Supplemental Digital Content is available in the text
Keywords: clinical parameters, lymphocyte-to-monocyte ratio, meta-analysis, ovarian cancer, overall survival, progression-free survival
Abstract
Background:
Lymphocyte-to-monocyte ratio (LMR) was recently proposed as a prognostic factor of ovarian cancer. However, prognostic value of the LMR in ovarian cancer remains inconclusive. The study aimed to assess prognostic value of the LMR in ovarian cancer.
Methods:
Seven common databases were comprehensively searched for relevant studies. The analyses were performed for overall survival (OS), progression-free survival (PFS) and clinical parameters. The hazard ratio (HR) and 95% confidence interval (CI) were used to analyze OS and PFS.
Results:
A total of 2343 patients with ovarian cancer were included in this meta-analysis. The results showed that a low LMR predicted shorter OS (HR = 1.81, 95% CI = 1.38–2.37, P < .01) and PFS (HR = 1.65 95% CI = 1.46–1.85, P < .01) when compared to a high LMR in ovarian cancer. Besides, a low LMR was significantly associated with advanced clinical stage (P < .01), earlier lymph node metastasis (P = .01), higher carbohydrate antigen-125 levels (P < .01), larger residual tumor (P < .01) and worse chemosensitivity (P < .01) when compared to a high LMR in ovarian cancer.
Conclusion:
Low LMR was associated with unfavorable survival in patients with ovarian cancer. LMR could serve as a prognostic biomarker of ovarian cancer.
1. Introduction
Ovarian cancer remains the most lethal gynecologic malignancy and the leading cause of cancer-related deaths in women.[1,2] There are 22,440 estimated new cases and 14,080 estimated deaths for ovarian cancer in 2017.[1] Although the cytoreductive surgery followed by platinum-based adjuvant chemotherapy prolongs the survival of patients with ovarian cancer, prognosis of most cases remains disappointing.[3,4] Given the poor prognosis, identification of optimal biomarkers to predict prognosis of ovarian cancer attracts a growing number of researchers’ attention.[5]
Inflammation has been proved to be associated with carcinogenesis and disease progression, and inflammation in tumor microenvironment can be reflected by a systemic inflammatory response.[6] Increasing evidence has shown that peripheral blood cells and relevant ratios are related to cancer prognosis, such as platelet-to-lymphocyte ratio[7] and neutrophil-to-lymphocyte ratio.[8,9] Similarly, absolute lymphocyte counts and monocyte counts have also been proved to be associated with prognosis of cancer.[10,11] Recently, a growing number of publications demonstrated that lymphocyte-to-monocyte ratio (LMR) was an independent prognostic factor for several types of cancer, such as colorectal cancer,[12] gastric cancer,[13] breast cancer,[14] and lung cancer.[15] Although many studies have explored clinical significance of the LMR in predicting prognosis of ovarian cancer, consensus has not been reached due to the inconsistent results of different studies.[16–22] Therefore, the aim of this meta-analysis was to explore prognostic significance of the LMR in ovarian cancer.
2. Materials and methods
2.1. Literature search
Seven common databases were comprehensively searched until July 21, 2018, including Scopus, PubMed, Web of Science, Embase, Cochrane Library, China National Knowledge Infrastructure, and Wanfang Database. The following keywords were used: (“lymphocyte-to-monocyte ratio” OR “monocyte-to-lymphocyte ratio” OR “LMR” OR “MLR”) AND (“ovarian cancer” OR “ovarian tumor” OR “ovarian neoplasm”). The references of retrieved studies were also checked to avoid missing relevant studies.
2.2. Inclusion criterion and exclusion criterion
Studies meeting the following criteria were eligible:
-
(1)
patients diagnosed as ovarian cancer by pathological examination;
-
(2)
focusing on prognostic value of the LMR in ovarian cancer;
-
(3)
patients divided into 2 groups (high LMR group vs low LMR group);
-
(4)
reporting overall survival (OS), progression-free survival (PFS), or clinical parameters;
-
(5)
having sufficient data.
