Abstract
Low-cost sensors provide a unique opportunity to continuously monitor patient progress during rehabilitation; however, these sensors have yet to demonstrate the fidelity and lack the calibration paradigms necessary to be viable tools for clinical research. The purpose of this study was to validate a low-cost wearable sensor ($35 US) that accurately measured peak knee extension during clinical exercises and needed no additional equipment for calibration. Sagittal plane knee motion was quantified using a 9-axis motion sensor and directly compared to motion capture data. The motion sensor measured the field strength of a strong earth magnet secured to the distal femur, which was correlated with knee angle during a simple calibration process. Peak knee motions and kinematic patterns were compared with motion capture data using paired t-tests and cross correlation, respectively. Peak extension values during seated knee extensions were accurate within 5 degrees across all subjects (root mean square error: 2.6 degrees, P = 0.29). Knee flexion during gait strongly correlated (0.84 ≤ rxy ≤ 0.99) with motion capture measurements but demonstrated peak flexion errors of 10 degrees. In this study, we present a low-cost sensor (< $XX) that accurately determines knee extension angle following a calibration procedure that did not require any other equipment. Our findings demonstrate that this sensor paradigm is a feasible tool to monitor patient progress throughout physical therapy. However, dynamic motions that are associated with soft-tissue artifact may limit the accuracy of this type of wearable sensor.
Keywords: wearable sensors, motion capture, inertial measurement unit, low-cost sensor
Introduction
Post-operative knee stiffness following total knee arthroplasty often leads to flexion contracture deformities that require aggressive therapies and revision surgery (Kim et al., 2004). Restoring knee motion within three months following joint replacement surgery mitigates the risk of flexion contracture and poor outcomes (Mitsuyasu et al., 2011; Scranton, 2001). While motion capture accurately quantifies knee flexion (Akbarshahi et al., 2010), such measurements are financially and logistically impractical for continuously monitoring post-operative patient progress. Knee flexion can also be accurately quantified with an inertial measurement unit (IMU) placed on both segments that comprise an articulating joint (Seel et al., 2014; Vitali et al., 2017). This approach requires multiple sensors, which can increase the complexities and costs associated with clinical research. Outpatient physical therapy is a common means of post-operative rehabilitative care. But this approach is costly, time-consuming, and ultimately depends on patient compliance. As such, there is a need for cost-effective means of remote, continuous tracking of patient knee motion following invasive surgery.
Therefore, the purpose of this study was to validate a novel implementation of a low-cost wearable sensor (Qu et al., 2018) by satisfying several criteria: 1) simple calibration procedure and 2) accurate measurement of peak knee extension during a clinical test for knee contracture. To that end, we compared the knee angle measurements derived from the wearable sensor to those calculated by motion capture during clinically relevant functional activities. Based on clinician input, we established a clinically acceptable peak-knee extension error of less than 5° during a knee-extension exercise compared to measurements using motion capture. A secondary aim of this device was to determine the measurement fidelity of the wearable sensor during walking. To do this, we compared peak knee flexion and shank angular velocities during walking at three different speeds and walking at a 10% grade.
Methods
Seven healthy young adults (4 males, 3 females; 26 ± 4 years; BMI 23.8 ± 3.7) participated and provided written informed consent in this study approved by the University of Pennsylvania IRB. Subjects wore athletic shorts, a tank top, and running shoes (Air Pegasus, Nike, Beaverton, OR). We placed 26 reflective markers on the lower extremities, which we tracked using a 12-camera motion capture system at 100 Hz (Raptor Series, Motion Analysis Corp, Santa Rosa, CA). We placed tracking markers on the thigh and shank segments (3 placed on each segment) and scaled the subject using anatomic landmarks: anterior and posterior iliac spines, medial and lateral knee condyles and ankle malleoli, calcaneus, and first and fifth metatarsal heads. Marker trajectories acquired during a standing trial were used to scale a constrained-kinematic lower-extremity model (Rajagopal et al., 2016). Subjects performed a clinically-relevant seated knee extension and walked on a treadmill for 2 minutes at three constant speeds (0.9, 1.2, and 1.5 m/s) and up a 10% grade (1.2 m/s). We measured incline walking to determine if the sensor had similar measurement fidelity during another common activity.
