or corticosterone in the blood, thus ruling out the implication of the aforementioned factors. Nausea or taste aversion could also explain the differential response to sCT. It has been shown that sCT does not cause any side effects like nausea, aversion or malaise (Mietlicki‐Baase et al. 2013). Moreover, in our experiments, sCT does not affect the preference per se in either CPP test, indicating that it does not condition for aversion in mice. Another tentative possibility might be that sCT reduces alcohol intake because of alcohol's caloric content, and indeed, we see that sCT reduces food intake in rats. However, in this study, we did not find an effect of sCT on peanut butter consumption in mice. The lack of effect on a highly caloric food led us to the hypothesis that the remarked effects of sCT on alcohol do not appear to be calorically regulated. On that note, results showing that sCT blocks amphetamine‐induced locomotor stimulation (Twery et al. 1986) do not support a role of caloric regulation but rather that of reward regulation. Another explanation for our results could lie in the fact that sCT affects other systems. Indeed, sCT has been proposed to act as an analgesic possibly by causing a CNS increase in beta‐endorphin levels (Franceschini et al. 1993), and previous studies have showed that antagonism of the signalling system of another gut–brain peptide, that of ghrelin, alters the levels of endogenous opioids (Engel, Nylander, & Jerlhag 2015); thus, upcoming studies could define other signalling systems involved in amylin‐regulated reward. Another explanation for our results could lie in the binding selectivity of sCT that could affect other receptor pathways. However, this appears less likely because research identifies that sCT binds selectively to calcitonin receptors, the core component of amylin receptors (Barwell et al. 2012) and irreversibly and with higher affinity on amylin receptors than amylin (Lutz et al. 2000); a selective binding on amylin receptors is also supported by the fact that the doses used in the present do not have an effect per se on locomotor activity and CPP in mice. Peripherally, sCT binds to calcitonin receptors on bone osteoclasts (Chesnut et al. 2008; Nicholson et al. 1986) and the kidney (Marx, Woodard, & Aurbach 1972), and it has been used for the treatment of bone metabolic diseases that involve these receptors, for example, osteoporosis (Munoz‐Torres, Alonso, & Raya 2004). It is well established that direct activation of calcitonin receptors by sCT on osteoclasts inhibits bone resorption and activation of renal receptors enhances calcium excretion. Thus, possible effects of the drug's binding to these peripheral receptors cannot be disregarded. However, inhibited bone resorption would not seem to explain the effects of sCT on the alcohol‐induced activation of the mesolimbic dopamine system, i.e. accumbal dopamine release, or the expression of alcohol‐induced CPP, which reflect reward processing (Bardo & Bevins 2000; Boileau et al. 2003). Another tentative explanation of our findings could be that enhanced calcium levels after sCT activation of renal calcitonin receptors reflect an increase in brain calcium levels consequently changing calcium influx/efflux in neuronal ion channels. On a similar note, a recent clinical study investigating the effects of acamprosate on alcohol‐dependent individuals attributed the drug's effects to calcium levels and not to a specific CNS target (Spanagel et al. 2014). Nevertheless, a number of studies investigating the effects of locally administered sCT on food intake accompanied by immunohistochemistry data have identified central sites of action, especially in areas of the mesolimbic dopamine system (Mietlicki‐Baase et al. 2013; Mietlicki‐Baase & Hayes 2014; Reiner et al. 2017). Thus, we argue that the present findings do not fall under the scope of calcium levels regulation, but they are a result of sCT's central action. Although there is no robust evidence that sCT crosses the blood–brain barrier effectively, there are data showing that peripheral sCT did not affect food intake in rats with a knocked down calcitonin receptor in the VTA but decreased food intake in control (Mietlicki‐Baase et al. 2015b). Moreover, a recent study showed that peripherally administered sCT decreased VTA‐evoked phasic dopamine release in the NAc (Whiting et al. 2017), supporting our notion that sCT acts centrally on the mesolimbic dopamine system to regulate dopamine neurotransmission. In line with the present results, we therefore suggest that sCT crosses the blood–brain barrier and reaches areas of the midbrain to regulate alcohol reinforcement. Thus, upcoming studies should explore and define the brain areas involved in the ability of sCT to regulate alcohol‐mediated behaviours. Moreover, in light of the findings that amylin receptor inhibition increases food intake as well as body adiposity in rats (Rushing et al. 2001; Reidelberger et al. 2004), future experiments exploring the possibility that an amylinergic receptor antagonist increases alcohol intake are warranted.
