Abstract
Strategies for altering the reaction pathway of reactive intermediates are of significant importance in diversifying organic synthesis. Enol silyl ethers, versatile enolate equivalents, are known to undergo one-electron oxidation to generate the radical cations that spontaneously form electrophilic α-carbonyl radicals via elimination of the silyl groups. Here, we demonstrate that close scrutiny of the property of the radical cations as strong C–H acids enables the identification of a catalyst system consisting of an iridium-based photosensitizer and 2,4,6-collidine for the generation of nucleophilic allylic radicals from enol silyl ethers through one-electron oxidation-deprotonation sequence under light irradiation without the desilylation of the radical cation intermediates. The resultant allylic radicals engage in the addition to electron-deficient olefins, establishing the selective allylic C-H alkylation of enol silyl ethers. This strategy is broadly applicable, and the alkylated enol silyl ethers can be transformed into highly functionalized carbonyl compounds by exploiting their common polar reactivity.
Subject terms: Catalytic mechanisms, Homogeneous catalysis, Photocatalysis, Synthetic chemistry methodology
Allylic C–H activation of enol silyl ethers via one-electron oxidation is usually accompanied by undesired desilylation. Here, under hybrid photoredox and Brønsted base catalysis, the authors show the mild functionalization of enol silyl ethers, providing a platform to access an array of complex carbonyl compounds.
Introduction
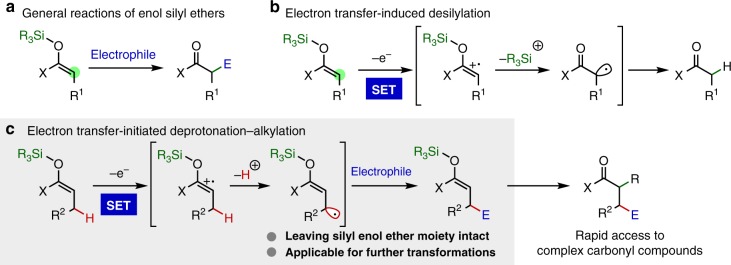
Enol silyl ethers and their analogs are one of the most versatile substrate classes and enjoy widespread applications in organic synthesis (Fig. 1a)1–3. They can be prepared from carbonyl compounds of all oxidation states, such as aldehydes, ketones, esters and amides, by reliable protocols and exhibit preeminent reactivity as enolate anion equivalents amenable to various catalysis manifolds. These distinct features render enol silyl ethers attractive yet powerful handles for selective α-functionalization of a wide array of carbonyl entities.
Fig. 1.
Transformations of enol silyl ethers. a General reaction pathway. b Known desilylation reaction initiated by single-electron oxidation. c The reaction design of allylic C–H alkylation
On the other hand, the allylic sp3-hybridized carbons of the enol silyl ethers are potential reaction sites as the electron-rich enol moiety contributes to decreasing bond-dissociation enthalpy of the allylic C–H bonds. In particular, selective bond formation at the allylic carbons4 with preservation of the enol silyl ether component offers an opportunity to harness the reactivity of the resulting functionalized enol silyl ethers for the conventional polar reactions, enabling access to α,β-difunctionalized carbonyl compounds5–7. However, despite their potential synthetic utility, only a few catalytic systems are available for direct allylic C–H functionalization of enol silyl ethers or their analogs, which rely on transition metal catalysis and synergistic photoredox-thiol catalysis8,9.
In addition to the common polar reactivity useful for a diverse set of transformations, it has long been recognized that enol silyl ethers undergo single-electron oxidation to generate the corresponding radical cations10. Yet, previous efforts for exploiting this radical reactivity in reaction development have been restricted to coupling with concomitantly generated heteroatom radicals11–15 or tethered olefins at the α-carbon of the carbonyl group; this is largely due to the strong propensity of the radical cation to undergo elimination of a silyl cation to produce parent carbonyls (Fig. 1b)10,16–22. Under these circumstances, we considered the intrinsic properties of this class of radical cations, specifically the presumed high acidity of allylic protons23–27. For instance, MacMillan28–31 reported that radical cations generated from aldehyde- or cyclic ketone-derived enamines underwent deprotonation to form allylic radicals that were susceptible to radical addition reactions for β-functionalizations of the parent carbonyls. With this profile in mind, we envisioned that the allylic proton of the radical cation derived from an enol silyl ether could be readily deprotonated to generate an allylic radical nucleophilic enough to be captured by an external electrophile (Fig. 1c). If the employment of an appropriate Brønsted base allows this proton abstraction event to occur predominantly over the desilylation, the single-electron oxidation–deprotonation sequence would provide a broadly applicable platform to execute bond construction exclusively at the allylic carbon, leaving the enol silyl ether component intact for further synthetic manipulations. Here, we disclose the successful implementation of this strategy through the development of a direct allylic C–H functionalization of a variety of enol silyl ethers with electron-deficient olefins under hybrid catalysis of an iridium-based photosensitizer and pyridine derivative with the irradiation of visible light. The utility of this unique C–H alkylation protocol is also demonstrated.
