Abstract
For last decade, low-intensity transcranial focused ultrasound (tFUS) has been rapidly developed for a myriad of successful applications in neuromodulation. tFUS possesses high spatial resolution, focality and depth penetration as a noninvasive neuromodulation tool. Despite the promise, confounding activation can be observed in rodents when stimulation parameters are not selected carefully. Here we summarize the existing classes of observations for ultrasound neuromodulation: ultrasound directly activates a localized area, or ultrasound indirectly activates auditory pathways, which further propagates to other cortical networks. We also present control in vivo animal studies, which suggest that underlying tFUS brain modulation is characterized by localized activation independent of auditory networks activations.
Keywords: neuromodulation, transcranial focused ultrasound, tFUS, low-intensity focused ultrasound, brain stimulation
Introduction
By using ultrasound waves (≥ 20 kHz) to penetrate the skull and target brain tissue in a focused way, low-intensity transcranial focused ultrasound (tFUS) has demonstrated its unprecedented capability in non-invasive neural stimulation [1,2], and unparalleled prospects for translational clinical application [3]. Different from high-intensity focused ultrasound (HIFU) [4], low-intensity tFUS can achieve reversible neural effects while complying with the Food and Drug Administration’s (FDA) [5] safety requirements, thus ensuring safety of human subjects.
The field of neuromodulation – interacting and modulating the nervous system through stimulation [6] – has grown significantly over the past decade, now encompassing techniques ranging from invasive neuromodulation through the direct delivery of electrical energy to brain networks through implantable stimulation technology, to the non-invasive transcranial delivery of electrical, magnetic, optical or acoustic energy [7]. These stimulation technologies can excite, inhibit, or disrupt brain network dynamics in a controlled way, depending on the stimulation parameters and application, and often offer superior specificity and reversibility to drug and surgical alternatives [8].
Among non-invasive neuromodulation approaches, the use of electrical or magnetic stimulation employed in transcranial current stimulation (TCS) [9,10], transcranial magnetic stimulation (TMS) [11,12], or transcranial static magnetic field stimulation (tSMS) [13], has been shown to have distributed, non-focal effects on various brain conditions. On the other hand, the high spatial resolution and specificity of tFUS provides the unique capacity to non-invasively target controlled stimulation volumes for both cortical and deep brain applications.
tFUS has demonstrated its robust neuromodulatory effects in numerous in vivo preparations of animals ranging from mice [14–18] and rats [19–23], to rabbits [24], swine [25,26], sheep [27], and even monkeys [28,29]. More recently it has been shown to be a safe and effective method for the transcranial stimulation of humans [30–36] with clinical applicability. Specifically, a recent study using ultrasonic thalamic stimulation suggested that this non-invasive tool may assist patients suffering from severe brain injury to recover consciousness [37].
Although tFUS has shown a wide range of practical neuromodulatory effects, there exists an ongoing debate over how tFUS interacts with the nervous system. While numerous observations initially suggested that tFUS directly stimulates the targeted neural tissue (Figure 1A) [15,20,24,25,27,38], two recent studies [39,40] have observed inadvertent activation of the auditory cortex via the mechanical conduction of ultrasound energy through the skull, leading to cortical activation (Figure 1B). By tuning ultrasound parameters and introducing a chemically-deafened rodent model, we provide further in vivo evidence that the target location and auditory cortex are activated independently (Figure 1C). Here we present three potential interpretations for how the brain responds to ultrasound energy deposition (Figure 1). Given the extensive electrophysiological [20,21,30,32], neurovascular [24,29,34,41], motor [14,16–18,22,23] and cognitive [32,35,36] evidences in support of tFUS-induced direct neural effects, we further examine whether the auditory pathway in the small brain volumes of rodent or mouse models dictates or impacts the observed activation patterns.
Figure 1.
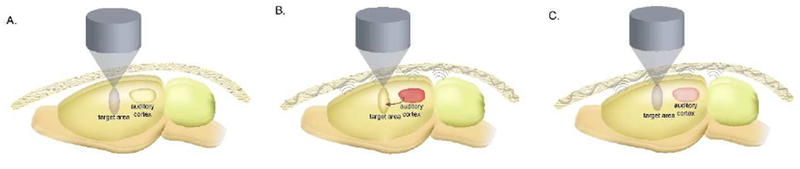
Possible activation pathways of brain activation by tFUS. A. tFUS directly stimulates the target area. B. tFUS leads to indirect activation of the auditory cortex, which propagates to the target area. C. tFUS directly stimulates the target area, with some auditory indirect stimulation, however the activations are independent of each other.
tFUS Directly Activates Local Brain Tissue
As early as 2010, William J. Tyler’s group brought excitement to the tFUS field through a series of experiments showing the direct local effects of tFUS [15]. Tufail et al. successfully demonstrated an immediate motor circuit response to a tone-burst pulsed tFUS (fundamental frequency FF: 500 kHz, pulse repetition frequency PRF: 1.5 kHz). Recordings of multi-unit activity (MUA) and local field potentials (LFPs) further demonstrated the direct and local brain response to the acoustic input (spatial-peak temporal-average intensity Ispta: 64.5 mW/cm2) [15]. Furthermore, deep brain modulation was achieved by utilizing a lower FF of 250 kHz with a PRF of 2 kHz, resulting in the promotion of expression of brain-derived neurotrophic factor compared to contralateral hippocampal regions [15]. These increased concentrations in combination with increased neuronal spiking and significant LFPs peaks within the targeted hippocampus also provided early but direct evidences of the in vivo brain response to low-intensity tFUS (Ispta: 84.32 mW/cm2) [15].
