Abstract
Background
Prostate cancer (PCa) is one of the most frequently diagnosed cancers in the world. Emerging evidence suggests that inflammatory cells such as M2 macrophages and regulatory T cells (Tregs) can contribute to cancer progression by suppressing the anti‐tumor immune response. This study investigated the number of CD163‐positive M2 macrophages in PCa tissue. It also investigated the correlation and interaction of M2 macrophages and Tregs.
Methods
This nested case‐control study included subjects from a cohort of men diagnosed with PCa as an incidental finding during transurethral resection of the prostate. The cases were 225 men who died from PCa, and the controls were 367 men who survived more than 10 years after PCa diagnosis without disease progression. Infiltrating CD163‐positive M2 macrophages and FOXP3/CD4‐positive Tregs in PCa tissue were identified using immunohistochemistry. The correlation and interaction of M2 macrophages and Tregs were assessed using Spearman's rank‐order correlation and a likelihood test, respectively. Logistic regression was used to estimate odds ratios (ORs) for lethal PCa and macrophage counts.
Results
The number of M2 macrophages and Tregs showed a significant correlation (P < 0.001) but no interactions. The OR for lethal PCa was 1.93 (95%CI: 1.23‐3.03) for men with high numbers of M2 macrophages. Also for cases with uncertain outcome (GS categories 3 + 4 and 4 + 3) high numbers of M2 macrophages does predict a poorer prognosis.
Conclusions
Our data showed that men with high numbers of M2 macrophages in the prostate tumor environment had increased odds of dying of PCa. It is possible that M2 macrophages, together with other suppressor cells such as Tregs, promote an immunosuppressive environment.
Keywords: CD163, FOXP3, Tregs, TAMs
1. INTRODUCTION
Prostate cancer (PCa) is the second most frequently diagnosed cancer in men worldwide, and recent studies have shown that specific immune cells in the tumor microenvironment play critical roles in disease progression.1, 2 Indeed, the immune system may influence cancer development in two opposing ways. On the one hand, specific immune cells can identify and eradicate transformed cells; on the other hand, immune cells can promote cancer progression. There may thus be prognostic value in identifying tumor‐promoting immune cells in patients with PCa.3
Tumor‐associated macrophages (TAMs) can have a tumor‐inhibiting M1 phenotype or a tumor‐promoting M2 phenotype, but they can also have mixed phenotypes, undergo phenotype switching upon stimulation, and develop into subpopulations that show differences in antigen expression.3, 4, 5, 6, 7 The presence of M2 macrophages has been associated with worse clinical outcome in several malignancies8, 9 and is thought to affect disease outcome by stimulating angiogenesis, metastasis, immune suppression and possibly by reducing the effectiveness of certain treatments.3, 4, 10, 11, 12 Previous studies of PCa tissue reported that high numbers of TAMs are associated with poor clinical outcome; however, contradicting results have been reported.7, 13, 14, 15, 16, 17, 18, 19 A major limitation of most of these studies is the use of CD68 to identify macrophages, since this marker cannot distinguish between M1 and M2 macrophages.7, 13, 14, 15, 16, 17 The scavenger receptor CD163 is associated with an anti‐inflammatory macrophage phenotype and is currently the most specific marker for identifying M2 macrophages.20 Recent studies found that high numbers of CD163‐positive M2 macrophages in prostate tumors were associated with poor outcome.18, 19
Regulatory T cells (Tregs) are another type of immune cell that contributes to tumor development by suppressing anti‐tumor immunity.21 Growing evidence supports the hypothesis that an elevated number of Tregs in PCa patients confers poor prognosis and decreases survival rates.22, 23, 24 Recently, when using the same PCa cohort we found that the number of Tregs was associated with a greater likelihood of death from PCa.25
Studies show a correlation between increased numbers of Tregs and macrophages in several types of cancer26, 27, 28, 29; however, to the best of our knowledge, this has not been investigated in prostate tumor progression. This study evaluated the prognostic value of M2 macrophages in a large PCa cohort with long‐term follow‐up. We also investigated the correlation and interaction of M2 macrophages and Tregs.
