Abstract
Background
Daratumumab is a human CD38‐directed monoclonal antibody indicated for the treatment of relapsed and refractory multiple myeloma (MM).
Methods
A multicenter, open‐label treatment protocol provided early access to daratumumab for patients who had progressive MM after they received ≥3 prior lines of therapy that included a proteasome inhibitor and an immunomodulatory agent or if they were refractory to both a proteasome inhibitor and an immunomodulatory agent. Patients received daratumumab 16 mg/kg weekly for 8 weeks, every other week for 16 weeks, and monthly until they developed disease progression, unacceptable toxicity, or 60 days after the drug gained US approval. Treatment‐emergent grade ≥3 adverse events (AEs), serious AEs, and AEs of special interest were collected.
Results
Three hundred forty‐eight patients were enrolled at 39 US sites between June and December 2015. Patients received study therapy for a median of 1.9 months (range, 0.03‐6.0 months). Fifty‐two percent of patients transitioned to commercially‐available daratumumab and 37% discontinued because of progressive disease. Grade ≥3 AEs occurred in 50% of patients, including thrombocytopenia (15%) and anemia (14%). Serious AEs occurred in 35% of patients (12% were drug‐related), including infections (11%). Infusion reactions occurred in 56%, 2%, and 2% of patients during the first, second, and all subsequent infusions, respectively; respiratory symptoms (cough, dyspnea, throat irritation, nasal congestion) were common. The infusion reaction rate for the first infusion was 38% in 50 patients at 2 sites who received montelukast as premedication for their first infusion and 59% in patients who did not receive montelukast.
Conclusions
The current findings are consistent with previously reported trials and confirm the safety profile of daratumumab in heavily pretreated US patients who have relapsed or refractory MM. Cancer 2018;124:000‐000.
Keywords: CD38, daratumumab, montelukast, monoclonal antibodies, multiple myeloma
Short abstract
An early access treatment protocol confirms the safety profile of daratumumab in patients with relapsed or refractory myeloma. Findings from 2 study sites (60 patients) that used montelukast premedication indicate reduced infusion reactions and infusion time associated with the first dose of daratumumab.
Introduction
Although proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs) have drastically changed the treatment landscape for multiple myeloma (MM), improving outcomes and survival rates in a patient population with limited treatment options,1, 2, 3, 4 there remains a high unmet need for effective and tolerable therapies for patients with relapsed and/or refractory disease.1 Daratumumab is a novel, human immunoglobulin Gκ monoclonal antibody targeting cluster of differentiation 38 (CD38 [also known as cyclic adenosine diphosphate ribose hydrolase]), which is heavily and uniformly expressed on myeloma cells.2, 5 The mechanisms of action of daratumumab include complement‐dependent cytotoxicity, antibody‐dependent cell‐mediated cytotoxicity, antibody‐dependent cellular phagocytosis, direct induction of apoptosis, and immunomodulation.5, 6, 7
Daratumumab initially exhibited antitumor activity as a single agent in a phase 1/2 trial in patients with myeloma that had relapsed after or was refractory to ≥2 prior therapy lines, yielding an overall response rate (ORR) of 36% in patients who received a 16‐mg/kg dose compared with 10% in those who received 8 mg/kg.8 In a subsequent, pivotal, single‐agent phase 2 trial of daratumumab 16 mg/kg, the ORR was 29% in heavily pretreated patients (those who received ≥3 prior therapy lines, including a PI and an IMiD, or who were refractory to both a PI and an IMiD).9 A pooled analysis of both monotherapy studies produced an ORR of 31% and a median overall survival of 20.1 months, demonstrating a durable response and clinical benefit in patients who had responses of stable disease or better.10 On the basis of these findings, daratumumab was approved in the US for use as monotherapy (16 mg/kg) for the treatment of patients with MM who have received ≥3 prior therapy lines, including a PI and an IMiD, or who are double‐refractory to a PI and an IMiD.11 In addition, experience from those studies provided a basis for managing monoclonal antibody‐associated infusion reactions (IRs) in patients with MM. CD38 is expressed on airway smooth muscle cells, and IRs reported in previous trials with daratumumab were commonly characterized by symptoms similar to those of allergic rhinitis (eg, cough, dyspnea, bronchospasm10, 12, 13). Anecdotal reports have suggested that premedication with montelukast, a leukotriene receptor antagonist known to reduce asthma attacks and allergic rhinitis, may reduce the IR rate associated with monoclonal antibodies,2, 14, 15, 16 and this observation was also reported by investigators in the phase 1/2 study that resulted in the initial approval of daratumumab.8
Herein, we present findings from the US cohort of a multicenter, open‐label, early access treatment protocol (EAP) conducted in patients with MM who received ≥3 prior therapy lines, including a PI and an IMiD, or who were double refractory to a PI and an IMiD. The study’s objectives were to provide early access to daratumumab for eligible patients before commercial approval and to collect additional safety and patient‐reported outcome (PRO) data.
