Abstract
Insulin resistance is a condition in which insulin sensitivity is reduced and the insulin signaling pathway is impaired. Although often expressed as an increase in insulin concentration, the disease is characterized by a decrease in insulin action. This increased workload of the pancreas and the consequent decompensation are not only the main mechanisms for the development of type 2 diabetes (T2D), but also exacerbate the damage of metabolic diseases, including obesity, nonalcoholic fatty liver disease, polycystic ovary syndrome, metabolic syndrome, and others. Many clinical trials have suggested the potential role of herbs in the treatment of insulin resistance, although most of the clinical trials included in this review have certain flaws and bias risks in their methodological design, including the generation of randomization, the concealment of allocation, blinding, and inadequate reporting of sample size estimates. These studies involve not only the single-flavored herbs, but also herbal formulas, extracts, and active ingredients. Numerous of in vitro and in vivo studies have pointed out that the role of herbal medicine in improving insulin resistance is related to interventions in various aspects of the insulin signaling pathway. The targets involved in these studies include insulin receptor substrate, phosphatidylinositol 3-kinase, glucose transporter, AMP-activated protein kinase, glycogen synthase kinase 3, mitogen-activated protein kinases, c-Jun-N-terminal kinase, nuclear factor-kappaB, protein tyrosine phosphatase 1B, nuclear factor-E2-related factor 2, and peroxisome proliferator-activated receptors. Improved insulin sensitivity upon treatment with herbal medicine provides considerable prospects for treating insulin resistance. This article reviews studies of the target mechanisms of herbal treatments for insulin resistance.
Keywords: herbal medicine, insulin resistance, signal transduction, pathway, mechanism
Introduction
Insulin resistance (IR) is a pathological condition in which target tissues (primarily skeletal muscle, liver, and adipose tissue) have an impaired biological response to insulin stimulation. During IR, the body’s compensatory release of excess insulin to maintain blood sugar stability causes hyperinsulinemia that can progress to type 2 diabetes mellitus (T2D). Prospective studies have highlighted the importance of IR in the pathogenesis of T2D and suggest that IR is the best predictor of future T2D diagnosis (Lillioja et al., 1993). IR and obesity are connected with chronic inflammation in metabolic tissues such as adipose tissue and the liver (Winer et al., 2016). Some studies have pointed out that body mass index is positively associated with IR (Li W. et al., 2014) and inflammation in visceral adipose tissue is a main driver of IR (Lumeng et al., 2007). Closely linked to the epidemic of obesity (Ng et al., 2014), the number of adults with diabetes increased from 108 million in 1980 to 422 million in 2014 (Zhou et al., 2016), and this figure is projected to rise to 642 million people by 2040. There are many vascular and nerve-related complications in diabetes such as diabetes-induced dysregulation of cardiac function, instability of microvasculature of the heart, and increased risk for heart failure (Riehle and Abel, 2016; Levelt et al., 2016; Hinkel et al., 2017). The risk of dementia, Alzheimer’s disease, and cognitive decline are elevated in people with IR (Biessels et al., 2006; Willette et al., 2015; Kullmann et al., 2016) and T2D; the global prevalence of diabetic foot pathologies is 6.3%, and 12.9 to 49.0 million people worldwide have a history of foot ulceration (Armstrong et al., 2017; Zhang P. et al., 2017). These complications bring a tremendous medical and socioeconomic burden. IR is associated with increased risk for other associated disorders, including polycystic ovary syndrome (PCOS), hepatitis C virus, nonalcoholic fatty liver disease (NAFLD), and metabolic syndrome (Diamanti-Kandarakis and Dunaif, 2012; Meex and Watt, 2017; Aytug et al., 2003). Improving IR may provide a therapeutic strategy for controlling T2D, obesity, and many other diseases. Current interventions for IR include intensive lifestyle interventions, thiazolidinedione, DPP-4 inhibitors, and metformin. However, IR is not well controlled and poses a threat to modern society (Kahn et al., 2006). Some herbal medicines such as Coptis chinensis Franch (Zhen et al., 2011), Ganoderma lucidum, and Panax ginseng C. A. Mey result in enhanced insulin sensitivity through modulation of diverse physiological and cellular pathways (Chang et al., 2015; Martel et al., 2017; Bai et al., 2018). For centuries, natural herbs and herbal formulae derived from systemic traditional Chinese medicine theory and practice have been used to treat many kinds of ailments in China. At present, Chinese medicine has received strong support from the World Health Organization and will be included in Chapter 26 of the 11th edition of the Global Medical Program. Chinese medicine also provides treatments for obesity and T2D (D, 2018). In the third century BC, Huang Di Nei Jing, the most classic book of Chinese medicine, recorded similar diseases related to diabetes and obesity and provided treatment principles. Now, traditional Chinese medicine is widely used to clinically treat IR. In this review, we explored whether herbs and their formulations or monomers can improve IR and the mechanisms of herbal compounds that increase insulin sensitivity.
Methodology
According to the Pharmacopoeia of the People’s Republic of China that was revised by the China Food and Drug Administration in 2015, herbal medicine is defined as therapy using herbs and materials derived from botanical herbal products and mineral and animal sources. Interventions that were used in this study include single-flavored herbs and their extracts, active ingredients, and herbal formulas. There were no geographical restrictions on the herbs included.
We reviewed literature (from PubMed) published between July 8, 2013 and July 6, 2018 on IR that had been treated with herbal medicine. The following combination of terms were used as search keywords: “herbal,” “phytochemical,” “phytomedicine,” “natural product,” and “insulin resistance” or “IR.” The search did not exclude articles based on language or status of the publication.
The specified exclusion criteria include: a) case reports, case series, editorials, reviews; b) interventions containing ingredients other than herbs; and c) relevant indicators of IR, such as homeostatic model assessment of IR (HOMA-IR), and IR-index, not involved in the primary and secondary outcomes of clinical trials.
Results
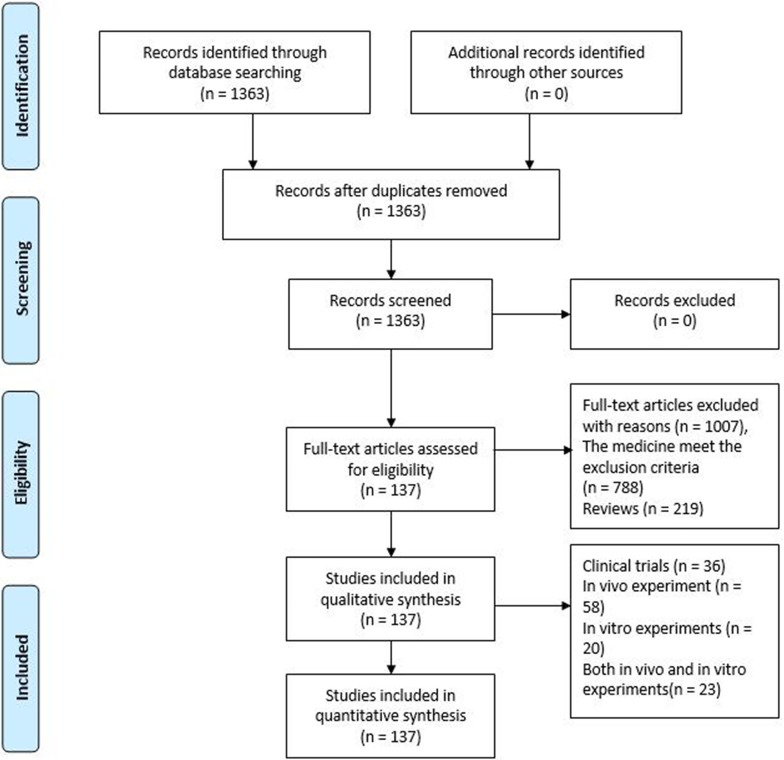
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart ( Figure 1 ) of article processing shows that our search yielded 1,363 articles, and 1,007 articles were excluded based on the exclusion criteria. After excluding these 1,007 articles, we included 137 articles, including 36 clinical trials, 58 in vivo experiments, 20 in vitro experiments, and 23 that were a combination of both in vitro and in vivo experiments. Based on further reading of this literature, we divided 101 in vivo and in vitro experiments into three parts based on the interventional drug used: active ingredients (31 articles), natural products (38 articles), and herbal formulas (32 articles). The results suggest that most clinical trials (30 articles) indicate that herbal active ingredients, natural products, and herbal formulas, such as JTTZ formula, Jinlida, and Curcumin, have a therapeutic effects on IR. There were a few clinical trials (six articles) that did not support the above results, such as those that used marjoram tea, hydroalcoholic extract of Juglans regia (walnut) leaves, Fraxinus excelsior L. seeds/fruit extract, garlic extract, bee propolis, red wine polyphenols. On the other hand, we concluded that the effects of herbal medicine on IR may be related to 11 important target molecules that affect insulin signaling, such as insulin receptor substrate, phosphatidylinositol 3-kinase, and glucose transporter.
Figure 1.
PRISMA 2019 Flow Diagram.
Clinical Trials to Improve Insulin Resistance
In clinical trials, participants in the treatment group should be restricted to using herbs alone. If medication other than herbs are being used, then the medication must be administered to both the treatment group and the control group. Participants in the control group should receive hypoglycemic agents, placebo, or life interventions. There is no limit to the number of predetermined herbs, recipes, sample sizes, or duration of treatment.
IR can be accurately assessed by clinical examination. It is generally believed that hyperinsulinemic-euglycemic clamps are the “gold standard” for determining IR, but the complex nature and inherent potential hazards of this technique limit its routine use (Park et al., 2015). Commonly used alternatives are primarily HOMA-IR and quantitative insulin sensitivity check index (QUICKI), which use fasting insulin and glucose concentrations to assess IR and correlate with the results of the clamp study (Matthews et al., 1985). In the 36 clinical trials ( Table 1 ) in the above table, most of the drugs examined were herbal formulas, including herbal extracts and active ingredients, and the diseases of concern included T2D, metabolic syndrome, obesity, impaired glucose tolerance, PCOS, and cirrhosis. Most studies have shown that herbal medicine can not only reduce IR but also improve blood sugar, blood lipids, glycosylated hemoglobin, and other biochemical indicators. Indicators of IR in these studies include HOMA-IR, QUICKI, area under the curve of insulin, and IR index. Among them, HOMA-IR was the indicator and was used in 29 studies (29/36, 80%). In order to objectively observe the therapeutic effect of herbs on IR, the changes in IR evaluation indicators before and after treatment in 36 clinical trials are listed in Table 2 .
Table 1.
Clinical trials related to insulin resistance.
| Compounds/formula | Design | Disease | Dose regimen | Duration | Case/control | Main outcome* | Side effect | Reference |
|---|---|---|---|---|---|---|---|---|
| JTTZ Formula | RCT | type 2 diabetic mellitus | 0.25g po bid | 12 weeks | 215/199 | HbA1c ↓, T ↓, weight ↓, HOMA-IR ↓ | not mentioned | (Yu X. et al., 2018) |
| Tangyiping Granules (TYP) | RCT | impaired glucose tolerance | 10g po bid | 3, 12, and 24 months | 60/60 | NGT ↑, 2hPG ↓, HbA1c ↓, HOMA-IR ↓, TG ↓ | not mentioned | (Huang et al., 2016) |
| Jinlida (JLD) | RCT | impaired glucose tolerance | one bag po tid | 12 weeks | 32/29 | HbA1c ↓, 2hPG ↓, HOMA-IR ↓ | no side effect is found | (Shi et al., 2016) |
| Qingxue Dan (QXD) | RCT | obesity | 900mg po qd | 8 week | 13/13 | BMI ↓, TG ↓, WC ↑, total cholesterol ↑, high-density lipoprotein cholesterol ↑, HOMA-IR ↓ | not mentioned | (Chung et al., 2016) |
| Artemisia dracunculus | RCT | impaired glucose tolerance | 1000mg po bid | 90 days | 12/12 | HbA1c ↓, AUC of insulin ↓, total insulin secretion ↓, HDL-C ↑, SBP ↓ | not mentioned | (Mendez-Del Villar et al., 2016) |
| Qingre Yangyin Recipe (QRYYR) | RCT | polycystic ovary syndrome | one dose po bid | 3 months | 30/30 | BMI ↓, FINS ↓, 2h INS ↓, HOMA-IR ↓, leptin ↓, LH ↓, PRL ↓, T ↓, APN ↑, FPG ↓, 2 hPG ↓ | not mentioned | (Zhang, 2015) |
| Sancaijiangtang powders | RCT | type 2 diabetic mellitus and vascular dementia | powders po tid | 12 weeks | 84/84 | fasting plasma glucose ↓, fasting insulin ↓, HbA1c ↓, HOMA-IR ↓, plasma nitric oxide ↓, endothelin-1 ↓ | not mentioned | (Qiang et al., 2015) |
| Cinnamon | RCT | type 2 diabetic mellitus | 1g po qd | 90 days | 35/35/35 | FPG ↓, 2h PG ↓, HOMA-IR ↓, | not mentioned | (Mirfeizi et al., 2016) |
| Jinlida | RCT | type 2 diabetic mellitus | 9g po tid+ metformin | 12 weeks | 92/94 | HbA1c ↓, FG ↓, 2h PG ↓, HOMA-β ↑, HOMA-IR ↓ | not mentioned | (Lian et al., 2015) |
| Zhenggan Tang decoction | RCT | HBV-induced cirrhotic | 125ml po bid | 3 months | 35/31 | leptin ↓, adiponectin ↓, IR-index ↓ | not mentioned | (Xu et al., 2015) |
| Marjoram tea | RCT | polycystic ovary syndrome | 1.3–1.5g po bid | 1 month | 14/11 | DHEA-S ↓, fasting insulin levels ↓, HOMA-IR was not reduced significantly | not mentioned | (Haj-Husein et al., 2016) |
| xin-ju-xiao-gao-fang (XJXGF) | RCT | obesity | 170ml po bid | 24 weeks | 59/55 | IR-index ↓, weight ↓ | not mentioned | (Zhou et al., 2014) |
| Yiqi Huaju Recipe (YHR) | RCT | hypertension patients with metabolic syndrome | 17.5g po bid | 12 weeks | 22/21 | WC ↓, WHR ↓, 2h PG ↓, FPI ↓, HOMA-IR ↓, average blood pressure amplitude ↑, blood pressure variability ↑, blood pressure load ↑ | not mentioned | (Chen et al., 2014) |
| Ginger (GG) | RCT | type 2 diabetic mellitus | 1g po tid | 8 weeks | 40/41 | FBS ↓, HbA1c ↓, HOMA-IR ↓, S% ↑, QUICKI ↑ | not mentioned | (Mozaffari-Khosravi et al., 2014) |
| Curcumin | RCT | type 2 diabetic mellitus | three capsules po bid | 6 months | 120/120 | pulse wave velocity ↓, adiponectin ↑, leptin ↓, HOMA-IR ↓, TG ↓, uric acid ↓, visceral fat ↓, total body fat ↓ | no side effect is found | (Chuengsamarn et al., 2014) |
| Hydroalcoholic extract of Juglans regia (walnut) leaves | RCT | type 2 diabetic mellitus | 100mg po qd | 8 weeks | 20/20 | weight ↓, body mass index ↓, systolic blood pressure ↓, no significant effect on blood glucose level and HOMA-IR | no side effect is found | (Rabiei et al., 2018) |
| Yangxin Tongmai Formula (YTF) | RCT | Graves’ disease with impaired glucose tolerance | 50ml po tid | 60 days | 20/20 | ISI ↓, HOMA-IR ↓, insulin receptor ↑, plasma insulin levels ↓ | not mentioned | (Luo et al., 2018) |
| Sancai powder | RCT | type 2 diabetic mellitus | 200 ml po bid | 12 weeks | 47/49 | HbA1c ↓, FPG ↓, 2hPG ↓, TG ↓, HOMA-β ↓, HOMA-IR ↓, ISI ↓ | not mentioned | (Guo et al., 2016) |
| Fraxinus excelsior L. seeds/fruits extract | RCT | non-diabetic overweight/obese | 1g po tid | 7 weeks | 11/6 | AUC of insulin ↓, 2hPG ↓, adiponectin: Leptin ratio ↑, fat mass ↓, serum fructosamine ↓, plasma glucagon ↑, no significant changes IR-index and Matsuda Index | no side effect is found | (Zulet et al., 2014) |
| Berberine | RCT | metabolic syndrome | 500mg po tid | 3 months | 12/12 | WC ↓,SBP ↓,TG ↓, AUC of insulin ↓, AUC of glucose ↓, insulinogenic index ↓,Matsuda index ↑ | no side effect is found | (Perez-Rubio et al., 2013) |
| Aloe vera gel complex | RCT | obese prediabetes | 1400mg po bid | 8 weeks | 60/62 | weight ↓, HOMA-IR ↓, FBG ↓, serum insulin level ↓ | not mentioned | (Choi et al., 2013) |
| Green tea extract | RCT | type 2 diabetes | 500mg po tid | 16 weeks | 39/38 | triglyceride ↓, high density lipoprotein cholesterol ↑, HOMA-IR ↓, Adiponectin ↑, apolipoprotein A1 ↑, apolipoprotein B100 ↑ | no side effect is found | (Liu C. et al., 2014) |
| Cinnamon | RCT | nonalcoholic fatty liver disease | 750mg po bid | 12 weeks | 23/22 | HOMA-IR ↓, FBS ↓, total cholesterol ↓, triglyceride ↓, ALT ↓, AST ↓, | not mentioned | (Askari et al., 2014) |
| Soybean leaf extracts (SLEs) | RCT | prediabetes | 2g po qd | 12 weeks | 15/15 | FBG ↓, HbA1c ↓, HOMA-IR ↓ | no side effect is found | (Choi et al., 2014) |
| Nigella sativa | RCT | type 2 diabetes mellitus | 2g po qd | 12 months | 57/57 | FBG ↓, HbA1c ↓, HOMA-IR ↓ | no side effect is found | (Kaatabi et al., 2015) |
| Chamomile tea | RCT | type 2 diabetes mellitus | 3g po tid | 8 weeks | 32/32 | serum insulin levels ↓, HbA1c ↓, HOMA-IR ↓, total cholesterol ↓, triglyceride ↓ | not mentioned | (Rafraf et al., 2015) |
| DLBS3233 | RCT | impaired glucose tolerance | 100mg po qd | 12 weeks | 38/36 | HOMA-IR ↓ | no side effect is found | (Manaf et al., 2016) |
| Garlic extract | RCT | type 2 diabetes mellitus | 1200mg po qd | 4 weeks | 13/13 | no significant changes in weight, SBP, DBP, total cholesterol, plasma HDL cholesterol, plasma triglycerides, HOMA-IR | indigestion | (Atkin et al., 2016) |
| Bee propolis | RCT | type 2 diabetes mellitus | 300mg po tid | 12 weeks | 30/27 | no significant difference in HOMA-IR | no side effect is found | (Samadi et al., 2017) |
| Artemisia Extract | RCT | gestational Diabetes Mellitus | 400mg po qd | 10 weeks | 64/65 | FPG ↓, serum insulin levels ↓, HOMA-IR ↓ | not mentioned | (Sun X. et al., 2016) |
| Red wine polyphenols | RCT | obesity | 300mg po bid | 8 weeks | 14/15 | no significant changes in HOMA-IR, LDL, HDL cholesterol or triglyceride levels | no side effect is found | (Woerdeman et al., 2018) |
| Fresh yellow onion | RCT | breast cancer | 30∼40g po qd | 8 weeks | 23/23 | FPG ↓, serum insulin levels ↓, HOMA-IR ↓ | no side effect is found | (Jafarpour-Sadegh et al., 2017) |
| Gymnema sylvestre | RCT | metabolic syndrome | 300mg po bid | 12 weeks | 12/12 | BMI ↓, VLDL ↓, AUC of insulin ↓ | no side effect is found | (Zuniga et al., 2017) |
| Artichoke leaf extract | RCT | metabolic syndrome | 1800mg po qd | 12 weeks | 33/35 | no significant in blood pressure, FPG, HOMA-IR ↓ | no side effect is found | (Ebrahimi-Mameghani et al., 2018) |
| Silybum marianum (L). Gaertn. (silymarin) extract | RCT | type 2 diabetes mellitus | 140mg po tid | 45 days | 20/20 | FPG ↓, serum insulin ↓, HOMA-IR ↓, triglyceride ↓, HDL-C ↓ | no side effect is found | (Ebrahimpour-Koujan et al., 2018) |
| Green coffee extract | RCT | metabolic syndrome | 400mg po bid | 8 weeks | 22/21 | FBS ↓, weight ↓, systolic blood pressure ↓, HOMA-IR ↓ | no side effect is found | (Roshan et al., 2018) |
AUC, area under the curve; APN, adiponectin; BMI, body mass index; BBT, basal body temperature; DHEA-S, dehydroepiandrosterone-sulphate; FSH, follicle stimulating hormone; FINS, fasting insulin; FPG, fasting blood glucose; FPI, fasting plasma insulin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; HbA1c, fasting glycosylated hemoglobin A1c; ISI, insulin sensitivity index; LH, luteinizing hormone; NGT, normal glucose tolerance; PRL, prolactin; QUICKI, quantitative insulin sensitivity check index; S%, SBP, systolic blood pressure; insulin sensitivity; T, testosterone; TG, triglyceride; WC, waist circumference; WHR, waist to hip ratio; 2h INS, postprandial 2 h insulin; 2h PG, 2 hours plasma glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 2.