Studies have presented that amylin receptor signalling in VTA, NAc and LDTg (Baisley & Baldo 2014; Mietlicki‐Baase et al. 2015b; Reiner et al. 2017) is involved in the control of food intake. Albeit these areas are part of the cholinergic–dopaminergic pathway, which is well established as an important pathway involved in reward‐related behaviours (Soderpalm & Ericson 2013), very little is known about the role of amylin receptor signalling within these areas in regard to artificial reinforcers, such as alcohol. It is now known that other gut peptides like ghrelin, glucagon‐like peptide‐1 and neuromedin U regulate both natural and artificial reward behaviours by acting on receptors located throughout this reward link (Egecioglu et al. 2010; Egecioglu et al. 2013b; Jerlhag & Engel 2011; Suchankova et al. 2013b; Vallof et al. 2016c). We therefore hypothesize that amylin receptors in these reward‐related brain areas could act as mediators of reward, potentially devaluating the incentive value of alcohol after their activation; however, local administration in sites of the mesolimbic dopamine pathway will reveal more about the neurobiological mechanisms involved.
In the present study, we show that acute administration of sCT affects alcohol, food and water intake as well as body weight and that this effect is more pronounced in high alcohol‐consuming than low alcohol‐consuming rats. sCT decreased short‐term (1‐hour values) food intake in rats, without altering the rats' weight in this low alcohol‐consuming group. However, sCT decreased both short‐term and long‐term (1‐ and 24‐hour values) food intake in high alcohol‐consuming rats, which was accompanied by a decrease in body weight. The short‐term effect of sCT on food intake in the low alcohol consumers is compatible with other food intake studies, where various doses of sCT suppress food intake in a dose‐dependent manner, showing the most robust effect on the first meal size (Bello et al. 2008). Moreover, the present results are in accordance with previous studies that have established an anorexigenic effect of the activation of amylin receptors (Reda et al. 2002). The remarked differences in food intake between the two groups show that high alcohol‐consuming rats are more sensitive to the anorexigenic effect of sCT, and possibly due to changes in the brain after long‐term high alcohol exposure. Importantly, we noted a robust decrease in the rat body weight after sCT administration in the high alcohol‐consuming group that was absent in the low alcohol‐consuming one. This is in corroboration with previous studies showing that peripheral sCT decreases 24‐hour body weight in outbred rats (Shah & Donald 1984) and causes decrease of body weight in diet‐induced obese rats (Feigh et al. 2011). Supportively, clinical data have showed that amylin analogues like pramlintide reduce body weight in overweight/obese and diabetic (type 2) patients compared with placebo, with the effect being more pronounced in markedly obese patients (Hollander et al. 2004). The discrepancy of the data between the two alcohol‐consuming groups strengthens the hypothesis that chronic exposure to high alcohol could possibly alter brain neurocircuits and increase the sensitivity to sCT in regard to body weight regulation. On a similar note, we found that sCT reduces alcohol intake at the first hour after administration, but not 24 hours later in the low alcohol‐consuming group; however, it dramatically decreased the 1‐ and 24‐hour alcohol intake in the high alcohol‐consuming rats. Indeed, the percentage of alcohol change is more robust in high compared with low alcohol‐consuming rats. In support for this robust effect in high consumers are the data showing that sCT reduces alcohol preference in these rats but not in low consumers. Moreover, sCT did not affect water and total fluid intake in the low alcohol‐consuming group, whereas it robustly increased it in the high alcohol‐consuming one. This increase in water intake could be attributed to the compensation of drinking behaviour in the high alcohol‐consuming group, as a consequence