Abstract
Targeted temperature management (TTM) is recommended as a standard of care for postcardiac arrest patients. Current TTM methods have significant limitations to be used in an ambulatory setting. We investigated the efficacy and safety of a novel noninvasive transnasal evaporative cooling device (CoolStat™). Eleven Yorkshire pigs underwent hypothermia therapy using the CoolStat device. CoolStat induces evaporative cooling by blowing dehumidified ambient air over the nasal turbinates in a unidirectional fashion. CoolStat's efficacy and safety were assessed by applying different cooling strategies (groups A, B and C). In group A (efficacy study; n = 5, TTM for 8 hours), time to achieve brain target temperature (2°C reduction from baseline), and the percentage of time in which the temperature ranged within ±0.5°C after reaching the target temperature were investigated. In the safety assessment (groups B and C), two worst-case therapy situations were reproduced: in group B (n = 3), continuous maximum air flow (65 L/min) was applied without temperature control and, in group C (n = 3), subjects underwent 24-hour TTM (prolonged therapy). Hemodynamic and respiratory parameters, nasal mucosa integrity (endoscopic assessment), and other therapy-related adverse effects were evaluated. Efficacy study: CoolStat cooling therapy successfully induced and sustained managed hypothermia in all subjects. Brain target temperature was achieved in 0.5 ± 0.6 hours and kept within a ±0.5°C range for the therapy duration (99.9% ± 0.1%). All animals completed the safety studies. Maximum air flow (group B) and 24-hour (group C) therapies were well tolerated and no significant damage was observed on nasal mucosa for neither of the groups. CoolStat was able to efficiently induce and maintain hypothermia using unidirectional high flow of dry air into the nostrils of porcine models. CoolStat therapy was well tolerated and no damage to nasal mucosa was observed under either maximum air flow or prolonged therapy.
Keywords: target temperature management, temperature control, hypothermia, cooling device, transnasal
Introduction
Cardiac arrest is a significant cause of mortality and morbidity in the developed world. Some degree of neurological disability is inevitable in the majority of cardiac arrest patients who survive to hospital discharge (Neumar et al., 2008). Poor cerebral perfusion triggers a deleterious chemical cascade that aggravates ischemic cerebral injury even after circulation has been restored (Lyon et al., 2010). Prior reports suggest that fever is an independent predictor of adverse outcome in patients with critical cerebral injury (Azzimondi et al., 1995; Hajat et al., 2000).
The beneficial effects of early and well-conducted targeted temperature management (TTM) have been fairly demonstrated in preclinical models (Kuboyama et al., 1993; Widmann et al., 1993; Colbourne and Corbett, 1994; Guan et al., 2007). TTM has been proven to positively impact postcardiac arrest neurological outcomes (Bernard et al., 2002; Holzer, 2002; Silfvast et al., 2003), and to benefit other patients with acute cerebral injury (Polderman, 2008). Furthermore, TTM also seems to improve neurological prognosis in neurocritical patients presenting fever (Diringer, 2004).
Several clinical temperature modulation methods are available for TTM, with distinct characteristics, advantages, and drawbacks (Seder and Van der Kloot, 2009). Cold fluid infusion is an extensively studied hypothermia induction strategy, however, lacks accurate temperature control and has not yet been validated for temperature maintenance (Kim et al., 2005; Polderman et al., 2005; Kliegel et al., 2007). Surface cooling methods are reasonably effective, but require special skin care, sedation, and are more prone to induce shivering (heat generation) (Mayer et al., 2004; Haugk et al., 2007; Hoedemaekers et al., 2007). Intravascular cooling techniques allow for accurate target temperature control, however, its use is limited to high complexity care units (Diringer, 2004) and is associated with risk of catheter-related infection and vascular complications (Polderman and Callaghan, 2006; Patel et al., 2013). Recently, transnasal perfluorocarbon (PFC) spray for evaporative cooling has shown promising results in clinical studies as a suitable out-of-hospital method for early TTM, whereas some safety concerns still taper its clinical acceptance (Abou-Chebl et al., 2011).
In this study, we report our experience with a novel transnasal cooling device called CoolStat™ (CoolTech, MD). CoolStat is a noninvasive device capable of delivering high flow of dehumidified air into the nasal mucosa. Preclinical data have demonstrated selective brain and core temperature reduction by evaporative cooling using transnasal unidirectional high flow of dry air into the nostrils of porcine models (Chava et al., 2017). Herein, we investigated the cooling efficacy of CoolStat device in induction and maintenance of hypothermia (primary objective). Additionally, we evaluated the safety of the device in two worst-case simulations (secondary objective).