Changes in insulin resistance evaluation indicators before and after treatment in clinical trials.
| Compounds/formula | HOMA-IR1, AUC of insulin2, IRS3, QUICKI4 | Baseline difference | ||
|---|---|---|---|---|
| BT | AT | p value** | ||
| JTTZ Formula1 | T: 1.58 ± 0.72 C: 1.5 ± 0.75 |
T: 1.39 ± 0.68 C: 1.35 ± 0.67 |
0.01 | NSD |
| Tangyiping Granules (TYP)1 | T: 4.02 ± 0.46 C: 3.87± 0.36 |
T: 3.59 ± 0.31 C: 3.83 ± 0.37 |
<0.05 | NSD |
| Jinlida (JLD)1 | T: 2.4(2.0,4.0) C: 2.5(2.1,3.0) |
T: 2.41(1.7,3.9) C: 3.0(2.1,3.3) |
0.029 | NSD |
| Qingxue Dan (QXD)1 | T: 164 ± 93 C: 226 ± 160 |
T: 150 ± 92 C: 205 ± 184 |
>0.05 | NSD |
| Artemisia dracunculus2 | T: 56,136 ± 27,426 C: 92,430 ± 55,920 |
T: 44,472 ± 23,370 C: 94,278 ± 43,230 |
<0.05 | NSD |
| Qingre Yangyin Recipe (QRYYR)1 | T: 3.48 ± 2.03 C: 3.81 ± 2.75 |
T: 2.83 ± 1.52 C: 2.69 ± 1.16 |
<0.05 | NR |
| Sancaijiangtang powders1 | T: 6.0 ± 0.8 C: 5.8 ± 1.2 |
T: 4.6 ± 1.3 C: 3.5 ± 0.8 |
<0.05 | NSD |
| Cinnamon1 | T: 8.82 ± 6.59 C: 7.06 ± 5.65 |
T: 5.58 ± 3.20 C: 5.8 ± 1.2 |
0.013 | NSD |
| Jinlida1 | T: 1.32 ± 0.79 C: 1.41 ± 0.79 |
T: 1.2 ± 0.66 C: 1.31 ± 0.67 |
0.824 | NSD |
| Zhenggan Tang decoction3 | T: 1.68 ± 0.21 C: 1.72 ± 0.32 |
T: 0.92 ± 0.18 C: 1.69 ± 0.44 |
<0.05 | NSD |
| Marjoram tea1 | T: 1.53(0.18) C: 1.5(0.25) |
T: 1.14(0.14) C: 1.68(0.28) |
0.06 | NSD |
| xin-ju-xiao-gao-fang (XJXGF)1 | T: 8.10 ± 5.32 C: 7.84 ± 5.18 |
T: 5.48 ± 1.05 C: 9.57 ± 1.45 |
0.77 | NSD |
| Yiqi Huaju Recipe (YHR)1 | T: 6.01 ± 4.05 C: 6.50 ± 4.15 |
T: 4.07 ± 2.80 C: 6.63 ± 4.02 |
<0.05 | NSD |
| Ginger (GG)4 | T: 0.316 ± 0.025 C: 0.324 ± 0.031 |
T: 0.337± 0.303 C: 0.333 ± 0.031 |
<0.005 | NSD |
| Curcumin1 | T: 6.12(2-24.1) C: 5.63(1.4-14.9) |
T: 5.92(1.4-14.9) C: 2.75(0.9-10.9) |
<0.01 | NSD |
| Hydroalcoholic extract of Juglans regia (walnut) leaves1 | T: 3.3 ± 2.7 C: 3.0 ± 1.7 |
T: 2.9 ± 2.2 C: 2.7 ± 1.4 |
0.186 | NSD |
| Yangxin Tongmai Formula (YTF)1 | T: 6.48 ± 1.05 C: 4.26 ± 0.74 |
T: 4.86 ± 0.54 C: 4.86 ± 0.69 |
<0.05 | NR |
| Sancai powder1 | T: 3.2 ± 0.6 C: 3.4 ± 0.6 |
T: 0.8 ± 0.5 C: 0.7 ± 0.5 |
<0.05 | NSD |
| Fraxinus excelsior L. seeds/fruits extract1 | T: 5.65(2.68) C: 5.32(3.15) |
T: 6.86(5.17) C: 6.05(3.36) |
>0.05 | NSD |
| Berberine2 | T: 92,056 ± 72,148 C: 67,605 ± 18,730 |
T: 67,407 ± 46,441 C: 86,852 ± 57,863 |
<0.01 | NSD |
| Aloe vera gel complex1 | T: 3.4 ± 1.6 C: 3.3 ± 1.2 |
T: 3.1 ± 0.2 C: 3.5 ± 0.2 |
<0.01 | NSD |
| Green tea extract1 | T: 5.4 ± 3.9 C: 5.9 ± 4.5 |
T: 3.5 ± 2.0 C: 4.7 ± 3.4 |
0.004 | NSD |
| Cinnamon1 | T: 2.7 ± 2.0 C: 3.0 ± 1.2 |
T: 1.7 ± 0.7 C: 3.0 ± 0.0 |
<0.001 | NSD |
| Soybean leaf extracts (SLEs)1 | T: 1.08 ± 0.06 C: 1.07 ± 0.08 |
T: 0.92 ± 0.12 C: 1.18 ± 0.08 |
<0.05 | NSD |
| Nigella sativa1 | T: 3.0 ± 0.24 C: 2.5 ± 0.17 |
T: 2.5 ± 0.18 C: 2.51 ± 0.15 |
0.004 | NSD |
| Chamomile tea1 | T: 7.05 ± 2.34 C: 5.24 ± 1.23 |
T: 4.24 ± 1.95 C: 5.55 ± 1.12 |
<0.001 | NSD |
| DLBS32331 | T: 3.00 ± 1.76 C: 2.76 ± 2.28 |
T: 2.16 ± 1.17 C: 2.28 ± 1.24 |
0.001 | NSD |
| Garlic extract1 | T: 1.89 ± 1.1 C: 2.5 ± 2.0 |
T: 1.7 ± 0.9 C: 2.0 ± 1.1 |
0.05 | NSD |
| Bee propolis4 | T: 0.37 ± 0.03 C: 0.36 ± 0.03 |
T: 0.34 ± 0.03 C: 0.03 ± 0.33 |
<0.001 | NSD |
| Artemisia Extract1 | T: 2.7 ± 1.8 C: 2.8 ± 1.4 |
T: 1.7 ± 2.4 C: 4.0 ± 1.5 |
0.031 | NSD |
| Red wine polyphenols1 | T: 3.2(2.0, 4.5) C: 2.3(1.4, 2.7) |
T: 2.9(2.1, 3.8) C: 2.2(1.5, 2.8) |
0.72 | NSD |
| Fresh yellow onion1 | T: 0.052 ± 0.011 C: 0.045 ± 0.01 |
T: 0.046 ± 0.006 C:0.051 ± 0.01 |
0.021 | NSD |
| Gymnema sylvestre2 | T: 61,626 ± 29,700 C: 64,314 ± 34,914 |
T: 60,468 ± 37,290 C: 90,816 ± 45,336 |
0.01 | NSD |
| Artichoke leaf extract1 | T: 3.53(1.44) C: 3.11(1.27) |
T: 3.30(1.47) C: 3.63(1.55) |
<0.05 | NSD |
| Silybum marianum (L). Gaertn. (silymarin) extract1 | T: 4.25 ± 2.43 C: 4.49 ± 2.94 |
T: 2.75 ± 1.19 C: 5.48 ± 3.51 |
0.008 | NSD |
| Green coffee extract1 | T: 5.04 ± 3.95 C: 4.71 ± 2.55 |
T: 3.62 ± 1.83 C: 5.94 ± 5.16 |
0.024 | NSD |
AT, after treatment; BT, before treatment; C, control group; NR, no reported; NSD, no significant difference; T, treatment group.
**p value: differences in treatment group before and after treatment. “1”, “2”, “3”, “4” indicate that the indicators of insulin resistance in the experiment are “HOMA-IR”, “AUC of insulin”, “IRS” and “QUICKI”.
Two evaluators independently assessed the risk of bias in each study and provided the methodological quality of inclusion in clinical trials according to predetermined criteria in the Cochrane Handbook ( Table 3 ). In general, the methodological quality was assessed to be poor. There are few reports of randomized sequence generation and allocation concealment. Fifteen studies (30/36, 83%) detailed how patients were randomized. Only six trials (6/36, 17%) in this study adequately reported the allocation of hidden methods. Twenty-five trials (25/36, 69%) used blinding on their subjects and investigators. Five trials (5/36, 14%) used blinding on their subjects, investigators, and outcome evaluators. The following restrictions should be considered before accepting the conclusion. First, most of the clinical trials included in this review have certain flaws and bias risks in their methodological design, including the generation of randomization, the concealment of allocation, blinding, and inadequate reporting of sample size estimates. Secondly, the duration of 21 trials (21/36, 58%) was greater than or equal to 12 weeks, and 3 trials (3/36, 8%) lasted longer than 6 months. The impact of duration on the results of the study cannot be ignored. Finally, outcome measures for evaluation of major adverse clinical events, such long-term follow-up, were not considered in this review. Therefore, this review indicates that the evidence for both the benefits and harms of herbal treatment for IR is not strong, and it is necessary to rigorously design further trials with high methodological quality to confirm the conclusion.
Table 3.
Risk of bias of the clinical trials.
| Compounds/formula | A | B | C | D | E | F | G | H | Reference |
|---|---|---|---|---|---|---|---|---|---|
| JTTZ Formula | ? | – | – | – | + | + | ? | ? | (Yu X. et al., 2018) |
| Tangyiping Granules (TYP) | + | + | + | + | ? | + | ? | ? | (Huang et al., 2016) |
| Jinlida (JLD) | + | – | – | – | ? | + | ? | ? | (Shi et al., 2016) |
| Qingxue Dan (QXD) | + | ? | + | + | – | + | ? | ? | (Chung et al., 2016) |
| Artemisia dracunculus | ? | – | + | + | – | + | ? | ? | (Mendez-Del Villar et al., 2016) |
| Qingre Yangyin Recipe (QRYYR) | – | – | + | – | – | – | ? | ? | (Zhang, 2015) |
| Sancaijiangtang powders | + | – | + | – | – | + | ? | ? | (Qiang et al., 2015) |
| Cinnamon | + | ? | + | + | + | + | ? | ? | (Mirfeizi et al., 2016) |
| Jinlida | + | + | + | + | + | + | – | ? | (Lian et al., 2015) |
| Zhenggan Tang decoction | + | – | – | – | – | + | ? | ? | (Xu et al., 2015) |
| Marjoram tea | + | – | + | + | + | + | ? | ? | (Haj-Husein et al., 2016) |
| xin-ju-xiao-gao-fang (XJXGF) | + | – | + | + | – | + | ? | ? | (Zhou et al., 2014) |
| Yiqi Huaju Recipe (YHR) | + | – | + | – | – | + | ? | ? | (Chen et al., 2014) |
| Ginger (GG) | + | – | + | + | ? | + | ? | ? | (Mozaffari-Khosravi et al., 2014) |
| Curcumin | + | + | + | + | – | + | ? | ? | (Chuengsamarn et al., 2014) |
| Hydroalcoholic extract of Juglans regia (walnut) leaves | + | + | + | + | – | + | ? | ? | (Rabiei et al., 2018) |
| Yangxin Tongmai Formula (YTF)1 | – | – | – | – | – | + | ? | ? | (Luo et al., 2018) |
| Sancai powder | + | – | + | – | – | + | ? | ? | (Guo et al., 2016) |
| Fraxinus excelsior L. seeds/fruits extract | ? | + | + | + | + | + | ? | ? | (Zulet et al., 2014) |
| Berberine | + | ? | + | + | – | + | ? | ? | (Perez-Rubio et al., 2013) |
| Aloe vera gel complex | + | – | + | + | – | + | ? | ? | (Choi et al., 2013) |
| Green tea extract | + | ? | + | + | – | + | ? | ? | (Liu C. et al., 2014) |
| Cinnamon | + | – | + | + | – | + | ? | ? | (Askari et al., 2014) |
| Soybean leaf extracts (SLEs) | + | – | – | – | – | + | ? | ? | (Choi et al., 2014) |
| Nigella sativa | + | – | + | – | – | + | ? | + | (Kaatabi et al., 2015) |
| Chamomile tea | + | – | + | – | – | + | ? | ? | (Rafraf et al., 2015) |
| DLBS3233 | ? | – | + | + | – | + | ? | ? | (Manaf et al., 2016) |
| Garlic extract | ? | – | + | + | – | + | ? | ? | (Atkin et al., 2016) |
| Bee propolis | ? | – | + | + | – | + | ? | ? | (Samadi et al., 2017) |
| Artemisia Extract | + | – | + | + | – | + | ? | ? | (Sun X. et al., 2016) |
| Red wine polyphenols | + | – | + | + | – | + | ? | ? | (Woerdeman et al., 2018) |
| Fresh yellow onion | + | – | + | + | + | + | ? | ? | (Jafarpour-Sadegh et al., 2017) |
| Gymnema sylvestre | + | – | + | + | – | + | ? | ? | (Zuniga et al., 2017) |
| Artichoke leaf extract | + | – | + | + | – | + | ? | ? | (Ebrahimi-Mameghani et al., 2018) |
| Silybum marianum (L). Gaertn. (silymarin) extract | + | – | + | + | – | + | ? | ? | (Ebrahimpour-Koujan et al., 2018) |
| Green coffee extract | + | + | + | + | ? | + | ? | ? | (Roshan et al., 2018) |
A, Adequate sequence generation; B, Concealment of allocation; C, Blinding (patient); D, Blinding (investigator); E, Blinding (assessor); F, Incomplete outcome data addressed (ITT analysis); G, Free of selective reporting; H, Other potential threat to validity; +, Low risk; –, High risk; ?, Unclear.
It must be mentioned that six clinical trials (Juglans regia leaves, marjoram tea, Fraxinus excelsior L. seeds/fruit extract, garlic extract, bee propolis, and red wine polyphenols) have not confirmed the therapeutic effect of herbs on IR. Possible reasons may include that, firstly, all herbal medicines are not necessarily effective for treating IR while also improving the metabolic index and secondly, these two herbs were not studied using conventional methods of their consuming (they were obtained from extraction using ethanol and soaking in boiling water, respectively). This may have altered the concentration and composition of the drug solution. Despite this, the potential therapeutic effects of herbs on IR are worthy of attention.
Insulin Signal Transduction
The physiology of insulin involves a complex network of signaling pathways that is activated by the insulin receptor (Samuel and Shulman, 2016). Insulin binding to an insulin receptor on a cell triggers autophosphorylation followed by phosphorylation of intracellular receptor substrates 1 and 2 (IRS-1/IRS-2) (Cheng et al., 2013). Several upstream and downstream key signaling molecules in the insulin signaling pathway have been identified, including the phosphoinositide 3-kinase (PI3K)/Akt pathway that is known to be involved in the translocation of glucose transporter 4 (GLUT-4) from intracellular vesicles to cells and promote glucose uptake to adipose tissue and skeletal muscle, eventually decreasing blood glucose levels. Other related pathways include the mitogen-activated protein kinase (MAPK), adenosine monophosphate-activated protein kinase (AMPK), and stress-activated c-Jun-N-terminal kinase (JNK) pathways (Belwal et al., 2017), among others, and these key players in signal transduction processes are potential targets for drug interventions in IR. IR is characterized by multiple defects, with decreases in receptor concentration and kinase activity, PI3K activity (Anitha et al., 2006), the concentration and phosphorylation of IRS-1 and IRS-2 (Hoehn et al., 2008), and glucose transporter translocation (Bogan, 2012). Previous studies have found that abnormalities in insulin signaling pathways caused by lipid metabolism disorders, inflammatory responses, oxidative stress, endoplasmic reticulum stress, and mitochondrial dysfunction lead to IR (Guilherme et al., 2008; Szendroedi et al., 2011; Gurzov et al., 2014; Park E. et al., 2014; Siwicki et al., 2016). Metabolic disorder and inflammation cause IR and promote leukocytes to secrete proinflammatory cytokines, including IL-6 and tumor necrosis factor-α (TNF-α) (Feve and Bastard, 2009; Wen et al., 2011; Wensveen et al., 2015), which provides a framework to understand how physiological stress, obesity, and diet promote IR. We have placed the experimental research on the treatment of IR by herbal medicine into three categories: active ingredients ( Table 4 ), natural product ( Table 5 ), and herbal formula ( Table 6 ). The active ingredient is a relatively single component, and research has proven to play a major role in the therapeutic effects of herbal medicine. The active ingredients listed in Table 4 are berberine, ginsenoside, astragaloside, polydatin, baicalin, maslinic acid, paeoniflorin, Lycium barbarum polysaccharide, dihydromyricetin, atractylenolide, etc. Natural products are also extracted from herbs, but the ingredients are relatively complex. The herbs involved in the natural products in the table include mulberry leaves, Coptis chinensis, litchi seed, red ginseng, and Gastrodia elata Blume. There are many herbs involved in herbal formulas. Some of these herbs have been used frequently, such as C. chinensis, mulberry leaves, Pueraria montana lobata, Salvia miltiorrhiza, and Astragalus membranaceus. Therapeutic targets for these herbs include: insulin receptor substrate, phosphatidylinositol 3-kinase, glucose transporter, AMP-activated protein kinase (AMPK), glycogen synthase kinase 3, MAPKs, JNK, nuclear factor-kappaB (NF-κB), protein tyrosine phosphatase 1B, nuclear factor-E2-related factor 2, and peroxisome proliferator-activated receptors. The results suggest that herbal interventions for IR are mostly multi-targeted, sometimes interfering with the same target through different pathways. Insulin receptor substrate signals transduction.
Table 4.
Active ingredients for improving insulin resistance.
| Type | Model | Monomer | Inducer | Animal/cell | Major findings | References |
|---|---|---|---|---|---|---|
| In vivo and in vitro | Insulin resistance | Baicalin | Diet-induced | Mice and 3T3-L1 cell | p38 MAPK, Akt, GLUT4 | (Fang et al., 2018) |
| In vivo | Diabetic myocardial hypertrophy | Polydatin | STZ | Mice | NF-κB, PPARβ | (Huang et al., 2015) |
| In vivo and in vitro | Diabetic and insulin resistance | Polydatin | High-fat and -sugar diet and streptozocin, palmitic acid | Rat and HepG2 cell | Akt, GSK-3β, IRS | (Hao et al., 2014) |
| In vivo and in vitro | Insulin resistance | Lycium barbarum polysaccharide (LBP) | High-fat diet | HepG2 cells and C57BL/6J mice | PI3K/Akt, Nrf2, GSK3β, JNK | (Yang Y. et al., 2014) |
| In vivo and in vitro | Adiposity and insulin resistance | Maslinic acid (MA) | High-fat diet | C57BL/6J mice and HepG2 cells | Akt, GSK3β | (Liu J. et al., 2014) |
| In vivo | Insulin resistance | LBP-4a | – | OLETF rats | PI3K, p38 MAPK, GLUT4 | (Zhao R. et al., 2014) |
| In vitro | Insulin resistance | Paeoniflorin | – | 3T3-L1 adipocytes | IRS-1, Akt | (Kong et al., 2013) |
| In vivo | Obesity | Berberine | High-fat diet | Rats | IRS-1 | (Liu D. et al., 2018) |
| In vivo | Obesity | Berberine | High-fat diet | Mice | AMPK | (Wang L. et al., 2018) |
| In vivo | Natural aging | Berberine | – | Rats | p-AMPK | (Yu Y. et al., 2018) |
| In vitro | Insulin resistance | Astragaloside IV | Glucose + insulin | HepG2 cells | AMPK | (Wang C. et al., 2018) |
| In vitro | Insulin resistance | Astragaloside IV | Palmitate | C2C12 myotubes | IRS1, Akt | (Zhu et al., 2016) |
| In vivo and in vitro | Obesity/insulin resistance | Ginsenoside Rb2 | High-fat diet/TNF-α | Mice/3T3-L1 adipocytes | IRS-1, PI3K/Akt, MAPK | (Dai et al., 2018) |
| In vivo | Obesity | Ginsenoside Rg3 | High-fat diet | C57BL/6 mice/3T3-L1 pre-adipocyte cell | PPAR | (Lee et al., 2017) |
| In vivo | Insulin resistance | Ginsenoside Re | High-fat diet | C57BL/6 mice | JNK | (Kim et al., 2017) |
| In vivo | Obesity | Ginsenoside Rg5 | High-fat diet | ICR mice | JNK | (Xiao et al., 2017) |
| In vivo | Insulin resistance | Dihydromyricetin (DMY) | High-fat diet | Male Sprague–Dawley (SD) rats | GLUT1, AMPK | (Le et al., 2016) |
| In vitro | Insulin resistance | Atractylenolide I (AT-I) and atractylenolide II (AT-II) | LY294002 | Mouse skeletal muscle, C2C12 cells | GLUT4, AMPK, PI3K/Akt | (Chao et al., 2016) |
| In vivo and in vitro | Insulin resistance | Fudan-Yueyang Ganoderma lucidum (FYGL) | – | ob/ob mice, L6 rat skeletal muscle cells | PTP1B, PI3K/Akt, GLUT4, IRS-1, | (Yang et al., 2018) |
| In vitro | Insulin resistance | α-Methyl artoflavanocoumarin (MAFC) | Insulin | HepG2 cells | PTP1B, PI3K/Akt, IRS-1, | (Jung et al., 2017) |
| In vitro | Insulin resistance | Anthocyanins from Purple Corn | DMEM | 3T3-L1 Adipocytes | PPARγ, IRS-1, GLUT4, Akt | (Luna-Vital et al., 2017) |
| In vitro | Insulin resistance | Geniposide | RPMI-1640 medium | HepG2 cells | NF-κB, GLUT-4 | (Jiang et al., 2017) |
| In vivo | Insulin resistance | Tartary buckwheat flavonoid fraction (TBF) | High fructose | Mice | Nrf2, GLUT-4, IRS-1, | (Hu et al., 2017) |
| In vitro and in vivo | Insulin resistance | Pectic bee pollen polysaccharide (RBPP-P) | High glucose and fatty acids/high-fat diet | HepG2 cells/mice | AMPK | (Li et al., 2017) |
| In vitro | Insulin resistance | Fucosterol | – | HepG2 cells | PTP1B | (Jung et al., 2016) |
| In vitro and in vivo | Insulin resistance | Mulberry anthocyanin extract (MAE) | High glucose plus palmitic acid | HepG2 cells/db/db mice | PI3K/Akt | (Yan et al., 2016) |
| In vitro | Insulin resistance | Tartary buckwheat flavonoids (TBF) | High glucose | HepG2 cells | MAPK, Nrf2, IRS-1, | (Hu et al., 2016) |
| In vivo | Insulin resistance | Corosolic acid | High-fat diet | C57BL/6 mice | IRS-1, AMPK, Akt | (Yang et al., 2016) |
| In vivo | Insulin resistance | 1-Deoxynojirimycin (DNJ) | – | db/db mice | PI3K/Akt, GLUT-4, IRS-1 | (Liu Q. et al., 2015) |
| In vivo | Insulin resistance | Rutin | S961 | C57BL/6 mice | GLUT4 | (Hsu et al., 2014) |
| In vitro | Insulin resistance | Total phenolic fraction of Anemarrhena asphodeloides | Macrophage-derived conditioned medium | Adipocytes | AMPK | (Zhao W. et al., 2014) |
Table 5.