Knee motion was quantified using a wearable magnet-based device (Qu et al., 2018) that was positioned on the right leg to measure knee flexion during functional activities. The magnetic field strength of a rare earth magnet (1.50”x0.50”x0.25” Neodymium Block Magnet, CMS Magnetics, Garland, TX) was measured using 9 degree-of-freedom IMU (LSM9DS0, FLORA 9-DOF, Adafruit, New York, NY). The magnet and sensor were secured to the distal-lateral thigh and proximal-lateral shank, respectively, approximately 10 cm away from the knee joint line, using fabric-backed tape and self-adhesive wrap. The accelerometer, gyroscope, and magnetometer ranges were set to ± 2g, ± 245°s−1, and 12 gauss, respectively, which were determined during pilot testing. Sensor data were sampled at 88 Hz and synchronized with the motion capture system via a digital pin on the microcontroller, which was worn on the shank using self-adhesive tape (FLORA – Wearable Platform, Adafruit, New York, NY) and logged to file (see appendix).
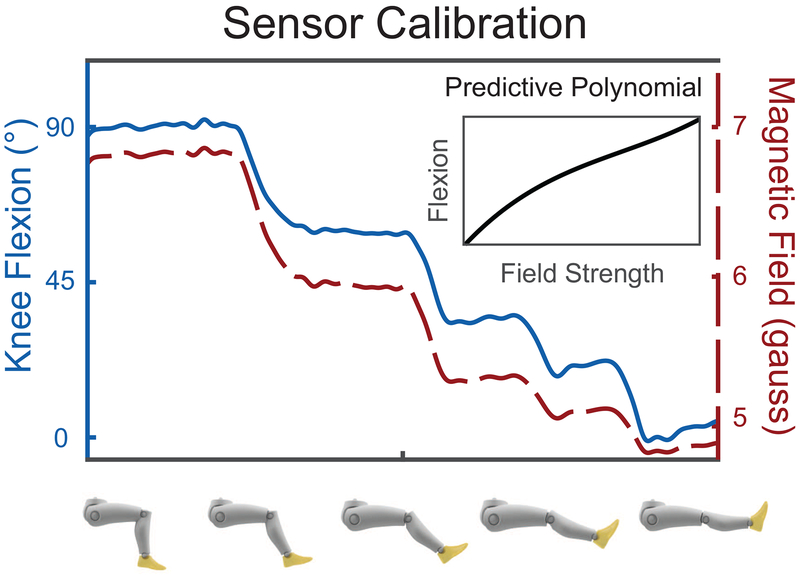
The wearable sensor was calibrated by modeling the flexion angle as a function of the magnetic field strength measured by the magnetometer during a series of quasi-static knee postures (Fig. 1). Sensor orientation on the shank was accounted for by calculating the accelerometer offset with respect to gravity and the gyroscope was tared while subjects stood with the shank vertically aligned to the ground. To calibrate the sensor and calculate knee flexion angle as a function of magnetometer measurement, subjects sat on a treatment table with the thigh parallel to the tabletop and positioned the knee in five arbitrary positions between 90 and 0 degrees knee flexion for several seconds. Based on the assumption that the thigh was parallel to the ground, we calculated knee angle based on the tilt of the shank segment using the accelerometer data. Subjects were instructed to bend their knee in five evenly spaced positions and hold at each position for several seconds. Next, knee flexion angle was approximated using the magnetometer data by fitting a 3rd order polynomial – which accurately characterizes magnetic fields (Qu et al., 2018) – to accelerometer-derived flexion angle as a function of magnetic field strength. The three magnetometer axes were calculated as a single magnitude and used in all calculations. The field strength of the rare earth magnet would exceed the measurement range of the magnetometer when the knee went into deep flexion and cause the sensor to ‘saturate’. Lower-leg angular velocities were collected during the quiet standing trial to tare the gyroscope signal. Marker trajectories and sensor data were collected during each activity.