Methods
Study design
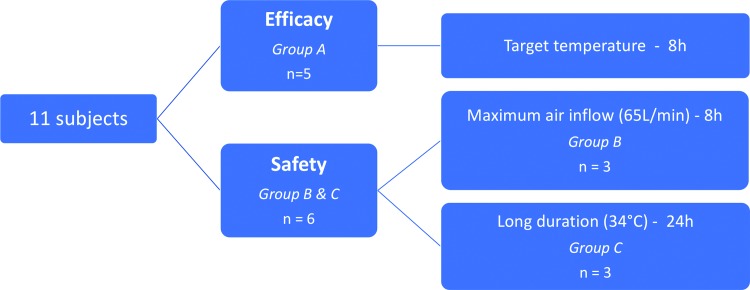
Eleven female Yorkshire pigs (30–40 kg) were subject to hypothermia induction and maintenance using the CoolStat device. As shown in Figure 1, five animals (group A) underwent an 8-hour efficacy study to assess the cooling efficacy of CoolStat's closed-loop control system in TTM (described in the following sessions). The remaining six animals were included in the safety study and divided into two groups according to the cooling strategy adopted (groups B and C). Three out of six animals (group B) underwent maximum air flow exposure for 8 hours to simulate worst-case air flow and the remaining three animals (group C) were tested for 24-hour exposure to a closed-loop control system to maintain a target core temperature of 34°C.
FIG. 1.
Study design. Color images are available online.
The study protocol was approved by Johns Hopkins University Animal Care and Use Committee and included only terminal experiments.
Animal preparation
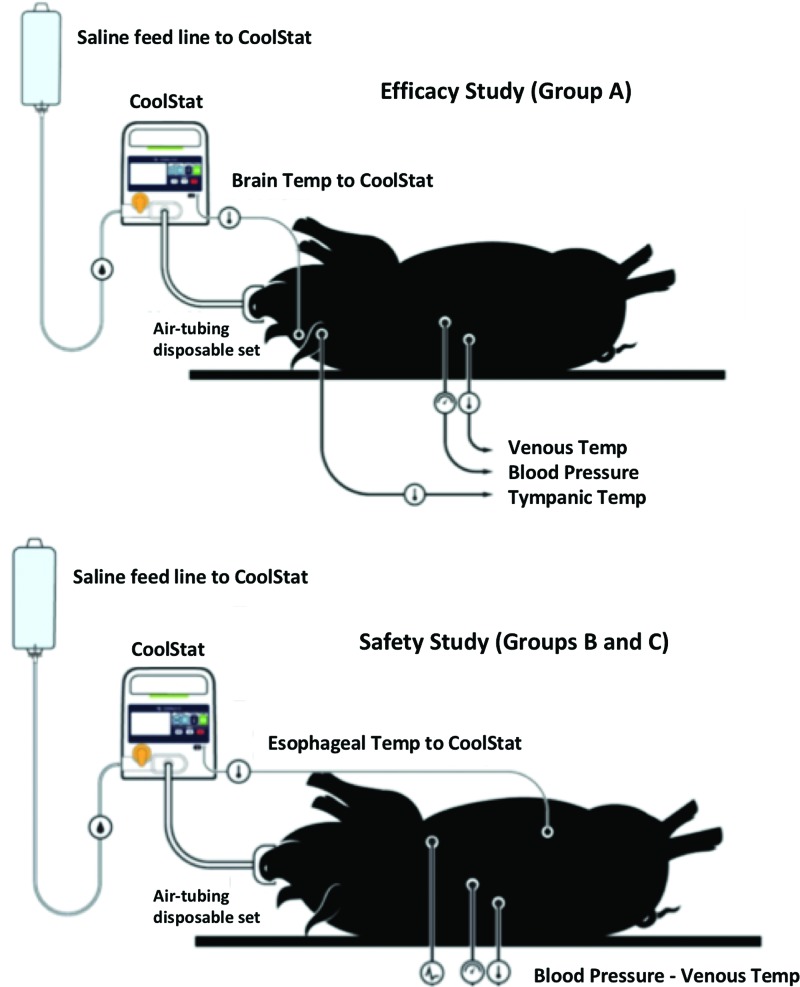
All animals were placed in supine position under general anesthesia, intubated, and kept sedated with isoflurane (1%) and 100% O2 support. Vascular access was obtained in femoral position (arterial and venous lines). Systemic blood pressure (given by mean arterial pressure [MAP]), heart rate (HR), ECG, respiratory rate, SPO2, and end-tidal CO2 (ETCO2) were continuously monitored and recorded (ADInstruments, NSW, Australia). Venous temperature was monitored and recorded in all animals. Temperature probe signals were measured using Neoptix™ Reflex 4-channel signal conditioner and recorded using Neoptix OptiLink™ (Neoptix, QC, Canada). Esophageal, intracranial (brain), and tympanic temperatures were also monitored depending on the cooling strategy (as described below and detailed in Fig. 2).
FIG. 2.
Animal preparation. In group A (top), brain temperature sensor provided the temperature feedback to CoolStat's closed-loop control system. In group B and C (bottom), the core temperature readout was given by an esophageal temperature sensor. Color images are available online.