Natural product that improves insulin resistance.
| Type | Model | Natural product | Inducer | Animal/cell | Major findings | References |
|---|---|---|---|---|---|---|
| In vitro | Insulin resistance | Oligonol | SF-MEM | HepG2 cells | PTP1B, IRS-1, PI3K/Akt, NF-κB | (Bhakta et al., 2017) |
| In vivo | Type 2 diabetic mellitus | Sang-Tong-Jian (STJ) | High-fat diet | KKAy mice | PI3K/Akt, GLUT2, GLUT4, IRS1 | (Kuai et al., 2016) |
| In vitro | Insulin resistance | Marein | High glucose | HepG2 cells | IRS-1, Akt, GLUT1, GSK-3β, AMPK | (Jiang B. et al., 2016) |
| In vivo | Metabolic syndrome | The fermented Red ginseng and Red ginseng extracts | High-fructose diet | Rats | IRS-1, GlUT4 | (Kho et al., 2016) |
| In vivo | Insulin resistance | Mulberry leaf extract and its Formulation | BW and high-energy diet | SD rats | IRS-1, JNK | (Liu et al., 2016) |
| In vivo | Insulin resistance | Parkinsonia aculeata (Caesalpineaceae) | High-fat diet | Mice | AMPKα | (Araujo et al., 2016) |
| In vivo | Insulin resistance | The fruit of Acanthopanax senticosus (Rupr. et Maxim). Harms | High-fat diet | Mice | AMPK | (Saito et al., 2016) |
| In vitro | Type 2 diabetic mellitus | Nymphaea nouchali Burm. f. (Family - Nymphaeaceae) | MDI | 3T3-L1 adipocytes | PPARγ, GLUT4 | (Parimala et al., 2015) |
| In vivo | Type 2 diabetic mellitus | Cordyceps militaris number 1 (CmNo1) | High-fat diet | Mice | IRS-1, Akt, GLUT4 | (Yu et al., 2015) |
| In vitro | Insulin resistance e | 6α-Hydroxylup-20(29)-en-3-on-28-oic acid (1) | Dexamethasone (DXM) | 3T3-L1 adipocytes | PI3K/Akt, GLUT4 | (Qin et al., 2015) |
| In vivo | Insulin resistance and hyperlipidemia | Ergostatrien-3β-ol (EK100) | High-fat diet | C57BL/6J mouse | GLUT4, AMPK | (Kuo et al., 2015) |
| In vitro | Insulin resistance | Coptidis Rhizoma extracts (CRE) | Tumor necrosis factor-α | 3T3-L1 adipocytes | PPAR-γ, IRS-1 | (Yuan et al., 2014) |
| In vivo | Metabolic syndrome | Gastrodia elata Blume (EGB) | High-fructose diet | Rats | AMPK | (Kho et al., 2014) |
| In vivo | Adipose dysfunction and insulin resistance | Cyclocarya paliurus leaves extracts (CPE) | Mac-CM | Mice | IRS-1, Akt | (Jiang et al., 2014) |
| In vivo and in vitro | Diabetic | Oroxylum indicum Vent. (OI) | STZ | Rats and 3T3-L1 adipocytes | GLUT4 | (Singh and Kakkar, 2013) |
| In vivo | Diabetic | Citrus sinensis fruit peel (CSMe) | High-fat diet and STZ | Rats | PPARγ, GLUT4 | (Sathiyabama et al., 2018) |
| In vivo | Type 2 diabetic mellitus | Nardostachys jatamansi DC extract (NJE) | Pelletized commercial chow diet | C57BL/KsJ-db/db mice | AMPK | (You et al., 2018) |
| In vivo and in vitro | Type 2 diabetic mellitus | Catalpol | High-fat diet and STZ/glucosamine administration | C57BL/6J mice/HepG2 cells | PI3K/Akt, AMPK | (Yan et al., 2018) |
| In vivo | Insulin resistance | Ethyl acetate fraction (EAF) | High-fat diet, STZ,nicotinamide | Rats | IRS1, Akt | (Ooi et al., 2018) |
| In vivo and in vitro | Obesity | Epigallocatechin-3-Gallate-Rich Green Tea Extract | High-fat diet | Male c57BL/6 mice/HepG2 cells | AMPK | (Bae et al., 2018) |
| In vivo | Type 2 diabetic mellitus | M. charantia ethanol extracts (MCE) | High-fat diet, streptozotocin (STZ) | Rats | JNK, GLUT4 | (Ma et al., 2017) |
| In vivo and in vitro | Insulin resistance | Caffeic acid phenethyl ester (CAPE) | – | Mice and HepG2 Cell | JNK, NF-κB, IRS1 | (Nie and Chang, 2017) |
| In vivo | Type 2 diabetic mellitus | Aged garlic extract (AGE) | – | Tsumura Suzuki mice | AMPK | (Miki et al., 2017) |
| In vivo and in vitro | Insulin resistance/type 2 diabetic mellitus | Sea buckthorn fruit oil is rich in palmitoleic acid (POA) | – | HepG2 cells/SD rats | PI3K/Akt, GSK-3β | (Gao et al., 2017) |
| In vitro | Insulin resistance | Opuntia ficus-indica var. saboten (OFS) | DMEM | L6 muscle cells | AMPK, p38 MAPK, GLUT4 | (Leem et al., 2016) |
| In vivo | Obesity | P. grandiflorus root ethanol extract (PGE) | High-fat diet | C57BL/6J mice | PPARα, PPARγ, | (Kim et al., 2016) |
| In vivo | Obesity | Parkinsonia aculeata (HEPa/EtOAc) | High-fat diet | C57BL/6J mice | AMPK | (Araujo et al., 2016) |
| In vivo and in vitro | Insulin resistance | Rhizoma Anemarrhenae extract (TFA) | STZ | Mice/3T3-L1 and Hela cells | AMPK | (Han et al., 2015) |
| In vivo and in vitro | Insulin resistance | Toona Sinensis leaf (TSL) | High-fat diet/AS160 | Mice/C2C12 myotubes | AMPK, PPARγ | (Liu H. et al., 2015) |
| In vitro and in vivo | Type 2 diabetes mellitus | Fumosorinone (FU) | – | HepG2 cells/KKAy mice | IRS2, Akt, GSK3β, PTP1B | (Liu Z. et al., 2015) |
| In vitro and in vivo | Insulin resistance | Ginseng berry extract (GBD) | DMEM | C57BL/6 mice/C2C12 cell | PPARγ. IRS1, Akt | (Yang et al., 2015) |
| In vivo | Type 2 diabetes mellitus | Bitter melon (BM; Momordica charantia) | High-fat diet | OLETF rats | NF-κB, JNK | (Seo et al., 2015) |
| In vivo | Obesity | Ginkgo biloba extract (GbE) | High-fat diet | Rats | IRS1, PTP1B | (Banin et al., 2014) |
| In vivo | Metabolic syndrome | Zingiber officinale | High-fat high-carbohydrate diet | Rats/L6 skeletal muscle cells | AMPK | (Li Y. et al., 2014) |
| In vitro and in vivo | Insulin resistance | M. koenigii (MK) | High-fat diet | Mice/L6 skeletal muscle cells | GLUT4, Akt | (Pandey et al., 2014) |
| In vivo | Obesity | Artemisia scoparia (SCO) and Artemisia santolinifolia (SAN) | High-fat diet | C57BL/6J mice | PPARγ | (Richard et al., 2014) |
| In vitro | Insulin resistance | Cinnamon extract (CE) | – | 3T3-L1 adipocytes and C2C12 myocytes | AMPK | (Shen et al., 2014) |
| In vivo | Obesity | Extracts of Artemisia santolinaefolia (SANT) and Artemisia scoparia (SCO) | High-fat diet | C57/B6J mice | AMPK | (Wang et al., 2013) |
Table 6.
Herbal formula that improves insulin resistance.
| Type | Model | Herbal formula | Inducer | Animal/cell | Major findings | References |
|---|---|---|---|---|---|---|
| In vivo | Type 2 diabetic mellitus | SGY preparation | High-fat diet and STZ | db/db mice | PI3K/Akt, IRS-1, GLUT4 | (Xing and Chen, 2018) |
| In vivo | Type 2 diabetic mellitus | Dai-Zong-Fang | − | db/db mice | Akt, IRS-1, AMPK, GLUT4 | (Zhu et al., 2018) |
| In vitro | Insulin resistance | Zengye Decoction (ZYD) | Insulin-induced | HepG2 cells | AMPK | (Liu Z. et al., 2018) |
| In vivo | Diabetic | Jia-Wei-Jiao-Tai-Wan (JWJTW) | STZ and a high-sucrose-high-fat diet | Rats | IRS, PI3K, GLUT4 | (Chen et al., 2017) |
| In vivo | Insulin resistance and nonalcoholic fatty liver disease | Seyoeum (SYE) | High-fat diet | C57BL/6 mice | IRS-1, IRS-2 | (Na et al., 2017) |
| In vitro | Insulin resistance | Wu-Mei-Wan | Palmitate | HepG2 cells | PI3K/Akt, GLUT4, IRS | (Yang et al., 2017) |
| In vivo and in vitro | Type 2 diabetic mellitus | Preparation JQ-R | Palmitic acid | KKAy mice and cells | NF-κB, PI3K/AKt, JNK, MAPK | (Liu et al., 2017) |
| In vivo | Type 2 diabetic mellitus | Fenugreek seed and mulberry leaf | High-fat diet and alloxan | Rats | GLUT4 | (Kan et al., 2017) |
| In vivo and in vitro | Insulin resistance | Erchen Decoction and Linguizhugan Decoction | High-fat diet | Rats | NF-κB, IRS-1 | (Zhang H. et al., 2017) |
| In vivo | Chronic partial sleep deprivation, obesity-resistant | Jiao-Tai-Wan (JTW) | High-fat, high-energy diet, environmental noise | SD rats | NF-κB | (Zou et al., 2017) |
| In vivo | Metabolic syndrome | Modified lingguizhugan decoction | High-fat diet | Rat | Akt | (Yao et al., 2017) |
| In vivo | Type 2 diabetic mellitus | Jiang Tang Xiao Ke (JTXK) granule | High-fat diet and STZ | KKAy mice | PI3K/Akt, IRS-1, GLUT4, GSK3β | (Yu et al., 2017) |
| In vivo | Insulin resistance | Jiangzhi Capsule | Liquid fructose | Rats | GLUT4, Akt | (Jiang L. et al., 2016) |
| In vivo | Type 2 diabetes mellitus | Liuwei Dihuang decoction | High-fat diets and STZ | SD rats | PI3K/Akt, IRS2 | (Dai et al., 2016) |
| In vivo | Type 2 diabetic mellitus | ZiBu PiYin Recipe (ZBPYR) | High-fat diets and STZ | Rat | GSK3β | (Sun Z. et al., 2016) |
| In vivo | Insulin resistance | Jinlida | High-fat diet | Mice | IRS-1 | (Jin et al., 2015) |
| In vivo | Polycystic ovary syndrome | Shouwu Jiangqi Decoction (SJD) | Sodium sulfate prasterone, high-fat diet | SD rats | IRS-1, PI3K | (Wang et al., 2016) |
| In vivo | Pre-diabetic | Tang-Nai-Kang (TNK) | Rats | AMPK, PPARγ | (Li et al., 2015) | |
| In vivo | Insulin resistance | Jinlida (JLD) | High-fat diet | Rats | JNK, p38MAPK | (Liu Y. et al., 2015) |
| In vitro | Insulin resistance | Modified Si-Miao-San (mSMS) | Conditioned medium derived from activated macrophages | 3T3-L1 adipocytes | NF-κB, AMPK, PI3K, IRS-1 | (Yang J. et al., 2014) |
| In vivo | Diabetic atherosclerosis | Gal-geun-dang-gwi-tang (GGDGT) | Western diet | (ApoE-/-) mice | IRS-1 | (Lee et al., 2014) |
| In vivo and in vitro | Insulin resistance | Gyeongshingangjeehwan 18 (GGEx18) | High-fat diet | C57BL/6J mice and 3T3-L1 adipocytes | AMPK, PPARα | (Oh et al., 2015) |
| In vivo and in vitro | Insulin resistance | Salvia-Nelumbinis naturalis (SNN) | HC diet | HepG2 cells and rats | Akt, IRS | (Zhang et al., 2014) |
| In vivo | Insulin resistance | Kangen-karyu and Salviae Miltiorrhizae Radix | − | Rats | PI3K/Akt, p38MAPK, NF-κB | (Park C. et al., 2014) |
| In vivo | Polycystic ovarian syndrome | Bushen Huatan Recipe (BHR) | Dehydroeplandrosterone | Rats | Akt, GSK-3β, GLUT4, IRS-1, PPAR-γ | (Hong and Wu, 2014) |
| In vivo and in vitro | Metabolic syndrome | Fu Fang Zhen Zhu Tiao Zhi formula (FTZ) | High insulin and high-fat diet | HepG2 cells and rats | PI3K, IRS-1 | (Hu et al., 2014) |
| In vivo | Type 2 diabetic mellitus | Fructus Mume formula and its separated prescription | High-fat diet and STZ | Rats | IRS-1, GlUT-4 | (Li et al., 2013) |
| In vivo | Insulin resistance | Refined-JQ (JQ-R) | High-fat diet | C57BL/6J mice | AMPK | (Gao et al., 2014) |
| In vivo | Type 2 diabetes mellitus | TZQ-F | High-fat diet | KKA(y) mice | PPARγ, IRS-1, IRS-2, GLUT1, PI3K | (Nan Xia et al., 2013) |
| In vivo | Type 2 diabetes mellitus | Jiaotai Pill (JTP) | STZ and high fat diet | Rats | PI3K, IRS-1, GLUT4 | (Dong et al., 2013) |
| In vivo | Polycystic ovary syndrome | Heqi San | Dehydroepiandrosterone | Female SD rats | PI3K/Akt, GLTU4 | (Zhao et al., 2017) |
| In vivo | Type 2 diabetes mellitus | Fructus Mume formula | High-fat diet and STZ | Rats | IRS-1, GLUT4 | (Li et al., 2013) |
Insulin Receptor Substrate Signal Transduction
The insulin receptor belongs to the subfamily of receptor tyrosine kinases, including insulin-like growth factor 1 receptors and insulin receptor-related receptors (White, 2003). Most insulin signals promote or regulate phosphorylation of IRS-1 or its homolog IRS-2 via tyrosine (Haeusler and Accili, 2008), and IRS-1 is the major substrate of the insulin receptor. IRS mediates insulin action differently in different tissues, with IRS-1 playing a prominent role in skeletal muscle and IRS-2 in the liver (Kido et al., 2000). Studies have shown that liver IRS-1 and IRS-2 have complementary effects in controlling liver metabolism; IRS-1 is more closely related to glucose homeostasis (Bouzakri et al., 2006), and IRS-2 is more closely related to lipid metabolism (Taniguchi et al., 2005). Insufficient expression of IRS-1 and IRS-2 can lead to IR (Tamemoto et al., 1994; Shimomura et al., 2000). Jinlida particles can raise insulin sensitivity in skeletal muscle in fat-induced insulin-resistant ApoE-/- mice by increasing the expression of IRS-1 mRNA and protein (Jin et al., 2015). Treating high insulin-induced HepG2 cells with FTZ in vitro upregulated the expression of IRS-1 protein while attenuating in vitro glucose levels (Hu et al., 2014). Abnormal phosphorylation of IRS is also an important mechanism of IR.
IRS1 and IRS2 appear to lack intrinsic catalytic activity but contain many serine and tyrosine phosphorylation sites (White, 2003). Serine/threonine phosphorylation of IRS-1 at the phosphorylation site Ser307 may inhibit insulin signaling (Rui et al., 2001) and attenuate tyrosine phosphorylation levels (Saad et al., 1992). Salvia-Nelumbinis naturalis (SNN) improves hepatic insulin sensitivity in rats and increases IRS phosphorylation (Zhang et al., 2014). Tumor necrosis factor-α (TNF-α) reduces insulin receptor substrate tyrosine phosphorylation and is an important mediator of IR in obesity and diabetes (Hotamisligil et al., 1996). Erchen decoction and Linguizhugan decoction reduce the level of TNF-α in diet-induced insulin-resistant rats to improve IR (Zhang H. et al., 2017), similar to the pharmacological action of thiazolidinedione (Peraldi et al., 1997). In addition, degenerative neuropathies such as Alzheimer’s disease (Talbot and Wang, 2014) and multiple system atrophy (Bassil et al., 2017) are also closely related to brain IR caused by blocked IRS signaling. Defects in IRS-1 may cause vascular damage and accelerate the progression of atherosclerosis (Abe et al., 1998), while IRS-2 delays neointimal formation under IR (Kubota et al., 2003). Gal-geun-dang-gwi-tang attenuates endothelial dysfunction by promoting nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling and improves insulin sensitivity in individuals with diabetic atherosclerosis. Gal-geun-dang-gwi-tang was also shown to be associated with restored expression of IRS-1 in the thoracic aorta and skeletal muscle (Lee et al., 2014).
PI3K/Akt Signaling Pathway Signal Transduction
Glucose is mainly metabolized in insulin-sensitive tissues by two pathways: the classical phosphatidylinositol 3-kinase (PI3K) pathway and the 5’-AMP activating kinase (AMPK) signal transduction pathway (Jeong et al., 2017). Tyrosine phosphorylation of the insulin receptor substrate activates PI3K, and activated PI3K catalyzes 4,5-2 phosphatidylinositol (PIP2) and produces PIP3, which acts as a second messenger that activates Akt (White, 2003). Activated Akt promotes downstream molecules that regulate metabolism. Liuwei Dihaung decoction can be used to treat IR by regulating the PI3K/Akt signaling pathway in the liver of rats with T2D, accompanied by phosphorylation and upregulation of PI3K/Akt pathway-associated proteins (Dai et al., 2016). PI3K is composed of a regulatory subunit p85 and a catalytic subunit p110 (Geering et al., 2007) and has dual activities of phosphatidylinositol kinase and serine/threonine (Ser/Thr) protein kinase. Heterozygous mutations in the PI3Kp85 regulatory subunit gene often result in metabolic disorders such as IR associated with decreased ability to activate PI3K in muscle and adipose tissue (Winnay et al., 2016). Significant damage to PI3K signaling in muscle often results in muscle IR and systemic glucose intolerance (Luo et al., 2006). Central glucagon-like peptide 2 can enhance hepatic insulin sensitivity by activating G3-2R-p85a interactions in PI3K signaling in proopiomelanocortin neurons (Shi et al., 2013). Different proportions of Jiaotai Pill enhance PI3K pathway insulin signaling by upregulating the expression of the PI3K p85 subunit in skeletal muscle, attenuating the development of diabetes in a rat model of T2D (Dong et al., 2013). Akt has three isoforms, of which Akt1 and Akt2 are highly expressed in skeletal muscle and have unique and overlapping functions (Matheny et al., 2018). Overexpression of Akt1 is associated with increased beta cell size and total islet mass (Tuttle et al., 2001), and Akt2 is more important for insulin-stimulated glucose metabolism (Bouzakri et al., 2006). Defects in signaling pathways caused by mutations in the protein kinase Akt2/PKB gene often impair the ability of insulin to lower blood glucose in the liver and skeletal muscle (George et al., 2004). Paeoniflorin improves TNF-α-induced IR in adipocytes and is associated with insulin-stimulated Akt phosphorylation recovery in adipocytes (Kong et al., 2013). Akt2 is involved in glucose uptake by insulin-regulated muscle and adipocytes by promoting the transport of GLUT-4 to the cell surface (Ng et al., 2008), as is metformin, which also increases liver Akt phosphorylation and promotes GLUT-4 translocation (Garabadu and Krishnamurthy, 2017). Jiangzhi capsule improved fructose-induced IR and repaired the damaged muscle fiber membrane GLUT-4 cycle by regulating the ratio of phosphorylated Akt to total Akt in the gastrocnemius muscle (Jiang L. et al., 2016). Akt2 is required for hepatic lipid accumulation in obese and insulin-resistant states induced by leptin deficiency or high-fat diet (HFD) (Leavens et al., 2009). Modified lingguizhugan decoction improves liver fat accumulation and IR in rats with metabolic syndrome by inhibiting abnormal increases in leptin and PKB in the liver (Yao et al., 2017).
