Summary
Asthma and obesity present rising incidence, and their concomitance is a reason for concern, as obese individuals are usually resistant to conventional asthma treatments and have more exacerbation episodes. Obesity affects several features in the lungs during asthma onset, shifting the T helper type 2 (Th2)/eosinophilic response towards a Th17/neutrophilic profile. Moreover, those individuals can present reduced atopy and delayed cytokine production. However, the impact of obesity on follicular helper T (Tfh) cells and B cells that could potentially result in antibody production disturbances are still unclear. Therefore, we aimed to assess the peripheral response to ovalbumin (OVA) in a concomitant model of obesity and asthma. Pulmonary allergy was induced, in both lean and obese female BALB/c mice, through OVA sensitizations and challenges. Mediastinal lymph nodes (MLNs) and spleen were processed for immunophenotyping. Lung was used for standard allergy analysis. Obese‐allergic mice produced less anti‐OVA IgE and more IgG2a than lean‐allergic mice. Dendritic cells (CD11c+ MHCII high) expressed less CD86 and more PDL1 in obese‐allergic mice compared with lean‐allergic mice, in the MLNs. Meanwhile, B cells (CD19+ CD40+) were more frequent and the amount of PDL1/PD1+ cells was diminished by obesity, with the opposite effects in the spleen. Tfh cells (CD3+ CD4+ CXCR5+ PD1+) expressing FoxP3 were more frequent in obese mice, associated with the predominance of Th (CD3+ CD4+) cells expressing interleukin‐4/GATA3 in the MLNs and interleukin‐17A/ROR γT in the spleen. Those modifications to the main components of the germinal centers could be resulting in the increased IgG2a production, which – associated with the Th17/neutrophilic profile – contributes to asthma worsening and represents an important target for future treatment strategies.
Keywords: asthma, follicular T cells, obesity, peripheral organs lymphoid
Abbreviations
- DCs
dendritic cells
- EPO
eosinophilic peroxidase
- HFD
high‐fat diet
- MHCII
major histocompatibility complex II
- MLNs
mediastinal lymph nodes
- MPO
myeloperoxidase
- NLRP3
NOD like receptor protein 3
- OVA
ovalbumin
- PD1
programmed death‐1
- PDL1
programmed death ligand 1
- Tfh
follicular T cells
- Tfr
follicular regulatory T cell
- Th1
T helper type 1
- TSLP
thymic stromal lymphopoietin
Introduction
Asthma is a highly debilitating chronic inflammatory disease of the airways, affecting >300 million people worldwide. There are several asthma phenotypes, among which allergic asthma can be highlighted as the most frequent.1 The variable degrees of bronchoconstriction, mucus hypersecretion and inflammatory process, triggered by exposure to the allergen, results in the most common symptoms – coughing, wheezing and shortness of breath.2
The allergic immune response begins with allergen contact with the epithelium in the lungs, promoting its activation and the release of thymic stromal lymphopoietin (TSLP), interleukin‐25 (IL‐25) and IL‐33.3 These cytokines trigger activation and migration to the peripheral lymphoid organs of dendritic cells (DCs), where they prime T cells to polarize to T helper type 2 (Th2)4, 5, 6 and follicular helper T (Tfh) profiles.7 The cooperative interaction between DCs, T cells and cognate B cells promotes Tfh cell differentiation and migration to germinal centers, in which they induce IgE production, by activating B cells and differentiating them into plasma cells.8 Altogether, the antibody production and T‐cell release of IL‐4, IL‐5 and IL‐13 result in mast cell activation, and eosinophil recruitment, which are the main effector cells of the classical Th2 allergic asthma.
Allergic asthma is heterogeneous, exhibiting several distinct immunological patterns according to the age, sex and obesity status of the patient.9, 10 In that regard, obesity, associated with hyperplasia and hypertrophy of the adipose tissue, sustains a low‐grade systemic inflammation, which interferes in many other tissues, such as the lungs.11 Therefore, the increasing rates of obese asthmatic individuals have become a serious burden, as obesity–asthma coexistence is related to resistance to conventional treatments and more frequent exacerbation episodes.12, 13 This profile develops due to an increased neutrophil influx, overcoming the classic asthmatic eosinophilia.14 It is already established that neutrophils are more resistant to glucocorticoids, contributing to the severity of the disease.15 Several mechanisms have been associated with this shift, as increased NLRP3 inflammasome activation,16 DC activation failure,17 macrophage profile modification18 and lower TSLP levels.19
We have previously demonstrated that obesity dampens the allergic response in mice at the epithelium by diminishing TSLP production; this shift was associated with the development of a delayed allergic immune response and lower allergen‐specific IgE titers.18 However, there is still some controversy about the role of obesity in antibody production during the allergic process. Clinical and experimental studies have demonstrated both the intensification and the reduction of IgE titers in obese asthmatic individuals.12, 18, 20 Despite the recent attention to the concomitance of obesity and asthma, there is still a lack of studies addressing the changes in lymphoid organs, mainly involving Tfh cells and antibody‐producing B cells.
Better comprehension of the particular events in the obese response to allergenic stimulus, can contribute to the development of new treatment strategies, suitable for patients that are usually resistant to conventional treatments.
Material and methods
Animals
Four‐ to six‐week‐old female BALB/c mice, obtained from the Federal University of Juiz de Fora, were maintained in a temperature‐controlled facility with 12/12‐hr light/dark cycle and were fed ad libitum. Mice were allowed 1 week of acclimation before the beginning of the protocol. The experiments were performed in accordance with the Brazilian Code for the Use of Laboratory Animals and approved by the UFJF Ethics Committee for the Use of Laboratory Animals (CEEA–UFJF No. 019/2017).
Induction of obesity and pulmonary allergy
Mice were randomly divided into four groups, according to their diet and asthma model induction. Obese groups (HFD and HFD‐OVA) were fed a high‐fat diet (HFD – 60% calories from fat; Prag Soluções Ind. Co. Ltd Jau, SP, Brazil), while the lean groups (CN and OVA) were fed a standard diet (10% calories from fat, Nuvilab‐CR1VR; Nuvital Nutrientes Ltd, Colombo, Brazil). Allergy induction was performed on the OVA and HFD‐OVA groups according to the following procedures. After 10 and 12 weeks of diet, mice were intraperitoneally sensitized with 3 μg of ovalbumin (OVA) (grade V; Sigma‐Aldrich Corp., St Louis, MO) in 1 mg alumen (Sigma‐Aldrich), and the challenges with aerosolized OVA 1% for 20 min were performed on the 21st, 23rd, 25th, 27th and 29th days after the first sensitization. Euthanasia occurred 24 hr and 48 hr after the last challenge (Fig. 1a).
Figure 1.
