Abstract
Background
Dopamine has been used in patients with cardiac dysfunction for more than five decades. Yet, no systematic review has assessed the effects of dopamine in critically ill patients with cardiac dysfunction.
Methods
This systematic review was conducted following The Cochrane Handbook for Systematic Reviews of Interventions. We searched for trials including patients with observed cardiac dysfunction published until 19 April 2018. Risk of bias was evaluated and Trial Sequential Analyses were conducted. The primary outcome was all‐cause mortality at longest follow‐up. Secondary outcomes were serious adverse events, myocardial infarction, arrhythmias, and renal replacement therapy. We used GRADE to assess the certainty of the evidence.
Results
We identified 17 trials randomising 1218 participants. All trials were at high risk of bias and only one trial used placebo. Dopamine compared with any control treatment was not significantly associated with relative risk of mortality (60/457 [13%] vs 90/581 [15%]; RR 0.91; 95% confidence interval 0.68‐1.21) or any other patient‐centred outcomes. Trial Sequential Analyses of all outcomes showed that there was insufficient information to confirm or reject our anticipated intervention effects. There were also no statistically significant associations for any of the outcomes in subgroup analyses by type of comparator (inactive compared to potentially active), dopamine dose (low compared to moderate dose), or setting (cardiac surgery compared to heart failure).
Conclusion
Evidence for dopamine in critically ill patients with cardiac dysfunction is sparse, of low quality, and inconclusive. The use of dopamine for cardiac dysfunction can neither be recommended nor refuted.
Editorial Comment
This systematic review and meta‐analysis shows that the evidence base for use of dopamine in critically ill adults with cardiac dysfunction is sparse with no firm evidence for benefit or harm. From this, routine use of dopamine in this population does not seem justified in this patient group.
1. INTRODUCTION
Dopamine is a natural catecholamine which has various cardiovascular effects throughout a dose‐dependent activation of dopaminergic, α‐ and β‐adrenergic receptors.1 Low‐dose dopamine (<4 µg·kg−1·min−1) is hypothesised to primarily provide mesenteric and renal arteriole vasodilation, moderate‐dose dopamine (4‐10 µg·kg−1·min−1) is hypothesised to have particularly positive inotropic and chronotropic effects, and high‐dose dopamine (>10 µg·kg−1·min−1) is considered a vasopressor due to the increase of systemic vascular resistance.1, 2 These doses are arbitrary as there is a wide interindividual variability of dopamine receptor sensitivity.2
Guidelines for treatment of heart failure mention dopamine among other drugs to treat acute heart failure.3, 4 Several randomised clinical trials (RCTs) have failed to show clinical benefits associated with use of dopamine in patients with acute heart failure5, 6, 7 and circulatory shock.8 Previous meta‐analyses advocate cautious use of high‐dose dopamine.9 Despite the decline in its use, dopamine is still the used inotrope in 25% of acute heart failure patients and in 14% of the patients undergoing cardiac surgery.10, 11
The debate about the benefits and harms of dopamine in critically ill patients with cardiac dysfunction remains.11, 12 Our objective was to conduct a systematic review with meta‐analyses and Trial Sequential Analyses (TSA) of RCTs comparing the benefits and harms of dopamine compared to placebo, no intervention, or any potentially active comparator in critically ill patients with cardiac dysfunction.
2. METHODS
This systematic review was conducted following our published protocol (CRD42016042867),13 the recommendations of The Cochrane Handbook for Systematic Reviews of Interventions,14 The Cochrane Hepato‐Biliary Group Module,15 and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.16
2.1. Eligibility criteria
We considered all RCTs eligible for inclusion irrespective of language, blinding, publication status, sample size, or control intervention(s) for assessment of benefits and harms. Quasi‐randomised and observational studies were included for assessment of potential harms and results were analysed separately.
Only RCTs with critically ill adult patients with cardiac dysfunction were included in our main analysis. Critical illness encompassed any clinical setting wherein patients with objectively measured cardiac dysfunction seemed to require intravenous dopamine without restrictions on dose or duration of administration. Cardiac dysfunction was defined as a left ventricular ejection fraction (LVEF) below 45% and/or a low cardiac output syndrome. Low cardiac output syndrome was defined as a pre‐existing or developing state of cardiac insufficiency with underlying left‐ or right‐ventricular systolic dysfunction seemed to require inotrope support to maintain a systolic blood pressure >90 mm Hg and a cardiac index >2.2 L·min−1·m−2.17 RCTs including both patients with and without cardiac dysfunction were included in the review only if the majority (more than 50%) of the included patients had cardiac dysfunction. During the selection process, we had to exclude a substantial number of trials because not all trials objectively measured cardiac dysfunction for each patient. We realised that our eligibility criteria may not reflect all the situations in which doctors decide to administer dopamine. To increase the external validity of our systematic review, we conducted a post hoc analysis including trials in which a substantial proportion of patients (more than 25%) were assumed to have cardiac dysfunction.
2.2. Outcomes
The primary outcome was all‐cause mortality. The secondary outcomes were serious adverse events (SAEs), myocardial infarction, arrhythmias (including supra‐ and ventricular tachycardia and fibrillation), and renal failure requiring renal replacement therapy. SAEs were defined according to the International Conference on Harmonisation of Good Clinical Practice definitions, excluding mortality to avoid double counts.18 Myocardial infarction, arrhythmias, and renal replacement therapy were defined according to the criteria used in the individual trials. We included data at longest follow‐up.
2.3. Search methods
We used a sensitive search strategy that was likely to include all clinical settings wherein cardiac dysfunction was prevalent: eg shock, heart failure, cardiac surgery (Appendix S1). We searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Web of Science, CINAHL, and Embase until 19 April 2018. We also searched the World Health Association's (WHO's) trial platform, ClinicalTrials.gov, and FDA and EMA homepages for ongoing trials. Last, we searched the references of the selected trials and previous meta‐analyses to identify further relevant trials.
2.4. Trial selection, data extraction, and bias risk assessments
Two authors independently identified trials for inclusion and extracted study, patient and intervention characteristics, evaluated outcomes, and risks of bias according to the domains of bias in The Cochrane Handbook for Systematic Reviews of Interventions.14 Trials with one or more of the risks of bias domains classified at high or unclear risk were considered trials at high risk of bias.14 The authors of the individual trials were contacted in case of any unclear or missing information.