Excited by the potential of tFUS as a noninvasive neuromodulation method, multiple groups have attempted to increase the spatial resolution and diminish the spatial focus of energy deposition of tFUS by tuning ultrasound parameters, namely the FF, especially when working with deep brain structures. Kamimura and colleagues targeted subcortical brain structures i.e. superior colliculus, pretectal nucleus, and hippocampus etc. with 1.9 MHz focused ultrasound (PRF: 1 kHz, Duty cycle: 50%, lateral focus 1.0 mm, axial focus 8.5 mm), reproducibly inducing eyeball movement and pupillary dilation [38]. The improved spatial specificity produced by the increased FF was demonstrated to lead to consistent behavioral responses compared to lower FF scenarios [38].
The consistent behavior patterns observed when tFUS is targeting distinct brain areas are deemed as strong evidences of direct and local brain activations by tFUS with increased FF. In another study, 2.9 MHz FF was directed to achieve a smaller focal spot of 0.65 mm in the mouse motor cortex, and the efficacy of tFUS was evaluated by electromyography (EMG) [14]. While it had been reported that high frequency ultrasound could elicit motor responses through increased ultrasound intensity, these responses were often inconsistent with the demonstrated increase in spatial resolution, and correlated with the expected increases in spatial specificity [14]. Through the systematic investigation of the effects of FF on efficacies in producing motor responses, frequency dependence was attributed to the mechanism of ultrasound neuromodulation, further establishing the parameter-dependent nature of tFUS as a neuromodulatory tool. In another study, 5 MHz tFUS successfully evoked brain activation with an “equivalent diameter of the stimulation region” of 0.29 ± 0.08 mm while being evaluated through EMG and motor response [17]. The brain was observed to have a shorter response latency for 5 MHz FF, when compared to 1 MHz [17].
The above studies suggest higher FF can achieve high spatial resolution and quicker responses; however, at high frequencies the skull becomes a significant barrier requiring the researchers to balance spatial resolution and acoustic penetration efficiency. One solution to this problem has been shown to be the use of two ultrasound transducers, driven by 2.25 MHz and 1.75 MHz FFs, respectively, producing a beat frequency of 500 kHz carried by a 2 MHz ultrasound wave with 1.5 kHz PRF [42] which can be focally deployed to stimulate or stepwise scan the cortex of mice brains. This modulated focused ultrasound (mFU) has been shown to induce a variety of movements with a 1-mm spatial selectivity. Meanwhile, the differentiation of motor responses throughout the step-wise scanning may also indicate a brain-region specific effect by tFUS. Besides the aforementioned studies evaluating the ultrasound evoked motor-related responses, very recently, 2.9 MHz focused ultrasound (FUS) was shown to also alter the local cerebral blood flow (CBF), monitored through laser speckle contrast imaging [41]. This cortical hemodynamic alteration by FUS provides further evidence in support of targeted activation through ultrasound. In using an optical imaging method, this study eliminates many possible artifacts recorded through electrical sensing, without necessitating a transgenic model, as is required for calcium imaging.
When researchers administered tFUS to the brains of large animals and humans, direct and local neural effects were reported without the observation of auditory effects [43]. Besides eliciting desired neuronal activity, the direct and local effects of tFUS can additionally suppress diseased neural activity. An early study [44] on a cat model provided experimental evidence for the ultrasound-induced attenuation of seizure activity and decreased morbidity through the use of a relatively high acoustic intensity of 840 W/cm2 with a fundamental acoustic frequency of 2.7 MHz. In epileptic models, researchers have demonstrated a reduction in occurrence of epileptic EEG bursts and improvement in Racine clinical scores in pentylenetetrazo-induced epileptigenic rats through low intensity tFUS sonication (Ispta: 130 mW/cm2) [21]. A further study successfully disrupted seizure activity using 0.35 MHz ultrasonic continuous wave [45].
These studies in various animal models and human subjects provided ample evidence supporting the hypothesis that tFUS can directly activate brain tissues targeted by ultrasonic beam, but do not rule out the possibility of secondary activation of the auditory cortex.
tFUS Indirectly Activates Auditory Cortex and Subsequently Propagates through Brain Networks
Since a lot of early tFUS work investigated motor responses to stimulation of the motor cortex, it is possible if not likely that the indirect actions of the auditory network have been overlooked, until now. The companion work, by Sato et al. [39] and Guo et al. [40], sheds new light on the possible side effects of ultrasound neuromodulation in rodents, identifying secondary activation pathways that indirectly interact with the auditory pathways.