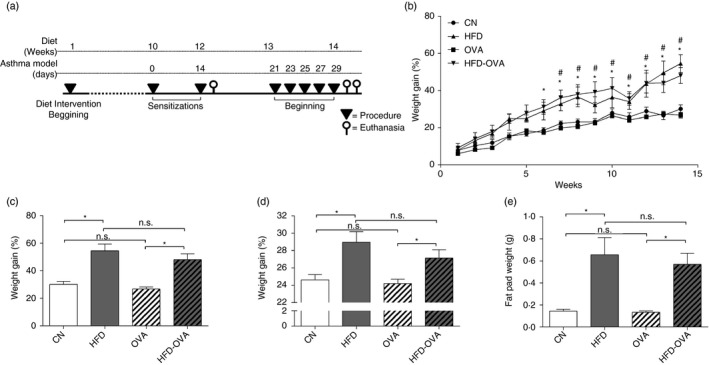
Experimental design and obesity establishment by high fat feeding. Experimental design, obesity induced by high‐fat diet (HFD) (60% of calories from fat). Pulmonary allergy induced by ovalbumin (OVA) on female BALB/c mice (a). Weight gain during the 14 weeks of the obesity protocol, significant differences between control (CN) and HFD groups are shown as #, and * for comparisons between OVA and HFD‐OVA groups (two‐way analysis of variance) (b). Weight gain was expressed as percentage of gained weight over the initial weight (c), weight by the end of the protocol (d) and perigonadal fat pad weight (e). Values are expressed as mean ± SEM. Different means are indicated as *, for P < 0·05. n = 6 for each group (analysis of variance).
Anti‐OVA‐specific antibodies evaluation
Serum was separated from blood samples obtained 24 hr and 48 hr after the last challenge. Anti‐OVA‐specific IgE and IgG2a were determined on serum, by optical density measurement at 492 nm in a microplate reader (SpectraMax 190; Molecular Devices, Sunnyvale, CA), as described elsewhere.21
Eosinophil quantification
Direct Red 80 (Sigma‐Aldrich) staining was used for eosinophil quantification per 100‐μm2 field in the peribronchovascular area. Slides were examined in blinded manner under an optical microscope (Zeiss, Hallbergmoos, Germany) at ×1000 magnification.
Measurement of cytokine and chemokine levels in the lungs
The lung tissue homogenate was prepared as described previously21 and the pellets were used in eosinophil peroxidase (EPO) and myeloperoxidase (MPO) activity assays. Cytokines and chemokine were detected using commercially available ELISA kits: IL‐4, IL‐5 (BD OptEIA; BD Biosciences, EUA); IL‐13, IL‐25, TSLP, eotaxin (CCL11) (R&D Systems, Minneapolis, MN); and IL‐17A (eBioscience, San Diego, CA). Assays were performed according to the manufacturer's instructions.
Eosinophil peroxidase and myeloperoxidase activity
Eosinophil peroxidase activity in lung homogenates was determined as described elsewhere.21 To evaluate MPO, activity equal parts of homogenate supernatant and O‐phenylenediamine solution in 10 mm citrate (pH 5·5), followed by the addition of H2O2 (20%). The reaction was stopped with H2SO4, and the absorbance was measured at 492 nm on a microplate reader (SpectraMax 190).
Flow cytometry
Lungs, spleen and mediastinal lymph nodes (MLNs) were macerated on extraction buffer (RPMI‐1640 medium supplemented with 5% fetal bovine serum and 0·075% EDTA) on cell strainers. Cells were counted after red blood cell lysis and plated at 2 × 105 cells/well. Fc receptor blockage was performed with FcBlock solution (1:100), followed by permeabilization, for intracellular staining. Anti‐CD19 (fluorescein isothiocyanate clone: 1D3), anti‐CD40 (allophycocyanin clone: 3/23), anti‐CXCR5 (phycoerythrin clone: 2G8); anti‐programmed death 1 (PD1) (BV 421 clone: J43); anti‐programmed death ligand 1 (PDL1) (phycoerythrin clone: MIH5), CD4 (Percep clone: RM4‐5), anti‐GATA3 (Alexa Fluor 647 clone: L50‐823), anti‐RORγT (BV 421 clone: Q31‐878); anti‐IL‐4 (BV 421 clone: 11B11), anti‐interferon‐γ (phycoerythrin‐Cy7 clone: XMG1.2), anti‐IL‐17 (Alexa 488 clone: TC11‐18H10); anti‐FOXP3 (Alexa Fluor 488 clone: MF23), anti‐CD11c (BV 510 clone: HL3), anti‐MHC‐II (allophycocyanin clone: AMS 321), anti‐CD80 (fluorescein isothiocyanate clone: 16‐10A1). Samples were fixed in paraformaldehyde after labeling. Data acquisition was performed in FACS‐Canto II (BD) and analyzed on flowjo (TreeStar, Ashland, OR).
Statistical analysis
Data were analyzed using graph pad prism 6.0 (San Diego, CA). Numerical data were analyzed using Kolmogorov–Smirnov normality test. Weight gain analysis along time was performed with two‐way analysis of variance, coupled with Tukey's multiple comparison test. For four‐column graphs, parametric data were analyzed with one‐way analysis of variance, followed by Bonferroni post‐test; for non‐parametric data a Kruskal–Wallis test was performed, followed by Dunn's test. For two‐column graphs, parametric data were analyzed using unpaired Student's t‐test and non‐parametric data were analyzed using Mann–‐hitney U‐test. The threshold significance level was set at P ≤ 0·05.
Results
HFD efficiently induces obesity
It has already been demonstrated that BALB/c mice are resistant to obesity induction;,22 however, several studies have already demonstrated how these animals present characteristic features of obese status.18, 23 Importantly, BALB/c mice are prone to develop allergic‐like Th2 immune responses,24 being more adequate for asthma models. In addition, clinical studies have demonstrated that obesity worsens asthma symptoms in women;10, 12, 25 therefore the present study was developed in a female model. We have shown that mice fed an HFD not only gained more weight than mice fed a standard diet (Fig. 1b–d), but also presented increased fat pad weight (Fig. 1e). Moreover, the asthma model induction, did not affect mouse weight or fat accumulation (Fig. 1b–e).
Obesity affects asthma‐related immunohistopathological parameters
To validate the model, some parameters, previously demonstrated to be affected by obesity during the asthma protocol,18 were analyzed in the present study. Initially, TSLP (Fig. 2a) and IL‐25 (Fig. 2b) levels were lower in lung homogenates of the obese‐allergic (HFD‐OVA) group, in comparison to the lean‐allergic (HFD) mice, 24 hr after the last challenge. At the same time‐point, HFD‐OVA also showed reduced CCL11 (Fig. 2c) and a tendency to reduce IL‐5 levels (Fig. 2d). These modifications were followed by a persistent reduction in eosinophil counts (Fig. 2e,f), associated with diminished EPO activity (Fig. 2g), at the 48 hr end point. In contrast, IL‐4 (Fig. 2h) and IL‐17A (Fig. 2i) levels were increased in HFD‐OVA, compared with the OVA group, associated with enhanced MPO activity, at 48 hr after the last challenge (Fig. 2j).
Figure 2.
