Abstract
Aims
Hypoxia-inducible factor-1 alpha (HIF-1α) is a key transcription factor responsible for the induction of genes that facilitate adaptation to hypoxia. To study HIF-1 signalling in the heart, we developed a mouse model in which an oxygen-stable form of HIF-1α can be inducibly expressed in cardiac myocytes, under the regulation of tetracycline.
Methods and results
Remarkably, expression of the transgene in mice generated two distinct phenotypes. One was the expected expression of HIF-regulated transcripts and associated changes in cardiac angiogenesis and contractility. The other was an unresponsive phenotype with much less expression of typical HIF-response genes and substantial expression of a zinc-finger protein, Protein Kinase C Binding Protein 1 (PRKCBP1). We have demonstrated that this second phenotype is due to an insertion of a fragment of DNA upstream of the PRKCBP1 gene that contains two additional canonical HIF binding sites and leads to substantial HIF binding, assessed by chromatin immunoprecipitation, and transcriptional activation. This insertion is found only in the FVB strain of mice that contributed the αMHC-tet binding protein transgene to these biallelic mice. In HEK293 cells transfected with oxygen-stable HIF-1α and PRKCBP1, we demonstrated inhibition of HIF-1 activity by a luciferase reporter assay. Using mouse primary cells and cell lines, we show that transfection with oxygen-stable HIF-1α and PRKCBP1 reduced expression of direct HIF-1 gene targets and that knockdown of PRKCBP1 removes that negative inhibition. Consistent with previous reports suggesting that PRKCBP1 modulates the chromatin landscape, we found that HL-1 cells transfected with oxygen-stable HIF-1α and PRKCBP1 have reduced global 5-methyl cytosine compared to HIF-1 alone.
Conclusion
We show genetic, transcriptional, biochemical, and physiological evidence that PRKCBP1 inhibits HIF activity. Identification of a new oxygen-dependent and previously unsuspected regulator of HIF may provide a target for new therapeutic approaches to ischaemic heart disease.
Keywords: HIF inhibition , Genetic variance , Zmynd8 , RACK7 , transcriptional repressor
1. Introduction
Hypoxia-inducible factor 1 (HIF-1) is a transcription factor that regulates hundreds of genes that mediate the cellular response to hypoxia. HIF is stabilized in areas of ischaemia and plays an important role in the physiology of hypoxic response, enhancing angiogenesis, glycolytic metabolism, and cell survival. Clinical pathologies involving HIF are equally broad, including abnormal embryonic vasculogenesis, Von Hippel-Lindau syndrome, ischaemic disease of the heart, extremities, and cerebrovasculature, and cancer angiogenesis.1,2 Given the important role of oxygen signalling pathways in the pathogenesis of these diseases, there is substantial clinical interest in factors that regulate HIF.
HIF-1 is a heterodimer of a labile HIF-1α subunit and the constitutively expressed HIF-1β subunit. Under hypoxic conditions, HIF-1α translocates to the nucleus, dimerizes with HIF-1β, and binds to hypoxia response elements (HREs) via basic helix-loop-helix domains in the promoters of oxygen-responsive genes.3 However, in the presence of oxygen HIF-1α is hydroxylated by prolyl hydroxylases at two Pro residues4–6 which leads to rapid degradation by the ubiquitin-proteasome pathway.7 An additional regulatory mechanism involves hydroxylation at an Asn of HIF-1α by the factor inhibiting HIF-1 (FIH-1), which reduces transcriptional activity of HIF.8 Thus, HIF-1α stability and its capacity to regulate downstream targets are tightly regulated by distinct but synergistic mechanisms.
The Prkcbp1 gene (known also as both Zmynd8 and Rack7) contains a variety of conserved motifs that are known to mediate interaction with DNA and transcription factors, including a plant homeodomain (PHD), bromodomain (BRD), zinc finger-MYND (myeloid, Nervy, and DEAF-1) domain, and nucleosome-binding PWWP (Pro-Trp-Trp-Pro) domain. Protein Kinase C Binding Protein 1 (PRKCBP1) was initially identified as a RACK (receptor for activated C-kinase) protein that binds to activated protein kinase C beta I.9 Subsequently, mutations and dysregulation of PRKCBP1 have been found in a variety of human cancers.10–13 Not surprisingly, PRKCBP1 was identified as the ninth strongest gene target of HIF-1α in MCF-7 breast cancer cells by chromatin immunoprecipitation (ChIP)-seq.14 Recently, it was found to play a tumour-suppressive role by directing histone methylation in the enhancer regions of genes associated with tumour growth, invasion, and migration.15 HIF-1α up-regulation and stabilization is a common feature of both cancer and myocardial ischaemia. Two studies have correlated differential regulation of PRKCBP1 with pathology in the human myocardium,16,17 however, its role in the heart has not been systematically evaluated.
Here, we present a potent and previously unsuspected regulatory mechanism of HIF action in the hypoxic heart. We have shown that PRKCBP1 is induced by ischaemia as well as HIF-1, it localizes with HIF in the nucleus, and its knockdown and over-expression alters downstream HIF-1-regulated genes. These findings incidentally suggest an explanation for the greater susceptibility of the FVB mouse strain to infarct-related heart failure, but more importantly imply that this protein can modify the pathophysiology of ischaemia.
2. Methods
2.1 Transgenic animal generation
All animal work was approved by and carried out in accordance with the University of Hawaii Institutional Animal Care and Use Committee (IACUC approval number 07-100-3), and complied with the standards stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Bethesda, MD, USA). The generation of our transgenic model has been previously described.18 Briefly it utilizes an αMHC-driven Tet-off binding protein (obtained in an FVB background), and a transgenic HIF-1α cDNA (in a C57Bl/6 strain) that had been mutagenized to substitute alanine for the two prolines that confer oxygen-destabilization, and the asparagine that is required for maximal transcriptional activity (Pro402, Pro564 Asn803 to Ala; denoted HIF-1α-PPN). The oxygen stable HIF-1α transgene included a carboxy-terminal HA tag. Double transgenic mice (tTA/HIF-1α-PPN) were maintained on 200 μg doxycycline per mL of 2.5% sucrose-water to suppress HIF-1α-PPN expression. All animals were treated with doxycycline from conception to 6 weeks. Thereafter, doxycycline was omitted for varying periods to assess effects of the mutated HIF-1α transgene. All experiments used 6–8-week-old male mice. Mice were euthanized by CO2, hearts were excised, frozen in liquid nitrogen, and stored at –80°C until processing for genomic DNA, RNA, or protein lysates.
2.2 Echocardiography
We evaluated left ventricular function in unsedated mice with transthoracic echocardiography (Sonos 5500 machine, Philips, Andover, MA, USA) using a S12 transducer (12 MHz). We examined both responsive and unresponsive HIF-1α-PPN transgenic mice at 7 days or 14 days after omitting doxycycline (n = 5 for each phenotype at each time point). Left ventricular parasternal short-axis views were obtained in M-mode imaging at the level of the papillary muscle. Two consecutive beats in three M-mode images (six beats total) were used for measurements of left ventricular end-diastolic internal diameter (LVEDD), left ventricular end-systolic internal diameter (LVESD), and posterior wall thickness. Fractional shortening (FS) was calculated as FS% = [(LVEDD−LVESD)/LVEDD] × 100.