Figure 1:
Sensor calibration was performed by placing the knee in 5 arbitrary positions between 0 and 90 degrees and fitting a 3rd order polynomial (inset) to the magnetometer-accelerometer data.
Lower extremity kinematics were calculated using a constrained-kinematic model that was scaled to each subject based on externally identified external landmarks (Opensim v3.3; (Delp et al., 2007; Rajagopal et al., 2016)). Marker trajectories and the wearable sensor measurements were filtered using a 6 Hz fourth-order Butterworth filter (Winter, 2009) before calculating knee kinematics. Heel strike events during gait were identified using a kinematic-based algorithm (Zeni et al., 2008) and strides were not analyzed during transitioning between speeds. Knee flexion angles and shank angular velocities were calculated for each stride and averaged across approximately 80 strides per subject. Angular velocities of the lower-leg were also calculated from segmental measurements calculated from the constrained-kinematic model and gyroscope.
Wearable sensor measurements were compared to motion capture measurements. The primary outcome was peak knee extension during a seated-knee extension with differences between measurement techniques of less than 5 degrees, which was considered clinically acceptable. Knee angle and shank angular velocity ninety-five percent confidence intervals were calculated for each movement using a bootstrap approach. Average peak knee extension during the seated-knee extension and flexion during gait for each subject were directly compared using paired t-tests and reported using RMSE (eq. 1). We decided not to correct for multiple comparisons (increased type I errors) to more readily detect possible differences in peak knee flexion measured using the sensor and motion capture methods.
| Eq 1. |
Where θsensor and θmocap are average peak knee angles calculated using the sensor and motion capture, respectively, and N is the number of subjects tested.
Kinematic patterns were compared using cross correlation analyses to quantify the similarities between the wearable sensor and motion capture measurements (Baxter et al., 2016). We tested for increases in shank angular velocities with increasing walking speed using one-way t-tests (slow-medium, medium-fast) and controlled for these 2 comparisons using a Bonferonni correction (P = 0.05 / 2).
Results
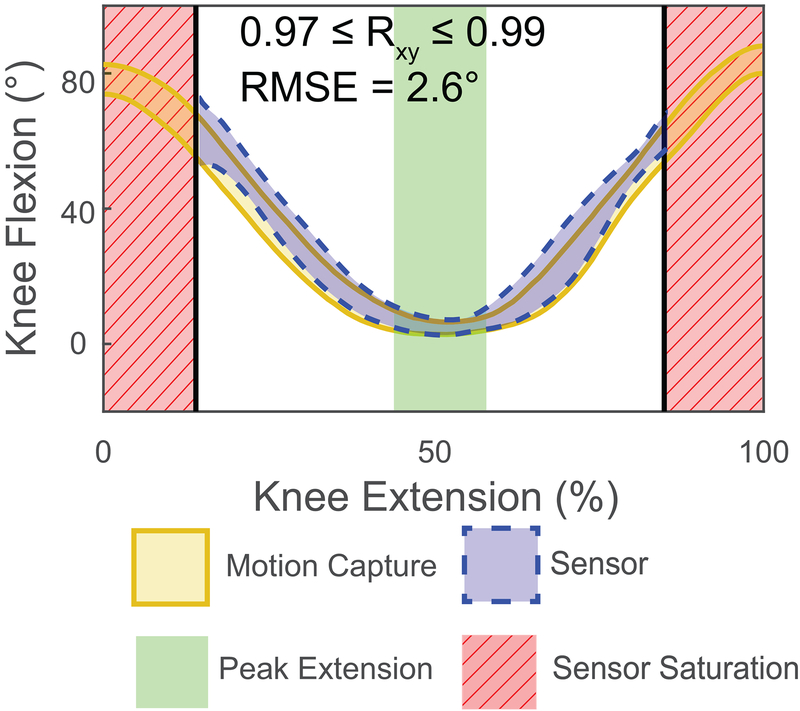
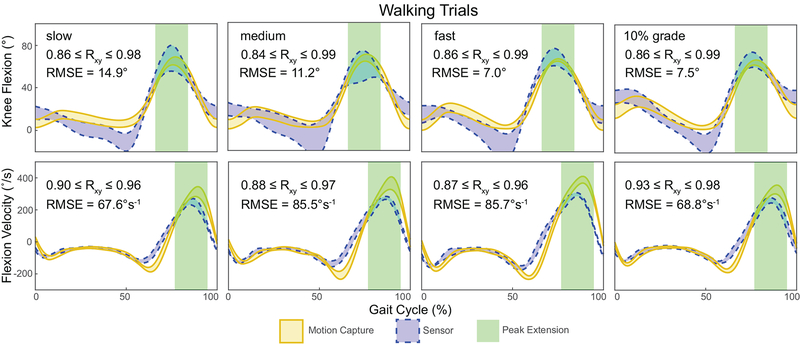
Seated-knee extension was accurate to within 5 degrees across all subjects (P = 0.229; root mean square (RMS) error: 2.6 degrees; Fig. 2). However, the wearable sensor saturated at approximately 65 degrees knee flexion (Fig. 2). Knee flexion angles and shank angular velocities strongly correlated with motion capture measurements during walking (0.84 ≤ rxy ≤ 0.99 and 0.87 ≤ rxy ≤ 0.98, respectively; Fig. 3). Despite similarities in kinematic patterns, the wearable sensor showed an average RMSE of 10.1 degrees peak knee flexion across all walking conditions. Walking faster and up an incline generated smaller errors (fast: P = 0.468, RMSE: 6.9 degrees; 10% grade: P = 0.377, RMSE: 7.5 degrees) compared to walking at slow and medium speeds generated (slow: P = 0.643, RMSE: 14.9; medium: P = 0.582, RMSE 11.2 degrees). Increased shank angular velocities were measured using the gyroscope approached statistical significance. These measurements demonstrated a 24.3 degree/s increase during medium compared to slow walking (P = 0.049) and a 19.9 degree/s increase during fast compared to medium pace walking (P = 0.026).