In the study conducted by Sato et al. [39], transgenic mice expressing fluorescent calcium indicators (Thy1-GCaMP6s) were prepared with thinned skulls and underwent simultaneous calcium imaging and ultrasound stimulation. Sato et al. stimulated the visual cortex using a single element FUS transducer at 500 kHz FF, PRF 1,500Hz, pulse duration of 200 μs, and sonication duration of 80 ms in a range of intensities (Ispta: 0.034 to 4.2 W/cm2). Across the range of tFUS intensities the authors observed significant activations of the auditory cortex, especially the contralateral hemisphere, at 200 to 1200 ms post sonication, while observing minimal activation of the visual cortex. When stimulating at high intensities, activation was first observed in the auditory cortex, with subsequent activations being seen in contralateral and ipsilateral somatosensory cortexes, similar to loud sound stimulation (108 dB). To investigate the contribution of auditory percepts on the response to tFUS, Sato et al. showed that stimulation at the visual cortex was able to induce motor responses. Furthermore, when chemically partial deafened mice were stimulated with tFUS on the right visual cortex, deafened subjects exhibited significantly decreased motor responses compared to control subjects.
This work provides a wide field fluorescent microscopy evidence on the propagation of neural activity in the mouse brain. However, it is difficult to compare these findings with the results reported by Tufail et al. [15] due to the low temporal and spatial resolution of the data presented (200 ms per frame, wide field). The underlying assumption of this study is that single trial tFUS stimulations will exhibit neural signal profiles which last much longer than the sonication duration, a premise which has been unfounded in other studies. Based on the results of previous work, the activation of the target area would only occur within the first 80 ms, which cannot be resolved in the 200 ms frame reported by Sato et al.
In the study conducted by Guo et al. [40], multi-electrode arrays are used to record from the primary auditory cortex (A1) of guinea pigs. tFUS stimulation is delivered at various locations using a single element focused transducer at 220 kHz FF, PRF of 10–1500 Hz, and pulse duration between 0.2 to 1 ms with pressures of 200, 1000, 2000 kPa (no intensity calculations were reported). Across different stimulation areas, they reported observations of activity in A1 and somatosensory cortex (S1) similar to activity derived from 70 dB sound stimulation. However, if the ultrasound transducer was uncoupled from the skull or if the auditory nerve was bilaterally severed, then little to no activation was observed in A1 or S1 in the post stimulus time histograms [40].
The work of Guo et al. elucidates the temporal specific effects of tFUS sonication not achieved in Sato et al. [39]. However, the comparison of these companion studies calls into question whether one can draw a fair comparison between the different stimulation parameters, such as FF or PRF, used by Sato et al. at 500 kHz FF and by Guo et al. at 220 kHz FF. Based on the work by Younan et al. [46], at 250 kHz FF, close to that used in Guo et al. [40], low-frequency tFUS stimulation can lead to significant standing waves in the head cavity enclosed by the rodent skull. These distributed local peaks are a source of concern when stimulating at low FF and it is possible that the activation of S1 and A1 during unrelated tFUS stimulation is the result of cochlear activation through the standing fields.
Another potential mechanism not precluded is the possible inhibitory effects from ultrasound, especially given the recording modality used in [39,40] is not sensitive to inhibitory modulations [43]. As reported by Min et al., ultrasound may induce a suppression of seizure activity [21] and EEG based visual evoked response potentials [47]. Since calcium imaging is an imaging modality that relies on neural depolarization, neural suppression, characterized by a lack of depolarization, is difficult to observe due to the lack of increase in intracellular calcium. Suppression is also difficult to observe in the MUAs presented by Guo et al. since baseline activities are unavailable to compare to. Although the field has not reached a consensus on what specific tFUS parameters result in neural suppression, the suppression effects offer another explanation as to why no local activities were observed at low ultrasound intensities, while at high ultrasound intensities the activation of auditory percepts may have dominated the observed activities.
Neural Imaging Shows Auditory Indirect Activation Depends on tFUS Parameters
The companion work presented above offered evidences of auditory activity during tFUS stimulation, however findings from other works suggest the presence of auditory effects depends on characteristics of the tFUS stimulation waveform. In contrast to the findings regarding the auditory pathway or side-effects of brain activation through tFUS, Daniels et al. [26] recently reported that in both in vivo rats and pigs auditory-evoked potentials (AEPs) induced by repetitive sounds can be suppressed by tFUS directed at the auditory pathway. Furthermore, these suppression effects were identified as a long-lasting effect through EEG recordings. In this study, a 1000-element focused ultrasound system (FF: 230 kHz) was employed to create a 3-mm focal energy deposition on the auditory pathway, and the directed low-intensity focused ultrasound (spatial-peak pulse-average intensity Isppa: 2.3 and 4.6 W/cm2) was confirmed to provide no heating or damage to brain tissue [26]. Additionally, the strength of the tFUS-induced suppression effects positively correlated with ultrasound intensity. The companion works [39,40] show indirect auditory activation due to the propagation of tFUS energy through skull coupling. If tFUS exhibited no direct local neural activation, one would expect there to be greater or equal magnitude of auditory activation when tFUS is directly applied to the auditory cortex. However, Daniel et al. observed suppression of AEP due to tFUS stimulation, which suggests there should be local direct neural effects of tFUS. While all of these works focused on the responses of the auditory system to tFUS stimulation, the discrepant effects observed between this study [26] and the companion works [39,40] may be due to the differences in animal models, procedures, and most importantly, the different tFUS parameters and administrative protocols used.