2. MATERIALS AND METHODS
2.1. Tissue samples
This study was nested within a cohort of men with localized PCa diagnosed in the Örebro and South East Health Care Regions of Sweden between 1977 and 1999. Patients were identified through the population‐based PCa quality database within these regions, and 1367 patients who were diagnosed with incidental PCa through transurethral resection of the prostate (TUR‐P) or adenoma enucleation, that is, category T1a‐b tumors, were identified. All patients were followed expectantly (watchful waiting) according to the standard treatment protocols in use at that time. Record linkages to the Swedish Death Register and Migration Register were used to follow the study cohort for cancer‐specific and all‐cause mortality until 1 March 2006. The cohort was described in detail previously.25, 30
Within this cohort, we utilized a nested study design that we described previously.25 The study included men who either died from or developed metastatic PCa during follow‐up (lethal PCa cases, N = 261) and men who survived at least 10 years following their diagnosis without disease progression (indolent PCa controls, N = 474). At the time of our study, there was sufficient high‐quality tissue available from 225 cases and 367 controls. The study was approved by the Ethical Review Boards in Örebro and Linköping, Sweden.
2.2. Immunohistochemistry
Tissue microarrays (TMAs) were created previously for this cohort using tissue cores with a diameter of 0.6 mm from each TUR‐P specimen. The TMA blocks included three tissue cores from each patient in order to address potential tumor heterogeneity. TMA sections of 4 μm were used for immunohistochemistry (IHC). The TMA sections were dried at 60°C, deparaffinized, rehydrated, and HIER‐treated (Diva solution, Decloaker™ Biocare Medical, Concord, CA). To reduce nonspecific binding, TMA sections were treated for 20 min with normal goat serum in PBS with 0.4% Triton X‐100, followed by a wash in PBS with 0.2% Triton X‐100 and 0.2 % BSA. For CD163 IHC staining, we used the monoclonal mouse anti‐human‐CD163 antibody (clone 10D6, Novocastra, Leica Microsystems, Newcastle, United Kingdom) diluted 1:100 in PBS with 0.1% Triton X‐100. Samples were incubated at room temperature for 1 h and then incubated with DAPI diluted 1:1000 and the secondary antibody mix (anti‐rabbit IgG AleaH488 and anti‐mouse IgG Alexa H555 antibodies Invitrogen, Thermo Fisher Scientific, Waltham, MA) diluted 1:400 in PBS with 0.1% Triton X‐100. Staining was performed using the automated Ventana Benchmark Ultra staining machine (Ventana ES, Ventana Inc., Tucson, AZ) as described previously.31 The tissue was counterstained using hematoxylin and mounted using Vectashield mounting media (Vector Laboratories, CA). The Panoramic 250 Flash II system (3DHISTECH, Budapest, Hungary) was used to convert the glass slides into high‐resolution digital slides (3Dhistech). The number of CD163‐positive M2 macrophages per TMA core was assessed by one observer who was blinded to all clinical data. All CD163 positive macrophages in each TMA core were counted by use of the CaseViewer program (3Dhistech). An experienced pathologist was consulted during counting in case of uncertain assessments. Based on the median number of 25 M2 macrophages/core for all patients (cases and controls), they were categorized as having either high numbers (>25 macrophages/core) or low numbers (≤25 macrophages/core).
2.3. Statistical analysis
The mean number of macrophages in three tissue cores from each patient was used for statistical analyses. To evaluate the association between clinical covariates and lethal case status versus indolent control status, the Student's t‐test was used for continuous variables, and the chi‐square test or chi‐square test for trend were used for categorical variables. To evaluate associations between clinical covariates and the number of M2 macrophages, the Mann‐Whitney U‐test or the Kruskal‐Wallis test were used. Treg data on the same cohort, published previously,25 were used to calculate the correlation and interaction of Tregs with M2 macrophages. Spearmańs rank‐order correlation was used to assess correlations between M2 macrophages and the tumor cell percentage of the tissue and the number of Tregs. A likelihood ratio test was used to test for interactions between M2 macrophages and Tregs. Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for lethal PCa according to M2 macrophage counts after adjusting for age at diagnosis, calendar year of diagnosis, Gleason score (GS) categories (5−6, 3 + 4, 4 + 3 and 8−10), T‐stage (T1a vs T1b), and the percentage of tumor in the tissue specimen. All data were analyzed using SPSS version 22 (IBM, Armonk, NY) and STATA release 14 (Stata Corp., College Station, TX).