Glucose Transporters Signal Transduction
There are currently 13 known sugar transporter proteins (GLUT1-12 and HMIT) encoded in the human genome (Joost and Thorens, 2001). Based on sequence similarity and characteristic elements, the extended GLUT family can be divided into three subfamilies, of which class I contains the glucose transporters GLUT1-4, which all show tissue/cell specific expression (Wood and Trayhurn, 2003). GLUT-4 is highly expressed in adipose tissue and skeletal muscle (Bogan, 2012). The main cellular mechanism for handling exogenous glucose load is insulin-stimulated glucose transport into skeletal muscle, and the primary glucose transporter that mediates this uptake is GLUT-4 (Huang and Czech, 2007). Oxidative stress caused by overnutrition often induces GLUT-4 inactivation by carbonylation and oxidation (Boden et al., 2015), and related gene variants (Stenbit et al., 1997) often lead to GLUT-4 deficiency and downregulation in skeletal muscle and adipose tissue (Zisman et al., 2000; Abel et al., 2001). Exercise and caloric restriction can upregulate GLUT-4 gene expression and increase insulin-induced GLUT-4 transport to the plasma membrane (Richter and Hargreaves 2013; Zanquetta et al., 2003). A novel botanical formula containing standardized extracts of mulberry leaf, fenugreek seed, and American ginseng can attenuate the decrease in GL UT-4 expression induced by an HFD and alloxan (Kan et al., 2017). A key step in the physiological role of GLUT-4 is translocation (Bai et al., 2007), in which GLUT-4 is redistributed from the intracellular pool to the plasma membrane under the regulation of the Akt substrate AS160 rab GTPaseactivating protein (Sano et al., 2007). Studies have shown that cold exposure therapy can significantly increase GLUT-4 translocation in basal skeletal muscle and may be a potential treatment for diabetes (Hanssen et al., 2015). Oroxylum indicum stem bark extract significantly enhanced insulin sensitivity in mature 3T3-L1 adipocytes, as evidenced by increased skeletal muscle GLUT-4 translocation (Singh and Kakkar, 2013). GLUT-2 is mainly found in the liver, intestine, kidney, and pancreatic beta cells (Wood and Trayhurn, 2003), and it plays an important role in glucose transport and energy metabolism because it promotes glucose uptake or liver efflux (Thorens et al., 1992). Sang-Tong-Jian, a new formulation of flavonoids and alkaloids from mulberry leaves, improves IR in KKAy mice, which is associated with upregulation of GLUT-2 (liver) gene, and protein expression (Kuai et al., 2016). GLUT-1 is expressed at the highest level in the endothelial tissues of barrier tissues such as blood vessels and blood-brain barriers, and moderate levels of expression are also observed in adipose tissue, muscle, and liver, which play an important role in supplying glucose to organs such as the brain (Deng et al., 2014; Tang et al., 2017). Chinese herbal formula TZQ-F treatment upregulates the expression of related proteins such as GLUT-1, which regulates the potency of insulin action and is beneficial for reducing hyperinsulinemia (Nan Xia et al., 2013).
AMP-Activated Protein Kinase Signal Transduction
AMPK is a conserved, ubiquitously expressed heterotrimeric serine/threonine protein kinase (Kahn et al., 2005) that plays a key role in regulating cellular energy metabolism (Lage et al., 2008). AMPK integrates nutrient and hormonal signals in peripheral tissues and the hypothalamus and is regulated by multiple hormones such as leptin, adiponectin, ciliary neurotrophic factor, and ghrelin (Minokoshi et al., 2002; Watt et al., 2006; Zhao L. et al., 2015). AMPK plays an important role in regulating food intake, body weight, glucose, and lipid metabolism (Minokoshi et al., 2004). In addition, AMPK can also be activated in response to cellular stress, exercise (Cantó et al., 2010), and drugs (Sasaki et al., 2009). Based on traditional Chinese medicine, Dai-Zong-Fang improves insulin sensitivity in db/db diabetic mice by inhibiting liver lipids and enhancing energy metabolism in skeletal muscle by inhibiting AMPK activation (Zhu et al., 2018). Under physiological conditions, AMPK is mainly present in an inactive form complexed with Mg-ATP, which is more abundant than AMP (Xiao et al., 2011). An increase in AMP concentration activates AMPK, and by phosphorylating the activation loop within the kinase domain (Li et al., 2011), AMPK transitions from an inactive form to a catalytic form: binding of AMP to the c-regulatory domain promotes phosphorylation of the upstream kinase and causes allosteric activation, and inhibition of the dephosphorylation of Thr172 in the kinase domain activation loop regulates AMPK phosphorylation levels (Xiao et al., 2007). Goka fruit supplements improve IR and liver lipid accumulation in mice with HFD-induced obesity by increasing AMPK phosphorylation in the liver. AMPK in adipocytes is critical for maintaining mitochondrial integrity, responding to pharmacological agents and heat stress, and protecting against nutrient overload-induced NAFLD and IR (Kishton et al., 2016; Mottillo et al., 2016). Modified Si-Miao-San positively regulates AMPK phosphorylation to promote basal glucose uptake by 3T3-L1 adipocytes and beneficially improves insulin signaling by inhibiting inflammation in adipocytes (Yang J. et al., 2014). Adiponectin is a major insulin-sensitized adipokine (Kadowaki and Yamauchi, 2011). It has been shown that adiponectin and its receptors AdipoR1 and AdipoR2 enhance glucose and fatty acid metabolism by activating AMPK in peripheral tissues (Kubota et al., 2007). The herbal composition GGEx18 can treat visceral obesity and visceral obesity-related IR by upregulating visceral fat expression of fatty acid oxidation genes. The results show that the expression of fatty acid oxidation genes, including genes encoding adiponectin, AMPK, and others, is significantly increased in mesenteric adipose tissue of 3T3-L1 adipocytes and obese mice (Oh et al., 2015).
Glycogen Synthase Kinase 3 Signal Transduction
GSK-3 is a ubiquitous cytosolic serine/threonine protein kinase expressed in mammalian tissues as two closely related isoforms: GSK-3α and GSK-3β (Dajani et al., 2001). GSK-3 is constitutively active under resting conditions and regulates human metabolism through phosphorylation of glycogen synthase and other substrates (Kaidanovich and Eldar-Finkelman, 2002). Inhibition of GSK-3 is required for insulin-stimulated glycogen and protein synthesis, and its inhibition is critical for the normal functioning of insulin-activated signaling pathways (Ali et al., 2001). The skeletal muscle GSK-3 activity and its expression level were significantly increased in patients with T2D (Saltiel and Kahn, 2001), and abnormally excessively elevated GSK-3 resulted in further inhibition of glycogen synthase activity. A large body of evidence indicates that GSK-3 inhibitors have therapeutic uses in neurodegenerative diseases, cancer, and T2D (Patel and Woodgett, 2008; Hur and Zhou, 2010; Martinez et al., 2002). Jiangtangxiaoke granules are an effective drug for T2D, since this treatment can regulate the expression of glycogen synthase kinase 3β (GSK3β) by regulating the PI3K/Akt signaling pathway in skeletal muscle of mice with T2D (Yu et al., 2017). Insulin-induced GSK-3 inhibition is mediated through its downstream target protein kinase B (PKB), which phosphorylates and inactivates GSK-3 at Ser9/21 (Cross et al., 1995; Frame et al., 2001). ZiBu PiYin recipe treatment alters insulin signaling in T2DM rats in association with inhibited GSK3β overexpression resulting from increased p-GSK3β levels in the pre-frontal cortex and hippocampus (Sun Z. et al., 2016). Abnormal overactivity of GSK-3 may also limit IR-mediated signaling through phosphorylation of IRS-1. Polydatin significantly increased phosphorylated GSK-3β and increased protein levels of phosphorylated IRS in liver and insulin-resistant HepG2 cells of diabetic rats (Hao et al., 2014). Therefore, GSK-3 inhibitors can be a promising new drug for diabetic IR.
P38 Mitogen-Activated Protein Kinase Signal Transduction
MAPKs and their downstream targets are important signaling modules for cellular responses to changes in the physical and chemical properties of the environment (Cuenda et al., 2017). It is known that MAPK has at least four subfamilies in mammalian cells: p38 kinase (p38α, β, γ, and δ), extracellular signal-regulated kinase (ERK1/2), ERK5, and Jun amino terminal kinase (JNK1-3) (Gehart et al., 2010). Insulin activates the PI3K/Akt pathway, which is responsible for glucose uptake, and the MAPK pathway, which is critical for IR (Saltiel and Kahn, 2001). MAPK is involved in a variety of processes that control hepatic metabolism (Lawan and Bennett, 2017). Obesity and inflammation-related stress responses in insulin-responsive tissues activate liver MAPKs, which are thought to impair insulin action and lipid metabolism (Hotamisligil and Davis, 2016). MAPK phosphatases (MKPs) can dephosphorylate MAPK to catalyze the inactivation of MAPK (Gehart et al., 2010). Baicalin plays an important role in reversing HFD-induced glucose intolerance and IR in diet-induced obese mice, and its mechanism is associated with downregulation of p-p38 MAPK levels (Fang et al., 2018). Reactive oxygen species (ROS)-mediated activation of p38MAPK stress response signaling has been recognized as one of the causes of insulin signaling damage and hepatic IR (Al-Lahham et al., 2016). Jinlida, a compound preparation based on traditional Chinese medicine, can attenuate oxidative stress and reduce phosphorylation of p38MAPK and JNK in high-fat fed rats, showing antioxidant effects and upregulation of insulin signaling (Liu Y. et al., 2015).
C-Jun-N-Terminal Kinase Signal Transduction
The JNK is a member of the MAPK family, and three JNK isoforms exist in mammals: JNK1, JNK2, and JNK3 (Lawan and Bennett, 2017). JNK1 and JNK2 are expressed in almost all celRls, including liver parenchymal cells, while JNK3 is mainly expressed in the brain, heart, and testis (Seki et al., 2012). Cytokines, ROS, endoplasmic reticulum stress, and free fatty acids activate JNK (Ozcan et al., 2004; Holzer et al., 2011; Zhao H. et al., 2015) and play a key role in metabolic disorders such as obesity, IR, and T2D (Vallerie and Hotamisligil, 2010). Lycium barbarum polysaccharide treatment effectively inhibits phospho-JNK levels in HFD-fed mice and reduces ROS levels via the PI3K/AKT/Nrf2 axis, acting as a novel anti-hyperlipide-induced IR oxidizer (Yang Y. et al., 2014). Stress factors such as non-esterified fatty acids are generally thought to induce inhibitory serine phosphorylation of IRS-1 through the JNK pathway and impair insulin signaling (Hirosumi et al., 2002; Gao et al., 2018). Studies have also confirmed that hepatocyte-specific deletion of JNK1 enhances IR (Sabio et al., 2009). Therefore, JNK may positively regulate hepatic insulin signaling, and in other insulin-sensitive organs, JNK negatively regulates insulin action, especially under stress conditions. This is the potential protective effect of mulberry leaf extract (MLE) and a formula consisting of MLE, fenugreek seed extract, and cinnamon cassia extract (MLEF) on hyperglycemia induced by high-energy diet and toxic chemicals in rats and recovery of insulin sensitivity, the most likely mechanism is the upregulation of phosphorylation of JNK and other related proteins in the liver to promote IRS-1 phosphorylation (Liu et al., 2016).
Nuclear Factor-kappaB Signal Transduction
NF-κB is a sequence-specific transcription factor that is a major regulator of inflammatory responses, including responses to inflammation and oxidative stress (Chiang et al., 2009). In the quiescent state, NF-κB binds to the inhibitor subunit IκB in an inactive form in the cytoplasm. IKK-β is required for activation during acute inflammation. Phosphorylation-activated IKKβ induces phosphorylation of IκB kinase α (Arkan et al., 2005) and IκBα phosphorylates IκB and leads to proteolysis of IκB, which exposes nuclear recognition sites for NF-κB. NF-κB is translocated into the nucleus, resulting in the expression of related target genes such as inflammatory cytokines (Cai et al., 2005). Thus, blocking NF-κB signaling improves IR and prevents the development of diabetes (Wang et al., 2014). Jiao-Tai-Wan, composed of Rhizome Coptidis and Cortex Cinnamomi, reversibly increases markers of systemic inflammation and IR caused by sleep loss in Sprague-Dawley rats, and these changes are related to downregulation of NF-κB mRNA expression in peripheral blood mononuclear cells (Zou et al., 2017). NF-κB may represent an attractive therapeutic target for obesity, IR, diabetes, and other complications associated with these diseases.
Protein Tyrosine Phosphatase 1B Signal Transduction
Protein tyrosine phosphatase 1B belongs to the protein tyrosine phosphatase (PTP) family (Tiganis, 2013) and has a catalytic domain characterized by an 11 amino acid sequence motif containing cysteine (Cys215) and arginine (Arg221) (Haque et al., 2011). These residues are critical to the catalytic activity of the enzyme. As a negative regulator of the insulin signaling cascade, PTP1B overexpression inhibits tyrosine phosphorylation of IR and IRS-1, enhances serine phosphorylation, thereby terminating insulin signaling (Johnson et al., 2002). Oligonol, a low molecular weight polyphenol mixture derived from lychee fruit, can significantly reduce PTP1B expression and reduce serine phosphorylation of IRS-1, improving insulin sensitivity in insulin-resistant HepG2 cells (Bhakta et al., 2017). In general, PTP1B inhibitors are a promising class of insulin sensitizers.
Nuclear Factor-E2-Related Factor 2 Signal Transduction
Nuclear factor erythrocyte 2-related factor 2 (Nrf2) is a key regulator of antioxidant signaling and plays a crucial role in maintaining redox homeostasis (Seo and Lee, 2013). Under physiological conditions, Nrf2 remains in the cytoplasm by binding to the endogenous inhibitor Keap1, which mediates rapid activation of the proteasome and subsequent degradation of Nrf2 (Zhang et al., 2015). Under exogenous and endogenous oxidative stress, Nrf2 becomes stable and released from the Keap1/Nrf2 complex, Nrf2 degradation is inhibited, and Nrf2 accumulates in the nucleus, synergistically enhancing the expression of various genes encoding antioxidant enzymes (Bhakkiyalakshmi et al., 2015). Many studies have shown that increased Nrf2 signaling can inhibit oxidative stress and improve insulin and leptin resistance (Yagishita et al., 2017). L. barbarum polysaccharide, an antioxidant from wolfberry, increases Nrf2 phosphorylation in livers of HFD-fed mice and HepG2 cells by inducing PI3K/AKT signaling and induces Nrf2/ARE signaling to reduce oxidative stress and maintain peripheral insulin sensitivity (Yang Y. et al., 2014).
Peroxisome Proliferator-Activated Receptor Signal Transduction
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors involved in the transcriptional control of genes encoding proteins involved in adipocyte differentiation, lipid and carbohydrate metabolism, and adipokine synthesis, including three isoforms encoded by different genes (Eldor et al., 2013). PPARα is highly expressed in liver, kidney, and skeletal muscle. PPARγ is also highly expressed in various cell types and organs, including fat cells, muscle cells, liver, and kidneys, and is considered to be a major regulator of glucose homeostasis (Haluzík and Haluzík, 2006). Studies have suggested that the production of future PPARα and γ double agonists will simultaneously bring about favorable changes in PPARα lipid mass spectrometry and blood glucose benefits of PPARγ agonists (Massaro et al., 2016). The existing insulin-sensitizing drug thiazolidinedione is a potent agonist of nuclear PPAR-γ (Hevener et al., 2003). Based on the traditional anti-diabetic formula, TZQ-F can improve IR in KKA(y) mice through its efficacy in regulating adipocyte differentiation and insulin action, and the results indicate that its therapeutic effect is related to the upregulation of PPARγ expression in liver tissue (Nan Xia et al., 2013) and PPARγ coactivator 1α (PGC1α), which is a PPAR-mediated transcriptional coactivator of fatty acid oxidation (Koo et al., 2004). Tang-Nai-Kang is a mixture of five herbal plant extracts that has been shown to improve glucose metabolism abnormalities in patients with pre-diabetes. Tang-Nai-Kang treatment can deacetylate PGC1α to activate it and synergize with PPAR expression to enhance fatty acid oxidation and improve insulin levels in rats (Li et al., 2015).
Conclusions and Perspectives
IR is a pathological condition common to many metabolic diseases; the most well known of which is T2D. Some surveys show that China is one of the countries with the highest incidence of T2D in the world, and the number of people with diabetes in the adult population have exceeded 113.9 million in 2010 (Zheng et al., 2018). The global trend of other metabolic diseases associated with IR is also not optimistic. From 2003 to 2012, the overall prevalence of metabolic syndrome in the United States was 33% (Aguilar et al., 2015). It is estimated that nearly 100 million people in the United States have NAFLD (Rinella, 2015). Global Burden of Disease studies have shown that the prevalence of obesity has doubled in 73 countries between 1980 and 2015 (Inoue et al., 2018). In addition, based on the 1990 National Institutes of Health standard, PCOS affects 6–10% of women worldwide (Goodarzi et al., 2011), and studies have indicated that 56.3% of Han women in China with PCOS have IR (Li et al., 2018). Therefore, in the face of such a large potential patient population, the harm caused by IR cannot be ignored. Herbs have been used in China for more than 2,000 years and are still considered effective drugs to prevent and treat various diseases. The discovery and application of artemisinin, a compound derived from Artemisia annua, is a good example. In recent years, research on Chinese medicine has gradually been standardized and systematized. China’s latest guidelines for preventing and treating T2D are also the first to include Chinese medicine treatment.
The information presented in this review shows that herbal formulas, active ingredients, and natural products can be effective to improve IR. Targets of herbal compounds that affect insulin signaling include insulin receptor substrate, phosphatidylinositol 3-kinase, glucose transporter, AMPK, glycogen synthase kinase 3, MAPKs, JNK, NF-κB, protein tyrosine phosphatase 1B, nuclear factor-E2-related factor 2, and peroxisome proliferator-activated receptors.
In Table 7 , we have listed the composition of the above herbal formula in detail. In addition, we have found that certain herbs have a good application prospect in the treatment of IR. Such single botanicals include Coptis, Pueraria, Mulberry, Salvia, and others. Effective extracts of herbal medicines include berberine and mulberry leaves. There are also effective active ingredients such as berberine, ginsenoside, astragaloside, and resveratrol. The effects of these drugs are not only limited to enhancing insulin sensitivity, but also can be beneficial for improving systemic metabolism, such as reducing fasting blood glucose and postprandial blood glucose, improving blood lipid metabolism, reducing body weight, lowering blood pressure, and regulating female hormone secretion. Therefore, the role of herbal medicine in the treatment of IR is not only beneficial for treating T2D, but also provides new ideas for treating obesity, metabolic syndrome, PCOS, and NAFLD.
Table 7.