of the robust alcohol intake decrease. The absence of long‐term effect of sCT on alcohol intake in the first experiment could be attributed to the half‐life of the drug, which has been shown to be rapidly absorbed with peak concentration in blood plasma within 30–60 minutes after subcutaneous administration (Sinko et al.). Nevertheless, the effect of sCT is very pronounced in the 24‐hour values in the high alcohol‐consuming group, indicating different sensitivity of the two consuming groups to sCT in regard to alcohol intake regulation. In accordance are studies showing that central administration of neuromedin U dose dependently decreases alcohol intake in high but not in low alcohol‐consuming rats (Vallof et al. 2016b). Moreover, administration of a ghrelin antagonist reduced alcohol intake more robustly in rats voluntarily exposed to alcohol for 5 months instead of 2 (Landgren et al. 2012). As previously mentioned, a tentative explanation could be that chronic exposure to high alcohol consumption can lead to alterations in the brain circuits involved in sCT alcohol reward regulation. Indeed, alcohol‐preferring and high alcohol‐drinking rats were found to have fewer calcitonin gene‐related peptide receptor‐binding sites in forebrain regions compared with non‐preferring and low alcohol‐drinking rats, respectively (Hwang et al. 1995). With focus on the present data, a speculative explanation could lie on the ability of sCT to more robustly decrease alcohol intake as a result of altered sensitivity in the brain. Nevertheless, gene expression studies and other molecular approaches would reveal more about these differences in the brain and are warranted in the future.
Interestingly, sCT administered in two different doses did not alter peanut butter intake in satiated mice. Our results are contradicting to previous studies showing that sCT decreases lever pressing for palatable reward (Morley et al. 1997) and palatable food intake in mice (Eiden et al. 2002). However, either these studies used high doses of amylin in the range of 100 to 200 μg/kg in an operant paradigm (Morley et al. 1997), or they were conducted in mice resistant to leptin after being scheduled on chocolate as a highly caloric substitute to chow for more than 40 days (Eiden et al. 2002). Another difference is that our experiments were conducted in novel cages; thus, novelty could be considered as a factor potentially influencing our data. It has been shown that palatable food consumption is inversely regulated in different energy statuses, as proved by studies with other gut–brain peptides. Given that the mice used in our experiments were satiated, our results are in accordance with studies showing that ghrelin does not increase palatable food intake in ad libitum fed mice, but it does so in fasted mice (Alen et al. 2013). This is corroborated by our previous data presenting that ghrelin does not increase peanut butter intake in satiated mice (Kalafateli et al. 2017) and that its administration in rats scheduled for palatable feeding decreases high‐fat‐diet consumption and enhances normal chow intake (Schéle et al. 2016; Bake, Hellgren, & Dickson 2017). It is therefore possible that the amylinergic mechanisms regulating food reward do not coincide with the ones mediating substance reinforcement.
Collectively, we show for the first time that a single peripheral injection of sCT attenuates alcohol‐mediated behaviours in rodents by decreasing alcohol's ability to activate the mesolimbic dopamine system. Importantly, amylin analogues like pramlintide for the treatment of diabetes and sCT products for the treatment of osteoporosis and Paget's disease are already commercially available. Providing that research on neurochemical mechanisms through which alcohol activates the mesolimbic dopamine reward link has led to identification of novel treatment targets (Edwards et al. 2011; Engel & Jerlhag 2014) and in combination with our preclinical data, the aforementioned or similar agents could tentatively be used as potential treatment of alcohol dependence as well as other addiction disorders.