3. RESULTS
3.1. Clinical characteristics and PCa outcome
The number of M2 macrophages were counted in prostate tissue samples from men who either died from or developed metastatic PCa during follow‐up (lethal PCa; cases) and men who survived at least 10 years following their diagnosis without disease progression (indolent PCa; controls). The clinical characteristics of the lethal cases (N = 225) and indolent controls (N = 367) are presented in Table 1.
Table 1.
Characteristics of the study population (N = 592)
| Indolent controls, N = 367 (N (%)) | Lethal cases, N = 225 (N (%)) | P‐value | |
|---|---|---|---|
| Age at diagnosis | |||
| Mean ± SD | 72.0 ± 6.6 | 74.8 ± 6.6 | <0.001 a |
| Calendar year of diagnosis | |||
| 1977‐1982 | 16 (4.4) | 13 (5.8) | 0.002 b |
| 1983‐1986 | 18 (4.9) | 4 (1.8) | |
| 1987‐1991 | 134 (36.5) | 113 (50.2) | |
| 1992‐1998 | 199 (54.2) | 95 (42.2) | |
| Tumor stage(1 missing) | |||
| T1a | 178 (48.6) | 56 (24.9) | <0.001 b |
| T1b | 188 (51.4) | 169 (75.1) | |
| Gleason score (1 missing) | |||
| ≤6 | 223 (60.9) | 45 (20.0) | <0.001 c |
| 3 + 4 | 81 (22.2) | 36 (16.0) | |
| 4 + 3 | 44 (12.0) | 44 (19.6) | |
| 8−10 | 18 (4.9) | 100 (44.4) | |
| Tumor percentage (55 missing) | |||
| ≤2 | 151 (44.9) | 37 (18.4) | <0.001 c |
| >2‐10 | 90 (26.8) | 50 (24.9) | |
| >10‐60 | 78 (23.2) | 79 (39.3) | |
| >60 | 17 (5.1) | 35 (17.4) |
SD, standard deviation.
t‐test.
χ 2‐ test.
χ 2‐test of trend.
3.2. IHC analysis of the M2 macrophage marker CD163
The IHC analysis of the M2 macrophage marker CD163 showed that M2 macrophages localized mainly in the stroma close to the tumor epithelial cells. The median number of M2 macrophages in PCa tissue were 30 and 24 for cases and controls, respectively. Representative images of PCa tissue cores with low and high number of CD163 positive macrophages, Figure 1. Selection of CD163 positive macrophages (M2 macrophages) for quantification, Figure 2.
Figure 1.
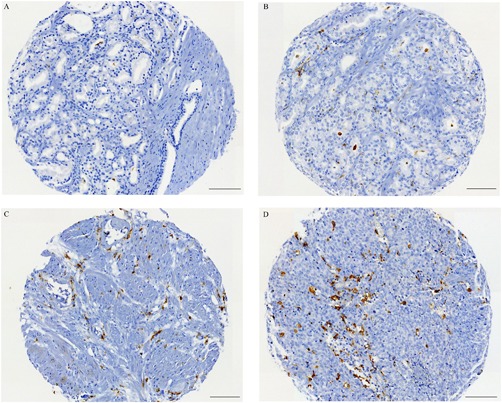
Representative immunohistochemical images of PCa tissue cores stained to visualize the M2 macrophage marker CD163 (brown). A‐B; low number of macrophages. C‐D; high number of macrophages. Scale bars 100 μm
Figure 2.
