Abstract
Kappa opioid receptor (KOPr) agonists have preclinical anti-cocaine and antinociceptive effects. However, adverse effects including dysphoria, aversion, sedation, anxiety and depression limit their clinical development. MP1104, an analogue of 3-iodobenzoyl naltrexamine, is a potent dual agonist at KOPr and delta opioid receptor (DOPr), with full agonist efficacy at both these receptors. In this study, we evaluate the ability of MP1104 to modulate cocaine-induced behaviors and side-effects preclinically. In male Sprague-Dawley rats trained to self-administer cocaine, MP1104 (0.3 and 1 mg/kg) reduced cocaine-primed reinstatement of drug-seeking behavior and caused significant downward shift of the dose-response curve in cocaine self-administration tests (0.3 and 0.6 mg/kg). The anti-cocaine effects exerted by MP1104 are in part due to increased dopamine (DA) uptake by the dopamine transporter (DAT) in the dorsal striatum (dStr) and nucleus accumbens (NAc). MP1104 (0.3 and 0.6 mg/kg) showed no significant anxiogenic effects in the elevated plus maze, pro-depressive effects in the forced swim test, or conditioned place aversion. Furthermore, pre-treatment with a DOPr antagonist, led to MP1104 producing aversive effects. This data suggests that the DOPr agonist actions of MP1104 attenuate the KOPr-mediated aversive effects of MP1104. The overall results from this study show that MP1104, modulates DA uptake in the dStr and NAc, and exerts potent anti-cocaine properties in self-administration tests with reduced side-effects compared to pure KOPr agonists. This data supports the therapeutic development of dual KOPr/DOPr agonists to reduce the side-effects of selective KOPr agonists.
Keywords: Self-administration, Cocaine, Drug-seeking, Behavioural pharmacology, Elevated plus maze, Conditioned place aversion
1. Introduction
Drug addiction is a chronic, relapsing disorder. It is accompanied by the compulsion to seek and take the drug, loss of control in limiting drug intake and developing a negative emotional state (dysphoria, anxiety) during withdrawal (Koob and Volkow, 2010; Koob and Le Moal, 1997). Despite decades of effort focussed on the development of anti-addiction pharmacotherapies, there are currently no Food and Drug Administration (FDA) approved drugs to treat psychostimulant addiction. In addition to the economic and social burden resulting from psychostimulant abuse (Grant et al., 2017), addiction to opioids has reached epidemic proportions (Rudd et al., 2016). Given the escalating addiction burden there is even greater need for the development of nonaddictive analgesics and effective anti-addiction pharmacotherapies.
It is well known that continued and repeated drug use upregulates the Kappa opioid receptor (KOPr) system and counteracts the effects of drugs of abuse (Wee and Koob, 2010). Activation of the endogenous KOPr ligand, dynorphin, opposes positive reinforcement (Shippenberg et al., 2007), and upregulation of the KOPr is also responsible for negative affects during drug withdrawal (Koob and Volkow, 2010). The anhedonia hypothesis suggests that dopamine (DA) in the brain plays a very important role in the motivation associated with positive rewards such as food, water, psychostimulants and opioids (Wise, 2008). Hence, modulating the effects of DA is a way of curbing the reinforcing effects of addictive drugs.
Modulation of the KOPr system, via both agonists and antagonists, have the potential to modulate different stages of addiction. KOPr antagonists such as nor-binaltorphimine (nor-BNI) and JDTic, have been shown to prevent behavioural adaptations related to stress (Beardsley et al., 2005; Jackson et al., 2013). Since there is a strong connection between stress and drug-relapse in humans (Koob, 2009), KOPr antagonists are being explored as potential pharmacotherapies for preventing stress-induced relapse (Beardsley et al., 2005; Bruchas et al., 2010; Redila and Chavkin, 2008). Whereas, KOPr agonists have anti-cocaine properties in preclinical models of drug use and attenuate the rewarding effects of psychostimulants (Ewald et al., 2017; Morani et al., 2009, 2012; Schenk et al., 1999; Shippenberg et al., 2007; Simonson et al., 2015) and these properties are being explored as possible formulations to reduce the abuse potential of pain medications (Naylor et al., 2015). The mechanisms through which they act are via reducing basal DA release (Carlezon et al., 2006; Spanagel et al., 1992; Spangler et al., 1997; Wee and Koob, 2010), drug-evoked DA release (Zhang et al., 2004a, 2004b) and by increasing DA reuptake via the dopamine transporter (DAT) (Kivell et al., 2014a; Simonson et al., 2015). However, the clinical utility of KOPr agonists is limited due to their dysphoric, aversive (Land et al., 2009), anxiogenic (Gillett et al., 2013), pro-depressive (Mague et al., 2003) and sedative effects (Gallantine and Meert, 2008). These KOPr mediated side-effects have been associated with decreases in dopaminergic neurotransmission (Brust et al., 2016; Crowley and Kash, 2015; Wee and Koob, 2010). Recently, regional specific effects have been identified that show KOPr activation in the ventral nucleus accumbens (NAc) shell elicits aversive-like behavior and the dorsal region of the NAc shell induces place preference (Al-Hasani et al., 2015). Therefore, finding ways of targeting these distinct KOPr expressing neuronal populations may be the key to develop KOPr agonists with reduced side-effects.
Another approach to overcome the undesirable effects of KOPr agonism is by targeting multiple opioid receptors simultaneously. Studies have shown that mixed opioid receptor agonists and/or antagonists may be a viable strategy to generate a more desirable drug profile (Anand and Montgomery, 2018; Ananthan, 2006; Balboni et al., 2002; Bidlack, 2014; Greedy et al., 2013; Majumdar and Devi, 2018; Morphy and Rankovic, 2009; Váradi et al., 2016; Wang et al., 2016). It has been well documented that mu opioid receptor (MOPr) and delta opioid receptor (DOPr) agonists are rewarding (Shippenberg et al., 2008), whereas, agonists with affinity at KOPr are aversive (Castro and Berridge, 2014; Herz, 1998; Suzuki and Misawa, 1997).
MP1104 (Fig. 1), an analogue of 3′-iodobenzoyl naltrexamine, is a novel mixed-opioid agonist that has high affinity for the KOPr in [125I] BNtxA competitive binding assays, with KOPr showing 3- and 13-fold higher binding affinity compared to that of MOPr and DOPr respectively (Váradi et al., 2015). Recently, the crystal structure of human KOPr in complex with MP1104 and an active-state-stabilizing nano-body has been explored to provide molecular insights into KOPr structure and function (Che et al., 2018). In functional [35S]GTPγS binding assays in Chinese hamster ovary (CHO) cells expressing cloned mouse opioid receptors, MP1104 showed 8- and 15-fold greater potency at KOPr compared to MOPr and DOPr, respectively (Váradi et al., 2015). Although, MP1104 showed full agonism at MOPr, the MOPr did not mediate the antinociceptive effects in the warm water tail-withdrawal assay in C57BL/6J mice. Analgesia was insensitive to the MOPr antagonist beta-funaltrexamine, and MOPr knock-out mice (Váradi et al., 2015). The reason for this may be due to the in vivo metabolism, which accounts for the differences between the in vitro and in vivo MOPr activity. We hypothesise that the rewarding properties of DOPr agonism (Devine et al., 1993) will negate the negative effects of KOPr agonism. Therefore, the aim of the present study was to investigate the anti-cocaine effects and side-effect profile of MP1104. Using a cocaine-prime induced reinstatement model of cocaine-seeking we evaluated the anti-cocaine effects of MP1104 in rats and evaluated the side-effects in models of motor-impairment, aversion, anxiety and depression.
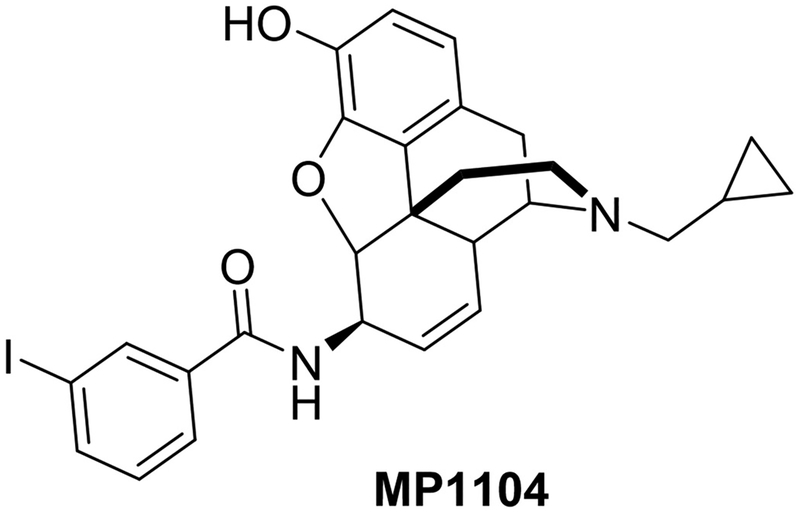
Fig. 1.
Structure of MP1104.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (Rattus norvegicus) weighing 250–400 g were housed 3–4 per cage (polycarbonate cage) depending on size, or individually for self-administration tests (n = 196). Animals were housed within the vivarium at the School of Biological Sciences, Victoria University of Wellington, New Zealand, in humidity (55%) and temperature (19–21 °C) controlled rooms. Lights were maintained on a 12-h light/12-h dark cycle with lights on at 07:00 h. Food and water were available ad libitum except during testing. All experimental procedures followed ARRIVE guidelines and were approved and conducted in accordance with the guidelines of the Animal Ethics Committee of Victoria University of Wellington, New Zealand; and adhere to the NIH guide for the care and use of laboratory animals. All efforts were made to minimize the number of animals used.