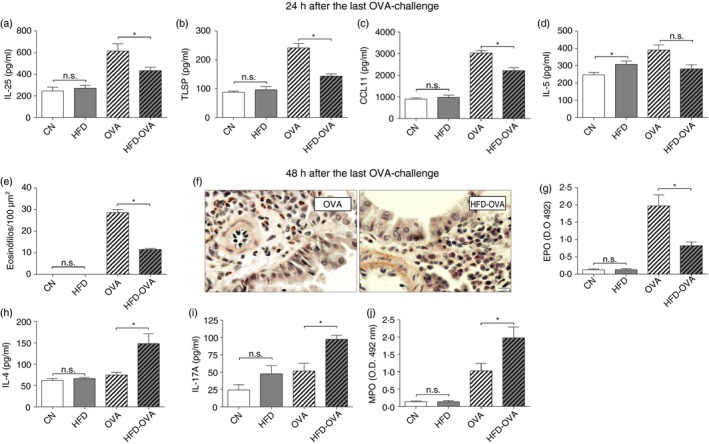
Experiment validation, obesity promotes greater neutrophil activity and early reduced Th2/eosinophilic response. Levels of the epithelial cytokines interleukin‐25 (IL‐25) (a) and thymic stromal lymphopoietin (TSLP) (b), 24 hr after the last OVA‐challenge. Eosinophil peroxidase (EPO) activity (c), and eosinophil counting on Sirius Red stain in peribronchovascular area (d and e), 48 hr after the last challenge. CCL11 (f) and IL‐5 (g) levels 24 hr after the last challenge. IL‐4, IL‐17A levels and myeloperoxidase (MPO) activity (h), 48 hr after the last challenge. Cytokine and chemokine levels were determined by ELISA and activity assay were used to determine EPO and MPO abundance, in lung homogenates. Values are expressed as mean ± SEM. Different means are indicated as *, for P < 0·05. n = 6 for each group.
Obesity affects serum OVA‐specific antibody titers
Obesity affected the standard allergic response, reducing and delaying several Th2 features; therefore we have assessed the anti‐OVA IgE and IgG2a serum levels, predominant on Th2 and Th1 immune responses, respectively. In the present work, IgE levels were lower in the HFD‐OVA group in comparison to the OVA group, at 24 hr and 48 hr after the last challenge (Fig. 3a). On the other hand, more IgG2a antibodies were produced in the HFD‐OVA group at both analyzed time‐points (Fig. 3b).
Figure 3.
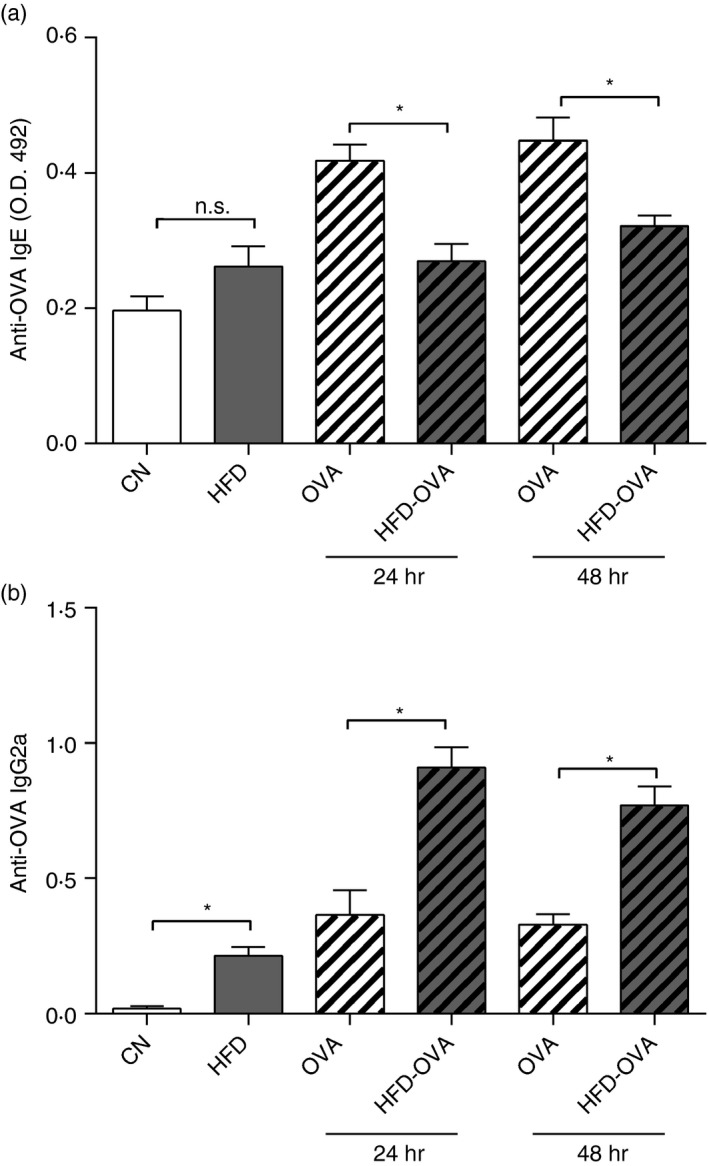
Obesity affects antibody production in response to allergen stimulation. ELISA‐determined anti‐ovalbumin (OVA) IgE (a) and IgG2a (b) levels, in the serum, 24 and 48 hr after the last challenge. Values are expressed as mean ± SEM. Different means are indicated as *, for P < 0·05. n = 6 for each group.
Obesity induces regulatory profile on DCs in the MLNs during pulmonary allergy
T‐cell priming in the peripheral lymphoid organs is an important step towards antibody production. DCs bearing the allergen trigger the following phases of allergic asthma onset.26 We have investigated DC activation in both lymph nodes and spleen, by measuring the expression of the activation and regulation markers, CD86 and PDL1, respectively. In the MLNs, DCs (CD11c+ MHCIIhigh) from the HFD‐OVA group presented diminished CD86 expression, in comparison to the OVA group (Fig. 4a). In addition, the HFD‐OVA group also presented higher PDL1 expression (Figs. 4b and S2). However, no difference was observed between the compared groups, regarding the expression of these markers, in the spleen (Fig. 4c,d).
Figure 4.
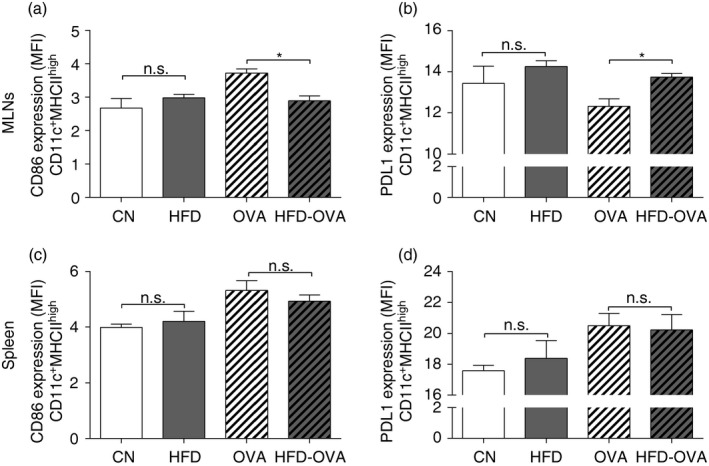
Obesity dampens dendritic cell activation on the mediastinal lymph nodes (MLNs). CD86 (a) and PDL1 (b) MFI, in CD11c+ MHC high, from the MLNs. CD86 (c) and PDL1 (d) MFI, in CD11c+ MHC high, from the spleen. 48 hr after the last challenge. Results were determined through immunophenotyping and flow cytometry. Values are expressed as mean ± SEM. Different means are indicated as *, for P < 0·05. n = 6 for each group.
