Abstract
Objective
Neuroanatomical differences in the cerebellum are among the most consistent findings in multiple system atrophy (MSA) patients. This study performed a detailed cerebellar morphology in MSA patients and its two subtypes: MSA-P (parkinson's symptoms predominate) and MSA-C (cerebellar symptoms predominant), and their relations to profiles of motor and cognitive deficits.
Materials and methods
Structure MRI data were acquired from 63 healthy controls and 61 MSA patients; voxel-based morphometry and the Spatially Unbiased Infratentorial Toolbox cerebellar atlas were performed to identify the cerebellar gray volume changes in MSA and its subtypes. Further, the gray matter changes were correlated with the clinical motor/cognitive scores.
Results
Patients with MSA exhibited widespread loss of cerebellar volume bilaterally, relative to healthy controls. In those with MSA-C, gray matter loss was detected from anterior (bilateral lobule IV-V) to posterior (bilateral crus I/II, bilateral lobule IX, left lobule VIII) cerebellar lobes. Lower anterior cerebellar volume negatively correlated with disease duration and motor performance, whereas posterior lobe integrity positively correlated with cognitive assessment. In patients with MSA-P, atrophy of anterior lobe (bilateral lobules IV-V) and posterior lobe in part (left lobule VI, bilateral IX) was evident; and in left cerebellar lobule IX, gray matter loss negatively correlated with motor scores. Direct comparison of MSA-P and MSA-C group outcomes showed divergence in right cerebellar crus II only.
Conclusions
Our data suggest that volumetric abnormalities of cerebellum contribute substantially to motor and cognitive performance in patients with MSA. In patients with MSA-P and MSA-C, affected regions of cerebellum differed.
Keywords: Multiple system atrophy, Cerebellum, SUIT, Motor, Cognitive
Abbreviations: MSA, multiple system atrophy; MSA-P, multiple system atrophy patients with predominant parkinsonism; MSA-C, multiple system atrophy patients with predominant cerebellar signs; GM, gray matter; SUIT, Spatially Unbiased Infratentorial Template; HC, Healthy Controls; VBM, voxel-based morphometry; MoCA, Montreal Cognitive Assessment; UMSARS, Unified Multiple System Atrophy Rating Scale; WM, white matter; CSF, cerebrospinal fluid; MNI, Montreal Neurological Institute; TIV, total intracranial volume; SD, standard deviation; FWHM, full width at half maximum; ANOVA, One-way analysis of variance; SMA, supply motor areas
Highlights
-
•
Cerebellum atrophy contributed substantially to motor and cognitive behavior in MSA.
-
•
Lower cerebellum IV-V volume was correlated with MSA-C disease duration and severity
-
•
Cerebellum atrophy in one side may imply symptoms onset on contralateral
1. Introduction
Multiple system atrophy (MSA) is a neurodegenerative disorder characterized by progressive declines in both motor and cognitive functions. Pathologically, there is selective neuronal loss, and glial cytoplasmic inclusions are present within the basal ganglia, pons, inferiorolivary nucleus, and cerebellum (Cykowski et al., 2015).
Given its anatomic and functional interconnectivity with the basal ganglia and much of the cortical mantle forming the cerebello-thalamo-cortical circuit, the cerebellum is a known arbiter of motor planning and execution, as well as a host of higher-order cognitive and emotional functions (Bostan et al., 2013; Buckner, 2013; Leggio and Molinari, 2015). Parallel lines of evidence also indicate that the cerebellum is a potential region of interest (ROI) for understanding motor and cognitive deficits in both parkinsonian (MSA-P) and cerebellar (MSA-C) MSA subtypes (Minnerop et al., 2007). In patients with MSA-C, for example, structural imaging data indicate that cerebellar volume is reduced in even early stages of MSA (Dash et al., 2018), and the related gray matter (GM) loss bears a significant association with cognitive profiles (Kim et al., 2015). Based on a three-dimensional gyrification index, Miyatake et al. (Miyatake et al., 2010) have further demonstrated that patients with the MSA-C variant show GM losses primarily in upper lobules and anterior lobes of cerebellum, determining a negative correlation between hemispheric volume and cerebellar ataxia scores. Cerebellar abnormalities are also common in the MSA-P variant, which is typically considered a basal ganglia disorder. Diminished cerebellar activation, in conjunction with reduced cortical activation, is reportedly reflected in both motor (Rosskopf et al., 2018) and cognitive scores (Wang et al., 2017). Because such structural and functional cerebellar differences are consistently reported in both types of MSA, cerebellar dysfunction may be an important etiologic factor in this disorder. Unfortunately, studies aimed at the relation between cerebellar volume loss and clinical motor/cognitive deficits in these two MSA variants are lacking.
Although most earlier publications have examined the cerebellum as a whole, the cerebellum is anatomically separable into anterior (lobules I-V) and posterior (lobules VI-X) lobes. Distinct cerebellar subregions project to unique cortical targets having specific roles in motor planning and execution, as well as in cognitive functions (Stoodley and Schmahmann, 2010). Despite similarities in the cerebellar dysfunction of patients with MSA-P and MSA-C, their clinical manifestations are distinct. By localizing respective structural disparities, the cerebellar findings in MSA may be more fully understood. However, the cerebellum is seated in posterior fossa, its cortical GM tightly folded with only thin layers of white matter (WM). These traits have proven technically problematic in gauging subregional GM volumetric flux. The current study was undertaken to better understand the relation between cerebellar morphology and clinical scores of patients with these two MSA variants. Herein, we used the novel Spatially Unbiased Infratentorial Template (SUIT; http://www.icn.ucl.ac.uk/motorcontrol/imaging/suit.htm), designed expressly for assessing cerebellar morphology. Limitations of the Talairach-Tournoux brain atlas are thus circumvented, enabling more accurate depiction than whole-brain methods offer. Previously conducted functional MRI studies relying on SUIT have successfully identified morphologic changes of cerebellar subregions in several neuropsychiatric diseases (Chen et al., 2018; Cocozza et al., 2017; de Azevedo et al., 2017; Piccinin et al., 2017).In addition, these studies showed that SUIT template preserves more anatomical detail of the cerebellum and allows for better localization of cerebellar findings than the commonly-used whole brain Voxel-based morphometry(VBM) MNI template.