Composition of herbal formula.
| JTTZ formula | Aloe vera, Coptis chinensis, Rhizoma Anemarrhenae, red yeast rice, Momordica charantia, Salvia miltiorrhiza, Schisandra chinensis, Zingiber oj-jicinale Rosc. |
| Tangyiping granules (TYP) | Astragalus mongholicus 30 g, Paeoniae Radix Alba 12 g, Coptis chinensis 15 g, Salvia miltiorrhiza 12 g, Pinellia ternate 9 g, Pueraria lobata 30 g. |
| Jinlida (JLD) | Panax ginseng C. A. Mey., puerarin, pale white atractylodes rhizome, Coptis chinensis, poria cocos, radix polygonati officinalis, and so on |
| Qingxue Dan (QXD) | Scutellaria baicalensis Georgi, Coptis chinensis Franch., Platycladus orientalis (Linn). Franco, Gardenia jasminoides Ellis, Rhizoma of Rheum palmatum Linne |
| Qingre Yangyin Recipe (QRYYR) | Cortex Lycii, Rehmannia glutinosa Libosch, Ophiopogon japonicus (Linn. f). Ker-Gawl.,Cynanchum otophyllum, Salvia miltiorrhiza,Poria cocos(Schw).Wolf, Acorus tatarinowii, Alisma plantago-aquatica Linn., Lycium barbarum L.,Cuscuta chinensis Lam.,Epimedium sagittatum, Rubus idaeus L. |
| Sancaijiangtang powders | Panax Ginseng,Asparagus Racemosus, Radix, Rehmanniae,dark plum fruit, Cortex, Cinnamomi, Rhizoma Coptidis, |
| Jinlida | Panax ginseng C. A. Mey., Fallopia multiflora (Thunb). Harald, Atractylodes lancea (Thunb). DC, Sophora flavescens, Ophiopogon japonicus (Linn. f). Ker-Gawl., Rehmannia glutinosa Libosch, Fallopia multiflora (Thunb). Harald, Cornus officinalis Sieb. et Zucc., Poria cocos (Schw). Wolf, Eupatorium fortunei Turcz.,Coptis chinensis Franch., Anemarrhena asphodeloides Bunge, Epimedii Folium, Salvia miltiorrhiza Bge., Pueraria thomsonii Benth, Litchi chinensis Sonn., Cortex Lycii |
| Zhenggan Tang decoction | Pseudostellaria heterophylla (Miq). Pax ex Pax et Hoffm., Atractylodes macrocephala Koidz., Astragalus mongholicus Bunge, Poria cocos (Schw). Wolf, Angelica sinensis (Oliv). Diels, Salvia miltiorrhiza Bge., Trionyx sinensis Wiegmann, Radix Bupleuri, Cynanchum otophyllum |
| xin-ju-xiao-gao-fang (XJXGF) | Rheum palmatum L., Coptis chinensis Franch.,Cassia tora Linn., Citrus aurantium L. |
| Yiqi Huaju Recipe (YHR) | Astragali Radix 10 g, Coptis chinensis Franch. 3 g, Typha angustifolia L. 10 g, Artemisia capillaris Thunb 10 g, Alisma plantago-aquatica Linn. 10 g |
| Yangxin Tongmai Formula (YTF) | Radix Ginseng 10 g, Radix Salviae Miltiorrhizae 15 g, Ramulus Cinnamomi 6 g, Fructus Aurantii Immaturus 10 g, Rhizoma Alismatis 10 g |
| Sancai powder | Radix Ginseng, Radix Asparagi, Cochinchinensis, Radix Rehmanniae, Rhizoma Coptidis, Cortex Cinnamomi Cassiae, Fructus Mume |
| SGY preparation | Morus alba L., Pueraria thomsonii Benth (Leguminous), Dioscoreae rhizoma (Dioscoreaceae), Momordica charantia L. |
| Dai-Zong-Fang | Rhizoma Coptidis, Fructus Aurantii Immaturus |
| Zengye Decoction (ZYD) | Radix Scrophulariae, Radix Rehmanniae, Radix Ophiopogonis |
| Jia-Wei-Jiao-Tai-Wan (JWJTW) | Cinnamomum cassia, Rhizoma coptidis, Astragalus membranaceus, Herba Gynostemmatis, Radix Puerariae Lobatae, Folium Mori, Semen Trigonellae |
| Seyoeum (SYE) | Coix lacryma-jobi, Oryza sativa, Sesamum indicum, Glycine max, Liriope platyphylla, Dioscorea batatas |
| Wu-Mei-Wan | Lycium barbarumL., Angelica dahurica (Fisch. ex Hoffm.)Benth. et Hook. f. ex Franch. et Sav, Zingiber oj-jicinale Rosc.,Rhizoma coptidis, Angelica sinensis (Oliv). Diels, Ziziphus jujuba Mill., Zanthoxylum bungeanum Maxim.,Cinnamomum cassia, Panax ginseng C. A. Mey., Platycladus orientalis (Linn). Franco |
| Preparation JQ-R | Rhizoma Coptidis, Astragalus membranaceus, Lonicera japonica |
| Fenugreek seed and mulberry leaf | Morus alba L., Trigonella foenum-graecum L., Panax quinquefolius L. |
| Erchen Decoction and Linguizhugan Decoction | Pinellia ternata, Pericarpium Citri Reticulatae, Poria cocos (Schw). Wolf, Glycyrrhiza uralensis Fisch./Poria cocos, cassia twig, Rhizoma Atractylodis Macrocephalae, and licorice |
| Jiao-Tai-Wan (JTW) | Rhizome Coptidis, Cortex Cinnamomi |
| Modified lingguizhugan decoction | Poria cocos (Schw). Wolf, Cinnamomum cassia Presl, Atractylodes lancea (Thunb). DC., Glycyrrhiza uralensis Fisch., Codonopsis pilosula (Franch). Nannf., d Rheum palmatum L |
| Jiang Tang Xiao Ke (JTXK) granule | Radix rehmanniae, Fructuscorni, Radix salviae miltiorrhizae, Rhizoma coptidis, Radix Puerariae Lobatae, etc. |
| Jiangzhi Capsule | Radix Astragali, Poria cocos (Schw). Wolf, Folium Nelumbinis, Rhizoma Alisma, Fructus Crataeg, Fructus Chaenomelis, Radix et Rhizoma Salviae Miltiorrhizae, Radix et Rhizoma Notoginseng, Pollen Typhae, Rhizoma et Radix Polygoni cuspidate, Herba Taraxaci, Radix Polygoni multiflori, Fructus Ligustri Lucidi |
| Liuwei Dihuang decoction | Rehmannia glutinosa Libosch, Cornus officinalis Sieb, Dioscorea opposite Thunb, Alisma orientale Juz, Poria cocos Wolf, Paeonia suffruticosa Andrews |
| ZiBu PiYin Recipe (ZBPYR) | Red Ginseng, Common Yam Rhizome, Poria cocos (Schw). Wolf, Cynanchum otophyllum, Salvia miltiorrhiza Bge., Dolicho Lablab L., Nelumbo nucifera Gaertn., Acorus tatarinowii, Polygala tenuifolia Willd., Santalum album linn, Pericarpium Citri Reticulatae, Glycyrrhiza uralensis Fisch. |
| Jinlida | Panax ginseng C. A. Mey., puerarin, pale white atractylodes rhizome, Coptis chinensis, poria cocos, radix polygonati officinalis, etc. |
| Shouwu Jiangqi Decoction (SJD) | Fallopia multiflora (Thunb)., Harald, Astragalus membranaceus (Fisch)., Bunge., roasted Bombyx Batryticatus, Common Yam Rhizome, Euonymus alatus (Thunb). Sieb., Cyperus rotundus L., etc. |
| Tang-Nai-Kang (TNK) | Fructus Ligustri Lucidi, Prunella vulgaris L., Saururus chinensis (Lour). Baill, Psidium guajava Linn., Panax ginseng C. A. Mey. |
| Jinlida (JLD) | Panax ginseng C. A. Mey., Polygonatum sibiricum, Atractylodes Lancea (Thunb). DC., Sophora flavescens, Ophiopogon japonicus (Linn. f). Ker-Gawl., Rehmannia glutinosa Libosch, Fallopia multiflora (Thunb). Harald, Cornus officinalis Sieb. et Zucc., Poria cocos (Schw). Wolf., Eupatorium fortunei Turcz., Coptis chinensis Franch., Anemarrhena asphodeloides Bunge, Epimedium brevicornu Maxim., Salvia miltiorrhiza Bge., Pueraria thomsonii Benth, Litchi chinensis Sonn., Cortex Lycii |
| Modified Si-Miao-San (mSMS) | Coptis chinensis Franch., Phellodendron amurense Rupr., Semen Coicis, Atractylodes Lancea (Thunb). DC. |
| Gal-geun-dang-gwi-tang (GGDGT) | Pueraria thomsonii Benth, Glycyrrhiza uralensis Fisch., Angelica sinensis (Oliv). Diels, Ophiopogon japonicus (Linn. f). Ker-Gawl., Cynanchum otophyllum, Cornus officinalis Sieb. et Zucc., Chaenomeles sinensis (Thouin) Koehne, Rehmannia glutinosa Libosch, Nelumbo nucifera Gaertn, Dark Plum Fruit, Schisandra chinensis, Anemarrhena asphodeloides Bunge, Ligusticum chuanxiong Hort., Asparagus cochinchinensis (Lour.) Merr., Trichosanthes kirilowii Maxim, Cyperus rotundus L. |
| Gyeongshingangjeehwan 18 (GGEx18) | Laminaria japonica, Rheum palmatum L., Ephedra sinica Stapf |
| Salvia-Nelumbinis naturalis (SNN) | Salvia miltiorrhiza Bge., Nehlmbo nucifera,Reynoutria japonica Houtt., Artemisia capillaris Thunb |
| Kangen-karyu and Salviae Miltiorrhizae Radix | Cynanchum otophyllum, Ligusticum chuanxiong Hort., Carthamus tinctorius L., Cyperus rotundus L. Radix Aucklandiae, Salvia miltiorrhiza Bge./Salvia miltiorrhiza Bge. |
| Bushen Huatan Recipe (BHR) | Epimedii Folium, Curculigo orchioides Gaertn, Atractylodes Lancea (Thunb). DC., Pinellia ternata (Thunb). Breit.,Pericarpium Citri Reticulatae, Acorus calamus L., Cyperus rotundus L., Ligusticum chuanxiong Hort., Alisma plantago-aquatica Linn., Cervus nippon Temminc, Arisaema heterophyllum Blume, Amomum villosum Lour., etc. |
| Fu Fang Zhen Zhu Tiao Zhi formula (FTZ) | Fructus Ligustri Lucidi, Atractylodes macrocephala Koidz., Salvia miltiorrhiza Bge., Coptis chinensis Franch., Panax notoginseng (Burk). F.H. Chen, Eucommia ulmoides, Cirsium japonicum Fisch. ex DC, Citrus medica L. var. sarcodactylis Swingle |
| Fructus Mume formula and its separated prescription | Prunus mume (Sieb). Sieb. et Zucc., Asarum heterotropoides, Zingiberis officinale Rosc, Coptidis chinensis Franch, Aconitum carmichaelii Debx., Angelicas Sinensis (Oliv). Diels, Zanthoxylum bungeanum Maxim., Cinnamomum cassia Presl, Panax ginseng C. A. Mey., Phellodendrom chinense Schneid |
| Refined-JQ (JQ-R) | Rhizoma Coptidis, Astragalus membranaceus, Lonicera japonica |
| TZQ-F | Morus alba L., Lotus Leaf,Salvia miltiorrhiza Bge., Grataegus pinnati fida Bge., Radix paeoniae rubra |
| Jiaotai Pill (JTP) | Coptis chinensis Franch., Cinnamomum cassia Presl |
| Heqi San | Schisandra chinensis (Turcz). Baill., Cynanchum otophyllum C. K. Schneid., Hordeum vulgare L |
| Fructus Mume formula | Prunus mume (Sieb).Sieb. et Zucc., Asarum heterotropoides Fr. Schmidt var. mandshuricum, Zingiberis officinale Rosc, Coptidis chinensis Franch, Aconitum carmichaelii Debx., Angelicas Sinensis (Oliv). Diels, Zanthoxylum bungeanum Maxim., Cinnamomum cassia Presl, Panax ginseng C. A. Mey., Phellodendrom chinense Schneid |
However, there were some shortcomings in our research. First, most of the interventions studied were herbal formulas and extracts. The diversity of ingredients in the herbal formulas and extracts results in complex potential therapeutic mechanisms. Not only does it make understanding the role of drugs difficult, but it also makes in-depth research impossible. However, the existing research also has the same shortcomings as our research. In addition, there are studies questioning the role of herbal medicine in the treatment of IR. The beneficial effects of ginsenosides on IR are listed in Table 4 , but clinical trials have shown that oral ginseng or ginsenosides do not improve insulin sensitivity in glucose-tolerant or obese/overweight subjects who are newly diagnosed with diabetes (Reeds et al., 2011). However, the clinical trial lasted only 8 weeks, and in most in vitro and in vivo tests, ginsenosides were administered by intraperitoneal injection. In clinical trials, the systemic utilization and metabolic processes have to be considered only after oral administration. Studies have indicated that ginsenoside is a precursor. The pharmacological action of ginsenoside is to activate intestinal deglycosylation and fatty acid esterification (Hasegawa, 2004). Therefore, the in vitro and in vivo levels of ginsenosides should not be the only focus of the trial.
In response to these shortcomings, we can make some improvements in future studies. For example, regarding the bioavailability of herbal medicines, we can use targeted drug delivery systems (TDDSs) to improve the way in which the active ingredients of herbal medicines are administered. Research on targeted preparations related to TDDSs has become a popular topic, especially for applications in the field of anti-cancer research. Targeted administration can be divided into liposomes, granules, nanoparticles, emulsions, and other similar preparations (Li et al., 2009). The combination of drugs and specific target carriers can play a synergistic and attenuating role. At present, research on herbal drug delivery systems is still in the exploration stage, and the design, synthesis, and quality evaluation of TDDS are more suitable for single herbal ingredients. Therefore, separating the active ingredients of herbs is particularly important, including those known or unknown. We can select safe and effective single herbs from the many herbal formulas obtained from the literature and experimental research and separate the active ingredients, such as monomers, by pharmacological methods. Then, based on the active ingredients of the herbal medicine, the effective target mechanism of the drug can be studied. Finally, the active ingredient, target, and TDDS are combined. This not only makes the research on herbal medicine more targeted and efficient, but also provides a good prospect for the development and application of anti-insulin drugs.
Author Contributions
JL and LB designed the work of review. JL, LB, and FW reviewed the literature available on this topic and wrote the paper. JZ, DW, YX, and WY contributed in the scientific writing of the manuscript. JL, LB, and JW revised the manuscript. All authors approved the paper for publication. JL, LB, FW, JZ, DW, YX, and WY contributed equally to this work. JL and LB contributed equally to this study and share first authorship.
Funding
This paper was supported by Central Health Research Project W2017BJ43.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abe H., Yamada N., Kamata K., Kuwaki T., Shimada M., Osuga J., et al. (1998). Hypertension, hypertriglyceridemia, and impaired endothelium-dependent vascular relaxation in mice lacking insulin receptor substrate-1. J. Clin. Invest. 101 (8), 1784–1788. 10.1172/JCI1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel E., Peroni O., Kim J., Kim Y., Boss O., Hadro E., et al. (2001). Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409 (6821), 729–733. 10.1038/35055575 [DOI] [PubMed] [Google Scholar]
- Aguilar M., Bhuket T., Torres S., Liu B., Wong R. J. (2015). Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 313 (19), 1973–1974. 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]
- Ali A., Hoeflich K., Woodgett J. (2001). Glycogen synthase kinase-3: properties, functions, and regulation. Chem. Rev. 101 (8), 2527–2540. 10.1021/cr000110o [DOI] [PubMed] [Google Scholar]
- Al-Lahham R., Deford J. H., Papaconstantinou J. (2016). Mitochondrial-generated ROS down regulates insulin signaling via activation of the p38MAPK stress response pathway. Mol. Cell Endocrinol. 419, 1–11. 10.1016/j.mce.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Anitha M., Gondha C., Sutliff R., Parsadanian A., Mwangi S., Sitaraman S. V., et al. (2006). GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J. Clin. Invest. 116 (2), 344–356. 10.1172/JCI26295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo T. G., de Oliveira A. G., Vecina J. F., Marin R. M., Franco E. S., Abdalla Saad M. J., et al. (2016). Parkinsonia aculeata (Caesalpineaceae) improves high-fat diet-induced insulin resistance in mice through the enhancement of insulin signaling and mitochondrial biogenesis. J. Ethnopharmacol. 183, 95–102. 10.1016/j.jep.2016.02.048 [DOI] [PubMed] [Google Scholar]
- Arkan M., Hevener A., Greten F., Maeda S., Li Z., Long J., et al. (2005). IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 11 (2), 191–198. 10.1038/nm1185 [DOI] [PubMed] [Google Scholar]
- Armstrong D. G., Boulton A. J. M., Bus S. A. (2017). Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 376 (24), 2367–2375. 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- Askari F., Rashidkhani B., Hekmatdoost A. (2014). Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr. Res. 34 (2), 143–148. 10.1016/j.nutres.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Atkin M., Laight D., Cummings M. H. (2016). The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. J. Diabetes Complications. 30 (4), 723–727. 10.1016/j.jdiacomp.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Aytug S., Reich D., Sapiro L. E., Bernstein D., Begum N. (2003). Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology 38 (6), 1384–1392. 10.1016/j.hep.2003.09.012 [DOI] [PubMed] [Google Scholar]
- Bae U. J., Park J., Park I. W., Chae B. M., Oh M. R., Jung S. J., et al. (2018). Epigallocatechin-3-gallate-rich green tea extract ameliorates fatty liver and weight gain in mice fed a high fat diet by activating the sirtuin 1 and AMP activating protein kinase pathway. Am. J. Chin. Med. 46 (3), 617–632. 10.1142/S0192415X18500325 [DOI] [PubMed] [Google Scholar]
- Bai L., Gao J., Wei F., Zhao J., Wang D., Wei J. (2018). Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front. Pharmacol. 9, 423. 10.3389/fphar.2018.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Wang Y., Fan J., Chen Y., Ji W., Qu A., et al. (2007). Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 5 (1), 47–57. 10.1016/j.cmet.2006.11.013 [DOI] [PubMed] [Google Scholar]
- Banin R. M., Hirata B. K., Andrade I. S., Zemdegs J. C., Clemente A. P., Dornellas A. P., et al. (2014). Beneficial effects of Ginkgo biloba extract on insulin signaling cascade, dyslipidemia, and body adiposity of diet-induced obese rats. Braz. J. Med. Biol. Res. 47 (9), 780–788. 10.1590/1414-431X20142983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil F., Canron M. H., Vital A., Bezard E., Li Y., Greig N. H., et al. (2017). Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain 140 (5), 1420–1436. 10.1093/brain/awx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belwal T., Nabavi S. F., Nabavi S. M., Habtemariam S. (2017). Dietary anthocyanins and insulin resistance: when food becomes a medicine. Nutrients 9 (10), 1–22. 10.3390/nu9101111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakkiyalakshmi E., Sireesh D., Rajaguru P., Paulmurugan R., Ramkumar K. M. (2015). The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacol. Res. 91, 104–114. 10.1016/j.phrs.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Bhakta H. K., Paudel P., Fujii H., Sato A., Park C. H., Yokozawa T., et al. (2017). Oligonol promotes glucose uptake by modulating the insulin signaling pathway in insulin-resistant HepG2 cells via inhibiting protein tyrosine phosphatase 1B. Arch. Pharm. Res. 40 (11), 1314–1327. 10.1007/s12272-017-0970-6 [DOI] [PubMed] [Google Scholar]
- Biessels G. J., Staekenborg S., Brunner E., Brayne C., Scheltens P. (2006). Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5 (1), 64–74. 10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- Boden G., Homko C., Barrero C., Stein T., Chen X., Cheung P., et al. (2015). Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci. Transl. Med. 7 (304), 304re307. 10.1126/scitranslmed.aac4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan J. S. (2012). Regulation of glucose transporter translocation in health and diabetes. Annu. Rev. Biochem. 81, 507–532. 10.1146/annurev-biochem-060109-094246 [DOI] [PubMed] [Google Scholar]
- Bouzakri K., Zachrisson A., Al-Khalili L., Zhang B. B., Koistinen H. A., Krook A., et al. (2006). siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab. 4 (1), 89–96. 10.1016/j.cmet.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Cai D., Yuan M., Frantz D., Melendez P., Hansen L., Lee J., et al. (2005). Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 11 (2), 183–190. 10.1038/nm1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C., Jiang L., Deshmukh A., Mataki C., Coste A., Lagouge M., et al. (2010). Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11 (3), 213–219. 10.1016/j.cmet.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. J., Lin C. S., Lu C. C., Martel J., Ko Y. F., Ojcius D. M., et al. (2015). Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 6, 7489. 10.1038/ncomms8489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C. L., Huang H. C., Lin H. C., Chang T. C., Chang W. L. (2016). Sesquiterpenes from Baizhu stimulate glucose uptake by activating AMPK and PI3K. Am. J. Chin. Med. 44 (5), 963–979. 10.1142/S0192415X16500531 [DOI] [PubMed] [Google Scholar]
- Chen G., Yang X., Yang X., Li L., Luo J., Dong H., et al. (2017). Jia-Wei-Jiao-Tai-Wan ameliorates type 2 diabetes by improving beta cell function and reducing insulin resistance in diabetic rats. BMC Complement. Altern. Med. 17 (1), 507. 10.1186/s12906-017-2016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Fu D. Y., Fu X. D., He Y. M., Wang W. J. (2014). Effect of yiqi huaju recipe combined with routine therapy in treating hypertension patients with metabolic syndrome: a clinical study. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34 (6), 680–687. [PubMed] [Google Scholar]
- Cheng K. C., Asakawa A., Li Y. X., Liu I. M., Amitani H., Cheng J. T., et al. (2013). Opioid mu-receptors as new target for insulin resistance. Pharmacol. Ther. 139 (3), 334–340. 10.1016/j.pharmthera.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Chiang S. H., Bazuine M., Lumeng C. N., Geletka L. M., Mowers J., White N. M., et al. (2009). The protein kinase IKKepsilon regulates energy balance in obese mice. Cell 138 (5), 961–975. 10.1016/j.cell.2009.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. C., Kim S. J., Son K. Y., Oh B. J., Cho B. L. (2013). Metabolic effects of aloe vera gel complex in obese prediabetes and early non-treated diabetic patients: randomized controlled trial. Nutrition 29 (9), 1110–1114. 10.1016/j.nut.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Choi M. S., Ryu R., Seo Y. R., Jeong T. S., Shin D. H., Park Y. B., et al. (2014). The beneficial effect of soybean (Glycine max (L). Food Funct. 5 (7), 1621–1630. 10.1039/c4fo00199k [DOI] [PubMed] [Google Scholar]