In 2010, Tufail and colleagues reported their findings on the spatial distributions of brain activation evoked by tFUS [15]. Although they did not point out potential ancillary neuronal activities of the auditory cortex, the functional activity maps of c-fos+ expression immediately after tFUS stimulation do suggest potential ancillary excitation, e.g. activations of auditory cortices (indicated by arrows in Figure 2A), in addition to the mouse brain response within the ultrasound stimulation zone (Figure 2A). Yu et al. [20] showed a similar presence of auditory activation in source imaging results estimated from the scalp EEG recordings (Figure 2B; 2.0 mm posterior to the bregma) in rat subjects during simultaneous tone-burst tFUS stimulation and EEG recordings.
Figure 2.
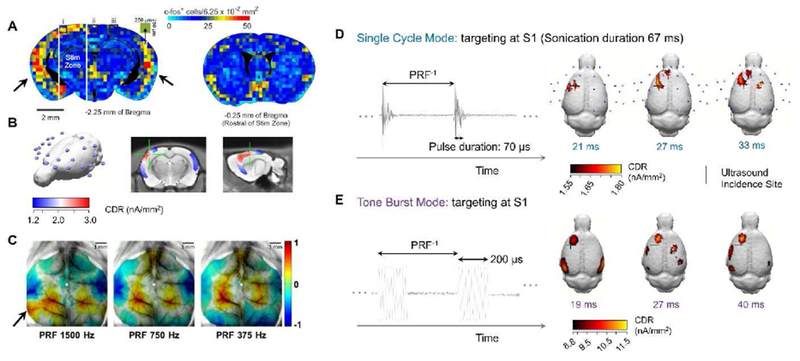
A. Psuedo-colored maps c-fos+ cell densities at three coronal brain sections. The brain areas between the two white lines were targeted with tFUS. The black arrows indicate the ancillary activations. (adapted from [15]) B. 24-channel rat EEG electrodes covering a 3-D reconstructed rat brain. The current source density reconstructed in electrophysiological source imaging (ESI) is displayed in a coronal section. The green cross indicates the stimulation target of the tone-burst tFUS shown in E. C. Shown as a principal component, local hemodynamic changes detected by optical imaging on awake mice suggested a dependence of brain activations on ultrasound PRF (adapted from [48]. D. The illustrated single cycle mode employs short pulse duration (fundamental frequency FF: 500 kHz). ESI-based global images localize the brain activation due to tFUS aiming at primary somatosensory cortex (S1) in this single-cycle mode. Measured ultrasound peak-to-peak pressure is 88.7 kPa, and the pulse duration is 70 μs. Maximal current density is shown at the local area targeted by tFUS in all ESI frames, while no significant auditory response can be seen among these images. E. The tone burst mode integrates multiple sinusoidal cycles for each pulse. Directing the tone burst mode tFUS onto S1 triggers brain responses at S1 and bilateral auditory cortices. In this mode, the pulse duration is elongated to 200 μs for 100 cycles per pulse.
Along with the studies described above, recent findings [48] suggest that the presence of extensive brain activation may depend on the tFUS wave features (e.g. pulse repetition frequency). Kim et al. applied optical intrinsic signal imaging (OISI) to simultaneously monitor wide-field cerebral hemodynamic changes during tFUS in awake head fixed mice (Figure 2C). The transcranial ultrasound (tUS) was applied unilaterally at 425 kHz in a non-targeted manner, to allow accommodation of cranial windows for light source access. The relative changes in both oxygenated and deoxygenated hemoglobin concentrations (i.e. HbO and RHb) is correlated with the in vivo brain responses to tUS. Similar to electrophysiological source imaging (ESI) [49], OISI has the ability to capture brain-wide activation and propagation of activities induced by tUS, in which global and local hemodynamic changes can be examined separately [48]. Unlike Sato et al.’s calcium imaging experiment, which monitored the tFUS targeted visual cortex, the unfocused application of tUS at PRFs of 375 and 750 Hz induced an increase of HbO signal mainly within non-auditory cortices. Similar to Sato et al.’s work high intensity stimulation (tUS at 1500 Hz PRF) led to an increased response of the auditory cortices (indicated with a black arrow in Figure 2C). These results suggest that only specific tUS PRFs induce activation of the auditory cortex. In this study, although OISI-based cortical mapping does not offer a direct measurement of neural activities, the indirect detection of activity, through the neurovascular coupling, suggests that the non-specific activation of the auditory pathway induced through transcranial ultrasound is parameter specific and potentially could be suppressed.