Results
Analysis of the property of enol silyl ether and its radical cation
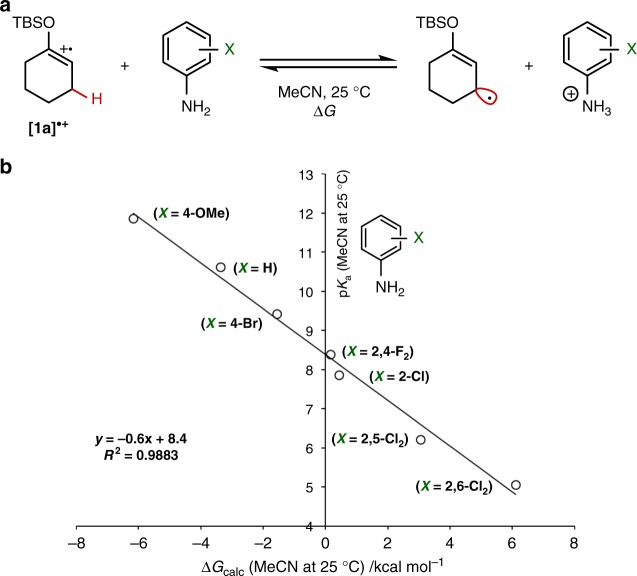
We selected the cyclohexanone-derived enol silyl ether 1a as a model substrate and investigated the physical properties requisite to establish a catalytic system for effecting the target allylic functionalization. First, the oxidation potential (Eox) of 1a was determined to be 1.52 V vs. saturated calomel electrode (SCE) by square wave voltammetry measurement in MeCN, which led us to use a IrIII photocatalyst bearing ligands suitable for imparting sufficient single-electron oxidation ability to the visible light-excited *IrIII to generate a radical cation from 1a. Second, the pKa value of the resulting radical cation [1a]•+ in MeCN was estimated from the calculated reaction free energy (ΔG) of deprotonation process. Since the direct determination of pKa from the calculated free energy is liable to give deviated value, we conducted statistical manipulation using experimentally available values. Specifically, the pKa of [1a]•+ was obtained by considering acid–base equilibria with a series of substituted anilines (Fig. 2), whose pKa values were experimentally determined (5–12 in MeCN)32. As shown in Fig. 2, the values for the reaction free energy calculated at the SMD(MeCN)-(U)CAM-B3LYP/6-311 + G(d,p) level and the experimental pKa values of anilines showed excellent linear correlation. The intercept of this plot corresponded to the pKa of [1a]•+ and was determined to be 8.4, which is close to that of TsOH [pKa(MeCN) = 8.6]33, meaning that [1a]•+ can be readily deprotonated by an appropriate Brønsted base. In view of structural tunability, we sought to employ an organic base with recognition that essential requirements for ensuring the function of the base are not only the pKa value but also the redox potential not to be involved in the single-electron redox processes operated by the photocatalyst.
Fig. 2.