The following studies were ineligible for this meta-analysis: reviews, letters, case reports, cell experiments, animal experiments, studies with duplicated patients, or studies without sufficient data. The study eligibility was independently assessed by 2 authors. Any disagreement was resolved by discussing with the third author.
2.3. Data extraction and quality assessment
The following items were extracted: the first author, the publication year, countries, the number of patients, histology, clinical stage, treatment, cut-off values, outcomes, analysis models of OS, and information for quality assessment. For prognostic items (eg, OS and PFS), hazard ratio (HR) and 95% confidence interval (CI) were directly obtained from the included studies. Besides, for the studies without HR or corresponding 95% CI, HR and 95% CI would be extracted from survival curves as described by Tierney et al[23] The quality of the included studies was evaluated using Newcastle–Ottawa scale (NOS).[24] Data extraction and quality assessment were completed by 2 authors independently. Any disagreement was resolved by discussing with the third author.
2.4. Statistical analysis
All analyses were performed with Review Manager 5.3 and Stata 12.0 for Windows. For prognostic variables (eg, OS and PFS), HR and corresponding 95% CI were used to detect the overall effects. While for clinicopathological parameters (eg, clinical stage and lymph node metastasis), odds ratio and corresponding 95% CI were used. Inter-study heterogeneity was evaluated by Chi-square test and I2 statistic. The I2 ≤50% showed there was no obvious heterogeneity among studies, hence, a fixed-effect model should be used. Or else, a random-effect model should be applied because of the obvious heterogeneity. Funnel plot was generated to assess the publication bias. Especially, the Begg test and Egger test were also performed to detect the publication bias for the meta-analysis of OS. Sensitivity analyses were conducted to check stability of the results. Galbraith plot was generated to explore the source of heterogeneity.
3. Results
3.1. The literature search and selection
As shown in Figure 1, a total of 191 papers were initially identified from electronic database search. After removal of duplicated papers, there were 84 papers left for further evaluation. Seventy papers were directly excluded by reading titles or abstracts. For the 14 remained papers, full-texts were carefully read and 7 papers were removed for following reasons: 4 papers irrelevant to this topic and 3 papers enrolled duplicated patients. Ultimately, 7 studies were included in this meta-analysis.[16–22]
Figure 1.
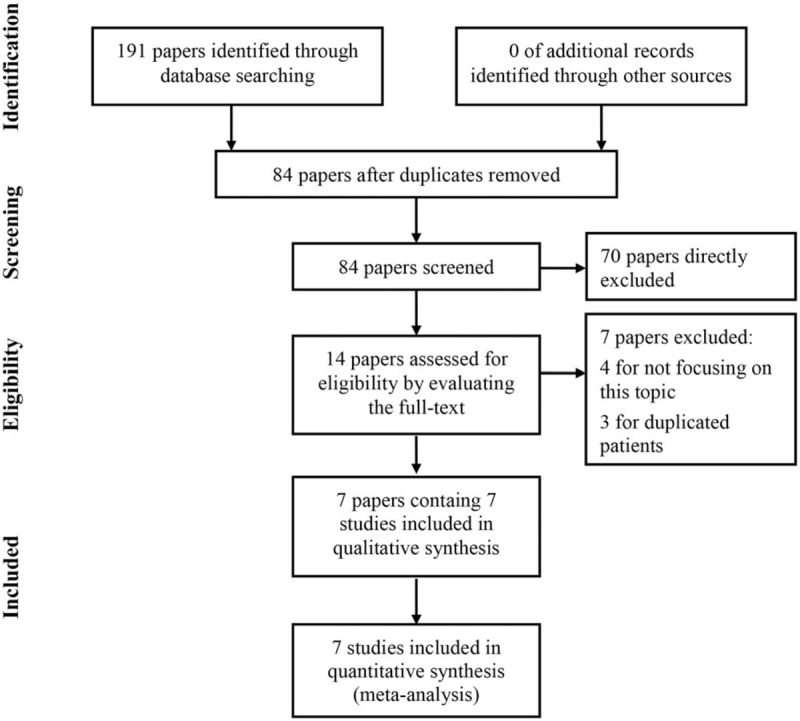
The flow chart of literature search and selection.