Figure 2:
Knee angles measured by the device (dashed blue) were compared to motion capture measurements (solid gold) during a clinically-relevant knee extension task. The sensor became saturated with increased knee flexion (red stripped area) using calculated root mean square errors (RMSE). Knee flexion is visualized as a 95% confidence interval of the tested subjects.
Figure 3:
Knee angles and shank angular velocities strongly agreed (rxy ≥ 0.84) between the device (dashed blue) and motion capture (solid gold). In addition, peak knee flexion measurements were compared at peak knee flexion (green area) using calculated root mean square errors (RMSE). Knee flexion is visualized as a 95% confidence interval of the tested subjects.
Discussion
This study tested a new paradigm for measuring clinically-relevant knee extension using a single low-cost sensor that does not need additional equipment to calibrate. Our results support the feasibility of this sensor paradigm for measuring knee extension during a seated-knee extension (Fig. 2). However, more dynamic motions like walking appear to be affected by soft tissue artifact and sensor limitations, which produce less accurate predictions of knee angle (Fig. 3). While this study focused on measuring knee motion, this paradigm could be adapted to work with other mostly-planar joints such as the elbow or ankle. Monitoring knee motion using a low-cost sensor provides new opportunities for providers to monitor patient progress and function outside of clinical visits.
This sensor paradigm has several strengths and weaknesses that should be considered. Utilizing a single sensor eliminates the need for additional componentry and communication protocols necessary for a second sensor. The simple calibration process (Fig. 1) permits convenient placement of the sensor and magnet on opposing sides of the knee joint. This sensor paradigm can be easily adapted to other joints by changing the strength and position of the magnet. The magnetic field of the rare earth magnet is not affected by total knee arthroplasty components. We confirmed this by placing a cobalt-chrome femoral component between the magnet and sensor, which produced no change in the magnetic field measured by the magnetometer. Because this sensor paradigm uses a strong magnetic field as a reference for distance, any change in the field strength is assumed to represent a change in knee flexion. This sensor paradigm has also been successfully implemented in large animal models (Qu et al., 2018), which reduces the number of sensors the animals can damage. Soft tissue artifact around the knee joint is well documented (Akbarshahi et al., 2010; Barré et al., 2013; Stagni et al., 2005) and was a likely cause of measurement error, especially during functional activities such as walking. Future research is aimed at developing correction algorithms that account for soft-tissue artifact to improve kinematic tracking. While well suited for planar joints, this paradigm is not appropriate for more multiplane joints, such as the hip or shoulder. Other low-cost sensors can also be combined to deliver additional measurements that have clinical relevance. For example, shank angular velocities provide insight into walking speed (Fig. 3) and could be continuously monitored and combined with knee flexion data to quantify physical activity.