Supporting information
Data S1. Supporting information
Acknowledgements
Britt‐Mari Larsson is gratefully acknowledged for expert and valuable technical assistance. The study is supported by grants from the Swedish Research Council (2015‐03219); Swedish Society for Medical Research; The Swedish Brain Foundation; LUA/ALF (grant no. 148251) from the Sahlgrenska University Hospital; Torsten Söderberg; Alcohol Research Council of the Swedish Alcohol Retailing Monopoly; and the foundations of Adlerbertska, Fredrik and Ingrid Thuring, Tore Nilsson, Längmanska, Wilhelm and Martina Lundgren, Knut and Alice Wallenberg, Magnus Bergvall, Anérs, Jeansons, Åke Wiberg, NovoNordisk and the Swedish Society of Medicine.
Disclosure/Conflict of Interest
EJ has received financial support from the Novo Nordisk Foundation. This does not alter the authors' adherence to any of the journal's policies on sharing data and materials. The remaining authors declare no conflict of interest.
Authors Contribution
ALK performed the hands‐on work, analysed data, wrote the manuscript and managed literature search; DV performed the hands‐on work and analysed data; JAE revised the content and contributed to the conception; EJ designed the study, contributed to the conception and interpretation, managed literature search, analysed data and wrote the manuscript. All authors contributed to and have approved the final manuscript.
Kalafateli, A. L. , Vallöf, D. , and Jerlhag, E. (2019) Activation of amylin receptors attenuates alcohol‐mediated behaviours in rodents. Addiction Biology, 24: 388–402. 10.1111/adb.12603.
References
- Abizaid A, Mineur YS, Roth RH, Elsworth JD, Sleeman MW, Picciotto MR, Horvath TL (2011) Reduced locomotor responses to cocaine in ghrelin‐deficient mice. Neuroscience 192:500–506. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N, Farnetti S, Domenicali M, D'Angelo C, Vonghia L, Mirijello A, Cardone S, Gasbarrini G (2006) Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res 30:1933–1937. [DOI] [PubMed] [Google Scholar]
- Adinoff B (2004) Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry 12:305–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Antwi DA (2008) Brain regulation of appetite and satiety. Endocrinol Metab Clin N Am 37:811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alen F, Crespo I, Ramírez‐López MT, Jagerovic N, Goya P, de Fonseca FR, de Heras RG, Orio L (2013) Ghrelin‐induced orexigenic effect in rats depends on the metabolic status and is counteracted by peripheral CB1 receptor antagonism. PLoS One 8:e60918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–2017. [DOI] [PubMed] [Google Scholar]
- Baisley SK, Baldo BA (2014) Amylin receptor signaling in the nucleus accumbens negatively modulates μ‐opioid‐driven feeding. Neuropsychopharmacology 39:3009–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake T, Hellgren KT, Dickson SL (2017) Acute ghrelin changes food preference from a high‐fat diet to chow during binge‐like eating in rodents. J Neuroendocrinol 29: n/a‐n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA (2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153:31–43. [DOI] [PubMed] [Google Scholar]
- Barwell J, Gingell JJ, Watkins HA, Archbold JK, Poyner DR, Hay DL (2012) Calcitonin and calcitonin receptor‐like receptors: common themes with family B GPCRs? Br J Pharmacol 166:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Kemm MH, Moran TH (2008) Salmon calcitonin reduces food intake through changes in meal sizes in male rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 295:R76–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, Söderpalm B (1993) The mesolimbic dopamine‐activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol 249:207–213. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse (New York, NY) 49:226–231. [DOI] [PubMed] [Google Scholar]
- Braegger FE, Asarian L, Dahl K, Lutz TA, Boyle CN (2014) The role of the area postrema in the anorectic effects of amylin and salmon calcitonin: behavioral and neuronal phenotyping. Eur J Neurosci 40:3055–3066. [DOI] [PubMed] [Google Scholar]