Transnasal cooling therapy
A nasal custom mask was placed on the animal's nose and secured in place using straps attached to subject's arms. The mask uses a standard nasal pillow designed to create an air seal at the outer surface of the nostrils and is connected to CoolStat through a customized plastic tubing set. Air flow (15–65 L/min) was automatically adjusted using a closed-loop control system as a target temperature was set (groups A and C); in group B, maximum air flow (∼65 L/min) was delivered without temperature control. To prevent severe hypothermia (no temperature control—group B), animals were covered by heating blankets. A mouth spreader was placed to keep the mouth open to allow for air outflow through the mouth.
CoolStat device
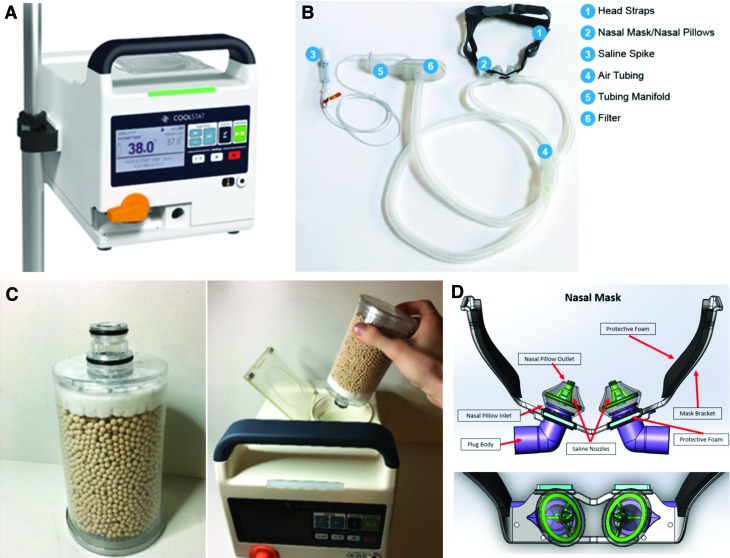
The CoolStat is an investigational device used to extract energy and induce temperature loss by transnasal evaporative cooling (Fig. 3A). The key components inside the device include a primary air blower, a replaceable desiccant cartridge, and in-line filters to dry and clean the air source, as well as flow, pressure, humidity, and temperature sensors, which are integrated into a closed-loop control system. A single-use custom nasal tubing set connects the device to the subject's nose (Fig. 3B). The blower inside the CoolStat is used to pump the inlet air across the desiccant cartridge to extract any moisture from the incoming air stream (dehumidification) (Fig. 3C). The CoolStat induces evaporative cooling by blowing dehumidified ambient air into the nose and over the nasal turbinates in a unidirectional fashion before flowing freely out of the mouth. The primary purpose of the turbinates is to warm and humidify inspired air before it reaches the sensitive tissue of the lungs. The heat transfer involved in liquid to gas phase change of nasal mucous results in heat loss, which in turn is proportional to the air flow rate setting and therapy duration (Chava et al., 2017).
FIG. 3.
Transnasal cooling therapy system. (A) CoolStat device. (B) Nasal mask and plastic tube set (disposable). (C) CoolStat™ disposable desiccant cartridge and loading in device. (D) Nasal mask in detail. Saline nozzles are placed inside the nasal pillows, throughout which small amounts of saline are periodically misted into the nostrils. Color images are available online.
To avoid desiccation of nasal mucosa while applying high flow of dry air, CoolStat mists a small amount of saline solution into the nose during operation. About four to five drops of saline are misted into the nose, about once every 10 to 15 seconds at air flow rates of about 60 L/min. The saline mist is proportional to the air flow rate (less saline is used at lower air flow rates), wherein a peristaltic pump controls saline solution delivery into the nose through custom misting nozzles embedded in the nasal mask (Fig. 3D).
The CoolStat device can be operated in either an open-loop or a closed-loop cooling mode. In the open-loop mode, the magnitude of the air flow of the device is set by the operator over a range of five flow settings (from low to high), limited by the maximum pressure supply of the device to ensure safety (30 cmH2O). In the closed-loop control mode, the device has a feedback control that uses the patient's temperature readout to control the temperature. The device continuously monitors both pressure and temperature that is supplied to the subject in real time and will reduce or turn air flow off to ensure that neither limit is exceeded.
Efficacy assessment (primary objective): group A
The primary objective of this study is to assess the efficacy of CoolStat transnasal cooling therapy in induction and maintenance of hypothermia using a closed-loop target temperature control.
Following animal preparation, a fiberoptic temperature probe (Neoptix) was advanced into the brain parenchyma under fluoroscopic guidance through a standard parietal burr hole to monitor and record brain temperature (parietal cortex). Target temperature was set as a 2°C drop in baseline temperature (minimum temperature limited at 33°C). Data collection (MAP, HR, ECG, SPO2 and brain, tympanic, and intravascular temperatures) was initiated 30 minutes before cooling therapy onset. The CoolStat was initiated and held running in closed-loop control mode for 8 hours. Saline bags and desiccant cartridge were replaced if necessary. After therapy termination, the animal was monitored for an additional 30 minutes and data were collected. CoolStat's efficacy was assessed by observing the time of therapy to achieve the target temperature and the percentage of time in which the temperature ranged within ±0.5°C after reaching the target temperature. Hemodynamic and respiratory test parameters were also evaluated.