Obesity affects B‐cell regulatory and activation markers in peripheral lymphoid organs during pulmonary allergy
Besides its well‐known role on antibody production, different B‐cell subtypes can also act as regulatory cells, negatively modulating T‐cell activation.27 Therefore we have investigated the expression of the regulatory markers PD1 and PDL1, in B cells at MLNs and spleen. Although the total cell count was similar between the OVA and HFD‐OVA groups (see Supplementary material, Fig. S1), B‐cell (CD19+ CD40+) frequency was higher in the HFD‐OVA MLNs and diminished in the spleen, in comparison to the OVA group (Fig. 5a,d). In addition, the frequency of B cells (CD19+ CD40+) expressing high levels of the regulation marker PDL1 (PDL1hi) was diminished in the MLNs (Fig. 5b). In contrast, the HFD‐OVA group presented increased PDL1high and PD1+ frequency in the spleen, when compared with OVA group (Fig. 5e,f).
Figure 5.
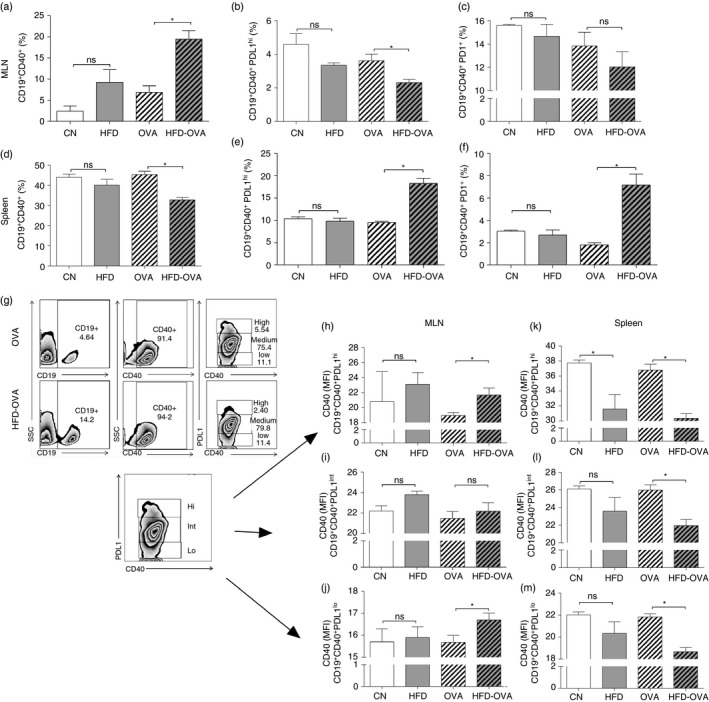
Obesity impacts B‐cell populations in both mediastinal lymph nodes (MLNs) and spleen. CD19+ CD40+ frequency (a, d), CD19+ CD40+ PDL1high frequency in CD19+ CD40+ (b, e), CD19+ CD40+ PD1+ frequency in CD19+ CD40+ (c, f), in both MLNs and spleen, determined by immunophenotyping and flow cytometry. Gating strategy for B cells and their discrimination by PDL1 expression levels, representative image from the MLNs (g). CD40 MFI in CD19+ CD40+ PDL1high (h,k), CD19+ CD40+ PDL1Int (i,l) and CD19+ CD40+ PDL1low (j,m), in both MLNs and spleen, determined by immunophenotyping and flow cytometry 48 hr after the last challenge. Values are expressed as mean ± SEM. Different means are indicated as *, for P < 0·05. n = 6 for each group.
The increase in CD40 expression is crucial for B‐cell activity and proliferation; therefore we have evaluated its expression in B‐cell populations expressing different levels of PDL1. In the MLNs, CD19+ CD40+ PDL1hi and CD19+ CD40+ PDL1lo populations from HFD‐OVA animals expressed more CD40 (mean fluorescence intensity) than the OVA group (Fig. 5h,j), in accordance with the higher B‐cell frequency in these lymph nodes (Fig. 5d). In contrast, the splenic CD40 expression in all PDL1 levels was low in the HFD‐OVA groups when compared with HFD mice (Fig. 5k–m), in agreement with the diminished B‐cell frequency in that organ.
Obesity affects the populations of both Th and Tfh cells in the peripheral lymphoid organs during pulmonary allergy
Tfh cells directly interact with B cells at germinal centers, promoting class switch and inducing antibody production. Nevertheless, Tfh can also express Foxp3 transcription factor, exhibiting regulatory effects over B cells.28, 29 Therefore, we have evaluated the frequency of Tfh (CD3+ CD4+ CXCR5+ PD1+) and T follicular regulatory (Tfr) cells (CD3+ CD4+ CXCR5+ PD1+ Foxp3+) in both MLNs and spleen. OVA and HFD‐OVA groups presented similar Tfh frequencies in the MLNs (Figs. 6a and S3). However, the HFD‐OVA group displayed an increased population of Tfr cells (Figs. 6c and S3). In addition, HFD‐OVA group splenic Tfh population was higher when compared with the OVA group (Figs. 6d and S4), whereas PD1 expression (mean fluorescence intensity) was reduced by obesity (Fig. 6e). Interestingly, obesity itself led to an increased splenic Tfr cell frequency, in comparison to the control animals (Fig. 6f), demonstrating the effects of obesity itself on the differentiation of these cells.
Figure 6.
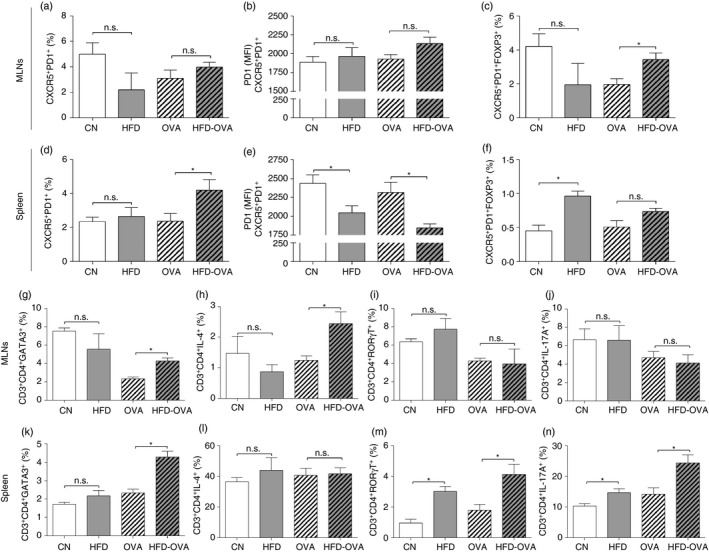
Obesity impacts follicular helper T (Tfh) cell population, promotes T helper type 2 (Th2) response in the mediastinal lymph nodes (MLNs) and Th17 profile in the spleen. Tfh cell evaluation. CXCR5+ PD1+ frequency in CD3+ CD4+ cells (a, d); CXCR5+ PD1+ Foxp3+ frequency in CD3+ CD4+ cells (b, e); PD1 MFI in CD3+ CD4+ CXCR5+ PD1+ cells (c, f). Th2 cell evaluation. GATA3+ cells (g, k) and IL‐4+ cells frequency in CD3+ CD4+ (h, l). Th17 cell evaluation. ROR γT+ cells (i, m) and IL17A+ cells frequency in CD3+ CD4+, in both MLNs and spleen, determined by immunophenotyping and flow cytometry. 48 hr after the last challenge. Values are expressed as mean ± SEM. Different means are indicated as *, for P < 0·05. n = 6 for each group.