2.2. Drugs
17-Cyclopropylmethyl-3-hydroxy-4,5α-epoxy-7,8-en-6-β-[(3′-iodo) benzamido]-morphinan (MP1104) and Naltrindole (NTI) (with > 98% purity) (Váradi et al., 2015) were provided by Dr. Susruta Majumdar (Memorial Sloan Kettering Cancer Centre, New York, USA). Trans-3,4-dichloro-N-methyl-N-(2–1-pyrrolidinyl)-cyclohexyl-benzeacetamide (U50,488) (Sigma-Aldrich, Auckland, New Zealand) and yohimbine (In Vitro Technologies Ltd., Auckland, NZ) were dissolved in dimethyl sulfoxide (DMSO)/Tween-80/water (Milli-Q) in a ratio of 2:1:7. All drugs were administered via intraperitoneal (i.p.) injections except NTI which was injected subcutaneously (s.c.). Cocaine HCl (BDH Ltd., Wellington, NZ) was dissolved in heparinised saline (3.0 U/mL) for intravenous (i.v) infusions and in physiological saline for i.p. injections. The KOPr antagonist nor-BNI (In Vitro Technologies) was dissolved in physiological saline and injected s.c. at a volume of 1 mL/kg 24h prior to experimental testing. Dopamine-HCl (Sigma-Aldrich) was dissolved in Milli-Q water. All drugs weights refer to the salt.
2.3. Surgery and self-administration training
Surgery to implant an indwelling i.v. cannula was performed as described previously (Ewald et al., 2017; Gabriele et al., 2012). Five days following surgery, rats underwent self-administration training in standard operant chambers equipped with two levers (Med Associates ENV-001, VT, USA). Depression of the active lever delivered a 12 s infusion of cocaine (0.1 mL; 0.5 mg/kg/i.v.) dissolved in physiological saline containing heparin (3.0 U/mL) accompanied by illumination of a light above the active lever. Rats were trained on a fixed response-1 (FR-1) schedule of reinforcement followed by FR-2 and subsequently to FR-5 for daily 2-h sessions, 6 days per week.
2.3.1. Cocaine-primed reinstatement
Once responding to FR-5 was stable, the effect of prior administration of MP1104 on drug-seeking behavior produced by cocaine was measured. The reinstatement test was conducted in three phases. Phase 1 consisted of cocaine self-administration on FR-5. Rats were moved to phase 2 when responses were within 20% of baseline responses over three consecutive days. Phase 2 was extinction phase where cocaine was replaced with heparinised saline (3 U/mL) and the light cue removed. Animals remained on this schedule until lever responses dropped below 20 active lever press in a single daily session for 3–4 days. Once extinction criteria were met, rats were subjected to a phase 3, reinstatement test, where they were injected with either vehicle or MP1104 (0.3, 1 mg/kg, i.p.) before receiving a priming injection of cocaine (20 mg/kg, i.p.) and the light cue restored. In order to measure whether the reduced drug-seeking effects were mediated via KOPr or DOPr, rats were pre-treated with KOPr (nor-BNI, 10 mg/kg, s.c.) or DOPr (NTI 15, mg/kg, s.c.) antagonists. All treatments were administered using a within-subject, Latin square design (n = 9/group) except for nor-BNI, which was administered last due to its long-lasting effects (Endoh et al., 1992).
2.3.2. Cocaine dose-response self-administration tests
Male Sprague-Dawley rats (n = 6) were trained on FR-2 schedule of cocaine reinforcement. Once the criteria for stable cocaine responding were met (less than 10% variability in number of active lever presses for 3 consecutive days), rats self-administered a full dose range of cocaine (0.03, 0.15, 0.5, 1 and 2 mg/kg per infusion) in a single session. Each comprised a 20 min component of self-administration at each dose, with a 20 min timeout period between cocaine doses. Before each daily cocaine self-administration test, a 30 min extinction period was included. The criteria for stable cocaine responding were a minimum of 10 mg/kg cocaine infusions per session, less than 10% variation in the total number of cocaine infusions for 3 consecutive days, and at least fivefold higher maximal response rates compared with those maintained during extinction (Song et al., 2012). Once all criteria were achieved rats were given i.p. injections of either vehicle or MP1104 (0.3 or 0.6 mg/kg) and tested for their effects.
2.4. Rotating disk electrode voltammetry
Rotating disk electrode voltammetry (RDEV) studies were carried out as previously described (Povlock and Schenk, 1997; Simonson et al., 2015). Briefly, tissue dissected from the dorsal striatum (dStr), and NAc were weighed, finely chopped in ice-cold Krebs buffer (130 mM NaCl,1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4·6H2O, 1.2 mM KH2PO4, 10 mM HEPES, 10 mM D-glucose, pH 7.4) and transferred into micro-centrifuge tubes. Tissue was washed eight times with 37 °C Krebs buffer aerated with carbogen gas (95% O2 & 5% CO2; BOC gasses, Wellington, New Zealand). Samples were placed in the RDEV chamber where the glassy carbon rotating electrode (Pine Instruments, AFMDO3GC, Durham, NC, USA) was lowered and rotated at 2000 rpm. A potential of +450 mV was applied relative to the Ag/AgCl reference electrode (eDAQ, Denistone, NSW, Australia). For the low to infinite trans model (Povlock and Schenk, 1997), multiple additions of increasing concentrations of DA (0.5–4 μM) were added with the current allowed to reach baseline between each addition. For the zero trans model, a single addition of DA (2 μM) was added once the current had reached baseline. For the nor-BNI or NTI additions, tissue was pre-incubated with 1 μM of the antagonist for 30 min in warm (37 °C) aerated Krebs buffer. Uptake data was collected for 10 s starting 1 s after addition of DA and linear regression calculated. Uptake was expressed as pmol/s/g of tissue.
2.5. Locomotor activity
The sedative effects of MP1104 were measured using spontaneous locomotor activity tests. Before testing, rats were habituated in the activity chambers for 30 min, then injected with either vehicle or MP1104 (0.3, 0.6 or 1 mg/kg, i.p.) and placed in the activity chamber for 60 min (n = 8 per group). Horizontal locomotor activity was measured for 60 min. Infrared beam-breaks were recorded as ambulatory counts (Med Associates: ENV-520; SOF-811). Testing was performed in dark between 9:00 and 17:00 h in the presence of white noise (67 dB).
2.6. Conditioned place aversion
Conditioned place aversion (CPA) was performed following previously described methods (Tejeda et al., 2013). The three-chamber apparatus (PanLab, Harvard Apparatus, USA) consisted of two large chambers (30 × 30 × 34 cm) connected by a smaller corridor (8 × 10 × 34 cm) separated by sliding doors. One large chamber had a smooth white floor with black walls and a white stripe pattern while the other had a texture black floor with white walls with a black dot pattern. The corridor was a neutral zone with grey walls and floor illuminated at an intensity of 70 lux and in each conditioning chamber the average light intensity was 20 lux. Experiments were conducted in the presence of white noise. The CPA procedure took place over 9 days. Day 0 was the habituation day where rats were habituated to the CPA apparatus for 15 min. Day 1 was the pre-conditioning day, where rats were allowed free access to both chambers for 15 min. All activities were tracked using SMART 3.0 software (PanLab). Animals that showed over 80% preference for a particular chamber or over 40% preference for the corridor were excluded from testing. On days 2–7 conditioning was conducted using a biased procedure, whereby injections (i.p.) of MP1104 (0.6 mg/kg) or U50,488 (10 mg/kg) was followed by confining the animal in the preferred chamber for 45 min. Vehicle injections were administered in the least preferred chamber on alternate days in a counterbalanced manner (n = 6–7 per group). The DOPr antagonist NTI (15 mg/kg, s.c.) was injected 15 min prior MP1104. The post-conditioning test was conducted on day 8, where rats were placed in the corridor and again allowed free access to both chambers for 15 min. Time spent in each chamber on preconditioning and post-conditioning days were compared to determine changes in preference.
2.7. Elevated plus maze
Time spent in the open arm of an elevated plus maze (EPM) is used as a preclinical model of anxiety (Walf and Frye, 2007). The EPM was made of black plastic and consisted of four arms (50 cm × 10 cm each) elevated 55 cm above the ground. The two open arms consisted a small parapet measuring 2.5 cm in height surrounding them and the two closed arms were enclosed by high black walls (40 cm). Animals were administered MP1104 (0.3 or 0.6 mg/kg), yohimbine (2.5 mg/kg) or vehicle and placed in the centre of the apparatus facing an open arm and activity recorded for 5 min (Sony HDR-SR5E digital camera recorder) (n = 10–13 per group). Time spent on each arm was calculated by the experimenter blinded to the treatment. Prior to testing, rats were habituated to conditions in the testing room for 60 min. Open arm time was calculated when rats had all four paws on the open arm.