3.2. Characteristics of the included studies
Characteristics of the included studies were listed in Table 1. Seven studies, containing 2343 patients, were analyzed in this paper.[16–22] The LMR was calculated using the lymphocyte count and monocyte count from routine blood test before treatment in all studies. There were 1240 patients in low LMR group and 1103 patients in high LMR group. Besides, 5 studies were conducted in China,[18–22] 1 study was performed in USA,[17] and 1 study was conducted in Korea.[17] Moreover, 6 studies reported histology of ovarian cancer[17–22] and all studies reported clinical stage of ovarian cancer based on the International Federation of Gynecology and Obstetrics (FIGO).[16–22] All patients received cytoreductive surgery with or without chemotherapy.[16–22] With regard to cut-off values, all studies provided cut-off values of the LMR, ranging from 2.22 to 4.35.[16–22] Regarding to outcomes, clinical parameters were reported by 5 studies,[16,18,19,21,22] PFS by 3 studies[16,21,22] and OS by 7 studies.[16–22] Furthermore, OS was assessed with multivariate analysis and univariate analysis in 6 studies[16–19,21,22] and 1 study,[20] respectively. The main adjusted factors in the multivariate analysis of OS are listed in Table 2, such as age, FIGO stage, lymph node metastasis, and so on. For quality assessment, NOS was equal to or greater than 6 in all studies, indicating that all studies had relatively high quality.[16–22]
Table 1.
The characteristics of included studies.

Table 2.
The main adjusted factors in the multivariate analysis of OS.

3.3. Association between the LMR and OS
Seven studies reported OS, which were included into the meta-analysis for the association between the LMR and OS[16–22] (Fig. 2). As shown in Figure 2a, the random-effect model was used because of obvious heterogeneity (I2 = 78%), and the results showed a low LMR was significantly associated with shorter OS compared to a high LMR (HR = 1.81, 95% CI = 1.38–2.37, P < .01). The sensitivity analysis indicated that the pooled results would not be altered by excluding any studies (Fig. 2b). There was no obvious publication bias among the included studies based on the funnel plot (Fig. 2c), Begg test and Egger test (Supplementary Fig. 1). Furthermore, Galbraith plot was generated to explore the source of heterogeneity, and the results showed Tang 2017,[18] Wang 2016,[19] and Li 2017[17] were the main sources of heterogeneity (Supplementary Fig. 2). The results confirmed the unfavorable prognostic role of the low LMR in ovarian cancer after removal of the 3 studies (Supplementary Fig. 3).
Figure 2.
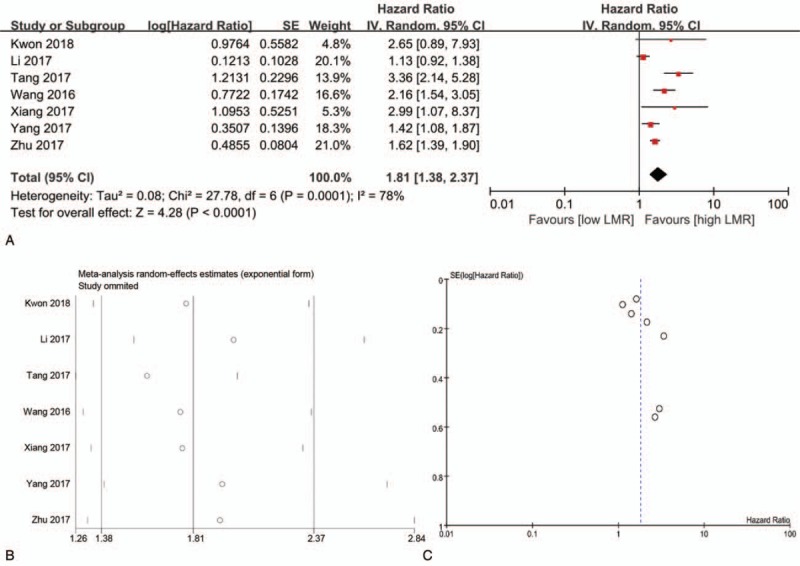
The meta-analysis for association between the LMR and OS (a, forest plot; b, sensitivity analysis; c, funnel plot). LMR = lymphocyte-to-monocyte ratio, OS = overall survival.