Besides their direct effect on allergy establishment, Th cells and their cytokines can also affect antibody production,30, 31 therefore we have evaluated the Th population in the peripheral lymphoid organs. In the MLNs there was a higher frequency of cells expressing the transcription factor GATA3 and producing IL‐4 in the HFD‐OVA group, when compared with the OVA group (Figs. 6g,h and S5). Although there was no difference between the groups regarding the splenic population of IL‐4‐producing Th cells, there were more cells expressing GATA3 in the HFD‐OVA group, compared with the OVA group (Fig. 5k,l). Regarding the RORγT‐expressing and IL‐17A‐producing Th cells, there was no difference between the OVA and HFD‐OVA groups, in the MLNs (Figs. 6i,j and S5). Nonetheless, those populations were increased in the HFD‐OVA group when compared with OVA animals, in the spleen. Still, this difference was already present in the HFD group compared with CN animals (Fig. 6m,n).
Obesity reduces Tfh population in peripheral lymphoid organs from the sensitization
To evaluate if obesity already affected allergy establishment from sensitization, we evaluated T‐cell populations 72 hr after the last sensitization. However, most of the parameters were similar between the HFD‐OVA and OVA groups (data not shown), except for the Tfh populations. Both conventional Tfh cells (CD3+ CD4+ CXCR5+ PD1+) and Tfr cells were more frequent in the OVA than in the HFD‐OVA group (Fig. 7).
Figure 7.
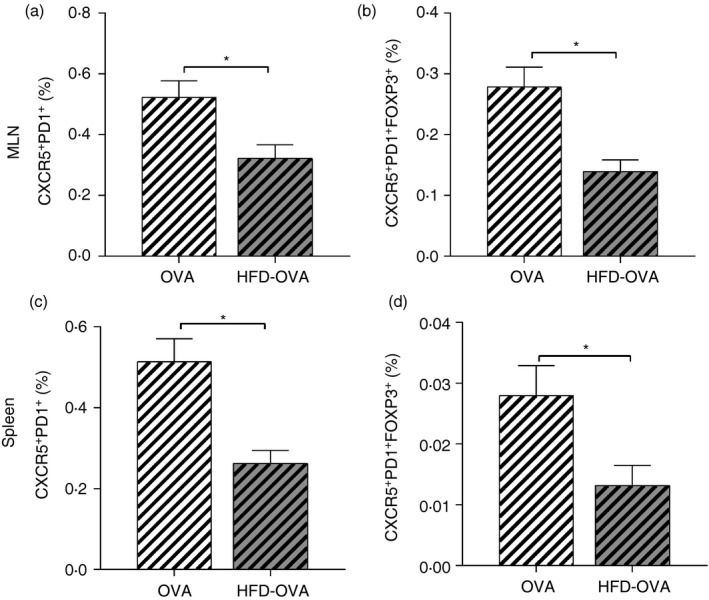
Obesity dampens follicular helper T (Tfh) cell differentiation from sensitization. Tfh cell evaluation; CXCR5+ PD1+ frequency in CD3+ CD4+ cells (a, c); CXCR5+ PD1+ FoxP3+ frequency in CD3+ CD4+ cells (b, d), determined by immunophenotyping and flow cytometry, 72 hr after the second ovalbumin sensitization. Values are expressed as mean ± SEM. Different means are indicated as *, for P < 0·05. n = 6 for each group.
Discussion
Due to the increasing prevalence of asthma, recent efforts have been directed to the investigation of its development and the occurrence of co‐morbidities. Although several studies have already demonstrated the critical role of obesity in the worsening of allergy‐associated diseases,12, 32 there are still blanks to be filled when it comes to its effect on the allergic phenotype of asthma. The present results and previously published data18 have demonstrated that obese‐allergic mice present a delayed immune response, as shown by the lower production of traditional Th2‐associated allergic parameters such as IgE, IL‐25, TSLP and CCL11, observed at 24 hr after the last challenge in obese allergic mice. These modifications could have led to the reduced eosinophil counting in the lungs and EPO activity, at 48 hr. Based on our previous data18 and on the present study, there is a late (48 hr) IL‐4 production in the obese‐allergic group, whereas, at the same time‐point, lean‐allergic mice present lower levels of this cytokine, in contrast with its early (24 hr) higher levels.18 Another study has already indicated that obesity can trigger a delayed Th2 response during pulmonary allergy, on C57BL/6 mice.33 On the other hand, there was a late increase (48 hr) of neutrophil‐associated features, IL‐17A and MPO, in obese‐allergic mice. This pro‐neutrophilic shift is associated with more exacerbation episodes and resistance to the conventional corticosteroid treatments.3, 4, 5, 6 Altogether these data indicated a phenotype observed in the clinic, that combines the delayed allergic response with the increased neutrophilia.34
We have demonstrated that obesity affects the production of the epithelial cytokines (IL‐25 and TSLP), important inducers of DC activation, that prime them to trigger the classical Th2 response.5, 6 It is known that in their absence or reduction, particularly TSLP, DCs will be more prone to polarize Th cells towards a Th17 profile.19, 35 In the present study, the diminished production of epithelial cytokines could have led to the reduced DC activation observed in the MLNs of obese‐allergic mice, resulting in the events downstream. It has already been demonstrated how DCs from obese mice present dampened activation in the lungs.36 In the MLNs, the main draining lymph nodes to the lungs, DCs displayed a regulatory profile, as shown by the reduced CD86 expression and increased PDL‐1 intensity. These changes could be promoting the dampened Th2 response,37 characteristic of the obese‐allergic mice and could also be creating a different environment from the lean‐allergic individuals in their peripheral organs.
The first sign of B‐cell activation is the increased antibody production; in that regard, allergic responses stand out for their excessive IgE production. Although obese‐allergic individuals usually present lower atopy, this reduction does not reflect in any attenuation of the disease.38, 39 In that sense, we have demonstrated reduced IgE titers in the serum of obese‐allergic mice, whereas these animals presented higher Th1 prototype antibody (IgG2a) levels. This shift, associated with the higher neutrophil counting, could potentially increase the severity of the disease, as IgG2a is known to trigger neutrophil‐dependent anaphylaxis, through FcγRIII engagement.40 The higher IgG2a titers could be a consequence of obesity‐triggered disturbances of the immune response setup on B‐cell populations from the peripheral lymphoid organs.41
B‐cell activation in the lymphoid organs is a critical point in antibody production, so the evaluation of their frequency can provide a good insight of how obesity may be affecting the general allergic response. In this context, the spleen is the main site for B cells to complete their maturation,42 nevertheless obesity itself can impact the splenic leukocyte function and proliferation, mainly B and T cells.43, 44, 45 Interestingly, our results demonstrate that OVA itself is not able to affect B‐cell populations in the spleen; however, when associated with obesity, the allergen affected not only their frequency, but also their activation status. Hence, the obese‐allergic splenic B‐cell population maintains a regulatory profile after the challenges, as shown by the high frequency of PDL1+ and PD1+ cells, associated with the lower CD40 expression, in contrast with the profile observed in the MLNs. This B‐cell activation on the MLNs could be supporting the antibody serum levels observed in the obese‐allergic animals. It is well established that the expression of PD1 and its ligand PDL1, on B cells, is an important mark of immune response regulation.27, 46 In accordance with the higher B‐cell (CD19+ CD40+) frequency, there was a reduction of their regulatory profile, as shown by the lower frequency of the PDL1high B cells. Differently, this regulatory population (CD19+ CD40+ PDL1high) frequency was increased in the spleen, leading to the reduced B‐cell population CD19+ CD40+.