- Chuengsamarn S., Rattanamongkolgul S., Phonrat B., Tungtrongchitr R., Jirawatnotai S. (2014). Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J. Nutr. Biochem. 25 (2), 144–150. 10.1016/j.jnutbio.2013.09.013 [DOI] [PubMed] [Google Scholar]
- Chung W., Ryu J., Chung S., Kim S. (2016). [Effect of Qingxue Dan on obesity and metabolic biomarker: a double-blind randomized-controlled pilot study]. J. Tradit. Chin. Med. 36 (3), 291–298. 10.1016/S0254-6272(16)30040-1 [DOI] [PubMed] [Google Scholar]
- Cross D., Alessi D., Cohen P., Andjelkovich M., Hemmings B. (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378 (6559), 785–789. 10.1038/378785a0 [DOI] [PubMed] [Google Scholar]
- Cuenda A., Lizcano J. M., Lozano J. (2017). Editorial: mitogen activated protein kinases. Front Cell Dev. Biol. 5, 80. 10.3389/fcell.2017.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D C. (2018). Why Chinese medicine is heading for clinics around the world. Nature 561, 448–450. 10.1038/d41586-018-06782-7 [DOI] [PubMed] [Google Scholar]
- Dai B., Wu Q., Zeng C., Zhang J., Cao L., Xiao Z., et al. (2016). The effect of Liuwei Dihuang decoction on PI3K/Akt signaling pathway in liver of type 2 diabetes mellitus (T2DM) rats with insulin resistance. J. Ethnopharmacol. 192, 382–389. 10.1016/j.jep.2016.07.024 [DOI] [PubMed] [Google Scholar]
- Dai S., Hong Y., Xu J., Lin Y., Si Q., Gu X. (2018). Ginsenoside Rb2 promotes glucose metabolism and attenuates fat accumulation via AKT-dependent mechanisms. Biomed. Pharmacother. 100, 93–100. 10.1016/j.biopha.2018.01.111 [DOI] [PubMed] [Google Scholar]
- Dajani R., Fraser E., Roe S., Young N., Good V., Dale T., et al. (2001). Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105 (6), 721–732. 10.1016/S0092-8674(01)00374-9 [DOI] [PubMed] [Google Scholar]
- Deng D., Xu C., Sun P., Wu J., Yan C., Hu M., et al. (2014). Crystal structure of the human glucose transporter GLUT1. Nature 510 (7503), 121–125. 10.1038/nature13306 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Dunaif A. (2012). Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 33 (6), 981–1030. 10.1210/er.2011-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Wang J. H., Lu F. E., Xu L. J., Gong Y. L., Zou X. (2013). Jiaotai Pill enhances insulin signaling through phosphatidylinositol 3-kinase pathway in skeletal muscle of diabetic rats. Chin. J. Integr. Med. 19 (9), 668–674. 10.1007/s11655-013-1560-1 [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Mameghani M., Asghari-Jafarabadi M., Rezazadeh K. (2018). TCF7L2-rs7903146 polymorphism modulates the effect of artichoke leaf extract supplementation on insulin resistance in metabolic syndrome: a randomized, double-blind, placebo-controlled trial. J. Integr. Med. 16 (5), 329–334. 10.1016/j.joim.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Ebrahimpour-Koujan S., Gargari B. P., Mobasseri M., Valizadeh H., Asghari-Jafarabadi M. (2018). Lower glycemic indices and lipid profile among type 2 diabetes mellitus patients who received novel dose of Silybum marianum (L). Phytomedicine 44, 39–44. 10.1016/j.phymed.2018.03.050 [DOI] [PubMed] [Google Scholar]
- Eldor R., DeFronzo R. A., Abdul-Ghani M. (2013). In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 36 Suppl 2, S162–174. 10.2337/dcS13-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P., Yu M., Min W., Han S., Shi M., Zhang Z., et al. (2018). Beneficial effect of baicalin on insulin sensitivity in adipocytes of diet-induced obese mice. Diabetes Res. Clin. Pract. 139, 262–271. 10.1016/j.diabres.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Feve B., Bastard J. P. (2009). The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 5 (6), 305–311. 10.1038/nrendo.2009.62 [DOI] [PubMed] [Google Scholar]
- Frame S., Cohen P., Biondi R. (2001). A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 7 (6), 1321–1327. 10.1016/S1097-2765(01)00253-2 [DOI] [PubMed] [Google Scholar]
- Gao L. H., Liu Q., Liu S. N., Chen Z. Y., Li C. N., Lei L., et al. (2014). A refined-JinQi-JiangTang tablet ameliorates prediabetes by reducing insulin resistance and improving beta cell function in mice. J. Ethnopharmacol. 151 (1), 675–685. 10.1016/j.jep.2013.11.024 [DOI] [PubMed] [Google Scholar]
- Gao S., Guo Q., Qin C., Shang R., Zhang Z. (2017). Sea buckthorn fruit oil extract alleviates insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus cells and rats. J. Agric. Food Chem. 65 (7), 1328–1336. 10.1021/acs.jafc.6b04682 [DOI] [PubMed] [Google Scholar]
- Gao W., Du X., Lei L., Wang H., Zhang M., Wang Z., et al. (2018). NEFA-induced ROS impaired insulin signalling through the JNK and p38MAPK pathways in non-alcoholic steatohepatitis. J. Cell Mol. Med. 22 (7), 3408–3422. 10.1111/jcmm.13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabadu D., Krishnamurthy S. (2017). Metformin attenuates hepatic insulin resistance in type-2 diabetic rats through PI3K/Akt/GLUT-4 signalling independent to bicuculline-sensitive GABAA receptor stimulation. Pharm. Biol. 55 (1), 722–728. 10.1080/13880209.2016.1268635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering B., Cutillas P., Nock G., Gharbi S., Vanhaesebroeck B. (2007). Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc. Natl. Acad. Sci. U.S.A. 104 (19), 7809–7814. 10.1073/pnas.0700373104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehart H., Kumpf S., Ittner A., Ricci R. (2010). MAPK signalling in cellular metabolism: stress or wellness? EMBO Rep. 11 (11), 834–840. 10.1038/embor.2010.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S., Rochford J., Wolfrum C., Gray S., Schinner S., Wilson J., et al. (2004). A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304 (5675), 1325–1328. 10.1126/science.1096706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi M. O., Dumesic D. A., Chazenbalk G., Azziz R. (2011). Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 7 (4), 219–231. 10.1038/nrendo.2010.217 [DOI] [PubMed] [Google Scholar]
- Guilherme A., Virbasius J. V., Puri V., Czech M. P. (2008). Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9 (5), 367–377. 10.1038/nrm2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Zhang H., Li M., Zhao Z., Luo Y., Luo Y., et al. (2016). Efficacy and safety of Sancai powder in patients with type 2 diabetes mellitus: a randomized controlled trial. J. Tradit. Chin. Med. 36 (5), 640–648. 10.1016/S0254-6272(16)30084-X [DOI] [PubMed] [Google Scholar]
- Gurzov E. N., Tran M., Fernandez-Rojo M. A., Merry T. L., Zhang X., Xu Y., et al. (2014). Hepatic oxidative stress promotes insulin-STAT-5 signaling and obesity by inactivating protein tyrosine phosphatase N2. Cell Metab. 20 (1), 85–102. 10.1016/j.cmet.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler R. A., Accili D. (2008). The double life of Irs. Cell Metab. 8 (1), 7–9. 10.1016/j.cmet.2008.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Husein I., Tukan S., Alkazaleh F. (2016). The effect of marjoram (Origanum majorana) tea on the hormonal profile of women with polycystic ovary syndrome: a randomised controlled pilot study. J. Hum. Nutr. Diet 29 (1), 105–111. 10.1111/jhn.12290 [DOI] [PubMed] [Google Scholar]
- Haluzík M., Haluzík M. (2006). Peroxisome proliferator activated receptors (PPAR) and insulin sensitivity: experimental studies. Cesk Fysiol. 55 (4), 163–168. [PubMed] [Google Scholar]
- Han J., Yang N., Zhang F., Zhang C., Liang F., Xie W., et al. (2015). Rhizoma Anemarrhenae extract ameliorates hyperglycemia and insulin resistance via activation of AMP-activated protein kinase in diabetic rodents. J. Ethnopharmacol. 172, 368–376. 10.1016/j.jep.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Hanssen M. J., Hoeks J., Brans B., van der Lans A. A., Schaart G., van den Driessche J. J., et al. (2015). Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 21 (8), 863–865. 10.1038/nm.3891 [DOI] [PubMed] [Google Scholar]
- Hao J., Chen C., Huang K., Huang J., Li J., Liu P., et al. (2014). Polydatin improves glucose and lipid metabolism in experimental diabetes through activating the Akt signaling pathway. Eur. J. Pharmacol. 745, 152–165. 10.1016/j.ejphar.2014.09.047 [DOI] [PubMed] [Google Scholar]
- Haque A., Andersen J. N., Salmeen A., Barford D., Tonks N. K. (2011). Conformation-sensing antibodies stabilize the oxidized form of PTP1B and inhibit its phosphatase activity. Cell 147 (1), 185–198. 10.1016/j.cell.2011.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H. (2004). Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J. Pharmacol. Sci. 95 (2), 153–157. 10.1254/jphs.FMJ04001X4 [DOI] [PubMed] [Google Scholar]
- Hevener A., He W., Barak Y., Le J., Bandyopadhyay G., Olson P., et al. (2003). Muscle-specific Pparg deletion causes insulin resistance. Nat. Med. 9 (12), 1491–1497. 10.1038/nm956 [DOI] [PubMed] [Google Scholar]
- Hinkel R., Howe A., Renner S., Ng J., Lee S., Klett K., et al. (2017). Diabetes Mellitus-Induced Microvascular Destabilization in the Myocardium. J. Am. Coll. Cardiol. 69 (2), 131–143. 10.1016/j.jacc.2016.10.058 [DOI] [PubMed] [Google Scholar]
- Hirosumi J., Tuncman G., Chang L., Gorgun C. Z., Uysal K. T., Maeda K., et al. (2002). A central role for JNK in obesity and insulin resistance. Nature 420 (6913), 333–336. 10.1038/nature01137 [DOI] [PubMed] [Google Scholar]
- Hoehn K. L., Hohnen-Behrens C., Cederberg A., Wu L. E., Turner N., Yuasa T., et al. (2008). IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 7 (5), 421–433. 10.1016/j.cmet.2008.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer R. G., Park E. J., Li N., Tran H., Chen M., Choi C., et al. (2011). Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 147 (1), 173–184. 10.1016/j.cell.2011.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y. L., Wu F. (2014). Effect of Bushen Huatan Recipe on the Akt signal pathway in polycystic ovarian syndrome model rats with insulin resistance: an experimental research. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34 (2), 230–234. [PubMed] [Google Scholar]
- Hotamisligil G. S., Davis R. J. (2016). Cell signaling and stress responses. Cold Spring Harb. Perspect. Biol. 8 (10), 1–20. 10.1101/cshperspect.a006072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G., Peraldi P., Budavari A., Ellis R., White M., Spiegelman B. (1996). IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271 (5249), 665–668. 10.1126/science.271.5249.665 [DOI] [PubMed] [Google Scholar]
- Hsu C. Y., Shih H. Y., Chia Y. C., Lee C. H., Ashida H., Lai Y. K., et al. (2014). Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 58 (6), 1168–1176. 10.1002/mnfr.201300691 [DOI] [PubMed] [Google Scholar]
- Hu X., Wang M., Bei W., Han Z., Guo J. (2014). The Chinese herbal medicine FTZ attenuates insulin resistance via IRS1 and PI3K in vitro and in rats with metabolic syndrome. J. Transl. Med. 12 ( (Electronic)), 47–56. 10.1186/1479-5876-12-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Hou Z., Liu D., Yang X. (2016). Tartary buckwheat flavonoids protect hepatic cells against high glucose-induced oxidative stress and insulin resistance via MAPK signaling pathways. Food Funct. 7 (3), 1523–1536. 10.1039/C5FO01467K [DOI] [PubMed] [Google Scholar]
- Hu Y., Hou Z., Yi R., Wang Z., Sun P., Li G., et al. (2017). Tartary buckwheat flavonoids ameliorate high fructose-induced insulin resistance and oxidative stress associated with the insulin signaling and Nrf2/HO-1 pathways in mice. Food Funct. 8 (8), 2803–2816. 10.1039/C7FO00359E [DOI] [PubMed] [Google Scholar]
- Huang B., Xue L., Wu Y., Jiang Q. S. (2015). Effect and mechanism of polydatin on diabetic myocardial hypertrophy in mice. Zhongguo Zhong Yao Za Zhi. 40 (21), 4256–4261. [PubMed] [Google Scholar]
- Huang S., Czech M. P. (2007). The GLUT4 glucose transporter. Cell Metab. 5 (4), 237–252. 10.1016/j.cmet.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Huang Y. Q., Yang Q. F., Wang H., Xu Y. S., Peng W., Jiang Y. H. (2016). Long-term clinical effect of Tangyiping Granules () on patients with impaired glucose tolerance. Chin. J. Integr. Med. 22 (9), 653–659. 10.1007/s11655-016-2512-3 [DOI] [PubMed] [Google Scholar]
- Hur E. M., Zhou F. Q. (2010). GSK3 signalling in neural development. Nat. Rev. Neurosci. 11 (8), 539–551. 10.1038/nrn2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Qin B., Poti J., Sokol R., Gordon-Larsen P. (2018). Epidemiology of obesity in adults: latest trends. Curr. Obes. Rep. 7 (4), 276–288. 10.1007/s13679-018-0317-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarpour-Sadegh F., Montazeri V., Adili A., Esfehani A., Rashidi M. R., Pirouzpanah S. (2017). Consumption of fresh yellow onion ameliorates hyperglycemia and insulin resistance in breast cancer patients during doxorubicin-based chemotherapy: a randomized controlled clinical trial. Integr. Cancer Ther. 16 (3), 276–289. 10.1177/1534735416656915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M., Park J., Youn D., Jung Y., Kang J., Lim S., et al. (2017). Albiflorin ameliorates obesity by inducing thermogenic genes via AMPK and PI3K/AKT in vivo and in vitro. Metab. Clin. Exp. 73, 85–99. 10.1016/j.metabol.2017.05.009 [DOI] [PubMed] [Google Scholar]
- Jiang B., Le L., Zhai W., Wan W., Hu K., Yong P., et al. (2016). Protective effects of marein on high glucose-induced glucose metabolic disorder in HepG2 cells. Phytomedicine 23 (9), 891–900. 10.1016/j.phymed.2016.05.004 [DOI] [PubMed] [Google Scholar]
- Jiang C., Yao N., Wang Q., Zhang J., Sun Y., Xiao N., et al. (2014). Cyclocarya paliurus extract modulates adipokine expression and improves insulin sensitivity by inhibition of inflammation in mice. J. Ethnopharmacol. 153 (2), 344–351. 10.1016/j.jep.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Jiang H., Ma Y., Yan J., Liu J., Li L. (2017). Geniposide promotes autophagy to inhibit insulin resistance in HepG2 cells via P62/NFkappaB/GLUT4. Mol. Med. Rep. 16 (5), 7237–7244. 10.3892/mmr.2017.7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Yao L., Yang Y., Ke D., Batey R., Wang J., et al. (2016). Jiangzhi capsule improves fructose-induced insulin resistance in rats: association with repair of the impaired sarcolemmal glucose transporter-4 recycling. J. Ethnopharmacol. 194, 288–298. 10.1016/j.jep.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Jin X., Zhang H. X., Cui W. W. (2015). Effect of Jinlida on DGAT1 in skeletal muscle in fat-induced insulin resistance ApoE -/- mice. Zhong Yao Cai. 38 (6), 1237–1241. [PubMed] [Google Scholar]
- Johnson T. O., Ermolieff J., Jirousek M. R. (2002). Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov. 1 (9), 696–709. 10.1038/nrd895 [DOI] [PubMed] [Google Scholar]
- Joost H., Thorens B. (2001). The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol. Membr. Biol. 18 (4), 247–256. 10.1080/09687680110090456 [DOI] [PubMed] [Google Scholar]
- Jung H. A., Bhakta H. K., Min B. S., Choi J. S. (2016). Fucosterol activates the insulin signaling pathway in insulin resistant HepG2 cells via inhibiting PTP1B. Arch. Pharm. Res. 39 (10), 1454–1464. 10.1007/s12272-016-0819-4 [DOI] [PubMed] [Google Scholar]
- Jung H. J., Seong S. H., Ali M. Y., Min B. S., Jung H. A., Choi J. S. (2017). Alpha-Methyl artoflavanocoumarin from Juniperus chinensis exerts anti-diabetic effects by inhibiting PTP1B and activating the PI3K/Akt signaling pathway in insulin-resistant HepG2 cells. Arch. Pharm. Res. 40 (12), 1403–1413. 10.1007/s12272-017-0992-0 [DOI] [PubMed] [Google Scholar]
- Kaatabi H., Bamosa A. O., Badar A., Al-Elq A., Abou-Hozaifa B., Lebda F., et al. (2015). Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One 10 (2), e0113486. 10.1371/journal.pone.0113486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Yamauchi T. (2011). Adiponectin receptor signaling: a new layer to the current model. Cell Metab. 13 (2), 123–124. 10.1016/j.cmet.2011.01.012 [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Alquier T., Carling D., Hardie D. G. (2005). AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1 (1), 15–25. 10.1016/j.cmet.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Kahn S. E., Hull R. L., Utzschneider K. M. (2006). Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444 (7121), 840–846. 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- Kaidanovich O., Eldar-Finkelman H. (2002). The role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Expert Opin. Ther.Targets. 6 (5), 555–561. 10.1517/14728222.6.5.555 [DOI] [PubMed] [Google Scholar]
- Kan J., Velliquette R. A., Grann K., Burns C. R., Scholten J., Tian F., et al. (2017). A novel botanical formula prevents diabetes by improving insulin resistance. BMC Complement. Altern. Med. 17 (1), 352. 10.1186/s12906-017-1848-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho M. C., Lee Y. J., Cha J. D., Choi K. M., Kang D. G., Lee H. S. (2014). Gastrodia elata ameliorates high-fructose diet-induced lipid metabolism and endothelial dysfunction. Evid. Based Complement. Alternat. Med. 2014, 101624. 10.1155/2014/101624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho M. C., Lee Y. J., Park J. H., Kim H. Y., Yoon J. J., Ahn Y. M., et al. (2016). Fermented red ginseng potentiates improvement of metabolic dysfunction in metabolic syndrome rat models. Nutrients 8 (6), 1–15. 10.3390/nu8060369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido Y., Burks D., Withers D., Bruning J., Kahn C., White M., et al. (2000). Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J. Clin. Invest. 105 (2), 199–205. 10.1172/JCI7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. M., Park C. H., Park S. K., Seung T. W., Kang J. Y., Ha J. S., et al. (2017). Ginsenoside Re ameliorates brain insulin resistance and cognitive dysfunction in high fat diet-induced C57BL/6 mice. J. Agric. Food Chem. 65 (13), 2719–2729. 10.1021/acs.jafc.7b00297 [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Choi J. Y., Ryu R., Lee J., Cho S. J., Kwon E. Y., et al. (2016). Platycodon grandiflorus root extract attenuates body fat mass, hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue. Nutrients 8 (9), 1–10. 10.3390/nu8090532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishton R., Barnes C., Nichols A., Cohen S., Gerriets V., Siska P., et al. (2016). AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab. 23 (4), 649–662. 10.1016/j.cmet.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong P., Chi R., Zhang L., Wang N., Lu Y. (2013). Effects of paeoniflorin on tumor necrosis factor-alpha-induced insulin resistance and changes of adipokines in 3T3-L1 adipocytes. Fitoterapia. 91, 44–50. 10.1016/j.fitote.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Koo S. H., Satoh H., Herzig S., Lee C. H., Hedrick S., Kulkarni R., et al. (2004). PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat. Med. 10 (5), 530–534. 10.1038/nm1044 [DOI] [PubMed] [Google Scholar]
- Kuai M., Li Y., Sun X., Ma Z., Lin C., Jing Y., et al. (2016). A novel formula Sang-Tong-Jian improves glycometabolism and ameliorates insulin resistance by activating PI3K/AKT pathway in type 2 diabetic KKAy mice. Biomed. Pharmacother. 84, 1585–1594. 10.1016/j.biopha.2016.10.101 [DOI] [PubMed] [Google Scholar]
- Kubota N., Yano W., Kubota T., Yamauchi T., Itoh S., Kumagai H., et al. (2007). Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 6 (1), 55–68. 10.1016/j.cmet.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Kubota T., Kubota N., Moroi M., Terauchi Y., Kobayashi T., Kamata K., et al. (2003). Lack of insulin receptor substrate-2 causes progressive neointima formation in response to vessel injury. Circulation 107 (24), 3073–3080. 10.1161/01.CIR.0000070937.52035.25 [DOI] [PubMed] [Google Scholar]
- Kullmann S., Heni M., Hallschmid M., Fritsche A., Preissl H., Haring H. U. (2016). Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol. Rev. 96 (4), 1169–1209. 10.1152/physrev.00032.2015 [DOI] [PubMed] [Google Scholar]
- Kuo Y. H., Lin C. H., Shih C. C. (2015). Ergostatrien-3beta-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation. J. Agric. Food Chem. 63 (9), 2479–2489. 10.1021/acs.jafc.5b00073 [DOI] [PubMed] [Google Scholar]