Materials and Methods
Patients
Patients were aged ≥18 years; had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0, 1, or 2; had documented MM with evidence of disease progression within 60 days of the most recent prior treatment regimen according to International Myeloma Working Group criteria17; either had received ≥3 prior therapy lines with a PI (≥2 cycles or 2 months of treatment) and an IMiD (≥2 cycles or 2 months of treatment) or had disease that was double refractory to a PI and an IMiD; and resided in areas where daratumumab was not yet commercially available through local health care providers, had not been enrolled in another daratumumab study, and were not eligible for or did not have access to enrollment in another ongoing clinical study of daratumumab. According to the protocol, patients were excluded if they had known chronic obstructive pulmonary disease with a forced expiratory volume in 1 second <50% of predicted normal; had known moderate/severe asthma within the past 2 years or currently uncontrolled asthma; or had clinically significant cardiac disease, cardiac arrhythmia, or clinically significant electrocardiogram abnormalities. Patients with an absolute neutrophil count ≤0.5 109/L, a hemoglobin level ≤7 g/dL (≤4 mmol/L), a platelet count <50 109/L, an alanine aminotransferase level ≥2.5 times the upper limit of normal, a total bilirubin level ≥2 times the upper limit of normal, a creatinine clearance ≤20 mL/minute/1.73 m2, a potassium level <3.0 mEq/L, or a corrected serum calcium level >14.0 mg/dL (3.5 mmol/L) also were excluded, as were those who had allergies, hypersensitivity, or intolerance to monoclonal antibodies or human proteins, sensitivity to mammalian‐derived products, or prior exposure to any anti‐CD38 monoclonal antibody.
Before any study‐related procedures were conducted, all patients provided written, informed consent to participate in the study, which was conducted according to the ethical principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. The protocol and amendments were approved by an Independent Ethics Committee or Institutional Review Board. The study was registered at clinicaltrials.gov (national clinical trials identifier NCT02477891).
Study Design
Eligible patients received daratumumab 16 mg/kg intravenously on days 1, 8, 15, and 22 of cycles 1 and 2 (weekly dosing); on days 1 and 15 of cycles 3 through 6 (every 2 weeks dosing); and on day 1 of cycle 7 and subsequent cycles (every 4 weeks dosing) until disease progression, unacceptable toxicity, loss of clinical benefit, or the end of the study (Fig. 1). All cycles were 28 days.
Figure 1.
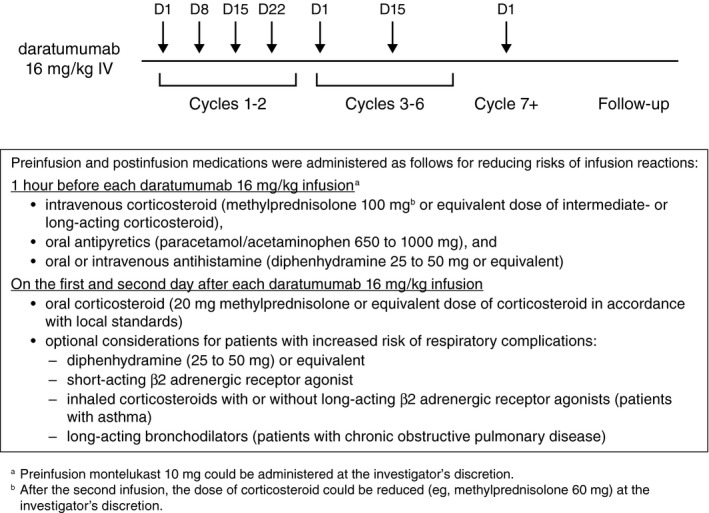
This is a schematic overview of daratumumab administration. D indicates day; IV, intravenous.