Estimation of pKa of radical cation [1a]•+. a Acid–base equilibria between [1a]•+ and substituted anilines. b Linear free energy plot for the acid–base equilibria
Design of catalysis and optimization of reaction conditions
Based on these initial results and considerations, we began to evaluate the viability of our strategy in the reaction of 1a with benzalmalononitrile (2a) as an acceptor in the presence of [Ir(dF(CF3)ppy)2(4,4′-dCF3bpy)]PF6 (4a, 2 mol%) (*E1/2red = 1.65 V vs. SCE)34 and 2,4,6-collidine (1 equiv) (pKa = 14.98 in MeCN, E1/2ox = ≥2.0 V vs. SCE)32 in MeCN at ambient temperature. Under the irradiation of a blue light-emitting diode (LED), bond formation occurred at the allylic carbon (β-position to the latent carbonyl) to give the alkylated enol silyl ether 3a in 78% nuclear magnetic resonance (NMR) yield (Table 1, entry 1). The nature of the Brønsted base was of critical importance as the use of the sterically demanding 2,6-di-tert-butyl-4-methylpyridine (DTBMP) substantially decreased the yield of 3a and inorganic bases, such as potassium phosphate, were totally ineffective (entries 2 and 3). Furthermore, attempted alkylations with IrIII complexes 4b or 4c, having different bipyridine ligands, resulted in low conversion or no reaction, respectively, indicating that the reduction potential of the IrIII complex in the excited state as well as the oxidation potential of the transient IrII complex have a significant impact on reaction efficiency (entries 4 and 5). Interestingly, solvent screening revealed that 1,2-dichloroethane (DCE) was optimal, allowing the reaction to proceed with higher efficiency under the influence of 4a and 2,4,6-collidine to afford 3a in 88% yield (entry 6). Although the catalytic use of the Brønsted base (10 mol%) appeared feasible, slight decrease in the product yield was inevitable because of undesired desilylated ketone formation (entry 7). This problem was overcome by performing the reaction at higher substrate and catalyst concentration; desilylation was completely suppressed and 3a was isolated almost quantitatively (entry 8). The direct allylic C–H alkylation protocol thus developed was scalable as demonstrated by the reaction of 0.42 g of 1a with 2a, which proceeded smoothly with a reduced amount of 4a (0.5 mol%) but with increased light intensity under otherwise identical conditions to yield 0.71 g of 3a (96%) (entry 9).
Table 1.
Optimization of conditions for reaction of enol silyl ether 1a with benzalmalononitrile (2a)
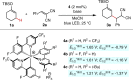
| ||||
|---|---|---|---|---|
| Entry | 4 | Base (mol%) | d.r.a | Yield (%)b |
| 1 | 4a | 2,4,6-collidine (100) | 1.7:1 | 78 |
| 2 | 4a | DTBMP (100) | 1.7:1 | 27 |
| 3 | 4a | K3PO4 (100) | – | 0 |
| 4 | 4b | 2,4,6-collidine (100) | 1.7:1 | 14 |
| 5 | 4c | 2,4,6-collidine (100) | – | 0 |
| 6c | 4a | 2,4,6-collidine (100) | 2.0:1 | 89 |
| 7c | 4a | 2,4,6-collidine (10) | 1.9:1 | 78 |
| 8d | 4a | 2,4,6-collidine (10) | 2.0:1 | 95 (98) |
| 9e | 4a | 2,4,6-collidine (10) | 1.8:1 | (96) |
Unless otherwise noted, the reactions were performed with 1a (0.1 mmol), 2a (1.2 equiv), base and 4 (2 mol%) in MeCN (1.0 mL) at 25 °C for 12 h under argon atmosphere with light irradiation (blue LED, 750 W m−2)
1H NMR proton nuclear magnetic resonance, d.r. diastereomeric ratios, LED light-emitting diode, DTBMP 2,6-di-tert-butyl-4-methylpyridine, TBS t-butyldimethylsilyl, DCE 1,2-dichloroethane
aDetermined by 1H NMR from crude reaction mixture
bNMR yield with mesitylene as an internal standard. The value within parentheses is an isolated yield
cThe reaction was conducted in DCE (1.0 mL)
dIn DCE (0.5 mL)
eCarried out with 1a (2.0 mmol, 0.42 g), 2a (1.1 equiv), 2,4,6-collidine (10 mol%) and 4a (0.5 mol%) in DCE (10 mL) at 25 °C for 18 h under argon atmosphere with light irradiation (blue LEDs, total 5000 W m−2)
To gain an insight into the reaction mechanism, especially the pathway for the generation of the reactive intermediate, Stern–Volmer luminescence quenching experiments were performed. Benzalmalononitrile (2a) and 2,4,6-collidine did not quench the excited state of photocatalyst 4a (Supplementary Fig. 3). In contrast, increasing the concentration of enol silyl ether 1a caused a significant decrease in emission intensity. These observations confirmed that the allylic C–H alkylation was initiated by the single-electron oxidation of 1a.