The presence of extensive brain activation may also be a function of the characteristics of tFUS pulses. Using the ESI [49], high temporal-resolution playback of in vivo whole-brain activities can be analyzed to further capture and uncover the dependence of auditory side effects on acoustic pulse parameters. We introduced single-cycle and tone-burst modes to directly compare their activation effects on the rats’ auditory cortex (Figure 2D, E). Utilizing ESI, the current source density mapping illustrates a focal initial activation of S1 during single-cycle mode tFUS. While this activation is observed to later propagate to the ipsilateral motor cortex and even the contralateral hemisphere (Figure 2E), no significant ultrasound-induced auditory side effects are induced by the single-cycle mode tFUS. However, when imaged using the same setup, the tone-burst mode of tFUS led to remarkable brain activation within the auditory cortices, with a commensurate current source density magnitude at the tFUS-targeting area (Figure 2E). Besides having different spatial patterns, the global current density produced by the tone burst tFUS was much larger, compared to that generated in the single-cycle mode. Although notable, this can most likely be attributed to the higher ultrasound intensity employed in the tone-burst mode. The difference between Figures 2D and 2E illustrates that it is possible to stimulate cortical regions in the rat brain using tFUS without eliciting direct auditory activation and that the auditory activation is mainly impacted by the stimulation waveform.
A 500 kHz fundamental frequency was utilized in our ESI study, similar to the study by Tufail et al. [15]. One should be cautious when applying even lower FFs (e.g. 220 kHz used by Guo et al.) in rodents. This is because in an in vivo setting with intact skulls, low-frequency tFUS (i.e. central frequency of 250 kHz, peak frequency of 320 kHz) may produce “considerable interference patterns with secondary and shifted peaks” [46]. Younan and colleagues concluded side-effect, non-focal activations may be due to standing waves formed inside the head [46]. Besides taking careful consideration in choosing a fundamental frequency, another critical concern is raised in the guidance of the ultrasound wave to the brain, i.e. the size of acoustic collimator outlet may need to be equivalent to one wavelength of the ultrasound wave. Guo et al. [40] used a 3-mm tip focusing cone to guide 220 kHz ultrasound wave (i.e. wavelength 6.8 mm in soft tissue). This may result in an ineffective way to deliver tFUS, leading to considerable ultrasound reflections (reverberations) inside the focusing cone. This issue can be seen from the Figure S1 in [40] in an ultrasound field map without the skull.
Control Studies Show Ultrasound Directly Modulating Target Areas in Deafened Rodents
The chemical deafening experiments by Sato et al. (2018) revealed side effect activations of the auditory cortex when researchers stimulated the visual cortex and measured responses in consequential motor behaviors. Although this study demonstrates the presence of an indirect activation pathway, we cannot preclude the presence of local activation at the tFUS target site.
To investigate whether the activation of the stimulation target is independent of auditory activation, we performed control studies in total of four animals, in which two animals’ cochlea is chemically deafened (Figure 3B). Similar to the study by Sato et al. on chemically deafened mice, in our study, the two rat subjects were injected with furosemide (175 mg/kg) and gentamicin (350 mg/kg). Instead of partially deafened over 3 hours, in our control study, animals were given 72 hours for the chemicals to reach maximal efficacy. Two additional rats were injected with normal saline as negative controls. Auditory brainstem response (ABR) tests [50] were performed before and 72-hours after injection to verify changes in hearing threshold (Figures 3A-C). After the two rats were deafened, tFUS was applied to S1, where local field potentials were obtained through an intracranial electrode array inserted through a craniotomy with diameter of 1.5 mm.
Figure 3.
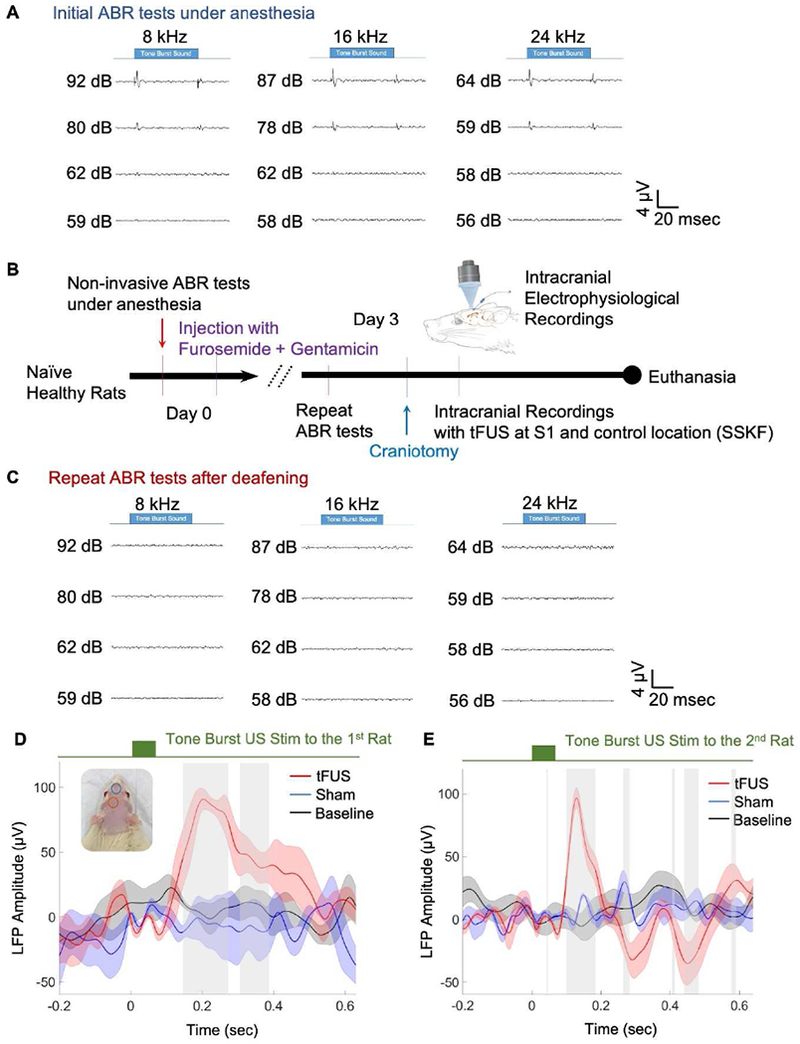
A. ABR tests in anesthetized rats before chemical deafening. B. The experimental protocol to induce deafening, conduct ABR tests, and subsequently the intracranial recording. C. ABR tests in anesthetized rats after chemical deafening. D and E. In two chemically-deafened rats, temporal waveforms of local field potentials (LFPs) recorded at S1 from baseline (averaged across 288 trials), tFUS at S1 and anterior control location (sham, both averaged across 318 trials). The ultrasound conditions are illustrated in the inset with corresponding colors for tFUS (orange circle) and sham (green circle). The LFPs are presented with the mean value (solid line) and standard error of the mean (shaded areas). The gray bars indicate significant differences between tFUS and sham conditions (p < 0.05).