The above‐mentioned results indicate that MLNs and spleen present contrasting results in obese‐allergic mice, probably due to a strong influence of obesity over the spleen,43 whereas the allergen preferentially affected the MLNs. Despite the lack of studies approaching the influence of obesity on B cells during the development of allergic responses, a disturbance of obese splenic B‐cell activation and antibody production has already been demonstrated in a murine influenza infection model41 and upon Staphylococcus aureus infection.47
There is crosstalk between B cells and DCs during antigen presentation and activation of T naive cells, promoting their differentiation into the different T‐cell profiles. Among them, Tfh cells are polarized by IL‐21 and IL‐6 stimuli, resulting in their increased CXCR5 expression, a chemokine receptor for CCL13, expressed in the germinal centers.8, 48 Tfh cells mutually interact with B cells contributing for high‐affinity antibody production in plasma cells.49 Additionally, recent studies have demonstrated that specific regulatory B cells dampen Tfh differentiation through their elevated PDL1 expression in the B‐cell marginal zone,27, 50 our data demonstrate this function, as the higher frequency of PDL1high B cells was accompanied by a reduced differentiation of Tfh cells in the spleen. However, in the MLNs the low frequency of regulatory B cells (CD19+ CD40+ PDL1high) did not result in a higher Tfh cell differentiation, possibly due to the regulatory profile of the interacting‐DCs (CD11c+ MHCII+ PDL1+). In fact, it has already been demonstrated that Tfh cell differentiation is dependent on CD86 co‐stimulation by DCs.51
Despite the higher number of splenic Tfh cells, they presented reduced PD1 expression. Although we have not evaluated the function of these cells, it has already been demonstrated that Tfh cells require PD1 expression to maintain cytokine production and their normal function.52 Hence, the impaired PD1 expression in Tfh cells could be affecting their interaction with B cells, contributing for their diminished activation. Moreover, the Tfh population was already reduced from the sensitizations, showing that their differentiation was already compromised, and may have contributed to the further response delays.
Recent studies have highlighted the role of Tfr Foxp3+ cells, derived from regulatory T cells, controlling reactions in the germinal center,29 and their reduction or impaired function is associated with the occurrence of autoantibody production.28 In that regard, PD1 and PDL1 deficiencies induce the Tfr cell differentiation, but do not affect the conventional Tfh cell population.53 Indeed, in the present work, the lower expression of these markers on B cells resulted in a greater Tfr cell frequency in the MLNs. In addition, the non‐regulatory role of these cells, which can trigger IL‐10‐dependent cell proliferation and IgG has been recently demonstrated.54, 55 Hence, the modifications observed in the antibody profile, with higher IgG2a and reduced IgE, in obese‐allergic animals, could be favored by the Tfr higher frequency. Meanwhile, the spleen did not present the same profile, and no difference was observed between the allergic groups regarding the Tfr.frequency.
T helper activity can also play an important role in antibody production through cytokine secretion. It is known that IL‐4, released by Th2 cells, can promote IgE production,56 while IL‐17A is among the factors that can trigger IgG2a synthesis.30 We have observed an increased frequency of IL4+/GATA3+ Th cells in the MLNs, while IL17A+/RORγT+ Th were more frequent in the spleen. These results suggest the establishment of a splenic Th17 profile, supported by obesity.
Although the above‐mentioned results give a good insight of what happens in terms of peripheral response during the pulmonary allergy in obese mice, new euthanasia points could give a better understanding of the kinetics of this response. In addition, the performance of similar analysis in other models of obesity–asthma, mainly those that indicate obesity as a booster for IgE production,57, 58 could help to elucidate the mechanisms promoting the difference between distinct groups of obese–asthmatic patients.
Taken together, our data show that obesity affects the peripheral response to the allergen, promoting antibody production disturbances and the differentiated profile of the main components of the germinal center. In the MLNs, there was a marked increase in B‐cell frequency, accompanied by an impairment of the regulatory profile, and associated with the higher Tfr cell population, in obese‐allergic mice. Meanwhile, the splenic response was characterized by a striking increase in IL17A+/RORγT+ Th cell population; but there was an apparent B‐cell regulation at this organ, as shown by the higher frequency of the PDL1high B‐cell population and the diminished total B‐cell frequency. The aforementioned results can potentially explain the shift to IgG2a, the diminished IgE production and the pro‐neutrophilic stage observed in the lungs of the obese‐allergic group. However, we did not find which of these organs was the source of each of the antibody isotype.
Although our data provide a new perspective about the pulmonary allergic immune response, showing some changes that support asthma worsening in the pulmonary tissue in obese individuals, one of its limitations is the use of an HFD obesity induction, leading to elevated low‐density lipoprotein, cholesterol, and fasting blood glucose.18 Future studies could seek to explain the contributions of weight gain, glucose tolerance, insulin resistance and blood lipid levels for the modifications observed in the immune response in the peripheral lymphoid organs. Additionally, the better understanding of the establishment of non‐Th2 features during the allergic response can help in the development of alternative treatment strategies, including, the use of specific antibodies that target IgG2a and IL‐17A, instead of IgE; gene therapy; and even the use of well‐established drugs that are known to target the neutrophilic/Th17 immune response.
Author contributions
EEO and FMCS designed the study, performed laboratory and statistical analyses and drafted the manuscript. MCA, MGEA and VPS performed laboratory analysis; GCM performed and analyzed flow cytometry; APF designed and supervised the project and edited the manuscript. All authors have read and approved the final content of the manuscript.
Disclosures
There is no conflict of interest to disclose in this study.
Supporting information
Figure S1. Obesity does not affect cell counting on the peripheral lymphoid organs. Cell counting on the MLNs 48 hr after the last OVA challegenge (a) and 72 hr after the second sensitization (b). Cell counting on the spleen 48 hr after the last OVA challegenge (c) and 72 hr after the second sensitization (d). Values are expressed as mean ± SEM. Different means are indicated as *, for P < 0·05. n = 6 for each group.
Figure S2. Representative flow cytometry gating strategy for DCs phenotyping in MLNs. Population selection, SSC x FSC (a), CD11c+MHCIIhigh gating (b), representative histogram for CD86 and PDL1 expression.
Figure S3. Representative flow cytometry gating strategy for Tfh phenotyping in MLNs. Population selection, SSC x FSC (a), CD3+CD4+CXCR5+PD1+ Foxp3+ gating strategy (b).
Figure S4. Representative flow cytometry gating strategy for Tfh phenotyping in the spleen. Population selection, SSC x FSC (a), CD3+CD4+CXCR5+PD1+ Foxp3+ gating strategy (b).
Figure S5. Representative flow cytometry gating strategy for Th phenotyping in the MLNs and spleen. Population selection, SSC x FSC (a). Representative CD3+CD4+IL4+/GATA3+ gating strategy in the MLNs (b). Representative CD3+CD4+IL17A+/RORγT+ gating strategy in the MLNs.