All data on the outcomes of all trials were assessed for the risks of systematic errors (‘bias’), the risks of other design errors, and the risks of random errors. The three‐dimensional Manhattan error matrix plot was used to facilitate the overview of available evidence at a glance.19 We used a funnel plot to explore small trial bias.14
2.5. Statistical methods
Results were presented as relative risks (RR), odds ratios (OR), and Peto's OR with 95% confidence interval (CI) when applicable. We used both a fixed‐effect model and a random‐effects model for our meta‐analyses and presented both models in case of discrepancy. Considering the anticipated clinical diversity, we emphasised the results from the random‐effects model as it provides the most conservative estimate of effect and/or CI. Heterogeneity was explored by inspection of forest plots and the chi‐squared test with significance set at P‐value of 0.10, and the quantity of heterogeneity was measured by I 2.20
We used TSA on all outcomes to control for the risks of random errors (“the play of chance”) and adjust the thresholds for statistical significance when few data are present or when tested repeatedly, comparable to interim analyses in a single RCT. TSA calculates a diversity‐adjusted required information size (RIS) which compares well to a sample size calculation for an RCT, and widens the thresholds for statistical significance before the RIS is accrued. The RIS was calculated based on an anticipated relative risk reduction (RRR) of 10% and appropriately adjusted for heterogeneity in terms of diversity (D 2) according to an overall type‐I error of 5% and a power of 90% considering early and repetitive testing.21 P‐values less than TSA‐adjusted significance levels were considered statistically significant.21 We explain the interpretation of a TSA‐graph in Figure S1. The concepts of TSA are explained in detail in the TSA Manual (http://www.ctu.dk/tsa) as well as in a recent overview.21 We used the software package Review Manager 5.3.5 for the meta‐analyses and the TSA program v.0.9.5.10 beta (http://www.ctu.dk/tsa) for the TSA.
2.6. Sensitivity and subgroup analyses
All outcomes were dichotomous. We constructed best‐worst and worst‐best case scenarios as sensitivity analyses for participants lost to follow‐up. Following our protocol, we conducted subgroup analyses to explore clinical heterogeneity according to: (a) risk of bias in trials; (b) control intervention (inactive compared to a potentially active control); (c) trials assessing a low dose (<4 µg·kg−1 .min−1) compared to a moderate (4‐10 µg·kg−1·min−1) or high dose (>10 µg·kg·−1·min−1); (d) clinical setting (patients having cardiac surgery compared to patients not having cardiac surgery).
2.7. GRADE assessments
We used the Grading of Recommendations Assessment, Development and Evaluations (GRADE) approach to rate and assess the quality of the body of evidence for each outcome and constructed a “Summary of findings” table.22
3. RESULTS
3.1. Study selection
After screening the literature search, titles and abstracts, 341 articles out of 10 858 hits remained (Figure 1). After assessment of full‐texts, 86 studies were included in our systematic review. Additional data was obtained from three studies.5, 6, 23 The main meta‐analysis included 17 RCTs with in total 1218 patients.5, 6, 7, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 Two observational studies were assessed for harmful outcomes.23, 38
Figure 1.
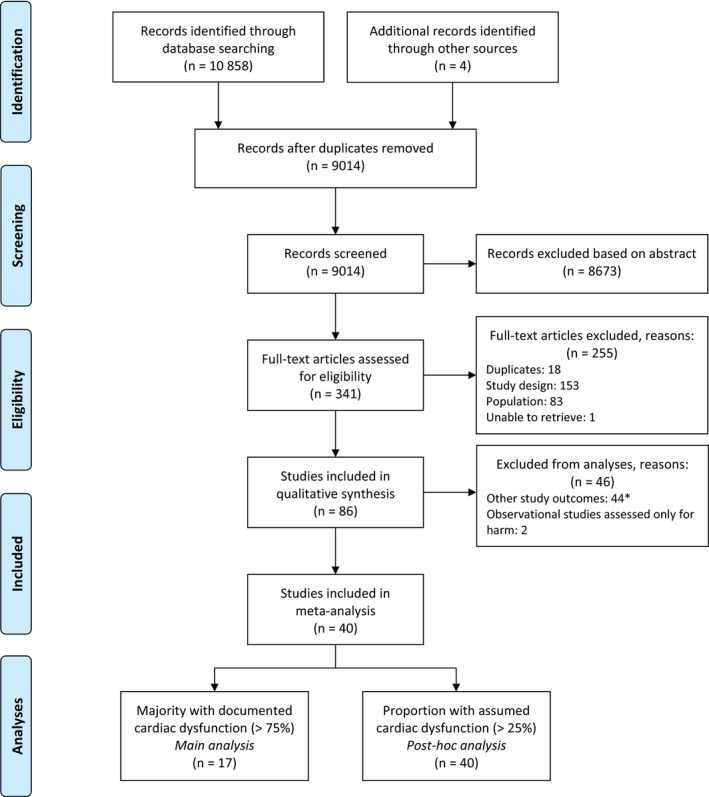
PRISMA flow diagram. *All authors from the studies published since 1990 were contacted for additional data in case of missing outcomes of interest [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Characteristics of included trials
The characteristics of the 17 trials included in our meta‐analyses are summarised in Table 1. In‐ and exclusion criteria of each trial are presented in Table S1. Nine trials had a two‐arm design, seven trials consisted of three treatment arms, and one administered four different treatments. One trial was placebo‐controlled,7 four trials used no intervention in the control group,6, 25, 30, 32 and 14 trials used a potentially active control intervention: eight trials administered an inotropic drug and six a diuretic drug. The administration duration of the study drugs varied from only during the perioperative period up to a maximum of 5 days. Seven of the 17 trials included solely patients who all had objectively verified cardiac dysfunction defined by an LVEF below 45% or a low cardiac output syndrome.25, 27, 29, 34, 36, 37 In a sensitivity analyses we only included these seven trials; findings were comparable to the analysis of 17 trials (e‐Table 2, Appendix S2).
Table 1.