In our study, we observed a significant peak in the LFP at the target site exclusively after tFUS activation in both deafened and negative control rats (negative control data not shown). During baseline recordings, when no tFUS was applied, or not during a sham condition, in which tFUS is coupled at a control location, no significant peaks in LFP were observed (Figure 3D-E). Control stimulation is delivered on an anterior skull location 8-10 mm away from the targeted S1 site, and aimed at the olfactory bulbs. Overall, the distinct temporal features of the LFP induced by tFUS in the deafened rats (Figure 3D-E) suggest that activation of the cochlea is not necessary for the ultrasound-mediated activation of S1 cortex. No motor responses were observed during these experiments, during which a 1.5% isoflurane was maintained at a constant anesthesia level throughout the experiments.
Summary
tFUS has shown significant neuromodulatory capabilities in in vivo animal models. Identifying the in vivo mechanistic pathways of tFUS activation is a key step in translating this promising technology to clinical utility. Recent studies illustrate new possibilities that auditory indirect activation propagates to cortical regions and may contribute to a perceived direct activation of cortical regions, in small animals. New evidence suggests the presence of auditory indirect activations is due to certain ultrasound stimulation parameters and may be present independently to the concurrent local, direct activations. We caution investigators to be careful in selecting ultrasound parameters in order to control for possible confounding effects due to auditory indirect activation, especially with respect to ultrasound pulse repetition frequencies, pulsed modes and fundamental frequencies. Further investigations are warranted to clarify whether or not the activation of a targeted brain region is dictated by the auditory excitation.
Acknowledgements
This work was supported in part by NIH grant MH114233, and NSF grant CBET-1450956. K.Y. was supported in part by an MnDRIVE Neuromodulation Fellowship and Doctoral Dissertation Fellowship at the University of Minnesota. The authors would also like to thank Daniel Suma for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Naor O, Krupa S, Shoham S: Ultrasonic neuromodulation. J Neural Eng 2016, 13:031003. [DOI] [PubMed] [Google Scholar]
- 2.Tyler WJ, Lani SW, Hwang GM: Ultrasonic modulation of neural circuit activity. Curr Opin Neurobiol 2018, 50:222–231. [DOI] [PubMed] [Google Scholar]
- 3.Bystritsky A, Korb AS: A Review of Low-Intensity Transcranial Focused Ultrasound for Clinical Applications. Current Behavioral Neuroscience Reports 2015, 2:60–66. [Google Scholar]
- 4.Quadri SA, Waqas M, Khan I, Khan MA, Suriya SS, Farooqui M, Fiani B: High-intensity focused ultrasound: past, present, and future in neurosurgery. Neurosurg Focus 2018, 44:E16. [DOI] [PubMed] [Google Scholar]
- 5.FDA: Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. Edited by Services USDHaH. Rockville, MD: Center for Devices and Radiological Health; 2008. [Google Scholar]
- 6.Krames ES, Hunter Peckham P, Rezai A, Aboelsaad F: Chapter 1 - What Is Neuromodulation? In Neuromodulation. Edited by Rezai ESKHPR: Academic Press; 2009:3–8. [Google Scholar]
- 7.Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, Roy A, Holt A, Lim KO, Carey JR, Vitek JL, et al. : Neuromodulation for Brain Disorders: Challenges and Opportunities. Ieee Transactions on Biomedical Engineering 2013, 60:610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelman BJ, Johnson N, Sohrabpour A, Tong S, Thakor N, He B: Systems Neuroengineering: Understanding and Interacting with the Brain. Engineering 2015, 1:292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horvath JC, Forte JD, Carter O: Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 2015, 66:213–236. [DOI] [PubMed] [Google Scholar]
- 10.Nitsche MA, Paulus W: Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology-London 2000, 527:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker AT, Jalinous R, Freeston IL: Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1:1106–1107. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi M, Pascual-Leone A: Transcranial magnetic stimulation in neurology. The Lancet Neurology 2003, 2:145–156. [DOI] [PubMed] [Google Scholar]
- 13.Oliviero A, Mordillo-Mateos L, Arias P, Panyavin I, Foffani G, Aguilar J: Transcranial static magnetic field stimulation of the human motor cortex. J Physiol 2011, 589:4949–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye PP, Brown JR, Pauly KB: Frequency Dependence of Ultrasound Neurostimulation in the Mouse Brain. Ultrasound Med Biol 2016, 42:1512–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates a frequency dependence of tFUS stimulation to generate motor responses, supports the presence of direct and local activations.