Integrating low-cost wearable sensors into the motion capture space is rapidly advancing. This sensor proposes a new paradigm: quantifying joint kinematics with a single sensor and a reference magnetic field, rather than two discrete IMUs. Previous studies utilized wearable sensors to quantify the joint level function that is provided by motion capture in various patient populations (Bolink et al., 2012; Tadano et al., 2016) but all rely on the use of multiple sensors per joint or a goniometer that crosses the joint (Toffola et al., 2012), which may provide greater accuracy during activities such as walking (Toffola et al., 2012). Additionally, many of these studies rely on complex calibration methods and additional tools to make their measurements (Picerno et al., 2008). By contrast, our study demonstrates the capacity to make these measures of joint level function with an easy to calibrate, low-cost device that is possible to incorporate gait event detection paradigms (Formento et al., 2014) without additional equipment or cost. Deciding on whether to use a single or multiple sensor paradigm should be based on the experimental constraints and measurement accuracy required for the research question. This single sensor paradigm is well-suited for knee motions that do not generate large amounts of soft-tissue artifact, such as seated knee extensions. However, other wearable paradigms that utilize absolute orientation sensors on each segment may provide greater measurement fidelity during multiplane joint or high-impact movements (Seel et al., 2014; Vitali et al., 2017).
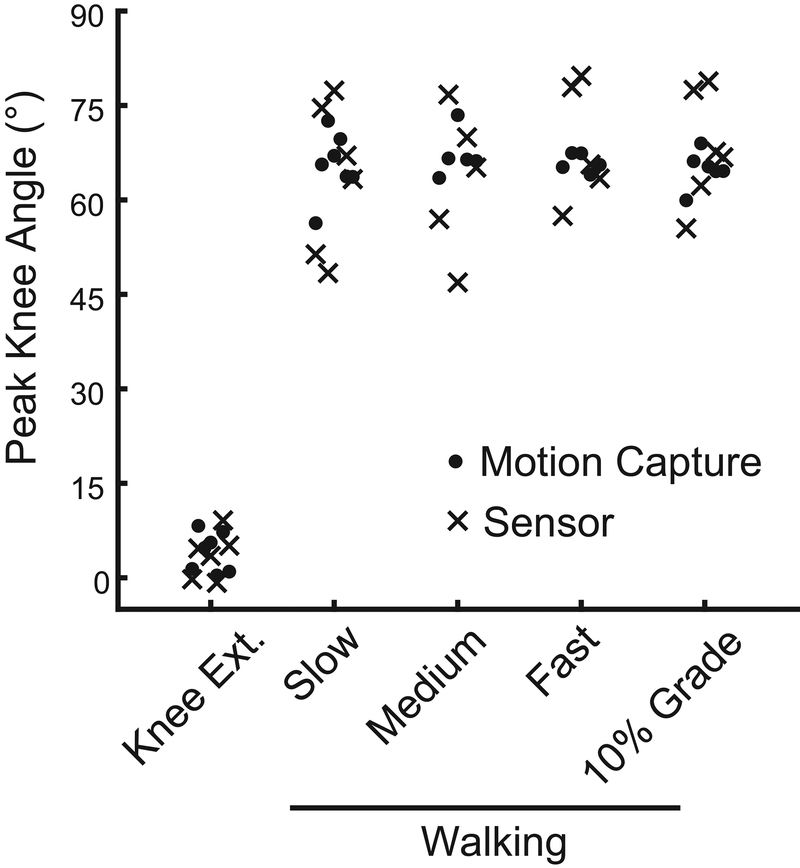
This study was affected by several limitations. Only healthy young adults participated in this study to establish the feasibility of the sensor and calibration paradigm. These subjects had a lower body mass index (23.8 ± 3.7) than many patient populations, including those undergoing total knee arthroplasty (Crowninshield et al., 2006) and a single investigator placed the sensor on all research subjects. Soft-tissue artifact around the knee joint (Akbarshahi et al., 2010) likely affected our measurements and other technical paradigms may be less sensitive to skin motion (Alonge et al., 2014; Papi et al., 2018). Due to the cubic decay of magnetic fields, sensor and magnet placement is important. Placement guides such as a disposable sticker, fabric sleeve, or brace may improve placement repeatability and accuracy. Due to the proof-of-concept nature of this work, our study had a small sample size (N=7). However, based on the increased variability and distribution of peak knee angles measured with the sensor (Fig. 4), we would expect that sampling a larger population would yield similarly insignificant differences between the motion capture and sensor measurements.