The subgroup analyses for the association between LMR and OS were also conducted based on the following categories: analysis models (multivariate analysis vs univariate analysis), countries (China vs other countries), sample size (<300 versus ≥300), cut-off values (<3.90 vs ≥3.90), and NOS (<8 vs ≥8) (Table 3). Significant relationship between the LMR and OS was observed in the majority subgroup analyses (P < .05) except for subgroup analyses of other countries (P = .34).
Table 3.
The subgroup analyses for the association between LMR and OS.

Subgroup analyses were also performed based on the adjusted factors in the multivariate analysis of OS (Table 4). There was obvious relationship between the LMR and OS in most subgroup analyses (P < .05) except for the subgroup analysis of adjusted carbohydrate antigen-125 (CA-125) (P = .24).
Table 4.
The subgroup analyses for the association between LMR and OS based on adjusted factors in multivariate analysis.

3.4. Association between the LMR and PFS
Three studies analyzed PFS and were included into this meta-analysis for association between the LMR and PFS (Fig. 3).[16,21,22] The fixed-effect model was employed for tiny heterogeneity (I2 = 5%), and the results showed that a low LMR predicted shorter PFS when compared to a high LMR (HR = 1.65, 95% CI = 1.46–1.85, P < .01) (Fig. 3a). There was no distinct publication bias among the included studies (Fig. 3b).
Figure 3.
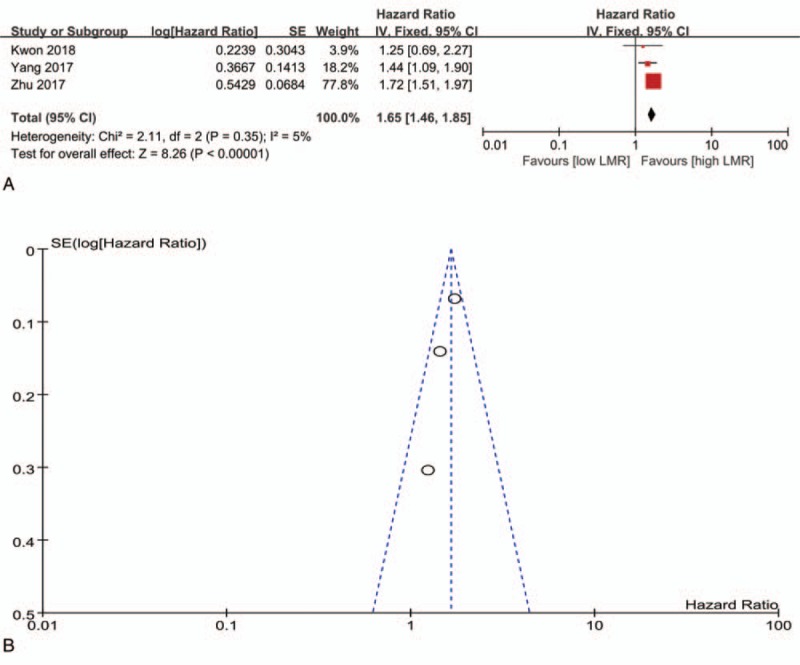
The meta-analysis for association between the LMR and PFS (a, forest plot; b, funnel plot). LMR = lymphocyte-to-monocyte ratio, PFS = progression-free survival.