Investigation of substrate scope
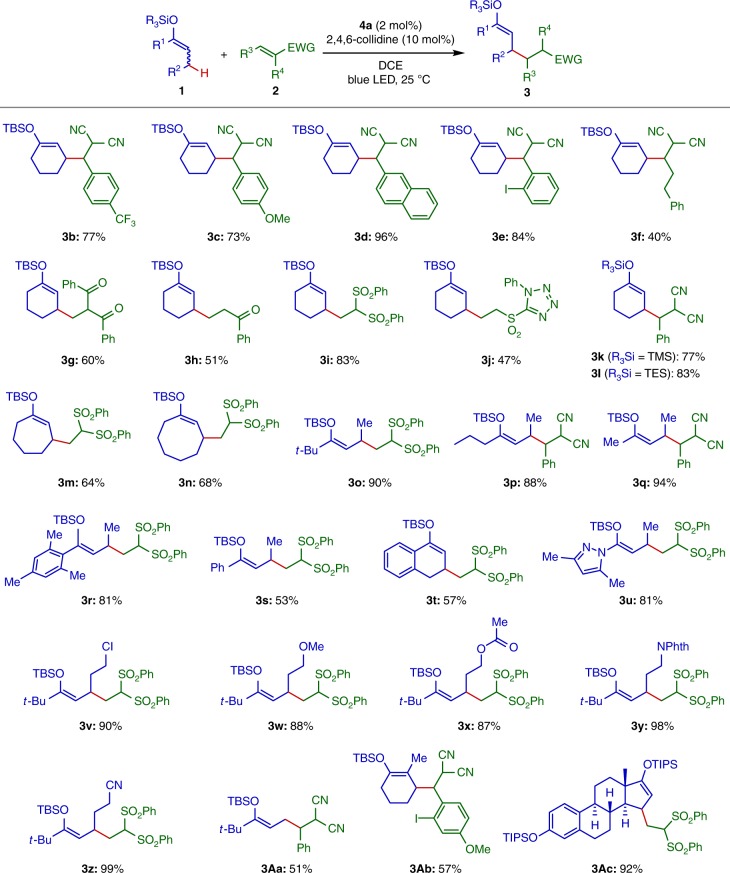
Having established the optimized reaction conditions, we explored the scope of this alkylation under photoredox-Brønsted base hybrid catalysis (Fig. 3). As demonstrated in the reactions of 1a, a range of arylidene malononitriles were employable as electrophilic acceptors and the corresponding alkylated enol silyl ethers 3b–3e were obtained in uniformly good yield. Alkylidene malononitrile could also be coupled with 1a to form 3f with moderate efficiency. Furthermore, α,β-unsaturated ketones and sulfones proved viable acceptors to furnish 3g–3j in moderate to good yield. With respect to enol silyl ether nucleophiles, it is important to note that trimethylsilyl and triethylsilyl derivatives underwent deprotonation–allylic alkylation to afford 3k and 3l in high yield, clearly indicating that the selectivity of deprotonation over desilylation does not rely on the steric demand of the silyl groups. In addition to the cyclohexanone derivatives, seven- and eight-membered cyclic enol silyl ethers smoothly reacted with 1,1-bis(phenylsulfonyl)ethylene to give good yields of 3m and 3n. The acyclic enol silyl ethers were also found to be suitable substrates; primary-alkyl ketone- and mesityl ketone-derived enol silyl ethers gave rise to the desired products 3o–3r in excellent yield, whereas the phenyl ketone-derived substrate provided 3s in lower yield (see below for theoretical investigations and associated discussion). Relatively low reactivity was also observed in the reaction of the α-tetralone derivative, resulting in the formation of 3t in 57% yield. Notably, butyryl pyrazole-derived ketene silyl hemiaminal was amenable to alkylation and afforded 3u in high yield. Enol silyl ethers with additional common functional groups, such as chlorine, ether, ester, imide and nitrile, were compatible with this protocol and could be converted into the corresponding alkylated enol silyl ethers 3v–3z with equally high efficiency. When the ethyl t-butyl ketone-derived enol silyl ether was subjected to the optimized conditions, the C–C bond formation occurred at the terminal, primary allylic carbon to yield 3Aa. On the other hand, 2-methylcyclohexanone-derived enol silyl ether was alkylated predominantly on the secondary allylic carbon to give 3Ab. An additional noteworthy aspect of this hybrid catalytic system was that it accommodated complex substrate setting; the allylic alkylation of the estrone derivative featuring a fused polycyclic framework delivered the enol silyl ether 3Ac in excellent yield.
Fig. 3.