Characteristics of the included trials
| Trial, year | N | Dopamine dose | Comparator(s) | Cardiac function | Outcomes |
|---|---|---|---|---|---|
| Acute heart failure | |||||
| Kamiya24 | 24 |
Low dose: 1.9 ± 0.8 µg·kg−1·min−1 |
Furosemide 17.1 ± 7.2 µg·kg−1·min−1 |
LVEF per group:
|
Mortality (in‐hospital) Serious adverse events Arrhythmias |
| Chen7 | 360 |
Low dose: 2.0 µg·kg−1·min−1 |
Placebo |
LVEF: 33% (IQR 22%‐50%) Proportion LVEF <50%: 74% |
Mortality (60 d) Serious adverse events Arrhythmias |
| Varriale25 | 20 |
Low dose: 2.0 µg·kg−1·min−1 |
Control |
Mean LVEF: 28.3% ± 9.1% Depressed LV‐function was an inclusion criterion |
Mortality (in‐hospital) Arrhythmias |
| Shah26 | 90 |
Low dose: 2.5 µg·kg−1·min−1 |
(1) Control (2) Furosemide 2dd 50 mg |
Mean LVEF: 33% |
Mortality (30 d) Serious adverse events |
| Arutiunov27 | 41 |
Low dose: 3.1 ± 0.2 µg·kg−1·min−1 |
Levosimendan (unknown dose) + ivabradine 2dd 5 mg |
Mean LVEF: 22% LVEF < 35% was an inclusion criterion |
Mortality (30 d) Myocardial infarction |
| Hsueh28 | 20 |
Moderate dose: 4.0 µg·kg−1·min−1 |
Dobutamine 4.0 µg·kg−1·min−1 |
LVEF: ±33% ± 10 LVEF < 45% was an inclusion criterion |
Mortality (72 h) Arrhythmias |
| Cotter29 | 20 |
Moderate dose: (1) 4.0 + furosemide 2dd 40 mg (2) 4.0 + furosemide 5 mg·kg−1 |
Furosemide 10 mg·kg−1·24 h−1 | LVEF > 40% was an exclusion criterion |
Mortality (in‐hospital) Arrhythmias |
| Giamouzis5 | 60 |
Moderate dose: 5.0 µg·kg−1·min−1 |
Furosemide 20 mg·h−1 |
LVEF: 36% ± 12% Proportion LVEF <40%: 70% |
Mortality (60 d) Serious adverse events |
| Triposkiadis6 | 161 |
Moderate dose: 5.0 µg·kg−1·min−1 |
(1) Control (2) Furosemide 20 mg h−1 |
LVEF: 31% (25%‐45%) Proportion LVEF <40%: 58% |
Mortality (1 y) Serious adverse events Arrhythmias Renal replacement therapy |
| Sindone30 | 67 | Not specified (abstract only) |
(1) Control (2) Dobutamine (3) Milrinone |
CI 1.9 ± 0.7 L·min−1·m−2 | Mortality (1 y) |
| Cardiac surgery | |||||
| Sirivella31 | 100 |
Low dose: (1 + 2) 2‐3 µg·kg−1·min−1 + mannitol + furosemide 0.6‐0.8 mg·kg−1 a (other inotropes were given) |
Furosemide 1.4‐3 mg·kg−1 + bumetadine 0.014 mg·kg−1
(other inotropes were given) |
LVEF: 35% Mean CO: 2.4 ± 0.2 L·min−1 |
Renal replacement therapy |
| Costa32 | 36 |
Low dose: (1) 2.5 µg·kg−1·min−1 (2) 2.5 µg·kg−1·min−1 + nitroprusside |
Control | Renal dysfunction was attributable to severe HF in all but three patients | Renal replacement therapy |
| Bove33 | 80 |
Low dose: 2.5 µg·kg−1·min−1 (65% received other inotropes) |
Fenaldopam 0.5 µg·kg−1·min−1
(68% received other inotropes) |
LVEF per group:
|
Mortality (in‐hospital) Renal replacement therapy |
| Rosseel34 | 70 |
Low dose: 3.1 ± 1.6 µg·kg−1·min−1 |
Dopexamine 1.2 ± 0.6 µg·kg−1·min−1 | Low cardiac output syndrome was an inclusion criterium |
Mortality (in‐hospital) Serious adverse events |
| Hausen35 | 41 |
Moderate dose: 5‐7 µg·kg−1·min−1 + glyceroltrinitrate (57% received adrenaline) |
(1) Enoximone 5‐20 µg·kg−1·min−1
(62% received adrenaline) (2) Piroximone 3‐6 µg·kg−1·min−1 (43% received adrenaline) |
A preoperative cardiac index <2.5 L·min−1·m−2 was an inclusion criterion |
Mortality (6 ± 3 mo) Myocardial infarction Arrhythmias |
| Oppizzi36 | 26 |
Moderate dose: 5‐10 µg·kg−1·min−1 (15% crossed over) |
Enoximone bolus 0.5 mg·kg−1, followed by 5‐10 µg·kg−1·min−1
(5% crossed over) |
LVEF <35% was an inclusion criterion |
Mortality (in‐hospital) Serious adverse events Myocardial infarction Arrhythmias |
| Tarr37 | 75 |
Moderate dose: 5‐10 µg·kg−1·min−1 (36% received other inotropes) |
(1) Enoximone 5‐10 µg·kg−1·min−1
(0% received other inotropes) (2) Dobutamine 7‐14 µg·kg−1·min−1 (12% received other inotropes) |
Cardiac index per group:
|
Mortality (in‐hospital) |
AHF, acute heart failure; LVEF, left‐ventricular ejection fraction.
Trials are sorted by setting and administered dose. We selected studies that provided data on cardiac function and accepted definitions of diagnoses according to criteria used in each individual RCT.
The timing of administering the experimental intervention differed between the treatment arms.
Table 2.
Risk and odds ratios of all outcomes with subgroups analyses