2.8. Forced swim test
The forced swim test (FST) was performed using a cylindrical swim chamber 44 cm in height and 20 cm in diameter. Water was maintained at a temperature of 25 ± 1 °C and filled to a depth of 35 cm. Rats were habituated to conditions in the experimental room 60 min before testing (n = 7–8 per group). Before the testing day, rats were habituated to forced swimming behavior for 15 min. On the test day rats were injected with MP1104 (0.3 or 0.6 mg/kg/i.p.) or vehicle before a 5-min testing session. All test sessions were recorded using a Sony HDRSR5E digital camera recorder. Test videos were scored as displaying either climbing, swimming or immobility using SMART 3.0 software (PanLab).
2.9. Statistical analysis
All statistical analyses were performed using GraphPad Prism software version 7 (GraphPad, La Jolla, CA). Data is expressed as the mean ± standard error of mean (SEM). For cocaine-primed reinstatement tests, data was analysed using one-way analysis of variance (ANOVA) with repeated measures. Data for multiple dose cocaine self-administration was analysed using two-way ANOVA followed by Bonferroni’s multiple comparisons test. DA uptake measured by single addition of DA using RDEV techniques were analysed using one-way ANOVA and post-hoc analysis determined by Fisher’s protected least significant difference (LSD). For the low to infinite trans model, uptake values were entered into GraphPad Prism and a nonlinear regression (one-site binding hyperbole) was fitted to each repeat and the Vmax values obtained. For locomotor activity tests, repeated measures oneway ANOVA was used to evaluate time course effects, and to compare total ambulatory counts and a one-way ANOVA was used to analyse EPM and FST experiments. A paired Students-t-test was used to analyse the preconditioning and post conditioning time in CPA. Values of P < 0.05 were considered to be statistically significant.
3. Results
3.1. Anti-cocaine effects of MP1104
Following extinction, cocaine-prime drug-seeking responses were significantly reduced upon MP1104 (0.3 and 1 mg/kg) administration. These effects were dose-dependent (Fig. 2A) (One-way ANOVA; F (5, 48) = 9.613; p < 0.0001). Pre-treatment of rats with the selective KOPr antagonist, nor-BNI (10 mg/kg, s.c.) prevented the attenuation of drug-seeking behavior observed with MP1104 alone (1 mg/kg), whereas, pretreatment with the DOPr antagonist, NTI (15 mg/kg, s.c.) did not attenuate the effects of MP1104 (1 mg/kg). Pretreatment with both nor-BNI and NTI in combination was no different to nor-BNI alone, indicating the effects of MP1104 are KOPr, and not DOPr mediated. MP1104 (0.3 and 0.6 mg/kg), dose-dependently inhibited cocaine self-administration and shifted the cocaine dose-response self-administration curve downward (Fig. 2B). Two-way ANOVA (F (2, 105) = 45.9; p < 0.0001) revealed a significant effect of treatment compared to the vehicle control group.
Fig. 2.
MP1104 attenuates cocaine prime-induced reinstatement of cocaine-seeking in rats and shifts cocaine dose-response self-administration curve downwards. (A) MP1104 (0.3 & 1 mg/kg, i.p.) dose-dependently attenuates cocaine-prime active lever responding compared to vehicle-treated controls which is inhibited by the pretreatment of nor-BNI (10 mg/kg, s.c.). NTI (15 mg/kg, s.c.) has no effect on MP1104-induced responding. Repeated measures ANOVA with Bonferroni’s post-test. ***p < 0.001, ****p < 0.0001, compared to the vehicle; #p < 0.05 compared to MP1104, 1 mg/kg dose, n = 9. (B) MP1104 (0.3 & 0.6 mg/kg, i.p.) significantly shifts the cocaine dose-response self-administration curve downwards. Two-way ANOVA with Bonferroni’s post-test. **p < 0.01, ****p < 0.0001 compared to the vehicle, n = 6. (C) Throughout the self-administration training, the number of active and inactive lever responses remained stable with an active:inactive lever ratio of ≥2:1. All values expressed as mean ± SEM. NTI = Naltrindole; nor-BNI = nor-binaltorphimine.
3.2. MP1104 increases DAT function in rat dorsal striatum and nucleus accumbens tissue
The effects of MP1104 in modulating DAT function was determined using RDEV, performed on minced tissue from the rat dStr and NAc.
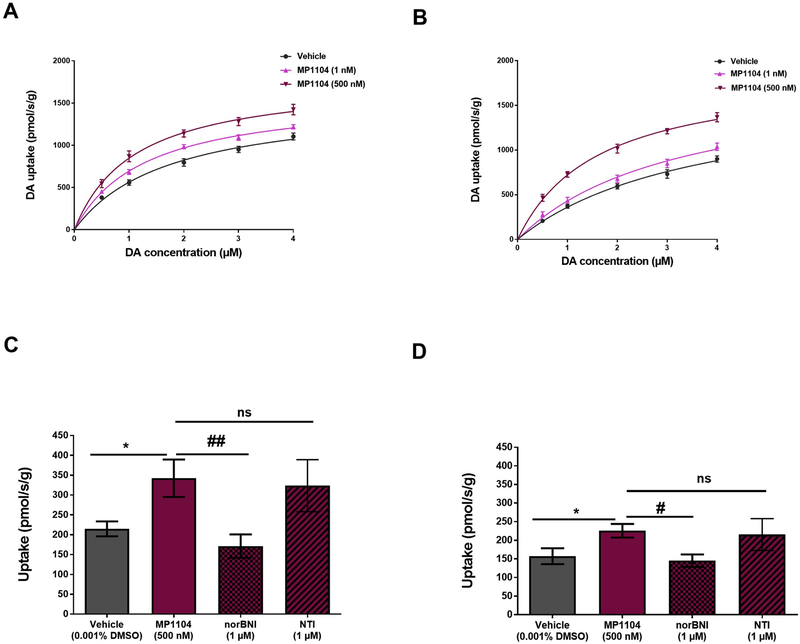
Tissue pre-incubated with MP1104 (1 nM or 500 nM) was evaluated for its ability to modulate DA uptake via DAT. MP1104 at both 1 nM (p < 0.0001) and 500 nM (p < 0.0001) significantly increased DA uptake compared to vehicle treated (0.001% DMSO) dStr tissue (Fig. 3A) (Two-way ANOVA with Bonferroni’s post-test). MP1104 significantly increased Vmax (expressed as pmol/s/g) at 1 nM (1598 ± 59.97, p < 0.0001 (mean ± SEM) and 500 nM (1790 ± 103.6, p < 0.0001) compared to the vehicle control (1533 ± 105.8). Similar effects were also seen in NAc tissue compared to vehicle treated tissue (Vmax: MP1104 at 1 nM (1833 ± 235.2, p < 0.01); 500 nM (1885 ± 110.8, p < 0.0001) compared to the vehicle control (1696 ± 222.3) (Fig. 3B). The effect of the KOPr antagonist, nor-BNI (1 μM) on the changes produced by MP1104 were evaluated using a single addition of DA (2 μM) using the RDEV zero trans model. Nor-BNI significantly inhibited the MP1104-induced increase DA uptake in both the rat dStr (Fig. 3C) and NAc (Fig. 3D); whereas NTI had no effect on MP1104 induced increases in DAT function in the dStr or NAc. One-way ANOVA with Fisher’s protected least significant difference (LSD) test (p < 0.05).
Fig. 3.
MP1104 shows increase of DA uptake in rat dStr and NAc tissues. DA uptake was measured using the low to infinite trans model. MP1104 (1 nM and 500 nM) showed a significant increase in DA uptake in dStr (A) and NAc (B) compared to vehicle-treated control (two-way ANOVA with Bonferroni’s post-test). In zero trans model, MP1104 (500 nM) increased DA uptake in the dStr (C) and NAc (D) when tested using a single 2 μM addition of DA. Pre-treatment of tissue suspensions with nor-BNI reversed the MP1104 induced increase in DA uptake, whereas NTI had no effect. One-way ANOVA with Fisher’s protected least significant difference (LSD) test, *p < 0.05 compared to the vehicle; #p < 0.05, ##p < 0.01 compared to MP1104 (500 nM). Values presented as mean ± SEM, n = 5–9 per group. DA = dopamine; dStr = dorsal striatum; NAc = nucleus accumbens; nor-BNI = nor-binaltorphimine; ns = non-significant; NTI = naltrindole.
3.3. Effects of MP1104 on locomotor activity
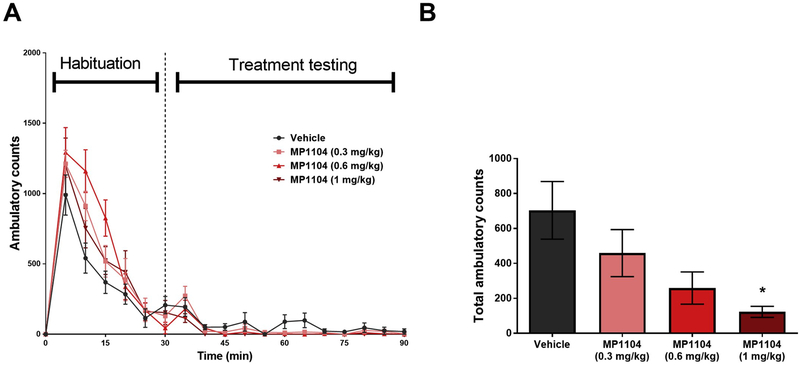
The time course effects of spontaneous locomotor activity showed a non-significant interaction between time and MP1104 (Fig. 4A, F (3,72) = 0.07611, p = 0.9727). Fig. 4B shows that MP1104 (0.3 mg/kg and 0.6 mg/kg) showed a non-significant trend in decreasing ambulatory counts in the 60 min locomotor activity test (p = 0.4517; and p = 0.0842; respectively). However, at 1 mg/kg, MP1104 shows significant sedative effects (p < 0.05; One-way ANOVA with Bonferroni’s post-test).