2.3 Myocardial infarctions
Myocardial infarctions were performed on 6–8-week-old C57Bl/6 and FVB mice by permanent ligation of the left anterior descending artery (LAD) (n = 5 per group), as previously described.19 Briefly, mice were anaesthetized with isoflurane (4%) and the left side of their chest shaved. Following endotracheal intubation, animals were placed supine on the surgical table over a heating pad and mechanically ventilated. Isoflurane (1.8%) was used to maintain general anaesthesia. A left-lateral thoracotomy between the 3rd and the 4th rib was used for access. The pericardial sac was opened and the LAD ligated with 8-0 Prolene suture (Ethicon, Norderstedt, Germany). Lead I and II electrocardiograms (ECG) were recorded continuously (Power Laboratory, AD Instruments, Mountain View, CA, USA). Visual blanching and ST segment elevation on continuous ECG display confirmed myocardial ischaemia. Three days after LAD ligation mice were sacrificed for further histological evaluation, as detailed below.
2.4 ChIP assays
After 1 or 3 days of induction of the HIF-1α transgene, responsive hearts were identified by obvious ventricular dilation and epicardial vascular dilation. Non-responsive hearts looked grossly normal. DNA was isolated from hearts with both phenotypes, subjected to ChIP, using Protein A magnetic beads according to manufacturer’s protocol (Magna ChIP, Millipore, Billerica, MA, USA) with an antibody directed against the HA-tag on the transgenic HIF protein (Roche, Mannheim, Germany). Fragments immunoprecipitated from the ChIP assay were ligated into pZEr0-1 plasmids, cloned into TOP 10 Escherichia coli (Invitrogen, Carlsbad, CA, USA), and sequenced using plasmid-specific T7 sequencing primers. Resulting sequences were mapped to the mouse genome (UCSC genome browser), following nucleotide BLAST analyses (NCBI).
2.5 Sequencing
After Prkcbp1 was implicated as important in the unresponsive phenotype by ChIP, the exons of Prkcbp1 were sequenced in both responsive and unresponsive mice. Sequence was generated until there was at least triple coverage on the complete region of interest. Sequences were aligned and consensus sequence was generated using Clustalx software.20 +4 KB and −2 KB of the untranslated regions were also generated via PCR and sequenced (Supplementary material online, Table S1). PCR primers were subsequently designed to interrogate the promoter region of PRKCBP1 for location (L set) and for the existence of the inserted sequence (E set) (Supplementary material online, Table S2).
2.6 HIF-inducible luciferase assay
Constitutive activity of the HIF-1α-PPN construct was assessed by transient transfection of a CMV-driven HIF-1α-PPN expression vector into a HEK-293 cell line that contained a luciferase expression construct downstream of a concatemerized HREs (n = 5; a gift from Rick Bruick).21 Modulation of the HIF-1 response was assessed by co-transfecting the HIF-1α-PPN expression vector with a CMV-driven PRKCBP1 expression vector (Open Biosystems, Huntsville, AL, USA). Luciferase activity was measured using Luciferase Reporter Assay System (Promega, Madison, WI, USA), following manufacturer’s instructions 20 h following transient transfection using the Lipofectamine 2000 transfection reagent (ThermoFisher, Waltham, MA, USA).
2.7 Western blotting
Individual whole mouse hearts were homogenized in RIPA-buffer and incubated for 1 h at 4°C. Western blots were performed on 30 μg total protein per lane with: a primary monoclonal anti-HA antibody (100 ng/mL) (Roche), a monoclonal anti-human-HIF-1α antibody (1:500) (Novus Biologicals, Littleton, CO, USA), a monoclonal anti-GAPDH (1:5000) (Sigma-Aldrich, St Louis, MO, USA), or a polyclonal rabbit anti-mouse PRKCBP1 antibody (1:1000). The anti-mouse Prkcbp1 antibody was custom developed using a BSA conjugated PRKCBP1 peptide (CAPAEKSKDSNPGSF) (GeneScript, Piscataway, NJ, USA). To reduce non-specific binding of primary antibodies, PVDF membranes were cut into 3 fractions, one containing the region 260–130 kDa for PRKCBP1, 130–60 kDa for HIF-1α, and 60–8 kDa for GAPDH, according to a pre-stained protein ladder (Li-Cor, Lincoln, NJ, USA). After incubation with secondary AlexFluor®-568-conjugated antibodies, Western blots were visualized with a Typhoon scanner (GE Lifesciences, Piscataway, NJ, USA) (535 nm excitation, 560LP emission, 500PMT). ImageJ software was used for densitometry analyses. Western blots were performed on mouse hearts of both responsive and unresponsive phenotype.
2.8 Immunohistochemistry and histology
Mice (n = 5 for each evaluation) were anaesthetized with Ketamine/Xylazine (100 mg/kg and 8 mg/kg), then perfused with PBS by cardiac puncture followed by fixation with 4% paraformaldehyde in PBS. For paraffin-embedded tissue, hearts were removed and immersed in 4% paraformaldehyde for 4 h, then transferred to 70% ethanol. Hearts were embedded in hot paraffin wax. Five micrometre sections were cut and kept on a heat block overnight. Polyclonal PRKCBP1 antibody (described above) was used as a primary antibody (1:100), with a secondary antibody of goat anti-rabbit IgG (H + L) conjugated with AlexFluor®-568 (1:250). HIF-1α antibody was a mouse monoclonal (1:200) (US Biologicals), used with a secondary antibody of sheep anti-mouse IgG (H + L) with AlexFluor®-488 (1:250). Fluorescence images were obtained using a Zeiss Axioscope 2 Plus microscope with AxioVision Release 4.7 software (Carl Zeiss MicroImaging, Thornwood, NY, USA).
2.9 Semi-quantitative real-time PCR
Total RNA from mouse hearts was isolated with TRIzol® reagent (Invitrogen) according to the manufacturer’s protocol. RNA from HL-1, MCF-7 and H9C2 cells was isolated using Qiagen RNeasy Mini Kit according to manufacturer’s protocol (Qiagen, Hilden, Germany). For real-time PCR, cDNA was produced from each individual heart (n = 3 for each time point) to allow assessment of inter-animal variation, or from cells (n = 6). Oligonucleotide primers were designed to cross an intron (Supplementary material online, Table S3). Real-time PCR was performed on cDNA representing 5 ng of total RNA. PCR was run in triplicate with QuantiTect SYBR Green (Qiagen). Tangerin (Ehbp1), which was stable on initial array analyses,18 was used as an internal control.
2.10 Neonatal cardiomyocyte isolation and culture
Neonatal (p1–p3) mice were euthanized by decapitation, hearts were harvested and washed in ice cold PBS. Atria were removed and ventricles were minced into small pieces and incubated in TrypLE (Thermo Fisher Scientific) for 15 min at RT. Tissue was pelleted by centrifugation at 500 × g for 5 min, washed with PBS and incubated with 0.08% collagenase type II (Worthington Biochemicals, Lakewood, NJ, USA) while subject to gentle rocking for 30 min at 37°C. Suspended cells were pelleted by centrifugation at 500 × g for 5 min and plated on a gelatine/fibronectin (0.04%/12.5 mg/mL) coated plate in Claycomb Media as described.22 After 24 h of culture, cells were transfected as outlined below (n = 3 per condition). Cells were placed in the hypoxia chamber 8 h after transfection.