Substrate scope. Isolated yields are shown. Diastereomeric ratios (d.r.) of the products with consecutive two stereocenters were 1.5:1–2.0:1. See the Supplementary Information for details
Analysis to rationalize the reactivity difference
The reactivity profile of the enol silyl ethers, particularly that observed with 1o (R1 = t-Bu), 1r (R1 = 2,4,6-Me3C6H2) and 1s (R1 = Ph), prompted us to conduct density functional theory calculations at the CAM-B3LYP/6-311 + G(d, p) level for these substrates in order to rationalize the differences in reactivity (Table 2). The pKa values of the corresponding radical cations in MeCN were calculated to be 8.4 (1o), 6.5 (1r) and 4.8 (1s), respectively (Supplementary Figs. 6–11). These values suggested that the deprotonation process to generate an allylic radical would be much easier for the 1s-derived radical cation ([1s]•+) than for the others, albeit the reaction of 1s provided the lowest yield (3s, 53%), indicating that acidity of the radical cation was not the sole factor governing the present radical addition reaction. We then calculated the singly occupied molecular orbital (SOMO) levels and spin density of 1o-, 1r- and 1s-derived allylic radicals as well as the activation barrier of their additions to 1,1-bis(phenylsulfonyl)ethylene (ΔG‡), respectively, and the results are summarized in Table 2. According to the frontier orbital theory, the reactivity of a radical generally depends on both the SOMO level and spin density at the reactive site. Considering almost the same SOMO level (=−5.77 eV), the reactivity difference between the three allylic radicals, [1o]•, [1r]• and [1s]•, would stem from differences in the spin density at the allylic C3 position. For [1o]•, from which the reaction proceeded with the lowest ΔG‡ of 21.2 kcal mol−1 to afford high yield of the product 3o (90%), a relatively large spin density (=0.70) was located at the allylic position. Although the larger spin density was estimated to be at the C1 position (=0.75), alkylation at this carbon would be highly unfavorable due to steric congestion, and in fact, the corresponding product was not detected experimentally. In contrast, the electron in the radical [1s]• can be delocalized over the phenyl group, decreasing the spin density at the C3 position (=0.51) compared to that of [1o]•. The difference in spin density was clearly reflected in the larger activation barrier (ΔG‡ = 23.3 kcal mol−1) and lower experimental yield of the product 3s (53%). The importance of spin localization for ensuring productive bond formation was also corroborated by the similar analysis with 1r in comparison to the outcome with 1s. Radical conjugation with the mesityl group is partially inhibited in [1r]• because of the torsional relationship between the allyl and mesityl moieties (Supplementary Fig. 12); this leads to a higher spin density (=0.64) at the C3 position and lower activation barrier (ΔG‡ = 21.6 kcal mol−1), thereby accounting for the experimentally observed higher efficiency in the formation of the product 3r (81% yield).
Table 2.
Summary of spin density and SOMO level of the radicals [1]• and activation barrier of their addition to 1,1-bis(phenylsulfonyl)ethylene
|
| |||||
|---|---|---|---|---|---|
| [1]• | Spin density | SOMOa (eV) | ΔG‡b (kcal mol−1) | Yield (%) of the reaction | |
| C1 | C3 | ||||
| [1o] • | 0.75 | 0.70 | −5.77 | 21.2 | 90 |
| [1r] • | 0.62 | 0.64 | −5.77 | 21.6 | 81 |
| [1s] • | 0.58 | 0.51 | −5.77 | 23.3 | 53 |
SOMO singly occupied molecular orbital
aCalculated at (U)CAM-B3LYP/6-311 + G(d, p)
bValues for Gibbs free energy (298.15 K and 1 atm) were calculated at SMD(DCE)-(U)CAM-B3LYP/6-311 + G(d, p)
Transformation of alkylated enol silyl ethers
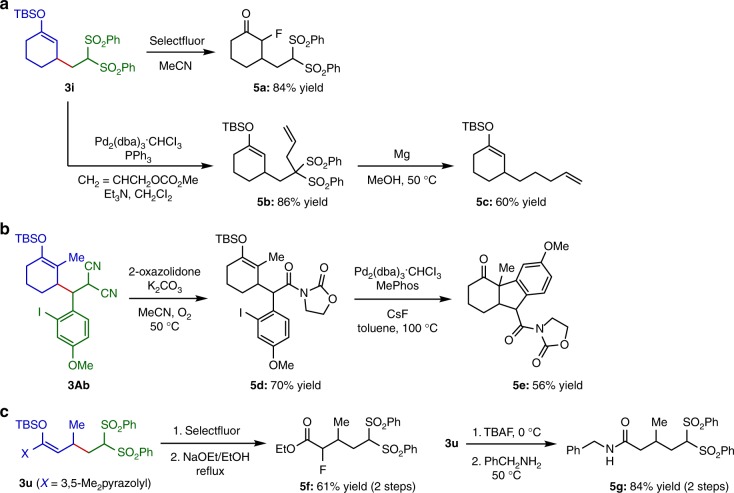