Figure 4:
Peak knee angles measured with motion capture (closed circles) and the wearable sensor (crosses) both showed normal distributions. However, sensor measurements demonstrated increased variability compared to motion capture measurements during walking.
Low-cost wearable sensors offer an viable alternative to traditional motion capture techniques for specific research needs (Alonge et al., 2014; Papi et al., 2018). In the current study, we present a unique implementation of an IMU in conjunction with a magnet that accurately measures a clinically-relevant knee extension task. Knee function during gait are less accurately measured with this sensor, a likely artifact of soft tissue motion. Low-cost sensors may address concerns of increasing costs and decreasing accessibility in healthcare. Establishing predictors of patient function and outcomes with low-cost wearable sensors may help detect adverse events, guide regenerative rehabilitation, and improve patient-specific treatments.
Supplementary Material
Acknowledgments:
We thank Ms. Annelise Slater for assistance with data collection.
Funding no funding has been provided for this research
Footnotes
Ethics Approval and Consent to Participate this study was approved by the Institutional Review Board at the University of Pennsylvania (#826667). Subjects provided written-informed consent.
Preprint a similar manuscript is posted on a pre-print server [doi: https://doi.org/10.1101/309328]
Conflicts of Interests
Feini Qu and Peter Gebhard are inventors on patent application US20170231533 and co-founders of Animotion, LLC. Feine Qu, Peter Gebhard, and Brendan Stoeckl have equity in Animotion, LLC, which is developing products related to the research described in this manuscript. All remaining co-authors have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarshahi M, Schache AG, Fernandez JW, Baker R, Banks S, Pandy MG, 2010. Non-invasive assessment of soft-tissue artifact and its effect on knee joint kinematics during functional activity. J. Biomech 43, 1292–1301. 10.1016/j.jbiomech.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Alonge F, Cucco E, D’Ippolito F, Pulizzotto A, 2014. The Use of Accelerometers and Gyroscopes to Estimate Hip and Knee Angles on Gait Analysis. Sensors 14, 8430–8446. 10.3390/s140508430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré A, Thiran JP, Jolles BM, Theumann N, Aminian K, 2013. Soft Tissue Artifact Assessment During Treadmill Walking in Subjects With Total Knee Arthroplasty. IEEE Trans. Biomed. Eng 60, 3131–3140. 10.1109/TBME.2013.2268938 [DOI] [PubMed] [Google Scholar]
- Baxter JR, Sturnick DR, Demetracopoulos CA, Ellis SJ, Deland JT, 2016. Cadaveric gait simulation reproduces foot and ankle kinematics from population-specific inputs. J. Orthop. Res 34, 1663–1668. 10.1002/jor.23169 [DOI] [PubMed] [Google Scholar]
- Bolink SAAN, Laarhoven SN van, Lipperts M, Heyligers IC, Grimm B, 2012. Inertial sensor motion analysis of gait, sit–stand transfers and step-up transfers: differentiating knee patients from healthy controls. Physiol. Meas 33, 1947 10.1088/0967-3334/33/11/1947 [DOI] [PubMed] [Google Scholar]
- Crowninshield RD, Rosenberg AG, Sporer SM, 2006. Changing Demographics of Patients with Total Joint Replacement. Clin. Orthop 443, 266–272. 10.1097/01.blo.0000188066.01833.4f [DOI] [PubMed] [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG, 2007. OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng 54, 1940–1950. 10.1109/TBME.2007.901024 [DOI] [PubMed] [Google Scholar]