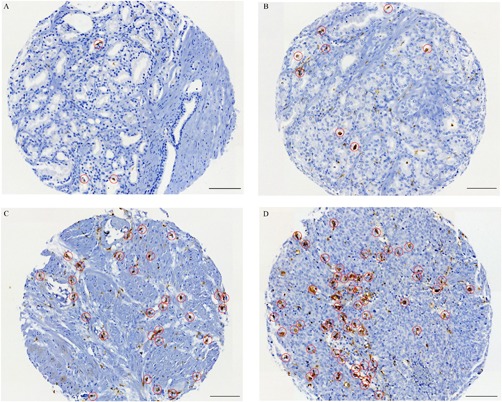
Representative immunohistochemical images of PCa tissue cores demonstrating the selection of CD163 positive cells (M2 macrophages) during quantification. A; N = 3 macrophages. B; N = 8 macrophages. C; N = 30 macrophages. D; N = 60 macrophages. Scale bars 100 μm
3.3. CD163‐positive macrophages and clinicopathological features
There was a significant correlation between the number of M2 macrophages and Tregs (P < 0.001), even though no interaction between the two cell types was found (P = 0.290). There was significantly greater infiltration of M2 macrophages in lethal cases than in indolent controls (P < 0.001). Our data also showed a strong association between the number of M2 macrophages and GS (P < 0.001), with higher numbers of CD163‐positive macrophages in more aggressive tumors (GS 8‐10). We found no association between tumor stage and the number of M2 macrophages (Table 2).
Table 2.
Association between the number of M2 macrophages (continuous) and clinicopathological features (N = 592)
| CD163+ M2 macrophages Median N (IQR) | ρ* | P‐value | |
|---|---|---|---|
| Total population | 25 (17‐40) | ||
| Cases vs controls | |||
| Indolent controls | 24 (15‐35) | <0.001 a | |
| Lethal cases | 30 (20‐44) | ||
| Gleason score (1 missing) | |||
| 5−6 | 23 (15‐38) | <0.001 b | |
| 3 + 4 | 25 (15‐31) | ||
| 4 + 3 | 25 (17‐39) | ||
| 8−10 | 36 (24‐51) | ||
| Tumor percentage (55 missing) | 0.001 | 0.547 c | |
| Tumor stage (1 missing) | |||
| T1a | 25 (17‐38) | 0.299 a | |
| T1b | 25 (17‐40) | ||
| FOXP3+ Treg 3 missing | 0.195 | <0.001 c |
IQR, interquartile range *Spearmańs rank‐order correlation coefficient.
Mann‐Whitney U‐test.
Kruskal‐Wallis test.
Spearmańs rank‐order correlation.
3.4. M2 macrophages and the risk of lethal PCa
The odds of dying of PCa were estimated in patients categorized as having high numbers (>25 macrophages/core) or low numbers (≤25 macrophages/core). In the unadjusted analyses, we observed that men with high numbers of M2 macrophages had a two‐fold increase in the odds of dying of PCa (OR: 2.05; 95%CI: 1.46‐2.88) compared to men with low numbers of M2 macrophages (Table 3). The odds of dying from the disease were significantly increased for men with a high number of M2 macrophages even after adjusting for age at diagnosis, calendar year of diagnosis, GS, tumor stage, and tumor percentage (OR: 1.93; 95%CI: 1.23‐3.03) (Table 3). When cases within GS categories 3 + 4 and 4 + 3 (N = 205) were analyzed it was demonstrated that men with >25 macrophages/core had a 2.05 times increased odds of lethal disease (OR:2.06; 95%CI: 1.04‐4.08) (Table 4).
Table 3.
Odds ratios (ORs) with 95% confidence intervals (CIs) for lethal prostate cancer
| CD163+ M2 macrophages | Model 1 OR (95%CI) | Model 2 OR (95%CI) | Model 3 OR (95%CI) |
|---|---|---|---|
| ≤25 cells | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >25 cells | 2.05 (1.46‐2.88) | 1.63 (1.08‐2.47) | 1.93 (1.23‐3.03) |
Model 1: Unadjusted.
Model 2: Adjusted for age at diagnosis, calendar year of diagnosis (1977‐1982, 1983‐1986, 1987‐1991, 1992‐1998), and Gleason score (5−6, 3 + 4,4 + 3, 8−10).
Model 3: Adjusted for model 2, tumor stage (T1a, T1b), and tumor percentage.
Table 4.