Safety assessment (secondary objective): groups B and C
As a secondary objective of the study, we assessed the safety of CoolStat cooling therapy under two worst-case simulations, wherein hemodynamic tolerance during cooling therapy and therapy-related adverse events were evaluated.
Instead of using brain and tympanic temperature sensors as described in a prior section (group A), in groups B and C an esophageal temperature probe (Zoll, MA) was properly placed and connected to CoolStat.
Animals were grouped according to the cooling therapy strategy adopted. In group B (three animals), an 8-hour study was performed with continuous maximum air inflow rate (∼65 L/min), driven by an open-loop cooling mode set (no temperature control). In group C (three animals), a 24-hour study was performed with variable flow rate, adjusted by a closed-loop temperature control mode (34°C). After the protocol, an endoscopic inspection of the nasal mucosa was performed by a board-certified otolaryngologist in all animals to evaluate damage to the nasal mucosa and collateral tissue structures, based on the Western Consortium for Cancer Nursing Research (WCCNR). Mucosal lesions greater than Grade 2 (WCCNR) would be forwarded to histological analysis.
Statistical methods
Continuous variables are expressed as either mean ± standard deviation. SPSS Version 24 (IBM, IL) was used for statistical analysis.
Results
Efficacy study: group A
All animals completed the protocol. The closed-loop control system successfully induced and sustained managed hypothermia.
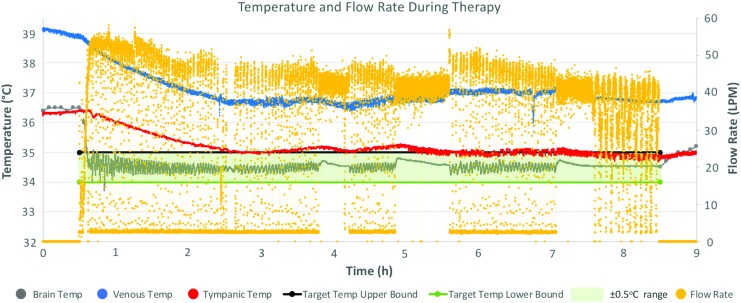
As shown in Table 1, target brain temperature was achieved in all subjects (0.5 ± 0.6 hours). After the target temperature was reached, CoolStat's closed-loop control system was able to maintain the temperature within a range of ±0.5°C (99.9% ± 0.1%) in all subjects. Temperature-based autoregulated air flow (26 ± 9.5 L/min) allowed for reliable control of brain temperature during cooling therapy. A 1.0°C ± 0.2°C increase in brain temperature was observed 30 minutes after cooling therapy was terminated. In Figure 4, the interaction among brain, tympanic, and vascular temperatures is illustrated for one of the subjects, with temperature fluctuations triggering air flow rate adjustments to achieve adequate cooling.
Table 1.
Results of Transnasal Cooling Therapy in All Subjects
| Efficacy study | Safety study | ||
|---|---|---|---|
| Group A (n = 5) | Group B (n = 3) | Group C (n = 3) | |
| Duration of therapy (hours) | 8 | 8 | 24 |
| Closed-loop temperature control | Enabled | Disabled | Enabled |
| Average airflow (L/min) | 26 ± 9.5 | 65a | 23.4 ± 5.3 |
| Target temperature readout | Brain | Esophagus | Esophagus |
| Time (hours) to target temperature | 0.5 ± 0.6 | NA | 1.5 ± 1.4 |
| % of time within ±0.5°C of target temperature after reached | 99.9 ± 0.1 | NA | 97.7 ± 3.3 |
| Brain temperature (°C) recovery at 30 minutes after therapy termination | 1.0 ± 0.2 | NA | NA |
| Subjects with identified nasal mucosal damage (WCCNR > Grade 1) | NA | 0 | 0 |
CoolStat's closed-loop temperature control was able to successfully induce and maintain targeted temperature management with minimal variation, regardless of the duration of therapy (groups A and C). Target temperature was achieved faster in the brain when compared with esophagus (core). A mean 1.0°C rise in brain temperature was observed 30 minutes after therapy offset. No significant mucosal damage was found in safety assessment (groups B and C). Values are given by mean ± standard deviation.
Fixed flow rate.
NA, not applicable; WCCNR, Western Consortium for Cancer Nursing Research.
FIG. 4.
Temperature and airflow rate during transnasal cooling therapy using closed-loop temperature control mode (group A, subject 5A). A high baseline temperature required a higher airflow rate during the first hour of therapy. During the fourth, fifth, and seventh hour of the study, a rise in brain temperature resulted in an upshift in flow rate scattering (higher density of yellow points along the upper flow rates). Once temperature control was properly reachieved, the flow rate distribution returned to previous pattern. We can also observe the close relationship between the tympanic and brain temperature readings and how the vascular temperature reflects their changes. Color images are available online.