The alkylation products have two different reactive sites, the enol silyl ether and active methine or methylene. This salient structural feature enables diverse transformations to access a variety of complex carbonyl compounds, providing a powerful demonstration of the utility of this method (Fig. 4). For instance, treatment of the alkylated enol silyl ether 3i with Selectfluor facilitated smooth fluorination of the enol silyl ether component to produce the α-fluoro-β-alkylated ketone 5a. On the other hand, selective functionalization of the bis(sulfonyl)methyl moiety of 3i was feasible by the palladium-catalyzed allylation with allyl carbonate, affording 5b in high yield. Subsequent exposure of 5b to magnesium metal in MeOH effected desulfonylation to give 5c. The malononitrile subunit of the enol silyl ether 3Ab could be selectively converted into imide by reaction with 2-oxazolidone under oxygen atmosphere35. The resulting 5d underwent intramolecular arylation under palladium catalysis to furnish the fused tricyclic ketone 5e, of which structure is often found in diterpenoids, such as taiwaniaquinol A36. Moreover, functionalized esters and amides, such as 5f and 5g, were accessible through the common derivatizations of pyrazole-substituted ketene silyl hemiaminal 3u; the reaction with Selectfluor followed by solvolysis in ethanol gave rise to α-fluoro-β-alkylated ester 5f, while desilylation and subsequent condensation with benzylamine proceeded with high efficiency to yield 5g.
Fig. 4.
Diverse transformations of alkylated products. a Derivatizations of alkylated enol silyl ether 3i. b Synthesis of complex fused tricyclic ketone 5e from alkylated enol silyl ether 3Ab. c Derivatizations of alkylated ketene silyl hemiaminal 3u
We have developed a strategy for the allylic C–H alkylation of enol silyl ethers and their derivatives, which relies heavily on the combined use of appropriate photoredox and Brønsted base catalysts for the generation of requisite allylic radicals while suppressing undesired desilylation process. Under the hybrid catalysis, a series of enol silyl ethers smoothly react with electron-deficient olefins to give the corresponding functionalized enol silyl ethers. This operationally simple protocol, in concert with the ready availability of enol silyl ethers and their conventional polar reactivity, provides rapid and reliable access to an array of complex carbonyl compounds and will find widespread use among practitioners of organic synthesis.
Methods
Representative procedure for allylic alkylation
To a flame-dried test tube were added 2a (18.5 mg, 0.12 mmol), [Ir(dFCF3ppy)2(4,4′-dCF3bpy)]PF6 (2.29 mg, 0.002 mmol, 2 mol%) and DCE (0.5 mL, 0.2 M). The reaction tube was sealed with a rubber septum and then evacuated in vacuo and backfilled with Ar five times. 1a (21.2 mg, 0.1 mmol) and 2,4,6-collidine (1.3 μL, 0.01 mmol, 10 mol%) were successively introduced. The whole reaction mixture was stirred at 25 °C under the irradiation of blue LED (448 nm, 750 W m−2) with a fan to keep the temperature. After 12 h, the reaction mixture was evaporated. Purification of the resulting crude residue by column chromatography on silica gel (hexane 100% to hexane/EtOAc = 5:1) afforded 3a in 98% yield.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was financially supported by CREST from JST (JPMJCR13L2:13418441), JSPS KAKENHI Grant Number JP17H06446 in Hybrid Catalysis for Enabling Molecular Synthesis on Demand and JP18H01972.
Author contributions
K.O. and T.O. conceived and designed the study, and co-wrote the paper. T.N. performed the experiments, and analyzed the data together with K.O. M.S. conducted the theoretical investigation. All authors discussed the results and commented on the manuscript.
Data availability
All relevant data are available on request from the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks Lei Gong and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-10641-y.