Odds ratios (ORs) with 95% confidence intervals (CIs) for lethal prostate cancer for GS 3 + 4 and GS 4 + 3 cases (N = 205)
| CD163+ M2 macrophages | Model 1 OR (95%CI) | Model 2 OR (95%CI) | Model 3 OR (95%CI) |
|---|---|---|---|
| ≤25 cells | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >25 cells | 1.45 (0.83‐2.55) | 1.55 (0.85‐2.81) | 2.06 (1.04‐4.08) |
Model 1: Unadjusted.
Model 2: Adjusted for age at diagnosis, calendar year of diagnosis (1977‐1982, 1983‐1986, 1987‐1991, 1992‐1998).
Model 3: Adjusted for model 2, tumor stage (T1a, T1b), and tumor percentage.
4. DISCUSSION
TAMs have been linked to worse clinical outcomes in several types of solid tumors, including PCa. This is probably due to the infiltration of TAMs with an M2 phenotype, which can promote tumor growth by suppressing the anti‐tumor immune response.4, 6, 20 M2 macrophages may induce Tregs in the tumor microenvironment, and together these cells can form a special niche that promotes tumor progression.32, 33 Here we found nearly two‐fold increased odds of lethal PCa for men with high infiltration of CD163‐positive M2 macrophages versus low infiltration. In addition, we could demonstrate that a high number of CD163 positive macrophages in cases with uncertain outcome (GS categories 3 + 4 and 4 + 3) does predict a poorer prognosis.
We also found a significant correlation between the number of CD163‐positive M2 macrophages and Tregs (P < 0.001).
TAMs in PCa are associated with poor prognosis, but this has mainly been investigated using the CD68 antigen,7, 13, 14, 15, 16, 17 which is expressed on both M1 and M2 macrophages.4, 6, 18 However, M2 macrophages can be distinguished from M1 macrophages using antibodies that recognize CD163. This is of great importance in studies that investigate the impact of macrophages in carcinogenesis, since M1 macrophages and M2 macrophages play opposing roles in the tumor environment.4, 6 In the present study, we examined the infiltration of CD163‐positive M2 macrophages in a large and clinically well‐defined PCa cohort, and our results demonstrated that men with high numbers of M2 macrophages had a nearly two‐fold increased odds of dying of PCa. This is in line with the results recently presented in a meta‐analysis showing that a higher density of CD68‐positive macrophages was associated with poorer overall survival.5
To our knowledge, only two previous studies have investigated CD163‐positive macrophages in PCa tissue. The first study, by Lanciotti et al, investigated the roles of M1 and M2 macrophages in 93 radical prostatectomy samples. They found that high infiltration of M2 macrophages was associated with poor prognosis and extracapsular extension.18 The second study, which evaluated 234 TUR‐P specimens, demonstrated an association between high numbers of M2 macrophages and poor clinical outcomes.19
M2 macrophages can induce Tregs in different cancer environments,32, 33 and increased numbers of Tregs and macrophages are suggested to correlate with reduced survival.26, 27, 28, 29 To our knowledge, the present study is the first to investigate both M2 macrophages and Tregs in PCa tissue, and our data indicate that this could also be the case in prostate tumors. We observed a positive correlation between the number of M2 macrophages and Tregs (P < 0.001), supporting our hypothesis that M2 macrophages and Tregs, together with other suppressor cells such as myeloid‐derived suppressor cells, promote an immunosuppressive environment in aggressive prostate tumors. This may be important for the clinical management of PCa patients, even though combining M2 macrophages and Tregs had no additional value in estimating prognosis compared to using each cell type separately.
One possible explanation for the correlation between M2 macrophages and Tregs found in the present study is that M2 macrophages can produce immunosuppressive cytokines and chemokines that are involved in recruiting lymphocytes and stimulating them to develop into Tregs.32, 33 In addition, Tregs produce high levels of IL‐10, IL32, and TGFβ that further suppress the anti‐tumor inflammatory response and stimulate M2 macrophages to increase their production of cytokines and chemokines that enable additional recruitment of Tregs. 34 Recently, using the same cohort as in the current study we found high iNOS expression in prostate tumor cells from men with aggressive PCa.35 One additional explanation for the high number of M2 macrophages and Tregs in tissue from patients with lethal PCa could be that a high level of iNOS consumes L‐arginine that could contribute to the activation and/or recruitment of myeloid‐derived suppressor cells, Tregs, and macrophages.36, 37 Our data support the hypothesis that M2 macrophages and Tregs form a niche in the tumor environment that promotes tumor progression. Based on this, therapeutic strategies may benefit from targeting both M2 macrophages and Tregs for an efficient treatment of PCa patients.