Fig. 4.
Sedative effects of MP1104. (A) Effects of MP1104 (0.3, 0.6 & 1 mg/kg, i.p.) on ambulatory counts during and following habituation are shown over time (One-way ANOVA). (B) MP1104 (0.3 & 0.6 mg/kg, i.p.) showed non-significant decrease in ambulatory counts compared to vehicle treatment during the treatment testing time (60 min). One-way ANOVA. *p < 0.05 compared to the vehicle. Values presented as mean ± SEM, n = 8 per group.
3.4. Effect of MP1104 on conditioned place aversion
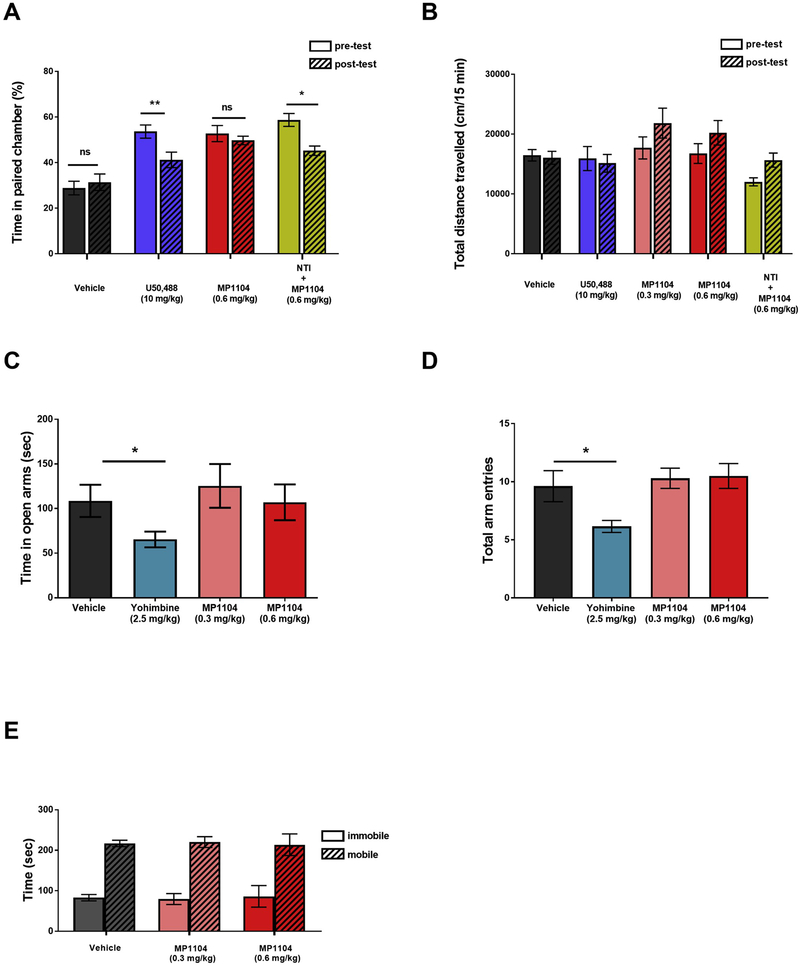
Rats treated with MP1104 (0.6 mg/kg, i.p.) produced no significant changes in time spent in the paired chamber (t (6) = 0.586, p = 1.385) unlike the KOPr agonist U50,488 (10 mg/kg, i.p.), used as a positive control, which showed that rats spent significantly less time in the U50,488 paired chamber (t (7) = 4.100, p = 0.0046) (Fig. 5A). To test whether the DOPr activity of MP1104 was responsible for negating the aversive-like effects we blocked the DOPr agonist activity of MP1104 using NTI (15 mg/kg, s.c.). Upon blockade with NTI, MP1104 showed significantly less time in the paired chamber (t (6) = 3.026, p = 0.0232).
Fig. 5.
MP1104 shows reduced aversive; anxiogenic and pro-depressive effects in rats. (A) MP1104 (0.6 mg/kg, i.p.) shows no change in time spent in the MP1104-paied chamber. Pre-treatment with the delta antagonist NTI (15 mg/kg) or treatment with selective KOPr agonist U50,488 decreases the time spent in the MP1104-paied chamber. Paired Student’s t-test *p < 0.05, **p < 0.01 compared to vehicle, n = 6–7 per group. (B) Total distance travelled in CPA tests showed no significant changes in locomotion following MP1104 (0.3 and 0.6 mg/kg, i.p.) administration. (C) In EPM tests, MP1104 (0.3 & 0.6 mg/kg, i.p.) showed no effect in open arm times or (D) the total number of arm entries. (C) Yohimbine (2.5 mg/kg, i.p) showed both a decrease in time spent in the open arm and (D) arm entries. One-way ANOVA; *p < 0.05 compared to vehicle, n = 10–13 per group. (E) In the FST, MP1104 (0.3 and 0.6 mg/kg, i.p.) showed no changes in mobility (p > 0.05) or immobility (p > 0.05); One-way ANOVA; n = 7–8 per group. All data expressed as mean ± SEM. CPA = conditioned place aversion; EPM = elevated plus maze; FST = forced swim test; ns = non-significant; NTI = naltrindole.
3.5. Effect of MP1104 on anxiety in the elevated plus maze
The effects of MP1104 (0.3 and 0.6 mg/kg) in the time spent in the open arms (Fig. 5C) and the total number of arm entries (Fig. 5D) of the EPM are compared to the vehicle. MP1104 at both 0.3 and 0.6 mg/kg showed no significant changes in time spent in the open arm, nor in the total arm entries (p = > 0.9999 for both time spent in the open arm and open arm entries at both 0.3 and 0.6 mg/kg doses, One-way ANOVA). In contrast, the anxiogenic drug, yohimbine (2.5 mg/kg), showed a significant decrease in time spent in the open arms compared to the vehicle-treated control group (*p < 0.05, One-way ANOVA) and the number of total arm entries (*p = 0.05, One-way ANOVA with Bonferroni’s post-test).
3.6. Effect of MP1104 in the forced swim test
The dose-response effects of MP1104 (Fig. 5E) on depressive-like behavior was evaluated in male Sprague-Dawley rats in the FST. MP1104 at both 0.3 mg/kg (t (6) = 0.1171, p = 0.9106) and 0.6 mg/kg (t (6) = 0.2783, p = 0.7901) showed no significant changes in the swimming behavior in the FST compared to vehicle-treated controls.
4. Discussion
KOPr agonists are known to counteract the reinforcing effects of cocaine (Kivell et al., 2014b; Morani et al., 2009; Schenk et al., 1999; Shippenberg et al., 2007). Acute administration of KOPr agonists reduce cocaine self-administration in rats (Glick et al., 1995; Schenk et al., 2000) and rhesus monkeys (Mello and Negus, 1998, 2000), and morphine self-administration in both rats (Glick et al., 1995) and mice (Kuzmin et al., 1997). Administration of KOPr agonists also decrease the rewarding properties of ethanol in rats (Logrip et al., 2009).
MP1104 is a mixed opioid agonist that binds to MOPr, DOPr and KOPr receptors, showing 3-fold and 13-fold greater affinity towards KOPr over MOPr and DOPr respectively (Váradi et al., 2015). Despite binding to MOPr, DOPr and KOPr, the analgesic effects have been shown to be mediated through activation of both KOPr and DOPr. MP1104 was shown to exhibit 15-fold greater antinociceptive potency (ED50 = 0.33 mg/kg) in C57BL/6J mice compared to morphine. In CD1 mice MP1104-induced antinociception was attenuated following administration of DOPr antagonists and also in KOPr knock-out mice. However, analgesia was not significantly altered in MOPr knock-out mice (Váradi et al., 2015). Together these data indicate that the anti-nociceptive effects of MP1104 in vivo are mediated via agonism at both KOPr and DOPr and that the anti-nociceptive effects of MP1104 are not mediated via actions at MOPr (Váradi et al., 2015). Further evaluation of MP1104 metabolism is required to evaluate these in vitro and in vivo effects. While it is possible that hydrolysis of the amide bond may lead to the formation of naltrexamine (a MOPr antagonist), there is currently no evidence for this.