References
- 1.Stork G, Hudrlik PF. Isolation of ketone enolates as trialkylsilyl ethers. J. Am. Chem. Soc. 1968;90:4462–4464. doi: 10.1021/ja01018a051. [DOI] [Google Scholar]
- 2.Kan SBJ, Ng KKH, Paterson I. The impact of the Mukaiyama aldol reaction in total synthesis. Angew. Chem. Int. Ed. 2013;52:9097–9108. doi: 10.1002/anie.201303914. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo J, Murakami M. The Mukaiyama aldol reaction: 40 years of continuous development. Angew. Chem. Int. Ed. 2013;52:9109–9118. doi: 10.1002/anie.201303192. [DOI] [PubMed] [Google Scholar]
- 4.Magnus P, Lacour J, Evans PA, Roe MB, Hulme C. Hypervalent iodine chemistry: new oxidation reactions using the iodosylbenzene−trimethylsilyl azide reagent combination. Direct α- and β-azido functionalization of triisopropylsilyl enol ethers. J. Am. Chem. Soc. 1996;118:3406–3418. doi: 10.1021/ja953906r. [DOI] [Google Scholar]
- 5.Tietze LF. Domino Reactions: Concepts for Efficient Organic Synthesis. Weinheim: Wiley; 2014. [Google Scholar]
- 6.Bhanja C, Jena S, Nayak S. Organocatalytic tandem Michael addition reactions: a powerful access to the enantioselective synthesis of functionalized chromenes, thiochromenes and 1,2-dihydroquinolines. Beilstein J. Org. Chem. 2012;8:1668–1694. doi: 10.3762/bjoc.8.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serrano-Molina D, Martín-Castro AM. Tandem sequences involving Michael additions and sigmatropic rearrangements. Synthesis. 2016;48:3459–3469. doi: 10.1055/s-0035-1562554. [DOI] [Google Scholar]
- 8.Davies HML, Ren P. Catalytic asymmetric C−H activation of silyl enol ethers as an equivalent of an asymmetric Michael reaction. J. Am. Chem. Soc. 2001;123:2070–2071. doi: 10.1021/ja0035607. [DOI] [PubMed] [Google Scholar]
- 9.Cuthbertson JD, MacMillan DWC. The direct arylation of allylic sp3 C–H bonds via organic and photoredox catalysis. Nature. 2015;519:74–77. doi: 10.1038/nature14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gassman PG, Bottorff KJ. Electron transfer induced desilylation of trimethylsilyl enol ethers. J. Org. Chem. 1988;53:1097–1100. doi: 10.1021/jo00240a034. [DOI] [Google Scholar]
- 11.Bhattacharya A, DiMichele LM, Dolling U-H, Grabowski EJJ, Grenda VJ. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) oxidation of silyl enol ethers to enones via DDQ-substrate adducts. J. Org. Chem. 1989;54:6118–6120. doi: 10.1021/jo00287a027. [DOI] [Google Scholar]
- 12.Bockman TM, Shukla D, Kochi JK. Photoinduced electron transfer from enol silyl ethers to quinone. Part 1. Pronounced effects of solvent polarity and added salt on the formation of α-enones. J. Chern. Soc. Perkin Trans. 1996;2:1623–1632. doi: 10.1039/P29960001623. [DOI] [Google Scholar]
- 13.Bockman TM, Kochi JK. Photoinduced electron transfer from enol silyl ethers to quinone. Part 2. Direct observation of ion-pair dynamics by time-resolved spectroscopy. J. Chern. Soc. Perkin Trans. 1996;2:1633–1643. doi: 10.1039/p29960001633. [DOI] [Google Scholar]
- 14.Rathore R, Kochi JK. α-Nitration of ketones via enol silyl ethers. Radical cations as reactive intermediates in thermal and photochemical processes. J. Org. Chem. 1996;61:627–639. doi: 10.1021/jo9515687. [DOI] [PubMed] [Google Scholar]
- 15.Ramsden CA, Smith RG. Reaction of silyl enol ethers with xenon difluoride in MeCN: evidence for a nonclassical radical cation intermediate. Org. Lett. 1999;1:1591–1594. doi: 10.1021/ol990231e. [DOI] [Google Scholar]
- 16.Hudson CM, Marzabadi MR, Moeller KD, New DG. Intramolecular anodic olefin coupling reactions: a useful method for carbon-carbon bond formation. J. Am. Chem. Soc. 1991;113:7372–7385. doi: 10.1021/ja00019a038. [DOI] [Google Scholar]
- 17.Snider BB, Kwon T. Oxidative cyclization of δ,ε- and ε,ζ-unsaturated enol silyl ethers and unsaturated siloxycyclopropanes. J. Org. Chem. 1992;57:2399–2410. doi: 10.1021/jo00034a038. [DOI] [Google Scholar]
- 18.Heidbreder A, Mattay J. PET-oxidative cyclization of unsaturated enol silyl ethers. Tetrahedron Lett. 1992;33:1973–1976. doi: 10.1016/0040-4039(92)88117-N. [DOI] [Google Scholar]
- 19.Hintz, S., Mattay, J., van Eldik, R. & Fu, W.-F. Regio‐ and stereoselective cyclization reactions of unsaturated silyl enol ethers by photoinduced electron transfer—mechanistic aspects and synthetic approach. Eur. J. Org. Chem. 1998, 1583–1596 (1998).