- 15.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SIH, Tyler WJ: Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron 2010, 66:681–694. [DOI] [PubMed] [Google Scholar]
- 16.King RL, Brown JR, Newsome WT, Pauly KB: Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med Biol 2013, 39:312–331. [DOI] [PubMed] [Google Scholar]
- 17.Li GF, Zhao HX, Zhou H, Yan F, Wang JY, Xu CX, Wang CZ, Niu LL, Meng L, Wu S, et al. : Improved Anatomical Specificity of Non-invasive Neuro-stimulation by High Frequency (5 MHz) Ultrasound. Sci Rep 2016, 6:24738. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates a difference in EMG response latency to tFUS stimulation at different FF, thus provides evidence of direct and local effects of tFUS.
- 18.Qiu W, Zhou J, Chen Y, Su M, Li G, Zhao H, Gu X, Meng, Wang C, Xiao Y, et al. : A Portable Ultrasound System for Non-Invasive Ultrasonic Neuro-Stimulation. IEEE Trans Neural Syst Rehabil Eng 2017, 25:2509–2515. [DOI] [PubMed] [Google Scholar]
- 19.Yoo SS, Kim H, Min BK, Franck E, Park S: Transcranial focused ultrasound to the thalamus alters anesthesia time in rats. Neuroreport 2011, 22:783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu K, Sohrabpour A, He B: Electrophysiological Source Imaging of Brain Networks Perturbed by Low-Intensity Transcranial Focused Ultrasound. IEEE Trans Biomed Eng 2016, 63:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that the single-cycle pulsed mode tFUS does not lead to significant auditory side effects by using non-invasive whole-brain EEG source imaging.
- 21.Min BK, Bystritsky A, Jung KI, Fischer K, Zhang Y, Maeng LS, Park SI, Chung YA, Jolesz FA, Yoo SS: Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci 2011, 12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulick DW, Li T, Kleim JA, Towe BC: Comparison of Electrical and Ultrasound Neurostimulation in Rat Motor Cortex. Ultrasound Med Biol 2017. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Chiu A, Lee SD, Fischer K, Yoo SS: Focused ultrasound-mediated non-invasive brain stimulation: examination of sonication parameters. Brain Stimul 2014, 7:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo SS, Bystritsky A, Lee JH, Zhang Y, Fischer K, Min BK, McDannold NJ, Pascual-Leone A, Jolesz FA: Focused ultrasound modulates region-specific brain activity. Neuroimage 2011, 56:1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dallapiazza RF, Timbie KF, Holmberg S, Gatesman J, Lopes MB, Price RJ, Miller GW, Elias WJ: Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. J Neurosurg 2018, 128:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates tFUS can modulate and map deep brain structures in pigs with no reported auditory effects.
- 26.Daniels D, Sharabi S, Last D, Guez D, Salomon S, Zivli Z, Castel D, Volovick A, Grinfeld J, Rachmilevich I, et al. : Focused Ultrasound-Induced Suppression of Auditory Evoked Potentials in Vivo. Ultrasound Med Biol 2018, 44:1022–1030. [DOI] [PubMed] [Google Scholar]; • Demonstrates in rats and pigs the ability to direct tFUS at the auditory pathway to suppress AEPs.