- Chesnut CH, 3rd , Azria M, Silverman S, Engelhardt M, Olson M, Mindeholm L (2008) Salmon calcitonin: a review of current and future therapeutic indications. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 19:479–491. [DOI] [PubMed] [Google Scholar]
- Clementi G, Busa L, de Bernardis E, Prato A, Drago F (1999) Effects of centrally injected amylin on sexual behavior of male rats. Peptides 20:379–382. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Rodriguez J, Schul D, Hughes S, Kniffin T, Hart N, Eitan S, Brunel L, Fehrentz JA, Martinez J, Wellman PJ (2012) Attenuation of cocaine‐induced locomotor sensitization in rats sustaining genetic or pharmacologic antagonism of ghrelin receptors. Addict Biol 17:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E (2011) The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol 340:80–87. [DOI] [PubMed] [Google Scholar]
- Edwards A, Abizaid A (2016) Driving the need to feed: insight into the collaborative interaction between ghrelin and endocannabinoid systems in modulating brain reward systems. Neurosci Biobehav Rev 66:33–53. [DOI] [PubMed] [Google Scholar]
- Edwards S, Kenna GA, Swift RM, Leggio L (2011) Current and promising pharmacotherapies, and novel research target areas in the treatment of alcohol dependence: a review. Curr Pharm Des 17:1323–1332. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E (2013a) The glucagon‐like peptide 1 analogue, exendin‐4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One 8:e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Jerlhag E, Salomé N, Skibicka KP, Haage D, Bohlooly‐Y M, Andersson D, Bjursell M, Perrissoud D, Engel JA, Dickson SL (2010) Preclinical study: full article: ghrelin increases intake of rewarding food in rodents. Addict Biol 15:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E (2013b) The glucagon‐like peptide 1 analogue exendin‐4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38:1259–1270. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Li TK, Lumeng L, Hwang BH, Jimenez P, Mathe AA (1999) Calcitonin gene‐related peptide (CGRP) levels and alcohol. Int J Neuropsychopharmacol 2:173–179. [DOI] [PubMed] [Google Scholar]
- Eiden S, Daniel C, Steinbrueck A, Schmidt I, Simon E (2002) Salmon calcitonin—a potent inhibitor of food intake in states of impaired leptin signalling in laboratory rodents. J Physiol 541:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E (2014) Role of appetite‐regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs 28:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JA, Nylander I, Jerlhag E (2015) A ghrelin receptor (GHS‐R1A) antagonist attenuates the rewarding properties of morphine and increases opioid peptide levels in reward areas in mice. Eur Neuropsychopharmacol 25:2364–2371. [DOI] [PubMed] [Google Scholar]
- Feigh M, Henriksen K, Andreassen KV, Hansen C, Henriksen JE, Beck‐Nielsen H, Christiansen C, Karsdal MA (2011) A novel oral form of salmon calcitonin improves glucose homeostasis and reduces body weight in diet‐induced obese rats. Diabetes Obes Metab 13:911–920. [DOI] [PubMed] [Google Scholar]
- Flood JF, Farr SA, Perry Iii HJ, Kaiser FE, Morley PMK, Morley JE (1995) Effects of amylin on appetite regulation and memory. Can J Physiol Pharmacol 73:1042–1046. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE (1992) Differential effects of amylin on memory processing using peripheral and central routes of administration. Peptides 13:577–580. [DOI] [PubMed] [Google Scholar]
- Franceschini R, Cataldi A, Cianciosi P, Garibaldi A, Corsini G, Barreca T, Rolandi E (1993) Calcitonin and beta‐endorphin secretion. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 47:305–309. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM‐5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiat 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD (2015) Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev 67:564–600. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M (2006) Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther 111:855–876. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Rada PV, Mark GP, Pothos EN (1999) Neural systems for reinforcement and inhibition of behavior: relevance to eating, addiction, and depression In: Kahneman D, Diener E, Schwarz N. eds. Well‐being: The Foundations of Hedonic Psychology, pp. 558–572. Russell Sage Foundation: New York, NY, US. [Google Scholar]