To our knowledge, this is the first use of SUIT to examine volumetric alterations of cerebellar subregions, comparing them with clinical parameters of patients with these MSA subtypes. We anticipated a link between diminished cerebellar volume and motor/cognitive impairment in this setting. We also envisioned both common and distinct subregional patterns of cerebellar atrophy in patients with MSA-P or MSA-C.
2. Materials and methods
2.1. Case selection
A total of 61 patients (28 MSA-C patients and 33 MSA-P patients) and 63 healthy controls (HC) were recruited from The First Hospital of China Medical University. Written informed consent was obtained from all of the participants. MSA patients were diagnosed by a neurologist and met the “probable” MSA clinical diagnostic criteria. All of the participants were right-handed Han Chinese. Potential participants were excluded when (a) obvious brain lesions were assessed on the basis of medical history and conventional MRI; (b) they had a history of other major neurological or psychiatric disorders, diabetes, or thyroid disease. Our study was approved by the medical research ethical committee of the First Affiliated Hospital of China Medical University, and informed consent was obtained from all of the participants.
2.2. Behavioral and neuropsychological assessment
Cognitive status was tested by administering the Montreal Cognitive Assessment (MoCA). Disease severity was measured by parts 2 and 4 of the Unified Multiple System Atrophy Rating Scale (UMSARS). Based on the predominant clinical symptomatology and consensus of the investigating neurologists, 33 patients qualified as MSA-P and 28 as MSA-C. A complete accounting of these assessments is shown in Table 1.
Table 1.
Demographic and clinical score in patients with multiple system atrophy and controls.
| Demographic variable | MSA (n = 61) | MSA-C (n = 28) | MSA-P (n = 33) | Control (n = 63) | p value |
|---|---|---|---|---|---|
| Age (mean ± SD) | 62.97 ± 8.53 | 63.83 ± 9.04 | 62.21 + 7.98 | 63.78 + 4.71 | 0.512 |
| Gender (M/F) | 26:35 | 13:15 | 14:19 | 25:38 | 0.742 |
| Education(years) | 12.03 ± 3.72 | 10.76 ± 3.54 | 11.09 ± 4.82 | 11.70 ± 2.53 | 0.559 |
| Duration of disease (years) | 3.37 ± 1.86 | 3.47 ± 1.60 | 3.27 ± 2.08 | – | 0.75# |
| MoCA | 22.39 ± 3.91 | 21.76 ± 4.06 | 22.39 ± 4.75 | 26.98 ± 1.09 | 0.00* |
| UMSARS | 30.43 ± 17.99 | 32.59 ± 20.79 | 30.12 ± 17.53 | – | 0.61# |
| Lateral onset(L/R/Nlo) | 16/33/12 | 5/16/7 | 11/17/5 | – | 0.149# |
SD, standard deviation; MSA: multiple system atrophy; MSA-P: MSA-parkinsonian variant; MSA-C: MSA-cerebellar variant; HC: healthy controls; UMSARS: Unified Multiple System Atrophy Rating Scale; MoCA: Montreal Cognitive Assessment; 0.00*: values <0.000; 0.75#: comparison between MSA-P and MSA-C; Nlo: Non lateralized onset.
2.3. MRI acquisition protocol
Images were generated using a 3.0 T MRI scanner (Verio; Siemens, Erlangen, Germany) equipped with a standard 32-channel head coil. High-revolution three-dimensional T1-weighted images were acquired in sagittal orientation employing a three-dimensional sagittal magnetization-prepared rapid acquisition gradient echo sequence with the following parameters: matrix size, 256 × 256; slice total, 176; TR, 5000 ms; TE, 2960 ms; FOV, 256 × 256 mm2; flip angle, 12°, slice thickness, 1 mm; distance factor, 0.5; and voxel size, 1.0 × 1.0 × 1.0 mm. All images were blindly reviewed by an experienced neuroradiologist to screen for other neurologic diseases and artifacts.
2.4. Whole-brain voxel-based morphometry analysis
Whole-brain VBM (patients and controls) was conducted, using Statistical Parametric Mapping version 12 (SPM12; Welcome Department of Imaging Neuroscience, London, UK) to analyzed GM abnormalities. Individual 3D T1 images were first examined for quality control, anterior commissure serving as origin. They were then segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) components for subsequent affine transformation into Montreal Neurological Institute (MNI) space and modulation. Standard smoothing of modulated images entailed an 8-mm full width at half maximum (FWHM) Gaussian kernel. A two-sample t-test was engaged, using age, sex, years of education, and total intracranial volume as covariates, to compare GM volumes of patient and HC subsets.
2.5. Cerebellar SUIT VBM analysis
Cerebellar data processing was performed using SUIT from SPM12. All images were visually inspected to ensure successful pre-processing and acceptable image quality. The images were processed using the following steps: 3DT1 images acquired in DICOM format were first transformed to NIfTI (dcm2nii software: (http://www.mccauslandcenter.sc.edu/mricro/mricron/dcm2nii.html). To minimize any error, the images were aligned along the anterior commissure and rotated in sagittal, coronal, and axial planes. The images were then isolated to infratentorial structures from surrounding tissue, and targeted maps were generated (GM, white matter, and cerebrospinal fluid). The segmented GM images were normalized to the SUIT template by using DARTEL (Welcome Department of Imaging Neuroscience)(Ashburner, 2007). Next, we conducted homogeneity tests using the images' covariance. Finally, the images were smoothed by a Gaussian filter kernel with 3 mm FWHM contained in SPM12 (http://www.fil.ion.ucl.ac.uk) to satisfy the normal distribution assumed for statistical analysis of regional differences. The generated statistical parametric map identifies regions with significant cerebellar differences.