| Trialsa | Patients | Events | RR or OR | 95% CI | Test for Interaction | |
|---|---|---|---|---|---|---|
| Mortality | 15 | 1038 | 150 | 0.91 | 0.68‐1.21 | P = 1.00 |
| (1) Placebo or control | 5 | 452 | 84 | 0.90 | 0.61‐1.33 | |
| (1) Potentially active control | 12 | 586 | 66 | 0.92 | 0.59‐1.43 | |
| (2) Low dose dopamine | 7 | 568 | 68 | 0.84 | 0.54‐1.30 | |
| (2) Moderate dose dopamine | 7 | 403 | 74 | 0.98 | 0.65‐1.47 | |
| (3) Acute heart failure | 10 | 746 | 132 | 0.90 | 0.67‐1.23 | |
| (3) Cardiac surgery | 5 | 292 | 18 | 0.93 | 0.35‐2.48 | |
| Serious adverse events | 6 | 582 | 113 | 1.20 | 0.91‐1.57 | P = 0.92 |
| (1) Placebo or control | 2 | 324 | 41 | 1.48 | 0.82‐2.67 | |
| (1) Potentially active control | 5 | 258 | 72 | 1.34 | 0.75‐2.40 | |
| (2) Low dose dopamine | 3 | 335 | 80 | 1.16 | 0.78‐1.71 | |
| (2) Moderate dose dopamine | 3 | 267 | 33 | 1.70 | 0.86‐3.39 | |
| (3) Acute heart failure | 4 | 486 | 59 | 1.54 | 0.94‐2.53 | |
| (3) Cardiac surgery | 2 | 96 | 54 | 1.45 | 0.43‐4.90 | |
| Myocardial infarction | 5 | 339 | 16 | 1.63 | 0.56‐4.71 | P = 0.99 |
| (1) Placebo or control | 1 | 83 | 2 | 2.00 | 0.12‐33.2 | |
| (1) Potentially active control | 5 | 256 | 14 | 1.57 | 0.50‐4.95 | |
| (2) Low dose dopamine | 2 | 111 | 8 | 1.68 | 0.15‐18.8 | |
| (2) Moderate dose dopamine | 3 | 228 | 8 | 1.99 | 0.47‐8.36 | |
| (3) Acute heart failure | 2 | 202 | 7 | 2.91 | 0.55‐15.3 | |
| (3) Cardiac surgery | 3 | 137 | 9 | 1.09 | 0.27‐4.33 | |
| Ventricular tachyarrhythmias | 8 | 538 | 24 | 1.46 | 0.52‐4.10 | P = 0.97 |
| (1) Placebo or control | 3 | 329 | 12 | 3.23 | 0.36‐28.6 | |
| (1) Potentially active control | 6 | 209 | 12 | 0.94 | 0.28‐3.15 | |
| (2) Low dose dopamine | 3 | 270 | 10 | 2.12 | 0.08‐55.3 | |
| (2) Moderate dose dopamine | 5 | 268 | 14 | 1.09 | 0.35‐3.43 | |
| (3) Acute heart failure | 6 | 471 | 21 | 1.29 | 0.38‐4.39 | |
| (3) Cardiac surgery | 2 | 67 | 3 | 2.18 | 0.17‐27.6 | |
| Renal replacement therapy | 4 | 371 | 51 | 0.44 | 0.07‐2.75 | P = 0.94 |
| (1) Placebo or control | 2 | 113 | 1 | 0.64 | 0.03‐15.3 | |
| (1) Potentially active control | 3 | 258 | 50 | 0.42 | 0.05‐3.67 | |
| (2) Low dose dopamine | 3 | 210 | 48 | 0.26 | 0.02‐3.43 | |
| (2) Moderate dose dopamine | 1 | 161 | 3 | 1.16 | 0.15‐9.15 | |
| (3) Acute heart failure | 1 | 161 | 3 | 1.16 | 0.15‐9.15 | |
| (3) Cardiac surgery | 3 | 210 | 48 | 0.26 | 0.02‐3.43 | |
| Atrial tachyarrhythmias | 2 | 181 | 3 | 1.16 | 0.14‐9.65 | P = 0.99 |
| (1) Placebo or control | 2 | 103 | 1 | 0.64 | 0.03‐16.2 | |
| (1) Potentially active control | 1 | 78 | 2 | 1.81 | 0.11‐30.2 | |
| (2) Low dose dopamine | 1 | 20 | 0 | — | — | |
| (2) Moderate dose dopamine | 1 | 161 | 3 | 1.16 | 0.14‐9.65 | |
| (3) Acute heart failure | 2 | 181 | 3 | 1.16 | 0.14‐9.65 | |
| (3) Cardiac surgery | 0 | 0 | 0 | — | — |
RR, relative risk; OR, odds ratio; CI, confidence interval.
Some trials compared dopamine with both a control intervention and a potentially active control (ie three‐arm design), which is why the combined number of trials in subgroup analysis 1 differ from the total amount.
3.3. Risk of bias
All 17 trials were at overall high risk of bias (Figure 2). Fourteen trials were at high risk of other bias, because nine trials (53%) did not provide a statement on conflicts of interest, two trials (12%) allowed cross‐over to another inotrope, and three trials (18%) were at risk of vested interests.
Figure 2.
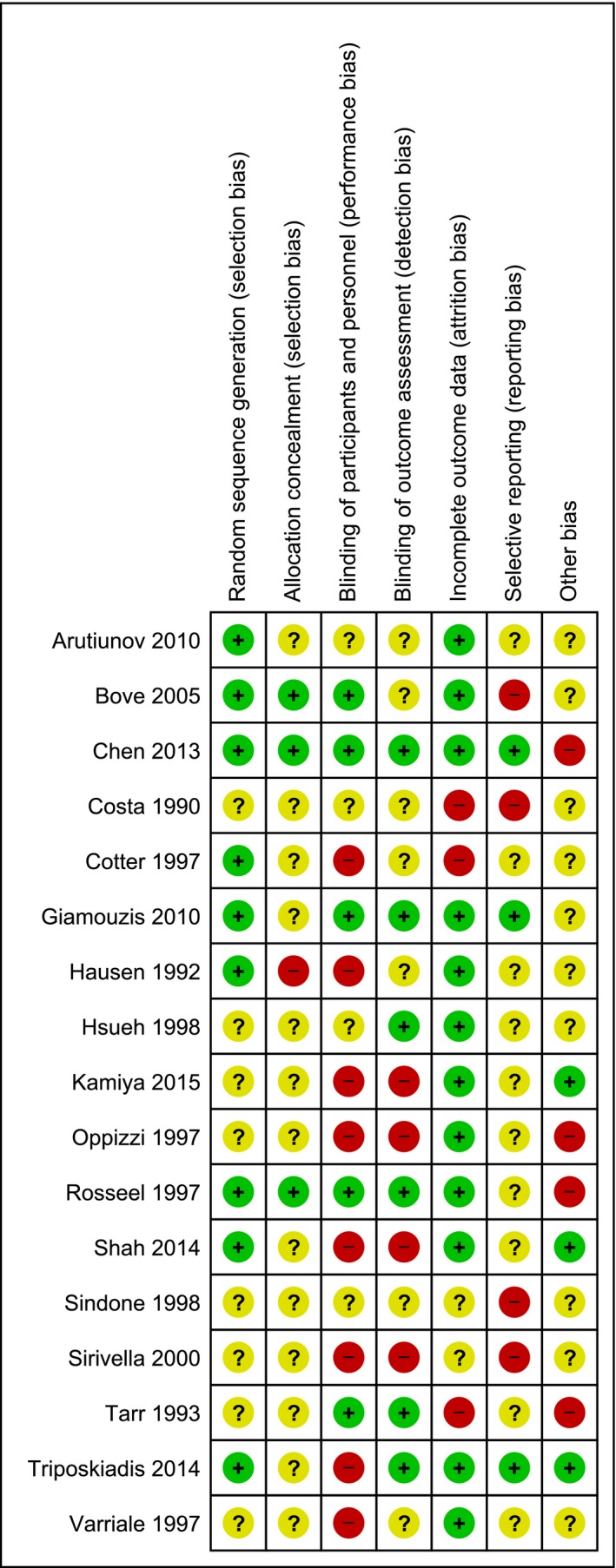
Risk of bias assessment. Red, high risk; yellow, unclear risk; green, low risk [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. Outcomes
Table 2 summarises the meta‐analysed intervention effect estimates. Due to absence of trials at overall low risk of bias and also due to absence of trials administering high‐dose dopamine, we were unable to conduct these predefined subgroup analyses. None of the comparisons or outcomes could be analysed with the TSA using our prespecified parameters. As a sensitivity analyses, we conducted a TSA with a type I error of 5%, type II error of 10%, and an RRR of 20% on our primary outcome mortality to evaluate the direction of the cumulative Z‐curve.