- Hollander P, Maggs DG, Ruggles JA, Fineman M, Shen L, Kolterman OG, Weyer C (2004) Effect of pramlintide on weight in overweight and obese insulin‐treated type 2 diabetes patients. Obes Res 12:661–668. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Kunkler PE, Lumeng L, Li TK (1995) Calcitonin gene‐related peptide (CGRP) content and CGRP receptor binding sites in discrete forebrain regions of alcohol‐preferring vs. ‐nonpreferring rats, and high alcohol‐drinking vs. low alcohol‐drinking rats. Brain Res 690:249–253. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA (2006) Ghrelin stimulates locomotor activity and accumbal dopamine‐overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol 11:45–54. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Engel JA (2010) Ghrelin receptor antagonism attenuates cocaine‐ and amphetamine‐induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology 211:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Engel JA (2011a) Glutamatergic regulation of ghrelin‐induced activation of the mesolimbic dopamine system. Addict Biol 16:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA (2009) Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A 106:11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Engel JA (2011) Ghrelin receptor antagonism attenuates nicotine‐induced locomotor stimulation, accumbal dopamine release and conditioned place preference in mice. Drug Alcohol Depend 117:126–131. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Landgren S, Egecioglu E, Dickson SL, Engel JA (2011b) The alcohol‐induced locomotor stimulation and accumbal dopamine release is suppressed in ghrelin knockout mice. Alcohol 45:341–347. [DOI] [PubMed] [Google Scholar]
- Kalafateli AL, Vallof D, Jornulf JW, Heilig M, Jerlhag E (2017) A Cannabinoid Receptor Antagonist Attenuates Ghrelin‐induced Activation of the Mesolimbic Dopamine System in Mice. Physiol Behav. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Bloom F (1988) Cellular and molecular mechanisms of drug dependence. Science 242:715–723. [DOI] [PubMed] [Google Scholar]
- Kraus T, Schanze A, Gröschl M, Bayerlein K, Hillemacher T, Reulbach U, Kornhuber J, Bleich S (2005) Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res 29:2154–2157. [DOI] [PubMed] [Google Scholar]
- Landgren S, Simms JA, Hyytia P, Engel JA, Bartlett SE, Jerlhag E (2012) Ghrelin receptor (GHS‐R1A) antagonism suppresses both operant alcohol self‐administration and high alcohol consumption in rats. Addict Biol 17:86–94. [DOI] [PubMed] [Google Scholar]
- Larsson A, Edstrom L, Svensson L, Soderpalm B, Engel JA (2005) Voluntary ethanol intake increases extracellular acetylcholine levels in the ventral tegmental area in the rat. Alcohol Alcohol 40:349–358. [DOI] [PubMed] [Google Scholar]
- Larsson A, Engel JA (2004) Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev 27:713–720. [DOI] [PubMed] [Google Scholar]
- Leggio L, Addolorato G, Cippitelli A, Jerlhag E, Kampov‐Polevoy AB, Swift RM (2011) Role of feeding‐related pathways in alcohol dependence: a focus on sweet preference, NPY, and ghrelin. Alcohol Clin Exp Res 35:194–202. [DOI] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, Kenna GA (2014) Intravenous ghrelin administration increases alcohol craving in alcohol‐dependent heavy drinkers: a preliminary investigation. Biol Psychiatry 76:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A (2012) Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol 17:513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA (2009) Control of food intake and energy expenditure by amylin‐therapeutic implications. Int J Obes 33:S24–S27. [DOI] [PubMed] [Google Scholar]