This study evaluates the anti-cocaine effects of MP1104 in rats to determine the effects of mixed opioid agonist activity on both cocaine-seeking and cocaine-taking behavior in rats using cocaine self-administration models. A summary of anti-cocaine effects and behavioural effects of MP1104 are presented in Table 1. Previously, a study in mice showed that MP1104 (1 mg/kg/s.c.) attenuated the rewarding effects of cocaine in conditioned place preference tests (Váradi et al., 2015). However, self-administration models are considered the gold standard model for studying drug abuse preclinically, due to high predictive validity for human drug use (O’connor et al., 2011). Studies using rat models of stress, cue and drug-primed reinstatement are increasingly utilised to understand drug use in humans (Bossert et al., 2013). The drug-primed reinstatement model utilised in this study measures the drug-seeking behavior in rats trained to self-administer cocaine. We found that animals reinstated cocaine-seeking behavior when exposed to a priming injection of cocaine (20 mg/kg, i.p.). The multiple cocaine dose response self-administration paradigm was used to measure the drug-taking behavior in rats and fully explore the effects of MP1104 on cocaine reward. Because the light stimulus is removed during extinction, and present during reinstatement, reinstatements tests measure a combination of both drug and light cue responding in this model. MP1104 dose-dependently (0.3 and 1 mg/kg) reduced cocaine-primed reinstatement of drug-seeking behavior following extinction, an effect that was found to be KOPr mediated (Fig. 2A). KOPr agonists attenuate the rewarding properties of drugs of abuse. However, DOPr agonists are known to increase the rewarding properties of psychostimulants (Pradhan et al., 2011). Our data shows that the DOPr agonist properties of MP1104 did not negate the KOPr-mediated attenuation of drug-seeking behaviors. This is likely because of higher KOPr potency (EC50 = 0.027 ± 0.002 nM) in comparison to DOPr (EC50 = 0.41 ± 0.11 nM). From this data we can conclude that delta opioid receptors do not contribute to effects of MP1104 on cocaine reinstatement. It is also noteworthy that combined KOPr and DOPr antagonism only partially blocked the effects of MP1104 on cocaine reinstatement, suggesting additional mechanisms, other than KOPr or DOPr may also be involved in attenuating cocaine-seeking behavior in the rat. While contributions of MOPr cannot be ruled out, MP1104 has not been shown to have any effects on MOPr in vivo (Váradi et al., 2015).
Table 1.
Summary of anti-cocaine, analgesic, and side effects produced by MP1104 in rats and mice.
| Effects in rats | Effects in mice | |
|---|---|---|
| Anti-cocaine effects | ||
| Cocaine-prime reinstatement | ↓ | nd |
| Cocaine self-administration | ↓ | nd |
| Cocaine place preference | nd | ↓ (Váradi et al., 2015) |
| Modulation of DAT function (rat tissue) | ||
| Dorsal striatum | ↑ | nd |
| Nucleus accumbens | ↑ | nd |
| Analgesic effects | ||
| Tail-flick | nd | ↑ ED50 = 0.33 mg/kg (Váradi et al., 2015) |
| Side effects | ||
| Aversion (CPA) | ne (0.6 mg/kg) | ne (1 mg/kg) (Váradi et al., 2015) |
| Anxiety (EPM) | ne (0.6 mg/kg) | nd |
| Depression (FST) | ne (0.6 mg/kg) | nd |
| Locomotor activity | ne (0.3, 0.6 mg/kg) ↓ (1 mg/kg) |
nd |
Summary of anti-cocaine, analgesic, and side effects produced by MP1104 in rats and mice.
↑ = Increased effect; ↓ = attenuation; n.e. = no effect; n.d. = not determined; DAT = dopamine transporter; CPA = conditioned place aversion; EPM = elevated plus maze; FST = forced swim test.
Previous studies have shown that, nor-BNI, when administered alone did not alter cocaine self-administration behavior in rats (10 mg/kg, s.c.) (Glick et al., 1995) or in rhesus monkeys at 3.2 mg/kg, i.v (Negus et al., 1997) or at 3.2 or 10 mg/kg, i.m. (Hutsell et al., 2016). Previous studies have administered intra-NAc infusions of NTI (1 μg) to male Sprague-Dawley rats trained to self-administer cocaine (0.75 mg/kg, 10 days, 6 h/day), and showed no significant effect on cocaine seeking behaviours (Dikshtein et al., 2013), suggesting that endogenous levels of KOPr and DOPr activating peptides have limited effects on cocaine-seeking behavior, although this concept warrants full investigation. We cannot rule out that antagonists administered in this study may inhibit contributions made by endogenous KOPr and DOPr ligands.
We further evaluated the effects of MP1104 on cocaine self-administration utilising the multiple-dose cocaine self-administration assay. Animals typically display an inverted U-shaped dose-response curve (Panlilio et al., 2006; Xi et al., 2010), whereby low doses and higher doses of cocaine have little response, whereas a mid-range of cocaine doses show high levels of self-administration. In this study, we found that cocaine infusions between 0.15 mg/kg and 0.5 mg/kg showed high levels of responding (Fig. 2B). Throughout the entire cocaine dose range tested (0.03–2 mg/kg/infusion), administration of MP1104 (0.3 and 0.6 mg/kg) resulted in a dose-dependent downward shift of the cocaine dose-response curve, indicating that the reinforcing effects of cocaine are attenuated by MP1104.
Having affirmed the anti-cocaine properties of MP1104, we further evaluated the effects of MP1104 on DA uptake by DAT in minced tissue preparations from the rat dStr and NAc. Simonson et al. (2015), using the same RDEV methods utilised in this study, showed that brain regions have varying rates of DA uptake, with the striatum showing the largest uptake, followed by the NAc, then the medial pre frontal cortex (Simonson et al., 2015). These results directly correlate with the amount of DAT that is expressed in each brain region, with the striatum having the highest DAT density, followed by the NAc and then the medial pre frontal cortex (Freed et al., 1995; Sesack et al., 1998; Thompson et al., 2000). Moreover, there is evidence of low DAT expression in the ventral tegmental area, as DAT is localised to synaptic regions (Shimada et al., 1992; Ciliax et al., 1995). Hence, in this study we chose to evaluate dStr and NAc brain regions for DA uptake and DAT functions.
Previous reports have shown that KOPr agonists attenuate DA release (Zhang et al., 2004a) and increase DA uptake via DAT (Thompson et al., 2000). Although these previous studies have utilised pure KOPr agonists, we sought to determine whether the mixed actions of MP1104 were sufficient to modulate DAT function. Here, we show that acute administration of MP1104 to tissue from the rat dStr and NAc increased DA uptake by increasing DAT function (Fig. 3). It is likely that these effects are due to direct interactions of KOPr and DAT, as studies evaluating co-expression of KOPr and DAT found that KOPr and DAT form interacting complexes in striatal synaptosomes. This KOPr and DAT interaction is enhanced by administration of KOPr agonists (Kivell et al., 2014a). Several studies have also reported that administration of KOPr agonists and endogenous KOPr activation reduces DA release (Carlezon et al., 2006; Gray et al., 1999), and there are differences in the regulation of DAT in NAc and dStr brain regions (Thompson et al., 2000). Moreover, DA regulation between these regions also vary (Richards and Zahniser, 2009; Wu et al., 2001). In this study, we showed MP1104 rapidly increases DAT function in both the dStr and NAc (Fig. 3A and B). This effect is due to an increase in Vmax, which indicates an increase in cell-surface DAT expression (Simonson et al., 2015). The MP1104-mediated increase in DA reuptake by DAT was nor-BNI reversible, indicating that the effect is KOPr mediated (Fig. 3C and D). MP1104 increased DAT function in both dStr and NAc tissue (Fig. 3A and B), an indication that DA levels are reduced in these regions following MP1104 addition. While further exploration of the cellular mechanisms underlying the ability of MP1104 to differentially regulate the anti-cocaine effects from other behavioural effects such as locomotion is warranted. Differential expression of regulators of KOPr and DOPr signalling such as G-proteins and regulators of G-proteins are likely to also play a role. It is also possible that different brain regions may display differences in signalling bias. Full evaluation of signalling bias at MOPr, KOPr and DOPr is needed in vivo to evaluate if this contributes to the behavioural effects observed in this study.
Many studies have reported that KOPr agonists attenuate the rewarding effects of drugs of abuse, however, side-effects limit their clinical development (Mello and Negus, 2000; Walsh et al., 2001a, 2001b). There are currently no FDA approved therapeutics for the treatment of psychostimulant abuse. Therefore, in this study, we also evaluated the side-effects of MP1104 to determine whether the mixed opioid actions reduced the aversive, anxiogenic, and pro-depressive effects typically seen with potent and selective KOPr agonists.
Traditional KOPr agonists such as U50,488 decrease spontaneous locomotor activity in rodents (Brust et al., 2016). Clinical studies have revealed that KOPr agonists also cause sedation (Knoll and Carlezon, 2010; Pfeiffer et al., 1986). In this study, a low dose of MP1104 (0.3 mg/kg and 0.6 mg/kg) showed a non-significant trend towards sedative effects, whereas MP1104 at the higher 1 mg/kg dose caused significant sedative effects in rats (Fig. 4B). Because baseline ambulatory counts in this test were low, a possible floor effect may limit observations of sedation. Therefore, we also evaluated additional measures of locomotor behavior. It is noteworthy that the total distance travelled in CPA (Fig. 5B) and the total arm entries in EPM (Fig. 5D) were not attenuated by MP1104 (0.3 and 0.6 mg/kg), additional supporting methods we utilised to evaluate motor behaviors. We therefore conclude that at the 0.3 mg/kg dose of MP1104 which attenuated drug-seeking behavior in rats, was not due to sedation or the inability of rats to perform the operant task. However, at higher doses the reduced drug-seeking behavior may be due to the sedative effects exerted by MP1104. The reduced rewarding effects of cocaine following KOPr agonist administration is believed to be due to their ability to cause aversion and/or dysphoric effects (Koob et al., 2014; Shippenberg et al., 2009). However, recently, it has become evident that it may be possible to separate the desirable therapeutic effects from side-effects, particularly with G-protein coupled receptors, with each chemical compound having unique effects on down-stream signalling pathways and, ultimately in vivo behavioural effects.