3.5. Comparison 1: all critically ill patients with cardiac dysfunction
3.5.1. All‐cause mortality
All‐cause mortality was reported in 15 of the 17 trials with a total of 1038 included patients. One trial reported mortality only during their 72‐hour study period, seven trials reported in‐hospital mortality, four trials 30‐ to 60‐day mortality, and three trials mortality after 6‐12 months of follow‐up (Table 1). Dopamine did not statistically significantly affect mortality when compared with any control intervention (60/457 [13%] vs 90/581 [15%]; RR 0.91; 95% CI 0.68‐1.21; I 2 0%), or when compared with an inactive control or with a potentially active control (Figure 3). TSA on all trials showed that 19% of the RIS data was accrued and that about another 4292 patients need to become randomised in RCTs before the RIS will be reached (Figure 4; RR 0.91; TSA‐adjusted CI 0.50‐1.67).
Figure 3.
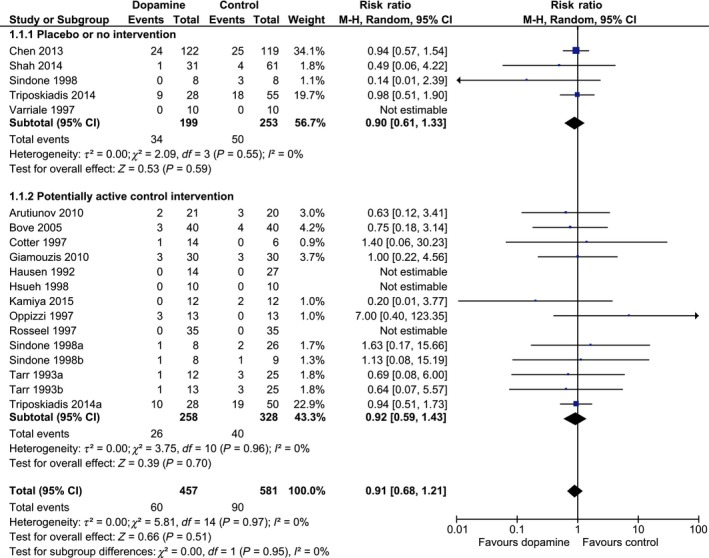
Forest plot of mortality in all trials stratified by intervention. Forest plot of all‐cause mortality in trials stratified by intervention. Size of squares for risk ratio (RR) reflects the weight of the trial in the meta‐analysis. Horizontal bars are 95% confidence intervals (CI) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.

Trial Sequential Analysis for all‐cause mortality. The Trial Sequential Analysis is based on 15 trials, which is the meta‐analysed effect of dopamine vs any (in)active comparator intervention. The blue cumulative z‐curve was constructed using a random‐effects model. The horizontal green dotted lines represent the conventional naïve boundaries for benefit (positive) or harm (negative). The red dotted lines represent the trial sequential boundaries for benefit (positive), harm (negative), or futility (middle triangular area) [Colour figure can be viewed at wileyonlinelibrary.com]
3.5.2. Serious adverse events
The occurrence of SAEs was reported in six trials with 582 included patients. Dopamine was not statistically significantly associated with SAEs when compared with any control intervention (62/268 [23%] vs 51/314 [16%]; RR 1.20; 95% CI 0.91‐1.57; I 2 2%; Figure 5). In a sensitivity analysis, we included mortality in our SAEs and found no statistically significant associations (122/457 [27%] vs 141/581 [24%]; RR 1.06; 95% CI 0.89‐1.27; I 2 0%). TSA on all trials showed that only 12% of the data was accrued and that about 4405 additional patients need to become randomised in RCTs before the RIS will be reached (RR 1.20; TSA‐adjusted CI 0.41‐3.41; Figure S2).
Figure 5.
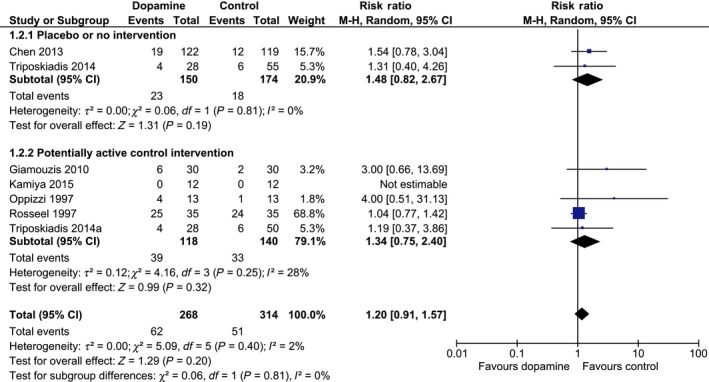
Forest plot of serious adverse events in trials stratified by intervention. Forest plot of serious adverse events in all trials stratified by intervention. Size of squares for risk ratio (RR) reflects the weight of the trial in the meta‐analysis. Horizontal bars are 95% confidence intervals (CI) [Colour figure can be viewed at wileyonlinelibrary.com]
3.5.3. Other outcomes
There were no significant differences in favour of any intervention on the other outcomes (Table 2). None of the outcomes could be analysed with TSA using our prespecified parameters because <5% of RIS was accrued.
3.6. Comparison 2: trials subdivided by dopamine dose (low compared to moderate)
3.6.1. All‐cause mortality
Seven trials administered low‐dose dopamine (ie <4 µg·kg−1·min−1) and seven trials a moderate dose (4‐10 µg·kg−1·min−1). Trials that studied low‐dose dopamine in patients with heart‐failure targeted to increase diuresis by improving renal perfusion, whereas low‐dose dopamine during cardiac surgery was used to preserve renal function. Moderate dose‐dopamine was administered in both patients with heart‐failure and cardiac surgery patients to increase renal perfusion and ameliorate cardiac function. One trial that reported mortality did not report on the dopamine dose.30 No statistically significant associations between different doses of dopamine and mortality were found (Table 2).
3.6.2. Serious adverse events
The occurrence of SAEs was recorded in three trials that administered low‐dose dopamine and in four trials administering moderate‐dose dopamine. No significant differences were found for either low‐ or moderate‐dose dopamine (Table 2).
3.6.3. Other outcomes
In the low‐dose dopamine group there was significant heterogeneity (I 2 90%, P = 0.002) due to one trial reporting use of renal replacement therapy in 36 of the 40 patients (90%) in the control group vs 2 of the 42 patients (5%) in the dopamine group. No significant differences were observed for any dose on any of the outcomes (Table 2).
3.7. Comparison 3: trials subdivided by setting (heart failure compared to cardiac surgery)
3.7.1. All‐cause mortality
Ten trials were conducted in patients admitted with acute heart failure and seven trials in patients undergoing cardiac surgery. Heart failure was often based on clinical symptoms classified by the New York Heart Association (NYHA) and a depressed LVEF (Table S1). The type of cardiac surgery varied between the trials: two trials included patients having cardiac artery bypass grafting,34, 36 two trials included patients having mitral valve surgery,35, 37 and three trials included patients having various cardiac surgeries.31, 32, 33 Subgroup analyses by clinical setting did not show any statistically significant associations on mortality (Table 2).