2.6. Statistical analysis
One-way analysis of variance (ANOVA), the chi-square test, the Kruskal–Wallis test, the Mann–Whitney test, and the two-sample t-test were performed for demographic variables and clinical data using SPSS22.0 software (IBM Software Analytics, New York, NY). p < .05 was considered to be statistically significant.
Statistical analysis of SUIT data was performed using SPM software. Two-sample t-tests with age, gender, years of education and the whole-brain volume (the sum of GM, WM, and CSF volumes) as covariates were performed to explore cerebellar volume differences between the MSA and HC groups. Subsequently, post hoc analysis was used to explore between-group cerebellum volume differences within a mask presenting statistical significance in the two-sample t-test results and sharing similar covariates For every contrast(MSA vs HC, MSA-C vs. HC, MSA-P vs. HC, MSA-C vs. MSA-P),regional differences were considered significant only if they survived after correction for multiple comparisons false discovery rate (FDR), FDR correction at cluster-level, p < .05 – clusters formed at uncorrected level with p < .001.According to the type of research data, a preliminary Spearman correlation analysis was conducted to investigate the correlations between UMSARS and MoCA scores and cerebellum values within MSA patients. Correlation analysis was also performed in present study in order to clarify how the disease duration acts over the cerebellar GM. A threshold of p < .05 was considered to be a significant difference by SPSS22 software.
3. Results
Major demographic and clinical characteristics of all study participants are presented in Table 1. Sixty-two patients with MSA (MSA-P, 33; MSA-C, 28; mean age, 62.97 ± 8.53 years) and 63 HC subjects (mean age, 63.78 ± 4.71 years) were enrolled. The MSA and HC subsets did not differ significantly by age, sex, or level of education. As expected, patients with MSA had lower MoCA scores than did HC group members. There were no significant differences between MSA-P and MSA-C patient subsets in terms of duration of illness, UMSARS scores, or laterality at onset.
3.1. Whole-brain VBM analysis
Both MSA-P and MSA-C patient subsets were examined for cerebellar GM declines. To summarize, patients with MSA-P (vs HC subjects) showed supratentorial (putamen) and infratentorial (cerebellar) loss of GM, whereas GM loss in patients with MSA-C was confined to cerebellum.
3.2. SUIT analysis
MSA patients showed significantly reduced GM in bilateral cerebellum crus I/II, bilateral lobule IX, bilateral lobule IV-V and right lobule VIII compared with the HC group. (Table 2; Fig. 1).
Table 2.
Brain regions with significant cerebellar GMV differences between MSA and HC group.
| Index brain region | Cluster size (voxels) |
Peak MNI Coordinates |
T values | ||
|---|---|---|---|---|---|
| X Y Z | |||||
| 1 Cerebellum crus IX_R (AAL) | 209 | 14 | −50 | −45 | −5.559 |
| 2 Cerebellum crus II_L (AAL) | 277 | −6 | −40 | −21 | −5.759 |
| 3 Cerebellum _IV-V_L (AAL) | 145 | −6 | −40 | −21 | −5.758 |
| 4 Cerebellum _IX_L (AAL) | 119 | −10 | −44 | −53 | −5.794 |
| 5 Cerebellum _VIII_R (AAL) | 111 | 6 | −74 | −41 | −5.104 |
| 6 Cerebellum crus II_R (AAL) | 135 | 32 | −76 | −39 | −4.490 |
| 7 Cerebellum crus I_R (AAL) | 98 | 36 | −70 | −25 | −4.274 |
| 8 Cerebellum crus I_L (AAL) | 193 | −28 | −54 | −31 | −4.158 |
| 9 Cerebellum_ IV-V _R (AAL) | 102 | 4 | −64 | −21 | −6.399 |
Clusters of GM identified by SPM analysis (FDR-corrected, p < .05).
Fig. 1.
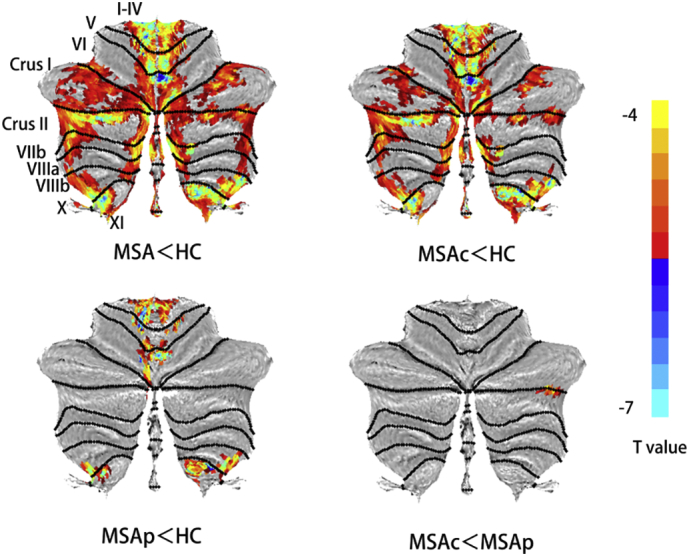
Group-wise structural atrophy of cerebellum (MSA vs HC subsets) Note: Map is color coded in overlay, with atrophy shown on surface-based flat maps determined by SUIT toolbox (Diedrichsen and Zotow, 2015).
Relative to HC subjects, MSA-C patients exhibited similarly decreased cerebellum GM volume in bilateral lobule IX, crus I/II; bilateral lobule IV-V and lobule VIII,. MSA-P patients showed decreased GM, including in bilateral lobule IX, left lobule VI, and bilateral lobule IV-V. Compared with the MSA-P subset, loss of right cerebellar crus II volume was notable in patients with MSA-C (Table 3; Fig. 1).