- 20.Wright DL, Whitehead CR, Sessions EH, Ghiviriga I, Frey DA. Studies on inducers of nerve growth factor: synthesis of the cyathin core. Org. Lett. 1999;1:1535–1538. doi: 10.1021/ol991032y. [DOI] [PubMed] [Google Scholar]
- 21.Ackermann L, Heidbreder A, Wurche F, Klärner F-G, Mattay J. Mechanistic studies on PET-oxidative cyclization of unsaturated silyl enol ethers: dependence of the regioselectivity on alcohol addition and pressure effects. J. Chern. Soc. Perkin Trans. 1999;2:863–869. doi: 10.1039/a807683i. [DOI] [Google Scholar]
- 22.Bunte JO, et al. Synthesis and PET oxidative cyclization of silyl enol ethers: build-up of quasi-steroidal carbocycles. Tetrahedron Lett. 2003;44:45–48. doi: 10.1016/S0040-4039(02)02491-7. [DOI] [Google Scholar]
- 23.Schmittel M, Burghart A. Understanding reactivity patterns of radical cations. Angew. Chem. Int. Ed. Engl. 1997;36:2550–2589. doi: 10.1002/anie.199725501. [DOI] [Google Scholar]
- 24.Nakajima K, Miyake Y, Nishibayashi Y. Synthetic utilization of α-aminoalkyl radicals and related species in visible light photoredox catalysis. Acc. Chem. Res. 2016;49:1946–1956. doi: 10.1021/acs.accounts.6b00251. [DOI] [PubMed] [Google Scholar]
- 25.Lee KN, Ngai M-Y. Recent developments in transition-metal photoredox-catalysed reactions of carbonyl derivatives. Chem. Commun. 2017;53:13093–13112. doi: 10.1039/C7CC06287G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou R, Liu H, Tao H, Yu X, Wu J. Metal-free direct alkylation of unfunctionalized allylic/benzylic sp3 C–H bonds via photoredox induced radical cation deprotonation. Chem. Sci. 2017;8:4654–4659. doi: 10.1039/C7SC00953D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McManus JB, Onuska NPR, Nicewicz DA. Generation and alkylation of α-carbamyl radicals via organic photoredox catalysis. J. Am. Chem. Soc. 2018;140:9056–9060. doi: 10.1021/jacs.8b04890. [DOI] [PubMed] [Google Scholar]
- 28.Pirnot MT, Rankic DA, Martin DBC, MacMillan DWC. Photoredox activation for the direct β-arylation of ketones and aldehydes. Science. 2013;339:1593–1596. doi: 10.1126/science.1232993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petronijević FR, Nappi M, MacMillan DWC. Direct β-functionalization of cyclic ketones with aryl ketones via the merger of photoredox and organocatalysis. J. Am. Chem. Soc. 2013;135:18323–18326. doi: 10.1021/ja410478a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terrett JA, Clift MD, MacMillan DWC. Direct β-alkylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 2014;136:6858–6861. doi: 10.1021/ja502639e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeffrey JL, Petronijević FR, MacMillan DWC. Selective radical–radical cross-couplings: design of a formal β-Mannich reaction. J. Am. Chem. Soc. 2015;137:8404–8407. doi: 10.1021/jacs.5b05376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaljurand I, et al. Extension of the self-consistent spectrophotometric basicity scale in acetonitrile to a full span of 28 pKa units: unification of different basicity scales. J. Org. Chem. 2005;70:1019–1028. doi: 10.1021/jo048252w. [DOI] [PubMed] [Google Scholar]
- 33.Kütt A, et al. A comprehensive self-consistent spectrophotometric acidity scale of neutral Brønsted acids in acetonitrile. J. Org. Chem. 2006;71:2829–2838. doi: 10.1021/jo060031y. [DOI] [PubMed] [Google Scholar]
- 34.Choi GJ, Zhu Q, Miller DC, Gu CJ, Knowles RR. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature. 2016;539:268–271. doi: 10.1038/nature19811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Lear MJ, Hayashi Y. Sterically demanding oxidative amidation of α-substituted malononitriles with amines using O2. Angew. Chem. Int. Ed. 2016;55:9060–9064. doi: 10.1002/anie.201603399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thommen C, Jana CK, Neuburger M, Gademann K. Syntheses of taiwaniaquinone F and taiwaniaquinol A via an unusual remote C–H functionalization. Org. Lett. 2013;15:1390–1393. doi: 10.1021/ol4003652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All relevant data are available on request from the authors.