2.11 Cell culture and siRNA
The murine HL-1 cell line was generated and provided to us by Dr Claycomb (Louisiana State University Medical Centre), and maintained in Claycomb Media as described.22 Rat ventricular cardiomyoblasts (H9C2) were maintained in DMEM containing 4 mM L-glutamine, 4500 mg/L glucose, 1 mM sodium pyruvate, and 1500 mg/L sodium bicarbonate with 10% FBS. Human mammary adenocarcinoma MCF-7 cells were maintained in DMEM with 10% FBS, as above, and supplemented with 0.01 mg/mL human recombinant insulin (Sigma). At 60% confluence, cells were transfected with a pcDNA expression vector for PRKCBP1 or the mutated oxygen-stable form of HIF-1α (HIF-1α-PPN) using FuGene HD (Promega) (Supplementary material online, Figure S2). Knockdown of PRKCBP1 was achieved by transfection of siRNA according to the manufacturer’s recommendations. siRNAprkcbp1 (Life Technologies, Carlsbad, CA, USA) was incubated with Lipofectamine RNAiMAX and Opti-MEM I Medium (Life Technologies) (Supplementary material online, Figure S3). In parallel, cells were transfected with siRNAscramble as control (Silencer® Select Negative Control No. 1 siRNA; Life Technologies) and/or a pcDNA expression vector for green fluorescent protein. In H9C2 cells that were transfected with both expression vectors and siRNA constructs, siRNA was first transfected, followed 1 h later by expression vectors. Cells were harvested after 48 h and processed for western blot or semi-quantitative real-time polymerase chain reaction analysis.
2.12 Cellular hypoxia
Cells were incubated at 37°C in 1% O2 and 5% CO2 using a Baker Ruskinn InvivO2 400 hypoxia workstation (Baker, Sanford, ME, USA). Cells were harvested after 24 h exposure, with lysis performed under hypoxia, and processed for downstream applications.
2.13 DNA methylation
The global DNA methylation status was determined on DNA isolated from HL-1 cells transfected with HIF-1α-PPN and PRKCBP1 (n = 4), and hearts of mice that were either induced or non-induced (n = 4 for each group) to express the HIF-1α transgene, using an ELISA assay (5-mC DNA ELISA kit, Zymo Research, Irvine, CA, USA). A 200 ng of genomic DNA purified from hearts was measured for 5-mC content according to the manufacturer's protocol. Assays were performed in triplicate.
2.14 Statistical analysis
Data are presented as mean ± standard deviation (SD) and were analysed by two-tailed Student’s t-test between two groups or by ANOVA when three or more groups were compared. A P value less than 0.05 was considered statistically significant.
3. Results
3.1 HIF-1α-PPN expression generates bimodal phenotypes
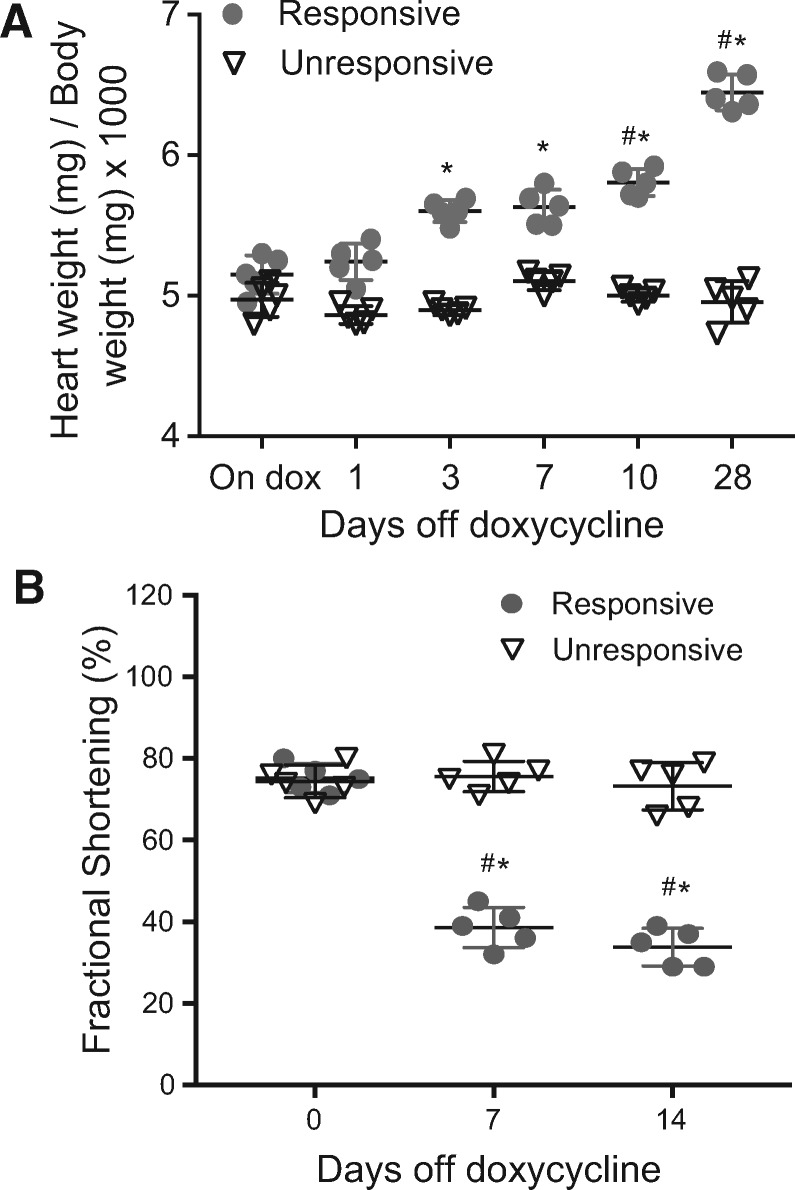
While evaluating the phenotype of tet-regulated expression of an oxygen-stable mutated HIF-1α, (Pro402, Pro564 Asn803 to Ala; denoted HIF-1α-PPN), in the hearts of transgenic mice, we recognized that a subset of genotype confirmed mice were not exhibiting phenotypic changes typically seen with HIF-1 expression and stabilization. The typical morphological effects of transgene expression in the heart include increased capillary density with increased VEGF expression as well as other components of a robust angiogenic response. We also typically see a marked deterioration in ventricular function with associated dilation.18 A portion of the bi-allelic HIF-1α-PPN mice removed from doxycycline did not show this prominent cardiac dilation nor a decline in cardiac function (Figure 1). Binding of HIF-1 to HRE in the promoter of its target genes facilitate the cellular response to low oxygen, and is responsible for the phenotypic changes seen with HIF-1 activation. Therefore, we were curious what HIF-1 was binding to in this HIF unresponsive phenotype, if not its classic gene targets. We investigated the phenotype further with ChIP for the HIF-1α transgene.
Figure 1.
HIF-1α-PPN transgene phenotypes. When induced to express the HIF-1α-PPN transgene by removal of doxycycline from the drinking water, some mice do not exhibit the expected phenotype, (A) as assessed by change in heart weight compared to body weight (n = 5 per group and time point), and (B) change in ventricular function (fractional shortening %; n = 5 per group and time point). Two-way ANOVA (A) and repeated measures two-way ANOVA (B) with Tukey’s post hoc test were used for statistical analysis. Data are expressed as mean ± SD; *#P < 0.05 (*between groups and #to baseline).
3.2 Chromatin Immunoprecipitation
We used ChIP assays to identify genes to which HIF-1α is directly bound in hearts with the typical phenotypic changes of HIF expression (responsive) and hearts lacking these findings (unresponsive). Four hundred and eighty of the recovered ChIP fragments were individually subcloned and sequenced. In hearts with obvious HIF response, including ventricular dilation and enlarged epicardial coronary arteries, ChIP revealed the expected binding location (HRE sites) in promoter fragments from known HIF-regulated genes. In mice lacking the strong phenotypic changes seen with HIF over-expression, ChIP results showed that HIF was predominantly associated with the promoter of the Prkcbp1 gene. Approximately 78% (374 out of 480 sequences) of the chromatin associated with HIF-1α transgene expression pulled down a fragment of the Prkcbp1 promoter in the unresponsive phenotype, compared to less than 1% in the responsive hearts (Table 1). This led us to question what the difference was between the Prkcbp1 gene in the two mouse phenotypes. We, therefore, sequenced the genomic region surrounding Prkcbp1 in the responsive and unresponsive phenotypes.