- Lutz TA (2012) Effects of amylin on eating and adiposity. Handb Exp Pharmacol 231–250. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E (1995) Amylin decreases meal size in rats. Physiol Behav 58:1197–1202. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E (2001a) The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord: Journal of the International Association for the Study of Obesity 25:1005–1011. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Tschudy S, Mollet A, Geary N, Scharrer E (2001b) Dopamine D2 receptors mediate amylin's acute satiety effect. Am J Physiol Regul Integr and Comp Physiol Ther 280:R1697–R1703. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Tschudy S, Rushing PA, Scharrer E (2000) Amylin receptors mediate the anorectic action of salmon calcitonin (sCT). Peptides 21:233–238. [DOI] [PubMed] [Google Scholar]
- Mack C, Wilson J, Athanacio J, Reynolds J, Laugero K, Guss S, Vu C, Roth J, Parkes D (2007) Pharmacological actions of the peptide hormone amylin in the long‐term regulation of food intake, food preference, and body weight. Am J Physiol Regul Integr Comp Physiol 293:R1855–R1863. [DOI] [PubMed] [Google Scholar]
- Marx SJ, Woodard CJ, Aurbach GD (1972) Calcitonin receptors of kidney and bone. Science 178:999. [DOI] [PubMed] [Google Scholar]
- Mietlicki‐Baase EG, Hayes MR (2014) Amylin activates distributed CNS nuclei to control energy balance. Physiol Behav 136:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki‐Baase EG, Olivos DR, Jeffrey BA, Hayes MR (2015a) Cooperative interaction between leptin and amylin signaling in the ventral tegmental area for the control of food intake. Am J Physiol Endocrinol Metab 308:E1116–E1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki‐Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ, Roitman MF, Hayes MR (2015b) Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology 40:372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki‐Baase EG, Rupprecht LE, Olivos DR, Zimmer DJ, Alter MD, Pierce RC, Schmidt HD, Hayes MR (2013) Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology 38:1685–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Suarez MD, Mattamal M, Flood JF (1997) Amylin and food intake in mice: effects on motivation to eat and mechanism of action. Pharmacol Biochem Behav 56:123–129. [DOI] [PubMed] [Google Scholar]
- Munoz‐Torres M, Alonso G, Raya MP (2004) Calcitonin therapy in osteoporosis. Treat Endocrinol 3:117–132. [DOI] [PubMed] [Google Scholar]
- Nicholson GC, Moseley JM, Sexton PM, Mendelsohn FA, Martin TJ (1986) Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization. The Journal of clinical investigation 78:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The Brain Stereotaxic Coordinates. Academic Press: New York. [Google Scholar]
- Potes CS, Lutz TA (2010) Brainstem mechanisms of amylin‐induced anorexia. Physiol Behav 100:511–518. [DOI] [PubMed] [Google Scholar]
- Qiu WQ (2017) Amylin and its G‐protein‐coupled receptor: a probable pathological process and drug target for Alzheimer's disease. Neuroscience 356:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WQ, Au R, Zhu H, Wallack M, Liebson E, Li H, Rosenzweig J, Mwamburi M, Stern RA (2014) Positive association between plasma amylin and cognition in a homebound elderly population. Journal of Alzheimer's disease: JAD 42:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Mark GP, Hoebel BG (1998) Galanin in the hypothalamus raises dopamine and lowers acetylcholine release in the nucleus accumbens: a possible mechanism for hypothalamic initiation of feeding behavior. Brain Res 798:1–6. [DOI] [PubMed] [Google Scholar]
- Reda TK, Geliebter A, Pi‐Sunyer FX (2002) Amylin, food intake, and obesity. Obes Res 10:1087–1091. [DOI] [PubMed] [Google Scholar]
- Reidelberger RD, Haver AC, Arnelo U, Smith DD, Schaffert CS, Permert J (2004) Amylin receptor blockade stimulates food intake in rats. Am J Physiol Regul Integr Comp Physiol 287:R568–R574. [DOI] [PubMed] [Google Scholar]