While many strategies have been explored to mitigate the side-effects of KOPr agonists, the recent concept includes the development of G-protein biased agonists (Kenakin, 2007; Kenakin and Christopoulos, 2013; Urban et al., 2007), due to the fact that β-arrestin-mediated signalling is largely responsible for the negative side-effects exerted by KOPr agonists (Bruchas and Chavkin, 2010; Bruchas and Roth, 2016; Chavkin et al., 2014; Kivell et al., 2014b). Previous reports using RB-64 have shown that sedative effects were mediated via β-arrestin, however, aversion in the CPA tests were present in both wild-type and β-arrestin KO mice indicating that the aversion is partly regulated through G-protein activation (White et al., 2015). Aversive effects have also been shown to be regulated via p38 mitogen activated protein kinase signalling pathways (Bruchas et al., 2007; Ehrich et al., 2015; Zan et al., 2016).
In order to avoid undesirable effects associated with activation of KOPr, simultaneous modulation of multiple opioid receptors with opposing actions is a strategy suggested by many researchers to be key in developing effective and safe KOPr medications. Many studies have investigated the utility of combinations of opioid receptor agonists and mixed opioid agonists (MOPr, KOPr and/or DOPr) to balance the therapeutic and the adverse effects (Balboni et al., 2002; Dietis et al., 2009; Morphy and Rankovic, 2009). For example, KOPr agonists are able to attenuate the tolerance effects of MOPr agonists such as morphine (Yamamato et al., 1988; Tao et al., 1994). However, only a few have investigated DOPr/KOPr combinations. For example, Taylor et al. (2015), showed that systemic administration of the KOPr agonist U50,488 (10 mg/kg, s.c.) to C57Bl/6J mice produced anti-nociception in the warm-water tail withdrawal assay through a stress induced mechanism. Moreover, this effect was blocked by distinct classes of anxiolytics, including the DOPr agonist SNC 80 (5 mg/kg, s.c.) (Taylor et al., 2015). This demonstrated that the anxiolytic effects of DOPr agonists can inhibit KOPr mediated stress effects. However, many DOPr agonists produce seizures which has limited combination approaches.
Few compounds possessing dual KOPr/DOPr activity have been reported previously. Tang et al. (2010) identified KDA-16, an analogue of ICI-199,441 that showed mixed KOPr and DOPr activity. KDA-16 displayed spinal antinociception via selective activation of KOPr-DOPr heteromers in mice (Tang et al., 2010). In contrast, KDN-21, which links KOPr antagonist, 5′- guanidinonaltrindole to DOPr antagonist, NTI, did not show antinociceptive effects in mice (Bhushan et al., 2004). However, it showed heterodimeric KOPr/DOPr interactions in human embryonic kidney 293 cells expressing rat DOPr and mouse KOPr (Xie et al., 2005) and in mouse spinal cord (Bhushan et al., 2004). KDAN-18, which links KOPr agonist, ICI-199,441 and DOPr antagonist, NTI, showed anti-nociceptive effects in tail-withdrawal assays in male mice (Daniels et al., 2005). Although these previous studies assessed the antinociceptive effects of dual KOPr/DOPr ligands, there no studies reporting the effects on reward. To address this gap, in this study we evaluated the anti-cocaine effects of our novel dual KOPr/DOPr agonist, MP1104 in male Sprague-Dawley rats.
To evaluate whether the dual KOPr and DOPr agonist properties of MP1104 reduced the aversive effects, MP1104 was evaluated in CPA tests. The prototypical KOPr agonist U50,488 showed significant reduction in time spent in the U50,488-paired chamber indicating aversive properties (Fig. 5A). These findings are consistent with previous literature reports in rats (Göktalay et al., 2006) and mice (Skoubis et al., 2001). However, rats treated with MP1104 showed no change in time spent in the MP1104-paired chamber, indicating it has no aversive properties at 0.6 mg/kg. Following antagonism of DOPr by the selective DOPr antagonist NTI (15 mg/kg, s.c), MP1104 showed significant aversive-like effects, with reduced time spent in the MP1104-paired chamber. This gives strong support for the development of dual KOPr/DOPr agonists as both non-addictive analgesics and anti-addiction therapies to reduce the side-effects seen with pure KOPr agonists.
To examine the effects of MP1104 on preclinical measures of anxiety we used the EPM test. A single administration of MP1104 (0.3 or 0.6 mg/kg) was shown to have no effect. In contrast, yohimbine, an α2 adrenoceptor antagonist showed a reduction in time spent in the open arm and the total number of arm entries in the EPM (Fig. 5C and D), effects consistent with previous reports on yohimbine in animals (Pellow et al., 1985). MP1104 produced no anxiogenic effects at the dose that was effective in attenuating cocaine-seeking (0.3 mg/kg). We also show that MP1104 showed no pro-depressive effects in the FST compared to vehicle-treated controls, unlike previous reports utilising pure KOPr agonists such as Salvinorin A and a structural analogue methoxymethyl ether Sal B (Carlezon et al., 2006; Morani et al., 2013). Both MP1104 doses (0.3 and 0.6 mg/kg) showed no changes in mobility or immobility properties (Fig. 5E). Previously, studies have reported that selective DOPr agonists have both anxiolytic and anti-depressant effects (Filliol et al., 2000; Saitoh et al., 2004; Torregrossa et al., 2006; Vergura et al., 2008). However, KOPr agonsits are involved in stress, fear, anxiety, and depressive-like behaviors (Bruchas et al., 2010; Gillett et al., 2013; Knoll and Carlezon, 2010). We have shown that MP1104, being a mixed opioid receptor agonist at both these receptors, attenuated behavioural side-effects, while maintaining potentially therapeutic anti-cocaine effects. It is important to note that DOPr agonists produce seizures (Comer et al., 1993; Lutz and Kieffer, 2013), which limits their clinical use. MP1104, however, produced no seizures at doses 30 times its antinociceptive ED50 value in mice (Váradi et al., 2015). We conclude that MP1104 has an improved side-effect profile compared to traditional KOPr agonists.
5. Conclusion
The mixed KOPr/DOPr agonist, MP1104 attenuates cocaine self-administration and produces a downward shift in cocaine dose-response curves in rats. One of the likely mechanisms we identified to be partly responsible for this effect is the ability of MP1104 to increase DA uptake via DAT in the NAc, a cellular mechanism that attenuates cocaine-induced increases in DA. We also show that MP1104 attenuates drug-seeking behavior in rats at doses that do not cause aversion, anxiety or depression. Moreover, we demonstrated for the first time that the DOPr agonist properties of MP1104 are able to negate the aversive effects produced by KOPr agonism. This study provides strong evidence for the development of mixed opioid agonists to reduce KOPr-mediated side-effects and has implications for the development of non-addictive KOPr analgesics without side-effects which limits current clinical utilisation of KOPr agonists.
HIGHLIGHTS.
MP1104 is a 3-Iodobenzoyl naltrexamine analogue with potent mixed kappa and delta opioid agonist activity in vivo.
MP1104 attenuates drug-primed reinstatement of drug-seeking via kappa opioid receptor dependent mechanisms.
MP1104 increases dopamine transporter uptake of dopamine in the dorsal striatum and nucleus accumbens.
Delta opioid receptor activity counteracts the kappa opioid side-effects of MP1104.
Funding sources
Health Research Council of New Zealand (Explorer; 2016 to BMK); Wellington Medical Research Foundation (2017 to BMK). Victoria University of Wellington provided a Postgraduate Research Scholarship (to DVA). NIH grants DA045884–01, DA046487–01, AA026949–01 and W81XWH-17–1-0256 (Office of the Assistant Secretary of Defense for Health Affairs through the (Peer Reviewed Medical Research Program)) and start-up funds from Center for Clinical Pharmacology, St. Louis College of Pharmacy and Washington University to SM, DA06241 from NIH to GWP. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. Funding agencies had no input into study design; collection, analysis or interpretation of data; or in the writing of this manuscript; or decision to submit the article for publication.
Abbreviations:
- KOPr
kappa opioid receptor
- DOPr
delta opioid receptor
- MOPr
mu opioid receptor
- DAT
dopamine transporter
- DA
dopamine
- dStr
dorsal striatum
- NAc
nucleus accumbens
- RDEV
rotating disk electrode voltammetry
- CPA
conditioned place aversion
- EPM
elevated plus maze
- FST
forced swim test
- nor-BNI
nor-binaltorphimine
- NTI
Naltrindole
- DMSO
dimethyl sulfoxide
Footnotes
This article is part of the Special Issue entitled ‘Opioid Neuropharmacology: Advances in treating pain and opioid addiction’.
Conflicts of interest
Both GWP and SM are co-founders of Sparian BioSciences. All authors report no other biomedical financial interests or potential conflicts of interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2019.02.010.