Patients received preinfusion and postinfusion medications to prevent IRs. Preinfusion medications, which were received approximately 1 hour before dosing, included acetaminophen (paracetamol) (650‐1000 mg), diphenhydramine (25‐50 mg) or an equivalent antihistamine drug, and methylprednisolone (100 mg for the first and second infusions and 60 mg for all subsequent infusions). Preinfusion dosing of montelukast (10 mg) was allowed at the investigator’s discretion. Methylprednisolone (20 mg) or equivalent was administered postinfusion for 2 days after all daratumumab infusions. For patients with a higher risk of respiratory complications, the following postinfusion medications were considered: diphenhydramine (25‐50 mg) or equivalent for 2 days after all daratumumab infusions, short‐acting β2 adrenergic receptor agonist (eg, salbutamol aerosol), inhaled corticosteroids with or without long‐acting β2 adrenergic receptor agonists for patients with asthma, and long‐acting bronchodilators (eg, tiotropium or salbutamol with or without inhaled corticosteroids) for patients with chronic obstructive pulmonary disease. Patients who continued to benefit from daratumumab at the study’s closure had the option to continue receiving the drug from commercial or other sources.
Safety Assessments
Patients were monitored for safety based on adverse events (AEs), ECOG performance status, vital sign measurements, physical examinations, and clinical laboratory tests (hematology, serum chemistry, and urinalysis). Only treatment‐emergent grade ≥3 or serious AEs (SAEs) or AEs of special interest (bronchospasm, IR, or any unscheduled laboratory abnormalities associated with these events) were collected. AEs that occurred between the time of signed, informed consent until 30 days after the last daratumumab dose were considered treatment‐emergent AEs, were reported by the patient (or other legally acceptable representative)—but monitored and evaluated by the investigator—and were graded according to National Cancer Institute Common Toxicity Criteria for Adverse Events (version 4.03; National Cancer Institute, Bethesda, MD).
Efficacy Assessments
This study did not include efficacy endpoints or assessments. To guide end‐of‐treatment decisions and to determine whether continued treatment with daratumumab was warranted, investigators assessed patients’ disease response (eg, disease progression or loss of clinical benefit) according to the local standard of care, as clinically indicated, from the start of daratumumab treatment until discontinuation. Progressive disease was determined based on investigator assessment.
Patient‐Reported Outcome Assessments
PRO assessments were collected at baseline; on day 1 of cycles 2, 3, 6, and every other cycle thereafter; and at the end‐of‐study visit. PRO tools included the European Organization for Research and Treatment of Cancer (EORTC) core quality‐of‐life questionnaire (QLQ‐C30), the EORTC Multiple Myeloma Module (QLQ‐MY20), and the European Quality of Life 5 Dimensions Questionnaire (EQ‐5D‐5L). These assessments were electronically recorded.
Statistical Analyses
The analysis population included all patients who received ≥1 daratumumab dose at sites in the US. Sample size was not determined by statistical calculations; rather, up to 2000 patients were expected to be enrolled globally, and the final number was determined by medical need and health authority approval. This study included no efficacy endpoints and no inferential statistics. Unless otherwise specified, all continuous endpoints were summarized using descriptive statistics, and categorical endpoints were summarized in terms of frequency.
Results
Baseline Demographics and Disease Characteristics
Enrollment occurred between June 23, 2015, and December 11, 2015; by January 16, 2016, all patients had either discontinued study participation or transitioned to commercial drug. The study period ended on February 29, 2016. In total, 400 patients were screened, 350 were enrolled from 39 US sites, and 348 received ≥1 daratumumab dose. Most patients were white (72%), were men (56%), and had a baseline ECOG performance status of 1 or 2 (74%). The median patient age was 65 years (range, 27‐94 years), and the median duration of follow‐up was 2.8 months (range, 0.1‐6.9 months). Table 1 provides information about additional patient demographics and baseline disease characteristics.
Table 1.
Patient Demographics and Baseline Characteristics: Daratumumab, N = 348
| Variable | No. of Patients (%) or Median [Range] |
|---|---|
| Age, y | 65 [27‐94] |
| Sex | |
| Men | 193 (56) |
| Race | |
| White | 251 (72) |
| Black or African American | 59 (17) |
| Unknown or not reported | 26 (8) |
| Asian | 7 (2) |
| Othera | 3 (1) |
| Multiple | 2 (1) |
| Weight, kg | 78 [43‐189] |
| ECOG performance status | |
| 0 | 90 (26) |
| 1 | 201 (58) |
| 2 | 57 (16) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Other races do not include American Indians, Alaska Natives, Native Hawaiians, or other Pacific Islanders.