Table 3.
Brain regions with significant cerebellar GMV differences between MSA subtypes and HC group.
| Index brain region | Cluster size |
Peak MNI coordinates |
T values | ||
|---|---|---|---|---|---|
| (voxels) | X | Y | Z | ||
| MSAc < HC | |||||
| 1 Cerebellum crus IX_R (AAL) | 140 | 14 | −-48 | −-47 | −-6.153 |
| 2 Cerebellum crus II_L (AAL) | 189 | −-40 | −-62 | −-43 | −-6.238 |
| 3 Cerebellum _IV-V_L (AAL) | 136 | −-4 | −-42 | −-15 | −-5.666 |
| 4 Cerebellum _IX_L (AAL) | 89 | −-10 | −-46 | −-47 | −-5.025 |
| 5 Cerebellum _VIII_L (AAL) | 71 | −-8 | −-74 | −-27 | −-5.615 |
| 6 Cerebellum _VIII_R (AAL) | 57 | 8 | −-72 | −-41 | −-5.405 |
| 7 Cerebellum crus II_R (AAL) | 105 | 34 | −-76 | −-39 | −-5.662 |
| 8 Cerebellum crus I_R (AAL) | 72 | 42 | −-64 | −-29 | −-4.774 |
| 9 Cerebellum crus I_L (AAL) | 53 | −-30 | −-52 | −-31 | −-4.536 |
| 10 Cerebellum_ IV_V _R (AAL) | 79 | 4 | −-64 | −-21 | −-6.057 |
| MSAp < HC | |||||
| 1. Cerebellum crus IX_L (AAL) | 41 | −-10 | −-44 | −-53 | −-5.197 |
| 2. Cerebellum crus IX_R (AAL) | 64 | 14 | −-50 | −-45 | −-4.889 |
| 3. Cerebellum _VI_L (AAL) | 23 | −-10 | −-68 | −-21 | −-4.631 |
| 4. Cerebellum _IV-V_R (AAL) | 41 | 14 | −-48 | −-23 | −-5.201 |
| 5. Cerebellum_ IV-V _L (AAL) | 59 | −-6 | −-50 | −-17 | −-4.938 |
| MSAc < MSAp | |||||
| 1. Cerebellum crus II_R (AAL) | 31 | 44 | −-70 | −-41 | −-4.480 |
Clusters of GM identified by SPM analysis (FDR-corrected, p < .05; threshold k > 20 voxels), MSA-P: MSA-parkinsonian variant; MSA-C: MSA-cerebellar variant; HC: healthy controls.
3.3. Correlation analyses between neuropsychological assessment and gray matter volume of cerebellum subfield
Compared to the HC group, MoCA scores were significantly lower level in the MSA group and showed positive correlation with the extent of atrophy in right lobule IX, right crus II, bilateral lobule IV-V, and right lobule VIII. The MSA group showed higher UMSARS scores and negative correlation with the extent of atrophy values in the bilateral lobule IV-V, right crus II.
In the MSA-C group, significant positive correlation between cerebellar integrity and MoCA scores were observed in bilateral lobule IX, bilateral crus I/II, bilateral lobule IV-V and bilateral lobule VIII, and significant negative correlation between cerebellar integrity and UMSARS scores were observed in bilateral lobule IV-V. Left lobule IV-V show negative correlation with disease duration.
In MSA-P, significant correlations were found for UMSARS scores (left lobule IX).
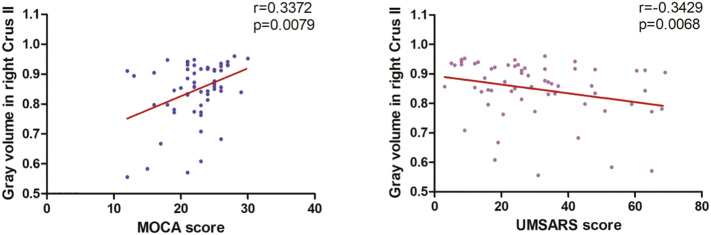
Compared to MSA-P, MSA-C patients showed GM loss only located in right crus II, GM atrophy in crus II show positive correlation with MoCA scores but inverse correlation with UMSARS scores (Tables 4; Fig. 2).
Table 4.
Regional correlations between cerebellar GM and behavioral (MoCA/UMSARS) scores or disease duration.
| Lobule | MoCA scores | UMSARS scores | Disease duration |
|---|---|---|---|
| MSA group | |||
| Cerebellum crus IX_R (AAL) | r = 0.285 (p = .026) | ||
| Cerebellum _IV-V_L (AAL) | r = 0.398(p = .002) | r = −0.395(p = .002) | |
| Cerebellum _VIII_R (AAL) | r = 0.298(p = .02) | ||
| Cerebellum crus II_ R (AAL) | r = 0.337(p = .008) | r = −0.343(p = .007) | |
| Cerebellum_ IV-V _R (AAL) | r = 0.374(p = .003) | r = −0.371(p = .003) | |
| MSA-C group | |||
| Cerebellum crus IX_R (AAL) | r = 0.560(p = .002) | ||
| Cerebellum crus II_L (AAL) | r = 0.433(p = .021) | ||
| Cerebellum _IV-V_L (AAL) | r = 0.508(p = .006) | r = −0.475(p = .011) | r = −0.440(p = .019) |
| Cerebellum _IX_L (AAL) | r = 0.519(p = .005) | ||
| Cerebellum _VIII_L (AAL) | r = 0.472(p = .011) | ||
| Cerebellum _VIII_R (AAL) | r = 0.527(p = .004) | ||
| Cerebellum crus II_R (AAL) | r = 0.415(p = .028) | ||
| Cerebellum crus I_R (AAL) | r = 0.409(p = .031) | ||
| Cerebellum crus I_L (AAL) | r = 0.471(p = .011) | ||
| Cerebellum_ IV-V _R (AAL) | r = 0.534(p = .003) | ||
| MSA-P group | |||
| Cerebellum _IX_L (AAL) | r = −0.413(p = .017) | ||
Fig. 2.