Acknowledgements
This study was supported by grants from Fundação de Amparo à Pesquisa de Minas Gerais – FAPEMIG UNIVERSAL (2012/APQ 00535‐12), FAPEMIG‐PPM‐Pesquisador Mineiro: 00269‐14; Conselho Nacional de Desenvolvimento Cientıfico e Tecnológico‐CNPq (Bolsa de Produtividade): 306575/2012‐4 and 306768/2015‐1; CNPQ/PVE (401332/2014‐4); Coordenação de Aperfeiçoamento Pessoal de Nível Superior – CAPES. Programa Pós Graduação em Saúde (PPGS) – Universidade Federal de Juiz de Fora. Juiz de Fora‐MG, Brasil. Programa de Pós Graduação em Ciências Biológicas (PPGCBio) ‐ Universidade Federal de Juiz de Fora, Juiz de Fora ‐ MG, Brasil.
References
- 1. GINA (2018) Global Strategy for Asthma Management and Prevention [Internet]. URL https://ginasthma.org/
- 2. McCracken JL, Veeranki SP, Ameredes BT, Calhoun WJ. Diagnosis and management of asthma in adults a review. JAMA 2017; 318:279–90. [DOI] [PubMed] [Google Scholar]
- 3. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012; 18:673–83. [DOI] [PubMed] [Google Scholar]
- 4. Besnard A‐G, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL‐33‐activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol 2011; 41:1675–86. [DOI] [PubMed] [Google Scholar]
- 5. Froidure A, Shen C, Gras D, Van Snick J, Chanez P, Pilette C. Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin‐mediated induction of Th2 and Th9 responses. Allergy 2014; 69:1068–76. [DOI] [PubMed] [Google Scholar]
- 6. Hongjia L, Caiqing Z, Degan L, Fen L, Chao W, Jinxiang W et al IL‐25 promotes Th2 immunity responses in airway inflammation of asthmatic mice via activation of dendritic cells. Inflammation 2014; 37:1070–7. [DOI] [PubMed] [Google Scholar]
- 7. Pattarini L, Trichot C, Bogiatzi S, Grandclaudon M, Meller S, Keuylian Z et al TSLP‐activated dendritic cells induce human T follicular helper cell differentiation through OX40‐ligand. J Exp Med 2017; 214:1529–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnett LG, Simkins HMA, Barnett BE, Korn LL, Johnson AL, Wherry EJ et al B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol 2014; 192:3607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang F, He XY, Baines KJ, Gunawardhana LP, Simpson JL, Li F et al Different inflammatory phenotypes in adults and children with acute asthma. Eur Respir J 2011; 38:567–74. [DOI] [PubMed] [Google Scholar]
- 10. Newson RB, Jones M, Forsberg B, Janson C, Bossios A, Dahlen SE et al The association of asthma, nasal allergies, and positive skin prick tests with obesity, leptin, and adiponectin. Clin Exp Allergy 2014; 44:250–60. [DOI] [PubMed] [Google Scholar]
- 11. Carpaij OA, van den Berge M. The asthma–obesity relationship. Curr Opin Pulm Med 2018; 24:42–9. [DOI] [PubMed] [Google Scholar]
- 12. Fitzpatrick S, Joks R, Silverberg JI. Obesity is associated with increased asthma severity and exacerbations, and increased serum immunoglobulin E in inner‐city adults. Clin Exp Allergy 2012; 42:747–59. [DOI] [PubMed] [Google Scholar]
- 13. Goleva E, Covar R, Martin RJ, Leung DYM. Corticosteroid pharmacokinetic abnormalities in overweight and obese corticosteroid resistant asthmatics. J Allergy Clin Immunol Pract 2016; 4:357–60.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Telenga ED, Tideman SW, Kerstjens HAM, Hacken NHTT, Timens W, Postma DS et al Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy Eur J Allergy Clin Immunol 2012; 67:1060–8. [DOI] [PubMed] [Google Scholar]
- 15. Wang M, Gao P, Wu X, Chen Y, Feng Y, Yang Q et al Impaired anti‐inflammatory action of glucocorticoid in neutrophil from patients with steroid‐resistant asthma. Respir Res 2016; 17:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim HY, Lee HJ, Chang Y, Pichavant M, Stephanie A, Fitzgerald KA et al IL‐17 producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity‐associated airway hyperreactivity. Nat Med 2014; 20:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pizzolla A, Oh DY, Luong S, Prickett SR, Henstridge DC, Febbraio MA et al High fat diet inhibits dendritic cell and T cell response to allergens but does not impair inhalational respiratory tolerance. PLoS ONE 2016; 11:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silva FMC, Oliveira EE, Gouveia ACC, Brugiolo ASS, Alves CC, Correa JOA et al Obesity promotes prolonged ovalbumin‐induced airway inflammation modulating T helper type 1 (Th1), Th2 and Th17 immune responses in BALB/c mice. Clin Exp Immunol 2017; 189:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yadava K, Massacand J, Mosconi I, Nicod LP, Harris NL, Marsland BJ. Thymic stromal lymphopoietin plays divergent roles in murine models of atopic and nonatopic airway inflammation. Allergy 2014; 69:1333–42. [DOI] [PubMed] [Google Scholar]
- 20. Gibeon D, Batuwita K, Osmond M, Heaney LG, Brightling CE, Niven R et al Obesity‐associated severe asthma represents a distinct clinical phenotype analysis of the British thoracic society difficult asthma registry patient cohort according to BMI. Chest 2013; 143:406–14. [DOI] [PubMed] [Google Scholar]
- 21. Gouveia ACC, Brugiolo ASS, Alves CCS, Silva FMC, Mesquita FP, Gameiro J et al Th2 responses in OVA‐Sensitized BALB/c mice are down‐modulated by Mycobacterium bovis BCG treatment. J Clin Immunol 2013; 33:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ et al Mouse strain‐dependent variation in obesity and glucose homeostasis in response to high‐fat feeding. Diabetologia 2013; 56:1129–39. [DOI] [PubMed] [Google Scholar]
- 23. Fearnside JF, Dumas ME, Rothwell AR, Wilder SP, Cloarec O, Toye A et al Phylometabonomic patterns of adaptation to high fat diet feeding in inbred mice. PLoS ONE 2008; 3:e1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sahu N, Morales JL, Fowell D, August A. Modeling susceptibility versus resistance in allergic airway disease reveals regulation by Tec kinase Itk. PLoS ONE 2010; 5:e11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muc M, Todo‐Bom A, Mota‐Pinto A, Vale‐Pereira S, Loureiro C. Leptin and resistin in overweight patients with and without asthma. Allergol Immunopathol 2014; 42:415–21. [DOI] [PubMed] [Google Scholar]
- 26. van Rijt LS, Jung S, KleinJan A, Vos N, Willart M, Duez C et al In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 2005; 201:981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD‐L1hi B cells are critical regulators of humoral immunity. Nat Commun 2015; 6:5997. [DOI] [PubMed] [Google Scholar]
- 28. Vaeth M, Müller G, Stauss D, Dietz L, Klein‐Hessling S, Serfling E et al Follicular regulatory T cells control humoral autoimmunity via NFAT2‐regulated CXCR29 expression. J Exp Med 2014; 211:545–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF et al Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 2011; 17:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Majewska‐Szczepanik M, Askenase PW, Lobo FM, Marcińska K, Wen L, Szczepanik M. Epicutaneous immunization with ovalbumin and CpG induces TH1/TH17 cytokines, which regulate IgE and IgG2a production. J Allergy Clin Immunol 2016; 138:262–73.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kubo M. T follicular helper and T H 2 cells in allergic responses. Allergol Int 2017; 66:377–81. [DOI] [PubMed] [Google Scholar]