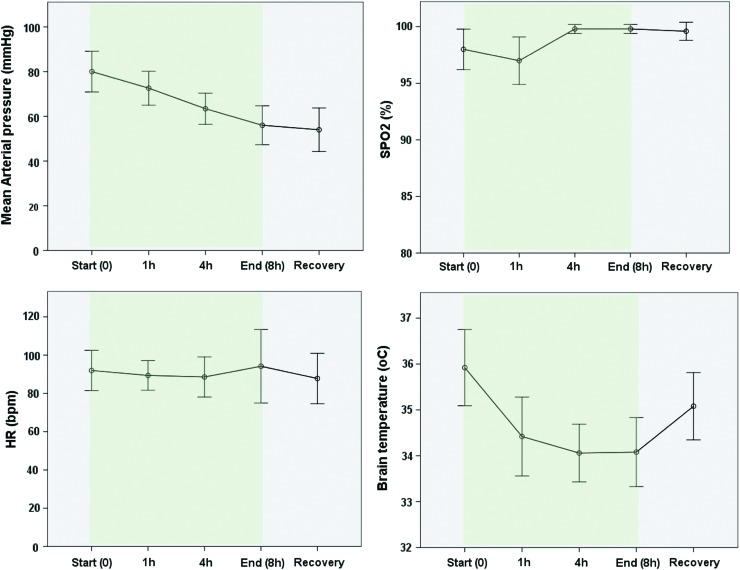
Despite small reduction in MAP along the study, no significant hemodynamic distress associated to transnasal cooling therapy was observed, and hemodynamic and respiratory parameters behaved as expected for prolonged use of general anesthesia (Fig. 5).
FIG. 5.
Hemodynamic and respiratory performances during CoolStat cooling therapy in group A (efficacy study). Mean arterial pressure drop is shown along the cooling therapy (prolonged anesthesia) without impact in heart rate (HR) or SPO2. CoolStat produced an important reduction in brain temperature during the first hour of therapy and remained stable during the remainder of the study. Termination of the therapy allowed for quick brain rewarming after 30 minutes recovery. Green shading area indicates the duration of cooling therapy. Color images are available online.
Safety study: groups B and C
All six animals in groups B and C (three in each group) completed the planned duration of CoolStat treatment (8 and 24 hours, respectively). Hypothermia was successfully induced in all subjects and generally well tolerated regardless the strategy employed. In concordance with the observations in group A, hemodynamic test parameters for all six subjects remained as expected during the study, considering the prolonged use of general anesthesia (up to 24 hours).
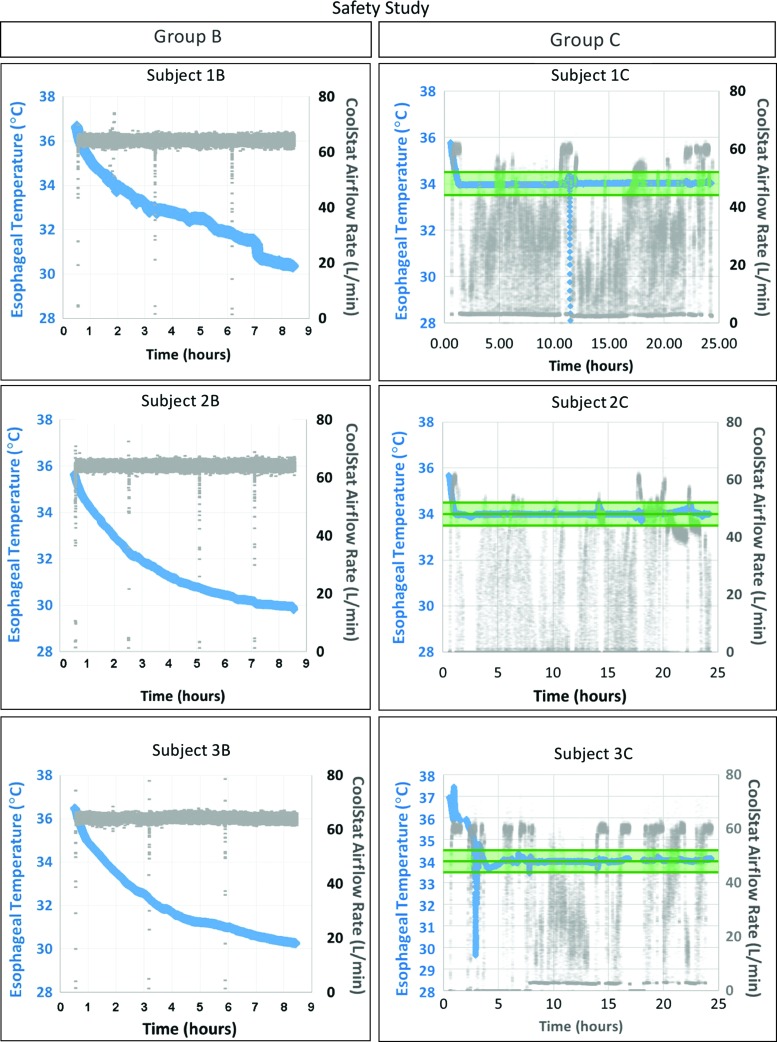
Shown in Figure 6 are the results from all safety studies (see also Table 1 for further details). In group B, high air flow rate (∼65 L/min) was successfully delivered to all subjects (open-loop temperature control mode), leading to a minimum core temperature of 30.2°C ± 0.2°C, despite the use of electric heating blankets to prevent severe hypothermia. In group C, target core temperature was achieved in all animals (1.5 ± 1.4 hours), with a mean airflow of 23.4 ± 5.3 L/min (closed-loop temperature control).