Scatter plots of GMV values of peak voxels and MoCA/UMSARS scores in the right cerebellum Crus II.
4. Discussion
To explore the contribution of cerebellar GM integrity toward motor and cognitive impairment in MSA patients, first, we compared cerebellar volumes between MSA patients and HCs. Then, based on the SUIT-VBM results, we searched for a relationship between volumes of cerebellar subfields and clinical measures. Compared to HCs, MSA patients showed widespread anterior (lobules IV-V) and posterior (lobules VI–IX) cerebellum GM atrophy. MSA-C patients had similar GM atrophy in anterior and posterior subregions, whereas MSA-P patients had GM atrophy in lobules IV-V and partly posterior cerebellum (lobules IX). Only right crus II atrophy was detected after a direct contrast between the two subtypes. The extent of atrophy in the anterior cerebellum was negatively correlated with UMSARS scores, and the extent of atrophy in the posterior cerebellum was positively correlated with MoCA scores, which was consistent with our hypotheses. These findings suggested that cerebellar volumetric abnormalities are likely to play an important role in the motor and cognitive performance in MSA patients. Furthermore, our results suggested that GM volume loss left lobule IV-V was correlated with disease duration and severity.
An intact cerebellum-to-cortex closed loop is integral to cerebellar regulation of motor and cognitive functions. Prior pathologic studies have implicated cerebellar GCIs in both MSA-P and MSA-C variants (Cykowski et al., 2015). Indeed, the structural and functional dysfunction observed in patients with MSA is linked to a profusion of GCIs. Despite an abundance of research focused on pathologic versus compensatory cerebellar engagement in MSA, results have been inconsistent (Payoux et al., 2010; Rosskopf et al., 2018). Both are seemingly increased, with diminished connectivity ostensibly driven by localized structural GM loss. In the present study, we found more widespread bilateral cerebellar GM decline in patients with MSA-C, rather than MSA-P. Thus, the cerebellar changes we observed are syndrome-specific in nature. It is noteworthy, however, that GM changes are late developments of MSA and therefore offer an incomplete picture of the disease. Studies addressing GM and WM changes are needed to better delineate the structural abnormalities of MSA.
Motor impairment is a prominent symptom of patients With MSA and is the main reason that Chinese patients seek medical treatment. Herein, we found a significant reduction in cerebellar volume in the anterior lobe of the cerebellum (bilateral lobules IV-V) in MSA patients compared to HCs. Cerebellum lobules IV-V are located in the anterior lobe and are known to control upper limb movement (Grodd et al., 2001; Nitschke et al., 1996; Schoch et al., 2006). Previous electrophysiological studies had demonstrated activation of single Purkinje cells within lobules IV-V during arm movements in trained monkeys(Nitschke et al., 1996). As expected, lower anterior cerebellum GM volume was also correlated with worse motor symptoms in MSA patients in our study. Our results refined the previous finding that pathological damage of cerebellum anterior lobe leads to abnormal movement in MSA patients (Ozawa et al., 2004). In the MSA-C patient subset, GM loss in left cerebellar lobules IV-V also showed a significant negative correlation with disease duration and severity. Using VBM methods, M. Minnerop et al. (Minnerop et al., 2007) and Yoko Shigemoto et al. (Shigemoto et al., 2013) have similarly documented cerebellar GM loss in both parkinsonian and cerebellar subtypes of MSA, demonstrating a negative correlation between MSA-C only and duration of illness. Hence, both our efforts and those of others underscore a direct relation between anterior cerebellar degeneration and MSA-C disease manifestations, although the same cannot be said of MSA-P. Consequently, the dwindling sizes of cerebellar lobules IV-V may constitute structural markers of disease progression in MSA-C. In patients with MSA-P, we observed a negative correlation between the extent of atrophy involving left lobule IX and severity of motor symptoms. Through functional continuity with caudate nuclei, medial prefrontal cortex, and precuneus, lobule IX is a known element in the DMN network (Buckner et al., 2011; Habas et al., 2009; Sang et al., 2012). Neuropathologically, MSA-P is characterized by widespread neuronal loss and gliosis of the basal ganglia (putamen, caudate nuclei), resulting in tremor and rigidity (Ubhi et al., 2011). GM loss in lobule IX may incite cerebellum-basal ganglia/DMN network neuromodulatory abnormalities, ultimately contributing to motor impairment in patients with MSA-P. However, the ramifications of atrophy in lobule IX have yet to be confirmed for such patients.
It has become increasingly apparent that MSA involves significant cognitive declines (Barcelos et al., 2018; Koga et al., 2017; Santangelo et al., 2018), which according to imaging studies may result from disrupted frontal predominance through atrophy or reduced fractional anisotropy values in left corpus callosum and cerebellum (Celebi et al., 2014; Chang et al., 2009; Hara et al., 2018; Lee et al., 2016). It is still debatable whether the cerebellum is involved in cognitive decline or in the differing cognitive impairment of MSA subtypes. Several studies have suggested that cognitive impairments in MSA may reflect a functionally interrupted cerebellum. For example, Kim et al. (Kim et al., 2015) have used VBM methodology to identify significant GM atrophy of bilateral basal ganglia and cerebellum in patients with MSA, also showing a significant correlation between loss of cerebellar volume and cognitive dysfunction. Conversely, other researchers have found that cognitive declines related to MSA emanate from impaired supratentorial structures (frontal lobe, in particular), so that cerebellar involvement in MSA may be limited to the motor domain (Gellersen et al., 2017). Herein, we employed a cerebellum-specific SUIT atlas to focus on cerebellar structures and minimize methodology-associated variance. The extent of cerebellar volume loss positively correlated with cognitive scores in study population, reinforcing the view that cerebellar atrophy is a factor in the cognitive declines shown by patients with MSA.