- Lage R., Dieguez C., Vidal-Puig A., Lopez M. (2008). AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 14 (12), 539–549. 10.1016/j.molmed.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Lawan A., Bennett A. M. (2017). Mitogen-activated protein kinase regulation in hepatic metabolism. Trends Endocrinol. Metab. 28 (12), 868–878. 10.1016/j.tem.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le L., Jiang B., Wan W., Zhai W., Xu L., Hu K., et al. (2016). Metabolomics reveals the protective of Dihydromyricetin on glucose homeostasis by enhancing insulin sensitivity. Sci. Rep. 6, 36184. 10.1038/srep36184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens K. F., Easton R. M., Shulman G. I., Previs S. F., Birnbaum M. J. (2009). Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 10 (5), 405–418. 10.1016/j.cmet.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. B., Yoon S. J., Lee S. H., Lee M. S., Jung H., Kim T. D., et al. (2017). Ginsenoside Rg3 ameliorated HFD-induced hepatic steatosis through downregulation of STAT5-PPARgamma. J. Endocrinol. 235 (3), 223–235. 10.1530/JOE-17-0233 [DOI] [PubMed] [Google Scholar]
- Lee S. M., Lee Y. J., Choi J. H., Kho M. C., Yoon J. J., Shin S. H., et al. (2014). Gal-geun-dang-gwi-tang improves diabetic vascular complication in apolipoprotein E KO mice fed a western diet. BMC Complement. Altern. Med. 14, 453. 10.1186/1472-6882-14-453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem K. H., Kim M. G., Hahm Y. T., Kim H. K. (2016). Hypoglycemic effect of Opuntia ficus-indica var. Nutrients 8 (12), 1–15. 10.3390/nu8120800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt E., Rodgers C. T., Clarke W. T., Mahmod M., Ariga R., Francis J. M., et al. (2016). Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur. Heart J. 37 (46), 3461–3469. 10.1093/eurheartj/ehv442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. C., Zhong X. K., Zeng Z. P., Jiang J. G., Li L., Zhao M. M., et al. (2009). Application of targeted drug delivery system in Chinese medicine. J. Control Release 138 (2), 103–112. 10.1016/j.jconrel.2009.05.008 [DOI] [PubMed] [Google Scholar]
- Li J. B., Xu L. J., Dong H., Huang Z. Y., Zhao Y., Chen G., et al. (2013). Effects of Chinese Fructus Mume formula and its separated prescription extract on insulin resistance in type 2 diabetic rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 33 (6), 877–885. 10.1007/s11596-013-1215-7 [DOI] [PubMed] [Google Scholar]
- Li L., Yoshitomi H., Wei Y., Qin L., Zhou J., Xu T., et al. (2015). Tang-Nai-Kang alleviates pre-diabetes and metabolic disorders and induces a gene expression switch toward fatty acid oxidation in SHR.Cg-Leprcp/NDmcr rats. PLoS One 10 (4), e0122024. 10.1371/journal.pone.0122024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Chen Q., Xie Y., Hu J., Yang S., Lin M. (2018). Prevalence and degree of insulin resistance in Chinese Han women with PCOS: Results from euglycemic-hyperinsulinemic clamps. Clin. Endocrinol. (Oxf). 90 (1), 138–144. 10.1111/cen.13860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Gong H., Yang S., Yang L., Fan Y., Zhou Y. (2017). Pectic bee pollen polysaccharide from Rosa rugosa alleviates diet-induced hepatic steatosis and insulin resistance via induction of AMPK/mTOR-mediated autophagy. Molecules 22 (5), 1–13. 10.3390/molecules22050699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Tran V. H., Kota B. P., Nammi S., Duke C. C., Roufogalis B. D. (2014). Preventative effect of Zingiber officinale on insulin resistance in a high-fat high-carbohydrate diet-fed rat model and its mechanism of action. Basic Clin. Pharmacol. Toxicol. 115 (2), 209–215. 10.1111/bcpt.12196 [DOI] [PubMed] [Google Scholar]
- Li Y., Xu S., Mihaylova M. M., Zheng B., Hou X., Jiang B., et al. (2011). AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13 (4), 376–388. 10.1016/j.cmet.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhang S., Liu H., Wang L., Zhang C., Leng J., et al. (2014). Different associations of diabetes with β-cell dysfunction and insulin resistance among obese and nonobese chinese women with prior gestational diabetes mellitus. Diabetes Care 37 (9), 2533–2539. 10.2337/dc14-0573 [DOI] [PubMed] [Google Scholar]
- Lian F., Tian J., Chen X., Li Z., Piao C., Guo J., et al. (2015). The efficacy and safety of Chinese herbal medicine Jinlida as add-on medication in type 2 diabetes patients ineffectively managed by metformin monotherapy: a double-blind, randomized, placebo-controlled, multicenter trial. PLoS One 10 (6), e0130550. 10.1371/journal.pone.0130550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Spraul M., Ferraro R., Foley J. E., Ravussin E., et al. (1993). Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 329 (27), 1988–1992. 10.1056/NEJM199312303292703 [DOI] [PubMed] [Google Scholar]
- Liu C. Y., Huang C. J., Huang L. H., Chen I. J., Chiu J. P., Hsu C. H. (2014). Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: a randomized, double-blinded, and placebo-controlled trial. PLoS One 9 (3), e91163. 10.1371/journal.pone.0091163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Zhang Y., Liu Y., Hou L., Li S., Tian H., et al. (2018). Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes 126 (8), 513–520. 10.1055/s-0043-125066 [DOI] [PubMed] [Google Scholar]
- Liu H. W., Huang W. C., Yu W. J., Chang S. J. (2015). Toona Sinensis ameliorates insulin resistance via AMPK and PPARgamma pathways. Food Funct. 6 (6), 1855–1864. 10.3390/molecules201219794 [DOI] [PubMed] [Google Scholar]
- Liu J., Wang X., Chen Y.-P., Mao L.-F., Shang J., Sun H.-B., et al. (2014). Maslinic acid modulates glycogen metabolism by enhancing the insulin signaling pathway and inhibiting glycogen phosphorylase. Chin. J. Nat. Med. 12 (4), 259–265. 10.1016/S1875-5364(14)60052-2 [DOI] [PubMed] [Google Scholar]
- Liu Q., Li X., Li C., Zheng Y., Peng G. (2015). 1-Deoxynojirimycin alleviates insulin resistance via activation of insulin signaling PI3K/AKT pathway in skeletal muscle of db/db mice. Molecules 20 (12), 21700–21714. 10.1039/C5FO00056D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Liu S., Gao L., Sun S., Huan Y., Li C., et al. (2017). Anti-diabetic effects and mechanisms of action of a Chinese herbal medicine preparation JQ-R in vitro and in diabetic KK(Ay) mice. Acta. Pharm. Sin B. 7 (4), 461–469. 10.1016/j.apsb.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li X., Xie C., Luo X., Bao Y., Wu B., et al. (2016). Prevention effects and possible molecular mechanism of mulberry leaf extract and its formulation on rats with insulin-insensitivity. PLoS One 11 (4), e0152728. 10.1371/journal.pone.0152728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Song A., Zang S., Wang C., Song G., Li X., et al. (2015). Jinlida reduces insulin resistance and ameliorates liver oxidative stress in high-fat fed rats. J. Ethnopharmacol. 162, 244–252. 10.1016/j.jep.2014.12.040 [DOI] [PubMed] [Google Scholar]
- Liu Z. Q., Liu T., Chen C., Li M. Y., Wang Z. Y., Chen R. S., et al. (2015). Fumosorinone, a novel PTP1B inhibitor, activates insulin signaling in insulin-resistance HepG2 cells and shows anti-diabetic effect in diabetic KKAy mice. Toxicol. Appl. Pharmacol. 285 (1), 61–70. 10.1016/j.taap.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Liu Z., Kuang W., Xu X., Li D., Zhu W., Lan Z., et al. (2018). Putative identification of components in Zengye decoction and their effects on glucose consumption and lipogenesis in insulin-induced insulin-resistant HepG2 cells. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1073, 145–153. 10.1016/j.jchromb.2017.12.019 [DOI] [PubMed] [Google Scholar]
- Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007). Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117 (1), 175–184. 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Vital D., Weiss M., Gonzalez de Mejia E. (2017). Anthocyanins from purple corn ameliorated tumor necrosis factor-alpha-induced inflammation and insulin resistance in 3T3-L1 adipocytes via activation of insulin signaling and enhanced GLUT4 translocation. Mol. Nutr. Food Res. 61 (12), 1–41. 10.1002/mnfr.201700362 [DOI] [PubMed] [Google Scholar]
- Luo J., Sobkiw C. L., Hirshman M. F., Logsdon M. N., Li T. Q., Goodyear L. J., et al. (2006). Loss of class IA PI3K signaling in muscle leads to impaired muscle growth, insulin response, and hyperlipidemia. Cell Metab. 3 (5), 355–366. 10.1016/j.cmet.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Luo Y. H., Zhu M., Wang D. G., Yang Y. S., Tan T., Zhu H., et al. (2018). Yangxin Tongmai formula ameliorates impaired glucose tolerance in children with Graves’ disease through upregulation of the insulin receptor levels. Acta. Pharmacol. Sin. 39 (6), 923–929. 10.1038/aps.2017.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Yu H., Xiao Y., Wang H. (2017). Momordica charantia extracts ameliorate insulin resistance by regulating the expression of SOCS-3 and JNK in type 2 diabetes mellitus rats. Pharm. Biol. 55 (1), 2170–2177. 10.1080/13880209.2017.1396350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaf A., Tjandrawinata R. R., Malinda D. (2016). Insulin sensitizer in prediabetes: a clinical study with DLBS3233, a combined bioactive fraction of Cinnamomum burmanii and Lagerstroemia speciosa. Drug Des. Devel. Ther. 10, 1279–1289. 10.2147/DDDT.S97568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel J., Ojcius D. M., Chang C. J., Lin C. S., Lu C. C., Ko Y. F., et al. (2017). Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 13 (3), 149–160. 10.1038/nrendo.2016.142 [DOI] [PubMed] [Google Scholar]
- Martinez A., Castro A., Dorronsoro I., Alonso M. (2002). Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Med. Res. Rev. 22 (4), 373–384. 10.1002/med.10011 [DOI] [PubMed] [Google Scholar]
- Massaro M., Scoditti E., Pellegrino M., Carluccio M. A., Calabriso N., Wabitsch M., et al. (2016). Therapeutic potential of the dual peroxisome proliferator activated receptor (PPAR)alpha/gamma agonist aleglitazar in attenuating TNF-alpha-mediated inflammation and insulin resistance in human adipocytes. Pharmacol. Res. 107, 125–136. 10.1016/j.phrs.2016.02.027 [DOI] [PubMed] [Google Scholar]
- Matheny R. W., Jr., Geddis A. V., Abdalla M. N., Leandry L. A., Ford M., McClung H. L., et al. (2018). AKT2 is the predominant AKT isoform expressed in human skeletal muscle. Physiol. Rep. 6 (6), e13652. 10.14814/phy2.13652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. (1985). Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28 (7), 412–419. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- Meex R. C. R., Watt M. J. (2017). Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 13 (9), 509–520. 10.1038/nrendo.2017.56 [DOI] [PubMed] [Google Scholar]
- Mendez-Del Villar M., Puebla-Perez A. M., Sanchez-Pena M. J., Gonzalez-Ortiz L. J., Martinez-Abundis E., Gonzalez-Ortiz M. (2016). Effect of Artemisia dracunculus administration on glycemic control, insulin sensitivity, and insulin secretion in patients with impaired glucose tolerance. J. Med. Food. 19 (5), 481–485. 10.1089/jmf.2016.0005 [DOI] [PubMed] [Google Scholar]
- Miki S., Inokuma K. I., Takashima M., Nishida M., Sasaki Y., Ushijima M., et al. (2017). Aged garlic extract suppresses the increase of plasma glycated albumin level and enhances the AMP-activated protein kinase in adipose tissue in TSOD mice. Mol. Nutr. Food Res. 61 (5), 1–22. 10.1002/mnfr.201600797 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y., Alquier T., Furukawa N., Kim Y., Lee A., Xue B., et al. (2004). AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428 (6982), 569–574. 10.1038/nature02440 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y., Kim Y., Peroni O., Fryer L., Müller C., Carling D., et al. (2002). Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415 (6869), 339–343. 10.1038/415339a [DOI] [PubMed] [Google Scholar]
- Mirfeizi M., Mehdizadeh Tourzani Z., Mirfeizi S. Z., Asghari Jafarabadi M., Rezvani H. R., Afzali M. (2016). Controlling type 2 diabetes mellitus with herbal medicines: a triple-blind randomized clinical trial of efficacy and safety. J. Diabetes 8 (5), 647–656. 10.1111/1753-0407.12342 [DOI] [PubMed] [Google Scholar]
- Mottillo E. P., Desjardins E. M., Crane J. D., Smith B. K., Green A. E., Ducommun S., et al. (2016). Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metab. 24 (1), 118–129. 10.1016/j.cmet.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari-Khosravi H., Talaei B., Jalali B. A., Najarzadeh A., Mozayan M. R. (2014). The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 22 (1), 9–16. 10.1016/j.ctim.2013.12.017 [DOI] [PubMed] [Google Scholar]
- Na H. Y., Seol M. H., Kim M., Lee B. C. (2017). Effect of seyoeum on obesity, insulin resistance, and nonalcoholic fatty liver disease of high-fat diet-fed C57BL/6 Mice. Evid. Based Complement. Alternat. Med. 2017, 4658543. 10.1155/2017/4658543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Xia J., Qin Zhang D., Du J., Wen J. (2013). Regulation effects of TZQ-F on adipocyte differentiation and insulin action. J. Ethnopharmacol. 150 (2), 692–699. 10.1016/j.jep.2013.09.038 [DOI] [PubMed] [Google Scholar]
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., et al. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384 (9945), 766–781. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y., Ramm G., Lopez J. A., James D. E. (2008). Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 7 (4), 348–356. 10.1016/j.cmet.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Nie J., Chang Y. (2017). Caffeic acid phenethyl ester (propolis extract) ameliorates insulin resistance by inhibiting JNK and NF-kappaB inflammatory pathways in diabetic mice and HepG2 cell models. J. Agric. Food Chem. 65 (41), 9041–9053. 10.1021/acs.jafc.7b02880 [DOI] [PubMed] [Google Scholar]
- Oh J., Lee H., Lim H., Woo S., Shin S. S., Yoon M. (2015). The herbal composition GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica inhibits visceral obesity and insulin resistance by upregulating visceral adipose genes involved in fatty acid oxidation. Pharm. Biol. 53 (2), 301–312. 10.3109/13880209.2014.917328 [DOI] [PubMed] [Google Scholar]
- Ooi J., Adamu H. A., Imam M. U., Ithnin H., Ismail M. (2018). Polyphenol-rich ethyl acetate fraction isolated from Molineria latifolia ameliorates insulin resistance in experimental diabetic rats via IRS1/AKT activation. Biomed. Pharmacother. 98, 125–133. 10.1016/j.biopha.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Ozcan U., Cao Q., Yilmaz E., Lee A., Iwakoshi N., Ozdelen E., et al. (2004). Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306 (5695), 457–461. 10.1126/science.1103160 [DOI] [PubMed] [Google Scholar]
- Pandey J., Maurya R., Raykhera R., Srivastava M. N., Yadav P. P., Tamrakar A. K. (2014). Murraya koenigii (L). J. Sci. Food Agric. 94 (11), 2282–2288. 10.1002/jsfa.6555 [DOI] [PubMed] [Google Scholar]
- Parimala M., Debjani M., Vasanthi H. R., Shoba F. G. (2015). Nymphaea nouchali Burm. J. Adv. Pharm. Technol. Res. 6 (4), 183–189. 10.4103/2231-4040.165013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. H., Kim D. H., Park M. H., Kim M. K., Kim N. D., Kim C. M., et al. (2014). Chinese prescription Kangen-karyu and Salviae Miltiorrhizae Radix improve age-related oxidative stress and inflammatory response through the PI3K/Akt or MAPK pathways. Am. J. Chin. Med. 42 (4), 987–1005. 10.1142/S0192415X14500621 [DOI] [PubMed] [Google Scholar]
- Park E. C., Kim S. I., Hong Y., Hwang J. W., Cho G. S., Cha H. N., et al. (2014). Inhibition of CYP4A reduces hepatic endoplasmic reticulum stress and features of diabetes in mice. Gastroenterology 147 (4), 860–869. 10.1053/j.gastro.2014.06.039 [DOI] [PubMed] [Google Scholar]
- Park S. E., Park C. Y., Sweeney G. (2015). Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit. Rev. Clin. Lab. Sci: 52 (4), 180–190. 10.3109/10408363.2015.1023429 [DOI] [PubMed] [Google Scholar]
- Patel S., Woodgett J. (2008). Glycogen synthase kinase-3 and cancer: good cop, bad cop? Cancer Cell. 14 (5), 351–353. 10.1016/j.ccr.2008.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraldi P., Xu M., Spiegelman B. (1997). Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling. J. Clin. Invest. 100 (7), 1863–1869. 10.1172/JCI119715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rubio K. G., Gonzalez-Ortiz M., Martinez-Abundis E., Robles-Cervantes J. A., Espinel-Bermudez M. C. (2013). Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 11 (5), 366–369. 10.1089/met.2012.0183 [DOI] [PubMed] [Google Scholar]
- Qiang G., Wenzhai C., Huan Z., Yuxia Z., Dongdong Y., Sen Z., et al. (2015). Effect of Sancaijiangtang on plasma nitric oxide and endothelin-1 levels in patients with type 2 diabetes mellitus and vascular dementia: a single-blind randomized controlled trial. J. Tradit. Chin. Med. 35 (4), 375–380. 10.1016/S0254-6272(15)30112-6 [DOI] [PubMed] [Google Scholar]
- Qin J. H., Ma J. Z., Yang X. W., Hu Y. J., Zhou J., Fu L. C., et al. (2015). A triterpenoid inhibited hormone-induced adipocyte differentiation and alleviated dexamethasone-induced insulin resistance in 3T3-L1 adipocytes. Nat. Prod. Bioprospect. 5 (3), 159–166. 10.1007/s13659-015-0063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiei K., Ebrahimzadeh M. A., Saeedi M., Bahar A., Akha O., Kashi Z. (2018). Effects of a hydroalcoholic extract of Juglans regia (walnut) leaves on blood glucose and major cardiovascular risk factors in type 2 diabetic patients: a double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 18 (1), 206. 10.1186/s12906-018-2268-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafraf M., Zemestani M., Asghari-Jafarabadi M. (2015). Effectiveness of chamomile tea on glycemic control and serum lipid profile in patients with type 2 diabetes. J. Endocrinol. Invest. 38 (2), 163–170. 10.1007/s40618-014-0170-x [DOI] [PubMed] [Google Scholar]
- Reeds D. N., Patterson B. W., Okunade A., Holloszy J. O., Polonsky K. S., Klein S. (2011). Ginseng and ginsenoside Re do not improve beta-cell functionor insulin sensitivity in overweight and obese subjects with impaired glucosetolerance or diabetes. Diabetes Care 34, 1071–1076. 10.2337/dc10-2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard A. J., Burris T. P., Sanchez-Infantes D., Wang Y., Ribnicky D. M., Stephens J. M. (2014). Artemisia extracts activate PPARgamma, promote adipogenesis, and enhance insulin sensitivity in adipose tissue of obese mice. Nutrition 30 (7–8 Suppl), S31–36. 10.1016/j.nut.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E., Hargreaves M. (2013). Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 93 (3), 993–1017. 10.1152/physrev.00038.2012 [DOI] [PubMed] [Google Scholar]
- Riehle C., Abel E. D. (2016). Insulin signaling and heart failure. Circ. Res. 118 (7), 1151–1169. 10.1161/CIRCRESAHA.116.306206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinella M. E. (2015). Nonalcoholic fatty liver disease: a systematic review. JAMA 313 (22), 2263–2273. 10.1001/jama.2015.5370 [DOI] [PubMed] [Google Scholar]
- Roshan H., Nikpayam O., Sedaghat M., Sohrab G. (2018). Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: a randomised clinical trial. Br. J. Nutr. 119 (3), 250–258. 10.1017/S0007114517003439 [DOI] [PubMed] [Google Scholar]
- Rui L., Aguirre V., Kim J., Shulman G., Lee A., Corbould A., et al. (2001). Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Invest. 107 (2), 181–189. 10.1172/JCI10934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M., Araki E., Miralpeix M., Rothenberg P., White M., Kahn C. (1992). Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J. Clin. Invest. 90 (5), 1839–1849. 10.1172/JCI116060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G., Cavanagh-Kyros J., Ko H., Jung D., Gray S., Jun J., et al. (2009). Prevention of steatosis by hepatic JNK1. Cell Metab. 10 (6), 491–498. 10.1016/j.cmet.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Nishida M., Saito M., Tanabe A., Eitsuka T., Yuan S. H., et al. (2016). The fruit of Acanthopanax senticosus (Rupr. Nutr. Res. 36 (10), 1090–1097. 10.1016/j.nutres.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Saltiel A., Kahn C. (2001). Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414 (6865), 799–806. 10.1038/414799a [DOI] [PubMed] [Google Scholar]