Our results provide some insights into the role of M2 macrophages and Tregs in PCa development. One strength of the study is the long follow‐up of the patients, which allowed us to track a substantial number of PCa deaths. A weakness of our study is its use of TMAs, because samples from tumors may not always be representative or may not include the most relevant tumor foci. However, to mitigate this issue, we used three core tissue samples from each tumor.
5. CONCLUSIONS
Infiltration of M2 macrophages identifies PCa patients who are at risk of having lethal disease. Regarding the underlying mechanism, it is possible that M2 macrophages, along with other suppressor cells such as Tregs, promote an immunosuppressive environment.
DISCLOSURE STATEMENT
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
This study was supported financially by the Örebro County Council Research Committee, by the Foundation for Medical Research at Örebro University Hospital Sweden, and by the Lions Cancer Foundation, Sweden.
Erlandsson A, Carlsson J, Lundholm M, et al. M2 macrophages and regulatory T cells in lethal prostate cancer. The Prostate. 2019;79: 363–369. 10.1002/pros.23742
Ove Andrén and Sabina Davidsson contributed equally.
REFERENCES
- 1. Sciarra A, Gentilucci A, Salciccia S, et al. Prognostic value of inflammation in prostate cancer progression and response to therapeutic: a critical review. J Inflamm (Lond). 2016;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strasner A, Karin M. Immune infiltration and prostate cancer. Front Oncol. 2015;5:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solinas G, Germano G, Mantovani A, Allavena P. Tumor‐associated macrophages (TAM) as major players of the cancer‐related inflammation. J Leukoc Biol. 2009;86:1065–1073. [DOI] [PubMed] [Google Scholar]
- 5. Cao J, Liu J, Xu R, Zhu X, Zhao X, Qian BZ. Prognostic role of tumour‐associated macrophages and macrophage scavenger receptor 1 in prostate cancer: a systematic review and meta‐analysis. Oncotarget. 2017;8:83261–83269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. [DOI] [PubMed] [Google Scholar]
- 7. Hu W, Qian Y, Yu F, et al. Alternatively activated macrophages are associated with metastasis and poor prognosis in prostate adenocarcinoma. Oncol Lett. 2015;10:1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao X, Qu J, Sun Y, et al. Prognostic significance of tumor‐associated macrophages in breast cancer: a meta‐analysis of the literature. Oncotarget. 2017;8:30576–30586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuan X, Zhang J, Li D, et al. Prognostic significance of tumor‐associated macrophages in ovarian cancer: a meta‐analysis. Gynecol Oncol. 2017;147:181–187. [DOI] [PubMed] [Google Scholar]
- 10. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. [DOI] [PubMed] [Google Scholar]
- 11. Escamilla J, Schokrpur S, Liu C, et al. CSF1 receptor targeting in prostate cancer reverses macrophage‐mediated resistance to androgen blockade therapy. Cancer Res. 2015;75:950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tumor‐infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445. [DOI] [PubMed] [Google Scholar]
- 14. Shimura S, Yang G, Ebara S, Wheeler TM, Frolov A, Thompson TC. Reduced infiltration of tumor‐associated macrophages in human prostate cancer: association with cancer progression. Cancer Res. 2000;60:5857–5861. [PubMed] [Google Scholar]
- 15. Nonomura N, Takayama H, Nakayama M, et al. Infiltration of tumour‐associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107:1918–1922. [DOI] [PubMed] [Google Scholar]
- 16. Gollapudi K, Galet C, Grogan T, et al. Association between tumor‐associated macrophage infiltration, high grade prostate cancer, and biochemical recurrence after radical prostatectomy. Am J Cancer Res. 2013;3:523–529. [PMC free article] [PubMed] [Google Scholar]
- 17. Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes‐Masson AM, Saad F. Characterization of the intra‐prostatic immune cell infiltration in androgen‐deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. [DOI] [PubMed] [Google Scholar]