In our study, both MSA-P and MSA-C showed cerebellar posterior lobe gray matter loss, However, MSA-C revealed GMV loss was more pronounced than in MSA-C, and the extent of volume loss in bilateral lobule IX, bilateral crus I/II, bilateral lobule IV-V and bilateral lobule VIII)demonstrate only positively correlated with cognitive assessment in MSA-C patients. The crus I/II regions share major connections with prefrontal and parietal areas and make contributions to parallel cortico-cerebellar loops involved in executive control and salience detection. Lobule IX, an established node of the DMN occupying posterior cerebellar lobe, is active in the processing of executive functions and shares connections with prefrontal cortical areas responsible for cognitive processes (Habas et al., 2009). Augmented cerebellar GM in lobules IV-V and VIII is also associated with better cognitive scores in measures of vocabulary, reading, and working memory (Moore et al., 2017). We may therefore assume that pathologic atrophy of the posterior lobe results in outflow dysfunction of the cerebellum-to-cortex circuit, the role of which is central in cognitive deficits of patients with MSA-C. Of note, we found no correlation between cognitive impairment and GM volume loss in patients with MSA-P, given that MoCA score reductions of MSA-P and MSA-C patient subsets were similar, relative to controls. The MSA-P variant is associated with striatocortical and cortical disruption. In conjunction with other past publications, our data indicate that a dysfunctional cerebellum-cortex circuit creates more pronounced cognitive impairment in the MSA-C (vs MSA-P) group (Kawai et al., 2008). However, our outcomes remain preliminary, because motor impairment may actually impede cognitive evaluations (Fiorenzato et al., 2017). In-depth investigation via multimodal imaging is thus warranted to clarify the relation between cerebellar mechanisms and cognitive impairment in patients with MSA.
Compared with the MSA-P group, our patients with MSA-C showed greater declines in cerebellar GM, largely in right crus II. A significant association was found between right crus II volume loss and not only cognitive but also motor performance. A positive correlation was evident between right crus II (cognitive cerebellum) and motor scores, which is not surprising. Crus II is a documented contributor to executive control (Habas et al., 2009), and impaired executive function is closely related to the development of clinical motor symptoms (eg, freezing of gait) in patients with MSA (Gurevich and Giladi, 2003). This relation is supported by previous morphologic evidence of cerebellar GM atrophy in the freezing of gait experienced by patients with Parkinson's disease (Jha et al., 2015). In recent studies, however, no significant GM subregional GM changes have been detected to differentiate between subtypes in early phases of MSA (Dash et al., 2018). These apparent contradictions may then be due to disparate data processing methods or varying courses of disease. GM changes reportedly emerge late in MSA, but the patients we studied were roughly at mid-stage (average duration: MSA-P, 3.27 years; MSA-C, 3.47 years). Importantly, reduced cerebellar integrity and related motor/cognitive correlations persisted after eliminating covariates of whole-brain atrophy. Our findings overall, including whole-brain VBM analysis, imply that volume deficits of right crus II are pivotal in this setting, potentially explaining motor and cognitive functional discrepancies in patients with MSA-P and MSA-C.
Compared with controls, a majority of our patients with MSA (49/61) showed left-dominant cerebellar GM loss. However, asymmetric pathologic change is not uncommon in this disease. There are many reports of patients with MSA displaying clear asymmetry of clinical symptoms (Hwang et al., 2015; Wu et al., 2012; Wüllner et al., 2007). Of the 61 patients we studied, 33 had right-onset disease, with left onset in 16. It has been reported that primary reciprocal connections between cerebral cortex and cerebellar hemispheres are contralaterally organized (Brodal, 1979). Prior diffusion tensor imaging studies have also confirmed that increasing fractional anisotropy in inferior, middle, and superior ipsilateral cerebellar peduncles correlates with contralateral symptoms of severe ataxia (Prakash et al., 2009). Mitsui et al. (Mitsui et al., 1996) have likewise reported a patient with clinically compatible symptoms of MSA-C and laterality in the pons, middle cerebellar peduncle, and cerebellum. Our results in general suggest that GM volume loss on one side of cerebellum is reflected clinically in contralateral motor symptoms. However, these conclusions are tentative, limited by a small sample size and indirect methods, and should be confirmed through direct studies.
5. Conclusion
In conclusion, we have ascertained that location and severity of cerebellar changes in patients with MSA bear a relation to motor and cognitive impairment. GM volume loss on one side of cerebellum is also related to contralateral motor symptom onset clinically, and syndrome-specific regional cerebellar abnormalities may help explain the differing clinical symptoms of patients with MSA subtypes.
6. Limitations
Our study has certain limitations. Even though our diagnosis of MSA-P or MSA-C is determined by symptom arrays, there is distinct symptomatic overlap in the two disease subtypes. Furthermore, subregional variations in cerebellar function are highly refined. A rough assessment of the relation between cerebellar atrophy and motor or cognitive impairment was achieved herein using the UMSARS and MoCA scales. More comprehensive and systematic quantification tools, such as the Scale for the Assessment and Rating of Ataxia (SARA) or the International Cooperative Ataxia Rating Scale (ICARS), would be helpful in future research.
Conflict of interests
None.
Ethical approval
All procedures involving human participants were conducted in accordance with ethical standards of the institutional and/or national research committee, adhering to the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants enrolled in this study.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101891.