FIG. 6.
CoolStat safety study. Group B (left column) shows constant maximum airflow and continued core temperature decrease. Group C (right column) shows high airflow until achievement of target temperature (34.0°C), followed by intermittent airflow to maintain core temperature in the target range (34.0°C ± 0.5°C; indicated by green shading). In subjects 1C and 3C, a sharp drop in esophageal temperature reading was associated to transient esophageal temperature probe dislodgement (not device related). Color images are available online.
No significant damage to the nasal mucosa or collateral structures was observed under endoscopic exam in neither of the groups (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ther). No mucosal damage Grade 2 or higher on WCCNR scale was found and, therefore, no histological samples were collected.
Discussion
This study demonstrates the efficacy of CoolStat device in inducing and consistently maintaining controlled hypothermia by unidirectional transnasal high flow dry air using a closed-loop temperature control system. Furthermore, in extreme-condition simulations, CoolStat was able to safely deliver dry air either in high flow rate (∼65 L/min) or during longer periods of time (24 hours) without any damage to the nasal mucosa.
TTM and its neuroprotective effects have been previously reported in preclinical and clinical studies, all collectively standing for reduction of neurological injury. Well-conducted randomized clinical trials have also shown the benefits of induced hypothermia in comatose survivors of cardiac arrest, supporting updated guideline recommendations of the American Heart Association for early TTM after cardiac arrest (Callaway et al., 2015). Along with the clinical validation of systemic (whole-body) hypothermia in global ischemic settings, selective brain cooling seems to emerge as a safe alternative in focal brain injury and has shown promising results in stroke animal models (Park et al., 1998; Fingas et al., 2007; Auriat et al., 2012). Local cooling allows for minor core temperature drop, which seems to reduce systemic adverse effects associated to whole-body hypothermia, such as shivering (Covaciu et al., 2008). Nevertheless, further investigation is still warranted to support its clinical use.
Several methods are clinically available for TTM, including noninvasive and invasive strategies; however, overall results remain suboptimal. Cold fluid infusion has fallen out of favor due to the risk of volume overload and rearrest (Grave et al., 2016). Intravascular cooling is more efficient than surface cooling, but associated with delay in hypothermia initiation, high cost, and access-related adverse events. As such, there is enthusiasm and a need for early hypothermia initiation device that is simple to use, effective, and can be used in an out of a hospital setting.
Transnasal evaporative cooling has emerged as a promising ambulatory TTM method. Intrinsic mechanisms of local blood flow redistribution and air flow rate variations into nasal mucosa can not only modulate brain temperature, but also contribute to blood and core temperature regulation. High-flow oxygen through the upper airways of intubated rats resulted in a flow-dependent decrease in brain temperature (Einer-Jensen and Khorooshi, 2000), which was later confirmed in porcine models using transnasal dry air (Chava et al., 2017). This study showed that the rate of brain cooling was significantly higher at higher airflow rates, independent of the air temperature, and was eliminated by humidifying the air, consistent with principles of evaporative cooling. In clinical settings, a 0.2°C reduction in brain temperature by a 5–10 L/min unilateral nasal oxygen inflow was demonstrated in trauma patients with hemorrhage (Mellergård, 1992). However, in a study enrolling 15 patients with the same characteristics, no change in brain parenchymal or subdural temperatures was induced by nasal humidified air inflow (cannula) (Andrews et al., 2005). Those inconsistent results may be explained by the employment of humidified air at low flow rates.
Evaporation of nasal mucus (energy consuming process) by the dry air seems to drive the cooling effect and is enhanced at higher flow rates. More recently, transnasal evaporative cooling using PFC nasal spray along with high-flow oxygen, delivered through long nasal prongs that are inserted deep into the nostrils, has shown encouraging results as a noninvasive/minimally invasive method for TTM (RhinoChill™) (Busch et al., 2008; Castren et al., 2010).