- 27.Lee W, Lee SD, Park MY, Foley L, Purcell-Estabrook E, Kim H, Fischer K, Maeng LS, Yoo SS: Image-Guided Focused Ultrasound-Mediated Regional Brain Stimulation in Sheep. Ultrasound Med Biol 2016, 42:459–470. [DOI] [PubMed] [Google Scholar]
- 28.Deffieux T, Younan Y, Wattiez N, Tanter M, Pouget P, Aubry JF: Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr Biol 2013, 23:2430–2433. [DOI] [PubMed] [Google Scholar]
- 29.Yang PF, Phipps MA, Newton AT, Chaplin V, Gore JC, Caskey CF, Chen LM: Neuromodulation of sensory networks in monkey brain by focused ultrasound with MRI guidance and detection. Sci Rep 2018, 8:7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, Tyler WJ: Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci 2014, 17:322–329. [DOI] [PubMed] [Google Scholar]
- 31.Mueller J, Legon W, Opitz A, Sato TF, Tyler WJ: Transcranial Focused Ultrasound Modulates Intrinsic and Evoked EEG Dynamics. Brain Stimul 2014. [DOI] [PubMed] [Google Scholar]
- 32.Lee W, Kim H, Jung Y, Song IU, Chung YA, Yoo SS: Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci Rep 2015, 5:8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legon W, Ai L, Bansal P, Mueller JK: Neuromodulation with single-element transcranial focused ultrasound in human thalamus. Hum Brain Mapp 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee W, Kim HC, Jung Y, Chung YA, Song IU, Lee JH, Yoo SS: Transcranial focused ultrasound stimulation of human primary visual cortex. Sci Rep 2016, 6:34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee W, Chung YA, Jung Y, Song IU, Yoo SS: Simultaneous acoustic stimulation of human primary and secondary somatosensory cortices using transcranial focused ultrasound. BMC Neurosci 2016, 17:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hameroff S, Trakas M, Duffield C, Annabi E, Gerace MB, Boyle P, Lucas A, Amos Q, Buadu A, Badal JJ: Transcranial ultrasound (TUS) effects on mental states: a pilot study. Brain Stimul 2013, 6:409–415. [DOI] [PubMed] [Google Scholar]
- 37.Monti MM, Schnakers C, Korb AS, Bystritsky A, Vespa PM: Non-Invasive Ultrasonic Thalamic Stimulation in Disorders of Consciousness after Severe Brain Injury: A First-in-Man Report. Brain Stimul 2016. [DOI] [PubMed] [Google Scholar]
- 38.Kamimura HAS, Wang S, Chen H, Wang Q, Aurup C, Acosta C, Carneiro AAO, Konofagou EE: Focused ultrasound neuromodulation of cortical and subcortical brain structures using 1.9 MHz. Medical Physics 2016, 43:5730–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato T, Shapiro MG, Tsao DY: Ultrasonic Neuromodulation Causes Widespread Cortical Activation via an Indirect Auditory Mechanism. Neuron 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Through calcium imaging, demonstrates the propagation of tFUS activation throughout the mouse cortical regions when stimulating at the visual cortex.
- 40.Guo H, Hamilton M II, Offutt SJ, Gloeckner CD, Li T, Kim Y, Legon W, Alford JK, Lim HH: Ultrasound Produces Extensive Brain Activation via a Cochlear Pathway. Neuron 2018. [DOI] [PubMed] [Google Scholar]; •• Demonstrated A1 is activated during tFUS stimulation even when tFUS is not directly targeting A1 through electrophysiology recordings.
- 41.Yuan Y, Zhao Y, Jia H, Liu M, Hu S, Li Y, Li X: Cortical Hemodynamic Responses Under Focused Ultrasound Stimulation Using Real-Time Laser Speckle Contrast Imaging. Frontiers in Neuroscience 2018, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehic E, Xu JM, Caler CJ, Coulson NK, Moritz CT, Mourad PD: Increased anatomical specificity of neuromodulation via modulated focused ultrasound. PLoS One 2014, 9:e86939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Airan RD, Butts Pauly K: Hearing out Ultrasound Neuromodulation. Neuron 2018, 98:875–877. [DOI] [PubMed] [Google Scholar]
- 44.Manlapaz JS, Astroem KE, Ballantine HT Jr., Lele PP: Effects of Ultrasonic Radiation in Experimental Focal Epilepsy in the Cat. Exp Neurol 1964, 10:345–356. [DOI] [PubMed] [Google Scholar]
- 45.Tufail Y, Yoshihiro A, Pati S, Li MM, Tyler WJ: Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat Protoc 2011, 6:1453–1470. [DOI] [PubMed] [Google Scholar]
- 46.Younan Y, Deffieux T, Larrat B, Fink M, Tanter M, Aubry JF: Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med Phys 2013, 40:082902. [DOI] [PubMed] [Google Scholar]
- 47.Kim H, Park MY, Lee SD, Lee W, Chiu A, Yoo SS: Suppression of EEG visual-evoked potentials in rats through neuromodulatory focused ultrasound. Neuroreport 2015, 26:211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim E, Anguluan E, Kim JG: Monitoring cerebral hemodynamic change during transcranial ultrasound stimulation using optical intrinsic signal imaging. Sci Rep 2017, 7:13148. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The presence of extensive hemodynamic changes depends on ultrasound pulse repetition frequencies when using unfocused tUS and optical imaging in awake headfixed mice.
- 49.He B, Sohrabpour A, Brown E, Liu Z: Electrophysiological Source Imaging: A Noninvasive Window to Brain Dynamics. Annu Rev Biomed Eng 2018, 20:171–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akil O, Oursler AE, Fan K, Lustig LR: Mouse Auditory Brainstem Response Testing. Bio Protoc 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]