Appendix A. Supplementary data
Supplementary material
References
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barcelos L.B., Saad F., Giacominelli C., Saba R.A., de Carvalho Aguiar P.M., SMA S. Neuropsychological and clinical heterogeneity of cognitive impairment in patients with multiple system atrophy. Clin. Neurol. Neurosurg. 2018;164:121–126. doi: 10.1016/j.clineuro.2017.10.039. [DOI] [PubMed] [Google Scholar]
- Bostan A.C., Dum R.P., Strick P.L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn. Sci. 2013;17(5):241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal P. The pontocerebellar projection in the rhesus monkey: an experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience. 1979;4(2):193–208. doi: 10.1016/0306-4522(79)90082-4. [DOI] [PubMed] [Google Scholar]
- Buckner R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebi O., Temuçin Ç.M., Elibol B., Saka E. Cognitive profiling in relation to short latency afferent inhibition of frontal cortex in multiple system atrophy. Parkinsonism Relat. Disord. 2014;20(6):632–636. doi: 10.1016/j.parkreldis.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Chang C.C., Chang Y.Y., Chang W.N., Lee Y.C., Wang Y.L., Lui C.C. Cognitive deficits in multiple system atrophy correlate with frontal atrophy and disease duration. Eur. J. Neurol. 2009;16(10):1144–1150. doi: 10.1111/j.1468-1331.2009.02661.x. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kumfor F., Landin-Romero R., Irish M., Hodges J.R., Piguet O. Cerebellar atrophy and its contribution to cognition in frontotemporal dementias. Ann. Neurol. 2018;84(1):98–109. doi: 10.1002/ana.25271. [DOI] [PubMed] [Google Scholar]
- Cocozza S., Petracca M., Mormina E., Buyukturkoglu K., Podranski K., Heinig M.M. Cerebellar lobule atrophy and disability in progressive MS. J. Neurol. Neurosurg. Psychiatry. 2017;88(12):1065–1072. doi: 10.1136/jnnp-2017-316448. [DOI] [PubMed] [Google Scholar]
- Cykowski M.D., Coon E.A., Powell S.Z., Jenkins S.M., Benarroch E.E., Low P.A. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain. 2015;138(Pt 8):2293–2309. doi: 10.1093/brain/awv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S.K., Stezin A., Takalkar T., George L., Kamble N.L., Netravathi M. Abnormalities of white and grey matter in early multiple system atrophy: comparison of parkinsonian and cerebellar variants. Eur. Radiol. 2018;29:716–724. doi: 10.1007/s00330-018-5594-9. [DOI] [PubMed] [Google Scholar]
- de Azevedo P.C., Guimarães R.P., Piccinin C.C., Piovesana L.G., Campos L.S., Zuiani J.R. Cerebellar gray matter alterations in Huntington disease: a voxel-based morphometry study. Cerebellum. 2017;16(5–6):923–928. doi: 10.1007/s12311-017-0865-6. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Zotow E. Surface-based display of volume-averaged cerebellar imaging data. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenzato E., Antonini A., Wenning G., Biundo R. Cognitive impairment in multiple system atrophy. Movement Disorders. 2017;32(9):1338–1339. doi: 10.1002/mds.27085. [DOI] [PubMed] [Google Scholar]
- Gellersen H.M., Guo C.C., O'Callaghan C., Tan R.H., Sami S., Hornberger M. Cerebellar atrophy in neurodegeneration-a meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2017;88(9):780–788. doi: 10.1136/jnnp-2017-315607. [DOI] [PubMed] [Google Scholar]
- Grodd W., Hülsmann E., Lotze M., Wildgruber D., Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum. Brain Mapp. 2001;13(2):55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich T., Giladi N. Freezing of gait in multiple system atrophy (MSA) Parkinsonism Relat. Disord. 2003;9(3):169–174. doi: 10.1016/s1353-8020(02)00049-4. [DOI] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29(26):8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K., Watanabe H., Bagarinao E., Kawabata K., Yoneyama N., Ohdake R. Corpus callosal involvement is correlated with cognitive impairment in multiple system atrophy. J. Neurol. 2018;265(9):2079–2087. doi: 10.1007/s00415-018-8923-7. [DOI] [PubMed] [Google Scholar]
- Hwang I., Sohn C.H., Kang K.M., Jeon B.S., Kim H.J., Choi S.H. Differentiation of parkinsonism-predominant multiple system atrophy from idiopathic Parkinson disease using 3T susceptibility-weighted MR imaging, focusing on Putaminal change and lesion asymmetry. AJNR Am. J. Neuroradiol. 2015;36(12):2227–2234. doi: 10.3174/ajnr.A4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha M., Jhunjhunwala K., Sankara B.B., Saini J., Kumar J.K., Yadav R. Neuropsychological and imaging profile of patients with Parkinson's disease and freezing of gait. Parkinsonism Relat. Disord. 2015;21(10):1184–1190. doi: 10.1016/j.parkreldis.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Suenaga M., Takeda A., Ito M., Watanabe H., Tanaka F. Cognitive impairments in multiple system atrophy: MSA-C vs MSA-P. Neurology. 2008;70(16 Pt 2):1390–1396. doi: 10.1212/01.wnl.0000310413.04462.6a. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Yang J.J., Lee D.K., Lee J.M., Youn J., Cho J.W. Cognitive impairment and its structural correlates in the parkinsonian subtype of multiple system atrophy. Neurodegener. Dis. 2015;15(5):294–300. doi: 10.1159/000430953. [DOI] [PubMed] [Google Scholar]
- Koga S., Parks A., Uitti R.J., van Gerpen J.A., Cheshire W.P., Wszolek Z.K. Profile of cognitive impairment and underlying pathology in multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2017;32(3):405–413. doi: 10.1002/mds.26874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.J., Shin J.H., Seoung J.K., Lee J.H., Yoon U., Oh J.H. Cognitive impairments associated with morphological changes in cortical and subcortical structures in multiple system atrophy of the cerebellar type. Eur. J. Neurol. 2016;23(1):92–100. doi: 10.1111/ene.12796. [DOI] [PubMed] [Google Scholar]