Upon study closure, 181 patients (52%) continued on commercially available daratumumab after US market authorization. At that time, 131 patients (38%) had discontinued because of progressive disease or disease relapse, 14 (4%) had discontinued because of an AE, 7 (2%) had withdrawn consent, 5 (1%) had died, 5 (1%) had discontinued for other reasons, 4 (1%) had discontinued based on their physicians’ decision, and 1 (0.3%) was lost to follow‐up.
Treatment Exposure
All 348 patients received ≥1 full or partial treatment cycle of daratumumab, and approximately one‐half (52%) received ≥3 cycles. Patients received a median of 8 daratumumab infusions (range, 1‐17 infusions). The median duration of daratumumab exposure was 1.9 months (range, 0.03‐6.01 months). Daratumumab‐related toxicities were managed primarily by dose delay; skipped infusion was reported in 60 patients (17%), including a skipped infusion associated with an AE in 42 patients (12%). Twenty patients (6%) experienced a cycle delay, including a cycle delay because of an AE in 13 patients (4%). Table 2 provides an overview of treatment exposure.
Table 2.
Treatment Exposure and Administration: Daratumumab, N = 348a
| Variable | Median (Range) |
|---|---|
| Duration of treatment, mo | 1.9 (0.03‐6.0) |
| No. treatment cycles | 3 (1‐7) |
| Total no. daratumumab infusions | 8 (1‐17) |
| Relative dose intensity, % | 100.7 (64.9‐108.9) |
| Duration of infusions, h | |
| First infusion | 7.4 (1.0‐24.0) |
| Second infusion | 4.4 (2.9‐16.3) |
| All subsequent infusions | 3.5 (0.8‐26.1) |
Doses include daratumumab delivered as part of investigational supply and not commercial supply. The duration of infusion includes both actual infusion time and interruption time, if any.
Efficacy
Investigator‐assessed objective responses (stringent complete response [sCR] + complete response [CR] + very good partial response [VGPR] + partial response [PR]) were observed in 23% of patients. Best responses of PR were reported in 62 patients (18%), a VGPR in 17 patients (5%), and a sCR in 2 patients (0.6%). Best responses of minimal response in 19 patients (6%), stable disease in 106 patients (31%), and progressive disease in 83 patients (24%) were reported. Fifty‐nine patients (17%) were not available/evaluable for response assessment.
Patient‐Reported Outcomes
Of 324 patients (93%) who completed the EQ‐5D‐5L assessment at baseline, 226 (65%) completed the assessment on day 1 of cycle 2. Of 326 patients (94%) who completed the EORTC QLQ‐C30 assessment at baseline, 228 (66%) completed the assessment on day 1 of cycle 2. In addition, at 3 study sites, 5 patient volunteers were interviewed (within 10 days of cycle 3 or within 10 days after the end of treatment). The data collected through these interviews provided qualitative insight into patient experiences and impressions regarding the use of daratumumab. Changes from baseline in EQ‐5D‐5L utility and visual analog scale scores and EORTC QLQ‐C30 values were minimal and are reported in Supporting Tables 1 and 2, respectively. The median EQ‐5D‐5L utility score was 0.79 (range, 0.1‐1.0), and the median visual analog score was 66 (range, 9‐100) at baseline; at the last assessment, the median scores were 0.79 (range, 0.0‐1.0) and 67 (0‐100), respectively.
Safety
In total, 227 patients (65%) had AEs determined by the investigator as related to daratumumab. Grade 3 and 4 AEs were reported in 175 patients (50%) and were considered to be daratumumab‐related in 85 (24%). SAEs were reported in 123 patients (35%), including 43 (12%) who had SAEs that were considered to be daratumumab‐related and 101 (29%) who had grade 3 or 4 SAEs. AEs leading to treatment discontinuation were reported for 32 patients (9%) and were considered to be daratumumab‐related for 10 (3%). AEs with an outcome of death were reported for 13 patients (4%), including 2 (0.6%) who had AEs (pyrexia, thrombocytopenia/subdural hematoma) that were considered to be daratumumab‐related.