3.7.2. Serious adverse events
Serious adverse events were reported in four trials that included patients with acute heart failure and in two trials that included patients undergoing cardiac surgery. There were no statistically significant associations on occurrence of SAEs in both settings (Table 2).
3.7.3. Other outcomes
There was no significant difference in favour of any intervention on the proportion of myocardial infarction, renal replacement therapy, and ventricular or atrial tachyarrhythmias (Table 2).
3.8. Post hoc meta‐analyses with broader inclusion criteria of cardiac dysfunction
These post hoc meta‐analyses included trials in which a substantial proportion of patients (>25%) were assumed to have cardiac dysfunction. This broader inclusion criterion added ten trials with patients suffering from shock (n = 1679) or septic shock (n = 444), who received high‐dose dopamine for treatment of hypotension. This meta‐analysis included 40 trials with 4182 patients and full details can be found in Appendix S2.
Dopamine seemed associated with increased mortality, increased SAEs, and increased tachyarrhythmias when compared with a potentially active control intervention (Table S2). The excess mortality was largely attributable to the trials which administered high‐dose dopamine and accounted for 87% of weight in the pooled effect (Figure S3). All but one of these trials compared dopamine with noradrenaline and two trials allowed other cardioactive co‐interventions with dobutamine or open‐label noradrenaline. TSA including all trials reporting on mortality showed that it is highly unlikely to show a beneficial effect of dopamine with further trials, as the cumulative Z‐curve would have to cross the futility area (Figure S4).
3.9. Observational studies
One quasi‐randomised study and one observational study were assessed for harms.23, 38 One study compared dopamine to levosimendan and recorded SAEs and arrhythmias38; the other evaluated dopamine to an intra‐aortic balloon pump and reported myocardial infarction and renal replacement therapy proportions.23 Dopamine did not significantly affect any of these outcomes (Table S3).
3.10. Quality of evidence
Based on GRADE, the certainty of the evidence on all outcomes was judged as ‘very low’ and was mainly attributable to serious risks of bias, serious indirectness, and very serious imprecision (Table 3). The Manhattan error matrix plots showed that there are lacunas in the evidence of dopamine regarding both systematic errors and random errors (Figure S5). The funnel plots showed no clear arguments for small trial bias including publication bias (Figure S6).
Table 3.
GRADEpro summary of finding table of the outcomes of interest
| Quality assessment | No of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Dopamine | Any (in)active comparator |
Relative (95% CI) |
Absolute (95% CI) |
||
| Mortality at maximum follow‐up | ||||||||||||
| 15 | RCTs | Seriousa | Not serious | Seriousb | Seriousc | None | 60/457 (13.1%) | 90/581 (15.5%) |
RR 0.91 (0.68‐1.21) |
14 fewer per 1.000 (from 33 more to 50 fewer) |
⨁◯◯◯ VERY LOW |
CRITICAL |
| Serious adverse events | ||||||||||||
| 6 | RCTs | Seriousa | Not serious | Seriousb | Seriousc | None | 62/268 (23.1%) | 51/314 (16.2%) |
RR 1.20 (0.91‐1.57) |
32 more per 1.000 (from 15 fewer to 93 more) |
⨁◯◯◯ VERY LOW |
CRITICAL |
| Myocardial infarction | ||||||||||||
| 5 | RCTs | Seriousa | Not serious | Seriousb | Very seriousd | None | 9/139 (6.5%) | 7/200 (3.5%) |
OR 1.63 (0.56‐4.71) |
21 more per 1.000 (from 15 fewer to 111 more) |
⨁◯◯◯ VERY LOW |
IMPORTANT |
| Ventricular tachyarrhythmias | ||||||||||||
| 8 | RCTs | Seriousa | Not serious | Seriousb | Very seriousd | None | 14/255 (5.5%) | 10/313 (3.2%) |
OR 1.46 (0.52‐4.10) |
14 more per 1.000 (from 15 fewer to 87 more) |
⨁◯◯◯ VERY LOW |
IMPORTANT |
| Renal replacement therapy | ||||||||||||
| 4 | RCTs | Seriousa | Seriouse | Seriousb | Seriousc | None | 9/174 (5.2%) | 42/197 (21.3%) |
RR 0.44 (0.07‐2.75) |
119 fewer per 1.000 (from 198 fewer to 373 more) |
⨁◯◯◯ VERY LOW |
IMPORTANT |
| Atrial tachyarrhythmias | ||||||||||||
| 2 | RCTs | Seriousa | Not serious | Seriousb | Very seriousd | None | 1/66 (1.5%) | 2/115 (1.7%) |
OR 1.16 (0.14‐9.65) |
3 more per 1.000 (from 15 fewer to 128 more) |
⨁◯◯◯ VERY LOW |
NOT IMPORTANT |
RCTs, randomised clinical trials; CI, confidence interval; RR, risk ratio; OR, odds ratio.
There were no trials at overall low risk of bias.
There was considerable difference in population types (ie heart failure, cardiac surgery) and both dosing and length of administration of the study drugs.
The confidence intervals include both appreciable harm and benefit and <5% of the required information size was accrued.
Odds ratios are based on very few events (<25).
There was considerable statistical heterogeneity (I 2 77%, P = 0.004), which was caused by one study at high risk of bias.
4. DISCUSSION
Our main meta‐analysis consisting of 17 trials with 1218 patients did not provide high‐quality evidence to support or refute the use of dopamine. All trials were at overall high risk of bias, only one trial compared dopamine with placebo, and TSA showed that further thousands of patients need to be randomised before firm conclusions can be drawn. The use of dopamine as preferred inotrope in up to 25% of heart failure patients lacks evidence from RCTs.
The largest trial on dopamine thus far observed that high‐dose dopamine, as compared with noradrenaline, is associated with increased 28‐day mortality in the subgroup of patients with cardiogenic shock.8 We could not include these patients in our main meta‐analysis because cardiac function was not measured in each patient and the randomisation procedure was not stratified for the cardiogenic shock subgroup. The increased mortality was supported by a meta‐analysis including trials randomising patients with cardiogenic shock receiving high‐dose dopamine.39 We were unable to include these trials because the meta‐analysis did not elaborate on cardiac function of each trial population and the full‐text manuscripts were inaccessible to us (ie the Wanfang and Weipu Database). Based on these studies, high‐dose dopamine for treatment of cardiogenic shock seems associated with increased harm.
Dopamine for treatment of cardiac dysfunction also seems harmful according to observational data.11 Nevertheless, the quality of current evidence on the possible benefits or harms of dopamine, milrinone, levosimendan, and probably all other inotropes is considered very low.40, 41 There is currently no high‐quality evidence on which inotrope should preferentially be administered to patients with cardiac dysfunction.