3.5. Association between the LMR and clinical parameters
As shown in Table 5, the LMR was obviously related to several clinical parameters, including FIGO stage (P < .01), lymph node metastasis (P = .01), CA-125 (P < .01), residual tumor (P < .01), and chemosensitivity (P < .01). However, there was no significant association between the LMR and other clinical parameters, including age (P = .22), histology (P = .71), and histologic grade (P = .45). There was no obvious publication bias among the included studies (Fig. 4).
Table 5.
The meta-analysis for the association between LMR and clinical parameters.

Figure 4.
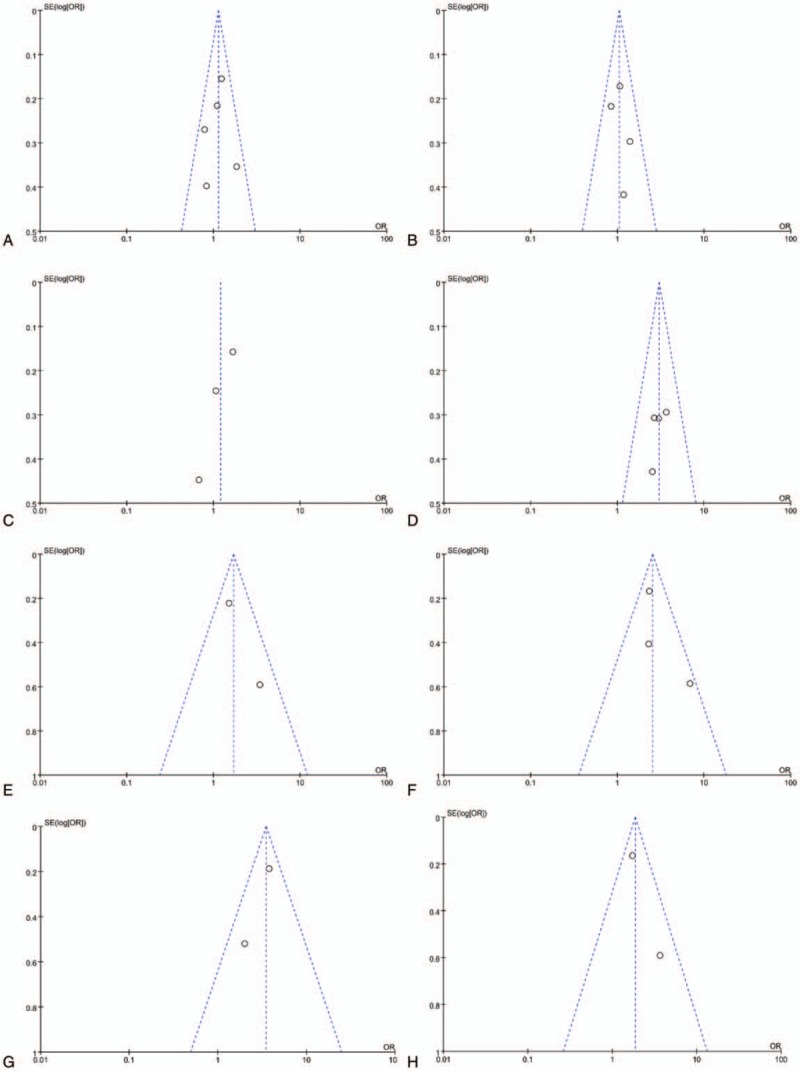
The funnel plots for association between the LMR and clinical parameters (a, age; b, histology; c, histologic grade; d, FIGO stage; e, lymph node metastasis; f, CA-125; g, residual tumor; h, chemosensitivity). CA-125 = carbohydrate antigen-125, FIGO = International Federation of Gynecology and Obstetrics, LMR = lymphocyte-to-monocyte ratio.