In our study, CoolStat promoted fast and reliable targeted brain and core hypothermia using ambient dehumidified air without any additional chemical components. The latent heat of vaporization of water is 25 times that of PFC, which allows for a more efficient, safe, and cost-effective cooling mechanism (Choi and Cho, 1999). Ambient air is captured, dehumidified, and safely propelled at a high flow rate with a lower upper limit of pressure (30 cmH2O), harnessing a physiological process of air humidification to extract energy from the nasal passages and subsequent body heat loss by convective cooling. This process, in porcine models, appears to selectively cool the brain before cooling the body and, thus, also provides a unique model to study the effects of selective brain cooling compared with whole-body cooling using this noninvasive approach.
CoolStat's closed-loop control system was able to efficiently manage target temperature regardless of the duration of treatment. Nasal mucosa integrity, respiratory parameters, and hemodynamic status remained preserved in all subjects, even when employed in extreme settings as maximum air inflow or long duration therapy. Finally, along with the efficacy and safety profiles, CoolStat portability may allow for intra-arrest cooling, which is currently not feasible with the available cooling technologies.
Limitations
A single-site monitoring of brain temperature was employed in this study. Thereby, cooling distribution along the brain during CoolStat TTM was not addressed and requires further investigation. Additionally, a rewarming strategy was not included in this study. Although the rate of rewarming can also be regulated by gradually adjusting the airflow rate, this feature was not specifically addressed in this study.
Conclusion
In this study, a novel device (CoolStat) was able to efficiently induce and maintain hypothermia using unidirectional high flow of dry air into the nostrils of porcine models. In addition to that, extreme-condition simulations (worst-case scenario) were well tolerated, accounting for the safety of CoolStat TTM.
Supplementary Material
Acknowledgments
The authors wish to acknowledge William DeMore, Chet Larrow, Sarah Fink, and Rick Tunin for their valuable contribution to this study.
This research was made possible by NIH SBIR (R44 HL108542) grant funding (H.T.) and in part through SBIR funding of the Marine Corps Systems Command. The content provided herein is solely the responsibility of the authors and does not necessarily represent the official views of the Marine Corps, Key Technologies, Inc, or the DoD.
Author Disclosure Statement
Harikrishna Tandri is the founder of CoolTech LLC, which is developing a transnasal device for hypothermia. The remainder of the authors declare no competing financial interests exist.
References
- Abou-Chebl A, Sung G, Barbut D, Torbey M. Local brain temperature reduction through intranasal cooling with the RhinoChill device: preliminary safety data in brain-injured patients. Stroke 2011;42:2164–2169 [DOI] [PubMed] [Google Scholar]
- Andrews P, Harris B, Murray G. Randomized controlled trial of effects of the airflow through the upper respiratory tract of intubated brain-injured patients on brain temperature and selective brain cooling. Br J Anaesth 2005;94:330–335 [DOI] [PubMed] [Google Scholar]
- Auriat AM, Klahr AC, Silasi G, Maclellan CL, Penner M, Clark DL, et al. Prolonged hypothermia in rat: a safety study using brain-selective and systemic treatments. Ther Hypothermia Temp Manag 2012;2:37–43 [DOI] [PubMed] [Google Scholar]
- Azzimondi G, Bassein L, Nonino F, Fiorani L, Vignatelli L, Re G, et al. Fever in acute stroke worsens prognosis. A prospective study. Stroke 1995;26:2040–2043 [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563 [DOI] [PubMed] [Google Scholar]
- Busch H-J, Janata A, Eichwede F, Födisch M, Wöbker G, Stephan T, et al. Abstract P63: safety and feasibility of a new innovative cooling approach for immediate induction of therapeutic hypothermia in patients after successful resuscitation. Trans-nasal cooling after cardiac arrest. Circulation 2008;118: S_1459 [Google Scholar]
- Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: post–cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S465–S482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010;122:729–736 [DOI] [PubMed] [Google Scholar]
- Chava R, Zviman M, Raghavan MS, Halperin H, Maqbool F, Geocadin R, et al. Rapid induction of therapeutic hypothermia using transnasal high flow dry air. Ther Hypothermia Temp Manag 2017;7:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Cho K. Thermal characteristics of a multichip module using PF-5060 and water. KSME Int J 1999;13:443–450 [Google Scholar]
- Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res 1994;654:265–272 [DOI] [PubMed] [Google Scholar]
- Covaciu L, Allers M, Enblad P, Lunderquist A, Wieloch T, Rubertsson S. Intranasal selective brain cooling in pigs. Resuscitation 2018;76:83–88 [DOI] [PubMed] [Google Scholar]
- Diringer MN. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med 2004;32:559–564 [DOI] [PubMed] [Google Scholar]