- Formento PC, Acevedo R, Ghoussayni S, Ewins D, 2014. Gait Event Detection during Stair Walking Using a Rate Gyroscope. Sensors 14, 5470–5485. 10.3390/s140305470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Nelson C, Lotke P, 2004. Stiffness after total knee arthroplasty. Prevalence of the complication and outcomes of revision. J. Bone Jt. Surg. Inc [PubMed] [Google Scholar]
- Mitsuyasu H, Matsuda S, Miura H, Okazaki K, Fukagawa S, Iwamoto Y, 2011. Flexion Contracture Persists If the Contracture is More Than 15° at 3 Months After Total Knee Arthroplasty. J. Arthroplasty 26, 639–643. 10.1016/j.arth.2010.04.023 [DOI] [PubMed] [Google Scholar]
- Papi E, Bo YN, McGregor AH, 2018. A flexible wearable sensor for knee flexion assessment during gait. Gait Posture 62, 480–483. 10.1016/j.gaitpost.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picerno P, Cereatti A, Cappozzo A, 2008. Joint kinematics estimate using wearable inertial and magnetic sensing modules. Gait Posture 28, 588–595. 10.1016/j.gaitpost.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Qu F, Stoeckl BD, Gebhard PM, Hullfish TJ, Baxter JR, Mauck RL, 2018. A Wearable Magnet-Based System to Assess Activity and Joint Flexion in Humans and Large Animals. Ann. Biomed. Eng 1–10. 10.1007/s10439-018-2105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal A, Dembia C, DeMers M, Delp D, Hicks J, Delp S, 2016. Full body musculoskeletal model for muscle-driven simulation of human gait. IEEE Trans. Biomed. Eng 9294, 1–1. 10.1109/TBME.2016.2586891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scranton PE, 2001. Management of knee pain and stiffness after Total Knee Arthoplasty. J. Arthroplasty [DOI] [PubMed] [Google Scholar]
- Seel T, Raisch J, Schauer T, Seel T, Raisch J, Schauer T, 2014. IMU-Based Joint Angle Measurement for Gait Analysis. Sensors 14, 6891–6909. 10.3390/s140406891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagni R, Fantozzi S, Cappello A, Leardini A, 2005. Quantification of soft tissue artefact in motion analysis by combining 3D fluoroscopy and stereophotogrammetry: a study on two subjects. Clin. Biomech 20, 320–329. 10.1016/j.clinbiomech.2004.11.012 [DOI] [PubMed] [Google Scholar]
- Tadano S, Takeda R, Sasaki K, Fujisawa T, Tohyama H, 2016. Gait characterization for osteoarthritis patients using wearable gait sensors (H-Gait systems). J. Biomech 49, 684–690. 10.1016/j.jbiomech.2016.01.017 [DOI] [PubMed] [Google Scholar]
- Toffola LD, Patel S, Ozsecen MY, Ramachandran R, Bonato P, 2012. A wearable system for long-term monitoring of knee kinematics. IEEE, pp. 188–191. 10.1109/BHI.2012.6211541 [DOI] [Google Scholar]
- Vitali R, Cain S, McGinnis R, Zaferiou A, Ojeda L, Davidson S, Perkins N, Vitali RV, Cain SM, McGinnis RS, Zaferiou AM, Ojeda LV, Davidson SP, Perkins NC, 2017. Method for Estimating Three-Dimensional Knee Rotations Using Two Inertial Measurement Units: Validation with a Coordinate Measurement Machine. Sensors 17, 1970. 10.3390/s17091970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA, 2009. Biomechanics and Motor Control of Human Movement. John Wiley & Sons. [Google Scholar]
- Zeni J, Richards J, Higginson JS, 2008. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 27, 710–714. 10.1016/j.gaitpost.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.