4. Discussion
To our knowledge, this is the first study to evaluate prognostic significance of the LMR in ovarian cancer. Our findings showed that a low LMR predicted shorter OS and PFS when compared to a high LMR in ovarian cancer. Besides, a low LMR was distinctly related to advanced FIGO stage, earlier lymph node metastasis, higher CA-125 levels, larger residual tumor, and worse chemosensitivity when compared to a high LMR. Therefore, the LMR might be a promising prognostic factor of ovarian cancer.
Subgroup analyses were also conducted for OS, and significant relationship between the LMR and OS was detected in the majority subgroup analyses. However, subgroup analyses demonstrated that the LMR was not significantly associated with OS in other countries except for China. It should be noted that only 2 studies were included into the analysis, and hence that conclusion might be affected by the small sample size. Therefore, more studies should be implemented to evaluate prognostic significance of the LMR in ovarian cancer in other countries.
Although a great number of publications have explored prognostic significance of the LMR in ovarian cancer, the underlying mechanism remains unclear.[16–22] The systematic inflammation status has been proved to be associated with progression and metastasis of ovarian cancer.[25,26] Plenty of studies have observed prognostic significance of tumor infiltrating immune cells in a variety of solid tumors.[14,16,27,28] The low LMR represented low lymphocyte or high monocyte levels in ovarian cancer. Lymphocytes can migrate into the tumor microenvironment and evolve into tumor-infiltrating lymphocytes.[29,30] CD4+ and CD8+ T lymphocytes play an important role in antitumor immune reaction through inducing cytotoxic cell death and inhibiting tumor cell proliferation and migration.[31,32] Moreover, several studies have demonstrated that lymphocytes prevent development of ovarian cancer.[27,33] Therefore, the decreased lymphocyte counts in blood and tumor stoma result in a weakened antitumor immune response.[32] On the other hand, numerous studies show that monocytes produce various cytokines, for instance, interleukin-6 and interleukin-10, which contribute to poor prognosis of cancer.[34,35] Furthermore, monocytes may promote the proliferation, angiogenesis, and metastatic potential of tumor cells by differentiating into tumor-associated macrophages (TAMs).[36,37] TAMs can facilitate angiogenesis, matrix breakdown, and tumor-cell motility, and then promote metastatic processes.[38,39] Besides, TAMs also produce many compounds to promote tumorigenesis, invasion, and metastasis of tumors, such as mutagenic oxygen and nitrogen radicals to angiogenic factors.[38] Monocytes/TAMs can promote solid-tumor progression and metastasis.[40,41] The aforementioned mechanisms may explain the unfavorable role of a low LMR in ovarian cancer. However, further studies should be conducted to investigate the exact mechanism.
To our knowledge, this is the first study to detect prognostic significance of the LMR in ovarian cancer. A total of 2343 patients were analyzed in our study, and the relatively large sample size could provide a more convincing conclusion. Moreover, subgroup analyses for OS were conducted in this study, which offered comprehensive results on prognostic significance of the LMR in ovarian cancer.
Several limitations should be considered when interpreting our results. First, the cut-off value for the LMR was not unified in our meta-analysis, which might affect the final conclusions. To lower the influence, we performed subgroup analyses based on the cut-off values and confirmed significant relationship between the LMR and OS. More studies should be carried to identify the optimal cut-off value of the LMR in future. Second, the heterogeneity in several analyses was moderate and even evident. As a result, a random-effect model was used, which might influence accuracy of the results. However, it should be noted that significant correlation between the LMR and OS was still observed after excluding 3 studies to eliminate the heterogeneity. Third, the sample size for the meta-analyses of specific clinical parameters was relatively small, which failed to provide strong evidence. Fourth, all the enrolled patients received cytoreductive surgery with or without chemotherapy, and patients receiving other treatment modalities were not analyzed in this meta-analysis. Fifth, most included studies were conducted in China. As a result, caution should be taken when the conclusion was applied to the population in other countries.
5. Conclusion
The LMR was closely associated with OS, PFS, FIGO stage, lymph node metastasis, CA-125, residual tumor, and chemosensitivity in ovarian cancer. Therefore, LMR could serve as a novel prognostic factor of ovarian cancer, especially in China.