- Reiner DJ, Mietlicki‐Baase EG, Olivos DR, McGrath LE, Zimmer DJ, Koch‐Laskowski K, Krawczyk J, Turner C, Noble EE, Hahn JD, Schmidt HD, Kanoski SE, Hayes MR (2017) Amylin acts in the lateral dorsal tegmental nucleus to regulate energy balance through GABA signaling. Biol Psychiatry . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushing PA, Hagan MM, Seeley RJ, Lutz TA, D'Alessio DA, Air EL, Woods SC (2001) Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology 142:5035. [DOI] [PubMed] [Google Scholar]
- Schéle E, Bake T, Rabasa C, Dickson SL (2016) Centrally administered ghrelin acutely influences food choice in rodents. PLoS One 11:e0149456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton MP, Paxinos G, Kenney MA, Wookey PJ, K. B (1994) In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience 62:14. [DOI] [PubMed] [Google Scholar]
- Shah NS, Donald AG (1984) Psychoneuroendocrine Dysfunction. Plenum: New York. [Google Scholar]
- Soderpalm B, Ericson M (2013) Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci 13:127–161. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Lof E, Ericson M (2009) Mechanistic studies of ethanol's interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry 42:S87–S94. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Vengeliene V, Jandeleit B, Fischer W‐N, Grindstaff K, Zhang X, Gallop MA, Krstew EV, Lawrence AJ, Kiefer F (2014) Acamprosate produces its anti‐relapse effects via calcium. Neuropsychopharmacology 39:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Jerlhag E, Jayaram‐Lindstrom N, Nilsson S, Toren K, Rosengren A, Engel JA, Franck J (2013a) Genetic variation of the ghrelin signalling system in individuals with amphetamine dependence. PLoS One 8:e61242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Steensland P, Fredriksson I, Engel JA, Jerlhag E (2013b) Ghrelin receptor (GHS‐R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long‐term voluntary alcohol consumption. PLoS One 8:e71284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twery MJ, Kirkpatrick B, Lewis MH, Mailman RB, Cooper CW (1986) Antagonistic behavioral effects of calcitonin and amphetamine in the rat. Pharmacol Biochem Behav 24:1203–1207. [DOI] [PubMed] [Google Scholar]
- Vadnie CA, Park JH, Abdel Gawad N, Ho AM, Hinton DJ, Choi DS (2014) Gut–brain peptides in corticostriatal‐limbic circuitry and alcohol use disorders. Front Neurosci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallof D, Maccioni P, Colombo G, Mandrapa M, Jornulf JW, Egecioglu E, Engel JA, Jerlhag E (2016a) The glucagon‐like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addict Biol 21:422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallof D, Ulenius L, Egecioglu E, Engel JA, Jerlhag E (2016b) Central Administration of the Anorexigenic Peptide Neuromedin U Decreases Alcohol Intake and Attenuates Alcohol‐induced Reward in Rodents. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallof D, Vestlund J, Engel JA, Jerlhag E (2016c) The anorexigenic peptide neuromedin U (NMU) attenuates amphetamine‐induced locomotor stimulation, accumbal dopamine release and expression of conditioned place preference in mice. PLoS One 11:e0154477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R (2008) Neuropharmacology of alcohol addiction. Br J Pharmacol 154:299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P, Andersson A, Westermark GT (2011) Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 91:795–826. [DOI] [PubMed] [Google Scholar]
- Whiting L, McCutcheon JE, Boyle CN, Roitman MF, Lutz TA (2017) The area postrema (AP) and the parabrachial nucleus (PBN) are important sites for salmon calcitonin (sCT) to decrease evoked phasic dopamine release in the nucleus accumbens (NAc). Physiol Behav 176:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang X, Wallack M, Li H, Carreras I, Dedeoglu A, Hur JY, Zheng H, Li H, Fine R, Mwamburi M, Sun X, Kowall N, Stern RA, Qiu WQ (2015) Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer's disease. Mol Psychiatry 20:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Xue X, Wang E, Wallack M, Na H, Hooker JM, Kowall N, Tao Q, Stein TD, Wolozin B, Qiu WQ (2017) Amylin receptor ligands reduce the pathological cascade of Alzheimer's disease. Neuropharmacology 119:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information