Table 1.
HIF-1α ChIP results from mouse hearts of responsive and unresponsive phenotypes
| HIF-responsive phenotype |
HIF-unresponsive phenotype |
||
|---|---|---|---|
| Gene | Percent of sequenced ChIP fragments (%) | Gene | Percent of sequenced ChIP fragments (%) |
| Eno1 | 2 | Prkcbp1 | 78 |
| Egln3 | 2 | Eno1 | 2 |
| Pfkp | 2 | Car9 | 2 |
| Abcg1 | 2 | Gpi1 | 2 |
| Pcca | 2 | Pdgfra | 2 |
| Car12 | 2 | Slc27a1 | 2 |
| A1bg | 2 | Paip1 | 1 |
| E2G 2 | 2 | Scd1 | 1 |
| Bazf | 2 | Ucp1 | 1 |
| Fscn1 | 2 | Usp3 | 1 |
| Mcmd6 | 2 | Plunc | >1 |
| Mki67 | 2 | Wisp1 | >1 |
| Vegfa | 2 | Vwf | >1 |
| Gstm5 | 2 | ||
| Ucp1 | 2 | ||
| Car9 | 1 | ||
| Gpi1 | 1 | ||
| Paip1 | 1 | ||
| Plunc | 1 | ||
| Scd1 | 1 | ||
| Slc27a1 | 1 | ||
| Wisp1 | 1 | ||
| LOC208820 | >1 | ||
| LOC68099 | >1 | ||
| LOC76862 | >1 | ||
| LOC269695 | >1 | ||
| LOC545279 | >1 | ||
| LOC211208 | >1 | ||
| Vwf | >1 | ||
| Prkcbp1 | >1 | ||
| Usp3 | >1 | ||
| Pdgfra | >1 | ||
| Eef1g | >1 | ||
| Rgs3 | >1 | ||
| Ncoa3 | >1 | ||
HIF-1 transgene induction resulted in two distinct phenotypes. HIF-1α-PPN was bound to promoters of typical HIF-1 target genes in the responsive phenotype. In the unresponsive phenotype, the transgene was bound predominantly to the promoter of Prkcbp1.
3.3 PRKCBP1 genomic sequence analysis
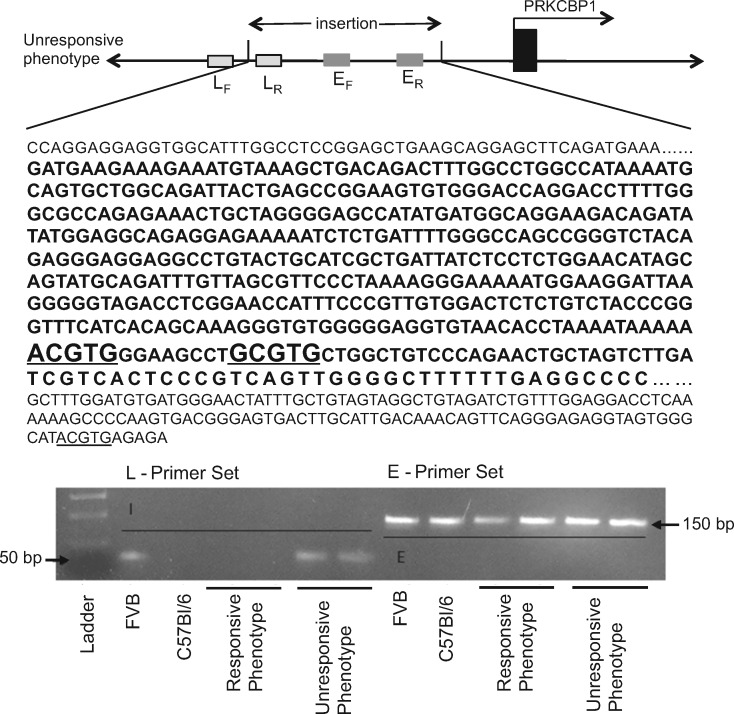
The coding sequence and +4KB/−2KB of the untranslated regions of four mice from each of the observed phenotypes were sequenced following PCR amplification. A 480 bp inserted region was identified +320 bp from the start of transcription of the Prkcbp1 gene relative to the C57Bl/6 reference sequence (Figure 2). The inserted fragment added two canonical HREs to the promoter region of Prkcbp1, 193 bp and 209 bp upstream of the known HRE. The inserted element demonstrated 90% similarity with the upstream promoter region of hypothetical protein, XP_896558, LOC626940 from chromosome 10 of Mus musculus strain C57BL/6J (NCBI Reference Sequence: NT_039500.7). To determine the origin of this inserted sequence, the existence (E), as well as location (L) of the sequence was evaluated in the transgenic as well as the two parental inbred strains that contribute to the heterogeneous background of the bi-allelic mice, namely C57Bl/6 and FVB/N (Figure 2 and Supplementary material online, Table S2). The parental and both the responsive and unresponsive mice had the inserted sequence present in the genome, however, at different locations. Only the unresponsive phenotype and the parental FVB inbred strain harbour the sequence in the 5’ UTR of Prkcbp1 (on chromosome 2).
Figure 2.
Genomic ‘FVB allele’ insertion. Illustration of genomic insertion and PCR identification of variant ‘FVB allele’. The specific insertion in the Prkcbp1 gene is only found in the FVB strain and unresponsive phenotype. The 480 bp inserted sequence is shown in bold and putative HREs are underlined.
3.4 PRKCBP1 expression profile
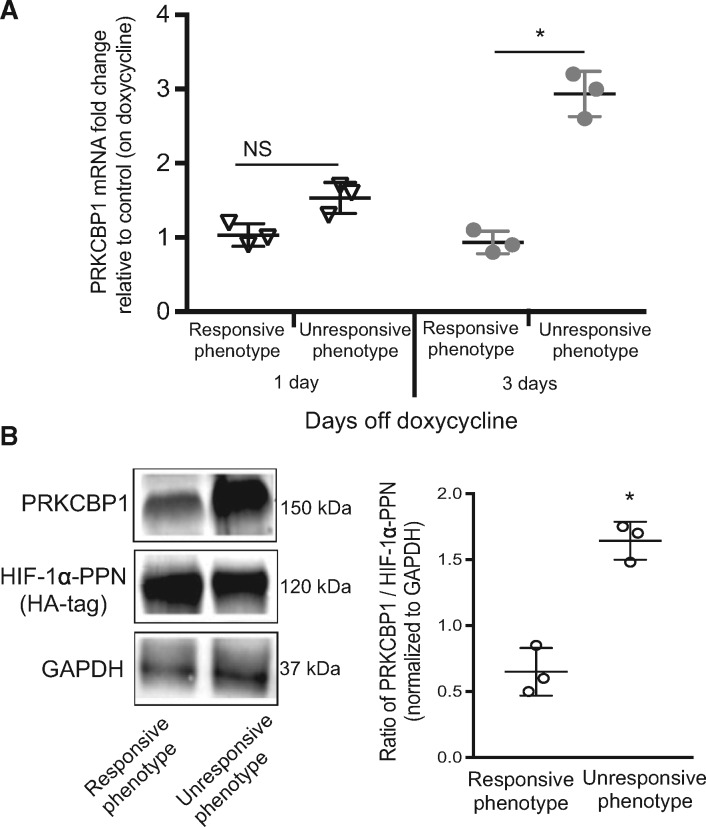
We next confirmed the sequence insert and ChIP data indicating that HIF was binding to and up-regulating PRKCBP1 in the unresponsive phenotype. Real-time PCR of cDNA from both phenotypes revealed increased Prkcbp1 transcript at day three of transgene expression in the unresponsive hearts (Figure 3A). Western blots confirmed that after seven days of transgene expression, both responsive and unresponsive hearts expressed approximately equal amounts of HIF-1α-PPN, however, unresponsive hearts had substantially higher expression of PRKCBP1 (Figures 3B and 4A). We next tested our hypothesis that the parental FVB strain contributed the inserted sequence, and that it was responsible for HIF-dependent induction of PRKCBP1. After myocardial infarction to stabilize HIF-1α, we found that PRKCBP1 expression was greater in the parental FVB than C57Bl/6 animals (Figure 4B). These data suggest that both transgenic and endogenous HIF-1α up-regulate PRKCBP1 with strain-specific variation (Supplementary material online, Figure S1).