The most frequently reported grade 3 and 4 AEs are listed in Table 3; the most frequently reported daratumumab‐related grade 3 and 4 AEs were thrombocytopenia (6%) and anemia (5%). Table 4 lists the most frequently reported SAEs, and the most common grade 3 and 4 SAEs were pneumonia and hypercalcemia (3% each) and thrombocytopenia (2%). The most frequently reported AEs leading to treatment discontinuation were thrombocytopenia (2%); hypercalcemia and acute kidney injury (1% each); and dyspnea, anemia, and pyrexia (0.9% each).
Table 3.
Most Frequently Reported Grade 3 or 4 Adverse Events: Daratumumab, N = 348a
| Adverse Event | No. of Patients (%) |
|---|---|
| Grade 3 or 4 adverse event | 175 (50) |
| Drug‐related | 85 (24) |
| Thrombocytopenia | 53 (15) |
| Anemia | 47 (14) |
| Neutropenia | 28 (8) |
| Lymphopenia | 22 (6) |
| Hypercalcemia | 17 (5) |
These were adverse events reported in ≥1% of patients.
Table 4.
Most Frequently Reported Serious Adverse Events: Daratumumab, N = 348a
| No. of Patients (%) | ||
|---|---|---|
| Adverse Event | All Grades | Grade 3 or 4 |
| Serious adverse event | 123 (35) | 101 (29) |
| Drug‐related | 43 (12) | |
| Hypercalcemia | 13 (4) | 10 (3) |
| Pneumonia | 12 (3) | 10 (3) |
| Dyspnea | 9 (3) | 6 (2) |
| Pyrexia | 9 (3) | 4 (1) |
| Urinary tract infection | 6 (2) | 6 (2) |
| Thrombocytopenia | 8 (2) | 8 (2) |
| Febrile neutropenia | 7 (2) | 6 (2) |
| Acute kidney injury | 7 (2) | 5 (1) |
| Anemia | 5 (1) | 5 (1) |
| Pancytopenia | 4 (1) | 4 (1) |
| Sepsis | 5 (1) | 5 (1) |
| Back pain | 4 (1) | 4 (1) |
| Renal failure | 5 (1) | 3 (1) |
| Cough | 4 (1) | 0 |
These were adverse events reported in ≥5% of patients.
Infusion modifications (ie, infusion interruption, decreased infusion rate, or infusion discontinuation) because of an AE were reported in 182 patients (52%) and were considered daratumumab‐related in 178 (51%), including chills (14%), cough (13%), IR not otherwise specified (NOS) (8%), dyspnea (8%), throat irritation (6%), and nausea (6%). Sixteen patients (5%) experienced AEs that led to discontinuation of that infusion; 15 were considered daratumumab‐related and included cough, dyspnea, or flushing (0.9% each) and chills and IR NOS (0.6% each).
IRs were reported in 195 patients (56%), including 24 (10%) who had IRs that were considered SAEs and 27 (8%) who had grade 3 or 4 IRs. The most frequently reported IRs were chills (15%), cough (14%), dyspnea (9%), IR NOS (8%), nausea (7%), throat irritation (6%), and nasal congestion (5%). All 195 patients (56%) experienced IRs during the first daratumumab infusion, 7 (2%) experienced IRs during the second infusion, and 6 (2%) experienced IRs during a subsequent infusion (Table 5). Six patients (2%) experienced bronchospasm, 1 of which was considered serious. In each instance, bronchospasm was considered related to daratumumab, occurred during the first infusion, and resolved after interruption and resumption of drug.
Table 5.
Infusion Reactions: Daratumumab, N = 348
| Reaction | All Patients, n = 348 | Montelukast Pretreated, n = 60 | No Montelukast, n = 288 |
|---|---|---|---|
| Patients with an infusion reaction, % | 56 | 33 | 61 |
| Grade 3‐4 | 8 | 2 | 9 |
| First infusiona | 56 | 38 | 59 |
| Second infusion | 2 | 4 | 2 |
| All subsequent infusions | 2 | 0 | 2 |
| Patients with respiratory or thoracic symptoms, % | 31 | 18 | 34 |
| Cough | 14 | 8 | 15 |
| Dyspnea | 9 | 5 | 9 |
| Throat irritation | 6 | 5 | 6 |
| Nasal congestion | 5 | 5 | 5 |
| Wheezing | 4 | 0 | 5 |
| Bronchospasm | 2 | 0 | 2 |
For the first infusion, only 50 patients received montelukast premedication.