Acknowledgments
We thank the authors of the included studies.
Author contributions
Study concepts and design: Huajing Yang; Literature search: Cong Lu, Long Zhou; Data extraction: Cong Lu, Jing Ouyang; Manuscript preparation and revision: Cong Lu, Huajing Yang, and Jing Ouyang. All authors have participated sufficiently in the study and approved the final version.
Conceptualization: Huajing Yang.
Data curation: Cong Lu, Jing Ouyang, Huajing Yang.
Formal analysis: Cong Lu, Huajing Yang.
Funding acquisition: Huajing Yang.
Investigation: Cong Lu, Long Zhou, Jing Ouyang, Huajing Yang.
Methodology: Cong Lu, Long Zhou, Jing Ouyang, Huajing Yang.
Resources: Huajing Yang.
Software: Cong Lu, Long Zhou, Jing Ouyang, Huajing Yang.
Validation: Cong Lu, Long Zhou.
Writing – original draft: Cong Lu, Long Zhou, Jing Ouyang, Huajing Yang.
Writing – review and editing: Cong Lu, Long Zhou, Jing Ouyang, Huajing Yang.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, FIGO = International Federation of Gynecology and Obstetrics, HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, NLR = neutrophil-to-lymphocyte ratio, NOS = Newcastle–Ottawa scale, OS = overall survival, PFS = progression-free survival.
As a meta-analysis, all the data and materials in the present study could be obtained from common databases.
The authors declare that they have no competing interests.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Rooth C. Ovarian cancer: risk factors, treatment and management. Br J Nurs 2013;22:S23–30. [DOI] [PubMed] [Google Scholar]
- [3].Davidson B, Trope CG. Ovarian cancer: diagnostic, biological and prognostic aspects. Womens Health (Lond) 2014;10:519–33. [DOI] [PubMed] [Google Scholar]
- [4].Raja FA, Chopra N, Ledermann JA. Optimal first-line treatment in ovarian cancer. Ann Oncol 2012;23Suppl 10:x118–27. [DOI] [PubMed] [Google Scholar]
- [5].Cannistra SA. Cancer of the ovary. N Engl J Med 2004;351:2519–29. [DOI] [PubMed] [Google Scholar]
- [6].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [7].Zhao Y, Si G, Zhu F, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget 2017;8:22854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhou Y, Wei Q, Fan J, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis containing 8252 patients. Cancer Immunol Immunother 2018;479:181–9. [DOI] [PubMed] [Google Scholar]
- [9].Chen S, Zhang L, Yan G, et al. Neutrophil-to-lymphocyte ratio is a potential prognostic biomarker in patients with ovarian cancer: a meta-analysis. Biomed Res Int 2017;2017:7943467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leibowitz-Amit R, Israel A, Gal M, et al. Association between the absolute baseline lymphocyte count and response to neoadjuvant platinum-based chemotherapy in muscle-invasive bladder cancer. Clin Oncol (R Coll Radiol) 2016;28:790–6. [DOI] [PubMed] [Google Scholar]
- [11].Rochet NM, Kottschade LA, Grotz TE, et al. The prognostic role of the preoperative absolute lymphocyte count and absolute monocyte count in patients with resected advanced melanoma. Am J Clin Oncol 2015;38:252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tan D, Fu Y, Tong W, et al. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: a meta-analysis. Int J Surg 2018;55:128–38. [DOI] [PubMed] [Google Scholar]
- [13].Ma JY, Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: a meta-analysis. Int J Surg 2018;50:67–71. [DOI] [PubMed] [Google Scholar]
- [14].Hu RJ, Liu Q, Ma JY, et al. Preoperative lymphocyte-to-monocyte ratio predicts breast cancer outcome: a meta-analysis. Clin Chim Acta 2018;484:1–6. [DOI] [PubMed] [Google Scholar]
- [15].Li W, Ma G, Wu Q, et al. Prognostic value of lymphocyte-to-monocyte ratio among Asian lung cancer patients: a systematic review and meta-analysis. Oncotarget 2017;8:110606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kwon BS, Jeong DH, Byun JM, et al. Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma. J Cancer 2018;9:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li Z, Hong N, Robertson M, et al. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep 2017;7:43001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tang Y, Li J, Xu F, et al. Association between monocyte-to-lymphocyte ratio and prognosis of patients with epithelial ovarian cancer. Chin J Obstet Gynecol Pediatr (Electron Ed) 2017;13:532–8. [Google Scholar]