Figure 3.
PRKCBP1 is more abundant in the unresponsive hearts. (A) Real-time PCR analysis of PRKCBP1 transcript. Hearts taken from the unresponsive mice have greater PRKCBP1 transcript than the hearts from responsive mice, assessed after 3 days of transgene induction. (B) HIF-1α and PRKCBP1 western blots and densitometry. Unresponsive hearts express significantly more PRKCBP1 with similar induction of HIF-1 transgene. HA-tag is used to assess HIF-1α-PPN expression. To reduce non-specific binding of primary antibodies, PVDF membranes were cut into three fractions, one containing the region 260–130 kDa for PRKCBP1 immunoblotting, 130–60 kDa for HIF-1α, and 60–8 kDa for GAPDH, according to pre-stained protein ladder. Two-tailed Student’s t-test was used for statistical analysis. Data are expressed as mean ± SD; n = 3 per group. *P < 0.05.
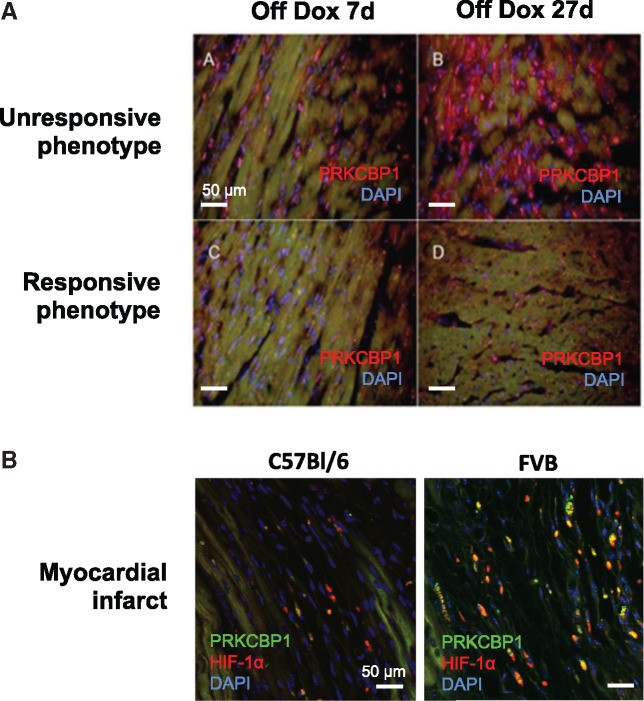
Figure 4.
Immunohistological evaluation of PRKCBP1. (A) PRKCBP1 expression after transgene expression. Unresponsive mice express more PRKCBP1 than responsive mice at 7 days and 27 days of HIF-1α transgene induction (red: PRKCBP1 and blue: nuclear DAPI). (B) PRKCBP1 expression after myocardial infarct. Three days after induced myocardial infarct, the parental FVB strain expresses more PRKCBP1 than C57Bl/6 mice in the infarct region. (red: HIF-1α, green: PRKCBP1, and blue: nuclear DAPI).
3.5 PRKCBP1 inhibits HIF-1 activity
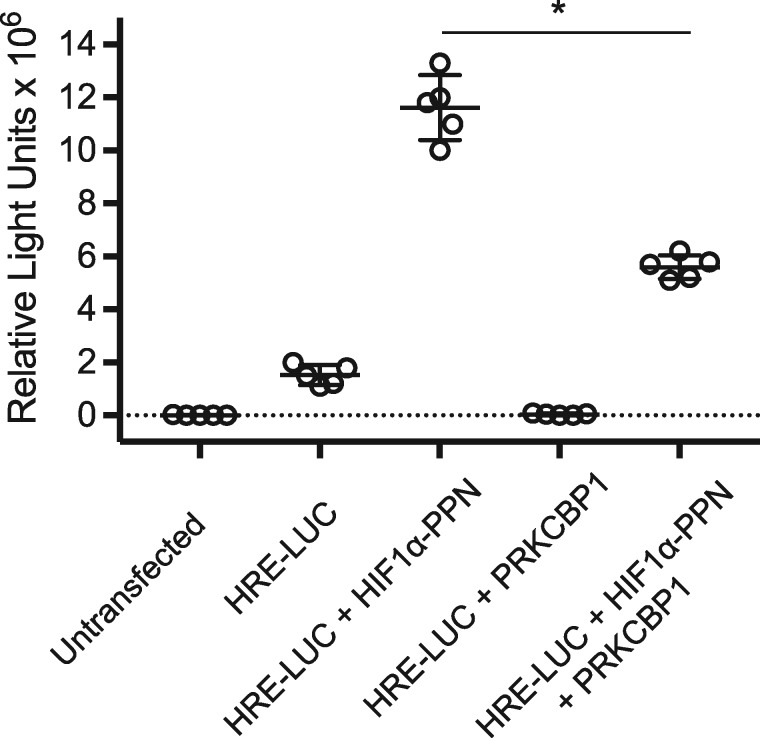
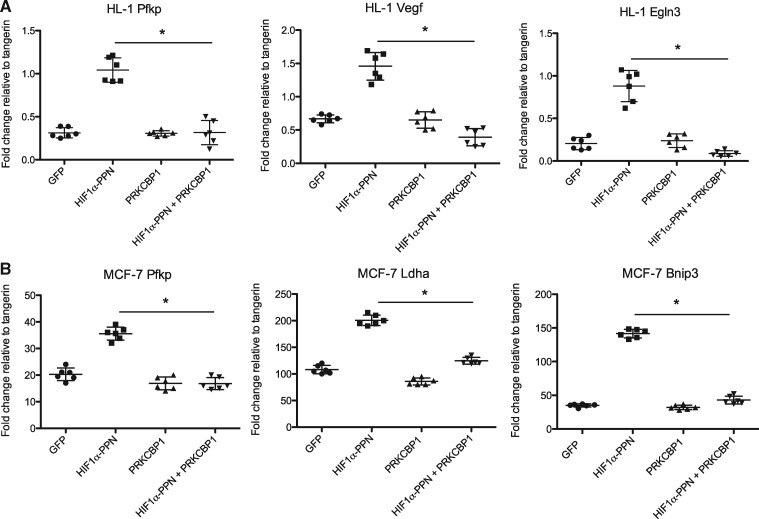
We next assessed whether the presence of increased PRKCBP1 was indeed affecting HIF-1 function. We constructed a HEK293 cell line with stable expression of a luciferase reporter downstream of concatamerized HRE. Transfection of an expression vector for HIF-1α-PPN produced the expected marked increase in luciferase reporter activity when it bound to the HRE’s. However, co-transfection of HIF-1α-PPN and a vector expressing PRKCBP1 substantially reduced this HIF-driven activity of the reporter (Figure 5). The effect of PRKCBP1 on endogenous downstream HIF-1α target-genes in murine cardiac HL-1 cells, rat ventricular H9C2 cardiomyoblasts, mouse primary cardiomyocytes, and human mammary adenocarcinoma MCF-7 cells was also investigated. We included the MCF-7 cell line in our analysis since the majority of recent investigation of PRKCBP1 has been performed in cancer cells. Semi-quantitative real-time PCR confirmed that transfection of the HIF-1α-PPN vector (or 1% O2 for primary cells) increased transcript of multiple known direct HIF-1α target genes, and that co-transfection with the PRKCBP1 expression vector reduced the abundance of target gene mRNAs in all four cell types (Figures 6A, B, 7D, and E). These in vitro experiments support our mouse in vivo findings that increased PRKCBP1 expression in the unresponsive phenotype leads to less HIF-1 target gene activation.