- Einer-Jensen N, Khorooshi M. Cooling of the brain through oxygen flushing of the nasal cavities in intubated rats: an alternative model for treatment of brain injury. Exp Brain Res 2000;130:244–247 [DOI] [PubMed] [Google Scholar]
- Fingas M, Clark DL, Colbourne F. The effects of selective brain hypothermia on intracerebral hemorrhage in rats. Exp Neurol 2007;208:277–284 [DOI] [PubMed] [Google Scholar]
- Grave M-S, Sterz F, Nürnberger A, Fykatas S, Gatterbauer M, Stättermayer AF, et al. Safety and feasibility of the RhinoChill immediate transnasal evaporative cooling device during out-of-hospital cardiopulmonary resuscitation: a single-center, observational study. Medicine 2016;95:e4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J, Tang W, Wang H, Tsai M-S, Li Y, Sun S, et al. Rapid induction of head cooling by the intranasal route during cardiopulmonary resuscitation improves survival and neurological outcomes. Circulation 2018;116:II_529–II_530 [Google Scholar]
- Hajat C, Hajat S, Sharma P. Effects of poststroke pyrexia on stroke outcome: A meta-analysis of studies in patients. Stroke 2000;31:410–414 [DOI] [PubMed] [Google Scholar]
- Haugk M, Sterz F, Grassberger M, Uray T, Kliegel A, Janata A, et al. Feasibility and efficacy of a new non-invasive surface cooling device in post-resuscitation intensive care medicine. Resuscitation 2007;75:76–81 [DOI] [PubMed] [Google Scholar]
- Hoedemaekers CW, Ezzahti M, Gerritsen A, van der Hoeven JG. Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care 2007;11:R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer M. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–556 [DOI] [PubMed] [Google Scholar]
- Kim F, Olsufka M, Carlbom D, Deem S, Longstreth WT, Jr, Hanrahan M, et al. Pilot study of rapid infusion of 2 L of 4 degrees C normal saline for induction of mild hypothermia in hospitalized, comatose survivors of out-of-hospital cardiac arrest. Circulation 2005;112:715–719 [DOI] [PubMed] [Google Scholar]
- Kliegel A, Janata A, Wandaller C, Uray T, Spiel A, Losert H, et al. Cold infusions alone are effective for induction of therapeutic hypothermia but do not keep patients cool after cardiac arrest. Resuscitation 2007;73:46–53 [DOI] [PubMed] [Google Scholar]
- Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med 1993;21:1348–1358 [DOI] [PubMed] [Google Scholar]
- Lyon RM, Robertson C, Clegg G. Therapeutic hypothermia in the emergency department following out-of-hospital cardiac arrest. Emerg Med J 2010;27:418–423 [DOI] [PubMed] [Google Scholar]
- Marine Corps Systems Command contract with Key Technologies, Inc., Contract No. M6785417C6550, Through MARCOR SYSCOM, August 2017 to February 2018, Topic # N171-002: Intranasal Cooling for Encephalopathy Prevention (ICEP)
- Mayer SA, Kowalski RG, Presciutti M, Ostapkovich ND, McGann E, Fitzsimmons BF, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med 2004;32:2508–2515 [DOI] [PubMed] [Google Scholar]
- Mellergård P. Changes in human intracerebral temperature in response to different methods of brain cooling. Neurosurgery 1991;31:671–677 [DOI] [PubMed] [Google Scholar]
- Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post–cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and Prognostication a consensus statement from the international liaison committee on resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008;118:2452–2483 [DOI] [PubMed] [Google Scholar]
- Park C-K, Jun S-S, Kim M-C, Kang J-K. Effects of systemic hypothermia and selective brain cooling on ischemic brain damage and swelling. In: Intracranial Pressure and Neuromonitoring in Brain Injury. Marmarou A, Bullock R, Avezaat C, Baethmann A, Becker D, Brock M, et al., ed. Vienna, Austria: Springer, 1998, pp. 225–228 [DOI] [PubMed] [Google Scholar]
- Patel N, Nair SU, Gowd P, Gupta A, Morris D, Geronilla GG, et al. Central line associated blood stream infection related to cooling catheter in cardiac arrest survivors undergoing therapeutic hypothermia by endovascular cooling. Conn Med 2013;77:35–41 [PubMed] [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet 2008;371:1955–1969 [DOI] [PubMed] [Google Scholar]
- Polderman KH, Callaghan J. Equipment review: cooling catheters to induce therapeutic hypothermia? Crit Care 2006;10:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman KH, Rijnsburger ER, Peerdeman SM, Girbes AR. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit Care Med 2005;33:2744–2751 [DOI] [PubMed] [Google Scholar]
- Seder DB, Van der Kloot TE. Methods of cooling: practical aspects of therapeutic temperature management. Crit Care Med 2009;37:S211–S222 [DOI] [PubMed] [Google Scholar]
- Silfvast T, Tiainen M, Poutiainen E, Roine RO. Therapeutic hypothermia after prolonged cardiac arrest due to non-coronary causes. Resuscitation 2003;57:109–112 [DOI] [PubMed] [Google Scholar]
- Widmann R, Miyazawa T, Hossmann KA. Protective effect of hypothermia on hippocampal injury after 30 minutes of forebrain ischemia in rats is mediated by postischemic recovery of protein synthesis. Journal of neurochemistry 1993;61:200–209 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.