- Samadi N., Mozaffari-Khosravi H., Rahmanian M., Askarishahi M. (2017). Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: a randomized, double-blind clinical trial. J. Integr. Med. 15 (2), 124–134. 10.1016/S2095-4964(17)60315-7 [DOI] [PubMed] [Google Scholar]
- Samuel V. T., Shulman G. I. (2016). The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 126 (1), 12–22. 10.1172/JCI77812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Eguez L., Teruel M. N., Fukuda M., Chuang T. D., Chavez J. A., et al. (2007). Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 5 (4), 293–303. 10.1016/j.cmet.2007.03.001 [DOI] [PubMed] [Google Scholar]
- Sasaki H., Asanuma H., Fujita M., Takahama H., Wakeno M., Ito S., et al. (2009). Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation 119 (19), 2568–2577. 10.1161/CIRCULATIONAHA.108.798561 [DOI] [PubMed] [Google Scholar]
- Sathiyabama R. G., Rajiv Gandhi G., Denadai M., Sridharan G., Jothi G., Sasikumar P., et al. (2018). Evidence of insulin-dependent signalling mechanisms produced by Citrus sinensis (L). Food Chem. Toxicol. 116 (Pt B), 86–99. 10.1016/j.fct.2018.03.050 [DOI] [PubMed] [Google Scholar]
- Seki E., Brenner D. A., Karin M. (2012). A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 143 (2), 307–320. 10.1053/j.gastro.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo E., Kim S., Lee S. J., Oh B. C., Jun H. S. (2015). Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice. Nutrients 7 (4), 3038–3053. 10.3390/nu7043038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H. A., Lee I. K. (2013). The role of Nrf2: adipocyte differentiation, obesity, and insulin resistance. Oxid. Med. Cell Longev. 2013, 184598. 10.1155/2013/184598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Honma N., Kobayashi K., Jia L. N., Hosono T., Shindo K., et al. (2014). Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS One 9 (2), e87894. 10.1371/journal.pone.0087894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Zhou F., Li X., Chang B., Li D., Wang Y., et al. (2013). Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab. 18 (1), 86–98. 10.1016/j.cmet.2013.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. L., Liu W. J., Zhang X. F., Su W. J., Chen N. N., Lu S. H., et al. (2016). Effect of Chinese herbal medicine Jinlida granule in treatment of patients with impaired glucose tolerance. Chin. Med. J. (Engl) 129 (19), 2281–2286. 10.4103/0366-6999.190676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I., Matsuda M., Hammer R., Bashmakov Y., Brown M., Goldstein J. (2000). Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 6 (1), 77–86. 10.1016/S1097-2765(05)00010-9 [DOI] [PubMed] [Google Scholar]
- Singh J., Kakkar P. (2013). Modulation of liver function, antioxidant responses, insulin resistance and glucose transport by Oroxylum indicum stem bark in STZ induced diabetic rats. Food Chem. Toxicol. 62, 722–731. 10.1016/j.fct.2013.09.035 [DOI] [PubMed] [Google Scholar]
- Siwicki M., Engblom C., Pittet M. J. (2016). Gal3 links inflammation and insulin resistance. Cell Metab. 24 (5), 655–656. 10.1016/j.cmet.2016.10.014 [DOI] [PubMed] [Google Scholar]
- Stenbit A., Tsao T., Li J., Burcelin R., Geenen D., Factor S., et al. (1997). GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat. Med. 3 (10), 1096–1101. 10.1038/nm1097-1096 [DOI] [PubMed] [Google Scholar]
- Sun X., Sun H., Zhang J., Ji X. (2016). Artemisia extract improves insulin sensitivity in women with gestational diabetes mellitus by up-regulating adiponectin. J. Clin. Pharmacol. 56 (12), 1550–1554. 10.1002/jcph.755 [DOI] [PubMed] [Google Scholar]
- Sun Z., Zhan L., Liang L., Sui H., Zheng L., Sun X., et al. (2016). ZiBu PiYin recipe prevents diabetes-associated cognitive decline in rats: possible involvement of ameliorating mitochondrial dysfunction, insulin resistance pathway and histopathological changes. BMC Complement. Altern. Med. 16, 200. 10.1186/s12906-016-1177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendroedi J., Phielix E., Roden M. (2011). The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 8 (2), 92–103. 10.1038/nrendo.2011.138 [DOI] [PubMed] [Google Scholar]
- Talbot K., Wang H. Y. (2014). The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement. 10 (1 Suppl), S12–25. 10.1016/j.jalz.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamemoto H., Kadowaki T., Tobe K., Yagi T., Sakura H., Hayakawa T., et al. (1994). Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372 (6502), 182–186. 10.1038/372182a0 [DOI] [PubMed] [Google Scholar]
- Tang M., Gao G., Rueda C. B., Yu H., Thibodeaux D. N., Awano T., et al. (2017). Brain microvasculature defects and Glut1 deficiency syndrome averted by early repletion of the glucose transporter-1 protein. Nat. Commun. 8, 14152. 10.1038/ncomms14152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi C., Ueki K., Kahn R. (2005). Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J. Clin. Invest. 115 (3), 718–727. 10.1172/JCI23187 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thorens B., Wu Y., Leahy J., Weir G. (1992). The loss of GLUT2 expression by glucose-unresponsive beta cells of db/db mice is reversible and is induced by the diabetic environment. J. Clin. Invest. 90 (1), 77–85. 10.1172/JCI115858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiganis T. (2013). PTP1B and TCPTP – nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J. 280 (2), 445–458. 10.1111/j.1742-4658.2012.08563.x [DOI] [PubMed] [Google Scholar]
- Tuttle R., Gill N., Pugh W., Lee J., Koeberlein B., Furth E., et al. (2001). Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat. Med. 7 (10), 1133–1137. 10.1038/nm1001-1133 [DOI] [PubMed] [Google Scholar]
- Vallerie S., Hotamisligil G. (2010). The role of JNK proteins in metabolism. Sci. Transl. Med. 2 (60), 60rv65. 10.1126/scitranslmed.3001007 [DOI] [PubMed] [Google Scholar]
- Wang C., Li Y., Hao M., Li W. (2018). Astragaloside IV inhibits triglyceride accumulation in insulin-resistant HepG2 cells via AMPK-induced SREBP-1c phosphorylation. Front. Pharmacol. 9, 345. 10.3389/fphar.2018.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Wang X., Yu X. Z., Xu W. T. (2016). Potent therapeutic effects of shouwu jiangqi decoction on polycystic ovary syndrome with insulin resistance in rats. Chin. J. Integr. Med. 22 (2), 116–123. 10.1007/s11655-015-2147-9 [DOI] [PubMed] [Google Scholar]
- Wang L., Ye X., Hua Y., Song Y. (2018). Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice. Biomed. Pharmacother. 105, 121–129. 10.1016/j.biopha.2018.05.110 [DOI] [PubMed] [Google Scholar]
- Wang X. A., Zhang R., She Z. G., Zhang X. F., Jiang D. S., Wang T., et al. (2014). Interferon regulatory factor 3 constrains IKKbeta/NF-kappaB signaling to alleviate hepatic steatosis and insulin resistance. Hepatology 59 (3), 870–885. 10.1002/hep.26751 [DOI] [PubMed] [Google Scholar]
- Wang Z. Q., Zhang X. H., Yu Y., Tipton R. C., Raskin I., Ribnicky D., et al. (2013). Artemisia scoparia extract attenuates non-alcoholic fatty liver disease in diet-induced obesity mice by enhancing hepatic insulin and AMPK signaling independently of FGF21 pathway. Metabolism 62 (9), 1239–1249. 10.1016/j.metabol.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M. J., Dzamko N., Thomas W. G., Rose-John S., Ernst M., Carling D., et al. (2006). CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat. Med. 12 (5), 541–548. 10.1038/nm1383 [DOI] [PubMed] [Google Scholar]
- Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M. T., et al. (2011). Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12 (5), 408–415. 10.1038/ni.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensveen F. M., Jelencic V., Valentic S., Sestan M., Wensveen T. T., Theurich S., et al. (2015). NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat. Immunol. 16 (4), 376–385. 10.1038/ni.3120 [DOI] [PubMed] [Google Scholar]
- White M. (2003). Insulin signaling in health and disease. Science 302 (5651), 1710–1711. 10.1126/science.1092952 [DOI] [PubMed] [Google Scholar]
- Willette A. A., Johnson S. C., Birdsill A. C., Sager M. A., Christian B., Baker L. D., et al. (2015). Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 11 (5), 504–510 e501. 10.1016/j.jalz.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer D. A., Luck H., Tsai S., Winer S. (2016). The intestinal immune system in obesity and insulin resistance. Cell Metab. 23 (3), 413–426. 10.1016/j.cmet.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Winnay J., Solheim M., Dirice E., Sakaguchi M., Noh H., Kang H., et al. (2016). PI3-kinase mutation linked to insulin and growth factor resistance in vivo. J. Clin. Invest. 126 (4), 1401–1412. 10.1172/JCI84005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerdeman J., Del Rio D., Calani L., Eringa E. C., Smulders Y. M., Serne E. H. (2018). Red wine polyphenols do not improve obesity-associated insulin resistance: a randomized controlled trial. Diabetes Obes. Metab. 20 (1), 206–210. 10.1111/dom.13044 [DOI] [PubMed] [Google Scholar]
- Wood I. S., Trayhurn P. (2003). Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br. J. Nutr. 89 (1), 3–9. 10.1079/BJN2002763 [DOI] [PubMed] [Google Scholar]
- Xiao B., Heath R., Saiu P., Leiper F. C., Leone P., Jing C., et al. (2007). Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 449 (7161), 496–500. 10.1038/nature06161 [DOI] [PubMed] [Google Scholar]
- Xiao B., Sanders M. J., Underwood E., Heath R., Mayer F. V., Carmena D., et al. (2011). Structure of mammalian AMPK and its regulation by ADP. Nature 472 (7342), 230–233. 10.1038/nature09932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N., Yang L. L., Yang Y. L., Liu L. W., Li J., Liu B., et al. (2017). Ginsenoside Rg5 inhibits succinate-associated lipolysis in adipose tissue and prevents muscle insulin resistance. Front. Pharmacol. 8, 43. 10.3389/fphar.2017.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Q., Chen Y. (2018). Antidiabetic effects of a Chinese herbal medicinal compound Sangguayin preparation via PI3K/Akt signaling pathway in db/db mice. Evid. Based Complement. Alternat. Med. 2018, 2010423. 10.1155/2018/2010423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Pan F., Xu H. (2015). [Effect of Zhenggan Tang decoction on the serum levels of leptin, adiponectin and insulin resistance on HBV-induced cirrhotic patients]. Zhonghua Liu Xing Bing Xue Za Zhi. 36 (4), 399–401. [PubMed] [Google Scholar]
- Yagishita Y., Uruno A., Fukutomi T., Saito R., Saigusa D., Pi J., et al. (2017). Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Rep. 18 (8), 2030–2044. 10.1016/j.celrep.2017.01.064 [DOI] [PubMed] [Google Scholar]
- Yan F., Dai G., Zheng X. (2016). Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. J. Nutr. Biochem. 36, 68–80. 10.1016/j.jnutbio.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Yan J., Wang C., Jin Y., Meng Q., Liu Q., Liu Z., et al. (2018). Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 130, 466–480. 10.1016/j.phrs.2017.12.026 [DOI] [PubMed] [Google Scholar]
- Yang J., Leng J., Li J. J., Tang J. F., Li Y., Liu B. L., et al. (2016). Corosolic acid inhibits adipose tissue inflammation and ameliorates insulin resistance via AMPK activation in high-fat fed mice. Phytomedicine 23 (2), 181–190. 10.1016/j.phymed.2015.12.018 [DOI] [PubMed] [Google Scholar]
- Yang J.-L., Wang J.-L., Huang F., Liu K., Liu B.-L. (2014). Modified Si-Miao-San inhibits inflammation and promotes glucose disposal in adipocytes through regulation of AMP-kinase. Chin. J. Nat. Med. 12 (12), 911–919. 10.1016/S1875-5364(14)60134-5 [DOI] [PubMed] [Google Scholar]
- Yang S. J., Choi J. M., Park S. E., Rhee E. J., Lee W. Y., Oh K. W., et al. (2015). Preventive effects of bitter melon (Momordica charantia) against insulin resistance and diabetes are associated with the inhibition of NF-kappaB and JNK pathways in high-fat-fed OLETF rats. J. Nutr. Biochem. 26 (3), 234–240. 10.1016/j.jnutbio.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Yang X., Li L., Fang K., Dong R., Li J., Zhao Y., et al. (2017). Wu-Mei-Wan reduces insulin resistance via inhibition of NLRP3 inflammasome activation in HepG2 cells. Evid. Based Complement. Alternat. Med. 2017, 7283241. 10.1155/2017/7283241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Li W., Li Y., Wang Q., Gao L., Zhao J. (2014). Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid. Med. Cell Longev. 2014, 145641. 10.1155/2014/145641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Wu F., He Y., Zhang Q., Zhang Y., Zhou G., et al. (2018). A novel PTP1B inhibitor extracted from Ganoderma lucidum ameliorates insulin resistance by regulating IRS1-GLUT4 cascades in the insulin signaling pathway. Food Funct. 9 (1), 397–406. 10.1039/C7FO01489A [DOI] [PubMed] [Google Scholar]
- Yao L., Wei J., Shi S., Guo K., Wang X., Wang Q., et al. (2017). Modified lingguizhugan decoction incorporated with dietary restriction and exercise ameliorates hyperglycemia, hyperlipidemia and hypertension in a rat model of the metabolic syndrome. BMC Complement. Altern. Med. 17 (1), 132. 10.1186/s12906-017-1557-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H. N., Park M. H., Hwang S. Y., Han J. S. (2018). Nardostachys jatamansi DC extract alleviates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice through the AMP-activated protein kinase signaling pathway. J. Med. Food. 21 (4), 324–331. 10.1089/jmf.2017.4015 [DOI] [PubMed] [Google Scholar]
- Yu N., Fang X., Zhao D., Mu Q., Zuo J., Ma Y., et al. (2017). Anti-diabetic effects of Jiang Tang Xiao Ke granule via PI3K/Akt signalling pathway in type 2 diabetes KKAy mice. PLoS One 12 (1), e0168980. 10.1371/journal.pone.0168980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. H., Chen S. Y., Li W. S., Dubey N. K., Chen W. H., Chuu J. J., et al. (2015). Hypoglycemic activity through a novel combination of fruiting body and mycelia of Cordyceps militaris in high-fat diet-induced type 2 diabetes mellitus mice. J. Diabetes Res. 2015, 723190. 10.1155/2015/723190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Xu L., Zhou Q., Wu S., Tian J., Piao C., et al. (2018). The efficacy and safety of the Chinese herbal formula, JTTZ, for the treatment of type 2 diabetes with obesity and hyperlipidemia: a multicenter randomized, positive-controlled, open-label clinical trial. Int. J. Endocrinol. 2018, 9519231. 10.1155/2018/9519231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Zhao Y., Teng F., Li J., Guan Y., Xu J., et al. (2018). Berberine improves cognitive deficiency and muscular dysfunction via activation of the AMPK/SIRT1/PGC-1a pathway in skeletal muscle from naturally aging rats. J. Nutr. Health Aging 22 (6), 710–717. 10.1007/s12603-018-1015-7 [DOI] [PubMed] [Google Scholar]
- Yuan Y., Wang X., Lu X., Marunaka Y., Wang X. (2014). Effect of Coptidis Rhizoma extracts in a water-based solution on insulin resistance in 3T3-L1 adipocytes. Biomed. Res. 35 (5), 321–327. 10.2220/biomedres.35.321 [DOI] [PubMed] [Google Scholar]
- Zanquetta M., Seraphim P., Sumida D., Cipolla-Neto J., Machado U. (2003). Calorie restriction reduces pinealectomy-induced insulin resistance by improving GLUT4 gene expression and its translocation to the plasma membrane. J. Pineal. Res. 35 (3), 141–148. 10.1034/j.1600-079X.2003.00067.x [DOI] [PubMed] [Google Scholar]
- Zhang L., Xu J., Song H., Yao Z., Ji G. (2014). Extracts from Salvia-Nelumbinis naturalis alleviate hepatosteatosis via improving hepatic insulin sensitivity. J. Transl. Med. 12 ( (Electronic)), 236–247. 10.1186/s12967-014-0236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Ta N., Chen P., Wang H. (2017). Erchen decoction and Linguizhugan decoction ameliorate hepatic insulin resistance by inhibiting IRS-1Ser307 phosphorylation in vivo and in vitro. Evid. Based Complement. Alternat. Med. 2017, 1589871. 10.1155/2017/1589871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Lu J., Jing Y., Tang S., Zhu D., Bi Y. (2017). Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann. Med. 49 (2), 106–116. 10.1080/07853890.2016.1231932 [DOI] [PubMed] [Google Scholar]
- Zhang T. (2015). Effect of Qingre Yangyin recipe on endocrine and metabolism of polycystic ovary syndrome patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 35 (10), 1175–1180. [PubMed] [Google Scholar]
- Zhang Z., Zhou S., Jiang X., Wang Y. H., Li F., Wang Y. G., et al. (2015). The role of the Nrf2/Keap1 pathway in obesity and metabolic syndrome. Rev. Endocr. Metab. Disord. 16 (1), 35–45. 10.1007/s11154-014-9305-9 [DOI] [PubMed] [Google Scholar]
- Zhao H., Huang X., Jiao J., Zhang H., Liu J., Qin W., et al. (2015). Protein phosphatase 4 (PP4) functions as a critical regulator in tumor necrosis factor (TNF)-alpha-induced hepatic insulin resistance. Sci. Rep. 5, 18093. 10.1038/srep18093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhou D., Chen Y., Liu D., Chu S., Zhang S. (2017). Beneficial effects of Heqi san on rat model of polycystic ovary syndrome through the PI3K/AKT pathway. Daru. 25 (1), 21. 10.1186/s40199-017-0188-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Fu Z., Wu J., Aylor K. W., Barrett E. J., Cao W., et al. (2015). Globular adiponectin ameliorates metabolic insulin resistance via AMPK-mediated restoration of microvascular insulin responses. J. Physiol. 593 (17), 4067–4079. 10.1113/JP270371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Qiu B., Li Q., Zhang T., Zhao H., Chen Z., et al. (2014). LBP-4a improves insulin resistance via translocation and activation of GLUT4 in OLETF rats. Food Funct. 5 (4), 811–820. 10.1039/C3FO60602C [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang M., Shao L., Liao M., Liu K., Huang F., et al. (2014). The total phenolic fraction of Anemarrhena asphodeloides inhibits inflammation and reduces insulin resistance in adipocytes via regulation of AMP-kinase activity. Planta Med. 80 (2–3), 146–152. 10.1055/s-0033-1360197 [DOI] [PubMed] [Google Scholar]
- Zhen Z., Chang B., Li M., Lian F. M., Chen L., Dong L., et al. (2011). Anti-diabetic effects of a Coptis chinensis containing new traditional Chinese medicine formula in type 2 diabetic rats. Am. J. Chin. Med. 39 (1), 53–63. 10.1142/S0192415X11008646 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Ley S. H., Hu F. B. (2018). Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14 (2), 88–98. 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- Zhou B., Lu Y., Hajifathalian K., Bentham J., Di Cesare M., Danaei G., et al. (2016). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4•4 million participants. Lancet 387 (10027), 1513–1530. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Chang B., Chen X. Y., Zhou S. P., Zhen Z., Zhang L. L., et al. (2014). Chinese herbal medicine for obesity: a randomized, double-blinded, multicenter, prospective trial. Am. J. Chin. Med. 42 (6), 1345–1356. 10.1142/S0192415X14500840 [DOI] [PubMed] [Google Scholar]
- Zhu L., Zhu X., Sun G., Meng X., Wang M., Cui H., et al. (2018). Dai-Zong-Fang, a traditional chinese herbal formula, ameliorates insulin resistance in db/db Mice. Front. Physiol. 9, 224. 10.3389/fphys.2018.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R., Zheng J., Chen L., Gu B., Huang S. (2016). Astragaloside IV facilitates glucose transport in C2C12 myotubes through the IRS1/AKT pathway and suppresses the palmitate-induced activation of the IKK/IkappaBalpha pathway. Int. J. Mol. Med. 37 (6), 1697–1705. 10.3892/ijmm.2016.2555 [DOI] [PubMed] [Google Scholar]
- Zisman A., Peroni O., Abel E., Michael M., Mauvais-Jarvis F., Lowell B., et al. (2000). Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 6 (8), 924–928. 10.1038/78693 [DOI] [PubMed] [Google Scholar]
- Zou X., Huang W., Lu F., Fang K., Wang D., Zhao S., et al. (2017). The effects of Jiao-Tai-Wan on sleep, inflammation and insulin resistance in obesity-resistant rats with chronic partial sleep deprivation. BMC Complement. Altern. Med. 17 (1), 165. 10.1186/s12906-017-1648-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulet M. A., Navas-Carretero S., Lara y Sanchez D., Abete I., Flanagan J., Issaly N., et al. (2014). A Fraxinus excelsior L. Phytomedicine 21 (10), 1162–1169. 10.1016/j.phymed.2014.04.027 [DOI] [PubMed] [Google Scholar]
- Zuniga L. Y., Gonzalez-Ortiz M., Martinez-Abundis E. (2017). Effect of Gymnema sylvestre administration on metabolic syndrome, insulin sensitivity, and insulin secretion. J. Med. Food. 20 (8), 750–754. 10.1089/jmf.2017.0001 [DOI] [PubMed] [Google Scholar]