Figure 5.
HIF-1 activity by luciferase reporter. HIF-1 regulated luciferase activity is reduced by co-transfection of HEK293 cells with CMV-driven expression vectors for HIF-1α-PPN and PRKCBP1. Two-tailed Student’s t-test was used for statistical analysis. Data are expressed as mean ± SD; n = 5 per group. *P < 0.05. HRE-LUC, hypoxia response elements concatamerized to a luciferase reporter
Figure 6.
In vitro co-transfection of HL-1 and MCF-7 cells. Transfection of HIF-1α-PPN leads to induction of HIF-1α target gene mRNA as assessed by semi-quantitative real-time PCR, in (A) HL-1, and (B) MCF-7. Co-transfection with a PRKCBP1 expression vector reduces HIF target gene transcript. One-way ANOVA with Bonferroni post hoc test was used for statistical analysis. n = 6. Data are expressed as mean ± SD. *P < 0.05.
Figure 7.
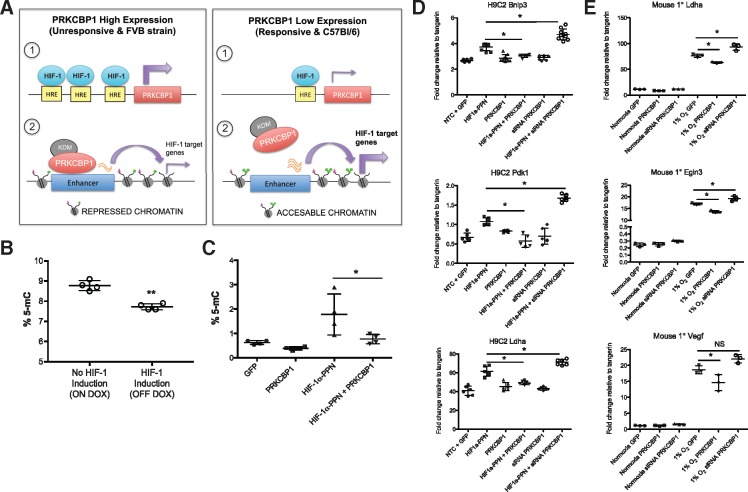
Proposed mechanism. (A) Proposed mechanism of HIF-induced PRKCBP1 expression, and PRKCBP1 mediated negative feedback of HIF-1 regulated genes. (B) Global DNA methylation is altered following cardiac HIF-1 induction in mice. After 3 days of HIF-1 induction, ELISA analysis confirmed significant reduction in the number of methylated CG residues compared to HIF-1 non-induced mice. n = 4 per group, **P = 0.0007. (C) DNA methylation is altered in response to HIF-1 and PRKCBP1 in HL-1 cells. ELISA analysis confirmed significant increase in the number of methylated CG residues in HL-1 HIF-1α-PPN transfected cells, and co-transfection with PRKCBP1 reduced % 5-mC to baseline levels. n = 4 per group, *P < 0.05. PRKCBP1 modulates the HIF response in (D) H9C2 cells and (E) mouse primary cardiomyocytes. Overexpression of PRKCBP1 in the presence of (D) HIF-1α-PPN or (E) 1% O2 reduces HIF-1 target gene transcript. siRNA mediated knockdown of PRKCBP1, in the presence of HIF-1α-PPN or hypoxia removed this negative inhibition (n = 6 H9C2; n = 3 mouse primary cells). Two-tailed Student’s t-test (B) and one-way ANOVA with Bonferroni post hoc test (C–E) was used for statistical analysis. Data are expressed as mean ± SD. *P < 0.05. Adapted from Shen et al. HRE, hypoxia response element; KDM, lysine demethylase.
3.6 Mechanistic hypothesis
Shen et al.15 recently reported that PRKCBP1 acts as a tumour suppressor by recruiting a histone demethylase which alters the chromatin landscape and ultimately represses transcription of genes associated with tumour growth. We propose that PRKCBP1 functions in a similar way in the heart, reducing the accessibility of HIF-1 target genes to HIF-dependent transcription (Figure 7A). To explore this hypothesis, we first aimed to learn about changes to the chromatin landscape in our transgenic mice due to HIF-1 induction and stabilization. It is well established that hypoxia alters the chromatin landscape,23,24 however, it is unknown whether HIF-1 alone has this effect. We found a decrease in global DNA methylation in hearts where the HIF-1α transgene was expressed, compared to mice that were not induced to express the transgene (Figure 7B). To address the effect of PRKCBP1 on methylation specifically in cardiomyocytes, we transfected HL-1 cells with HIF and/or PRKCBP1 expression vectors and found that HIF up-regulates DNA methylation and PRKCBP1 has an opposing effect (Figure 7C). This in vitro data, discordant with the in vivo findings, nonetheless supports the notion that HIF-1α can independently affect DNA methylation. The observation that PRKCBP1 reduces the HIF-induced increase further supports a role for PRKCBP1 in modulating the HIF response at the epigenetic level. To further characterize the effect of PRKCBP1 on HIF-1 activity we used siRNA to knockdown PRKCBP1, obtaining an 85% reduction of protein in cardiac H9C2 cells and nearly 60% reduction of transcript in mouse primary cardiomyocytes, (Supplementary material online, Figure S3). In siRNA-treated H9C2 cells subject to HIF-1α-PPN expression for 48 h, HIF-1 target gene expression was significantly increased compared to scramble control or HIF-1α-PPN expression alone (Figure 7D). This effect was also apparent in siRNA treated mouse primary cardiomyocytes subject to 1% O2 for 24 h (Figure 7E). Thus, inhibition of PRKCBP1 released the negative regulation of HIF-1 target genes. Importantly, knockdown of PRKCBP1 in the absence of HIF-1 activation had no effect on HIF-1 target genes, suggesting that the effects of PRKCBP1 are only revealed during episodes of HIF stabilization.
4. Discussion
We previously created a mouse model of cardiac HIF action by expressing an oxygen-stable form of HIF-1α in cardiac myocytes of transgenic mice using a tet-regulated system.18 This is a bi-allelic system with a tet-off regulated transcriptional activator driven by the myosin heavy chain promoter that is crossed into a strain with the tet-response element upstream of a HIF-1α cDNA that has been mutagenized for stability in normoxia (Pro402, Pro564 Asn803 to Ala; denoted HIF-1α-PPN). Therefore, the genetic background of these animals incorporates genomic variation from the C57Bl/6 and FVB strains that contained the two alleles. The typical physiological effects of HIF-1α transgene expression in the heart include increased capillary density with increased VEGF expression, as well as other components of a robust angiogenic response. We also typically see a marked deterioration in ventricular function and associated dilation. A portion of our mice that express the mutated HIF nonetheless did not manifest the typical phenotype of HIF over-expression. We then evaluated whether a modifying allele might be responsible for the dichotomous phenotypes.