Previous systematic reviews on dopamine in critically ill adult patients differ in design; all studied dopamine in patients with cardiogenic,39, 42 hypotensive,9 or septic shock.43, 44, 45, 46, 47 Some identified a potentially harmful effect of dopamine on mortality and occurrence of arrhythmias,39, 43, 44, 46 while others were inconclusive.9, 42, 45, 47 These systematic reviews used different inclusion criteria and most studied high‐dose dopamine.9, 39, 43, 44, 45, 46, 47 The main analysis of our systematic review included fewer patients (n = 1218) compared to five of the other reviews (n = 510,39 n = 70,42 n = 1400,9 n = 2043,44 n = 1408,43, 47 n = 3819,45 n = 171846) due to our more stringent inclusion criteria on cardiac dysfunction. We selected patients with objectively measured cardiac dysfunction because these patients would presumably benefit the most from an inotropic drug based on a pathophysiological reasoning. Critically ill patients with a normal cardiac function probably benefit less from the inotropic effects of dopamine and are more likely to only suffer potential harms.
4.1. Limitations and strengths
Potential biases may have arisen during the review process. Our systematic review mainly included small trials (ie <100 patients per trial) that used haemodynamic variables as their primary outcome. Therefore, our effect estimates may contain covariate imbalances and the included trials were individually underpowered for our outcomes.48 Such problems with imbalance and power are, however, best mitigated through the conduct of meta‐analyses.
It can be debated whether our inclusion criteria fully reflect daily clinical practise. We were interested in patients with cardiac dysfunction based on cardiac index and LVEF measurements, which are operator dependent and may have considerable interobserver variability.49, 50 Though, these are currently the advocated measures to quantify left‐ventricular function and often used as trigger to start inotropic treatments.51
Although statistical heterogeneity was often absent, our meta‐analyses had considerable clinical heterogeneity because (a) not all trials included patients who all have objectively verified cardiac dysfunction and (b) dopamine was administered in different doses to patients in different clinical settings, based on different assumed pathophysiological mechanisms. In fact, very few of the included trials had objective haemodynamic targets to direct infusion of dopamine and other inotropes. We probably cannot move forward understanding the role of inotropes before we understand the pathophysiology of shock on organ level.
More insight is needed into the pathophysiology of shock on organ level with bridging to haemodynamic goals to achieve optimal organ function support in critically ill patients. To detect possible sources of clinical heterogeneity, we first conducted subgroup analyses on dopamine dose, clinical setting, and a sensitivity analysis of trials exclusively including patients with cardiac dysfunction. Second, we conducted post hoc meta‐analyses with a broader inclusion criterion for cardiac dysfunction.
5. CONCLUSIONS
Evidence for dopamine in critically ill adults with cardiac dysfunction is sparse and of low quality due to high risks of systematic errors and random errors. The use of dopamine in patients with cardiac dysfunction can neither be recommended nor refuted.
CONFLICT OF INTEREST
TWLS received honoraria from Edwards Lifesciences and Masimo Inc (Irvine, California, USA) for consulting and for giving lectures. TWLS received honoraria from Pulsion Medical Systems SE for giving lectures. JW and CG are members of a task force at the Copenhagen Trial Unit to develop theory and software to do Trial Sequential Analysis which, including a manual, is freeware at www.ctu.dk/tsa. Authors BH, GK, JCJ, FK and ICCvdH declare no conflicts of interest.
Supporting information
ACKNOWLEDGEMENTS
We thank Sjoukje van der Werf, medical information specialist of Central Medical Library of the UMCG, for her assistance with the search strategy.
Hiemstra B, Koster G, Wetterslev J, et al. Dopamine in critically ill patients with cardiac dysfunction: A systematic review with meta‐analysis and trial sequential analysis. Acta Anaesthesiol Scand. 2019;63:424–437. 10.1111/aas.13294
PROSPERO registration number: CRD42016042867
REFERENCES
- 1. Bangash MN, Kong ML, Pearse RM. Use of inotropes and vasopressor agents in critically ill patients. Br J Pharmacol. 2012;165:2015‐2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jentzer JC, Coons JC, Link CB, Schmidhofer M. Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J Cardiovasc Pharmacol Ther. 2015;20:249‐260. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:240. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;2016(37):2129‐2200. [DOI] [PubMed] [Google Scholar]
- 5. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD‐HF) Trial. J Card Fail. 2010;16:922‐930. [DOI] [PubMed] [Google Scholar]
- 6. Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD‐HF II) trial. Int J Cardiol. 2014;172:115‐121. [DOI] [PubMed] [Google Scholar]
- 7. Chen HH, Anstrom KJ, Givertz MM, et al. Low‐dose dopamine or low‐dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779‐789. [DOI] [PubMed] [Google Scholar]
- 9. Gamper G, Havel C, Arrich J, et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2016;2:CD003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thongprayoon C, Cheungpasitporn W, Harrison AM, et al. Temporal trends in the utilization of vasopressors in intensive care units: an epidemiologic study. BMC. Pharmacol Toxicol. 2016;17:z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mebazaa A, Motiejunaite J, Gayat E, et al. Long‐term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology Heart Failure Long‐Term Registry. Eur J Heart Fail. 2018;20:332‐341. [DOI] [PubMed] [Google Scholar]
- 12. da Costa BR, Juni P. Systematic reviews and meta‐analyses of randomized trials: principles and pitfalls. Eur Heart J. 2014;35:3336‐3345. [DOI] [PubMed] [Google Scholar]
- 13. van der Horst I, Keus F, Hiemstra B, Koster G, Wetterslev J, Gluud C. Dopamine for cardiac dysfunction in critically ill adult patients: a systematic review with meta‐analysis and trial sequential analysis.PROSPERO.2016; http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016042867. Accessed July 13, 2016.
- 14. Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. www.cochrane-handbook.org
- 15. Gluud C, Nikolova D, Klingenberg SL.Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)). 2015:Art. No.: LIVER.