Use of Montelukast Premedication
Sixty patients (17%) received montelukast 10 mg before at least 1 infusion. Of these, 50 patients (14%) at 2 study sites received montelukast before the first daratumumab infusion, 49 of whom received montelukast on same day more than 30 minutes before daratumumab infusion. Based on those 50 patients, the IR rates at first infusion were 38% and 59% (respiratory symptoms, 20% and 33%), respectively, in those who did or did not receive montelukast. Furthermore, the incidence of grade 3 and 4 IRs was 2% and 9% in patients who did (n = 60) or did not (n = 298) receive montelukast, respectively (Table 5). Gastrointestinal symptoms were observed in 4% and 11% of patients who did or did not receive montelukast, respectively, whereas chills were observed in 14% of patients in both groups. The median duration of the first daratumumab infusion was 6.7 hours (range, 1.0‐10.7 hours) for the 50 patients who received montelukast before their first infusion versus 7.6 hours (range, 1.4‐24.0 hours) for those who did not.
Discussion
The findings of this EAP study confirm the safety profile of daratumumab in patients with MM who have received ≥3 prior therapy lines, including a PI and an IMiD, or who are refractory to both a PI and an IMiD. The safety profile in this study was consistent with results observed in the initial phase 1/2 study and the subsequent pivotal phase 2 study, and no new safety signals were identified.8, 9, 10, 11 Similar to previous studies of daratumumab monotherapy, approximately one‐half of patients experienced an IR at the first daratumumab infusion, but IR rates rapidly declined over subsequent infusions, decreasing to only 2% of patients at the second infusion. The majority of IRs were grade 1 and 2, with grade 3 and 4 IRs observed in 8% of patients. Although the rate of grade 3 and 4 IRs was low, the slightly higher rate in the current study, compared with previous studies, may have been because of the broader range of study centers (including community‐based sites) that treated patients in our trial. Similar to previously described findings, respiratory symptoms were commonly observed during IRs and included cough (14%), dyspnea (9%), throat irritation (6%), and nasal congestion (5%).
CD38 is expressed on upper airway cells, and previous studies observed increased respiratory symptoms, such as cough, dyspnea, and bronchospasm, as components of IRs to daratumumab. There are published case reports using montelukast (a leukotriene receptor antagonist used to treat asthma and allergies) in resensitization protocols for patients who have hypersensitivity reactions to monoclonal antibodies. In the current study, montelukast was used primarily at only 2 large academic centers that had the most prior experience with daratumumab, which may have affected the IR observation rate. To our knowledge, however, this is the first clinical trial report of montelukast premedication in patients who received a monoclonal antibody. Patients who received montelukast premedication before daratumumab experienced a lower rate of IRs at the first daratumumab infusion than those who did not receive montelukast, including a lower incidence of grade 3 and 4 IRs. We note that montelukast premedication generally was prescribed in addition to existing premedication and postmedication guidelines, as outlined in prescribing information for daratumumab11 and used in previous clinical trials, which reported similar IR rates with both single‐agent daratumumab8, 9, 10 and combinations using standard‐of‐care regimens.12, 13 Patients who received montelukast had fewer respiratory and gastrointestinal symptoms than those who did not receive montelukast, whereas systemic symptoms, such as chills, were similar in both groups. This positive effect may have been caused by generalized histamine secretion and local inflammation inhibition by montelukast, as leukotriene receptors are present in both the lung and the gut. Moreover, interpatient differences in receptors or drug metabolism may explain why not all patients benefited from montelukast. The small sample size of montelukast‐premedicated patients and the uncontrolled nature of this observation limit any conclusion about the impact of montelukast preinfusion on IRs.
Although efficacy was not an endpoint of this EAP study, investigator assessment of response to therapy was reported at the time of study discontinuation for each patient. The observed ORR of 23% in this study was similar to the ORR of 29% in the pivotal phase 2 study.9 While these findings support the available data from previous daratumumab clinical studies, particularly given the broad range of sites with various degrees of daratumumab experience, the utility of response data is limited by numerous factors (eg, investigator report of response at study discontinuation, short length of therapy, incomplete characterization of prior therapies, unknown time from diagnosis to study enrollment). Moreover, no response duration, progression‐free survival, or overall survival data were collected because of the nature of the EAP study and limited follow‐up period. Of note, this is the first daratumumab study to capture PROs, and no negative impact on PROs was observed, which is noteworthy when considering the heavily pretreated patient population.