- 18. Lanciotti M, Masieri L, Raspollini MR, et al. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. Biomed Res Int. 2014;2014:486798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lundholm M, Hägglöf C, Wikberg ML, et al. Secreted Factors from Colorectal and Prostate Cancer Cells Skew the Immune Response in Opposite Directions. Sci Rep. 2015;5:15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Komohara Y, Hirahara J, Horikawa T, et al. AM‐3K, an anti‐macrophage antibody, recognizes CD163, a molecule associated with an anti‐inflammatory macrophage phenotype. J Histochem Cytochem. 2006;54:763–771. [DOI] [PubMed] [Google Scholar]
- 21. Chen X, Du Y, Lin X, Qian Y, Zhou T, Huang Z. Regulatory T cells in tumor immunity. Int Immunopharmacol. 2016;34:244–249. [DOI] [PubMed] [Google Scholar]
- 22. Ebelt K, Babaryka G, Frankenberger B, et al. Prostate cancer lesions are surrounded by FOXP3+, PD‐1+ and B7‐H1+ lymphocyte clusters. Eur J Cancer. 2009;45:1664–1672. [DOI] [PubMed] [Google Scholar]
- 23. Valdman A, Jaraj SJ, Comperat E, et al. Distribution of Foxp3‐, CD4‐ and CD8‐positive lymphocytic cells in benign and malignant prostate tissue. APMIS. 2010;118:360–365. [DOI] [PubMed] [Google Scholar]
- 24. Davidsson S, Andren O, Ohlson AL, et al. FOXP3+ regulatory T cells in normal prostate tissue, postatrophic hyperplasia, prostatic intraepithelial neoplasia, and tumor histological lesions in men with and without prostate cancer. Prostate. 2018;78:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davidsson S, Ohlson AL, Andersson SO, et al. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3(+) regulatory T cells with respect to lethal prostate cancer. Mod Pathol. 2013;26:448–455. [DOI] [PubMed] [Google Scholar]
- 26. Zhu Q, Wu X, Wang X. Differential distribution of tumor‐associated macrophages and Treg/Th17 cells in the progression of malignant and benign epithelial ovarian tumors. Oncol Lett. 2017;13:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wartenberg M, Zlobec I, Perren A, et al. Accumulation of FOXP3+T‐cells in the tumor microenvironment is associated with an epithelial‐mesenchymal‐transition‐type tumor budding phenotype and is an independent prognostic factor in surgically resected pancreatic ductal adenocarcinoma. Oncotarget. 2015;6:4190–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dannenmann SR, Thielicke J, Stöckli M, et al. Tumor‐associated macrophages subvert T‐cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology. 2013;2: 23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waniczek D, Lorenc Z, Śnietura M, Wesecki M, Kopec A, Muc‐Wierzgoń M. Tumor‐Associated Macrophages and Regulatory T Cells Infiltration and the Clinical Outcome in Colorectal Cancer. Arch Immunol Ther Exp (Warsz). 2017;65:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidsson S, Fiorentino M, Andrén O, et al. Inflammation, focal atrophic lesions, and prostatic intraepithelial neoplasia with respect to risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edin S, Wikberg ML, Dahlin AM, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS ONE. 2012;7: 47045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. [DOI] [PubMed] [Google Scholar]
- 33. Cao Q, Wang Y, Zheng D, et al. IL‐10/TGF‐beta‐modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21:933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4 + CD25 + Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Erlandsson A, Carlsson J, Andersson S‐O, Andrén O, Davidsson S. Rider R J. High iNOS in prostate tumor epithelium is associated with lethal prostate cancer. Scand J Urol. 2018;8:1–5. [DOI] [PubMed] [Google Scholar]
- 36. Idorn M, Køllgaard T, Kongsted P, Sengeløv L, Thor Straten P. Correlation between frequencies of blood monocytic myeloid‐derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration‐resistant metastatic prostate cancer. Cancer Immunol Immunother. 2014;63:1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SH, Roszik J, Grimm EA, Ekmekcioglu S. Impact of l‐Arginine Metabolism on Immune Response and Anticancer Immunotherapy. Front Oncol. 2018;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]