- Leggio M., Molinari M. Cerebellar sequencing: a trick for predicting the future. Cerebellum. 2015;14(1):35–38. doi: 10.1007/s12311-014-0616-x. [DOI] [PubMed] [Google Scholar]
- Minnerop M., Specht K., Ruhlmann J., Schimke N., Abele M., Weyer A. Voxel-based morphometry and voxel-based relaxometry in multiple system atrophy-a comparison between clinical subtypes and correlations with clinical parameters. NeuroImage. 2007;36(4):1086–1095. doi: 10.1016/j.neuroimage.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Mitsui Y., Tanaka H., Nakamura Y., Yagi Y., Takahashi M. Neuroradiological findings of sporadic olivopontocerebellar atrophy with marked laterality and degenerative changes in the corticopontine tract. Rinsho shinkeigaku = Clinical neurology. 1996;36(10):1150–1154. [PubMed] [Google Scholar]
- Miyatake S., Mochizuki H., Naka T., Ugawa Y., Tanabe H., Kuzume D. Brain volume analyses and somatosensory evoked potentials in multiple system atrophy. J. Neurol. 2010;257(3):419–425. doi: 10.1007/s00415-009-5338-5. [DOI] [PubMed] [Google Scholar]
- Moore D.M., D'Mello A.M., McGrath L.M., Stoodley C.J. The developmental relationship between specific cognitive domains and grey matter in the cerebellum. Developmental cognitive neuroscience. 2017;24:1–11. doi: 10.1016/j.dcn.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke M.F., Kleinschmidt A., Wessel K., Frahm J. Somatotopic motor representation in the human anterior cerebellum. A high-resolution functional MRI study. Brain : a journal of neurology. 1996;119(Pt 3):1023–1029. doi: 10.1093/brain/119.3.1023. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Paviour D., Quinn N.P., Josephs K.A., Sangha H., Kilford L. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain : a journal of neurology. 2004;127(Pt 12):2657–2671. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- Payoux P., Brefel-Courbon C., Ory-Magne F., Regragui W., Thalamas C., Balduyck S. Motor activation in multiple system atrophy and Parkinson disease: a PET study. Neurology. 2010;75(13):1174–1180. doi: 10.1212/WNL.0b013e3181f4d78f. [DOI] [PubMed] [Google Scholar]
- Piccinin C.C., Campos L.S., Guimarães R.P., Piovesana L.G., MCA D.S., Azevedo P.C. Differential pattern of cerebellar atrophy in tremor-predominant and Akinetic/rigidity-predominant Parkinson's disease. Cerebellum. 2017;16(3):623–628. doi: 10.1007/s12311-016-0834-5. [DOI] [PubMed] [Google Scholar]
- Prakash N., Hageman N., Hua X., Toga A.W., Perlman S.L., Salamon N. Patterns of fractional anisotropy changes in white matter of cerebellar peduncles distinguish spinocerebellar ataxia-1 from multiple system atrophy and other ataxia syndromes. NeuroImage. 2009;47(Suppl. 2):T72–T81. doi: 10.1016/j.neuroimage.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Rosskopf J., Gorges M., Müller H.P., Pinkhardt E.H., Ludolph A.C., Kassubek J. Hyperconnective and hypoconnective cortical and subcortical functional networks in multiple system atrophy. Parkinsonism Relat. Disord. 2018;49:75–80. doi: 10.1016/j.parkreldis.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Sang L., Qin W., Liu Y., Han W., Zhang Y., Jiang T. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage. 2012;61(4):1213–1225. doi: 10.1016/j.neuroimage.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Santangelo G., Cuoco S., Pellecchia M.T., Erro R., Barone P., Picillo M. Comparative cognitive and neuropsychiatric profiles between Parkinson's disease, multiple system atrophy and progressive supranuclear palsy. J. Neurol. 2018;265(11):2602–2613. doi: 10.1007/s00415-018-9038-x. [DOI] [PubMed] [Google Scholar]
- Schoch B., Dimitrova A., Gizewski E.R., Timmann D. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. NeuroImage. 2006;30(1):36–51. doi: 10.1016/j.neuroimage.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Shigemoto Y., Matsuda H., Kamiya K., Maikusa N., Nakata Y., Ito K. In vivo evaluation of gray and white matter volume loss in the parkinsonian variant of multiple system atrophy using SPM8 plus DARTEL for VBM. NeuroImage. Clinical. 2013:2491–2496. doi: 10.1016/j.nicl.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex; a journal devoted to the study of the nervous system and behavior. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K., Low P., Masliah E. Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci. 2011;34(11):581–590. doi: 10.1016/j.tins.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Edmiston E.K., Luo X., Yang H., Chang M., Wang F. Comparing abnormalities of amplitude of low-frequency fluctuations in multiple system atrophy and idiopathic Parkinson's disease measured with resting-state fMRI. Psychiatry research. Neuroimaging. 2017;269:73–81. doi: 10.1016/j.pscychresns.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Wu Y.T., Shyu K.K., Jao C.W., Liao Y.L., Wang T.Y., Wu H.M. Quantifying cerebellar atrophy in multiple system atrophy of the cerebellar type (MSA-C) using three-dimensional gyrification index analysis. NeuroImage. 2012;61(1):1–9. doi: 10.1016/j.neuroimage.2012.02.057. [DOI] [PubMed] [Google Scholar]
- Wüllner U., Schmitz-Hübsch T., Abele M., Antony G., Bauer P., Eggert K. Features of probable multiple system atrophy patients identified among 4770 patients with parkinsonism enrolled in the multicentre registry of the German competence network on Parkinson's disease. J. Neural Transm. 2007;114(9):1161–1165. doi: 10.1007/s00702-007-0746-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material