Daratumumab has been subsequently approved in the US and the European Union for use in combination (with either lenalidomide and dexamethasone or bortezomib and dexamethasone) for the treatment of patients with MM who have received ≥1 prior therapy, based on 2 randomized phase 3 studies.11 The CASTOR study compared daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone. The addition of daratumumab resulted in a 61% reduction in the risk of disease progression or death and an ORR of 83%.12 In the POLLUX study, daratumumab was studied in combination with lenalidomide and dexamethasone versus lenalidomide and dexamethasone in patients with MM. The investigators reported a 63% reduction in the risk of disease progression or death and an ORR of 93%.13
Furthermore, 2 additional randomized phase 3 studies in relapsed MM have been activated: pomalidomide and dexamethasone with or without daratumumab (APOLLO) and daratumumab in combination with carfilzomib, lenalidomide, and dexamethasone (CANDOR). Three registration‐directed studies of daratumumab in combination with PI‐based and/or IMiD‐based therapy with and without stem cell transplantation in patients with newly diagnosed MM also have been conducted. The first of these studies, the ALCYONE study of daratumumab in combination with bortezomib, melphalan, and prednisone, indicated that the addition of daratumumab to a standard, frontline regimen yields similar efficacy benefit, with an acceptable safety profile, as in the relapsed setting.18 Furthermore, registration studies of daratumumab are ongoing in high‐risk, smoldering MM and light‐chain amyloidosis, thereby exploring the potential of daratumumab across plasma cell dyscrasias.
In conclusion, this EAP study confirms the safety profile of daratumumab monotherapy in a large cohort of patients with relapsed and refractory MM that was similar to the initial population indicated for daratumumab in the US. The findings also indicate that further investigation of montelukast premedication to reduce IRs is warranted, and formal studies of montelukast as a premedication for daratumumab are ongoing, as are those investigating a subcutaneous daratumumab formulation. The large safety sample size is of value, and EAPs offer an ethical, compliant means of addressing unmet needs in patients with limited treatment options, or who have exhausted all available options, by making a drug accessible to those who are not eligible for ongoing trials and before the drug is commercially available.19
Funding Support
This study was supported by Janssen Research & Development, LLC.
Conflict of interest disclosures
Ajai Chari reports personal fees research funding, and membership on an advisory board from Amgen, Celgene, Janssen, Novartis, and Takeda; personal fees from Bristol Myers Squibb and The Binding Site outside the submitted work; research funding from Array Biopharma and Pharmacyclics outside the submitted work; and membership on an advisory board for Seattle Genetics and Adaptive Biotechnology outside the submitted work. Sagar Lonial reports personal fees from Amgen, Bristol‐Myers Squibb, Celgene, Janssen, Merck, Millennium, Novartis, and Onyx outside the submitted work. Tomer M. Mark reports research funding from Amgen and Celgene and membership on a board of directors or an advisory committee for Celgene, Janssen, and Takeda, all outside the submitted work. Amrita Y. Krishnan reports personal fees from Celgene, Onyx, Janssen, and Takeda outside the submitted work; serves on a scientific advisory board for Sutro; and owns stock in Celgene. Keith E. Stockerl‐Goldstein reports personal fees from Janssen outside the submitted work. Saad Z. Usmani reports research funding from Amgen, Array, BioPharma, Bristol‐Myers Squibb, Celgene, Janssen, Merck, Millennium, Novartis, Onyx, Pharmacyclics, Sanofi, Skyline, and Takeda; personal fees from Amgen, BioPharma, Bristol‐Myers Squibb, Celgene, Janssen, Merck, Millennium, Novartis, Onyx, Sanofi, Skyline, and Takeda; and serves on a board of directors or advisory committee for BioPharma, Celgene, Janssen, Millennium, Onyx, Sanofi, Skyline, and Takeda, all during the conduct of the study. Jon Ukropec and Thomas S. Lin are employees of Janssen Scientific Affairs, LLC. Anil Londhe, Delores Etheredge, Sarah Fleming, and Baolian Liu are employees of Janssen Research & Development, LLC. Ajay K. Nooka reports personal fees from Janssen Pharmaceuticals during the conduct of the study and from Novartis, Onyx, Spectrum, Celgene, and Adaptive Technologies and serving on an advisory board for Adaptive technologies, Amgen, BMS Pharmaceuticals, Celgene, GSK, Novartis, Spectrum, and Takeda. outside the submitted work. Sundar Jagannath reports personal fees from Celgene, Bristol‐Myers Squibb, Novartis, Janssen, and Merck outside the submitted work.