ChIP revealed that HIF-1α was binding to previously identified gene targets as expected in the responsive mice. The relative abundance of these represented genes was as expected given that HIF-1α has over 300 gene targets, and that we individually subcloned and sequenced only a portion (480 fragments) of the precipitated fragments.14 However, in the group that did not manifest the typical phenotype, HIF-1α was bound primarily to the HREs upstream of Prkcbp1, with over 75% of the precipitated fragments coming from this gene. Sequencing of the promoter region of the Prkcbp1 gene revealed an unexpected 480 base pair insert in the HIF-unresponsive animals, containing two canonical HIF binding sites. We found that the parental FVB mice contain the promoter insert as well and thus refer to it as the FVB allele. This suggests that in the unresponsive phenotype, HIF-1α-PPN is driving the over-expression of PRKCBP1. This in turn somehow interferes with HIF-1 binding to the promoters of traditional HIF-1 regulated genes, while HIF binding to the promoter of Prkcbp1 is somehow insulated from this effect. Although, we do not know how PRKCBP1 redirects HIF promoter specificity, recent findings on its role in other cell types suggests a mechanism.15,25
One possibility might be that insertional modification of the promoter for Prkcbp1 in the unresponsive phenotype enhances transcription by remaining accessible to HIF, (by an as yet unknown mechanism), thereby providing a simple and effective feedback for inhibition of HIF transcriptional activity. The additional HREs provided by the insertion are likely to play a role in this effect. Other mechanisms of HIF modification by PRKCBP1 are suggested by the domain structure of the PRKCBP1 protein. The domains that PRKCBP1 shares with p300, the bromodomain and PHD finger, suggest that PRKCBP1 may compete with p300 and prevent HIF-1 target gene activation. PRKCBP1 also contains a MYND-DNA binding domain, which may competitively dislodge HIF-1 from its traditional HRE sites when bound. The bromodomain and PHD finger are known to bind acetylated histones, and are key domains in the HIF co-activator p300. For these reasons, we speculate that PRKCBP1 is not outcompeting HIF-1 for HRE sites, but rather interfering with the interaction of HIF-1α with HRE’s, either by interfering with formation of the HIF transcription complex or the recognition of HREs by that complex once formed. The newly described role of PRKCBP1 in modifying chromatin structure by directing histone methylation15,26 would be an elegant and parsimonious explanation for how HIF-sensitive promoter regions could be made inhospitable to HIF activation. Moreover, our mouse hearts induced to express oxygen-stable HIF-1α show significant alterations in genome-wide DNA methylation, further supporting the role of chromatin modification in response to not just hypoxia,23,24 but HIF-1 specifically. Interestingly, we found that HIF-1α consistently down-regulated DNA methylation in mouse hearts in vivo but up-regulated DNA methylation in HL-1 cardiomyocytes in vitro. As described by multiple groups, this may be due to differences between the in vivo and in vitro models, or may reflect cell type specific responses to changes in oxygen tension.27,28 Nevertheless, this data support the notion that HIF-1 can independently affect DNA methylation and that PRKCBP1 can modify the HIF-induced changes, further supporting a role for PRKCBP1 in modulating the HIF response at the epigenetic level. Also, importantly, the initial observation that PRKCBP1 inhibits HIF action of a mutated HIF-1α, proves that the mechanism of inhibition does not act through the hydroxylation sites previously implicated in HIF regulation (and removed from the mutated protein). Nonetheless, PRKCBP1 is inhibiting HIF action in response to oxygen levels, and we suggest that this is an indirect result of the oxygen-dependent transcriptional regulation of Prkcbp1 by HIF. Moreover, enhanced PRKCBP1 expression is able to overcome the transgenic expression of uninhibitable transgenic HIF expressed under the very strong α-MHC promoter. This suggests that it would also be able to inhibit HIF action during the milder induction of intrinsic oxygen-labile HIF during physiological induction by hypoxia, for example after infarction.
We examined the effect of PRKCBP1 on HIF transcriptional activity in transient transfection assays in a HEK293 cell line that contained a reporter construct for HIF action. Transient transfection of the oxygen-stable HIF-1α-PPN construct produced 12-fold induction of the reporter in these cells. Co-transfection with PRKCBP1 substantially inhibited the activity of HIF-1α-PPN. We confirmed this functional inhibition of PRKCBP1 on HIF activity by expressing the mutated HIF in murine atrial HL-1 cardiomyocytes, rat ventricular H9C2 cardiomyocytes, and human mammary adenocarcinoma MCF-7 cells, and investigated the influence of PRKCBP1 on the endogenous downstream target genes of HIF-1. We investigated transcript of six classic HIF-1 target genes that are robustly activated in the heart during myocardial infarct, and for each cell line we show results from 3 representative genes, with commonality of genes between cell lines to demonstrate consistency between species and cell type. As expected, we saw a robust increase in transcript of the target genes when transfected with the HIF-1 construct, and a substantial decrease in their message when cells were co-transfected with PRKCBP1. This inhibition of HIF-1 action was lost when we used siRNA to knockdown PRKCBP1 in cardiac H9C2 cells. Importantly, knockdown of PRKCBP1 without a hypoxic or HIF-1 challenge did not alter HIF-1 gene targets, suggesting that the inhibitory effect of PRKCBP1 on downstream target genes is HIF dependent.
A previous study demonstrated important differences in mouse strains in outcomes following myocardial infarction.29 It has been recognized that the FVB strain, which contributes the modified Prkcbp1 allele (and HIF-resistant phenotype) in our studies, was particularly susceptible to early, lethal, heart failure after infarction. These findings may support a beneficial physiological role for HIF action in the immediate post-infarct period. Other cardiac pathology has strong strain-dependent variation, for example the hypertrophic response to aortic banding,30 and given the pleotropic roles of HIF it is possible that genetic variation in its regulation or its sequence could modulate a variety of cardiac processes.
Ultimately, it will be important to determine whether PRKCBP1 plays a role in the susceptibility of humans to acute or chronic myocardial ischaemia, wherein HIF-1 is stabilized and active. For example, one could imagine that PRKCBP1 polymorphisms in the human population might modify the hypoxic response, as has been inferred for Egln1 polymorphisms in high versus low altitude populations.31,32 Multiple SNPs have been identified in the human and mouse PRKCBP1 gene, however, the functional consequences are incompletely characterized. Investigation into the abundance, location, and variation of PRKCBP1 has largely been examined in the cancer setting, however, we suggest that PRKCBP1 may also play a novel role in modifying HIF-1 responses in the heart.
5. Conclusions
Our data suggest that PRKCBP1 may be acting in a negative feed-back mechanism to shut off HIF-1-directed gene expression. While the role of PRKCBP1 in response to hypoxia remains to be fully elucidated, the phenotypic changes seen with interaction with oxygen-stable HIF suggests that it may prove a useful target for anti-ischaemic and anti-angiogenic therapies.
Supplementary Material
Acknowledgements
The authors would like to thank Karen Chang for her technical assistance, Steffen Oeser from the JABSOM Genomics Core Facility as well as Mingxin Tang and Aaron Tuia from the JABSOM mouse phenotyping core for infarction surgeries and animal breeding, respectively.
Conflict of interest: none declared.