References
- O’connor EC, Chapman K, Butler P, Mead AN, 2011. The predictive validity of the rat self-administration model for abuse liability. Neurosci. Biobehav. Rev 35, 912–938. 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR, 2015. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87, 1063–1077. 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand JP, Montgomery D, 2018. Multifunctional opioid ligands. Handb. Exp. Pharmacol 10.1007/164_2018_104. [DOI] [PubMed] [Google Scholar]
- Ananthan S, 2006. Opioid ligands with mixed μ/δ opioid receptor interactions: an emerging approach to novel analgesics. AAPS J 8, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni G, Guerrini R, Salvadori S, Bianchi C, Rizzi D, Bryant SD, Lazarus LH, 2002. Evaluation of the Dmt− Tic pharmacophore: conversion of a potent δ-Opioid receptor antagonist into a potent δ agonist and ligands with mixed properties. J. Med. Chem 45, 713–720. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI, 2005. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology 183, 118–126. 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS, 2004. A bivalent ligand (KDN-21) reveals spinal δ and κ opioid receptors are organized as heterodimers that give rise to δ1 and κ 2 phenotypes. selective targeting of δ− κ heterodimers. J. Med. Chem 47 (12), 2969–2972. 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- Bidlack JM, 2014. Mixed kappa/mu partial opioid agonists as potential treatments for cocaine dependence. Adv. Pharmacol 69, 387–418. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y, 2013. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berlin) 229, 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C, 2010. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berlin) 210, 137–147. 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Roth BL, 2016. New technologies for elucidating opioid receptor function. Trends Pharmacol. Sci 37, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C, 2007. Stress-induced p38 mitogen-activated protein kinase activation mediates κ-opioid-dependent dysphoria. J. Neurosci 27, 11614–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas M, Land B, Chavkin C, 2010. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L,Stahl EL, Cameron MD, Scarry SM, Aubé J, Jones SR, Martin TJ, Bohn LM, 2016. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci. Signal 9, ra117 10.1126/scisignal.aai8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM, 2006. Depressive-like effects of the κ-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J. Pharmacol. Exp. Ther 316, 440–447. 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Castro DC, Berridge KC, 2014. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J. Neurosci 34, 4239–4250. 10.1523/JNEUROSCI.4458-13.2014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Schattauer SS, Levin JR, 2014. Arrestin-mediated activation of p38 MAPK:molecular mechanisms and behavioral consequences. Handb. Exp. Pharmacol 219, 281–292. 10.1007/978-3-642-41199-1_14. [DOI] [PubMed] [Google Scholar]
- Che T, Majumdar S, Zaidi SA, Ondachi P, McCorvy JD, Wang S, Mosier PD, Uprety R, Vardy E, Krumm BE, Han GW, Lee MY, Pardon E, Steyaert J, Strachan RT, Tribo AR, Pasternak GW, Carroll F,I, Stevens RC, Cherezov V, Katritch V, Wacker D, Roth BL, 2018. Structure of the nanobody-stabilized active state of the kappa opioid receptor. Cell 172, 55–67. e15. 10.1016/j.cell.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI, 1995. The dopamine transporter: immunochemical characterization and localization in brain. J. Neurosci 15 (3), 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Hoenicke E, Sable A, McNutt R, Chang K, De Costa B, Mosberg H, Woods J, 1993. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J. Pharmacol. Exp. Ther 267, 888–895. [PubMed] [Google Scholar]
- Crowley NA, Kash TL, 2015. Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 62, 51–60. 10.1016/j.pnpbp.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS, 2005. A bivalent ligand (KDAN-18) containing δ-antagonist and κ-agonist pharmacophores bridges δ2 and κ1 opioid receptor phenotypes. J. Med. Chem 48 (6), 1713–1716. 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise R, 1993. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J. Pharmacol. Exp. Ther 266, 1236–1246. [PubMed] [Google Scholar]
- Dietis N, Guerrini R, Calo G, Salvadori S, Rowbotham D, Lambert D, 2009. Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile. Br. J. Anaesth 103, 38–49. 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- Dikshtein Y, Barnea R, Kronfeld N, Lax E, Roth-Deri I, Friedman A, Gispan I, Elharrar E, Levy S, Ben-Tzion M, Yadid G, 2013. β-endorphin via the delta opioid receptor is a major factor in the incubation of cocaine craving. Neuropsychopharmacology 38 (12), 2508–2514. 10.1038/npp.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, Zweifel LS, Kieffer BL, Phillips PE, Chavkin C, 2015. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J. Neurosci 35, 12917–12931. 10.1523/JNEUROSCI.2444-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H, 1992. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch. Int. Pharmacodyn. Ther 316, 30–42. [PubMed] [Google Scholar]
- Ewald AW, Bosch PJ, Culverhouse A, Crowley RS, Neuenswander B, Prisinzano TE, Kivell BM, 2017. The C-2 derivatives of salvinorin A, ethoxymethyl ether Sal B and β-tetrahydropyran Sal B, have anti-cocaine properties with minimal side- effects. Psychopharmacology (Berlin) 234, 2499–2514. 10.1007/s00213017-4637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL, 2000. Mice deficient for δ-and μ-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet 25 (2), 195 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Freed C, Revay R, Vaughan RA, Kriek E, Grant S, Uhl GR, Kuhar MJ, 1995. Dopamine transporter immunoreactivity in rat brain. J. Comp. Neurol 359 (2), 340–349. 10.1002/cne.903590211. [DOI] [PubMed] [Google Scholar]
- Gabriele A, Pacchioni AM, See RE, 2012. Dopamine and glutamate release in the dorsolateral caudate putamen following withdrawal from cocaine self-administration in rats. Pharmacol. Biochem. Behav 103 (2), 373–379. 10.1016/j.pbb.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF, 2008. Antinociceptive and Adverse Effects of μ-and κ-Opioid Receptor Agonists: A Comparison of Morphine and U50488-H. Basic Clin. Pharmacol. Toxicol 103, 419–427. 10.1111/j.1742-7843.2008.00306.x. [DOI] [PubMed] [Google Scholar]
- Gillett K, Harshberger E, Valdez GR, 2013. Protracted withdrawal from ethanol and enhanced responsiveness stress: regulation via the dynorphin/kappa opioid receptor system. Alcohol 47, 359–365. 10.1016/j.alcohol.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Sydney A, 1995. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res 681, 147–152. 10.1016/0006-8993(95)00306-B. [DOI] [PubMed] [Google Scholar]
- Göktalay G, Cavun S, Levendusky MC, Hamilton JR, Millington WR, 2006. Glycyl-glutamine inhibits nicotine conditioned place preference and withdrawal. Eur. J. Pharmacol 530, 95–102. 10.1016/j.ejphar.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS, 2017. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatr 74, 911–923. 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF, 1999. The K-Opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J. Neurochem 73, 1066–1074. [DOI] [PubMed] [Google Scholar]
- Greedy BM, Bradbury F, Thomas MP, Grivas K, Cami-Kobeci G, Archambeau A, Bosse K, Clark MJ, Aceto M, Lewis JW, Traynor JR, Husbands SM, 2013. Orvinols with mixed kappa/mu opioid receptor agonist activity. J. Med. Chem 56, 3207–3216. 10.1021/jm301543e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A, 1998. Opioid reward mechanisms: a key role in drug abuse? Can. J. Physiol. Pharmacol 76, 252–258. 10.1139/y98-017. [DOI] [PubMed] [Google Scholar]
- Hutsell BA, Cheng K, Rice KC, Negus SS, Banks ML, 2016. Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addict. Biol 21 (2), 360–373. 10.1111/adb.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K, McLaughlin J, Carroll F, Damaj M, 2013. Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berlin) 226, 763–768. 10.1007/s00213-012-2716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T, 2007. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol. Sci 28, 407–415. 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A, 2013. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat. Rev. Drug Discov 12, 205–216. 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- Kivell B, Uzelac Z, Sundaramurthy S, Rajamanickam J, Ewald A, Chefer V, Jaligam V, Bolan E, Simonson B, Annamalai B, Mannangatti P, Prisinzano TE, Gomes I, Devi LA, Jayanthi LD, Sitte HH, Ramamoorthy S, Shippenberg TS, 2014a. Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology 86, 228–240. 10.1016/j.neuropharm.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivell BM, Ewald AW, Prisinzano TE, 2014b. Salvinorin A analogs and other kappa-opioid receptor compounds as treatments for cocaine abuse. Adv. Pharmacol 69, 481–511. 10.1016/B978-0-12-420118-7.00012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA, 2010. Dynorphin, stress, and depression. Brain Res 1314, 56–73. 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2009. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56, 18–31. 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 1997. Drug abuse: hedonic homeostatic dysregulation. Science 278, 52–58. [DOI] [PubMed] [Google Scholar]
- Koob G, Volkow N, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW Jr., George O, 2014. Addiction as a stress surfeit disorder. Neuropharmacology 76, 370–382. 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin AV, Semenova S, Gerrits MA, Zvartau EE, Van Ree JM, 1997. κ-Opioid receptor agonist U50, 488H modulates cocaine and morphine self-administration in drug-naive rats and mice. Eur. J. Pharmacol 321 (3), 265–271. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C, 2009. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc. Natl. Acad. Sci 106, 19168–19173. 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D, 2009. Blockade of ethanol reward by the kappa opioid receptor agonist U50, 488H. Alcohol 43, 359–365. 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz P-E, Kieffer BL, 2013. Opioid receptors: distinct roles in mood disorders. Trends Neurosci 36, 195–206. 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jones RM, Portoghese PS, Carlezon WA, 2003. Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther 305, 323–330. 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Devi LA, 2018. Strategy for making safer opioids bolstered. Nature 553, 286–288. 10.1038/d41586-018-00045-1. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, 1998. Effects of kappa opioid agonists on cocaine-and food-maintained responding by rhesus monkeys. J. Pharmacol. Exp. Ther 286, 812–824. [PubMed] [Google Scholar]