- 32. Schatz M, Hsu J‐WY, Zeiger RS, Chen W, Dorenbaum A, Chipps BE et al Phenotypes determined by cluster analysis in severe or difficult‐to‐treat asthma. J Allergy Clin Immunol 2014; 133:1549–56. [DOI] [PubMed] [Google Scholar]
- 33. Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol 2010; 159:617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pelikan Z. Delayed type of asthmatic response to allergen challenge and cytokines in the peripheral blood. Respiration 2012; 84:385–95. [DOI] [PubMed] [Google Scholar]
- 35. Barlow JL, Flynn RJ, Ballantyne SJ, McKenzie ANJ. Reciprocal expression of IL‐25 and IL‐17A is important for allergic airways hyperreactivity. Clin Exp Allergy 2011; 41:1447–55. [DOI] [PubMed] [Google Scholar]
- 36. Pizzolla A, Yuan OhD, Luong S, Prickett SR, Henstridge DC, Febbraio MA et al High fat diet inhibits dendritic cell and T cell response to allergens but does not impair inhalational respiratory tolerance. PLoS ONE 2016; 11:e0160407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gouveia ACC, Braga FG, Mota M, Silva FMC, Brugiolo ASS, Oliveira EE et al Enhanced expression of PD‐L1 and IFN‐γ on dendritic cells is associated with BCG‐induced Th2 inhibition. Cytokine 2017; 99:163–72. [DOI] [PubMed] [Google Scholar]
- 38. Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE. Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol 2006; 118:1284–91. [DOI] [PubMed] [Google Scholar]
- 39. Fenger RV, Gonzalez‐Quintela A, Vidal C, Gude F, Husemoen LL, Aadahl M et al Exploring the obesity‐asthma link: do all types of adiposity increase the risk of asthma? Clin Exp Allergy 2012; 42:1237–45. [DOI] [PubMed] [Google Scholar]
- 40. Beutier H, Gillis CM, Iannascoli B, Godon O, England P, Sibilano R et al IgG subclasses determine pathways of anaphylaxis in mice. J Allergy Clin Immunol 2017; 139:269–80.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA et al B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon Murine influenza infection. J Immunol 2017; 198:4738–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood 2007; 109:2339–45. [DOI] [PubMed] [Google Scholar]
- 43. Gheorghe A, Pérez de Heredia F, Hunsche C, Redondo N, Díaz LE, Hernández O et al Oxidative stress and immunosenescence in spleen of obese mice can be reversed by 2‐hydroxyoleic acid. Exp Physiol 2017; 102:533–44. [DOI] [PubMed] [Google Scholar]
- 44. Gotoh K, Inoue M, Masaki T, Chiba S, Shimasaki T, Ando H et al A novel anti‐inflammatory role for spleen‐derived interleukin‐10 in obesity‐induced inflammation in white adipose tissue and liver. Diabetes 2012; 61:1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sato Mito N, Suzui M, Yoshino H, Kaburagi T, Sato K. Long term effects of high fat and sucrose diets on obesity and lymphocyte proliferation in mice. J Nutr Health Aging 2009; 13:602–6. [DOI] [PubMed] [Google Scholar]
- 46. Guan H, Wan Y, Lan J, Wang Q, Wang Z, Li Y et al PD‐L1 is a critical mediator of regulatory B cells and T cells in invasive breast cancer. Sci Rep 2016; 6:35651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Farnsworth CW, Shehatou CT, Maynard R, Nishitani K, Kates SL, Zuscik MJ et al A humoral immune defect distinguishes the response to Staphylococcus aureus infections in mice with obesity and type 2 diabetes from that in mice with type 1 diabetes. Infect Immun 2015; 83:2264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baumjohann D, Baumjohann D, Ansel KM. (2013) Identification of T follicular helper (Tfh) cells by flow cytometry. Protoc Exch. URL http://www.nature.com/protocolexchange/protocols/2707
- 49. Reinhardt RL, Liang H‐E, Locksley RM. Cytokine‐secreting follicular T cells shape the antibody repertoire. Nat Immunol 2009; 10:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nus M, Sage AP, Lu Y, Masters L, Lam BYH, Newland S et al Marginal zone B cells control the response of follicular helper T cells to a high‐cholesterol diet. Nat Med 2017; 23:601–10. [DOI] [PubMed] [Google Scholar]
- 51. Watanabe M, Fujihara C, Radtke AJ, Chiang YJ, Bhatia S, Germain RN et al Co‐stimulatory function in primary germinal center responses: CD40 and B7 are required on distinct antigen‐presenting cells. J Exp Med 2017; 214:2795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Good‐Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD‐1 regulates germinal center B cell survival and the formation and affinity of long‐lived plasma cells. Nat Immunol 2010; 11:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD‐1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 2013; 14:152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu H, Chen Y, Liu H, Xu L‐L, Teuscher P, Wang S et al Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur J Immunol 2016; 46:1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xie MM, Dent AL. Unexpected help: follicular regulatory T cells in the germinal center. Front Immunol 2018; 9:1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lin AA, Freeman AF, Nutman TB. IL‐10 indirectly downregulates IL‐4‐induced IgE production by human B cells. ImmunoHorizons 2018; 2:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Diaz J, Warren L, Helfner L, Xue X, Chatterjee PK, Gupta M et al Obesity shifts house dust mite‐induced airway cellular infiltration from eosinophils to macrophages: effects of glucocorticoid treatment. Immunol Res 2016; 63:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol 2005; 115:103–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Obesity does not affect cell counting on the peripheral lymphoid organs. Cell counting on the MLNs 48 hr after the last OVA challegenge (a) and 72 hr after the second sensitization (b). Cell counting on the spleen 48 hr after the last OVA challegenge (c) and 72 hr after the second sensitization (d). Values are expressed as mean ± SEM. Different means are indicated as *, for P < 0·05. n = 6 for each group.
Figure S2. Representative flow cytometry gating strategy for DCs phenotyping in MLNs. Population selection, SSC x FSC (a), CD11c+MHCIIhigh gating (b), representative histogram for CD86 and PDL1 expression.
Figure S3. Representative flow cytometry gating strategy for Tfh phenotyping in MLNs. Population selection, SSC x FSC (a), CD3+CD4+CXCR5+PD1+ Foxp3+ gating strategy (b).
Figure S4. Representative flow cytometry gating strategy for Tfh phenotyping in the spleen. Population selection, SSC x FSC (a), CD3+CD4+CXCR5+PD1+ Foxp3+ gating strategy (b).
Figure S5. Representative flow cytometry gating strategy for Th phenotyping in the MLNs and spleen. Population selection, SSC x FSC (a). Representative CD3+CD4+IL4+/GATA3+ gating strategy in the MLNs (b). Representative CD3+CD4+IL17A+/RORγT+ gating strategy in the MLNs.