Author Contributions
Ajai Chari: Acquisition/collection of data, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Sagar Lonial: Conceptualization, design, acquisition/collection of data, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Tomer M. Mark: Acquisition/collection of data, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Amrita Y. Krishnan: Acquisition/collection of data, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Keith E. Stockerl‐Goldstein: Conceptualization, design, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Saad Z. Usmani: Acquisition/collection of data, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Anil Londhe: Conceptualization, design, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Delores Etheredge: Acquisition/collection of data, writing–original draft, and writing–review and editing. Sarah Fleming: Conceptualization, design, writing–original draft, and writing–review and editing. Baolian Liu: Acquisition/collection of data, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Jon Ukropec: Conceptualization, design, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Thomas Lin: Conceptualization, design, formal analysis/interpretation of data, writing–original draft, and writing–review and editing. Sundar Jagannath: Conceptualization, design, writing–original draft, and writing–review and editing. Ajay K. Nooka: Acquisition/collection of data, formal analysis/interpretation of data, writing–original draft, and writing–review and editing.
Supporting information
References
- 1. Usmani SZ, Rodriguez‐Otero P, Bhutani M, Mateos MV, Miguel JS. Defining and treating high‐risk multiple myeloma. Leukemia. 2015;29:2119‐2125. [DOI] [PubMed] [Google Scholar]
- 2. Moreau P, van de Donk NW, San Miguel J, et al. Practical considerations for the use of daratumumab, a novel CD38 monoclonal antibody, in myeloma. Drugs. 2016;76:853‐867. [DOI] [PubMed] [Google Scholar]
- 3. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385:2197‐2208. [DOI] [PubMed] [Google Scholar]
- 5. Laubach JP, Tai YT, Richardson PG, Anderson KC. Daratumumab granted breakthrough drug status. Expert Opin Investig Drugs. 2014;23:445‐452. [DOI] [PubMed] [Google Scholar]
- 6. de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840‐1848. [DOI] [PubMed] [Google Scholar]
- 7. Overdijk MB, Verploegen S, Bogels M, et al. Antibody‐mediated phagocytosis contributes to the anti‐tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207‐1219. [DOI] [PubMed] [Google Scholar]
- 9. Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment‐refractory multiple myeloma (SIRIUS): an open‐label, randomised, phase 2 trial. Lancet. 2016;387:1551‐1560. [DOI] [PubMed] [Google Scholar]
- 10. Usmani SZ, Weiss BM, Plesner T, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darzalex [prescribing information]. Horsham, PA: Janssen Biotech, Inc; 2016. [Google Scholar]
- 12. Palumbo A, Chanan‐Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754‐766. [DOI] [PubMed] [Google Scholar]
- 13. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319‐1331. [DOI] [PubMed] [Google Scholar]
- 14. Arora A, Bhatt VR, Liewer S, Armitage JO, Bociek RG. Brentuximab vedotin desensitization in a patient with refractory Hodgkin's lymphoma. Eur J Haematol. 2015;95:361‐364. [DOI] [PubMed] [Google Scholar]
- 15. Quercia O, Emiliani F, Foschi FG, Stefanini GF. Adalimumab desensitization after anaphylactic reaction. Ann Allergy Asthma Immunol. 2011;106:547‐548. [DOI] [PubMed] [Google Scholar]
- 16. Lebel E, Ben‐Yehuda D, Bohbot E, Dranitzki Z, Shalit M, Tal Y. Hypersensitivity reactions to rituximab: 53 successful desensitizations in 7 patients with severe, near‐fatal reactions. J Allergy Clin Immunol Pract. 2016;4:1000‐1002. [DOI] [PubMed] [Google Scholar]
- 17. Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691‐4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378:518‐528. [DOI] [PubMed] [Google Scholar]
- 19. Patil S. Early access programs: benefits, challenges, and key considerations for successful implementation. Perspect Clin Res. 2016;7:4‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