Time for primary review: 16 days
Funding
This work was supported by the National Institutes of Health (HL080532, HL073449, RR016453, RR003061, MD007601, G12MD007601, and P30HL107251) and the Hawaii Community Foundation (17ADVC-86291).
References
- 1. Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 1998;8:588–594. [DOI] [PubMed] [Google Scholar]
- 2. Shohet RV, Garcia JA.. Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia. J Mol Med 2007;85:1309–1315. [DOI] [PubMed] [Google Scholar]
- 3. Wang GL, Jiang BH, Rue EA, Semenza GL.. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995;92:5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masson N, Ratcliffe PJ.. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J Cell Sci 2003;116:3041–3049. [DOI] [PubMed] [Google Scholar]
- 5. Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ.. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J 2001;20:5197–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Srinivas V, Zhang LP, Zhu XH, Caro J.. Characterization of an oxygen/redox-dependent degradation domain of hypoxia-inducible factor alpha (HIF-alpha) proteins. Biochem Biophys Res Commun 1999;260:557–561. [DOI] [PubMed] [Google Scholar]
- 7. Salceda S, Caro J.. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 1997;272:22642–22647. [DOI] [PubMed] [Google Scholar]
- 8. Mahon PC, Hirota K, Semenza GL.. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 2001;15:2675–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fossey SC, Kuroda S, Price JA, Pendleton JK, Freedman BI, Bowden DW.. Identification and characterization of PRKCBP1, a candidate RACK-like protein. Mamm Genome 2000;11:919–925. [DOI] [PubMed] [Google Scholar]
- 10. Eichmuller S, Usener D, Dummer R, Stein A, Thiel D, Schadendorf D.. Serological detection of cutaneous T-cell lymphoma-associated antigens. Proc Natl Acad Sci USA 2001;98:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panagopoulos I, Micci F, Thorsen J, Haugom L, Buechner J, Kerndrup G, Tierens A, Zeller B, Heim S.. Fusion of ZMYND8 and RELA genes in acute erythroid leukemia. PLoS One 2013;8:e63663.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuroyanagi J, Shimada Y, Zhang B, Ariyoshi M, Umemoto N, Nishimura Y, Tanaka T.. Zinc finger MYND-type containing 8 promotes tumour angiogenesis via induction of vascular endothelial growth factor-A expression. FEBS Lett 2014;588:3409–3416. [DOI] [PubMed] [Google Scholar]
- 13. Park J, Betel D, Gryfe R, Michalickova K, Di Nicola N, Gallinger S, Hogue CW, Redston M.. Mutation profiling of mismatch repair-deficient colorectal cancers using an in silico genome scan to identify coding microsatellites. Cancer Res 2002;62:1284–1288. [PubMed] [Google Scholar]
- 14. Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ.. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem 2009;284:16767–16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen H, Xu W, Guo R, Rong B, Gu L, Wang Z, He C, Zheng L, Hu X, Hu Z, Shao ZM, Yang P, Wu F, Shi YG, Shi Y, Lan F.. Suppression of enhancer overactivation by a RACK7-histone demethylase complex. Cell 2016;165:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fam NP, Arab S, Billia F, Han R, Proteau G, Latter D, Errett L, Bonneau D, Dunne R, Liu PP, Stewart DJ.. Increased myocardial expression of angiopoietin-2 in patients undergoing urgent surgical revascularization for acute coronary syndromes. Can J Cardiol 2010;26:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al Turki S, Manickaraj AK, Mercer CL, Gerety SS, Hitz MP, Lindsay S, D'Alessandro LC, Swaminathan GJ, Bentham J, Arndt AK, Louw J, Breckpot J, Gewillig M, Thienpont B, Abdul-Khaliq H, Harnack C, Hoff K, Kramer HH, Schubert S, Siebert R, Toka O, Cosgrove C, Watkins H, Lucassen AM, O'Kelly IM, Salmon AP, Bu'lock FA, Granados-Riveron J, Setchfield K, Thornborough C, Brook JD, Mulder B, Klaassen S, Bhattacharya S, Devriendt K, Fitzpatrick DF, Consortium UK, Wilson DI, Mital S, Hurles ME.. Rare variants in NR2F2 cause congenital heart defects in humans. Am J Hum Genet 2014;94:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bekeredjian R, Walton CB, MacCannell KA, Ecker J, Kruse F, Outten JT, Sutcliffe D, Gerard RD, Bruick RK, Shohet RV.. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS One 2010;5:e11693.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S.. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 2004;16:349–360. [DOI] [PubMed] [Google Scholar]
- 20. Thompson JD, Gibson TJ, Higgins DG.. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics 2002;Chapter 2:Unit 2.3. [DOI] [PubMed] [Google Scholar]
- 21. Erbel PJ, Card PB, Karakuzu O, Bruick RK, Gardner KH.. Structural basis for PAS domain heterodimerization in the basic helix–loop–helix-PAS transcription factor hypoxia-inducible factor. Proc Natl Acad Sci USA 2003;100:15504–15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Claycomb WC, Lanson NA Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ Jr.. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 1998;95:2979–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thienpont B, Steinbacher J, Zhao H, D’Anna F, Kuchnio A, Ploumakis A, Ghesquière B, Van Dyck L, Boeckx B, Schoonjans L, Hermans E, Amant F, Kristensen VN, Koh KP, Mazzone M, Coleman ML, Carell T, Carmeliet P, Lambrechts D.. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 2016;537:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watson JA, Watson CJ, McCann A, Baugh J.. Epigenetics, the epicenter of the hypoxic response. Epigenetics 2010;5:293–296. [DOI] [PubMed] [Google Scholar]
- 25. Chen Y, Zhang B, Bao L, Jin L, Yang M, Peng Y, Kumar A, Wang JE, Wang C, Zou X, Xing C, Wang Y, Luo W.. ZMYND8 acetylation mediates HIF-dependent breast cancer progression and metastasis. J Clin Invest 2018;128:1937–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li N, Li Y, Lv J, Zheng X, Wen H, Shen H, Zhu G, Chen TY, Dhar SS, Kan PY, Wang Z, Shiekhattar R, Shi X, Lan F, Chen K, Li W, Li H, Lee MG.. ZMYND8 reads the dual histone mark H3K4me1-H3K14ac to antagonize the expression of metastasis-linked genes. Mol Cell 2016;63:470–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watson CJ, Collier P, Tea I, Neary R, Watson JA, Robinson C, Phelan D, Ledwidge MT, McDonald KM, McCann A, Sharaf O, Baugh JA.. Hypoxia-induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Hum Mol Genet 2014;23:2176–2188. [DOI] [PubMed] [Google Scholar]
- 28. Varley KE, Gertz J, Bowling KM, Parker SL, Reddy TE, Pauli-Behn F, Cross MK, Williams BA, Stamatoyannopoulos JA, Crawford GE, Absher DM, Wold BJ, Myers RM.. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res 2013;23:555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM.. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res 2009;84:273–282. [DOI] [PubMed] [Google Scholar]
- 30. Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW.. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 2007;292:H2119–H2130. [DOI] [PubMed] [Google Scholar]
- 31. Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT, Ge R.. Genetic evidence for high-altitude adaptation in Tibet. Science 2010;329:72–75. [DOI] [PubMed] [Google Scholar]
- 32. Aggarwal S, Negi S, Jha P, Singh PK, Stobdan T, Pasha MA, Ghosh S, Agrawal A, Prasher B, Mukerji M.. EGLN1 involvement in high-altitude adaptation revealed through genetic analysis of extreme constitution types defined in Ayurveda. Proc Natl Acad Sci USA 2010;107:18961–18966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.