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006‐1012. [DOI] [PubMed] [Google Scholar]
- 17. Rao V, Ivanov J, Weisel RD, Ikonomidis JS, Christakis GT, David TE. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg. 1996;112:38‐51. [DOI] [PubMed] [Google Scholar]
- 18. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH) adopts consolidated guideline on good clinical practice in the conduct of clinical trials on medicinal products for human use. Int Dig Health Legis. 1997; 48:231‐234. [PubMed] [Google Scholar]
- 19. Keus F, Wetterslev J, Gluud C, van Laarhoven CJ. Evidence at a glance: error matrix approach for overviewing available evidence. BMC Med Res Methodol. 2010;10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 21. Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta‐analysis. BMC Med Res Methodol. 2017;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guyatt GH, Oxman AD, Kunz R, et al. What is "quality of evidence" and why is it important to clinicians? BMJ. 2008;336:995‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mao CT, Chen TH, Chen DY, Tsai ML, Lin YS, Wang SH. Comparison of intra‐aortic balloon pump and dopamine for patients with acute myocardial infarction and unstable haemodynamics undergoing percutaneous coronary intervention. EuroIntervention. 2015. [Google Scholar]
- 24. Kamiya M, Sato N, Nozaki A, et al. Renal effects of added low‐dose dopamine in acute heart failure patients with diuretic resistance to natriuretic peptide. J Cardiovasc Pharmacol. 2015;65:282‐288. [DOI] [PubMed] [Google Scholar]
- 25. Varriale P, Mossavi A. The benefit of low‐dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: does it protect renal function? Clin Cardiol. 1997;20:627‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shah RA, Subban V, Lakshmanan A, et al. A prospective, randomized study to evaluate the efficacy of various diuretic strategies in acute decompensated heart failure. Indian Heart J. 2014;66:309‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arutiunov GP, Arutiunov AG, Volkova AL. Study evaluating the impact of a combination of inotropic support and heart rate control on prognosis and stabilization rate in patients with decompensated chronic heart failure (LEGION). Ter Arkh. 2010;82:47‐52. [PubMed] [Google Scholar]
- 28. Hsueh CW, Lee WL, Chen CK, et al. Dopamine and dobutamine have different effects on heart rate variability in patients with congestive heart failure. Zhonghua Yi Xue Za Zhi (Taipei). 1998;61:199‐209. [PubMed] [Google Scholar]
- 29. Cotter G, Weissgarten J, Metzkor E, et al. Increased toxicity of high‐dose furosemide versus low‐dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther. 1997;62:187‐193. [DOI] [PubMed] [Google Scholar]
- 30. Sindone A, MacDonald P, Keogh AM. Haemodynamic, neurohumoral and symptomatic effects of dobutamine, dopamine and milrinone in severe heart failure. Aust N Z J Med. 1998;28:113. [Google Scholar]
- 31. Sirivella S, Gielchinsky I, Parsonnet V. Mannitol, furosemide, and dopamine infusion in postoperative renal failure complicating cardiac surgery. Ann Thorac Surg. 2000;69:501‐506. [DOI] [PubMed] [Google Scholar]
- 32. Costa P, Ottino GM, Matani A, et al. Low‐dose dopamine during cardiopulmonary bypass in patients with renal dysfunction. J Cardiothorac Anesth. 1990;4:469‐473. [DOI] [PubMed] [Google Scholar]
- 33. Bove T, Landoni G, Calabro MG, et al. Renoprotective action of fenoldopam in high‐risk patients undergoing cardiac surgery: a prospective, double‐blind, randomized clinical trial. Circulation. 2005;111:3230‐3235. [DOI] [PubMed] [Google Scholar]
- 34. Rosseel PM, Santman FW, Bouter H, Dott CS. Postcardiac surgery low cardiac output syndrome: dopexamine or dopamine? Intensive Care Med. 1997;23:962‐968. [DOI] [PubMed] [Google Scholar]
- 35. Hausen B, Heublein B, Vogelpohl J, von der Leyen H, Haverich A. Comparison of enoximone and piroximone in patients after mitral valve operation: a prospective and controlled clinical study. J Cardiovasc Pharmacol. 1992;19:299‐307. [DOI] [PubMed] [Google Scholar]
- 36. Oppizzi M, Montorsi E, Tosoni A, et al. The effectiveness of enoximone in patients with serious left ventricular dysfunction submitted for aorto‐coronary bypass. Minerva Anestesiol. 1997;63:17‐27. [PubMed] [Google Scholar]
- 37. Tarr TJ, Moore NA, Frazer RS, Shearer ES, Desmond MJ. Haemodynamic effects and comparison of enoximone, dobutamine and dopamine following mitral valve surgery. Eur J Anaesthesiol Suppl. 1993;8:15‐24. [PubMed] [Google Scholar]
- 38. Dzhaiani NA, Kositsyna IV, Gnidkina NA, Tereshchenko SN. Efficacy of levosimendan vs dopamine in patients with resistant cardiac failure. Ter Arkh. 2011;83:53‐59. [PubMed] [Google Scholar]
- 39. Rui Q, Jiang Y, Chen M, Zhang N, Yang H, Zhou Y. Dopamine versus norepinephrine in the treatment of cardiogenic shock: a PRISMA‐compliant meta‐analysis. Medicine (Baltimore). 2017;96:e8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koster G, Bekema HJ, Wetterslev J, Gluud C, Keus F, van der Horst IC. Milrinone for cardiac dysfunction in critically ill adult patients: a systematic review of randomised clinical trials with meta‐analysis and trial sequential analysis. Intensive Care Med. 2016;42:1322‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koster G, Wetterslev J, Gluud C, et al. Effects of levosimendan for low cardiac output syndrome in critically ill patients: systematic review with meta‐analysis and trial sequential analysis. Intensive Care Med. 2015;41:203‐221. [DOI] [PubMed] [Google Scholar]
- 42. Schumann J, Henrich EC, Strobl H, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2018;1:CD009669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta‐analysis*. Crit Care Med. 2012;40:725‐730. [DOI] [PubMed] [Google Scholar]
- 44. Vasu TS, Cavallazzi R, Hirani A, Kaplan G, Leiby B, Marik PE. Norepinephrine or dopamine for septic shock: systematic review of randomized clinical trials. J Intensive Care Med. 2012;27:172‐178. [DOI] [PubMed] [Google Scholar]
- 45. Zhou F, Mao Z, Zeng X, Kang H, Liu H, Pan L, Hou PC. Vasopressors in septic shock: a systematic review and network meta‐analysis. Ther Clin Risk Manag. 2015;11:1047‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Avni T, Lador A, Lev S, Leibovici L, Paul M, Grossman A. Vasopressors for the treatment of septic shock: systematic review and meta‐analysis. PLoS ONE. 2015;10:e0129305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oba Y, Lone NA. Mortality benefit of vasopressor and inotropic agents in septic shock: a Bayesian network meta‐analysis of randomized controlled trials. J Crit Care. 2014;29:706‐710. [DOI] [PubMed] [Google Scholar]
- 48. Turner RM, Bird SM, Higgins JP. The impact of study size on meta‐analyses: examination of underpowered studies in Cochrane reviews. PLoS ONE. 2013;8:e59202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cole GD, Dhutia NM, Shun‐Shin MJ, et al. Defining the real‐world reproducibility of visual grading of left ventricular function and visual estimation of left ventricular ejection fraction: impact of image quality, experience and accreditation. Int J Cardiovasc Imaging. 2015;31:1303‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pellikka PA, She L, Holly TA, et al. Variability in ejection fraction measured by echocardiography, gated single‐photon emission computed tomography, and cardiac magnetic resonance in patients with coronary artery disease and left ventricular dysfunction. JAMA Network Open. 2018;1:e181456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233‐270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