- Mello NK, Negus SS, 2000. Interactions between kappa opioid agonists and cocaine: preclinical studies. Ann. N. Y. Acad. Sci 909, 104–132. 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- Morani AS, Kivell B, Prisinzano TE, Schenk S, 2009. Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol. Biochem. Behav 94, 244–249. 10.1016/j.pbb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morani AS, Schenk S, Prisinzano TE, Kivell B, 2012. Single injection of novel kappa opioid receptor agonist salvinorin A attenuates expression of cocaine induced behavioral sensitization in rats. Behav. Pharmacol 23, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morani AS, Ewald A, Prevatt-Smith KM, Prisinzano TE, Kivell BM, 2013. The 2-methoxy methyl analogue of salvinorin A attenuates cocaine-induced drug seeking and sucrose reinforcements in rats. Eur. J. Pharmacol 720, 69–76. 10.1016/j.ejphar.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphy R, Rankovic Z, 2009. Designing multiple ligands-medicinal chemistry strategies and challenges. Curr. Pharm. Des 15 (6), 587–600. [DOI] [PubMed] [Google Scholar]
- Naylor J, Prisinzano T, Freeman K, 2015. Self-administration of oxycodone alone or as a mixture with the kappa agonist, salvinorin a, by monkeys under a progressive ratio schedule of reinforcement. Drug Alcohol Depend 146, e48–e49. 10.1016/j.drugalcdep.2014.09.503. [DOI] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin C-E, 1997. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J. Pharmacol. Exp. Therapeut 282 (1), 44–55. [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW, 2006. Cocaine self-administration under variable-dose schedules in squirrel monkeys. Pharmacol. Biochem. Behav 84, 235–243. 10.1016/j.pbb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M, 1985. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14, 149–167. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM, 1986. Psychotomimesis mediated by kappa opiate receptors. Science 233, 774–776. [DOI] [PubMed] [Google Scholar]
- Povlock SL, Schenk JO, 1997. A multisubstrate kinetic mechanism of dopamine transport in the nucleus accumbens and its inhibition by cocaine. J. Neurochem 69, 1093–1105. 10.1046/j.1471-4159.1997.69031093.x. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL, 2011. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol. Sci 32, 581–590. 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Chavkin C, 2008. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berlin) 200, 59–70. 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Zahniser NR, 2009. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J. Neurochem 108, 1575–1584. 10.1111/j.1471-4159.2009.05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM, 2016. Increases in drug and opioid overdose deaths—United States, 2000–2014. Am. J. Transplant 64, 1378–1382. 10.1111/ajt.13776. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J, 2004. Potential anxiolytic and antidepressant-like activities of SNC80, a selective. DELTA.-opioid agonist, in behavioral models in rodents. J. Pharmacol. Sci 95, 374–380. 10.1254/jphs.FPJ04014X. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS, 1999. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology 144, 339–346. 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS, 2000. Reinstatement of extinguished drug-taking behavior in rats: effect of the kappa-opioid receptor agonist, U69593. Psychopharmacology (Berlin) 151, 85–90. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI, 1998. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci 18 (7), 2697–2708. 10.1523/JNEUROSCI.18-07-02697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Walther D, Uhl G, 1992. Dopamine transporter mRNA: dense expression in ventral midbrain neurons. Mol. Brain Res 13 (4), 359–362. 10.1016/0169-328X(92)90220-6. [DOI] [PubMed] [Google Scholar]
- Shippenberg T, Zapata A, Chefer V, 2007. Dynorphin and the pathophysiology of drug addiction. Pharmacol. Ther 116, 306–321. 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg T, LeFevour A, Chefer V, 2008. Targeting endogenous mu-and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol. Disord. -Drug Targets 7, 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Thompson AC, 2009. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol. Psychiatry 65, 169–174. 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson B, Morani AS, Ewald AW, Walker L, Kumar N, Simpson D, Miller JH, Prisinzano TE, Kivell BM, 2015. Pharmacology and anti-addiction effects of the novel kappa opioid receptor agonist Mesyl Sal B, a potent and long-acting analogue of salvinorin A. Br. J. Pharmacol 172, 515–531. 10.1111/bph.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoubis P, Matthes H, Walwyn W, Kieffer B, Maidment N, 2001. Naloxone fails to produce conditioned place aversion in μ-opioid receptor knock-out mice. Neuroscience 106, 757–763. 10.1016/S0306-4522(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaál J, Xi ZX, Gardner EL, 2012. YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict. Biol 17, 259–273. 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS, 1992. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl Acad. Sci 89, 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Zhou Y, Maggos CE, Schlussman SD, Ho A, Kreek MJ, 1997. Prodynorphin, proenkephalin and κ opioid receptor mRNA responses to acute “binge” cocaine. Mol. Brain Res 44, 139–142. 10.1016/S0169-328X(96)00249-5. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Misawa M, 1997. Opioid receptor types and dependence. Nihon Yakurigaku Zasshi 109, 165–174. [DOI] [PubMed] [Google Scholar]
- Tang Y, Yang J, Lunzer MM, Powers MD, Portoghese PS, 2010. A κ opioid pharmacophore becomes a spinally selective κ-δ agonist when modified with a basic extender arm. ACS Med. Chem. Lett 2 (1), 7–10. 10.1021/ml1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao PL, Hwang CL, Chen CY, 1994. U-50,488 blocks the development of morphine tolerance and dependence at a very low dose in guinea pigs. Eur. J. Pharmacol 256 (3), 281–286. 10.1016/0014-2999(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Taylor A, Roberts K, Pradhan A, Akbari H, Walwyn W, Lutfy K, Carroll FI, Cahill CM, Evans C, 2015. Anti-nociception mediated by a κ opioid receptor agonist is blocked by a δ receptor agonist. Br. J. Pharmacol 172 (2), 691–703. 10.1111/bph.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Bäckman CM, Chefer V, O’Donnell P, Shippenberg TS, 2013. Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38, 1770–1779. 10.1038/npp.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Vaughan RA, Sharpe LG, Shippenberg TS,2000. κ-Opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J. Neurosci 20 (24), 9333–9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Jutkiewicz EM, Mosberg HI, Balboni G, Watson SJ, Woods JH, 2006. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res 1069 (1), 172–181. 10.1016/j.brainres.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, Von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB, 2007. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther 320, 1–13. 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Eans SO, Ganno ML, Subrath JJ, LeRouzic V, Hunkele A, Pasternak GW, McLaughlin JP, Majumdar S, 2015. Synthesis and characterization of a dual kappa-delta opioid receptor agonist analgesic blocking cocaine reward behavior. ACS Chem. Neurosci 6, 1813–1824. 10.1021/acschemneuro.5b00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A, Pagirsky J, Eans SO, Medina JM, Xu J, Pan YX, Borics A, Pasternak G,W, McLaughlin JP, Majumdar S, 2016. Mitragynine/Corynantheidine pseudoindoxyls as opioid analgesics with mu agonism and delta antagonism, which do not recruit β-Arrestin-2. J. Med. Chem 59, 8381–8397. 10.1021/acs.jmedchem.6b00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, Trapella C, Lazarus LH, Regoli D, Guerrini R, Salvadori S, Caló G, 2008. Anxiolyticand antidepressant-like activities of H-Dmt-Tic-NH-CH (CH 2-COOH)-Bid (UFP- 512), a novel selective delta opioid receptor agonist. Peptides 29, 93–103. 10.1016/j.peptides.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA, 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc 2, 322–328. 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE, 2001a. Enadoline and butorphanol: evaluation of κ-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J. Pharmacol. Exp. Ther 299, 147–158. [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE, 2001b. Enadoline, a selective kappa opioid agonist:comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berlin) 157, 151–162. [DOI] [PubMed] [Google Scholar]
- Wang Q, Long Y, Hang A, Zan G-Y, Shu X-H, Wang Y-J, Liu J-G, 2016. The anxiolytic-and antidepressant-like effects of ATPM-ET, a novel κ agonist and μ partial agonist, in mice. Psychopharmacology (Berlin) 233, 2411–2418. 10.1007/s00213-016-4292-z. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF, 2010. The role of the dynorphin–κ opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berlin) 210, 121–135. 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL, 2015. The G protein–biased κ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J. Pharmacol. Exp. Ther 352, 98–109. 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, 2008. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox. Res 14, 169–183. 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA, 2001. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J. Neurosci 21, 6338–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR Jr., 2010. Inhibition of NAALADase by 2-PMPA attenuates cocaine- induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J. Neurochem 112, 564–576. 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Bhushan RG, Daniels DJ, Portoghese PS, 2005. Interaction of bivalent ligand KDN21 with heterodimeric δ-κ opioid receptors in HEK293 cells. Mol. Pharmacol 68 (4), 1079–1086. 10.1124/mol.105.012070. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ohno M, Ueki S, 1988. A selective κ-opioid agonist, U-50,488 H, blocks the development of tolerance to morphine analgesia in rats. Eur. J. Pharmacol 156 (1), 173–176. 10.1016/0014-2999(88)90162-8. [DOI] [PubMed] [Google Scholar]
- Zan GY, Wang Q, Wang YJ, Chen JC, Wu X, Yang CH, chai JR, Li M, Liu Y, Hu XW, Shu XH, Liu JG, 2016. p38 mitogen-activated protein kinase activation in amygdala mediates κ opioid receptor agonist U50, 488H-induced conditioned place aversion. Neuroscience 320, 122–128. 10.1016/j.neuroscience.2016.01.052. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ, 2004a. Effect of the endogenous κ opioid agonist dynorphin A (1–17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berlin) 172, 422–429. 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ, 2004b. Effect of the κ opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berlin) 173, 146–152. 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]