- [19].Wang XJ, Yuan ZF, Qiu HF, et al. The relationship between preoperative blood lymphocyte-to-monocyte ratio and the prog-nostic of epithelial ovarian cancer. Prog Obstet Gynecol 2016;25:654–7. [Google Scholar]
- [20].Xiang J, Zhou L, Li X, et al. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol 2017;10:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang HM, Lou G. The relationship of preoperativelymphocyte-monocyte ratio and the clinicopathological characteristics and prognosis of patients with epithelial ovarian cancer. Zhonghua Zhong Liu Za Zhi 2017;39:676–80. [DOI] [PubMed] [Google Scholar]
- [22].Zhu JY, Liu CC, Wang L, et al. Peripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer 2017;8:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [25].Clendenen TV, Lundin E, Zeleniuch-Jacquotte A, et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 2011;20:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rei M, Goncalves-Sousa N, Lanca T, et al. Murine CD27(−) Vgamma6(+) gammadelta T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A 2014;111:E3562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007;104:3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li B, Zhou P, Liu Y, et al. Platelet-to-lymphocyte ratio in advanced cancer: review and meta-analysis. Clin Chim Acta 2018;483:48–56. [DOI] [PubMed] [Google Scholar]
- [29].Santoiemma PP, Powell DJ., Jr Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther 2015;16:807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Webb JR, Milne K, Watson P, et al. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res 2014;20:434–44. [DOI] [PubMed] [Google Scholar]
- [31].Zikos TA, Donnenberg AD, Landreneau RJ, et al. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother 2011;60:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang Y, Wang L, Liu Y, et al. Preoperative neutrophil-lymphocyte ratio before platelet-lymphocyte ratio predicts clinical outcome in patients with cervical cancer treated with initial radical surgery. Int J Gynecol Cancer 2014;24:1319–25. [DOI] [PubMed] [Google Scholar]
- [33].Abiko K, Matsumura N, Hamanishi J, et al. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer 2015;112:1501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Anand M, Chodda SK, Parikh PM, et al. Abnormal levels of proinflammatory cytokines in serum and monocyte cultures from patients with chronic myeloid leukemia in different stages, and their role in prognosis. Hematol Oncol 1998;16:143–54. [DOI] [PubMed] [Google Scholar]
- [35].Ishii H, Takahara M, Nagato T, et al. Monocytes enhance cell proliferation and LMP1 expression of nasal natural killer/T-cell lymphoma cells by cell contact-dependent interaction through membrane-bound IL-15. Int J Cancer 2012;130:48–58. [DOI] [PubMed] [Google Scholar]
- [36].Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263–6. [DOI] [PubMed] [Google Scholar]
- [37].Wang X, Zhao X, Wang K, et al. Interaction of monocytes/macrophages with ovarian cancer cells promotes angiogenesis in vitro. Cancer Sci 2013;104:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Galdiero MR, Bonavita E, Barajon I, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013;218:1402–10. [DOI] [PubMed] [Google Scholar]
- [39].Mhawech-Fauceglia P, Wang D, Ali L, et al. Intraepithelial T cells and tumor-associated macrophages in ovarian cancer patients. Cancer Immun 2013;13:1. [PMC free article] [PubMed] [Google Scholar]
- [40].Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science 2014;344:921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011;9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


